Abstract
Aims
Although comprehensive assessment of right ventricular (RV) function using multiple echocardiographic parameters is recommended for management of patients with non‐ischaemic dilated cardiomyopathy (DCM), it is unclear which RV parameters to combine. Additionally, normalization of RV parameters by estimated pulmonary artery systolic pressure (PASP), in consideration of RV–pulmonary artery coupling, may be clinically significant. The aim of our study was to elucidate the best combination of echocardiographic RV functional parameters, with or without indexing for PASP, to predict outcome in patients with heart failure with reduced ejection fraction secondary to DCM.
Methods and results
We retrospectively analysed 109 DCM patients with left ventricular ejection fraction <40%. RV size was assessed by RV end‐diastolic area (RVEDA) and RV end‐systolic area (RVESA) from RV‐focused apical four‐chamber view. RV function was assessed by fractional area change (FAC) and tricuspid annular plane systolic excursion (TAPSE) and by RV longitudinal strain (RVLS) using two‐dimensional speckle‐tracking echocardiography. All functional parameters were also indexed for estimated PASP. Cox analyses were used to evaluate the association of RV morphology and functional parameters with 1 year outcome (composite of left ventricular assist device implantation and all‐cause death). Area under the curve was used to compare prognostic values. Mean age was 44 ± 14 years, and 76 (69.7%) were men. Mean left ventricular ejection fraction was 21.9%, median RVEDA was 22.1 cm2, FAC was 27.0%, TAPSE was 15.0 mm, and RVLS was −12.5%. Forty‐one (37.6%) patients experienced the primary outcome. Multivariate Cox analysis revealed that RVEDA, RVESA, FAC, TAPSE, RVLS, FAC/PASP, and RVLS/PASP were independent predictors for primary outcome (all P < 0.05). However, normalization with PASP did not improve area under the curve for any RV functional parameters. When we evaluate hazard ratios according to the combination of two echocardiographic parameters of RV function, patients with impairment of both FAC (<27%) and RVLS (>−8.6%) had significantly higher hazard ratio than those with either impairment alone (11.3 vs. 3.4, P < 0.001); the other combinations did not improve prognostic value.
Conclusions
Normalizing echocardiographic RV parameters for PASP did not improve the prognostic values for our population. Meanwhile, combined evaluation of FAC and RVLS improved risk stratification in patients with heart failure with reduced ejection fraction secondary to DCM.
Keywords: Dilated cardiomyopathy, Right ventricular function, Echocardiography, Speckle‐tracking echocardiography, Pulmonary artery
Introduction
Non‐ischaemic dilated cardiomyopathy (DCM) is characterized by left ventricular (LV) dilatation and systolic dysfunction. 1 However, it is widely recognized that patients with DCM frequently have not only LV systolic dysfunction but also right ventricular (RV) systolic dysfunction. 2 Moreover, RV systolic function is an important prognostic predictor of outcome in these patients. 1 , 3 , 4 While cardiovascular magnetic resonance is the gold‐standard technique for the assessment of RV function, echocardiography plays an essential role in the assessment of RV function in a clinical setting.
Right ventricular systolic function is evaluated by echocardiography using several parameters, including fractional area change (FAC), tricuspid annular plane systolic excursion (TAPSE), and RV longitudinal strain (RVLS). 5 , 6 In particular, recent studies have shown the excellent prognostic value of RVLS, which provides angle‐independent assessment of RV function based on two‐dimensional speckle‐tracking echocardiography, compared with the conventional echocardiographic parameters of RV function. 7 , 8 , 9 , 10 Because there are intrinsic limitations for each echocardiographic RV parameter, comprehensive evaluation by multiple RV parameters is ideal. 5 , 6 However, it is not yet clear which parameters should be combined for efficient estimation of RV function.
Right ventricular function is sensitive to change in afterload, known as RV–pulmonary artery coupling, and it is often a challenge to distinguish between a change in RV function due to increased afterload and intrinsic RV myocardial dysfunction in heart failure (HF). 11 , 12 , 13 Recent studies have indicated that load‐independent parameters for assessing RV function such as TAPSE/pulmonary artery systolic pressure (PASP) are useful as prognostic markers in HF. 14 , 15 , 16 , 17 However, direct comparisons of the prognostic values of various RV parameters with or without normalization for PASP are scarce for patients with DCM.
Therefore, the aim of our study was to elucidate the best combination of echocardiographic RV parameters, with or without indexing for PASP, to predict outcome in patients with HF with reduced ejection fraction (HFrEF) secondary to DCM.
Methods
Study subjects
We retrospectively obtained data on patients who underwent echocardiography under the diagnosis of DCM from January 2011 to December 2017. DCM was defined by the presence of LV dilatation and systolic dysfunction in the combined absence of coronary artery disease, specific heart muscle disease, and active myocarditis at endomyocardial biopsy. Coronary artery disease was assessed by invasive coronary angiography, coronary computed tomography, or cardiac magnetic resonance imaging. There were 305 patients who underwent echocardiography with a diagnosis of DCM and had an LV ejection fraction (LVEF) <40%. Exclusion criteria were patients after heart transplant (n = 7); patients who have not undergone coronary angiography or myocardial biopsy (n = 81); patients with diseases other than DCM (n = 31); patients after device implantation (patients with intra‐aortic balloon pump, percutaneous cardiopulmonary support, or LV assist device; implantation with a cardiac resynchronization device in the previous 6 months; n = 51); patients who could not be followed for one year (n = 16); and lack of measurement of tricuspid regurgitation peak gradient (TRPG) or RV parameters (n = 10). Considering the possibility that cardiac function may not be stable immediately after implantation of a cardiac resynchronization device, patients <6 months after implantation of a cardiac resynchronization device were excluded. We included 109 patients with DCM who underwent echocardiography and management of cardiomyopathy in the final statistical analysis (Figure 1 ). Most patients had stable haemodynamic condition and were at well‐compensated phase treated with best of the recommended therapy according to the recent guidelines at the time of echocardiography. 18 Clinical characteristics, laboratory parameters, and echocardiographic parameters were collected in all patients. The median difference between the day of transthoracic echocardiography and the day of laboratory test was 0 days (inter‐quartile range 0–1 days). Body mass index (BMI) was calculated by the formula BMI (kg/m2) = weight (kg)∕[height (m)]2. Estimated glomerular filtration rate was calculated by the following formula: estimated glomerular filtration rate (mL/min/1.73 m2) = 194 × serum creatinine−1.094 × age−0.287 × 0.739 (if female). 19 The primary outcome was defined as a composite of LV assist device implantation and all‐cause death within 1 year. The investigation conforms to the principles outlined in the Declaration of Helsinki, and our data collection protocol was approved by the institutional review board of the University of Tokyo Hospital.
Figure 1.
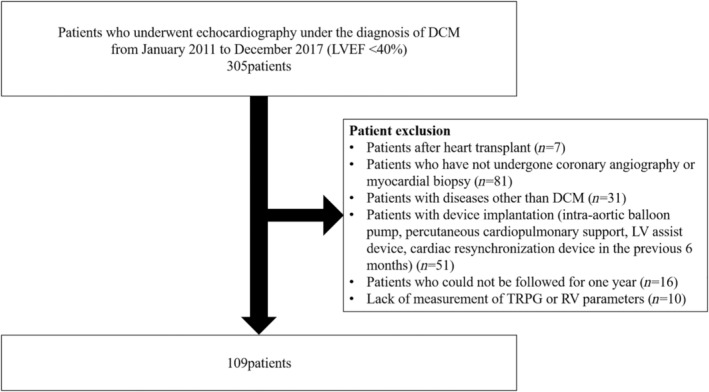
Patient flow diagram. We included 109 patients with dilated cardiomyopathy (DCM) who underwent echocardiography and management of cardiomyopathy in the final statistical analysis. LV, left ventricular; LVEF, left ventricular ejection fraction; RV, right ventricular; TRPG, tricuspid regurgitation peak gradient.
Echocardiographic examination
Participants underwent two‐dimensional echocardiography performed by experienced operators in accordance with the guidelines of the American Society of Echocardiography. 5 Our echocardiography laboratory is maintained according to the guidelines of the Japanese Society of Echocardiography. 20 LV mass was calculated by the following formula: LV mass (g) = 0.8 × {1.04 × [(IVST + LVEDD + PWT)3 − (LVEDD)3]} + 0.6, where IVST is interventricular septum thickness, LVEDD is LV end‐diastolic diameter, and PWT is posterior wall thickness. 5 LV mass was indexed for body surface area.
Left ventricular ejection fraction was evaluated by Simpson's biplane method. We used pulsed‐wave Doppler echocardiography in the apical four‐chamber view to assess early diastolic transmitral flow velocity (E). The early diastolic peak tissue Doppler imaging velocity (e′) was measured in the apical four‐chamber view as an average of septal and lateral wall. The ratio of the E‐wave to the e′ velocity (E/e′) was calculated to evaluate LV filling pressure. 21 Left atrial (LA) volume was usually calculated from apical four‐chamber and two‐chamber views using Simpson's biplane method; when that was not possible, LA volume was measured only from the four‐chamber view. LA volume was then indexed for body surface area. The severity of mitral regurgitation (MR) and tricuspid regurgitation (TR) was assessed using colour flow imaging. Because quantitative assessments of MR and TR were not performed in all patients, qualitative assessments by experienced echocardiologists were conducted. RV size was assessed by RV end‐diastolic area (RVEDA) and RV end‐systolic area (RVESA) from RV‐focused apical four‐chamber view.
Right ventricular contractility was evaluated by FAC, TAPSE, and RVLS according to the guidelines. 5 , 6 FAC was calculated from the RV‐focused apical four‐chamber view by the following formula: FAC (%) = (RVEDA − RVESA)∕RVEDA × 100. TAPSE was measured on the M‐mode tracing obtained from the RV‐focused apical four‐chamber view using the distance of systolic excursion of the RV annular segment along its longitudinal plane. TRPG was calculated from the continuous‐wave Doppler tricuspid valve regurgitant velocity by the simplified Bernoulli equation. PASP was calculated by the following formula: PASP = 4 × (peak velocity of TR)2 + estimated right atrial pressure. Right atrial pressure was based on inferior vena cava diameter and collapsibility, following the guidelines. 5
Speckle‐tracking analysis was performed offline using apical four‐chamber view images. Semi‐automated border detection was performed using vendor‐independent commercially available software (2D Strain Analysis; TOMTEC Imaging System, Unterschleissheim, Germany), and RV borders were tracked throughout the entire cardiac cycle. We performed manual correction when inaccurate endocardium was detected. RVLS was evaluated by longitudinal peak systolic strain of the RV free wall and is represented as a negative number; lower values of RVLS indicate better RV function. A representative image of RVLS is shown in Figure 2 . RV functional parameters were also evaluated as variables adjusted by PASP (i.e. FAC/PASP, TAPSE/PASP, and RVLS/PASP). The reproducibility of RVLS was assessed with intraclass correlation coefficients and Bland–Altman analysis. Excellent correlations were observed in the inter‐observer and intra‐observer variabilities of RVLS in 10 randomly selected patients: r = 0.95 and r = 0.99 for RVLS. In the Bland–Altman analysis, the inter‐observer and intra‐observer variabilities were −0.06 ± 3.22% and −0.31 ± 1.14% for RVLS (mean ± 1.96 SD, respectively).
Figure 2.
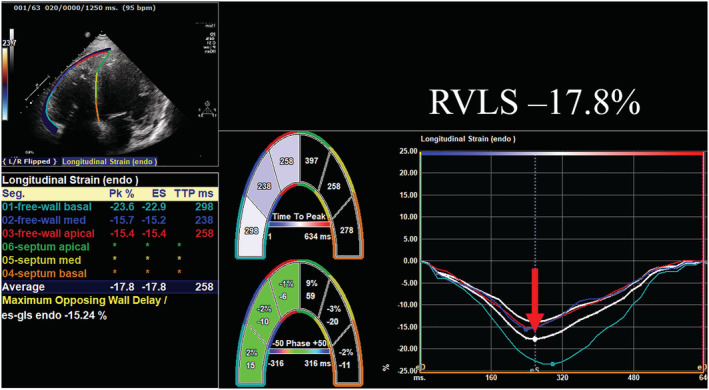
A representative image of right ventricular longitudinal strain (RVLS) in a dilated cardiomyopathy patient. RVLS was reduced to −17.8% suggesting impaired right ventricular systolic function in this case.
Statistical analysis
All data were displayed as the mean ± standard deviation, median (inter‐quartile range), or the number (%) of patients as appropriate. We used Student's t‐test or Wilcoxon test to assess differences between the mean or median values of continuous variables, respectively. We used the χ 2 test for comparisons between groups for categorical variables.
Univariate and multivariate Cox proportional hazards regression analyses were used to evaluate the association between RV parameters and 1 year outcome in a stepwise fashion in three models (entry P‐value of a maximum of 0.05): Model 1: adjustment for age, BMI, New York Heart Association (NYHA) class, systolic blood pressure (BP), and heart rate; Model 2: adjustment for variables as in Model 1 plus haemoglobin (Hb) and use of diuretics; and Model 3: adjustment for variables as in Model 2 plus LVEF, LA volume index (LAVI), and MR severity. Receiver operating characteristic (ROC) curve analysis was used to compare the prognostic value and optimal dichotomous threshold of RV function. Cox analysis was used to compare the hazard ratio of single parameters with the combination of RV parameters. A probability value of P < 0.05 was used to indicate statistical significance. All statistical analyses were conducted utilizing JMP Version 14.0 (SAS Institute, Cary, NC, USA). The validity of these statistical analyses was reviewed by a statistical expert (Tomohiro Shinozaki).
Results
Table 1 shows clinical characteristics of this study population according to primary outcome status. During the first year, 41 (37.6%) patients experienced the primary outcome. Mean LVEF was 21.9%, median RVEDA was 22.1 cm2, FAC was 27.0%, TAPSE was 15.0 mm, and RVLS was −12.5%. Among the clinical and echocardiographic variables, RVEDA, RVESA, FAC, TAPSE, RVLS, FAC/PASP, TAPSE/PASP, and RVLS/PASP were significantly different in patients with and without primary outcome (all P < 0.05). Age, BMI, NYHA class, systolic BP, diastolic BP, LV size, LVEF, MR ≥ moderate, LAVI, TRPG, PASP, total bilirubin, Hb, and diuretics were also significantly different in patients with and without primary outcome (all P < 0.05). RVEDA, RVESA, FAC, TAPSE, RVLS, FAC/PASP, TAPSE/PASP, RVLS/PASP, age, BMI, NYHA class, systolic BP, diastolic BP, heart rate, LVEF, MR ≥ moderate, LAVI, TRPG, PASP, Hb, and diuretics were predictors of prognosis in the univariate Cox proportional hazard model (Table 2 ).
Table 1.
Clinical characteristics
| Clinical characteristics | Primary outcome (n = 41) | No primary outcome (n = 68) | P‐value |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 40.0 ± 14.1 | 46.6 ± 13.9 | 0.02* |
| Male sex, n (%) | 29 (71) | 47 (69) | 1.00 |
| BMI (kg/m2) | 20.9 (18.4–23.2) | 22.7 (19.5–25.8) | 0.03* |
| NYHA Class III or IV, n (%) | 31 (76) | 33 (49) | 0.009* |
| Systolic BP (mmHg) | 86 (80–91) | 97 (90–113) | <0.001* |
| Diastolic BP (mmHg) | 53 (49–61) | 60 (53–71) | <0.001* |
| Heart rate (b.p.m.) | 77 (70–97) | 72 (60–89) | 0.06 |
| ICD or CRTD implantation, n (%) | 15 (37) | 13 (19) | 0.07 |
| Clinical history | |||
| Diabetes mellitus, n (%) | 4 (10) | 10 (15) | 0.56 |
| Hyperlipidaemia, n (%) | 15 (37) | 20 (29) | 0.53 |
| Echocardiographic parameters | |||
| LVDd (cm) | 7.3 ± 1.2 | 6.9 ± 0.9 | 0.03* |
| LVDs (cm) | 6.8 ± 1.3 | 6.2 ± 1.0 | 0.006* |
| LVEDV (mL) | 265.0 (205.0–310.0) | 215.5 (180.3–286.0) | 0.04* |
| LVESV (mL) | 221.0 (150.0–266.5) | 167.0 (127.0–217.0) | 0.01* |
| LV mass index (g/m2) | 135.0 (113.7–171.7) | 132.4 (110.2–161.8) | 0.89 |
| LV ejection fraction (%) | 18.9 ± 7.5 | 23.8 ± 7.3 | 0.001* |
| E/e′ ratio | 13.3 (11.2–19.6) | 12.7 (8.9–18.4) | 0.31 |
| MR ≥ moderate, n (%) | 16 (39) | 8 (12) | 0.002* |
| LA volume index (mL/m2) | 69.0 (53.0–82.3) | 49.0 (35.0–67.0) | <0.001* |
| RVEDA (cm2) | 26.2 (22.2–32.8) | 20.2 (17.4–24.8) | <0.001* |
| RVESA (cm2) | 21.1 (17.9–27.1) | 14.4 (10.0–17.5) | <0.001* |
| FAC (%) | 21.0 (14.5–26.5) | 32.0 (23.0–40.0) | <0.001* |
| TAPSE (mm) | 13.0 (10.0–18.0) | 16.0 (13.0–20.3) | <0.001* |
| RVLS (%) | −9.9 (−14.5 to −7.0) | −13.8 (−21.1 to −10.8) | <0.001* |
| TR ≥ moderate, n (%) | 2 (2.9) | 4 (9.8) | 0.20 |
| TRPG (mmHg) | 27.0 (18.5–38.0) | 22.5 (16.0–29.8) | 0.04* |
| PASP (mmHg) | 38.0 (25.0–48.0) | 27.5 (21.0–34.0) | 0.003* |
| FAC/PASP (%/mmHg) | 0.51 (0.37–0.99) | 1.19 (0.73–1.66) | <0.001* |
| TAPSE/PASP (mm/mmHg) | 0.32 (0.24–0.53) | 0.56 (0.36–0.87) | <0.001* |
| RVLS/PASP (%/mmHg) | −0.26 (−0.52 to −0.16) | −0.53 (−0.72 to −0.32) | <0.001* |
| Laboratory parameters | |||
| AST (U/L) | 21 (18–26) | 23 (19–29) | 0.37 |
| ALT (U/L) | 19 (14–34) | 21 (15–33) | 0.75 |
| Total bilirubin (mg/dL) | 1.0 (0.8–1.4) | 0.8 (0.7–1.1) | 0.04* |
| Hb (g/dL) | 13.0 ± 1.7 | 13.9 ± 1.7 | 0.01* |
| eGFR (mL/min/1.73 m2) | 61.1 (47.6–85.1) | 64.7 (53.2–77.3) | 0.62 |
| Medications | |||
| Beta‐blocker, n (%) | 38 (93) | 64 (94) | 1.00 |
| ACEI or ARB, n (%) | 38 (93) | 57 (84) | 0.24 |
| MRA, n (%) | 31 (76) | 42 (62) | 0.15 |
| Diuretics, n (%) | 39 (95) | 55 (81) | 0.045* |
ACEI, angiotensin‐converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin II receptor blocker; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; CRTD, cardiac resynchronization therapy defibrillator; eGFR, estimated glomerular filtration rate; FAC, fractional area change; Hb, haemoglobin; ICD, implantable cardioverter defibrillator; LA, left atrial; LV, left ventricular; LVDd, left ventricular end‐diastolic diameter; LVDs, left ventricular end‐systolic diameter; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; RVEDA, right ventricular end‐diastolic area; RVESA, right ventricular end‐systolic area; RVLS, right ventricular longitudinal strain; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; TRPG, tricuspid regurgitation pressure gradient.
P < 0.05.
Table 2.
Univariate Cox proportional hazard model
| Variables | HR | 95% CI | P‐value |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 0.98 | 0.96–1.00 | 0.03* |
| Male sex | 1.07 | 0.55–2.10 | 0.83 |
| BMI (kg/m2) | 0.91 | 0.84–0.98 | 0.01* |
| NYHA Class III or IV | 2.82 | 1.38–5.75 | 0.002* |
| Systolic BP (mmHg) | 0.95 | 0.92–0.97 | <0.001* |
| Diastolic BP (mmHg) | 0.96 | 0.93–0.98 | <0.001* |
| Heart rate (b.p.m.) | 1.02 | 1.00–1.03 | 0.02* |
| ICD or CRTD implantation | 1.88 | 1.00–3.55 | 0.06 |
| Clinical history | |||
| Diabetes mellitus | 0.68 | 0.24–1.90 | 0.44 |
| Hyperlipidaemia | 1.21 | 0.64–2.29 | 0.55 |
| Echocardiographic parameters | |||
| LV mass index, g/m2 (HR per 10 g/m2 increase) | 1.00 | 0.94–1.07 | 0.90 |
| LV ejection fraction (%) | 0.92 | 0.88–0.97 | <0.001* |
| E/e′ ratio | 1.02 | 0.98–1.06 | 0.29 |
| MR ≥ moderate | 3.12 | 1.66–5.87 | <0.001* |
| LA volume index (mL/m2) | 1.03 | 1.01–1.04 | <0.001* |
| RVEDA (cm2) | 1.08 | 1.04–1.11 | <0.001* |
| RVESA (cm2) | 1.09 | 1.06–1.13 | <0.001* |
| FAC (%) | 0.92 | 0.89–0.95 | <0.001* |
| TAPSE (mm) | 0.87 | 0.81–0.94 | <0.001* |
| RVLS (%) | 1.15 | 1.08–1.24 | <0.001* |
| TR ≥ moderate | 2.59 | 0.92–7.28 | 0.11 |
| TRPG (mmHg) | 1.04 | 1.01–1.06 | 0.007* |
| PASP (mmHg) | 1.04 | 1.02–1.06 | <0.001* |
| FAC/PASP (%/mmHg) | 0.20 | 0.09–0.39 | <0.001* |
| TAPSE/PASP (mm/mmHg) | 0.10 | 0.02–0.36 | <0.001* |
| RVLS/PASP (%/mmHg) | 18.60 | 4.64–93.53 | <0.001* |
| Laboratory parameters | |||
| AST, U/L (HR per 10 U/L) | 0.95 | 0.70–1.24 | 0.74 |
| ALT, U/L (HR per 10 U/L) | 1.00 | 0.86–1.11 | 1.00 |
| Total bilirubin (mg/dL) | 1.25 | 0.81–1.71 | 0.27 |
| Hb (g/dL) | 0.80 | 0.66–0.95 | 0.01* |
| eGFR (mL/min/1.73 m2) | 1.01 | 0.99–1.02 | 0.39 |
| Medications | |||
| Beta‐blocker | 0.66 | 0.20–2.13 | 0.51 |
| ACEI or ARB | 2.11 | 0.65–6.83 | 0.17 |
| MRA | 1.77 | 0.87–3.62 | 0.10 |
| Diuretics | 3.65 | 0.88–15.14 | 0.03* |
ACEI, angiotensin‐converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin II receptor blocker; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; CI, confidence interval; CRTD, cardiac resynchronization therapy defibrillator; eGFR, estimated glomerular filtration rate; FAC, fractional area change; Hb, haemoglobin; HR, hazard ratio; ICD, implantable cardioverter defibrillator; LA, left atrial; LV, left ventricular; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; RVEDA, right ventricular end‐diastolic area; RVESA, right ventricular end‐systolic area; RVLS, right ventricular longitudinal strain; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; TRPG, tricuspid regurgitation pressure gradient.
P < 0.05.
Multivariate Cox proportional hazard analyses were performed with each RV parameter tested separately for possible association with primary outcome. When we adjusted for age, BMI, NYHA class, systolic BP, and heart rate, all RV functional variables were independent predictors of prognosis (Table 3 , Model 1). In the multivariate analysis adjusted for variables as in Model 1 plus Hb and diuretics, FAC, TAPSE, RVLS, FAC/PASP, and RVLS/PASP remained independent predictors of prognosis (Table 3 , Model 2). In the full‐adjusted model including variables in Model 2 plus LVEF, LAVI, and severity of MR, this result persisted (Table 3 , Model 3). In terms of RV size, RVEDA and RVESA were also independently associated with primary outcome in the multivariable model.
Table 3.
Association of RV parameters, one at a time with primary outcome
| Hazard ratio | 95% CI | P‐value | |
|---|---|---|---|
| Model 1 | |||
| RVEDA | 1.08 | 1.04–1.12 | <0.001* |
| RVESA | 1.10 | 1.06–1.14 | <0.001* |
| FAC | 0.92 | 0.89–0.96 | <0.001* |
| TAPSE | 0.90 | 0.82–0.97 | 0.007* |
| RVLS | 1.11 | 1.03–1.21 | 0.005* |
| FAC/PASP | 0.23 | 0.09–0.50 | <0.001* |
| TAPSE/PASP | 0.19 | 0.03–0.82 | 0.02* |
| RVLS/PASP | 8.71 | 1.82–51.79 | 0.005* |
| Model 2 | |||
| RVEDA | 1.08 | 1.04–1.12 | <0.001* |
| RVESA | 1.10 | 1.06–1.14 | <0.001* |
| FAC | 0.93 | 0.89–0.96 | <0.001* |
| TAPSE | 0.91 | 0.83–0.98 | 0.02* |
| RVLS | 1.10 | 1.02–1.20 | 0.01* |
| FAC/PASP | 0.24 | 0.10–0.54 | <0.001* |
| TAPSE/PASP | 0.23 | 0.04–1.04 | 0.06 |
| RVLS/PASP | 7.45 | 1.45–46.23 | 0.02* |
| Model 3 | |||
| RVEDA | 1.09 | 1.05–1.14 | <0.001* |
| RVESA | 1.10 | 1.06–1.15 | <0.001* |
| FAC | 0.93 | 0.89–0.96 | <0.001* |
| TAPSE | 0.91 | 0.82–0.99 | 0.03* |
| RVLS | 1.11 | 1.02–1.21 | 0.01* |
| FAC/PASP | 0.30 | 0.12–0.71 | 0.006* |
| TAPSE/PASP | 0.37 | 0.06–1.71 | 0.21 |
| RVLS/PASP | 6.79 | 1.17–48.64 | 0.03* |
BMI, body mass index; BP, blood pressure; CI, confidence interval; FAC, fractional area change; Hb, haemoglobin; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; RVEDA, right ventricular end‐diastolic area; RVESA, right ventricular end‐systolic area; RVLS, right ventricular longitudinal strain; TAPSE, tricuspid annular plane systolic excursion.
Model 1: adjusted for age, BMI, NYHA ≥ III, systolic BP, and heart rate. Model 2: adjusted for variables as in Model 1 plus Hb and diuretics. Model 3: adjusted for variables as in Model 2 plus LVEF, LAVI, and MR ≥ moderate.
P < 0.05.
We compared the predictive value of RV functional parameters with and without PASP normalization by ROC curve analyses (Table 4 ). There was no significant difference between the AUC of FAC and that of FAC/PASP (0.78 vs. 0.77, P = 0.70); similar results were found between TAPSE and TAPSE/PASP (0.70 vs. 0.73, P = 0.43) and between RVLS and RVLS/PASP (0.73 vs. 0.75, P = 0.66). Among FAC, TAPSE, and RVLS, the AUC of FAC was the highest and the AUC of TAPSE was the lowest, but there was no significant difference between FAC and TAPSE (0.78 vs. 0.70, P = 0.15).
Table 4.
Comparison of AUCs
| Variable | Sensitivity | Specificity | AUC | Variable | Sensitivity | Specificity | AUC | P‐value |
|---|---|---|---|---|---|---|---|---|
| FAC | 0.80 | 0.65 | 0.78 | FAC/PASP | 0.54 | 0.88 | 0.77 | 0.70 |
| TAPSE | 0.44 | 0.86 | 0.70 | TAPSE/PASP | 0.72 | 0.71 | 0.73 | 0.43 |
| RVLS | 0.49 | 0.91 | 0.73 | RVLS/PASP | 0.64 | 0.81 | 0.75 | 0.66 |
AUC, area under the curve; FAC, fractional area change; PASP, pulmonary artery systolic pressure; RVLS, right ventricular longitudinal strain; TAPSE, tricuspid annular plane systolic excursion.
Figure 3 shows hazard ratios according to the combination of two echocardiographic parameters of RV function. FAC < 27%, TAPSE < 12 mm, and RVLS > −8.6% were defined as the best cut‐off values for identifying patients who experienced a 1 year primary outcome by ROC curve analysis. When stratified by FAC and RVLS, we found that patients with impaired FAC and RVLS had a significantly higher hazard ratio compared with patients with impaired FAC or RVLS (11.3 vs. 3.4, P < 0.001) (Figure 3 A ). On the other hand, when stratified by FAC and TAPSE, patients with impaired FAC and TAPSE did not have a significantly higher hazard ratio compared with patients with impaired FAC or TAPSE (7.2 vs. 3.8, P = 0.08) (Figure 3 B ). When stratified by TAPSE and RVLS, a similar result was found (6.9 vs. 3.2, P = 0.08) (Figure 3 C ).
Figure 3.
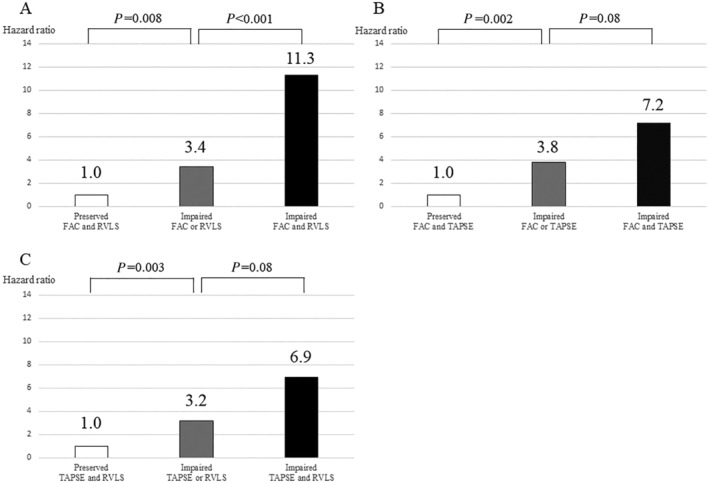
Comparison of hazard ratios between single parameters and the combination of conventional parameters and right ventricular longitudinal strain (RVLS). (A) When stratified by fractional area change (FAC) and RVLS, we found that patients with impaired FAC and RVLS had a significantly higher hazard ratio compared with patients with impaired FAC or RVLS (11.3 vs. 3.4, P < 0.001). (B) On the other hand, when stratified by FAC and tricuspid annular plane systolic excursion (TAPSE), patients with impaired FAC and TAPSE did not have a significantly higher hazard ratio compared with patients with impaired FAC or TAPSE (7.2 vs. 3.8, P = 0.08). (C) When stratified by TAPSE and RVLS, a similar result was found (6.9 vs. 3.2, P = 0.08). Combined evaluation of RV function with FAC and RVLS showed excellent predictive value of outcome compared with other single or combined parameters.
Discussion
Main findings
The main findings of this study are that (i) FAC, TAPSE, and RVLS were independent predictors of prognosis; (ii) normalization for PASP did not significantly improve the prognostic value of RV functional parameters; and (iii) the combination of FAC and RVLS was a better prognostic marker than other single or combined parameters in severe DCM patients.
Prognostic value of right ventricular parameters with and without indexed for pulmonary artery systolic pressure
Several investigators reported the prognostic values of RV parameters with normalization for PASP such as FAC/PASP, TAPSE/PASP, and RVLS/PASP in HF patients, 14 , 15 , 16 , 17 , 22 although conflicting results were observed. Recently, TAPSE/PASP has gained attention not only as a prognostic factor in patients with HF but also as a prognostic factor in patients with coronavirus infectious disease 2019 complicated by an acute respiratory distress syndrome. 23 Guazzi et al. reported that TAPSE/PASP predicted cardiovascular deaths in patients with HFrEF and HF with preserved ejection fraction. 14 Another study showed independent prognostic value of RVLS/PASP in 657 patients with HFrEF and HF with preserved ejection fraction. 16 On the other hand, Frea et al. showed that neither TAPSE/PASP nor FAC/PASP predict cardiovascular outcome in acute decompensation of advanced chronic HF (mean LVEF 22.2%, mean FAC 27.4%, and mean TAPSE 15.1 mm). 22 In our population, RV parameters without normalization for PASP predicted primary outcome including LV assist device implantation and all‐cause death. This finding was consistent with previous reports. 3 , 4 , 7 , 8 , 9 , 24 , 25 , 26 , 27 , 28 However, normalization of RV parameters for PASP did not improve their predictive values. This might be partially explained by the different population enrolled in our study; our patients had more advanced LV and RV dysfunction and did not have ischaemic aetiology. Furthermore, patients included in the present study had relatively lower PASP compared with the previous studies, 14 , 16 , 22 which might attenuate the predictive values of RV parameters with normalization for PASP.
Clinical utility of the combination of right ventricular parameters
The prognostic value of FAC, TAPSE, or RVLS alone in HF was previously reported, 3 , 4 , 7 , 8 , 9 , 24 , 25 , 26 , 27 , 28 and the clinical utility of assessing RV function in HF has been established. However, the prognostic value of combined echocardiographic RV parameters remained unclarified, despite comprehensive evaluation by multiple echocardiographic parameters being recommended for the assessment of RV function. We therefore evaluated the prognostic value of combinations of RV parameters in the current study. We found that patients with impairment of FAC (<27%) and RVLS (>−8.6%) had significant higher hazard ratio compared with patients with impaired FAC or RVLS alone. Although the combination of FAC and TAPSE and of RVLS and TAPSE tended to increase the prognostic value, there was no statistically significant difference.
Prihadi et al. evaluated the incremental prognostic value of RVLS in patients with significant functional TR. 10 They found that RVLS significantly improved prognostic value over FAC. This finding was consistent with our results. A possible explanation is that FAC quantifies radial as well as longitudinal shortening and RVLS is angle independent and provides a more global assessment of RV function, whereas TAPSE is an angle dependent and regional parameter. 4 , 6 , 16 , 29 , 30 , 31 In patients with severe DCM, the combination of RVLS and FAC, providing assessment of both longitudinal and radial RV contraction, may have better prognostic value than RVLS or FAC alone. Moreover, RV global longitudinal strain (RVGLS) that includes longitudinal strain measurements of the interventricular septum is susceptible of LV systolic motion, whereas RVLS is less susceptible of LV systolic function. 32 Thus we did not use RVGLS but RVLS. Similar to RVGLS, FAC is affected by septal motion. By combining FAC and RVLS, each may be able to compensate for the other's limitation.
Clinical implications
In the present study, the prognostic values of FAC, TAPSE, and RVLS alone were not significantly different from each other with or without normalization for PASP. However, by measuring both FAC and RVLS, we could stratify the high‐risk group in a way that could not be obtained with evaluation of a single parameter of RV function. The assessment of both FAC and RVLS may well have clinical implications for better management of DCM.
Limitations
Our study has several limitations. First, this study included only a relatively small number of patients in a single centre. In addition, the number of patients implanted with implantable cardioverter defibrillator is relatively small compared with previous studies particularly those targeting at ischaemic HF patients, which might not allow generalization to patients with different demographic composition and risk profiles. Second, this was a retrospective study, and a prospective observational study is needed to confirm this result. Third, there may have been patient selection bias. In our hospital, we manage a large number of advanced HF patients and perform the highest number of heart transplantations among Japanese hospitals. As a result, young patients who need heart transplants are often brought to our hospital; this may be why younger age was significantly associated with worse outcome in this study. Fourth, complete quantitative evaluation of valvular regurgitation has not been performed in all patients, which might lead to misclassification of the severity of MR/TR, although it was evaluated by experienced echocardiologists. Furthermore, severe TR can cause underestimation of PASP, which might affect our observations. Finally, RV function was not evaluated by three‐dimensional echocardiography. Because the right ventricle has a complex geometry, it is ideal to perform a three‐dimensional evaluation.
Conclusions
In conclusion, FAC, TAPSE, and RVLS were independent predictors of prognosis even after adjustment for clinical and echocardiographic variables in advanced DCM patients. On the other hand, there was no significant difference in the diagnostic accuracies between RV parameters with and without normalization for PASP. Combined evaluation using FAC and RVLS serves as a better prognostic predictor than other single or combined parameters for stratifying high risk in patients with DCM.
Conflict of interest
None declared.
Funding
This work was supported by a Grant‐in‐Aid for Scientific Research C (18K12098) from the Japan Society for the Promotion of Science (M.D.).
Author contributions
J.I., M.D., K.N., and T.S. (Tadafumi Sugimoto) contributed to the conception or design of the work. J.I., M.D., K.N., T.K., E.A., M.H. (Masaru Hatano), H.M., and I.K. contributed to the acquisition, analysis, or interpretation of data for the work. J.I. drafted the manuscript. T.S. (Tomohiro Shinozaki) designed and reviewed the statistical part of this study. M.D., K.N., T.S. (Tadafumi Sugimoto), T.K., T.S. (Tomohiro Shinozaki), T.N., M.H. (Megumi Hirokawa), N.S., Y.Y. (Yuriko Yoshida), E.A., M.H. (Masaru Hatano), H.M., Y.Y. (Yutaka Yatomi), and I.K. critically revised the manuscript. All the authors gave final approval and agreed to be accountable for all aspects of work, ensuring its integrity and accuracy.
Ishiwata, J. , Daimon, M. , Nakanishi, K. , Sugimoto, T. , Kawata, T. , Shinozaki, T. , Nakao, T. , Hirokawa, M. , Sawada, N. , Yoshida, Y. , Amiya, E. , Hatano, M. , Morita, H. , Yatomi, Y. , and Komuro, I. (2021) Combined evaluation of right ventricular function using echocardiography in non‐ischaemic dilated cardiomyopathy. ESC Heart Failure, 8: 3947–3956. 10.1002/ehf2.13519.
This study was performed in the University of Tokyo Hospital, Tokyo, Japan.
References
- 1. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008; 29: 270–276. [DOI] [PubMed] [Google Scholar]
- 2. Gulati A, Ismail TF, Jabbour A, Alpendurada F, Guha K, Ismail NA, Raza S, Khwaja J, Brown TD, Morarji K, Liodakis E, Roughton M, Wage R, Pakrashi TC, Sharma R, Carpenter JP, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation 2013; 128: 1623–1633. [DOI] [PubMed] [Google Scholar]
- 3. Venner C, Selton‐Suty C, Huttin O, Erpelding ML, Aliot E, Juillière Y. Right ventricular dysfunction in patients with idiopathic dilated cardiomyopathy: prognostic value and predictive factors. Arch Cardiovasc Dis 2016; 109: 231–241. [DOI] [PubMed] [Google Scholar]
- 4. Kawata T, Daimon M, Kimura K, Nakao T, Lee SL, Hirokawa M, Kato TS, Watanabe M, Yatomi Y, Komuro I. Echocardiographic assessment of right ventricular function in routine practice: which parameters are useful to predict one‐year outcome in advanced heart failure patients with dilated cardiomyopathy? J Cardiol 2017; 70: 316–322. [DOI] [PubMed] [Google Scholar]
- 5. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–271. [DOI] [PubMed] [Google Scholar]
- 6. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. [DOI] [PubMed] [Google Scholar]
- 7. Guendouz S, Rappeneau S, Nahum J, Dubois‐Randé JL, Gueret P, Monin JL, Lim P, Adnot S, Hittinger L, Damy T. Prognostic significance and normal values of 2D strain to assess right ventricular systolic function in chronic heart failure. Circ J 2012; 76: 127–136. [DOI] [PubMed] [Google Scholar]
- 8. Cameli M, Righini FM, Lisi M, Bennati E, Navarri R, Lunghetti S, Padeletti M, Cameli P, Tsioulpas C, Bernazzali S, Maccherini M, Sani G, Henein M, Mondillo S. Comparison of right versus left ventricular strain analysis as a predictor of outcome in patients with systolic heart failure referred for heart transplantation. Am J Cardiol 2013; 112: 1778–1784. [DOI] [PubMed] [Google Scholar]
- 9. Carluccio E, Biagioli P, Alunni G, Murrone A, Zuchi C, Coiro S, Riccini C, Mengoni A, D'Antonio A, Ambrosio G. Prognostic value of right ventricular dysfunction in heart failure with reduced ejection fraction: superiority of longitudinal strain over tricuspid annular plane systolic excursion. Circ Cardiovasc Imaging 2018; 11: e006894. [DOI] [PubMed] [Google Scholar]
- 10. Prihadi EA, van der Bijl P, Dietz M, Abou R, Vollema EM, Marsan NA, Delgado V, Bax JJ. Prognostic implications of right ventricular free wall longitudinal strain in patients with significant functional tricuspid regurgitation. Circ Cardiovasc Imaging 2019; 12: e008666. [DOI] [PubMed] [Google Scholar]
- 11. Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute Working Group on cellular and molecular mechanisms of right heart failure. Circulation 2006; 114: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 12. Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation 2012; 126: 975–990. [DOI] [PubMed] [Google Scholar]
- 13. Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014; 35: 3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, Temporelli PL, Arena R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Circ Physiol 2013; 305: H1373–H1381. [DOI] [PubMed] [Google Scholar]
- 15. Guazzi M, Dixon D, Labate V, Beussink‐Nelson L, Bandera F, Cuttica MJ, Shah SJ. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging 2017; 10: 1211–1221. [DOI] [PubMed] [Google Scholar]
- 16. Bosch L, Lam CSP, Gong L, Chan SP, Sim D, Yeo D, Jaufeerally F, Leong KTG, Ong HY, Ng TP, Richards AM, Arslan F, Ling LH. Right ventricular dysfunction in left‐sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail 2017; 19: 1664–1671. [DOI] [PubMed] [Google Scholar]
- 17. Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, Temporelli PL, Rossi A, Faggiano P, Traversi E, Vriz O, Dini FL. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail 2017; 19: 873–879. [DOI] [PubMed] [Google Scholar]
- 18. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016; 37: 2129–2200.27206819 [Google Scholar]
- 19. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 20. Daimon M, Akaishi M, Asanuma T, Hashimoto S, Izumi C, Iwanaga S, Kawai H, Toide H, Hayashida A, Yamada H, Murata M, Hirano Y, Suzuki K, Nakatani S. Guideline from Japanese Society of Echocardiography: 2018 focused update incorporated into guidance for the management and maintenance of echocardiography equipment. J Echocardiogr 2018; 16: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321–1360. [DOI] [PubMed] [Google Scholar]
- 22. Frea S, Pidello S, Bovolo V, Iacovino C, Franco E, Pinneri F, Galluzzo A, Volpe A, Visconti M, Peirone A, Morello M, Bergerone S, Gaita F. Prognostic incremental role of right ventricular function in acute decompensation of advanced chronic heart failure. Eur J Heart Fail 2016; 18: 564–572. [DOI] [PubMed] [Google Scholar]
- 23. D'Alto M, Marra AM, Severino S, Salzano A, Romeo E, De Rosa R, Stagnaro FM, Pagnano G, Verde R, Murino P, Farro A, Ciccarelli G, Vargas M, Fiorentino G, Servillo G, Gentile I, Corcione A, Cittadini A, Naeije R, Golino P. Right ventricular‐arterial uncoupling independently predicts survival in COVID‐19 ARDS. Crit Care 2020; 24: 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zornoff LA, Skali H, Pfeffer MA, St John Sutton M, Rouleau JL, Lamas GA, Plappert T, Rouleau JR, Moyé LA, Lewis SJ, Braunwald E, Solomon SD. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol 2002; 39: 1450–1455. [DOI] [PubMed] [Google Scholar]
- 25. Merlo M, Gobbo M, Stolfo D, Losurdo P, Ramani F, Barbati G, Pivetta A, Di Lenarda A, Anzini M, Gigli M, Pinamonti B, Sinagra G. The prognostic impact of the evolution of RV function in idiopathic DCM. JACC Cardiovasc Imaging 2016; 9: 1034–1042. [DOI] [PubMed] [Google Scholar]
- 26. Ghio S, Recusani F, Klersy C, Sebastiani R, Laudisa ML, Campana C, Gavazzi A, Tavazzi L. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol 2000; 85: 837–842. [DOI] [PubMed] [Google Scholar]
- 27. Kjaergaard J, Akkan D, Iversen KK, Køber L, Torp‐Pedersen C, Hassager C. Right ventricular dysfunction as an independent predictor of short‐ and long‐term mortality in patients with heart failure. Eur J Heart Fail 2007; 9: 610–616. [DOI] [PubMed] [Google Scholar]
- 28. Dini FL, Demmer RT, Simioniuc A, Morrone D, Donati F, Guarini G, Orsini E, Caravelli P, Marzilli M, Colombo PC. Right ventricular dysfunction is associated with chronic kidney disease and predicts survival in patients with chronic systolic heart failure. Eur J Heart Fail 2012; 14: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee JZ, Low SW, Pasha AK, Howe CL, Lee KS, Suryanarayana PG. Comparison of tricuspid annular plane systolic excursion with fractional area change for the evaluation of right ventricular systolic function: a meta‐analysis. Open Heart 2018; 5: e000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alsoos F, Khaddam A. Echocardiographic evaluation methods for right ventricular function. J Echocardiogr 2015; 13: 43–51. [DOI] [PubMed] [Google Scholar]
- 31. Focardi M, Cameli M, Carbone SF, Massoni A, de Vito R, Lisi M, Mondillo S. Traditional and innovative echocardiographic parameters for the analysis of right ventricular performance in comparison with cardiac magnetic resonance. Eur Heart J Cardiovasc Imaging 2015; 16: 47–52. [DOI] [PubMed] [Google Scholar]
- 32. Hamada‐Harimura Y, Seo Y, Ishizu T, Nishi I, Machino‐Ohtsuka T, Yamamoto M, Sugano A, Sato K, Sai S, Obara K, Yoshida I, Aonuma K. Incremental prognostic value of right ventricular strain in patients with acute decompensated heart failure. Circ Cardiovasc Imaging 2018; 11: e007249. [DOI] [PubMed] [Google Scholar]


