Abstract
Aims
Atrial fibrillation (AF) after heart transplantation (HTX) is associated with worse clinical outcomes. The current study aimed to analyse the association between AF before HTX and AF within 30 days after HTX.
Methods and results
This study included 639 adults who received HTX at Heidelberg Heart Center. Patients were subdivided into four groups depending on the status of AF before and after HTX. Analyses comprised recipient and donor data, medication, echocardiographic features, permanent pacemaker implantation, stroke, and mortality after HTX. Three hundred thirty‐two patients (52.0%) had neither AF before nor after HTX, 15 patients (2.3%) had no AF before HTX but showed AF after HTX, 219 patients (34.3%) showed AF before HTX but had no AF after HTX, and 73 patients (11.4%) had AF before and after HTX. Patients with AF before and after HTX had a higher 1 year post‐transplant mortality (39.7%) than patients without AF before or after HTX (18.1%, P < 0.01). Secondary outcomes showed a higher percentage of enlarged atria, ventricular dysfunction, mitral regurgitation, 1‐year stroke, and 1‐year permanent pacemaker implantation in patients with AF before and after HTX. Multivariate analysis revealed a six‐fold elevated risk for post‐transplant AF in patients with AF before HTX (hazard ratio: 6.59, confidence interval: 3.72–11.65; P < 0.01). Further risk factors for post‐transplant AF were higher donor age and prolonged ischaemic time, whereas total orthotopic HTX was associated with a two‐fold lower risk for post‐transplant AF.
Conclusions
Atrial fibrillation before HTX is a risk factor for post‐transplant AF, permanent pacemaker implantation, and mortality after HTX.
Keywords: Atrial fibrillation, Heart transplantation, Pacemaker, Stroke, Survival
Introduction
Early post‐transplant atrial fibrillation (AF) has been associated with increased morbidity and mortality in patients after heart transplantation (HTX). 1 , 2 , 3 , 4 Besides increasing the risk of thromboembolic events and stroke, post‐transplant AF has been linked to graft rejection, graft failure, and increased mortality after HTX. 4 , 5 , 6 , 7 Even short episodes of post‐transplant AF have been shown to increase the risk of adverse outcomes. 3
Previous reported frequencies of early post‐transplant AF range from 7.9% to 18.2% depending on the observed time interval after HTX. 2 , 3 A recently published Cochrane Review of 5393 HTX recipients reported an incidence of early post‐transplant AF of 9.7%, implicating that one out of ten HTX recipients is affected by early post‐transplant AF. 1
Several risk factors have been identified to facilitate the development of AF in the early stage after HTX including the choice of surgical HTX technique (biatrial, bicaval, or total orthotopic HTX), distorted atrial anatomy with enlarged atrial cavities, proarrhythmic scar tissue, sinus node injury, mitral regurgitation, prolonged ischaemic time, cardiac allograft rejection, chronic obstructive pulmonary disease, increased pulmonary vascular resistance, and older donor age. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17
Although it is generally assumed that the donor atria are electrically isolated from the remnants of the recipient atria by suture lines, several case reports have reported recipient‐to‐donor atrioatrial conduction across the suture lines in HTX recipients. 18 , 19 , 20 , 21 , 22 , 23 Patients with AF before HTX may therefore be more likely to develop early post‐transplant AF as an expression of a chronic disease state. By contrast, early post‐transplant AF in patients who did not suffer from AF before HTX may rather result from graft rejection or graft failure indicating an acute disease process. However, data on the association between AF before HTX and AF in the early stage after HTX are limited to case reports and have not been studied systematically. 18 , 19 , 20 , 21 , 22 , 23
We therefore sought to investigate the association between AF before HTX and early post‐transplant AF within 30 days after HTX as well as the underlying risk factors and clinical outcomes.
Patients and methods
Patients
We performed this study in accordance with the ethical principles of the Declaration of Helsinki. Institutional review board approval was granted by the University of Heidelberg (ethical approval number: S‐286/2015, Version 1.2, 28‐07‐2020) prior to the start of the study. Written informed consent was obtained from patients for inclusion in the Heidelberg HTX Registry allowing the clinical and scientific use of data. According to the ethical approval, no additional written informed consent was required for this observational study as only data of the clinical routine were used. 4 , 15 , 16 , 24 , 25 , 26
Our study included all adult patients (≥ 18 years) who received first HTX at Heidelberg Heart Center, Heidelberg, Germany, between 1989 and 2019. Patients with a second HTX were not included. Heart rhythm was assessed in each patient by electrocardiogram (ECG) before HTX. After HTX and during the initial hospital stay, patients were routinely monitored via telemetry. Resting 12‐lead ECG was periodically performed, and 24 h Holter was performed prior to discharge. Diagnosis of AF before HTX as well as diagnosis of AF ≤ 30 days after HTX was based upon all available records pertaining to heart rhythm. 4 , 15 , 16 , 24 , 25 , 26
At Heidelberg Heart Center, cardiac surgeons are able to perform all three HTX techniques (biatrial, bicaval, and total orthotopic HTX). Regarding these three HTX techniques, total orthotopic HTX was mostly used (277 HTX), followed by bicaval HTX (198 HTX) and biatrial HTX (164 HTX) in this study. The choice of surgical technique had not been preassigned. Factors influencing the choice of HTX technique were surgeon's preference, anatomical characteristics, and previous open‐heart surgery. 4
Based upon status of AF before and ≤ 30 days after HTX, we stratified patients into the following four groups: patients without AF before and after HTX, patients without AF before HTX but with AF ≤ 30 days after HTX, patients with AF before HTX but without AF ≤ 30 days after HTX, and patients with AF before and after HTX.
Follow‐up
Follow‐up of patients after HTX was performed in accordance with the standard of care at Heidelberg Heart Center. After the initial hospital stay, patients were seen monthly during the first 6 months after HTX, then bimonthly until the end of the first year, and thereafter usually three to four times per year (or if clinically indicated) at our HTX outpatient clinic. Routine follow‐up included medical history, physical examination, resting 12‐lead ECG, echocardiography, endomyocardial biopsy, and blood tests including immunosuppressive drug levels. 4 , 15 , 16 , 24 , 25 , 26 , 27
Restoration of sinus rhythm in patients with early post‐transplant AF was successfully achieved with pharmacological (amiodarone) or electrical cardioversion (defibrillator) if AF did not spontaneously convert to sinus rhythm. As patients with AF have a higher risk for thromboembolic complications such as stroke, 28 , 29 , 30 patients with AF after HTX routinely received appropriate anticoagulation therapy, mostly in form of unfractionated heparin or low molecular weight heparin. In stable patients without major bleeding risk, vitamin K antagonists or directly acting oral anticoagulants were also used.
Medication and immunosuppression
Post‐transplant medication including immunosuppressive drug therapy was administered in accordance with the standard of care at Heidelberg Heart Center. Patients routinely received an anti‐thymocyte globulin‐based immunosuppression induction therapy after HTX. Cyclosporine A and azathioprine were used as standard immunosuppressive drug therapy at the beginning of the study period. From 2001 onward, azathioprine was subsequently replaced by mycophenolate mofetil, and cyclosporine A was consecutively substituted by tacrolimus from 2006 onward. During the first post‐transplant months, steroids (prednisolone) were tapered incrementally and were finally discontinued 6 months after HTX (if clinically possible). 4 , 15 , 16 , 24 , 25 , 26
Statistical analysis
We performed data analysis with SAS (Version 9.4, SAS Institute, Cary, NC, USA). Data were expressed as count (n) with percentage (%) or as mean ± standard deviation (SD). χ 2 test/Fisher's exact test was used for categorical variables and analysis of variance (ANOVA)/Kruskal–Wallis test, respectively Student's t test/Mann–Whitney U test for continuous variables, as appropriate. Survival after HTX was graphically displayed with the Kaplan–Meier estimator and compared using log‐rank test. A P value of < 0.05 was considered statistically significant. 4 , 15 , 16 , 24 , 25 , 26
Univariate analyses were performed to examine differences between groups including recipient data, previous open‐heart surgery, principal diagnosis for HTX, donor data, transplant sex mismatch, perioperative data, medication including immunosuppressive drug therapy, echocardiographic features, graft rejections, heart rates, permanent pacemaker (PPM) implantation, transient ischaemic attack (TIA), and stroke after HTX. Causes for 1‐year mortality after HTX were grouped into the following categories: graft failure, acute rejection, infection/sepsis, malignancy, and thromboembolic event/bleeding. Analysis of 1 year mortality after HTX also included a multivariate analysis (Cox regression model) with the following seven clinically relevant parameters based on a predetermined model: AF ≤ 30 days after HTX (in total), recipient age (years), cyclosporine A (in total), azathioprine (in total), donor age (years), ischaemic time (min), and total orthotopic HTX (in total). In order to avoid biased regression coefficients and to ensure a stable number of events per analysed variable, we decided not to include further variables in the multivariate analysis. 4 , 15 , 16 , 24 , 25 , 26
The primary outcome of this study was 1 year mortality after HTX including causes of death. Secondary outcomes comprised graft rejection, TIA, stroke, echocardiographic features, bradycardia, and PPM implantation after HTX. As we sought to investigate a possible association between AF before HTX and AF ≤ 30 days after HTX, we performed a second multivariate analysis (Cox regression model) to estimate the impact of the following seven variables on AF ≤ 30 days after HTX: AF before HTX (in total), recipient age (years), cyclosporine A (in total), azathioprine (in total), donor age (years), ischaemic time (min), and total orthotopic HTX (in total). Given the long study period, we additionally performed a sensitivity analysis to test the robustness of our results and to examine a possible era effect using a subgroup of patients with tacrolimus and mycophenolate mofetil as the immunosuppressive drug regimen was changed from 2006 onward. 4 , 15 , 16 , 24 , 25 , 26
Results
Atrial fibrillation before and after heart transplantation
This study included 639 HTX recipients, 292 patients (45.7%) showed AF before HTX and 347 patients (54.3%) had no AF before HTX. Within 30 days after HTX, 88 patients (13.8%) showed AF, while 551 patients (86.2%) had no AF ≤ 30 days after HTX.
Stratified by status of AF before and ≤ 30 days after HTX, 332 patients (52.0%) had no AF before or ≤ 30 days after HTX, 15 patients (2.3%) had no AF before HTX but showed AF ≤ 30 days after HTX, 219 patients (34.3%) had AF before HTX but showed no AF ≤ 30 days after HTX, and 73 patients (11.4%) showed AF before and ≤ 30 days after HTX.
Stratified by timing of post‐transplant AF, we observed in this study a post‐transplant AF rate of 38.6% between Day 0 and Day 4 after HTX, 27.3% between Day 5 and Day 9 after HTX, 18.2% between Day 10 and Day 14 after HTX, 4.5% between Day 15 and Day 19 after HTX, 5.7% between Day 20 and Day 24 after HTX, and 5.7% between Day 25 and Day 30 after HTX.
Demographics and medication after heart transplantation
Analysis of baseline characteristics showed a significantly lower recipient age (50.6 ± 10.9 years, P < 0.01) and donor age (39.6 ± 12.7 years, P < 0.01) in patients without AF before and ≤ 30 days after HTX compared to the three other groups. Patients without AF before and ≤ 30 days after HTX (28.6%) as well as patients with AF before HTX but without AF ≤ 30 days after HTX (18.3%) had a significantly lower percentage of biatrial HTX technique (P = 0.01), whereas patients with AF before and ≤ 30 days after HTX had a significantly lower percentage of total orthotopic HTX technique (24.7%, P < 0.01) and a significantly longer ischaemic time (253.6 ± 66.5 min, P < 0.01). There were no statistically significant differences between groups in regard to the remaining recipient data, previous open‐heart surgery, principal diagnosis for HTX, donor sex, donor body mass index, transplant sex mismatch, or bicaval HTX technique (all P ≥ 0.05). Baseline characteristics are presented in Table 1 .
Table 1.
Baseline characteristics
| Parameter | No AF before HTX | No AF before HTX | AF before HTX | AF before HTX | P value |
|---|---|---|---|---|---|
| No AF after HTX | AF after HTX | No AF after HTX | AF after HTX | ||
| (n = 332) | (n = 15) | (n = 219) | (n = 73) | ||
| Recipient data | |||||
| Age (years), mean ± SD | 50.6 ± 10.9 | 53.0 ± 9.8 | 54.2 ± 8.6 | 52.2 ± 11.4 | <0.01* |
| Male sex, n (%) | 253 (76.2%) | 13 (86.7%) | 176 (80.4%) | 56 (76.7%) | 0.56 |
| Body mass index (kg/m2), mean ± SD | 25.0 ± 3.9 | 24.8 ± 3.5 | 24.8 ± 3.9 | 25.1 ± 4.4 | 0.95 |
| Arterial hypertension, n (%) | 177 (53.3%) | 7 (46.7%) | 125 (57.1%) | 41 (56.2%) | 0.75 |
| Dyslipidaemia, n (%) | 205 (61.7%) | 10 (66.7%) | 146 (66.7%) | 45 (61.6%) | 0.67 |
| Diabetes mellitus, n (%) | 101 (30.4%) | 6 (40.0%) | 81 (37.0%) | 27 (37.0%) | 0.35 |
| Renal insufficiency a , n (%) | 182 (54.8%) | 7 (46.7%) | 135 (61.6%) | 44 (60.3%) | 0.33 |
| eGFR (mL/min/1.73 m2), mean ± SD | 62.1 ± 21.7 | 64.5 ± 18.7 | 57.2 ± 21.5 | 60.1 ± 21.7 | 0.06 |
| Previous open‐heart surgery | |||||
| Overall open‐heart surgery, n (%) | 91 (27.4%) | 5 (33.3%) | 66 (30.1%) | 28 (38.4%) | 0.31 |
| CABG surgery, n (%) | 46 (13.9%) | 2 (13.3%) | 22 (10.0%) | 8 (11.0%) | 0.59 |
| Other surgery b , n (%) | 27 (8.1%) | 2 (13.3%) | 32 (14.6%) | 10 (13.7%) | 0.10 |
| VAD surgery, n (%) | 23 (6.9%) | 2 (13.3%) | 18 (8.2%) | 12 (16.4%) | 0.06 |
| Principal diagnosis for HTX | |||||
| Ischaemic CMP, n (%) | 113 (34.0%) | 4 (26.7%) | 66 (30.1%) | 26 (35.6%) | 0.69 |
| Non‐ischaemic CMP, n (%) | 167 (50.3%) | 9 (60.0%) | 128 (58.4%) | 35 (47.9%) | 0.20 |
| Valvular heart disease, n (%) | 16 (4.8%) | 1 (6.7%) | 10 (4.6%) | 7 (9.6%) | 0.38 |
| Cardiac amyloidosis, n (%) | 36 (10.8%) | 1 (6.7%) | 15 (6.8%) | 5 (6.8%) | 0.37 |
| Donor data | |||||
| Age (years), mean ± SD | 39.6 ± 12.7 | 41.7 ± 14.0 | 41.2 ± 14.2 | 46.6 ± 12.6 | <0.01* |
| Male sex, n (%) | 146 (44.0%) | 5 (33.3%) | 103 (47.0%) | 24 (32.9%) | 0.16 |
| Body mass index (kg/m2), mean ± SD | 24.8 ± 4.1 | 24.5 ± 3.8 | 24.9 ± 4.2 | 24.8 ± 3.7 | 0.98 |
| Transplant sex mismatch | |||||
| Mismatch, n (%) | 143 (43.1%) | 8 (53.3%) | 94 (42.9%) | 38 (52.1%) | 0.45 |
| Donor (m) to recipient (f), n (%) | 18 (5.4%) | 0 (0.0%) | 10 (4.6%) | 3 (4.1%) | 0.77 |
| Donor (f) to recipient (m), n (%) | 125 (37.7%) | 8 (53.3%) | 84 (38.3%) | 35 (47.9%) | 0.26 |
| Perioperative data | |||||
| Ischaemic time (min), mean ± SD | 214.0 ± 65.2 | 198.3 ± 73.4 | 229.4 ± 69.7 | 253.6 ± 66.5 | <0.01* |
| Biatrial HTX, n (%) | 95 (28.6%) | 6 (40.0%) | 40 (18.3%) | 23 (31.5%) | 0.01* |
| Bicaval HTX, n (%) | 93 (28.0%) | 5 (33.3%) | 68 (31.1%) | 32 (43.8%) | 0.07 |
| Total orthotopic HTX, n (%) | 144 (43.4%) | 4 (26.7%) | 111 (50.7%) | 18 (24.7%) | <0.01* |
Abbreviations: AF, atrial fibrillation; CABG, coronary artery bypass graft; CMP, cardiomyopathy; f, female; eGFR, estimated glomerular filtration rate; HTX, heart transplantation; m, male; n, number; SD, standard deviation; VAD, ventricular assist device.
eGFR < 60 mL/min/1.73 m2.
Congenital, valvular, or ventricular surgery.
Statistically significant (P < 0.05).
Comparison of the immunosuppressive drug therapy showed a significantly higher percentage of cyclosporine A (66.7%, P < 0.01) and azathioprine (60.0%, P < 0.01) in patients without AF before HTX but with AF ≤ 30 days after HTX, while patients with AF before and ≤ 30 days after HTX had a significantly higher percentage of tacrolimus (57.5%, P < 0.01) and mycophenolate mofetil (67.1%, P < 0.01). We found no statistically significant differences between the four groups regarding the administration of acetylsalicylic acid, beta blockers, ivabradine, calcium channel blockers, angiotensin‐converting‐enzyme inhibitors/angiotensin II receptor blockers, or statins (all P ≥ 0.05). Medication after HTX is shown in Table 2 .
Table 2.
Medication after HTX
| Parameter | No AF before HTX) | No AF before HTX | AF before HTX | AF before HTX | P value |
|---|---|---|---|---|---|
| No AF after HTX | AF after HTX | No AF after HTX | AF after HTX | ||
| (n = 332) | (n = 15) | (n = 219) | (n = 73) | ||
| Immunosuppressive drug therapy | |||||
| Cyclosporine A, n (%) | 208 (62.7%) | 10 (66.7%) | 98 (44.7%) | 31 (42.5%) | <0.01* |
| Tacrolimus, n (%) | 124 (37.3%) | 5 (33.3%) | 121 (55.3%) | 42 (57.5%) | <0.01* |
| Azathioprine, n (%) | 161 (48.5%) | 9 (60.0%) | 73 (33.3%) | 24 (32.9%) | <0.01* |
| Mycophenolate mofetil, n (%) | 171 (51.5%) | 6 (40.0%) | 146 (66.7%) | 49 (67.1%) | <0.01* |
| Steroids, n (%) | 332 (100.0%) | 15 (100.0%) | 219 (100.0%) | 73 (100.0%) | n. a. |
| Concomitant medication | |||||
| ASA, n (%) | 35 (10.5%) | 2 (13.3%) | 25 (11.4%) | 6 (8.2%) | 0.87 |
| Beta blocker, n (%) | 53 (16.0%) | 4 (26.7%) | 40 (18.3%) | 17 (23.3%) | 0.38 |
| Ivabradine, n (%) | 31 (9.3%) | 1 (6.7%) | 25 (11.4%) | 4 (5.5%) | 0.49 |
| Calcium channel blocker, n (%) | 92 (27.7%) | 4 (26.7%) | 55 (25.1%) | 20 (27.4%) | 0.93 |
| ACE inhibitor/ARB, n (%) | 139 (41.9%) | 6 (40.0%) | 109 (49.8%) | 24 (32.9%) | 0.06 |
| Diuretic, n (%) | 332 (100.0%) | 15 (100.0%) | 219 (100.0%) | 73 (100.0%) | n. a. |
| Statin, n (%) | 127 (38.3%) | 4 (26.7%) | 91 (41.6%) | 32 (43.8%) | 0.53 |
| Gastric protection a , n (%) | 332 (100.0%) | 15 (100.0%) | 219 (100.0%) | 73 (100.0%) | n. a. |
Abbreviations: ACE inhibitor, angiotensin‐converting‐enzyme inhibitor; ARB, angiotensin II receptor blocker; AF, atrial fibrillation; ASA, acetylsalicylic acid; HTX, heart transplantation; n, number; n. a., not applicable.
Gastric protection defined as proton pump inhibitor or histamine receptor (H2) blocker.
Statistically significant (P < 0.05).
Primary outcome after heart transplantation
Assessment of survival showed a significantly inferior 1 year post‐transplant survival in patients with AF before HTX [213 of 292 (72.9%)] compared with patients without AF before HTX [279 of 347 (80.4%), P = 0.04]. Similarly, patients with AF ≤ 30 days after HTX had a significantly lower 1 year post‐transplant survival [51 of 88 (58.0%)] in comparison with patients without AF ≤ 30 days after HTX [441 of 551 (80.0%), P < 0.01]. Kaplan–Meier survival curves are displayed in Figures 1 and 2 .
Figure 1.
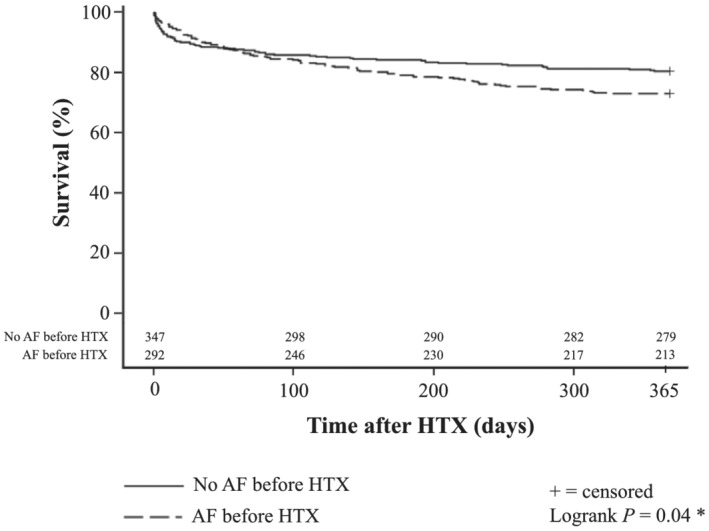
One year survival after HTX in patients with and without AF before HTX (Kaplan–Meier estimator). Patients with AF before HTX showed a statistically significant inferior 1 year post‐transplant survival [213 of 292 (72.9%)] in comparison with patients without AF before HTX [279 of 347 (80.4%), P = 0.04]. Abbreviations: AF, atrial fibrillation; HTX, heart transplantation; *statistically significant (P < 0.05).
Figure 2.
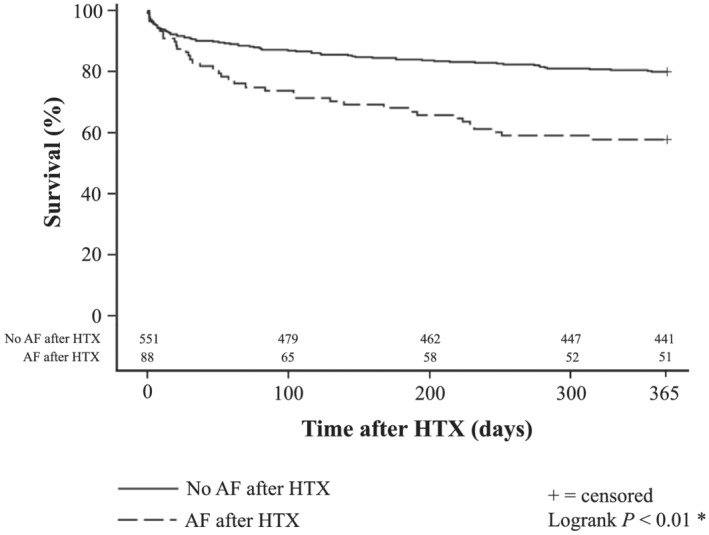
One year survival after HTX in patients with and without AF ≤ 30 days after HTX (Kaplan–Meier estimator). Patients with AF ≤ 30 days after HTX showed a statistically significant inferior 1 year post‐transplant survival [51 of 88 (58.0%)] in comparison with patients without AF ≤ 30 days after HTX [441 of 551 (80.0%), P < 0.01]. Abbreviations: AF, atrial fibrillation; HTX, heart transplantation; *statistically significant (P < 0.05).
Stratified by status of AF before and ≤ 30 days after HTX, patients without AF before and after HTX had the highest 1 year post‐transplant survival [272 of 332 (81.9%)], followed by patients with AF before HTX but without AF ≤ 30 days after HTX [169 of 219 (77.2%)], and patients with AF before and after HTX [44 of 73 (60.3%)]. Patients without AF before HTX but with AF ≤ 30 days after HTX showed the lowest 1 year post‐transplant survival of all groups [7 of 15 (46.7%), P < 0.01]. Survival of patients stratified by status of AF before and ≤ 30 days after HTX is shown in Figure 3 .
Figure 3.
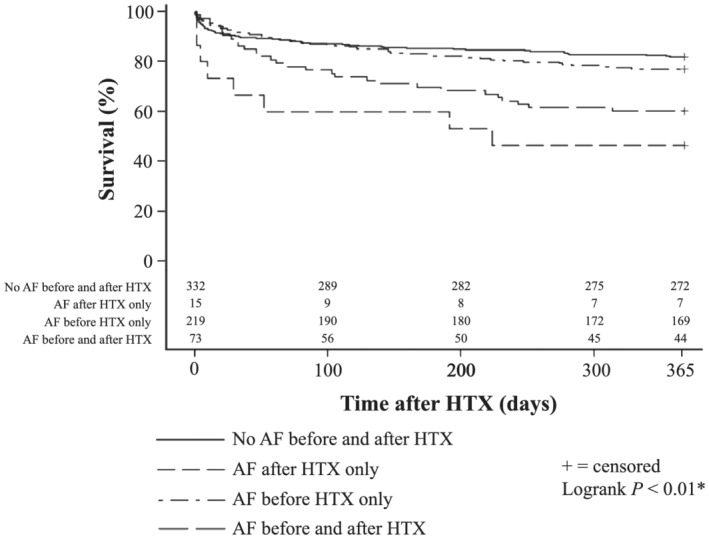
One year survival after HTX in patients with and without AF before and ≤ 30 days after HTX (Kaplan–Meier estimator). Stratification of patients by status of AF before and ≤ 30 days after HTX showed that patients without AF before and after HTX had the highest 1 year post‐transplant survival [272 of 332 (81.9%)], followed by patients with AF before HTX but without AF ≤ 30 days after HTX [169 of 219 (77.2%)], and patients with AF before and after HTX [44 of 73 (60.3%)]. Patients without AF before HTX but with AF ≤ 30 days after HTX showed the lowest 1 year post‐transplant survival of all groups [7 of 15 (46.7%), P < 0.01]. Abbreviations: AF, atrial fibrillation; HTX, heart transplantation; *statistically significant (P < 0.05).
Considering the causes of death within the first year after HTX, significantly more patients died from graft failure in the ‘no AF before HTX but AF ≤ 30 days after HTX’ group (33.3%, P < 0.01), while significantly more patients died from infection/sepsis in the ‘AF before and after HTX’ group (24.7%, P < 0.01). There were no statistically significant differences between the four groups regarding causes of death related to acute rejection, malignancy, or thromboembolic event/bleeding (all P ≥ 0.05). Causes of death within the first year after HTX are given in the first part of Table 3 .
Table 3.
Primary and secondary outcomes after HTX
| Parameter | No AF before HTX | No AF before HTX | AF before HTX | AF before HTX | P value |
|---|---|---|---|---|---|
| No AF after HTX | AF after HTX | No AF after HTX | AF after HTX | ||
| ( n = 332) | (n = 15) | (n = 219) | (n = 73) | ||
| Mortality within 1 year after HTX | |||||
| All causes, n (%) | 60 (18.1%) | 8 (53.3%) | 50 (22.8%) | 29 (39.7%) | <0.01* |
| Causes of death within 1 year after HTX | |||||
| Graft failure, n (%) | 29 (8.7%) | 5 (33.3%) | 14 (6.4%) | 7 (9.6%) | <0.01* |
| Acute rejection, n (%) | 3 (0.9%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 0.79 |
| Infection/sepsis, n (%) | 23 (6.9%) | 3 (20.0%) | 31 (14.2%) | 18 (24.7%) | <0.01* |
| Malignancy, n (%) | 1 (0.3%) | 0 (0.0%) | 1 (0.5%) | 1 (1.4%) | 0.67 |
| Thromboembolic event/bleeding, n (%) | 4 (1.2%) | 0 (0.0%) | 3 (1.4%) | 3 (4.1%) | 0.30 |
| Secondary outcomes after HTX | |||||
| 30 day ≥ 1 graft rejection, n (%) | 74 (22.3%) | 8 (53.3%) | 30 (13.7%) | 6 (8.2%) | <0.01* |
| 30 day bradycardia a , n (%) | 12 (3.6%) | 0 (0.0%) | 6 (2.7%) | 3 (4.1%) | 0.81 |
| 30 day PPM implantation, n (%) | 2 (0.6%) | 0 (0.0%) | 2 (0.9%) | 2 (2.7%) | 0.38 |
| 30 day TIA, n (%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 0.59 |
| 30 day stroke, n (%) | 6 (1.8%) | 1 (6.7%) | 4 (1.8%) | 4 (5.5%) | 0.17 |
| 1 year PPM implantation, n (%) | 3 (0.9%) | 1 (6.7%) | 4 (1.8%) | 4 (5.5%) | 0.03* |
| 1 year TIA, n (%) | 0 (0.0%) | 0 (0.0%) | 3 (1.4%) | 1 (1.4%) | 0.19 |
| 1 year stroke, n (%) | 6 (1.8%) | 1 (6.7%) | 6 (2.7%) | 6 (8.2%) | 0.03* |
Abbreviations: AF, atrial fibrillation; HTX, heart transplantation; n, number; PPM, permanent pacemaker; TIA, transient ischaemic attack.
Bradycardia defined as mean weekly heart rate < 60 beats per minute.
Statistically significant (P < 0.05).
Secondary outcomes after heart transplantation
Analysis of 30 day episodes of graft rejection showed a significantly higher percentage of graft rejection (53.3%, P < 0.01) in patients without AF before HTX but with AF ≤ 30 days after HTX. There were no statistically significant differences regarding 30 day bradycardia, 30 day PPM implantation, 30 day TIA, or 30 day stroke between the four groups (all P ≥ 0.05). Within the first year after HTX, we found a significantly higher percentage of 1 year PPM implantation in patients with AF before and ≤ 30 days after HTX (5.5%) as well as in patients without AF before HTX but with AF ≤ 30 days after HTX (6.7%, P = 0.03). In addition, patients with AF before and ≤ 30 days after HTX (8.2%) as well as patients without AF before HTX but with AF ≤ 30 days after HTX (6.7%) more often suffered from stroke within the first year after HTX (P = 0.03). Secondary outcomes after HTX are presented in the second part of Table 3 .
Assessment of post‐transplant echocardiographic features showed that patients with AF before and ≤ 30 days after HTX as well as patients without AF before HTX but with AF ≤ 30 days after HTX had a significantly lower percentage of normal sized right atrial (P < 0.01), left atrial (P < 0.01), right ventricular (P < 0.01), and left ventricular end‐diastolic diameter (P = 0.01) along with a higher rate of reduced right (P < 0.01) and left ventricular function (P < 0.01). Moreover, patients with AF before and ≤ 30 days after HTX as well as patients without AF before HTX but with AF ≤ 30 days after HTX had a higher rate of mitral (P < 0.01) and tricuspid regurgitation (P = 0.01). Echocardiographic features after HTX are given in Table 4 .
Table 4.
Echocardiographic features after HTX
| Parameter | No AF before HTX | No AF before HTX | AF before HTX | AF before HTX | P value |
|---|---|---|---|---|---|
| No AF after HTX | AF after HTX | No AF after HTX | AF after HTX | ||
| (n = 332) | (n = 15) | (n = 219) | (n = 73) | ||
| 30 day end‐diastolic diameter | |||||
| Normal RA (< 35 mm), n (%) | 201 (60.5%) | 5 (33.3%) | 131 (59.8%) | 22 (30.1%) | <0.01* |
| Normal RV (< 30 mm), n (%) | 286 (86.1%) | 9 (60.0%) | 185 (84.5%) | 43 (58.9%) | <0.01* |
| Normal LA (< 40 mm), n (%) | 173 (52.1%) | 4 (26.7%) | 121 (55.3%) | 23 (31.5%) | <0.01* |
| Normal LV (< 55 mm), n (%) | 305 (91.9%) | 11 (73.3%) | 209 (95.4%) | 64 (87.7%) | 0.01* |
| 30 day right ventricular function | |||||
| Normal, n (%) | 283 (85.2%) | 9 (60.0%) | 180 (82.2%) | 43 (58.9%) | <0.01* |
| Reduced, n (%) | 49 (14.8%) | 6 (40.0%) | 39 (17.8%) | 30 (41.1%) | <0.01* |
| Mild, n (%) | 17 (5.1%) | 1 (6.7%) | 21 (9.6%) | 12 (16.4%) | |
| Moderate, n (%) | 7 (2.1%) | 0 (0.0%) | 4 (1.8%) | 6 (8.2%) | |
| Severe, n (%) | 25 (7.5%) | 5 (33.3%) | 14 (6.4%) | 12 (16.4%) | |
| 30 day left ventricular function | |||||
| Normal, n (%) | 302 (91.0%) | 10 (66.7%) | 206 (94.1%) | 61 (83.6%) | <0.01* |
| Reduced, n (%) | 30 (9.0%) | 5 (33.3%) | 13 (5.9%) | 12 (16.4%) | <0.01* |
| Mild, n (%) | 5 (1.5%) | 1 (6.7%) | 6 (2.7%) | 7 (9.6%) | |
| Moderate, n (%) | 6 (1.8%) | 1 (6.7%) | 0 (0.0%) | 2 (2.7%) | |
| Severe, n (%) | 19 (5.7%) | 3 (20.0%) | 7 (3.2%) | 3 (4.1%) | |
| 30 day tricuspid regurgitation | |||||
| No, n (%) | 231 (69.6%) | 8 (53.3%) | 130 (59.4%) | 37 (50.7%) | 0.01* |
| Yes, n (%) | 101 (30.4%) | 7 (46.7%) | 89 (40.6%) | 36 (49.3%) | 0.01* |
| Mild, n (%) | 56 (16.9%) | 2 (13.3%) | 61 (27.9%) | 15 (20.5%) | |
| Moderate, n (%) | 30 (9.0%) | 2 (13.3%) | 17 (7.8%) | 13 (17.8%) | |
| Severe, n (%) | 15 (4.5%) | 3 (20.0%) | 11 (5.0%) | 8 (11.0%) | |
| 30 day mitral regurgitation | |||||
| No, n (%) | 259 (78.0%) | 9 (60.0%) | 170 (77.6%) | 39 (53.4%) | <0.01* |
| Yes, n (%) | 73 (22.0%) | 6 (40.0%) | 49 (22.4%) | 34 (46.6%) | <0.01* |
| Mild, n (%) | 69 (20.8%) | 5 (33.3%) | 47 (21.5%) | 32 (43.8%) | |
| Moderate, n (%) | 3 (0.9%) | 1 (6.7%) | 2 (0.9%) | 2 (2.7%) | |
| Severe, n (%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
Abbreviations: AF, atrial fibrillation; HTX, heart transplantation; LA, left atrium; LV, left ventricle; n, number; RA, right atrium; RV, right ventricle.
Statistically significant (P < 0.05).
Multivariate analysis for 1 year mortality after heart transplantation
Multivariate analysis showed a more than two‐fold increased risk for 1 year mortality after HTX in patients with AF ≤ 30 days after HTX [hazard ratio (HR): 2.06, confidence interval (CI): 1.40–3.05; P < 0.01]. In addition, higher donor age was also associated with increased 1 year post‐transplant mortality (HR: 1.02, CI: 1.00–1.03; P = 0.01), whereas the other five analysed variables (recipient age, use of cyclosporine A after HTX, use of azathioprine after HTX, ischaemic time, and use of total orthotopic HTX technique) had no statistically significant effect on post‐transplant 1 year mortality (all P ≥ 0.05). Multivariate analysis for 1 year mortality after HTX is shown in the first part of Table 5 .
Table 5.
Multivariate analysis
| Variable | Hazard Ratio | 95% CI | P value |
|---|---|---|---|
| 1 year mortality after HTX | |||
| Atrial fibrillation ≤ 30 days after HTX (in total) | 2.06 | 1.40–3.05 | <0.01* |
| Recipient age (years) | 1.02 | 0.99–1.03 | 0.11 |
| Cyclosporine A (in total) | 0.69 | 0.37–1.30 | 0.25 |
| Azathioprine (in total) | 1.82 | 0.93–3.55 | 0.08 |
| Donor age (years) | 1.02 | 1.00–1.03 | 0.01* |
| Ischaemic time (min) | 1.00 | 0.99–1.01 | 0.11 |
| Total orthotopic HTX (in total) | 1.02 | 0.72–1.45 | 0.91 |
| 30 day atrial fibrillation after HTX | |||
| Atrial fibrillation before HTX (in total) | 6.59 | 3.72–11.65 | <0.01* |
| Recipient age (years) | 0.99 | 0.97–1.01 | 0.16 |
| Cyclosporine A (in total) | 1.40 | 0.63–3.08 | 0.41 |
| Azathioprine (in total) | 1.50 | 0.64–3.51 | 0.35 |
| Donor age (years) | 1.03 | 1.01–1.05 | <0.01* |
| Ischaemic time (min) | 1.01 | 1.00–1.01 | 0.01* |
| Total orthotopic HTX (in total) | 0.39 | 0.24–0.65 | <0.01* |
Abbreviations: CI, confidence interval; HTX, heart transplantation.
Statistically significant (P < 0.05).
Association between atrial fibrillation before and after heart transplantation
χ 2 test was used to investigate the association between AF before HTX and AF ≤ 30 days after HTX by comparing the corresponding expected and observed frequencies. A total of 83.0% of patients with AF ≤ 30 days after HTX already had AF prior to HTX, whereas only 17.0% of patients with AF ≤ 30 days after HTX had no AF before HTX (P < 0.01). Multivariate analysis further showed a more than six‐fold elevated risk for AF ≤ 30 days after HTX in patients with AF before HTX (HR: 6.59, CI: 3.72–11.65; P < 0.01). Other risk factors for AF ≤ 30 days after HTX were higher donor age (HR: 1.03, CI: 1.01–1.05; P < 0.01) and prolonged ischaemic time (HR: 1.01, CI: 1.00–1.01; P = 0.01), whereas total orthotopic HTX was associated with a more than two‐fold lower risk for AF ≤ 30 days after HTX (HR: 0.39, CI: 0.24–0.65; P < 0.01). The other three analysed variables (recipient age, use of cyclosporine A after HTX, and use of azathioprine after HTX) showed no statistically significant effect on AF ≤ 30 days after HTX (all P ≥ 0.05). Multivariate analysis for AF ≤ 30 days after HTX is given in the second part of Table 5 .
Sensitivity analysis
In order to investigate a possible era effect and to examine the robustness of our results, we performed a sensitivity analysis with a subgroup of patients [292 of 639 patients (45.7%)] who were administered tacrolimus and mycophenolate mofetil as immunosuppressive drug therapy.
This analysis provided similar results in terms of the primary outcome (1 year mortality after HTX) and the secondary outcomes (graft rejections, bradycardia, TIA, stroke, echocardiographic features, bradycardia, and PPM implantation after HTX) backing the robustness of our results and minimizing a possible era effect.
Discussion
Frequency and significance of atrial fibrillation before and after heart transplantation
Atrial fibrillation is the most common heart rhythm disorder in patients with heart failure and its prevalence increases with heart failure severity. 31 , 32 Up to 50% of patients with advanced heart failure suffer from AF, and the presence of AF in patients with advanced heart failure has been linked to increased mortality. 31 , 32 , 33 Moreover, AF in the early stage after HTX has been associated with increased morbidity and mortality after HTX. 1 , 2 , 3 , 4 However, the association between AF before HTX and AF in the early stage after HTX has yet not been investigated. We therefore conducted this large study with a total of 639 HTX recipients to analyse the association between AF before HTX and early post‐transplant AF within 30 days after HTX as well as the underlying risk factors and clinical outcomes.
Post‐transplant AF is often mistaken for post‐operative AF as both occur after cardiac surgery. However, there are important differences between post‐operative and post‐transplant AF. Post‐operative AF often occurs after coronary artery bypass graft (CABG) surgery or valvular surgery as these patients suffer from structural heart disease and many of these patients already had AF before surgery. Given this susceptibility to AF, the rate of post‐operative AF is quite high with reported frequencies of 30% after CABG surgery, 40% after valvular surgery, and even 50% after combined CABG and valvular surgery. 34 , 35 In contrast, during HTX, the old, morbid heart is replaced by a new, healthy heart. Therefore, the rate of post‐transplant AF is markedly lower with a reported frequency of 9.7% in a recently published Cochrane Review of 5393 HTX recipients. 1
In addition, there is a difference in timing between post‐operative and post‐transplant AF. Post‐operative AF mainly occurs within 4 days after cardiac surgery, with a peak at Day 2 after cardiac surgery and is rarely seen after the first post‐operative week, while post‐transplant AF can occur anytime in the early period after HTX. 34 , 35
Almost half of the patients in our study were affected by AF before HTX (45.7%) emphasizing the clinical relevance of AF in patients with advanced heart failure. 31 , 32 , 33 In terms of early post‐transplant AF, 88 patients (13.8%) had AF ≤ 30 days after HTX which is comparable with previous reports ranging between 7.9% and 18.2%. 2 , 3 Further stratification by status of AF before and AF ≤ 30 days after HTX showed 332 patients (52.0%) without AF before or after HTX, 15 patients (2.3%) without AF before HTX but with AF after HTX, 219 patients (34.3%) with AF before HTX but without AF after HTX, and 73 patients (11.4%) with AF before and after HTX. These numbers indicate that most patients (83.0%) with early post‐transplant AF ≤ 30 days after HTX already had AF prior to HTX.
Risk factors for atrial fibrillation before and after heart transplantation
Given the vast number of risk factors for AF, 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 we performed an in‐depth analysis of risk factors for early post‐transplant AF. Higher recipient and donor age have both been reported as risk factors for AF in the early stage after HTX. 1 , 2 Accordingly, patients without AF before and ≤ 30 days after HTX had a lower recipient (P < 0.01) and donor age (P < 0.01) in this study. Besides older age, the use of biatrial HTX has been associated with higher rates of early post‐transplant AF. 1 , 4 Biatrial HTX results in enlarged atrial cavities with distorted anatomy and potentially proarrhythmic scar tissue, which can facilitate the development of AF. 11 , 12 , 13 , 14 We also found a significantly higher percentage of biatrial HTX (P = 0.01) in patients with AF before and after HTX as well as in patients without AF before HTX but with AF ≤ 30 days after HTX. By contrast, patients without AF before and after HTX as well as patients with AF before HTX but without AF ≤ 30 days after HTX showed a significantly higher rate of total orthotopic HTX (P < 0.01). In comparison with biatrial HTX, total orthotopic HTX excises most of the recipient atria and preserves the geometry and conduction system of the donor atria, thus minimizing proarrhythmogenic scar tissue. 4 Another perioperative risk factor for early post‐transplant AF is prolonged ischaemic time. Ischaemic damage and subsequent endocardial fibrosis can provide a substrate for AF. 17 In our study, patients with AF before and ≤ 30 days after HTX showed a significantly longer ischaemic time (P < 0.01).
Besides surgical parameters, cardiac graft rejection has been associated with supraventricular arrhythmias. 17 , 36 , 37 , 38 In this context, the underlying immunosuppressive drug therapy that plays a key role as a tacrolimus‐based immunosuppressive regimen has been demonstrated to be superior to a cyclosporine A‐based immunosuppressive drug therapy in the prevention of graft rejection. 39 In our study, significantly more patients without AF before HTX but with AF after HTX had a cyclosporine A‐based immunosuppressive drug therapy in combination with azathioprine.
Fluid volume imbalance, electrolyte disorders, or the use of inotropic agents have also been linked to the occurrence of AF. 6 , 17 , 29 , 30 , 36 However, in this study, we did not observe an association between these parameters and early post‐transplant AF.
As several risk factors may have an impact on early post‐transplant AF, 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 we performed a multivariate analysis for AF within 30 days after HTX, which showed higher donor age and prolonged ischaemic time as significant risk factors. Emphasizing the importance of HTX technique, total orthotopic HTX was associated with a more than two‐fold lower risk, while the other three analysed variables (recipient age, cyclosporine A, and azathioprine) had no statistically significant effect on early post‐transplant AF. Most importantly, patients with AF before HTX had a more than six‐fold elevated risk for AF within 30 days after HTX (HR: 6.59, CI: 3.72–11.65; P < 0.01) indicating that AF before HTX is an important risk factor for early post‐transplant AF, independently of the other investigated risk factors.
Clinical outcomes of atrial fibrillation after heart transplantation
Early post‐transplant AF has been associated with worse clinical outcomes including graft rejection, graft failure, and increased mortality after HTX. 1 , 2 , 3 , 4 , 5 , 6 , 7 In our study, patients with AF within 30 days after HTX had a significantly lower 1 year post‐transplant survival (P < 0.01). Multivariate analysis additionally showed a more than two‐fold increased risk for 1 year mortality after HTX in patients with AF within 30 days after HTX (HR: 2.06, CI: 1.40–3.05; P < 0.01). These findings are in line with previous studies reporting an increased mortality in patients with post‐transplant AF. 1 , 2 , 3 , 4
Further stratification of patients by status of AF before and ≤ 30 days after HTX revealed that patients with AF before and after HTX had a significantly higher 1 year post‐transplant mortality (39.7%) than patients without AF before or after HTX (18.1%, P < 0.01). In terms of causes of death, patients with AF before and after HTX more often died from infection/sepsis (P < 0.01). Early post‐transplant AF has been linked to pulmonary infections, as alterations in the pulmonary veins due to pulmonary hypertension, hypoxemia, and acidosis can provoke AF, and atypical foci of AF are more frequent in patients with chronic obstructive pulmonary disease and elevated pulmonary vascular resistance. 15 , 16 , 40 , 41 , 42 By contrast, patients without AF before HTX but with AF ≤ 30 days after HTX mainly died from graft failure (P < 0.01) and showed a significantly higher percentage of graft rejection (53.3%, P < 0.01). As post‐transplant AF was primarily associated with graft rejection in patients without former AF, this may explain why previous studies did not observe a statistically significant association between graft rejection and post‐transplant AF in general. 2 , 3 , 4
Concerning further clinical outcomes, it is well‐known that patients with AF have a higher risk for thromboembolic complications such as stroke. 28 In accordance, we found a significantly higher percentage of stroke within the first year after HTX in patients with AF before and ≤ 30 days after HTX (8.2%) as well as in patients without AF before HTX but with AF ≤ 30 days after HTX (6.7%). Additionally, AF has been associated with bradycardia and need for PPM implantation. 43 , 44 , 45 Especially in patients with sinus node dysfunction, successful treatment of AF and restoration of sinus rhythm may manifest in severe bradycardia requiring PPM implantation. 4 , 24 In our study, we also found a significantly higher percentage of 1 year PPM implantation after HTX in patients with AF before and ≤ 30 days after HTX (5.5%) as well as in patients without AF before HTX but with AF ≤ 30 days after HTX (6.7%) indicating an association between post‐transplant AF and the need for PPM implantation after HTX.
Regarding echocardiographic features, we found a higher percentage of enlarged atria, ventricular dysfunction, and mitral regurgitation, in patients with AF before and after HTX as well as in patients without AF before HTX but with AF ≤ 30 days after HTX. Mitral regurgitation can provoke the development of AF by inducing rapidly discharging triggers and jet‐induced stretching of the left atrium. 4 Furthermore, the presence of mitral regurgitation may be related to dilated atrial size affecting valvular integrity and function. 46 Mitral regurgitation and enlarged left atrial diameter after HTX as risk factors for post‐transplant AF highlight the importance of preserved anatomic integrity. 4
Association between atrial fibrillation before and after heart transplantation
The majority of patients with post‐transplant AF within 30 days after HTX (83.0%) already had AF prior to HTX indicating an association between AF before and after HTX (P < 0.01). In addition, multivariate analysis showed a more than six‐fold elevated risk for AF within 30 days after HTX in patients with AF before HTX (HR: 6.59, CI: 3.72–11.65; P < 0.01). Furthermore, our findings suggest two different groups of patients with post‐transplant AF. The first and larger group of patients with early post‐transplant AF (83.0%) already had AF prior to HTX, received older donor hearts, had a longer ischaemic time, had the lowest percentage of total orthotopic HTX, and mainly died from infection/sepsis. The second and smaller group of patients with early post‐transplant AF (17.0%) had no AF prior to HTX, mainly received cyclosporine A and azathioprine as immunosuppressive drug therapy, showed a higher percentage of early graft rejection, and predominantly died from graft failure. Of note, both groups, patients with AF before and after HTX as well as patients without AF before HTX but with AF ≤ 30 days after HTX, had a significantly higher requirement for 1 year PPM implantation and significantly more often suffered from stroke within the first year after HTX.
In conclusion, our data indicate an association between AF before and AF after HTX. As patients with AF before HTX are more likely to develop AF after HTX and patients with AF are at higher risk for stroke, 28 close heart rhythm follow‐ups including ECG, monitor‐telemetry and 24 h Holter recording as well as adequate oral anticoagulation in case of post‐transplant AF is required in order to prevent stroke and other worse clinical outcomes.
Study limitations
Our findings are derived from a single‐center registry (Heidelberg HTX Registry). This design has certain limitations and results therefore have to be interpreted carefully. However, in contrast to several multicenter studies or registries, we could include the highly detailed data of 639 HTX recipients in this study which is comparable in sample size with many multicenter studies. Furthermore, these patients received a standardized treatment and follow‐up, reducing the likelihood of potential selection bias and confounders. 4 , 15 , 16 , 24 , 25 , 26
Due to of the long study period (1989–2019), a possible era effect as a consequence of changes in surgical approaches, medical care, diagnostic, and treatment options may have influenced our results. We therefore performed a sensitivity analysis with patients who were administered tacrolimus and mycophenolate mofetil, as the immunosuppressive drug therapy was switched from 2006 onward. This analysis showed similar results substantiating the robustness of our findings. 4 , 15 , 16 , 24 , 25 , 26
Our results should be regarded as hypothesis generating, especially in the context of early post‐transplant AF or mortality, as different factors may negatively affect these outcomes. Furthermore, our data merely indicated an association between AF before HTX and AF ≤ 30 days after HTX but can neither proof nor disproof a causal relationship. In this context, further large multicenter trials are required to investigate the association between AF before and after HTX.
Conclusions
Early post‐transplant AF has been associated with worse clinical outcomes. We therefore investigated the association between AF before HTX and AF within 30 days after HTX. Our study comprised a total of 639 HTX recipients including 332 patients (52.0%) without AF before or ≤ 30 days after HTX, 15 patients (2.3%) without AF before HTX but with AF ≤ 30 days after HTX, 219 patients (34.3%) with AF before HTX but without AF ≤ 30 days after HTX, and 73 patients (11.4%) with AF before and ≤ 30 days after HTX.
Our analysis showed two different groups of patients with early post‐transplant AF, each with specific characteristics. Patients with AF before and after HTX received older donor hearts, had a longer ischaemic time, had the lowest percentage of total orthotopic HTX, and mainly died from infection/sepsis, while patients without AF before HTX but with AF ≤ 30 days after HTX primarily received cyclosporine A and azathioprine as immunosuppressive drug therapy, showed a higher percentage of early graft rejection, and predominantly died from graft failure.
Importantly, both groups, patients with AF before and after HTX as well as patients without AF before HTX but with AF ≤ 30 days after HTX, had a significantly higher 1 year mortality, a significantly higher 1 year PPM implantation rate, and a significantly higher 1 year stroke rate than patients without AF ≤ 30 days after HTX.
Multivariate analysis showed higher donor age and prolonged ischaemic time as significant risk factors for early post‐transplant AF, whereas total orthotopic HTX was associated with a two‐fold lower risk for AF ≤ 30 days after HTX. Most importantly, patients with AF before HTX had a more than six‐fold elevated risk for AF within 30 days after HTX (HR: 6.59, CI: 3.72–11.65; P < 0.01) indicating that AF before HTX is an important risk factor for early post‐transplant AF, independently of the other investigated risk factors.
Conflict of interest
None declared.
Funding
This work was supported by the Fondation Coeur—Daniel Wagner, Fondation de Luxembourg (FFD), the Physician‐Scientist‐Program of the Faculty of Medicine, University of Heidelberg (FFD and RR), the German Society of Internal Medicine (AKR), and the German Heart Foundation/German Foundation of Heart Research (RR).
Acknowledgements
We thank Viola Deneke and Berthold Klein for their assistance and advice.
Darche, F. F. , Helmschrott, M. , Rahm, A.‐K. , Thomas, D. , Schweizer, P. A. , Bruckner, T. , Ehlermann, P. , Kreusser, M. M. , Warnecke, G. , Frey, N. , and Rivinius, R. (2021) Atrial fibrillation before heart transplantation is a risk factor for post‐transplant atrial fibrillation and mortality. ESC Heart Failure, 8: 4265–4277. 10.1002/ehf2.13552.
References
- 1. Chokesuwattanaskul R, Bathini T, Thongprayoon C, Preechawat S, O'Corragain OA, Pachariyanon P, Ungprasert P, Cheungpasitporn W. Atrial fibrillation following heart transplantation: a systematic review and meta‐analysis of observational studies. J Evid Based Med 2018; 11: 261–271. [DOI] [PubMed] [Google Scholar]
- 2. Dasari TW, Pavlovic‐Surjancev B, Patel N, Williams AA, Ezidinma P, Rupani A, Sinacore JL, Heroux AL. Incidence, risk factors, and clinical outcomes of atrial fibrillation and atrial flutter after heart transplantation. Am J Cardiol 2010; 106: 737–741. [DOI] [PubMed] [Google Scholar]
- 3. Pavri BB, O'Nunain SS, Newell JB, Ruskin JN, William G. Prevalence and prognostic significance of atrial arrhythmias after orthotopic cardiac transplantation. J Am Coll Cardiol 1995; 25: 1673–1680. [DOI] [PubMed] [Google Scholar]
- 4. Rivinius R, Helmschrott M, Ruhparwar A, Erbel C, Gleissner CA, Darche FF, Thomas D, Bruckner T, Katus HA, Doesch AO. The influence of surgical technique on early posttransplant atrial fibrillation – comparison of biatrial, bicaval, and total orthotopic heart transplantation. Ther Clin Risk Manag 2017; 13: 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohn WE, Gregoric ID, Radovancevic B, Wolf RK, Frazier OH. Atrial fibrillation after cardiac transplantation: experience in 498 consecutive cases. Ann Thorac Surg 2008; 85: 56–58. [DOI] [PubMed] [Google Scholar]
- 6. Ferretto S, Giuliani I, Sanavia T, Bottio T, Fraiese AP, Gambino A, Tarzia V, Toscano G, Iliceto S, Gerosa G, Leoni L. Atrial fibrillation after orthotopic heart transplantatation: Pathophysiology and clinical impact. Int J Cardiol Heart Vasc 2021; 32: 100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scott CD, Dark JH, McComb JM. Arrhythmias after cardiac transplantation. Am J Cardiol 1992; 70: 1061–1063. [DOI] [PubMed] [Google Scholar]
- 8. Shumway NE, Lower RR, Stofer RC. Transplantation of the heart. Adv Surg 1966; 2: 265–284. [PubMed] [Google Scholar]
- 9. Sievers HH, Weyand M, Kraatz EG, Bernhard A. An alternative technique for orthotopic cardiac transplantation, with preservation of the normal anatomy of the right atrium. Thorac Cardiovasc Surg 1991; 39: 70–72. [DOI] [PubMed] [Google Scholar]
- 10. Dreyfus G, Jebara V, Mihaileanu S, Carpentier AF. Total orthotopic heart transplantation: an alternative to the standard technique. Ann Thorac Surg 1991; 52: 1181–1184. [DOI] [PubMed] [Google Scholar]
- 11. Dell'Aquila AM, Mastrobuoni S, Bastarrika G, Praschker BL, Agüero PA, Castaño S, Herreros J, Rabago G. Bicaval versus standard technique in orthotopic heart transplant: assessment of atrial performance at magnetic resonance and transthoracic echocardiography. Interact Cardiovasc Thorac Surg 2012; 14: 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Locali RF, Matsuoka PK, Cherbo T, Gabriel EA, Buffolo E. Should biatrial heart transplantation still be performed?: A meta‐analysis. Arq Bras Cardiol 2010; 94: 829–840. [DOI] [PubMed] [Google Scholar]
- 13. Morgan JA, Edwards NM. Orthotopic cardiac transplantation: comparison of outcome using biatrial, bicaval, and total techniques. J Card Surg 2005; 20: 102–106. [DOI] [PubMed] [Google Scholar]
- 14. Schnoor M, Schäfer T, Lühmann D, Sievers HH. Bicaval versus standard technique in orthotopic heart transplantation: a systematic review and meta‐analysis. J Thorac Cardiovasc Surg 2007; 134: 1322–1331. [DOI] [PubMed] [Google Scholar]
- 15. Rivinius R, Helmschrott M, Ruhparwar A, Schmack B, Darche FF, Thomas D, Bruckner T, Katus HA, Ehlermann P, Doesch AO. Chronic obstructive pulmonary disease in patients after heart transplantation is associated with a prolonged hospital stay, early post‐transplant atrial fibrillation, and impaired post‐transplant survival. Clin Epidemiol 2018; 10: 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rivinius R, Helmschrott M, Ruhparwar A, Schmack B, Darche FF, Thomas D, Bruckner T, Doesch AO, Katus HA, Ehlermann P. Elevated pre‐transplant pulmonary vascular resistance is associated with early post‐transplant atrial fibrillation and mortality. ESC Heart Fail 2020; 7: 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thajudeen A, Stecker EC, Shehata M, Patel J, Wang X, McAnulty JH Jr, Kobashigawa J, Chugh SS. Arrhythmias after heart transplantation: mechanisms and management. J Am Heart Assoc 2012; 1: e001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lefroy DC, Fang JC, Stevenson LW, Hartley LH, Friedman PL, Stevenson WG. Recipient‐to‐donor atrioatrial conduction after orthotopic heart transplantation: surface electrocardiographic features and estimated prevalence. Am J Cardiol 1998; 82: 444–450. [DOI] [PubMed] [Google Scholar]
- 19. Dahu MI, Hutchinson MD. What is the mechanism of the atrial arrhythmia in a patient after orthotopic heart transplantation? J Cardiovasc Electrophysiol 2012; 23: 225–227. [DOI] [PubMed] [Google Scholar]
- 20. Hayek A, Gardey K, Dulac A, Bessiere F, Chevalier P. Atrial arrhythmia in a patient after bicaval heart transplantation: Evidence for recipient‐to‐donor conduction. HeartRhythm Case Rep 2019; 6: 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johner N, Namdar M, Buttu A, Perenet S, Meyer P, Shah DC. Recipient atrial fibrillation manifesting as an irregular monomorphic atrial tachycardia in the donor heart. Can J Cardiol 2017; 33: 1336.e5–1336.e8. [DOI] [PubMed] [Google Scholar]
- 22. Landolina M, De Ferrari GM, Cantù F, Campana C. Donor‐to‐recipient decremental conduction of atrial fibrillation following orthotopic heart transplantation: insights into the mechanism of atrioatrial conduction. J Cardiovasc Electrophysiol 2000; 11: 1043–1047. [DOI] [PubMed] [Google Scholar]
- 23. Ribbing M, Mönnig G, Wasmer K, Breithardt G, Eckardt L. Catheter ablation of atrial tachycardia due to recipient‐to‐donor transatrial conduction after orthotopic heart transplantation. Europace 2004; 6: 215–219. [DOI] [PubMed] [Google Scholar]
- 24. Rivinius R, Helmschrott M, Rahm AK, Darche FF, Thomas D, Bruckner T, Doesch AO, Ehlermann P, Katus HA, Zitron E. Risk factors and survival of patients with permanent pacemaker implantation after heart transplantation. J Thorac Dis. 2019; 11: 5440–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rivinius R, Helmschrott M, Rahm AK, Darche FF, Thomas D, Bruckner T, Doesch AO, Ehlermann P, Katus HA, Zitron E. Combined amiodarone and digitalis therapy before heart transplantation is associated with increased post‐transplant mortality. ESC Heart Fail 2020; 7: 2082–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rahm AK, Helmschrott M, Darche FF, Thomas D, Bruckner T, Ehlermann P, Kreusser MM, Warnecke G, Frey N, Rivinius R. Newly acquired complete right bundle branch block early after heart transplantation is associated with lower survival. ESC Heart Fail 2021. 10.1002/ehf2.13494. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu‐Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005; 24: 1710–1720. [DOI] [PubMed] [Google Scholar]
- 28. Hohnloser SH, Pajitnev D, Pogue J, Healey JS, Pfeffer MA, Yusuf S, Connolly SJ, ACTIVE W Investigators . Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol 2007; 50: 2156–2161. [DOI] [PubMed] [Google Scholar]
- 29. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, ESC Scientific Document Group . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021; 42: 373–498. [DOI] [PubMed] [Google Scholar]
- 30. Knaut M, Sindt M, Madej T. Postoperatives Vorhofflimmern. Z Herz‐ Thorax‐ Gefäßchir 2017; 31: 95–109. [Google Scholar]
- 31. Piccini JP, Allen LA. Heart failure complicated by atrial fibrillation: Don't Bury the Beta‐blockers just yet. JACC Heart Fail 2017; 5: 107–109. [DOI] [PubMed] [Google Scholar]
- 32. Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014; 64: 710–721. [DOI] [PubMed] [Google Scholar]
- 33. Ziff OJ, Carter PR, McGowan J, Uppal H, Chandran S, Russell S, Bainey KR, Potluri R. The interplay between atrial fibrillation and heart failure on long‐term mortality and length of stay: insights from the, United Kingdom ACALM registry. Int J Cardiol 2018; 252: 117–121. [DOI] [PubMed] [Google Scholar]
- 34. Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, Collins JJ Jr, Cohn LH, Burstin HR. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation 1996; 94: 390–397. [DOI] [PubMed] [Google Scholar]
- 35. Echahidi N, Pibarot P, O'Hara G, Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol 2008; 51: 793–801. [DOI] [PubMed] [Google Scholar]
- 36. Cohn WE, Gregoric ID, Radovancevic B, Wolf RK, Frazier OH. Atrial fibrillation after cardiac transplantation: experience in 498 consecutive cases. Ann Thorac Surg 2008; 85: 56–58. [DOI] [PubMed] [Google Scholar]
- 37. Cui G, Tung T, Kobashigawa J, Laks H, Sen L. Increased incidence of atrial flutter associated with the rejection of heart transplantation. Am J Cardiol 2001; 88: 280–284. [DOI] [PubMed] [Google Scholar]
- 38. Scott CD, Dark JH, McComb JM. Arrhythmias after cardiac transplantation. Am J Cardiol 1992; 70: 1061–1063. [DOI] [PubMed] [Google Scholar]
- 39. Helmschrott M, Beckendorf J, Akyol C, Ruhparwar A, Schmack B, Erbel C, Gleissner CA, Akhavanpoor M, Ehlermann P, Bruckner T, Katus HA, Doesch AO. Superior rejection profile during the first 24 months after heart transplantation under tacrolimus as baseline immunosuppressive regimen. Drug Des Devel Ther 2014; 8: 1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gasparova I, Kubatka P, Opatrilova R, Caprnda M, Filipova S, Rodrigo L, Malan L, Mozos I, Rabajdova M, Nosal V, Kobyliak N, Valentova V, Petrovic D, Adamek M, Kruzliak P. Perspectives and challenges of antioxidant therapy for atrial fibrillation. Naunyn Schmiedebergs Arch Pharmacol 2017; 390: 1–14. [DOI] [PubMed] [Google Scholar]
- 41. Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol 2007; 115: 135–143. [DOI] [PubMed] [Google Scholar]
- 42. Ogi H, Nakano Y, Niida S, Dote K, Hirai Y, Suenari K, Tonouchi Y, Oda N, Makita Y, Ueda S, Kajihara K, Imai K, Sueda T, Chayama K, Kihara Y. Is structural remodeling of fibrillated atria the consequence of tissue hypoxia? Circ J 2010; 74: 1815–1821. [DOI] [PubMed] [Google Scholar]
- 43. John RM, Kumar S. Sinus node and atrial arrhythmias. Circulation 2016; 133: 1892–1900. [DOI] [PubMed] [Google Scholar]
- 44. Semmler V, von Krogh F, Haller B, Reents T, Bourier F, Telishevska M, Kottmaier M, Kornmayer M, Brooks S, Koch‐Büttner K, Lennerz C, Brkic A, Grebmer C, Blazek P, Weigand S, Hessling G, Kolb C, Deisenhofer I. The incidence, indications and predictors of acute pacemaker implantation after ablation of persistent atrial fibrillation. Clin Res Cardiol 2019; 108: 651–659. [DOI] [PubMed] [Google Scholar]
- 45. Wagner L, Darche FF, Thomas D, Lugenbiel P, Xynogalos P, Seide S, Scholz EP, Katus HA, Schweizer PA. Cryoballoon pulmonary vein isolation‐mediated rise of sinus rate in patients with paroxysmal atrial fibrillation. Clin Res Cardiol 2021; 110: 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Angermann CE, Spes CH, Tammen A, Stempfle HU, Schütz A, Kemkes BM, Theisen K. Anatomic characteristics and valvular function of the transplanted heart: transthoracic versus transesophageal echocardiographic findings. J Heart Transplant 1990; 9: 331–338. [PubMed] [Google Scholar]


