Abstract
Aims
This study aimed to explore the rapid effects of dapagliflozin in heart failure with reduced ejection fraction (HFrEF).
Methods and results
We studied the functional, echocardiographic, electrophysiological, lung ultrasound, ambulatory blood pressure (BP), microvascular and macrovascular function, and biochemical effects of 2 week treatment with dapagliflozin in 19 type 2 diabetic HFrEF patients in a double‐blind, crossover, placebo‐controlled trial. Dapagliflozin had no significant effect on clinical, functional, or quality of life parameters. Dapagliflozin reduced systolic BP [114 (105, 131) vs. 106 (98, 113) mmHg, P < 0.01] and diastolic BP [71 (61, 78) vs. 62 (55, 70) mmHg, P < 0.01]. There was no effect on cardiac chamber size, ventricular systolic function, lung ultrasound, or arterial wave reflection. Dapagliflozin increased creatinine [117 (92, 129) vs. 122 (107, 135) μmol/L, P < 0.05] and haemoglobin [135 (118, 138) vs. 136 (123, 144) g/L, P < 0.05]. There was a reduction in ventricular ectopy [1.4 (0.1, 2.9) vs. 0.2 (0.1, 1.4) %, P < 0.05] and an increase in standard deviation of normal heart beat intervals [70 (58, 90) vs. 74 (62, 103), P < 0.05]. Unexpectedly, dapagliflozin increased high‐sensitivity troponin T [25 (19, 37) vs. 28 (20, 42) ng/L, P < 0.01] and reduced reactive hyperaemia index [1.29 (1.21, 1.56) vs. 1.40 (1.23, 1.84), P < 0.05].
Conclusions
After 2 weeks, while multiple parameters supported BP reduction and haemoconcentration with dapagliflozin, reduction in cardiac filling pressure, lung water, and functional improvement was not shown. Reduced ventricular ectopic burden suggests an early antiarrhythmic benefit. The small increase in troponin T and the reduction in the reactive hyperaemia index warrant further mechanistic exploration in this treatment of proven mortality benefit in HFrEF.
Keywords: HFrEF, Dapagliflozin, Mechanism, Acute effects
Introduction
While sodium–glucose cotransporter 2 (SGLT2) inhibitors have morbidity and mortality benefits in heart failure (HF) with reduced ejection fraction (HFrEF), 1 , 2 , 3 the mechanisms responsible for their protective effect remain speculative. 4 Postulated benefits include diuresis, renal protection, weight loss, improved myocardial energetics, reduced myocardial fibrosis, reduced blood pressure (BP) and arterial stiffness, and attenuation of endothelial dysfunction. 4 Intriguingly from a mechanistic viewpoint, the benefit appears to manifest within weeks. 3 , 5 Rapid salient effects of this magnitude in HFrEF suggest haemodynamic attenuation of the pathophysiological HF state, an antiarrhythmic effect, or improved myocardial function. The latter two have generally proven to be mutually exclusive in the pharmacological management of HFrEF through experiences with inotropic agents. 6 Finally, a non‐cardiovascular end‐organ benefit in HFrEF may improve short‐term outcome (HF hospitalization), with the most likely candidate organs being the lungs or kidneys.
We explored the very early (2 weeks) haemodynamic, cardiac energetics and arrhythmic effects of dapagliflozin in type 2 diabetes mellitus (T2DM) HFrEF subjects to inform and direct future mechanistic studies.
Methods
Nineteen T2DM subjects [HBA1c > 7.0%, age 73 (63, 81) years, 26% female] with chronic HFrEF [left ventricular ejection fraction 35% (25, 40)], on stable, maximally tolerated doses of guideline directed medical therapy for at least 2 weeks, were studied (Supporting Information, Figure S1 ). Subjects were at least 2 weeks distant from a hospitalization for decompensated HF. All subjects were taking angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers/angiotensin receptor neprilysin inhibitors and beta‐blockers, and 95% were taking mineralocorticoid receptor antagonists. After baseline investigations, subjects received double‐blind dapagliflozin 10 mg daily or matching placebo for 2 weeks before investigations were repeated. After a further 2 week washout period, subjects were crossed over for two more weeks of therapy after which investigations were repeated.
Investigations were composed of (i) functional assessment with 6 min walk test (6‐MWT), quality of life score EQ‐5D‐5L (sum score of five domains: mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression), and visual analogue scale of ‘health today’ (EuroQol Research Foundation, Rotterdam, the Netherlands); (ii) clinical assessment with New York Heart Association functional classification, HF clinical signs score [crepitations (0–3 based on severity), jugular venous pressure (JVP) elevation (0–3), and peripheral oedema (0–3)], waist circumference, and body weight; (iii) haemodynamic assessment with ambulatory 24 h BP monitor, central aortic haemodynamic assessment with radial artery applanation tomography 7 (SphygmoCor, AtCor Medical, Sydney, Australia), and microvascular endothelial function assessment following 5 min of cuff brachial artery occlusion using reactive hyperaemia peripheral arterial tonometry (EndoPAT, Itamar Medical, Caesarea, Israel) 8 ; (iv) echocardiographic assessment of cardiac chamber size, chamber filling pressure [mitral E‐wave velocity to mitral annular velocity ratio (E:e′), tricuspid regurgitation gradient, and inferior vena cava size], and systolic function (left ventricular ejection fraction, left ventricular outflow tract velocity time integral, left ventricular global longitudinal strain, tricuspid annular plane systolic excursion, and right ventricular S‐wave velocity); (v) lung ultrasound assessment of extravascular lung water (Kerley B line quantification) 9 ; (vi) cardiac rhythm assessment of 24 h average heart rate, ventricular ectopy frequency (percent of total heart beats), and heart rate variability [standard deviation of normal heart beat intervals (SDNN)]; and (vii) biochemical assessment of cardiac parameters [N‐terminal fragment of pro‐brain natriuretic peptide (NT‐proBNP) and high‐sensitivity troponin T], renal parameters (sodium, potassium, bicarbonate, and creatinine), hepatic parameters (aspartate aminotransferase and gamma‐glutamyl transferase), haematological parameters (haemoglobin), inflammatory parameters (C‐reactive protein), and mixed‐organ origin parameters (lactate dehydrogenase, ferritin, albumin, and urate). All investigations and clinical staff were blinded to treatment. Compliance was confirmed by pharmacist reconciliation of returned drug (average compliance 96%).
At the time of recruitment, SGLT2 inhibitors were not supported as a therapy for HFrEF in Australia. They were prescribed in this cohort on the basis of type 2 diabetes mellitus. Trial participation resulted in participants being denied dapagliflozin for 6 weeks of the 8 week trial duration. This period was assessed as being too short to impact the well‐being of a type 2 diabetic. The study complies with the Declaration of Helsinki and was approved by the Southern Adelaide Clinical Human Research Ethics Committee, approval—203.18. Informed consent was obtained from all subjects.
Given the exploratory nature of this study, the sample size was determined based on the change in 6‐MWT, a validated HFrEF functional endpoint that incorporates several potential pathophysiological changes of interest. Our power calculation for 6‐MWT, the primary endpoint, suggested that 22 subjects were required to detect an effect size of 30 m (standard deviation 50 m) with 80% power and a level of significance of 0.05. The study was stopped at 19 subjects following the release of data from a much larger study of empagliflozin in HFrEF (n = 315) in February 2021, showing no significant benefit in 6‐MWT. 10 As data were not normally distributed, results are displayed as median (25th, 75th percentile) or number (%) and compared with Wilcoxon signed‐rank test for continuous variables, P < 0.05 reported as statistically significant. To account for baseline variability, our analysis compared the difference between baseline and placebo vs. difference between baseline and treatment. In comparing treatment effects between treatment periods for these parameters, no carry‐over effect between Period 1 and Period 2 was observed. No correction for multiple comparisons was made because of the relatively small subject numbers and the desire to explore the many potential impacts of dapagliflozin in HFrEF.
Results
Dapagliflozin did not alter 6‐MWT distance after 2 weeks [338 (281, 397) m] vs. placebo [327 (208, 389) m, P = 0.118] (Figure 1 ). Dapagliflozin had no significant effect on clinical, functional, or quality of life parameters except body weight, which fell marginally [90 (73, 111) vs. 89 (73, 110) kg, P < 0.05]. Dapagliflozin reduced systolic BP [114 (105, 131) vs. 106 (98, 113) mmHg, P < 0.01] and diastolic BP [71 (61, 78) vs. 62 (55, 70) mmHg, P < 0.01]. While radial artery‐derived central arterial pulse pressure fell with dapagliflozin [42 (34, 54) vs. 38 (28, 43) mmHg, P < 0.01], peripheral capillary‐derived reactive hyperaemia index also fell [1.40 (1.23, 1.84) vs. 1.29 (1.21, 1.56), P < 0.05]. There was no effect on cardiac chamber sizes, ventricular systolic function, lung ultrasound detection of extravascular lung water, NT‐proBNP, or wave reflection (augmentation index). Dapagliflozin increased serum creatinine [117 (92, 129) vs. 122 (107, 135) μmol/L, P < 0.05], haemoglobin [135 (118, 138) vs. 136 (123, 144) g/L, P < 0.05], and albumin [37 (35, 39) vs. 39 (36, 41) g/L, P < 0.01]. Dapagliflozin did not change average heart rate; however, there was a reduction in the frequency of ventricular ectopy [1.4 (0.1, 2.9) vs. 0.2 (0.1, 1.4) %, P < 0.05] and an increase in the heart rate variability marker of SDNN [70 (58, 90) vs. 74 (62, 103), P < 0.05]. Unexpectedly, dapagliflozin increased high‐sensitivity troponin T [25 (19, 37) vs. 28 (20, 42) ng/L, P < 0.01] (Table 1 ).
Figure 1.
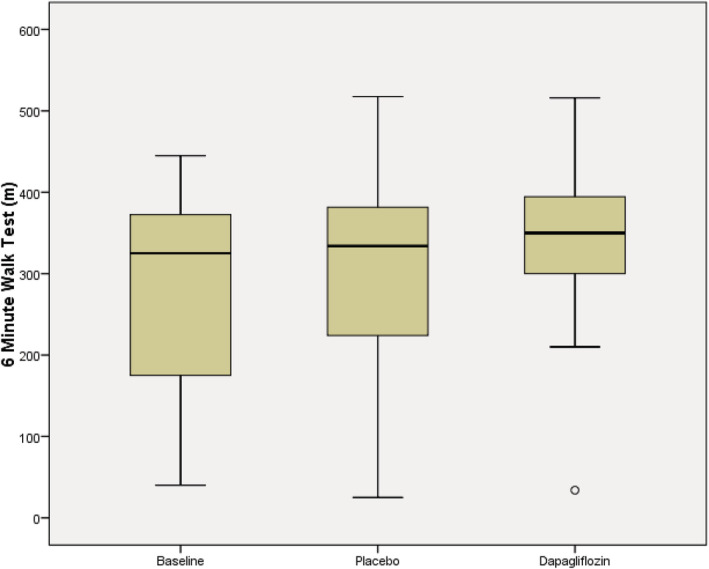
Six minute walk test. n = 19, 6 min walk test distance (m), box and whisker plot (median, quartiles, and maximum/minimum) of baseline, placebo (2 week treatment), and dapagliflozin (2 week treatment). The difference between baseline–placebo and baseline–dapagligflozin was compared with no significant difference shown, P = 0.118.
Table 1.
Results of investigations: (i) clinical parameters (NYHA class, clinical score, body weight, and waist circumference); (ii) functional assessment (6 min walk test and quality of life score); (iii) haemodynamic assessment (24 h BP monitor radial artery applanation tomography and peripheral artery tonometry); (iv) cardiac rhythm assessment of 24 h Holter; (v) echocardiographic assessment; (vi) lung ultrasound assessment of extravascular lung water; and (vii) biochemical parameters
| Baseline | Placebo | Dapagliflozin | P value b | |
|---|---|---|---|---|
| Clinical parameters | ||||
| 6‐MWT (m) a | 325 (153, 385) | 327 (208, 389) | 338 (281, 397) | 0.118 |
| NYHA class, n (II, III) | 14, 5 | 15, 4 | 16, 3 | NS |
| Clinical score d | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | NS |
| Body weight (kg) | 90 (73, 110) | 90 (73, 111) | 89 (73, 110) | <0.05 c |
| Waist circumference (cm) | 110 (94, 124) | 107 (98, 123) | 106 (95, 124) | NS |
| Quality of life (sum score) | 8 (5, 10) | 8 (5, 10) | 7 (5, 9) | NS |
| Quality of life (visual analogue score) | 75 (70, 85) | 76 (70, 90) | 80 (75, 90) | NS |
| 24 h BP monitor | ||||
| Systolic BP (mmHg) | 112 (100, 128) | 114 (105, 131) | 106 (98, 113) | <0.01 c |
| Diastolic BP (mmHg) | 69 (61, 76) | 71 (61, 78) | 62 (55, 70) | <0.01 c |
| Radial artery applanation tomography | ||||
| Systolic BP (mmHg) | 113 (99, 119) | 111 (95, 130) | 101 (95, 110) | <0.05 c |
| Diastolic BP (mmHg) | 66 (60, 74) | 69 (61, 76) | 67 (62, 72) | NS |
| Pulse pressure (mmHg) | 43 (32, 55) | 42 (34, 54) | 38 (28, 43) | <0.01 c |
| Augmentation index | 20 (16, 24) | 24 (15, 29) | 18 (10, 28) | NS |
| Peripheral arterial tonometry | ||||
| Reactive hyperaemia index | 1.39 (1.09, 1.71) | 1.40 (1.23, 1.84) | 1.29 (1.21, 1.56) | <0.05 c |
| Holter monitor | ||||
| Average HR (b.p.m.) | 61 (51, 70) | 65 (51, 73) | 62 (52, 69) | NS |
| Ventricular ectopy (%) | 0.2 (0.03, 2.6) | 1.4 (0.1, 2.9) | 0.2 (0.09, 1.4) | <0.05 c |
| SDNN | 65 (52, 88) | 70 (58, 90) | 74 (62, 103) | <0.05 c |
| Echocardiography | ||||
| LVEDD (cm) | 5.5 (5, 6.3) | 5.5 (5.2, 6.2) | 5.5 (5.1, 6.4) | NS |
| LVESV (mL) | 82 (46, 110) | 69 (45, 106) | 75 (53, 118) | NS |
| LV ejection fraction (%) | 43 (31, 48) | 42 (35, 52) | 44 (36, 51) | NS |
| LV velocity time integral (cm) | 17 (15, 19) | 19 (16, 22) | 17 (16, 19) | NS |
| LV global longitudinal strain (%) | −9 (−11, −8) | −11 (−12, −9) | −10 (−13, −9) | NS |
| LA size (mL/m2) | 56 (44, 76) | 53 (40, 67) | 49 (39, 83) | NS |
| E:e′ | 14 (12, 16) | 14 (13, 20) | 14 (12, 19) | NS |
| RVs′ (cm/s) | 8 (7, 9) | 8 (6, 10) | 8 (6, 11) | NS |
| TAPSE (cm) | 1.7 (1.5, 2) | 1.6 (1.3, 1.8) | 1.4 (1.3, 1.9) | NS |
| Tricuspid regurgitation gradient (mmHg) | 27 (23, 34) | 28 (24, 33) | 27 (22, 37) | NS |
| Inferior vena cava size (cm) | 1.1 (0.8, 1.3) | 1.1 (0.9, 1.2) | 1.1 (0.8, 1.3) | NS |
| Lung ultrasound | ||||
| Kerley B lines (n) | 8 (3, 12) | 5 (3, 7) | 3 (2, 8) | NS |
| Haematology/biochemistry | ||||
| Haemoglobin (g/L) | 135 (115, 138) | 135 (118, 138) | 136 (123, 144) | <0.05 c |
| Sodium (mmol/L) | 140 (137, 141) | 139 (137, 140) | 139 (138, 142) | NS |
| Potassium (mmol/L) | 4.3 (4.2, 4.6) | 4.3 (4, 4.7) | 4.4 (4.2, 4.5) | NS |
| Bicarbonate (mmol/L) | 27 (25, 30) | 27 (24, 29) | 27 (26, 30) | NS |
| Creatinine (μmol/L) | 112 (93, 132) | 117 (92, 129) | 122 (107, 135) | <0.05 c |
| Albumin (g/L) | 37 (34, 39) | 37 (35, 39) | 39 (36, 41) | <0.01 c |
| Urate (mmol/L) | 0.46 (0.34, 0.53) | 0.36 (0.3, 0.46) | 0.38 (0.34, 0.46) | NS |
| Aspartate aminotransferase (U/L) | 22 (20, 27) | 22 (19, 26) | 24 (20, 27) | NS |
| Gamma‐glutamyl transferase (U/L) | 34 (20, 58) | 33 (19, 55) | 33 (19, 49) | NS |
| Lactate dehydrogenase (U/L) | 196 (175, 229) | 197 (160, 233) | 195 (164, 225) | 0.058 |
| C‐reactive protein (mg/L) | 2 (1, 6.4) | 2 (0.8, 11) | 1 (0.7, 6) | NS |
| Ferritin (μg/L) | 193 (159, 598) | 276 (105, 682) | 243 (127, 855) | NS |
| Iron saturation (%) | 24 (20, 32) | 24 (18, 31) | 25 (17, 31) | NS |
| Troponin T (ng/L) | 24 (18, 39) | 25 (19, 37) | 28 (20, 42) | <0.01 c |
| NT‐proBNP (ng/L) | 1043 (562, 1792) | 815 (537, 2045) | 803 (454, 2107) | NS |
6‐MWT, 6 min walk test; BP, blood pressure; E:e′, mitral E‐wave velocity to mitral annular velocity ratio; HR, heart rate; LA, left atrial; LV, left ventricular; LVEDD, left ventricular end‐diastolic diameter; LVESV, left ventricular end‐systolic volume; NT‐proBNP, N‐terminal fragment of pro‐brain natriuretic peptide; NYHA, New York Heart Association; RVs′, right ventricular systolic excursion velocity; SDNN, standard deviation of normal heart beat intervals; TAPSE, tricuspid annular plane systolic excursion.
Data are presented as median (25th, 75th percentile). There were missing data for the primary endpoint of 6‐MWT in two subjects at baseline and another subject on treatment; of the other endpoints reported, three had more than four missing data points: urate, tricuspid regurgitation gradient, and SDNN. There were no subject dropouts.
Primary endpoint.
Comparison between difference between baseline and placebo vs. difference between baseline and treatment.
Nominally statistically significant.
Sum of score based on clinical signs of heart failure; lung crepitations (0: none; 1: bases of lung fields only; 2: lower ½; and 3: over ½), JVP elevation (0: not elevated at 45°; 1: 1–4 cm elevated; 2: 4–8 cm; and 3: at ear lobes), and peripheral oedema (0: none; 1: to level of ankles; 2: calves; and 3: thighs).
Discussion
Consistent with the early beneficial effects in large clinical trials with SGLT2 inhibitors in HFrEF, we have demonstrated several salutatory changes in physiological and pathophysiological parameters following 2 weeks of therapy with dapagliflozin in T2DM HFrEF patients.
A relatively large BP reduction was demonstrated with dapagliflozin with evidence to support a reduction in central arterial stiffness (central arterial pulse pressure reduction). Surprisingly, there was a concomitant signal for reduced microvascular endothelial‐derived vasodilatation with dapagliflozin (reduced reactive hyperaemia index). Clinical trial data to explore endothelial function changes with SGLT2 inhibitors in T2DM patients are limited and conflicting. 11 , 12 Moreover, data in exclusive HFrEF populations are absent. Endothelial dysfunction is associated with T2DM, HFrEF, and several co‐morbidities present in our cohort; it is a dynamic state but one that is associated with poor cardiovascular outcome. Because SGLT2 inhibitors are of proven benefit in HFrEF, 1 , 2 , 3 the demonstration of attenuated of microvascular endothelial‐derived vasodilatation at 2 weeks in this cohort warrants confirmation and further exploration.
Two weeks of dapagliflozin therapy demonstrated evidence to support diuresis and haemoconcentration with increased haemoglobin, serum creatinine, and albumin concentrations, as well as total body weight reduction. We did not observe evidence of an associated reduction in cardiac filling pressure (by either echocardiography or with NT‐proBNP), cardiac chamber size, pulmonary interstitial lung water, and functional or clinical improvement arguing against a significant acute haemodynamic benefit through simple diuresis.
Our finding of a small increase in high‐sensitivity troponin T was unexpected. We cannot discount the reduction in renal function with dapagliflozin as an explanation for the increment in high‐sensitivity troponin T, although an ability to demonstrate this in our cohort would seem unlikely. Troponin T release from the myocardium is generally regarded as a marker of poorer prognosis, and this small magnitude increase appears counter‐intuitive with the proven long‐term morbidity and mortality benefits of the SGLT2 inhibitors. These incongruent observations may be mechanistically related through inhibition of the myocardial sodium–hydrogen exchange (NHE) pump. 13 NHE inhibitors have shown protective effects in models of cardiac damage by defending against intracellular acidosis, where they reduce cellular Na+ and Ca2+ concentrations and increase mitochondrial Ca2+. 14 In diabetic and failing hearts, cellular energetics are impaired, because ATP generation is directly linked to mitochondrial Ca2+, the increase in mitochondrial Ca2+ resulting from SGLT2 inhibition has been postulated to improve cardiac energetics. 15 This increased myocardial contraction may be associated with a small increase in high‐sensitivity troponin T, akin to what has been recently described with the positive inotropic agent omecamtiv mecarbil, which also does not increase intracellular Ca2+, 16 unlike traditional inotropic agents. 6 Although not examined in our study, this small troponin elevation seen after initiation of dapagliflozin is unlikely to be associated with adverse long‐term prognosis.
Reduced ventricular ectopic burden and increased heart rate variability (SDNN) support an early antiarrhythmic benefit of dapagliflozin in HFrEF. SGLT2 inhibitors have theoretical antiarrhythmic potential, as an elevation of intracellular Ca2+ concentration favours the opening probability of the ryanodine receptor channels with the consequent release of Ca2+ from the sarcoplasmic reticulum. This in turn leads to transient inward currents that are responsible for delayed after‐depolarizations. 17 This phenomenon typically triggers cardiac arrhythmias. Although the inhibitory effect of SGLT2 inhibitors towards NHE, with consequent reduced intracellular Ca2+ and arrhythmogenesis in the failing heart, supports the recently described observation of reduced atrial fibrillation with dapagliflozin, 18 many other potential SGLT2 inhibitor antiarrhythmic mechanisms are proposed. 19
The major limitation of the study is the relatively small number of participants. While the crossover design increases the trial power to show true effects, the unexpected changes and repeated comparisons design demand caution in interpretation and should be viewed as hypothesis generating.
In summary, significant BP lowering and evidence of haemoconcentration and weight loss are detectable after only 2 weeks of therapy with dapagliflozin in T2DM subjects with HFrEF. The signal of an antiarrhythmic effect supports an emerging early additional benefit of dapagliflozin in HFrEF. The apparent worsening of microvascular endothelial function and the small increase in troponin T warrant confirmation and further characterization in a larger cohort.
Conflict of interest
C.G.D.P. has received consultation fees from AstraZeneca, Boehringer Ingelheim, Lily, Pfizer, Sanofi, and Janssen and speaking honoraria from AstraZeneca, Boehringer Ingelheim, Sanofi, and Pfizer. He is a director of Flinders Cardiac/Heart and Vascular. D.P.C.'s institution has received a research grant form AstraZeneca Australia. He is a Matthew Flinders Fellow and a Professor of Cardiology at Flinders University. J.B.S. has received research grant support from Biotronik, Bayer, Sanofi, Actelion, Novartis, and Circle Cardiovascular Imaging. He has been on the advisory board of Sanofi, Faraday, and Recardio. He is a speaker bureau from AstraZeneca, Boehringer Ingelheim, Novartis, Abbot, Sanofi, Biotronik, Circle Cardiovascular Imaging, and Shire. He is a Professor of Cardiology at Flinders University, Director of Cardiac Imaging Southern Area Health Service, and Director of South Australian Health and Medical Research Institute. S.S. has received speakers fees from Lilly, Sanofi, and AstraZeneca and participated in advisory boards for AstraZeneca in the last 3 years. In the more distant past, he has received speaker's fees from Novo Nordisk and Boehringer Ingelheim and participated in advisory boards for Sanofi and the BI–Lilly alliance. He is the current President of the Australian Diabetes Society and Director of Southern Adelaide Diabetes and Endocrine Services. A.A.M. has attended workshops sponsored by Boehringer Ingelheim and received research grants from medac GmbH. He is a strategic professor of clinical pharmacology and senior consultant physician at Flinders Medical Centre. F.I. has no affiliations or disclosures to declare and is a consultant cardiologist at SA Health, South Australia. S.L.T. has no affiliations or disclosures and is a consultant cardiologist at Alfred Hospital, Melbourne, Victoria. L.J. has no affiliations or disclosures and is a consultant geriatrician at Northern Adelaide Local Health Network, South Australia. M.H. has no affiliations or disclosures. He is the clinical data manager at Flinders University and business intelligence coach at Flinders Medical Centre. T.H. has no affiliations or disclosures and is a senior research cardiac sonographer at Flinders Medical Centre. A.S. has no affiliations or disclosures and is a senior cardiac research sonographer at Flinders Medical Centre. F.W. has no affiliations or disclosures and is the manager of Flinders Cardiology Research Department at Flinders Medical Centre. S.T. has no affiliations or disclosures and is a clinical trials nurse at Flinders Cardiology Research Department at Flinders Medical Centre. W.C. has no affiliations or disclosures and is a senior pharmacist at Flinders Medical Centre. C.D.P. has no affiliations or disclosures and is a cardiology research assistant at Flinders Medical Centre.
Funding
The work was partially supported by a donated funding grant administered through the Flinders Medical Centre Foundation.
Supporting information
Figure S1. Consort diagram.
Acknowledgements
Study drug and matching placebo were supplied by AstraZeneca. Ambulatory blood pressure and Holter monitor equipment was supplied by Flinders Cardiac/Heart and Vascular.
Ilyas, F. , Jones, L. , Tee, S. L. , Horsfall, M. , Swan, A. , Wollaston, F. , Hecker, T. , De Pasquale, C. , Thomas, S. , Chong, W. , Stranks, S. , Mangoni, A. A. , Selvanayagam, J. B. , Chew, D. P. , and De Pasquale, C. G. (2021) Acute pleiotropic effects of dapagliflozin in type 2 diabetic patients with heart failure with reduced ejection fraction: a crossover trial. ESC Heart Failure, 8: 4346–4352. 10.1002/ehf2.13553.
References
- 1. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand SI, Belohlavek J, Bohm M, Chiang C, Chopra V, DeBoer RA, Desai SA, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde A. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 2. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Bohm M, Choi DJ, Chopra V, Chuquiiure E, Giannetti N, Janssens S, Zhang J, Juanatey JRG, Kaul S, Rocca HB, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 3. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med 2020; 10.1056/NEJMoa2030183. Published online ahead of print November 16 2020. [DOI] [PubMed] [Google Scholar]
- 4. Verma S. Potential mechanisms of sodium‐glucose co‐transporter 2 inhibitor‐related cardiovascular benefits. Am J Cardiol 2019; 24: S36–S44. [DOI] [PubMed] [Google Scholar]
- 5. Packer M, Anker S, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M, Miller A, Pehrson S, Teerlink JR, Bruieckmann M, Jamal W, Zeller C, Schnaidt S, Zannad F. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: The emperor‐reduced trial. Circulation; 143:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hasenfuss G, Teerlink J. Cardiac inotropes: current agents and future directions. Eur Heart J 2011; 32: 1838–1845. [DOI] [PubMed] [Google Scholar]
- 7. O'Rourke M, Adji A. Noninvasive generation of aortic pressure from radial pressure waveform by applanation tonometry, brachial cuff calibration, and generalised transfer function. Am J Hypertens 2013; 27: 143–145. [DOI] [PubMed] [Google Scholar]
- 8. Erre GL, Piga M, Fedele AL, Mura S, Piras A, Cadoni ML, Cangemi I, Dessi M, DiSante G, Tolusso B, Gremese I, Cauli A, Mangoni AA, Saba PS, Carru C, Ferraccioli G, Mathieu A, Passiu G. Prevalence and determinants of peripheral microvascular endothelial dysfunction in rheumatoid athritis patients: A multicenter cross‐ sectional study. Mediat Inflamm 2018; 2018: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T, International Liaison Committee on Lung Ultrasound (ILC‐LUS) for International Consensus Conference on Lung Ultrasound (ICC‐LUS) . International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med 2012; 38: 577–591. [DOI] [PubMed] [Google Scholar]
- 10. Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, Filippatos G, Gniot J, Fu M, Gullestad L, Howlett JG, Nicholls SJ, Redon J, Schenkenberger I, Silva‐Cardoso J, Störk S, Krzysztof Wranicz J, Savarese G, Brueckmann M, Jamal W, Nordaby M, Peil B, Ritter I, Ustyugova A, Zeller C, Salsali A, Anker SD. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J 2021; 42: 700–710. [DOI] [PubMed] [Google Scholar]
- 11. Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, Bruno RM. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol 2017; 16: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanaka A, Shimabukuro M, Machii N, Teragawa H, Okada Y, Shima KR, Takamura T, Taguchi I, Hisauchi I, Toyoda S, Matsuzawa Y, Timiyama H, Yamaoka‐ Tojo M, Yoshida H, Sato Y, Ikehara Y, Ueda S, Higashi Y, Node K. Effect of empagliflozin on endothelial function in patients with type 2 diabetes and cardiovascular disease: results from the multicenter, randomized, placebo‐ controlled, double‐blind EMBLEM trial. Diabetes Care 2019; 42: 159–161. [DOI] [PubMed] [Google Scholar]
- 13. Uthman L, Baartscheer A, Schumacher CA, Fiolet JWT, Kuschma MC, Hollmann MW, Coronel R, Weber NC, Zuurbier CJ. Direct cardiac actions of sodium glucose cotransporter 2 inhibitors target pathogenic mechanisms underlying heart failure in diabetic patients. Front Physiol 2018; 9: 1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karmazyn M, Gan XT, Humphreys RA, Yoshida H, Kusumoto K. The myocardial Na1‐H1 exchange structure, regulation, and its role in heart disease. Circ Res 1999; 85: 777–786. [DOI] [PubMed] [Google Scholar]
- 15. Bertero E, Maack C. Calcium signaling and reactive oxygen species in mitochondria. Circ Res 2018; 122: 1460–1478. [DOI] [PubMed] [Google Scholar]
- 16. Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias‐Mendoza A, Biering‐Sørensen T, Böhm M, Bonderman D, Cleland JGF, Corbalan R, Crespo‐Leiro MG, Dahlström U, Echeverria LE, Fang JC, Filippatos G, Fonseca C, Goncalvesova E, Goudev AR, Howlett JG, Lanfear DE, Li J, Lund M, Macdonald P, Mareev V, Momomura SI, O'Meara E, Parkhomenko A, Ponikowski P, Ramires FJA, Serpytis P, Sliwa K, Spinar J, Suter TM, Tomcsanyi J, Vandekerckhove H, Vinereanu D, Voors AA, Yilmaz MB, Zannad F, Sharpsten L, Legg JC, Varin C, Honarpour N, Abbasi SA, Malik FI, Kurtz CE; GALACTIC‐HF Investigators . Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021; 384: 105–116. [DOI] [PubMed] [Google Scholar]
- 17. Baartscheer A, Schumacher CA, Belterman CNW, Coronel R, Fiolet JWT. SR calcium handling and calcium after‐transients in a rabbit model of heart failure. Cardiovasc Res 2003; 58: 99–108. [DOI] [PubMed] [Google Scholar]
- 18. Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Budaj A, Kiss RG, Padilla F, Gause‐Nilsson I, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of dapagliflozin on atrial fibrillation in patient with type 2 diabetes mellitus. insights from the DECLARE‐TIMI 58 trial. Circ 2020; 141: 1227–1234. [DOI] [PubMed] [Google Scholar]
- 19. Zelniker TA, Braunwald E. Mechanisms of Cardiorenal Effects of Sodium‐Glucose Cotransporter 2 Inhibitors. J Am Coll Cardiol 2020; 75: 422–434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Consort diagram.


