Abstract
Aims
The TOPCAT trial showed no benefit for spironolactone in heart failure patients with preserved ejection fraction (HFpEF). Post‐hoc, spironolactone helped participants from the Americas, but not Eastern Europe. Determining which patients with HFpEF could respond like TOPCAT's responders should help guide their care. We aimed to develop a TOPCAT Trial Score (TS) as a composite metric to identify such patients.
Methods and results
From the TOPCAT individual‐level data, we calculated a TS of age, body mass index, systolic blood pressure, heart rate, creatinine, potassium, glucose, left ventricular ejection fraction, and left atrial volume for each participant as a weighted distance in multidimensional space from the theoretical perfectly average Americas participant. Logistic regression was used to measure TS and spironolactone as predictors of TOPCAT's primary outcome. The relationship between TS and the H2FPEF score was also determined in TOPCAT and a registry cohort of real‐world patients in the U.S. with HFpEF. A bimodal distribution of TS separated American (n = 1766) and Eastern European (n = 1,677) participants. Those with lower TS showed no significant response to spironolactone. Spironolactone's benefit rose with rising TS [βinteraction = ‐0.28 (P < 0.01)]. Significantly more American participants had benefit from spironolactone based on higher TS (> 1.14), in addition to higher likelihood of HFpEF based on higher H2FPEF scores (≥3). The cohort of real‐world patients with HFpEF had even higher TS than American TOPCAT participants.
Conclusions
Patients with HFpEF can be quantified by the TS to capture the likelihood of benefit from spironolactone.
Keywords: Heart failure; HFpEF; Spironolactone; Outcomes; Real‐world
Introduction
Over 6.2 million American adults are afflicted with heart failure (HF), and over half these patients have HF with preserved ejection fraction (HFpEF). 1 , 2 There are few consensus medical treatments for HFpEF despite numerous clinical trials, as most showed no benefit to the trialled intervention. 3 A key proposed reason for recurrent neutral trials is the difficulty in accurately diagnosing, and thus enrolling, patients with HFpEF. This difficulty has been particularly evident in analyses of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. 4 , 5 , 6 , 7 Recently, more accurate diagnostic scores and criteria have been developed, but there are limitations to the retrospective application of these criteria to prior randomized controlled trials. 8 , 9
Spironolactone has been regarded as a promising medication for the treatment of HFpEF because of its observed positive effects on the cellular, serum biomarker, and echocardiographic level. 10 , 11 , 12 , 13 In the TOPCAT trial, patients with HFpEF were randomized to spironolactone versus placebo, and no significant difference in the primary composite outcome was found. 4 However, further analyses indicated significant improvement in the patients enrolled in the United States, Canada, Brazil and Argentina (i.e. Americas) when compared with those enrolled in Russia and the Republic of Georgia (i.e. Eastern Europe). 14 , 15 The reason for this difference has included concerns over accurate diagnosis and enrolment of patients with true HFpEF in the Eastern Europe group, as well as suspicion, based on serum measurements of the spironolactone metabolite canrenone, that Eastern European patients randomized to spironolactone were not actually taking the medication. 14 , 15 , 16 Overall, TOPCAT provides an opportunity, based on non‐uniformity of the participants, to identify the types of patients with HFpEF that would respond positively to spironolactone.
Even without the caveats affecting the TOPCAT trial, applications of clinical trials to individual patients have proven difficult. A specific patient may be phenotypically similar to the trial participants, but not strictly fulfil all of the inclusion criteria, and conversely, a patient may have characteristics that would have allowed for trial enrolment yet still have an overall phenotype dissimilar to the trial population. The development of a Trial Score (TS) to assess an individual patient's representation within a clinical trial was first described and validated in a secondary analysis of the Systolic Blood Pressure Intervention (SPRINT) trial. 17 Due to the heterogeneous nature of HFpEF as a syndrome and the need to identify patients more likely to benefit from spironolactone, TOPCAT is well positioned for development of a TS as a metric that captures multiple characteristics of TOPCAT participants concurrently to allow for tangible comparison to individual patients.
We now understand that HFpEF is a heterogeneous diagnosis, treatment requires accurate diagnosis, and TOPCAT had significant limitations that likely prevented it from being a positive trial. Therefore, we aimed to identify, quantitatively, which patients, based on the results of TOPCAT, were best represented as most likely to respond to spironolactone. We developed and demonstrated a composite metric to easily identify these patients.
Methods
De‐identified individual‐level data from the TOPCAT trial were provided by the US National Heart, Lung, and Blood Institute via the BioLincc data repository. 18 , 19 This study was deemed exempt by the University of Chicago Institutional Review Board, and the data were obtained after a signed data use agreement. Statistical analysis, data synthesis and model creation were performed between July 2019 and April 2020. Study design, baseline characteristics and primary outcomes of the TOPCAT trial have been described in detail previously. 4 , 14 , 20 , 21
A conceptual framework was created to define and succinctly quantify the difference between an individual patient and the theoretical average participant enrolled in the TOPCAT trial. This difference was termed the TS. This method of assessing patients' baseline characteristics, regardless of trial inclusion or exclusion criteria, was previously described in a secondary analysis of the SPRINT trial. 21
Baseline characteristics collected for calculation of the TS included age, body mass index (BMI), systolic blood pressure (SBP), heart rate (HR), creatinine, potassium, glucose, left ventricular ejection fraction (LVEF) and left atrial volume (LAV) for each American participant. Each baseline characteristic was chosen because it is a continuous variable, sufficiently distinct from the others, and a standard clinical measure for a patient with HFpEF. 8 We did not include brain natriuretic peptide (BNP) or N‐terminal‐proBNP in the TS as not all patients enrolled in the trial were required to have one or the other; the differing assays for BNP combined with excess absent measurements prevented its inclusion. On the other hand, we included LVEF and LAV, which were not available for every study participant, as these were still the most available echocardiographic measures and are associated with the diagnosis of HFpEF. 8
The TS is a weighted distance in multidimensional space from the theoretical individual with exactly average values for each measure. Conceptually, as opposed to considering each baseline characteristic of a trial on its own, the TS is a number that captures how similar or dissimilar a given participant (or patient) is from the ‘perfectly average’ trial participant—a theoretical individual possessing the exact mean value for all characteristics—when considering multiple baseline characteristics all at once. For each variable, weighting was applied separately to values above vs. below the average value after normalization, respectively, by positive or negative deviation (a unidirectional variation of standard deviation). See description of weighting in Statistical analysis. The TS also corrects for missing variables by normalizing to the square root of the number of available variables for each participant. The formula for the positive and negative deviations and the formula for the weighted TS are provided in Supporting Information.
Using the formula derived from the American participants, the TS was also calculated for the Eastern European participants. TS and treatment arm were then incorporated into regression analysis as predictors of the trial's primary outcome—cardiovascular mortality, aborted cardiac arrest or heart failure hospitalization—as the dependent variable.
We also calculated the TOPCAT TS for a de‐identified clinical registry cohort of patients with HFpEF (n = 420) recruited prospectively from the outpatient clinic of the Northwestern University HFpEF Program between March 2008 and May 2011 as part of a systematic observational study of HFpEF (ClinicalTrials.gov identifier NCT01030991), which has been described in detail previously. 22 , 23 All patients were recruited after hospitalization for HF, and all had an LVEF > 50%. Besides a prior hospitalization for HF, patients were required to have evidence of either significant diastolic dysfunction (Grade 2 or 3) on echocardiography, evidence of elevated left ventricular filling pressures on invasive hemodynamic testing or BNP > 100 pg/mL to ensure the diagnosis of HFpEF. Patients with greater than moderate valvular disease, prior cardiac transplantation, history of reduced LVEF < 40% (i.e. recovered EF) or diagnosis of constrictive pericarditis were excluded. All study participants gave written informed consent, and the institutional review board at Northwestern University approved the study. Patients with creatinine ≥ 2.5 mg/dL were removed from this cohort as spironolactone is contraindicated in these patients (and were excluded from TOPCAT), 3 , 4 resulting in 377 total patients included in the present analysis.
Finally, we calculated the H2FPEF score in all TOPCAT patients to determine the relationship between accurate HFpEF diagnosis and benefit from spironolactone.
Statistical analysis
The differences between an individual patient's baseline characteristic and the average were calculated and weighted as part of the TS calculation. Logistic regression was used to measure baseline characteristics and spironolactone as predictors of the trial's primary outcome. Weighting was determined by two factors: (1) the association of each characteristic with the primary outcome and (2) the association with benefit from spironolactone. Missing data were not imputed. The TS is normalized by the number of variables available for each participant (equalizing missingness between analysed groups did not significantly change the TS). 𝜒2 tests were used to determine significance of group differences (Americas vs. Eastern Europe vs. registry) in TS and H2FPEF score.
Results
Trial score calculation and response to spironolactone
The TS was calculated with data from the participants enrolled in TOPCAT from the Americas (n = 1766). First, the mean and deviations in both directions of nine distinct clinical and echocardiographic characteristics were calculated. Next, the deviations of each of those nine characteristics were weighted in the TS calculation based on their individual association with the primary outcome and with benefit from spironolactone (see Table 1 ). The strongest weighted characteristics were older age, high BMI, low creatinine, high creatinine, low potassium, large left atrium and high LVEF; extreme values in those characteristics would increase the TS the most. All weights were incorporated into the full TS formula (Supporting Information).
Table 1.
Trial Score component variables
| Clinical measure | Mean | Positive deviation | Negative deviation | Weight for values above mean | Weight for values below mean |
|---|---|---|---|---|---|
| Age | 71.5 years | +9.1 years | −10.3 years | 1.95 | 1.37 |
| BMI | 33.8 kg/m2 | +9.6 kg/m2 | −6.8 kg/m2 | 2.35 | 1.15 |
| Creatinine | 1.17 mg/dL | +0.39 mg/dL | −0.30 mg/dL | 2.09 | 2.19 |
| K | 4.19 mEq/L | +0.39 mEq/L | −0.47 mEq/L | 0.66 | 1.70 |
| Glucose | 116 mg/dL | +78 mg/dL | −27 mg/dL | 0.90 | 1.52 |
| HR | 69 per min | +12 per min | −10 per min | 1.46 | 1.43 |
| SBP | 127.5 mm Hg | +15.2 mm Hg | −16.6 mm Hg | 1.11 | 1.28 |
| LVEF | 59.6% | +6.8% | −8.8% | 2.72 | 1.07 |
| LA volume | 61.4 mL | +34.3 mL | −20.7 mL | 1.94 | 0.23 |
The nine Trial Score input components of the Americas participants. Shown are mean values, the positive and negative deviations from the mean (unidirectional versions of standard deviation; formula in Supporting Information) and the weighting of each variable, depending on whether a participant's value falls above or below the mean.
BMI, body mass index; HR, heart rate; K, potassium; LA volume, left atrial volume; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure.
The distribution of the TS for the Americas participants is shown in Figure 1 . The theoretical Americas participant possessing the exact average value for all of the nine characteristics would have a TS of zero. The median American TS was 1.49 (IQR 1.19–1.83), with 97.5% of TS values being less than 2.63. Using the formula derived from the Americas participants on the Eastern European participants (n = 1677) reveals that their TS were significantly lower [median 1.24 (IQR 1.02–1.50), 97.5th percentile 2.08; compared with Americas, P < 1 × 10−15]. In other words, by this score, Eastern European participants were more similar to the theoretical American participant possessing perfectly average characteristics than the American participants themselves, who had a greater tendency to be outliers (Figure 1 A ).
Figure 1.
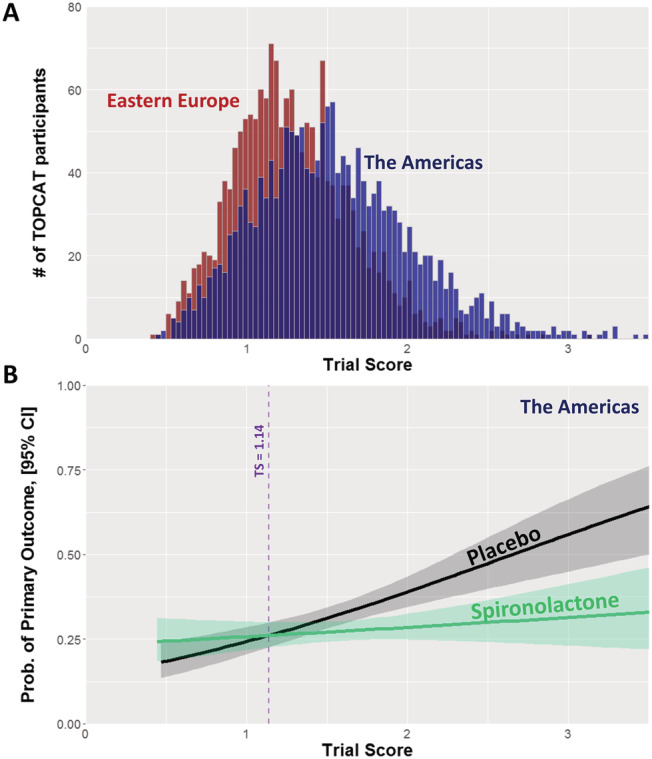
The Trial Score in TOPCAT. (A) Distribution of trial score (TS) in the TOPCAT participants from the Americas (blue) and Eastern Europe (red). (B) TOPCAT Americas primary event rate—cardiovascular mortality, aborted cardiac arrest and heart failure hospitalization—as a function of treatment arm and TS. The line estimate curves start to diverge at TS = 1.14, with the rate on placebo rising as TS rises, whereas the rate on spironolactone does not.
Analysis of the American participants' primary event rate as a function of TS and spironolactone treatment indicated that TS began to predict benefit from spironolactone at TS 1.14 or greater (Figure 1 B ). Participants with TS < 1.14 did not, as a group, derive benefit. In the placebo arm, events were more frequent with higher TS, but in the spironolactone arm, that relationship was nearly flattened. Thus, the benefit from spironolactone rose with increasing TS, confirmed by logistic regression of treatment arm and TS as predictors of the primary event (βinteraction = −0.28, P < 0.01). Because the majority of events occurred in the Americas subset, the treatment effect of spironolactone on the total TOPCAT population is very similar to the Americas (Figure S1 ). There were far fewer events in the Eastern European subset, so no relationship can be identified, and this is in line with the overall observation that these participants had far lower‐than‐expected event rates and low adherence to the trial intervention (Figures S2 and S3 ). 14 , 15 , 16
Real‐world demonstration of trial score
We demonstrated the TS in a real‐world clinical registry of patients with confirmed HFpEF and a prior hospitalization for HF. Of the 420 patients, 43 were not included in this analysis due to creatinine ≥ 2.5 mg/dL, because this is a relative contraindication for spironolactone and because this population is excluded in both TOPCAT and current ACC/AHA heart failure guidelines. 3 , 4 The distribution of TS for the remaining patients (n = 377) was more similar to the Americas subgroup than the Eastern Europe subgroup, but an even greater proportion were in a range consistent with spironolactone benefit (Figure 2 ), with 90% possessing a TS > 1.14 [median 1.76 (IQR 1.38–2.15); compared with Americas, P < 1x10‐15, and to Eastern Europe, P < 1 × 10−15].
Figure 2.
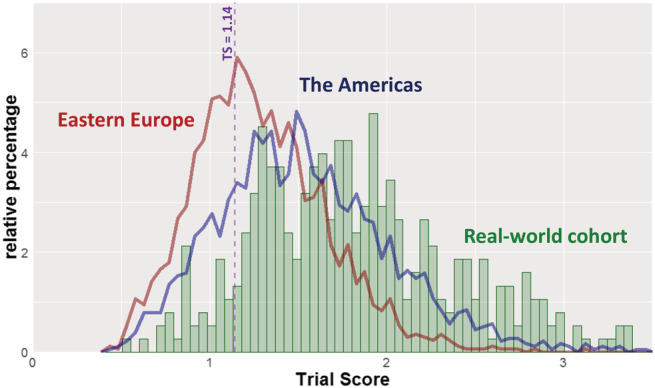
Real‐world TOPCAT Trial Scores compared with TOPCAT participants. Distribution of trial score (TS) in a validated real‐world cohort of patients with HFpEF (green), shown in the context of the American (blue) and Eastern European (red) TOPCAT participants. In the TOPCAT Americas cohort, the benefit of spironolactone occurred once TS exceeded 1.14.
Patient stratification by accurate HFpEF diagnosis and predicted spironolactone response
We calculated the H2FPEF score on all participants enrolled in TOPCAT, as well as the patients in the real‐world cohort, as an orthogonal way to estimate appropriateness of spironolactone therapy by estimated probability of ‘true HFpEF’. 8 A H2FPEF score ≥ 3 correlates with >50% likelihood of HFpEF, and a H2FPEF score ≤ 2 is consistent with <40% likelihood of HFpEF. TOPCAT participants and registry patients were stratified into four zones based on the TS and H2FPEF score (Figure 3 ). In Zone 1, HFpEF is unlikely (H2FPEF score ≤ 2); in Zones 2–4, HFpEF is likely (H2FPEF score ≥ 3), with Zone 2 meaning the patient was well represented but too close to ‘perfectly average’ in TOPCAT (TS < 1.14) and thus less likely to receive benefit from spironolactone; Zone 3 meaning the patient was reasonably well represented in TOPCAT but with TS between 1.14 and 2.63 and thus more likely to receive benefit from spironolactone; and Zone 4 meaning that although HFpEF is likely, the patient was not well represented in TOPCAT (TS > 2.63, the 97.5th percentile), so the benefit of spironolactone is predicted, yet unsupported by sufficient numbers of trial participants. In this schema, Zone 3 is the most ideal for considering spironolactone for a patient with suspected HFpEF. TS 1.14‐2.63 is associated with response to spironolactone, and Zone 3 participants (TS 1.14‐2.63 with H2FPEF score > 2) have elevated risk on placebo but not on spironolactone (Tables S1 and S2 ). All of this supports spironolactone mitigating the elevated risk of those with true HFpEF and TS 1.14–2.63.
Figure 3.
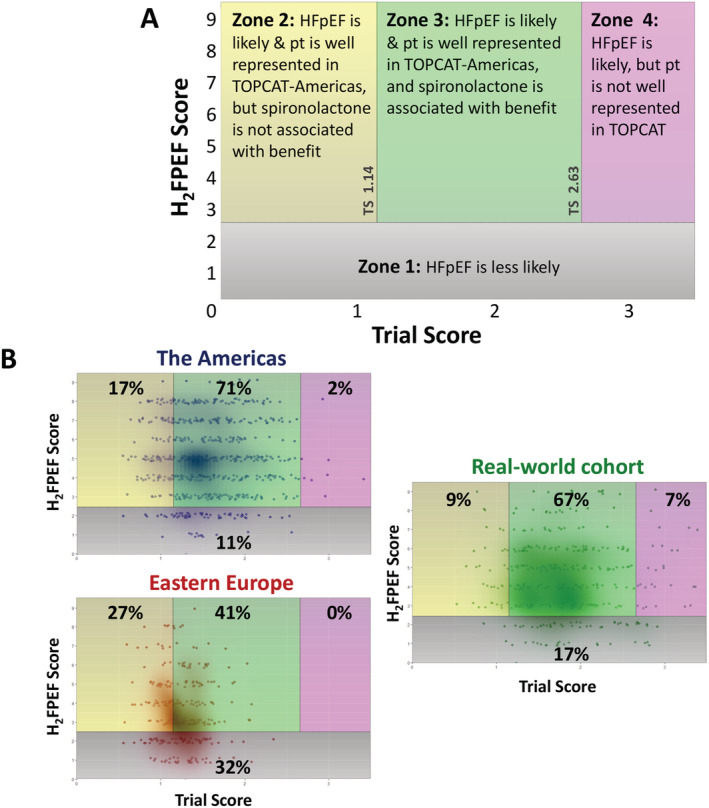
Categorizing patients by zones according to Trial Score and H2FPEF score. (A) Zone definitions for categorizing patients by concomitant TS and H2FPEF score: Zone 1: HFpEF is unlikely (H2FPEF score ≤ 2). Zone 2: HFpEF is likely (H2FPEF score ≥ 3), and the patient is well represented in TOPCAT but unlikely benefit from spironolactone (TS < 1.14). Zone 3: HFpEF is likely (H2FPEF score ≥ 3), and the patient is well represented in TOPCAT and likely benefit from spironolactone (1.14 ≤ TS < 2.63). Zone 4: HFpEF is likely (H2FPEF score ≥ 3), but the patient is not well represented in TOPCAT, so there is unclear benefit from spironolactone (TS ≥ 2.63). (B) Distributions across zones for TOPCAT participants from the Americas and Eastern Europe and also for the validated HFpEF patient registry. Only those with echocardiographic data, required for H2FPEF score, were included. Each individual is shown along with a density plot overlay. Percentages in each zone are denoted. All three population distributions were significantly different from each other (P < 0.0001 by χ2 for overall comparison and for each pair), although the patient registry more closely resembled the TOPCAT Americas participants.
Seventy‐one percent of TOPCAT Americas participants were in Zone 3, meaning they had likely HFpEF, were more likely to benefit from spironolactone based on TS > 1.14 and were reasonably well represented in the trial (TS < 2.63). On the other hand, participants from Eastern Europe only had 41% in Zone 3 and had substantial proportions in Zone 1 (HFpEF less likely) and Zone 2 (spironolactone non‐benefit). Similar to the Americas subgroup, patients from the Northwestern registry cohort were most frequently in Zone 3 (67%) but also had a larger proportion in the more ‘data‐sparse’ Zone 4. These latter patients would be predicted to benefit from spironolactone but are not well represented by TOPCAT. Even though the patient registry closely resembled the TOPCAT Americas participants in some respects, all three population distributions were significantly different from each other (P < 0.0001 by 𝜒2 for overall comparison and for each pair).
Discussion
HFpEF is a very heterogeneous clinical syndrome. The difficulty in accurately classifying therapeutically homogeneous subgroups of this syndrome is likely a major reason that all large pharmacological treatment trials, to date, have been neutral. Subgroup analyses and post hoc analyses are hypothesis‐generating; they have been helpful in understanding the neutral trials and guiding designs of future studies, but it is difficult to apply these data prospectively to patients. In our study, we successfully created a multidimensional TS that characterizes similarity to the TOPCAT Americas subgroup and determines the likelihood of a beneficial response to spironolactone. The TS provides distinct information, additive to the validated H2FPEF score, by supplementing HFpEF likelihood with a determination of likely spironolactone benefit. Finally, we were able to demonstrate the TS is easily obtained in real patients from an established HFpEF registry.
The main purpose of the TS is to capture multiple characteristics all at once, expressed as a distance from the theoretical perfectly average participant in the trial. Every clinical trial, upon publication, presents multiple distances, each in one dimension, from the mean or median—this is the essence of the typical Table 1 of baseline characteristics. Conceptually, distance in multiple dimensions is a logical extension of this—instead of considering each characteristic one by one, treat them as we would a patient, all at the same time. In the TS used for this study, for each characteristic, deviations from the mean in either direction were treated separately. These details can be seen in Supporting Information. The TS is meant to allow clinicians to see the similarities and differences, particularly as they pertain to potential spironolactone benefit, between patients they see and the TOPCAT participants. The TS is not designed or claimed to be a prognostic marker, but rather as a way to characterize how an individual's baseline characteristics compare to the trial population and to potentially predict treatment effect.
Patient examples from the validated HFpEF registry demonstrate how the score could be applied and interpreted (Figure 4 ). In these examples, three patients meet all major TOPCAT inclusion criteria, and they all have H2FPEF scores consistent with likely HFpEF, but based on TS, they are in different zones for confidence about spironolactone benefit, with only one in the most ideal Zone 3. A fourth patient is also in this ideal zone and is highly similar to the types of TOPCAT participants who benefitted from spironolactone, but this patient would have been excluded from the trial based solely on age. The TS could, after accounting for trial exclusions based on safety concerns, be used to apply the lessons learned from the trial to patients, such as this one, who were not a perfect fit by inclusion criteria. A tool is provided to allow for quick TOPCAT TS calculation and visualization (Supporting Information, The TOPCAT Trial Score Calculator). Importantly, spironolactone will affect potassium and creatinine, both of which are routinely considered when prescribing this medication, and safety warnings are included in the calculator, which will alert the user and not return a score if potassium equals or exceeds 5 mEq/L or if the serum creatinine equals or exceeds 2.5 mg/dL, which are generally considered contraindications for spironolactone and were also exclusion criteria in TOPCAT (Figure S4 ).
Figure 4.
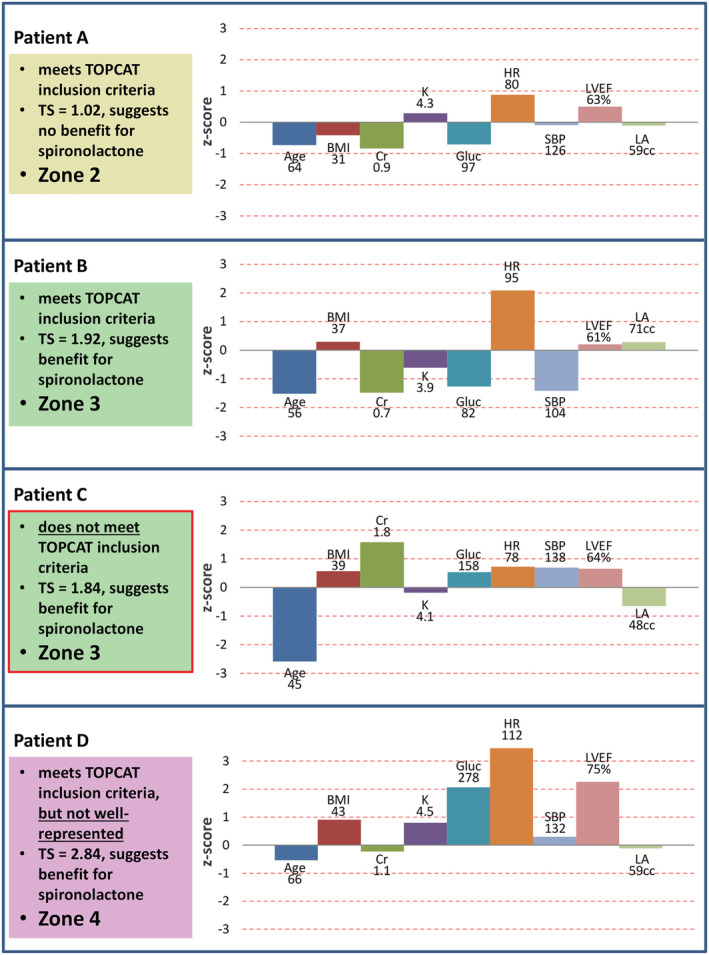
Patient examples for application and interpretation of Trial Scores. Patient examples from the validated HFpEF registry showing application and interpretation of TS. Patients A, B and D all meet TOPCAT inclusion criteria, and they all have H2FPEF scores consistent with likely HFpEF, but based on TS, they are in different zones for confidence about spironolactone benefit. Patient C is well represented and would be predicted to derive benefit from spironolactone based on TS but would have been excluded from TOPCAT based solely on age. The TS components along with z‐scores are shown to the right of each patient; the more these components deviate from the TOPCAT averages, the higher the TS.
There have been persistent questions, and multiple hypotheses, regarding the reason for the regional variation between the Americas and Eastern European cohorts in TOPCAT. Leading explanations include inadequate adherence to the study drug and likely inaccurate diagnoses of HFpEF in the Eastern European participants. 14 , 15 , 16 In our study, no group of Eastern Europe patients showed a positive response to spironolactone based on the TS developed. This is most likely due to the markedly lower event rates in this group compared with the Americas group (~4‐fold lower), which calls into question the accuracy of the HFpEF diagnosis of the Eastern European patients and also supports post hoc analyses focusing on just the Americas group. Therefore, the TS should only be used in patients with likely HFpEF, and not only H2FPEF scores, but also other objective measures of filling pressures and diastolic function, can be used to establish the diagnosis (for instance, most of the registry patients with H2FPEF scores ≤ 2 had their HFpEF confirmed by these other objective measures). Only once the diagnosis is deemed likely, the TS could hone in on those who were well represented in TOPCAT with benefit from spironolactone.
Recently, a latent‐class analysis of TOPCAT stratified participants into three phenogroups based on clinical, echocardiographic, vascular and serum biomarker data. The phenogroup with the most significant response to spironolactone was notable for its high prevalence of obesity, diabetes and chronic kidney disease. 24 Indeed, in our analysis, highly weighted TS component variables included high BMI and high creatinine, but additional highly weighted factors, including low creatinine, high LVEF and large left atrium, emerged. The phenogroup study sheds light on the pathophysiology of the various aetiologies of HFpEF enrolled in a single trial. However, the phenogroups are not easily directly applied to an individual patient, especially as many of the serum biomarkers used are not routinely available. 24 On the other hand, our TS is able to take routine clinical and echocardiographic data and determine the likelihood of spironolactone response to an individual patient.
Additional, randomized controlled studies are needed to assess the benefit of spironolactone in a cohort of accurately diagnosed HFpEF, some of which are currently ongoing. 25 , 26 In the meantime, it is important to apply the knowledge we have to this patient population, which has a dearth of evidence‐based treatment options. The TOPCAT TS provides an opportunity, based on a clinical trial with some of the strongest evidence to date for HFpEF therapy, to individualize patient care for this heterogeneous patient population.
Limitations
The TS is limited to baseline characteristics that are continuous variables. Although categorical variables like sex and race are not included, these factors often influence cardiovascular risk through many of the variables that were included. Another limitation of our study is that this is a retrospective analysis of the TOPCAT trial. Though we demonstrated our TS through application to a validated HFpEF clinical registry, it has not yet been prospectively studied in a trial.
Conclusions
Although TOPCAT was a neutral trial overall, spironolactone was beneficial in a substantial subgroup of this heterogeneous population. Patients with HFpEF can be quantified by the TS, a composite metric that captures how similar they are to the TOPCAT Americas cohort and how likely they are to benefit from spironolactone.
Conflict of interest
M.N.B. and J.E.B. have no disclosures or conflicts of interest.
Funding
S.J.S. is supported by grants from the National Institutes of Health (R01 HL140731, R01 HL120728, R01 HL107577 and R01 HL149423), the American Heart Association (#16SFRN28780016 and #15CVGPSD27260148); Actelion, AstraZeneca, Corvia, Novartis and Pfizer and has received consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer‐Ingelheim, Cardiora, CVRx, Cyclerion, Cytokinetics, Eisai, Eli Lilly, Ionis, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Sanofi, Shifamed, Tenax and United Therapeutics. F.J.A. is supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (R03 HL154292), the Harold S. Geneen Charitable Trust Awards Program for Coronary Heart Disease Research and the American Heart Association (#20TPA35490264).
Supporting information
Table S1. Primary Event Rates by TS grouping, TOPCAT Americas.
Table S2. Primary Event Rates by Zone, TOPCAT participants with Echocardiographic Data.
Figure S1. TOPCAT primary event rate—cardiovascular mortality, aborted cardiac arrest, or heart failure hospitalization—as a function of treatment arm and TS for all TOPCAT participants (Americas and Eastern Europe combined).
Figure S2. TOPCAT primary event rate—cardiovascular mortality, aborted cardiac arrest, or heart failure hospitalization—as a function of treatment arm and TS for Eastern European TOPCAT participants. Event rates among the Eastern European participants did not correlate with spironolactone treatment or TS, but were also quite low.
Figure S3. TOPCAT primary event rate—cardiovascular mortality, aborted cardiac arrest, or heart failure hospitalization—as a function of geographic location and TS. Event rates among the Eastern European participants were quite low compared to participants in the Americas.
Figure S4. Example screenshots of the TOPCAT Trial Score calculator. The calculator will accept input for each of the 9 component baseline characteristics of the score. If a characteristic is not available, it can be left blank. The score, text guidance, and plots will auto‐refresh with any change in the characteristics. If a K ≥ 5 mEq/L or Cr ≥ 2.5 mg/dL are entered, the score will not be provided, and a notice will specify that these are generally contraindications to spironolactone
Data S1. Formulas for deviations, z‐score variables, and Trial Score.
Data S2. The TOPCAT Trial Score Calculator – Microsoft Excel tool for calculating and visualizing the TS for specific patients.
Belkin, M. N. , Blair, J. E. , Shah, S. J. , and Alenghat, F. J. (2021) A composite metric for predicting benefit from spironolactone in heart failure with preserved ejection fraction. ESC Heart Failure, 8: 3495–3503. 10.1002/ehf2.13523.
References
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, Van Wagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics‐2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56‐e528. [DOI] [PubMed] [Google Scholar]
- 2. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591‐602. [DOI] [PubMed] [Google Scholar]
- 3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136:e137‐e161. [DOI] [PubMed] [Google Scholar]
- 4. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383‐1392. [DOI] [PubMed] [Google Scholar]
- 5. Kelly JP, Mentz RJ, Mebazaa A, Voors AA, Butler J, Roessig L, Fiuzat M, Zannad F, Pitt B, O'Connor CM, Lam CSP. Patient selection in heart failure with preserved ejection fraction clinical trials. J Am Coll Cardiol 2015;65:1668‐1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel RB, Shah SJ, Fonarow GC, Butler J, Vaduganathan M. Designing Future Clinical Trials in Heart Failure With Preserved Ejection Fraction: Lessons From TOPCAT. Curr Heart Fail Rep 2017;14:217‐222. [DOI] [PubMed] [Google Scholar]
- 7. Zakeri R, Cowie MR. Heart failure with preserved ejection fraction: controversies, challenges and future directions. Heart Br Card Soc 2018;104:377‐384. [DOI] [PubMed] [Google Scholar]
- 8. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence‐Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation 2018;138:861‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske‐Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297‐3317. [DOI] [PubMed] [Google Scholar]
- 10. Mitter SS, Shah SJ. Spironolactone for Management of Heart Failure with Preserved Ejection Fraction: Whither to After TOPCAT? Curr Atheroscler Rep 2015;17:64. [DOI] [PubMed] [Google Scholar]
- 11. Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the Randomized Aldosterone Antagonism in Heart Failure with Preserved Ejection Fraction trial (RAAM‐PEF). J Card Fail 2011;17:634‐642. [DOI] [PubMed] [Google Scholar]
- 12. Edelmann F, Wachter R, Schmidt AG, Kraigher‐Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Löffler M, Düngen HD, Tschöpe C, Herrmann‐Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B; Aldo‐DHF Investigators . Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo‐DHF randomized controlled trial. JAMA 2013;309:781‐791. [DOI] [PubMed] [Google Scholar]
- 13. MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res 1997;35:30‐34. [DOI] [PubMed] [Google Scholar]
- 14. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34‐42. [DOI] [PubMed] [Google Scholar]
- 15. McMurray JJV, O'Connor C. Lessons from the TOPCAT trial. N Engl J Med 2014;370:1453‐1454. [DOI] [PubMed] [Google Scholar]
- 16. de Denus S, O'Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, Jutras M, Lavoie J, Solomon SD, Pitt B, Pfeffer MA, Rouleau JL. Spironolactone Metabolites in TOPCAT ‐ New Insights into Regional Variation. N Engl J Med 2017;376:1690‐1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laffin LJ, Besser SA, Alenghat FJ. A data‐zone scoring system to assess the generalizability of clinical trial results to individual patients. Eur J Prev Cardiol 2019;26:569‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ross JS, Ritchie JD, Finn E, Desai NR, Lehman RL, Krumholz HM, Gross CP. Data sharing through an NIH central database repository: a cross‐sectional survey of BioLINCC users. BMJ Open 2016;6:e012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. BioLINCC ‐ Biologic Specimen and Data Repository Information Coordinating Center. Accessed May 6, 2020. https://biolincc.nhlbi.nih.gov/home/
- 20. Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O'Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J 2011;162:966‐972.e10. [DOI] [PubMed] [Google Scholar]
- 21. Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B, Boineau R, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Lewis EF, Markov V, O'Meara E, Kobulia B, Shaburishvili T, Solomon SD, Pitt B, Pfeffer MA, Li R. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail 2013;6:184‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015;131:269‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah SJ, Cogswell R, Ryan JJ, Sharma K. How to Develop and Implement a Specialized Heart Failure with Preserved Ejection Fraction Clinical Program. Curr Cardiol Rep 2016;18:122. [DOI] [PubMed] [Google Scholar]
- 24. Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, Yarde M, Wang Z, Bhattacharya PT, Chirinos DA, Prenner S, Zamani P, Seiffert DA, Car BD, Gordon DA, Margulies K, Cappola T, Chirinos JA. Clinical Phenogroups in Heart Failure With Preserved Ejection Fraction: Detailed Phenotypes, Prognosis, and Response to Spironolactone. JACC Heart Fail 2020;8:172‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure With Preserved Ejection Fraction ‐ Full Text View ‐ ClinicalTrials.gov. Accessed May 6, 2020. https://clinicaltrials.gov/ct2/show/NCT02901184
- 26. German Centre for Cardiovascular Research: DZHK‐Studie. Accessed June 18, 2020. https://spirit‐hf.dzhk.de/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primary Event Rates by TS grouping, TOPCAT Americas.
Table S2. Primary Event Rates by Zone, TOPCAT participants with Echocardiographic Data.
Figure S1. TOPCAT primary event rate—cardiovascular mortality, aborted cardiac arrest, or heart failure hospitalization—as a function of treatment arm and TS for all TOPCAT participants (Americas and Eastern Europe combined).
Figure S2. TOPCAT primary event rate—cardiovascular mortality, aborted cardiac arrest, or heart failure hospitalization—as a function of treatment arm and TS for Eastern European TOPCAT participants. Event rates among the Eastern European participants did not correlate with spironolactone treatment or TS, but were also quite low.
Figure S3. TOPCAT primary event rate—cardiovascular mortality, aborted cardiac arrest, or heart failure hospitalization—as a function of geographic location and TS. Event rates among the Eastern European participants were quite low compared to participants in the Americas.
Figure S4. Example screenshots of the TOPCAT Trial Score calculator. The calculator will accept input for each of the 9 component baseline characteristics of the score. If a characteristic is not available, it can be left blank. The score, text guidance, and plots will auto‐refresh with any change in the characteristics. If a K ≥ 5 mEq/L or Cr ≥ 2.5 mg/dL are entered, the score will not be provided, and a notice will specify that these are generally contraindications to spironolactone
Data S1. Formulas for deviations, z‐score variables, and Trial Score.
Data S2. The TOPCAT Trial Score Calculator – Microsoft Excel tool for calculating and visualizing the TS for specific patients.


