Abstract
Aims
Although aging is strongly associated with both heart failure and a decline in gait speed, a definition of slowness incorporating an age‐related decline has yet to be developed. We aimed to define an event‐driven cut‐off for the relative decline in gait speed against age‐adjusted reference values derived from the general population and evaluate its prognostic implications.
Methods and results
Standardized gait speed (SGS) was defined as the median gait speed stratified by age, sex, and height in 3777 elderly (age ≥ 65 years) individuals without a history of cardiovascular diseases (Tokyo Metropolitan Institute of Gerontology‐Longitudinal Interdisciplinary Study on Aging: general population cohort). The mortality event‐driven optimal cut‐off of the SGS ratio (actual gait speed divided by the respective SGS) was defined using FRAGILE‐HF cohort data and externally validated using Kitasato cohort data, comprising 1301 and 1247 hospitalized elderly patients with heart failure, respectively. Using FRAGILE‐HF data, the optimal SGS ratio cut‐off was determined as 0.527. In the Kitasato cohort, SGS ratio < 0.527 was associated with a higher 1 year [hazard ratio (HR): 1.70, 95% confidence interval (CI): 1.07–2.72, P = 0.024] and long‐term (HR: 1.46, 95% CI: 1.05–2.02, P = 0.024) mortality rate, independent of pre‐existing covariates.
Conclusions
Gait speed was significantly declined in patients with heart failure, even after taking age and sex‐related decline into account. A SGS ratio of 0.527 is a validated cut‐off for slowness independently associated with mortality in patients with heart failure age ≥65.
Keywords: Frailty, Gait speed, Geriatrics, Mortality
Introduction
Heart failure is a global pandemic affecting more than 26 million people worldwide, with one million patients hospitalized due to heart failure each year. 1 There is a strong association between aging and the development of heart failure. For instance, the National Health and Nutrition Examination Survey reported that the proportions of men with heart failure are 6.6% and 10.6% for those aged 60–79 and ≥80 years, respectively; the respective proportions in women are 4.8% and 13.5%. 2
Gait speed, a metric of slowness, is considered a valid measure of frailty and is associated with functional status and mortality in the general population. 3 , 4 Furthermore, as frailty plays a crucial prognostic role in heart failure, gait speed has also been shown to be associated with the prognosis in numerous studies. 5 , 6 , 7 However, most previous studies defined slowness using a single cut‐off value derived from the general population, regardless of age; no age‐specific cut‐offs have been proposed, even though there is a clear age‐related decline in gait speed. 8 , 9 , 10 As the heart failure population is highly saturated with elderly patients, it is unclear whether older age or heart failure itself contributes the substantial number of patients with heart failure with low gait speed. This further raises questions regarding whether the prognostic impact of gait speed in patients with heart failure is truly independent of the age‐related decline, even though most previous studies used age as an adjustment variable. Moreover, pre‐existing cut‐offs have not been defined according to their association with adverse events. The development of cut‐offs using an event‐driven approach in elderly patients with heart failure is relevant, not only in terms of clinical research but also in terms of individualized risk stratification.
Against these backdrops, we sought to define an event‐driven cut‐off for the relative decline in gait speed compared with age‐adjusted reference values derived from the general population and evaluate its prognostic implications.
Methods
The Tokyo Metropolitan Institute of Gerontology‐Longitudinal Interdisciplinary Study on Aging (TMIG‐LISA) cohort was used to define reference gait speed values in the general population stratified by age, sex, and height. We applied the obtained reference values to the FRAGILE‐HF cohort, which comprised hospitalized patients with heart failure aged ≥65 years, to determine the gait speed in patients with heart failure relative to that in the general cohort. Next, we defined the optimal cut‐off of the proportional decline in gait speed relative to the respective reference value, in terms of prognostication. Lastly, we tested the external validity of slowness defined by this event‐driven optimal cut‐off in an independent cohort (the Kitasato cohort), which included hospitalized patients with heart failure.
TMIG‐LISA cohort (general population)
The TMIG‐LISA cohort was established by the TMIG‐LISA Research Group to determine risk factors of geriatric diseases or chronic medical conditions and to identify factors that accelerate or decelerate aging, in representative samples of older Japanese adults. We utilized a pooled data set of 4683 participants from six TMIG cohort studies: the Nangai Cohort Study, Itabashi Cohort Study 2002, Yoita Longitudinal Study, Kusatsu Longitudinal Study, Hatoyama Cohort Study, and Itabashi Cohort Study 2011 (ITABASHI11). The participants of each cohort are detailed elsewhere. 11 Of note, four out of the six cohorts included participants aged ≥65 years, one cohort included participants aged ≥71 years, and one included participants aged ≥70 years. Patients who were aged ≤65 years or had a self‐reported history of heart disease were excluded from the present analysis. Usual gait speeds were measured over 5 m with a dynamic start, except for one cohort (the ITABASHI 11 cohort), which measured usual gait speeds over 10 m. Participants stood with their feet behind, but just touching, a starting line marked with tape at 0 m. Upon receiving the tester's command, they started walking at their usual pace along a 11 m (16 m in the ITABASHI 11 cohort) course. The actual walking time was measured over 5 m, starting when the body trunk passed the 3 m mark, and ending when the body trunk passed the 8 m (13 m in the ITABASHI 11) mark. The measurement was performed once.
FRAGILE‐HF cohort (derivation heart failure cohort)
The FRAGILE‐HF cohort study was a multicentre observational study, which included 1332 hospitalized patients with decompensation of heart failure, aged ≥65 years, who could ambulate at discharge. Patients with missing data were excluded from the present analysis. The main results of the FRAGILE‐HF study have been published elsewhere. 12 Briefly, the main objective of the FRAGILE‐HF study was to evaluate the prevalence and prognostic impact of multifrailty domains in patients with heart failure. Exclusion criteria were as follows: (i) previous heart transplantation or treatment with a left ventricular assist device, (ii) on either chronic peritoneal dialysis or haemodialysis, and (iii) acute myocarditis. Patients with missing brain natriuretic peptide (BNP) or N‐terminal‐proBNP data, and patients with a BNP level <100 pg/mL or N‐terminal‐proBNP level <300 pg/mL at admission, were also excluded as the diagnosis could be unclear. Heart failure with reduced and preserved ejection fraction was enrolled.
The FRAGILE‐HF investigation conforms with the principles outlined in the Declaration of Helsinki and Japanese Ethical Guidelines for Medical and Health Research involving Human Subjects. Because it was an observational study without invasive procedures or interventions, written informed consent was not required under the Ethical Guidelines for Medical and Health Research Involving Human Subjects, issued by the Japanese Ministry of Health, Labor, and Welfare. Study information, including the objectives, inclusion and exclusion criteria, primary outcomes, and names of the participating hospitals, were published in the publicly available University Hospital Medical Information Network (UMIN‐CTR, unique identifier: UMIN000023929), before the first patient was enrolled.
In the FRAGILE‐HF study, gait speed was measured with a static start and 4 m distance, as part of a brief physical performance battery. Patients were asked to walk along a 4 m corridor at their usual speed without running. Each patient started in a standing position at the start line. They were permitted to use walking aids, such as canes or walkers. A standard digital stopwatch was used to time the travel between the first footstep after the 0 m line and the first footstep after the 4 m line. The patients performed the test twice, and whichever time was shorter was adopted as the patient's gait speed.
Kitasato cohort (external validation heart failure cohort)
The Kitasato cohort was a single‐centre retrospective observational study that included 1247 elderly patients with heart failure whose usual gait speed was investigated during inpatient cardiac rehabilitation at the Kitasato University Hospital between 1 January 2008 and 31 December 2017. Patients at high risk of adverse events during gait measurements and those with difficulty in understanding instructions were selectively excluded. Data for all evaluated variables and clinical characteristics, including the primary endpoint, were obtained from electronic medical records. Patients with missing data were excluded from the present analysis.
The Kitasato cohort study was approved by the Research Ethics Committee of Kitasato University Hospital (B20‐030) and was performed following the tenets of the Declaration of Helsinki. Participants were informed that they could refuse to participate by opting out.
In the Kitasato cohort, gait speed was measured near the date of discharge. Patients were asked to walk at their usual speed for 16 m, and the time to cover the middle 10 m was determined using a stopwatch. There were no restrictions on the use, or type, of walking aids. Patients at high risk of falling were assisted by medical staff, with light hand contact, or were monitored nearby, as needed. The gait speed was calculated as the time required per meter.
Statistical analysis
Data are expressed as the mean and standard deviation for normally distributed variables and as the median with quartiles for non‐normally distributed data. Categorical data are expressed as numbers and percentages. Variables with non‐normal distribution by histogram evaluation were transformed into a logarithmic scale for further analyses. Group differences were evaluated using the Student's t‐test or Mann–Whitney U test for continuous variables and the chi‐squared or Fisher's exact tests for categorical variables.
We stratified all participants into subgroups according to age (65–69, 70–74, 75–79, 80–84, and ≥85 years), height (above/below median for each sex), and sex, as the age‐related decline in gait speed has been shown to significantly interact with sex. 11 We then calculated the median gait speed value in each category, which was defined as the age, sex, and height‐standardized gait speed (SGS). Next, we applied the SGS to each patient enrolled in the FRAGILE‐HF study and calculated the SGS ratio by dividing the actual gait speed by the respective SGS. We obtained 95% confidence intervals (CIs) of the median SGS ratio via boot strapping (2000 samples) using the percentile method.
To define the event‐driven optimal cut‐off value of the SGS ratio in terms of predicting mortality in patients with heart failure, a receiver operating characteristic curve analysis was performed, with the cut‐off value defined based on the Youden index. 13 Internal validity of the derived cut‐off value was examined by stratifying the FRAGILE‐HF cohort into slow and non‐slow groups according to the cut‐off and by determining whether the slow group was associated with worsened 1 year mortality, independent of pre‐existing prognostic factors, in univariate/multivariable Cox regression analyses. The Meta‐Analysis Global Group in Chronic (MAGGIC) heart failure risk score and log‐transformed BNP were used as the adjustment variables for the outcome of all‐cause death, as their associations with mortality have been well validated in Japanese patients with heart failure. 14
Finally, the external validity and long‐term prognostic impact of slowness as defined using the derived cut‐off value was evaluated using data from another heart failure cohort (the Kitasato cohort). The SGS ratio was used to define slow and non‐slow groups. Multivariable Cox regression analysis was performed to determine whether the slow group was associated with worsened 1 year and long‐term mortality, independent of pre‐existing prognostic factors. In this analysis, variables comprising the MAGGIC score (i.e. age, male sex, body mass index, current smoker, systolic blood pressure, New York Heart Association class at discharge, left ventricular ejection fraction, history of diabetes, chronic obstructive pulmonary disease, or heart failure, creatinine at discharge, and prescription of a beta‐blocker or angiotensin converting enzyme inhibitor/angiotensin II receptor blocker at the time of discharge) and the log‐transformed BNP were used as the adjustment variables, rather than the MAGGIC risk score, as information on heart failure duration, which is needed to calculate the original MAGGIC score, was lacking. 15
A two‐tailed P value < 0.05 was considered statistically significant. Statistical analyses were performed using R Version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria; ISBN 3‐900051‐07‐0, URL http://www.R‐project.org).
Results
The study flowchart is shown in Figure S1 . After excluding patients with a history of cardiovascular disease or missing data, 3777 participants from the TMIG‐LISA cohort were used to define the SGS stratified according to age, sex, and height. Additionally, 1301 and 1247 patients from the FRAGILE‐HF and Kitasato cohorts, respectively, were used to derive and externally validate, respectively, the SGS ratio cut‐off, after excluding those with missing gait speed or height data. A box‐whisker plot of the gait speed in all three groups, stratified by age category, is shown in Figure 1 . In all three cohorts, gait speed significantly declined with increasing age. Additionally, the heart failure cohorts (i.e. FRAGILE‐HF and Kitasato cohorts) showed lower gait speed compared with that in the TMIG‐LISA cohort (general population cohort) for all age categories.
Figure 1.
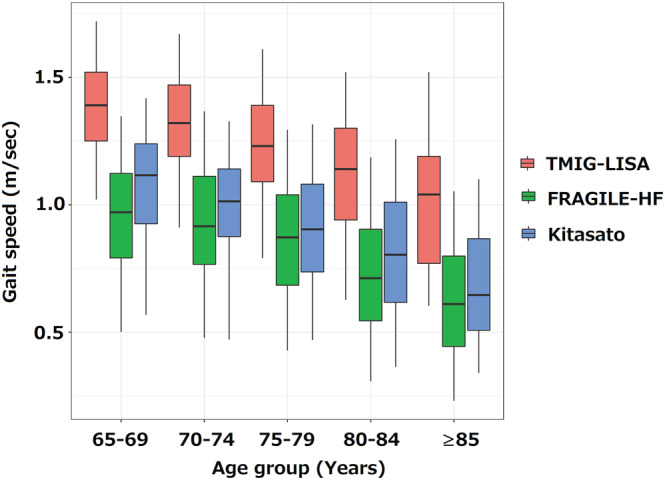
Box‐whisker plot of gait speed stratified by cohort and age categories. All three cohorts showed a significant association between age and decline in gait speed (P < 0.001 for all). TMIG‐LISA, Tokyo Metropolitan Institute of Gerontology‐Longitudinal Interdisciplinary Study on Aging.
According to the gait speed standardized for age, sex, and height derived from the TMIG‐LISA cohort (Table S1 ), the SGS ratio was calculated for all patients from the FRAGILE‐HF and Kitasato cohorts, and the density plot is shown in Figure 2 . The median SGS ratio was slightly, but significantly, lower in the FRAGILE‐HF cohort than in the Kitasato cohort [0.65 (quartiles: 0.50–0.82) vs. 0.74 (quartiles: 0.59–0.87), respectively, P < 0.001], even though the gait speed distributions visually overlapped. The 95% CIs of the median SGS ratio were 0.64–0.67 (P < 0.001) for the FRAGILE‐HF cohort and 0.73–0.76 (P < 0.001) for the Kitasato cohort. As the CIs were both below a value of 1, the gait speed was significantly lower in the heart failure cohorts than in the general population cohort used to define the SGS. In addition, the SGS ratio decreased with aging in both FRAGILE‐HF cohort and Kitasato cohort (Figure S2 ).
Figure 2.
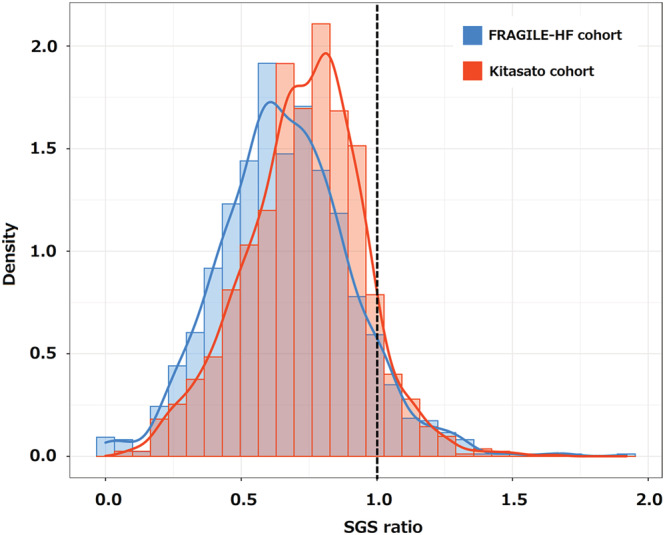
Density plot of the SGS ratio from the FRAGILE‐HF and Kitasato cohorts. The dotted line represents an SGS ratio of 1. SGS, standardized gait speed.
In total, 156 deaths occurred within 1 year of discharge in the FRAGILE‐HF cohort. The receiver operating characteristic analysis for all‐cause death identified an SGS ratio of 0.527 as the event‐driven optimal cut‐off for predicting mortality. For internal validation, the FRAGILE‐HF cohort was divided into two groups: the non‐slow group (SGS ratio > 0.527) and slow group (SGS ratio ≤ 0.527). Table 1 shows the baseline characteristics of the two groups. The slow group was associated with older age, female sex, severe symptoms, lower haemoglobin, albumin, and blood urea nitrogen, and higher left ventricular ejection fraction. The BNP level at discharge was significantly higher in slow group than in the non‐slow group; however, no significant differences were observed for heart failure medication prescriptions at discharge, except for beta‐blockers (lower in the slow group). The slow group was associated with significantly higher mortality in Kaplan–Meier analysis (Figure 3 A ). Likewise, the slow group was significantly associated with mortality in the univariate and multivariable Cox regression analyses, even after adjustment for the MAGGIC score and log‐transformed BNP (Table 2 ).
Table 1.
Baseline characteristics at discharge in the FRAGILE‐HF and Kitasato cohorts stratified by group (non‐slow vs. slow)
| Variable | FRAGILE‐HF cohort | Kitasato cohort | ||||
|---|---|---|---|---|---|---|
| Non‐slow group | Slow group | P value | Non‐slow group | Slow group | P value | |
| N = 943 | N = 358 | N = 1034 | N = 213 | |||
| Age (years) | 80 [73, 86] | 82 [77, 87] | <0.001 | 75 [71, 81] | 78 [74, 84] | <0.001 |
| Male (%) | 556 (59.0) | 184 (51.4) | 0.016 | 615 (59.5) | 109 (51.2) | 0.027 |
| BMI | 21.4 ± 3.7 | 21.2 ± 4.2 | 0.224 | 21.7 ± 4.0 | 22.0 ± 4.7 | 0.298 |
| SBP (mmHg) | 114 ± 17 | 113 ± 17 | 0.300 | 120 ± 29 | 112 ± 26 | <0.001 |
| DBP (mmHg) | 62 ± 11 | 62 ± 11 | 0.676 | 67 ± 19 | 62 ± 16 | 0.002 |
| HR (bpm) | 71 ± 13 | 72 ± 16 | 0.307 | 79 ± 20 | 78 ± 21 | 0.302 |
| Current smoker (%) | 102 (11.0) | 23 (7.0) | 0.015 | 119 (11.6) | 14 (6.8) | 0.038 |
| NYHA (%) | <0.001 | <0.001 | ||||
| I | 435 (46.1) | 130 (36.3) | 31 (3.3) | 6 (3.1) | ||
| II | 402 (42.6) | 143 (39.9) | 687 (73.6) | 77 (39.7) | ||
| III | 104 (11.0) | 84 (23.5) | 213 (22.8) | 106 (54.6) | ||
| IV | 2 (0.2) | 1 (0.3) | 3 (0.3) | 5 (2.6) | ||
| Left ventricular ejection fraction (%) | 45 ± 17 | 48 ± 17 | 0.009 | 47 ± 17 | 46 ± 17 | 0.258 |
| Comorbidities (%) | ||||||
| Hypertension | 668 (70.8) | 262 (73.2) | 0.442 | 805 (77.9) | 160 (75.1) | 0.418 |
| Diabetes mellitus | 327 (34.7) | 137 (38.3) | 0.253 | 518 (50.1) | 104 (48.8) | 0.764 |
| Atrial fibrillation | 434 (46.0) | 145 (40.5) | 0.084 | 306 (29.6) | 69 (32.4) | 0.413 |
| COPD | 103 (11.0) | 40 (11.0) | 0.921 | 61 (5.9) | 15 (7.0) | 0.529 |
| Prior history of heart failure | 506 (54.0) | 210 (59.0) | 0.119 | 521 (50.4) | 123 (57.7) | 0.051 |
| Laboratory data | ||||||
| Albumin | 3.5 ± 0.5 | 3.3 ± 0.5 | <0.001 | 3.6 [3.2, 3.9] | 3.2 [2.9, 3.6] | <0.001 |
| Haemoglobin | 11.9 ± 2.0 | 11.5 ± 2.0 | <0.001 | 11.6 [10.2, 13.2] | 10.9 [9.7, 12.4] | <0.001 |
| Creatinine | 1.4 ± 0.9 | 1.4 ± 0.7 | 0.359 | 1.1 [0.9, 1.6] | 1.3 [0.9, 1.8] | 0.002 |
| eGFR | 53 ± 22 | 52 ± 22 | 0.627 | 45 [30, 59] | 40 [24, 54] | <0.001 |
| BNP | 258 [132, 462] | 326 [150, 600] | 0.007 | 328 [154, 709] | 516 [240, 1005] | <0.001 |
| Medication at discharge (%) | ||||||
| ACE‐I/ARB | 640 (67.9) | 236 (65.9) | 0.547 | 839 (81.1) | 167 (78.4) | 0.391 |
| MRA | 79 (8.4) | 27 (7.5) | 0.705 | 427 (41.3) | 90 (42.3) | 0.819 |
| Beta blocker | 706 (74.9) | 241 (67.3) | 0.008 | 746 (72.1) | 156 (73.2) | 0.801 |
| Loop diuretics | 818 (86.7) | 318 (88.8) | 0.360 | 818 (79.1) | 167 (78.4) | 0.853 |
| Gait speed (m/s) | 0.88 [0.73, 1.05] | 0.46 [0.34, 0.55] | <0.001 | 0.99 [0.84, 1.15] | 0.51 [0.38, 0.58] | <0.001 |
ACE‐I/ARB, angiotensin converting enzyme inhibitor/angiotensin II receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HR, heart rate; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; SBP, systolic blood pressure.
Figure 3.
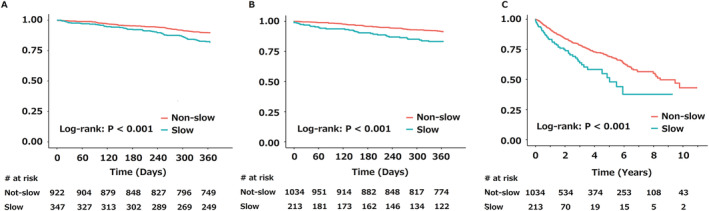
Kaplan–Meier curves for mortality. The 1 year mortality in the FRAGILE‐HF cohort (A), and the 1 year (B) and long‐term mortality (C) in the Kitasato cohort are shown. HF, heart failure.
Table 2.
Unadjusted and adjusted Cox regression analysis for 1 year and long‐term mortality in the (A) FRAGILE‐HF and (B) Kitasato cohorts
| (A) | ||||||
|---|---|---|---|---|---|---|
| Groups | Unadjusted | Adjusted model a | ||||
| HR | 95% CI | P‐value | HR | 95% CI | P value | |
| Non‐slow group | 1 (Reference) | 1 (Reference) | ||||
| Slow group | 1.80 | 1.30–2.48 | <0.001 | 1.54 | 1.09–2.18 | 0.014 |
| (B) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | 1 year mortality | Long‐term mortality | ||||||||||
| Unadjusted | Adjusted model b | Unadjusted | Adjusted model b | |||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Non‐slow group | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||||||
| Slow group | 2.17 | 1.42–3.31 | <0.001 | 1.70 | 1.07–2.72 | 0.024 | 1.87 | 1.39–2.51 | <0.001 | 1.46 | 1.05–2.02 | 0.024 |
CI, confidence interval; HR, hazard ratio.
Adjusted for MAGGIC risk score and log‐transformed BNP at discharge.
Adjusted for age, male sex, body mass index, current smoker or not, systolic blood pressure, New York Heart Association class at discharge, left ventricular ejection fraction, history of diabetes, chronic obstructive pulmonary disease, and heart failure, creatinine at discharge, prescription of a beta‐blocker, or angiotensin converting enzyme inhibitor/angiotensin II receptor blocker at the time of discharge.
For external validation, the Kitasato cohort was divided into two groups: the non‐slow group (SGS ratio > 0.527) and slow group (SGS ratio ≤ 0.527). The baseline characteristics of each group are shown in Table 1 . The slow group was associated with older age, a history of heart failure, lower albumin and haemoglobin, and estimated glomerular filtration rate. Although significance was not reached, the slow group was marginally associated with female sex and higher left ventricular ejection fraction. No differences were observed in heart failure medication prescriptions. In total, 107 deaths occurred within 1 year from discharge, and 282 deaths occurred during the entire follow‐up period (median: 693 days; quartiles: 322–1349 days). The slow group was associated with higher 1 year and long‐term mortality (log‐rank: P < 0.001 for both) (Figure 3 B,C ). Furthermore, these associations retained significance even after adjustment for other covariates at discharge (Table 2 ).
Discussion
The present study evaluated the decline in gait speed in elderly patients with heart failure by applying reference gait speeds derived from a general population cohort. We clarified the extent to which gait speed was declined in patients with heart failure, aged ≥65 years, and compared it with elderly individuals without heart failure, after taking the age‐related decline into consideration. We further determined the event‐driven optimal cut‐off for the SGS ratio to identify patients at high risk for 1 year mortality and validated the obtained ratio in an independent cohort of elderly patients with heart failure. To our knowledge, the present study is the first to demonstrate that the gait speed in elderly patients with heart failure is significantly declined relative to that in general population, independent of the age‐related decline. Furthermore, we showed that evaluating the relative decline in gait speed using the SGS ratio provides additive prognostic information.
In contrast to the prognostic impact of gait speed, 5 , 16 the ‘normal value’ of gait speed has not received much attention in studies on patients with heart failure. Even though gait speed is one of the validated metrics of physical performance and is incorporated into most of the definitions of sarcopenia from major societies or groups, a single cut‐off value is typically used, irrespective of age, sex, and height. 17 , 18 , 19 , 20 Indeed, a value of 0.8 m/s is frequently used to define slowness in studies involving patients with heart failure. 5 , 7 , 21 However, the use of this cut‐off value in patients with heart failure is not recommended for several reasons. First, this value was mainly derived from community‐dwelling individuals and not from patients with heart failure. Second, even though this cut‐off value has been recommended for defining sarcopenia in current consensus/guidelines, sarcopenia associated with heart failure is generally regarded as secondary sarcopenia. Third, previously suggested or proposed cut‐off values were not originally event‐driven, and most were derived from cross‐sectional studies of the general population using a median, quantile, or percentile value. Fourth, using a single cut‐off value irrespective of age and sex does not reflect the impact of aging, which is a powerful driver of heart failure. In the present study, we defined an event‐driven cut‐off value for patients with heart failure using age‐standardized reference values of gait speed derived from the general population, incorporating the impact of the age‐related decline. Moreover, we externally validated the obtained cut‐off value and showed its additive prognostic values over a pre‐existing prognostic model.
Thus, for the first time, we demonstrated that gait speed in patients with heart failure was declined relative to that of normal individuals, even after considering the fact that the heart failure population was highly saturated by elderly patients. The decline in gait speed in two heart failure cohorts, relative to that of the general population after adjusting for age, sex, and height, was 35–40% according to the SGS ratio. We also showed that the decline in gait speed in patients with heart failure relative to that in the general population was accelerated by aging; however, this association could be still underestimated, as older patients with heart failure with a lower gait speed are more likely to die than are those without heart failure at the same age, and thus, were excluded from analysis.
The development of age‐adjusted and sex‐adjusted and event‐driven cut‐offs values for gait speed may contribute not only to patient care but also to future clinical research in patients with heart failure. Referring to disease‐specific and patient‐specific reference values for gait speed would enable healthcare providers to estimate the future risk of adverse events in individual patients with heart failure in clinical practice. This is clinically relevant, as the slowness threshold cannot be the same for a 65‐year‐old male and a 90‐year‐old female, which is clearly supported by the present data. In terms of scientific research, the reference values and event‐driven cut‐off value derived in the present study may aid in defining the individualized target gait speed in interventional studies using gait speed as a surrogate endpoint. However, whether interventions increasing gait speed subsequently improve the prognosis should be clarified in future studies.
Several limitations of the present study should be acknowledged. The most important limitation is the difference in testing procedures among the three cohorts in the present study, even though all the cohort studies measured the usual gait speed. The TMIG‐LISA cohort used a dynamic start with a distance of 10 or 5 m, the FRAGILE‐HF cohort used a static start with a distance of 4 m, and the Kitasato cohort used a dynamic start with a distance of 10 m. However, two systematic reviews and a meta‐analysis comprising 48 studies (7000 patients) and 46 studies (18428 patients), respectively, showed that the type of start (static vs. dynamic) and walking distance (short vs. long) did not significantly affect the gait speed value. 22 , 23 Nevertheless, this limitation should be carefully considered, as it could have impacted the study results. Although we considered the SGS ratio stratified according to age, gender, and height, as these factors supposedly impact gait speed and/or an age‐related decline in gait speed, there may be other factors that were not taken into account and may affect the results of our study. Regarding participants from the general population cohort, we excluded those with cardiovascular comorbidities; however, the medical history was obtained by questionnaire, and no further evaluations were performed to exclude potential comorbidities. This implies that our general cohort may have included some patients with cardiovascular disease. Furthermore, although the two heart failure cohorts performed a gait speed assessment at a steady state, the difference in the timing of the test might have impacted the study results. Finally, we observed a difference between the FRAGILE‐HF and Kitasato cohorts in the prevalence of patients with slow gait speed, which may be explained by a difference in age between the cohorts, as we found a significant interaction between age and SGS ratio in both the FRAGILE and Kitasato cohorts (with similar coefficients).
In conclusion, gait speed is significantly declined in patients with heart failure relative to that in the general population, independent from the age‐related decline. The age‐adjusted and sex‐adjusted, event‐driven, and disease‐specific cut‐off proposed in the present study was independently associated with mortality. The present study findings may contribute to both clinical practice and future research on physical function in elderly patients with heart failure.
Conflict of interest
Dr Y.M. and Dr T.K. are affiliated with a department endowed by Philips Respironics, ResMed, Teijin Home Healthcare, and Fukuda Denshi. Dr Y.M. received an honorarium from Otsuka Pharmaceutical Co. Dr K.K. has received research funding from Eiken Chemical Co., Ltd. Dr N.K. is affiliated with a department funded by Philips Healthcare; Asahi KASEI Corporation; Inter Reha Co., Ltd; and Toho Holdings Co., Ltd based on collaborative research agreements. All other authors have nothing to declare.
Funding
FRAGILE‐HF was supported by Novartis Pharma Research Grants and Japan Heart Foundation Research Grant. This work was also partially supported by JSPS KAKENHI (grant 18K15862). TMIG‐LISA study was supported by grants from the Tokyo Metropolitan Government, the Japan Arteriosclerosis Prevention Fund, the Research Institute of Science and Technology for Society, the Japan Science and Technology Agency, International Life Sciences Institute of Japan, Grants‐in‐Aid for Scientific Research (B) (2) 14370150, (B) 17390194, (B) 21390212, and a Grant‐in‐Aid for Exploratory Research 17659192 from the Japan Society for the Promotion of Science, and Health Labour Sciences Research Grants H14‐Chouju‐006, H15‐Ganyobo‐065, H15‐Seisaku‐017, H16‐Chouju‐031, H23‐Chouju‐Ippan‐001, and H23‐Chouju‐Ippan‐002 from the Ministry of Health, Labour and Welfare of Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supporting information
Table S1 Median gait speed (m/sec) in the TMIG‐LISA cohort, stratified by age, sex, and height.
Figure S1 Study flowchart.
Figure S2 Relationship between age and the SGS ratio.
Ozawa, T. , Yamashita, M. , Seino, S. , Kamiya, K. , Kagiyama, N. , Konishi, M. , Saito, H. , Saito, K. , Ogasahara, Y. , Maekawa, E. , Kitai, T. , Iwata, K. , Jujo, K. , Wada, H. , Kasai, T. , Momomura, S. , Hamazaki, N. , Nozaki, K. , Kim, H. , Obuchi, S. , Kawai, H. , Kitamura, A. , Shinkai, S. , and Matsue, Y. (2021) Standardized gait speed ratio in elderly patients with heart failure. ESC Heart Failure, 8: 3557–3565. 10.1002/ehf2.13392.
References
- 1. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, De Ferranti S, Després JP, Fullerton HJ, Howard VJ. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016; 133: 447–454. [DOI] [PubMed] [Google Scholar]
- 3. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M. Gait speed and survival in older adults. JAMA 2011; 305: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette‐Guyonnet S, Inzitari M, Nourhashemi F. Gait speed at usual pace as a predictor of adverse outcomes in community‐dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009; 13: 881–889. [DOI] [PubMed] [Google Scholar]
- 5. Lo AX, Donnelly JP, McGwin G Jr, Bittner V, Ahmed A, Brown CJ. Impact of gait speed and instrumental activities of daily living on all‐cause mortality in adults >/=65 years with heart failure. Am J Cardiol 2015; 115: 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dunlay SM, Redfield MM, Weston SA, Therneau TM, Long KH, Shah ND, Roger VL. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol 2009; 54: 1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaudhry SI, McAvay G, Chen S, Whitson H, Newman AB, Krumholz HM, Gill TM. Risk factors for hospital admission among older persons with newly diagnosed heart failure: findings from the Cardiovascular Health Study. J Am Coll Cardiol 2013; 61: 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim MJ, Yabushita N, Tanaka K. Exploring effective items of physical function in slow walking speed and self‐reported mobility limitation in community‐dwelling older adults. Geriatr Gerontol Int 2012; 12: 50–58. [DOI] [PubMed] [Google Scholar]
- 9. Lee A, Bhatt T, Smith‐Ray RL, Wang E, Pai YC. Gait speed and dynamic stability decline accelerates only in late life: a cross‐sectional study in community‐dwelling older adults. J Geriatr Phys Ther 2019; 42: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age‐associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003; 95: 1851–1860. [DOI] [PubMed] [Google Scholar]
- 11. Seino S, Shinkai S, Fujiwara Y, Obuchi S, Yoshida H, Hirano H, Kim HK, Ishizaki T, Takahashi R, TMIG‐LISA Research Group . Reference values and age and sex differences in physical performance measures for community‐dwelling older Japanese: a pooled analysis of six cohort studies. PLoS One 2014; 9: e99487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsue Y, Kamiya K, Saito H, Saito K, Ogasahara Y, Maekawa E, Konishi M, Kitai T, Iwata K, Jujo K, Wada H. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILE‐HF cohort study. Eur J Heart Fail 2020; 22: 2112–2119. [DOI] [PubMed] [Google Scholar]
- 13. Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut‐point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology 2005; 16: 73–81. [DOI] [PubMed] [Google Scholar]
- 14. Sawano M, Shiraishi Y, Kohsaka S, Nagai T, Goda A, Mizuno A, Sujino Y, Nagatomo Y, Kohno T, Anzai T, Fukuda K. Performance of the MAGGIC heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC Heart Fail 2018; 5: 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013; 34: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 16. Kamiya K, Hamazaki N, Matsue Y, Mezzani A, Corrà U, Matsuzawa R, Nozaki K, Tanaka S, Maekawa E, Noda C, Yamaoka‐Tojo M. Gait speed has comparable prognostic capability to six‐minute walk distance in older patients with cardiovascular disease. Eur J Prev Cardiol 2018; 25: 212–219. [DOI] [PubMed] [Google Scholar]
- 17. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 19. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, van Kan GA, Andrieu S, Bauer J, Breuille D, Cederholm T. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011; 12: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo GI, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) "cachexia‐anorexia in chronic wasting diseases" and "nutrition in geriatrics". Clin Nutr 2010; 29: 154–159. [DOI] [PubMed] [Google Scholar]
- 21. Pandey A, Kitzman D, Whellan DJ, Duncan PW, Mentz RJ, Pastva AM, Nelson MB, Upadhya B, Chen H, Reeves GR. Frailty among older decompensated heart failure patients: prevalence, association with patient‐centered outcomes, and efficient detection methods. JACC Heart Fail 2019; 7: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graham JE, Ostir GV, Kuo YF, Fisher SR, Ottenbacher KJ. Relationship between test methodology and mean velocity in timed walk tests: a review. Arch Phys Med Rehabil 2008; 89: 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci 2013; 68: 39–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Median gait speed (m/sec) in the TMIG‐LISA cohort, stratified by age, sex, and height.
Figure S1 Study flowchart.
Figure S2 Relationship between age and the SGS ratio.


