Abstract
Pemphigus is a rare disease characterized by bullous lesions of the skin and mucous membranes. The aetiology is autoimmune and related to the formation of IgG autoantibodies against desmogleins, which are structural proteins of desmosomes that ensure the stability of contacts between cells. Cardiac involvement in patients with pemphigus is poorly documented. We report the data in the literature on this topic and a case of pemphigus‐associated autoimmune myocarditis with damage of intercalated disc responding to immunosuppressive therapy. The occurrence of cardiomyopathy with left ventricular dysfunction in patients affected by pemphigus should be appropriately screened with endomyocardial biopsy as it could be the myocardial extension of a potentially reversible autoimmune disorder.
Keywords: Pemphigus, Autoimmune myocarditis, Cardiomyopathy
Introduction
Pemphigus is a rare disease characterized by bullous lesions of the skin and mucous membranes. In Central Europe, the annual incidence is two cases per million inhabitants. 1 The aetiology is autoimmune and related to the formation of IgG autoantibodies against desmogleins, which are structural proteins of desmosomes belonging to the cadherin family that provide stable intercellular bonds. The binding of these autoantibodies to desmogleins alters the structural integrity of the desmosome and consequently the mechanical stability of the epidermis and mucous membranes, causing detachment of the cells through acantholysis and the formation of bubbles, vesicles, and erosions. 2 Similarly to most autoimmune disorders, the pathogenesis of pemphigus is likely to be linked to a complex interaction between environmental factors, genetic predisposition, and characteristics of the immune system. There are three major clinical forms of pemphigus: vulgaris, foliaceus, and paraneoplastic.
Pemphigus vulgaris, responsible of 70% of cases, represents the most severe form with prevalent clinical onset between 40 and 60 years and very painful ulcerative lesions of the skin and mucous membranes. 2 Pemphigus foliaceus can be sporadic or endemic and equally affects men and women. Endemic areas are Brazil, Colombia, and Perù. Skin lesions are erythematous and crusted blisters spread over the scalp, the face, and the trunk, sometimes painful. Mucosal involvement is rare.
Pemphigus has been associated to autoimmune disorders such as vasculitis and type 1 diabetes mellitus and haematological, oropharyngeal, gastrointestinal, and lung neoplasias. 3
The disease is characterized by the production of IgG autoantibodies against desmogleins, in particular desmogleins 1 and 3, which are membrane proteins of the desmosome promoting extracellular binding to adjacent cells. The autoantibodies produced against these proteins cause the detachment of keratinocytes in the epidermis resulting in the formation of blisters and erosions. In patients with pemphigus, not only the skin but also other organs may be involved. Indeed, systemic involvement including renal and cardiovascular disease occurs in about one‐third of patients with the foliaceus variant of pemphigus. 4
Therefore, a molecular mimicry mechanism can be hypothesized that determines the interaction of autoantibodies against other tissues including cardiomyocytes.
The identification of an associated cardiomyopathy in patients with pemphigus is poorly documented and investigated in terms of pathogenesis. We report the data in the literature on this topic and identification by endomyocardial biopsy of a case of autoimmune myocarditis responding to immunosuppressive therapy.
Cardiac involvement in patients with pemphigus
Cardiac involvement in pemphigus is limited in the literature to few and often single case studies, although the third cause of death in pemphigus patients is cardiovascular after pneumonia and septicemia. 5
Abreu‐Velez et al. 6 described the presence of cardiac autoantibodies in patients affected by a new form of endemic pemphigus foliaceus found in El‐Bagre, Colombia, South America, with high incidence of sudden cardiac death, occurring in about 14% of cases before than 50 years. 7 , 8 Patients with the variant ‘El‐Bagre’ of endemic pemphigus foliaceus share several autoantigens with patients affected with paraneoplastic pemphigus. 9 The authors tested the presence of cardiac autoantibodies in serum of 15 patients with the variant ‘El‐Bagre’ of endemic pemphigus foliaceus and 15 controls utilizing human heart samples from autopsies of affected patients and beef, mouse, and rat heart samples. They described the presence of cardiac autoantibodies in 46% of patients with the variant ‘El‐Bagre’ of endemic pemphigus foliaceus. The autoantibodies were polyclonal and reactive against numerous desmosome adhesion proteins (such as desmoplakins I and II and connexin 43) and co‐localized in blood vessels, sarcomeric fibres, and Purkinje system. No lymphocytic infiltration in the autopsy human heart of patients affected by the variant ‘El‐Bagre’ of endemic pemphigus foliaceus was detected. However, the fibrolipomatous substitution of 15% of the myocardium of the right ventricle was observed. The authors conclude that sudden death in these patients could be related to the presence of autoantibodies against the heart conduction system and desmosomes. Moreover, some patients with the variant ‘El‐Bagre’ of endemic pemphigus foliaceus have autoantibodies against the ‘bullous pemphigoid antigen 1’ that is a plakin; it has been documented that mutation of the ‘bullous pemphigoid antigen 1’ induces skin weakness and neuromuscular deterioration in mice skin. 10 , 11 So it can be postulated that autoantibodies in these patients can react against the cardiac conduction system.
In another issue, Abreu‐Velez et al. 12 reported cardiac rhythm abnormalities in 30 patients with the variant ‘El‐Bagre’ of endemic pemphigus foliaceus compared with 30 controls. Heart rhythm abnormalities were more frequent in these patients, including sinus bradycardia, left bundle branch block, left anterior branch block, and left posterior branch block. Moreover, the authors tested the serum of the patient population and controls for cardiac autoantibodies and found in the patients with the variant ‘El‐Bagre’ of endemic pemphigus foliaceus cardiac autoantibodies reactive against adherence junctions of nodal cells and His bundle in cow heart samples. Therefore, the authors hypothesized that autoantibodies in patients with this form of pemphigus may be polyclonal and reactive against the heart conduction tissue. In their commentary editorial, Lee and Melduni 13 underline some interesting aspects of the study of Abreu‐Velez and colleagues, 12 although the sample size is quite small and a definite causative correlation between autoimmunity and cardiac arrhythmias is lacking. First, the variant ‘El‐Bagre’ of endemic pemphigus foliaceus is limited to the State of Colombia, and this suggests a possible interaction between genes and the environment. Second, the presence of polyclonal autoantibodies against structural desmosomal proteins against the heart could be related to the development of cardiomyopathy and electrical instability.
In a further report, Abreu‐Velez et al. 14 demonstrated that patients with the variant ‘El‐Bagre’ of endemic pemphigus foliaceus have polyclonal autoantibodies reactive against the areae compositae that are a special type of cardiac intercellular junctions with the role of stabilizing the intercellular junctions in the heart. Patients with reactive autoantibodies against the areae compositae also showed ventricular hypertrophy at echocardiography; therefore, the authors hypothesized that the rupture of these junctions, due to an autoimmune process, may be implicated in the development of myocardial hypertrophy as a compensatory mechanism.
Finally, a report of autoimmune myocarditis has been obtained in a single case, 15 where the final diagnosis of myocarditis was supposed and not confirmed by an endomyocardial biopsy.
Immunosuppression is the cornerstone of the treatment of pemphigus. The association between steroids and immunosuppressive drugs is useful to limit side effects of steroids. In fact, steroid pulse therapy may be associated with cardiac dysfunction as suggested by the reduction of global longitudinal strain by speckle‐tracking 2D echocardiography and QT dispersion on the surface electrocardiogram. 16
Immune‐mediated mechanisms are associated with cardiovascular disorders. 17 Herein, we report the manifestation of progressive cardiac dilatation and dysfunction, which pathologic substrate is characterized by an autoimmune myocarditis and damage of intercalated disc responding to immunosuppressive therapy.
Case report
A 31‐year‐old man, who was previously in good health, was hospitalized for acute heart failure associated to oral (Figure 1 A ) and genital ulcerative lesions and blisters of trunk, abdomen, and limb skin (Figure 1 B ) appeared 3 weeks earlier. He appeared in New York Heart Association (NYHA) functional class III, symptomatic for dyspnoea due to mild exertion and with peripheral oedema. Routine laboratory exams were within limits except white blood cells (12.160/UL, 75% neutrophils), C‐reactive protein (30 mg/mL, nv 0–0.5 mg/dL), high‐sensitive cardiac troponin (0.5 mcg/L, nv < 0.014 mcg/L), and N‐terminal pro‐B‐type natriuretic peptide (458.7 pg/mL, nv 0–254 pg/mL). The evaluation of oral and genital ulcers was negative for autoimmune and viral aetiology. HLA‐B51, screened to investigate Behcet's disease, was negative while patient serology revealed an elevated antibody titre for desmoglein 3.
Figure 1.
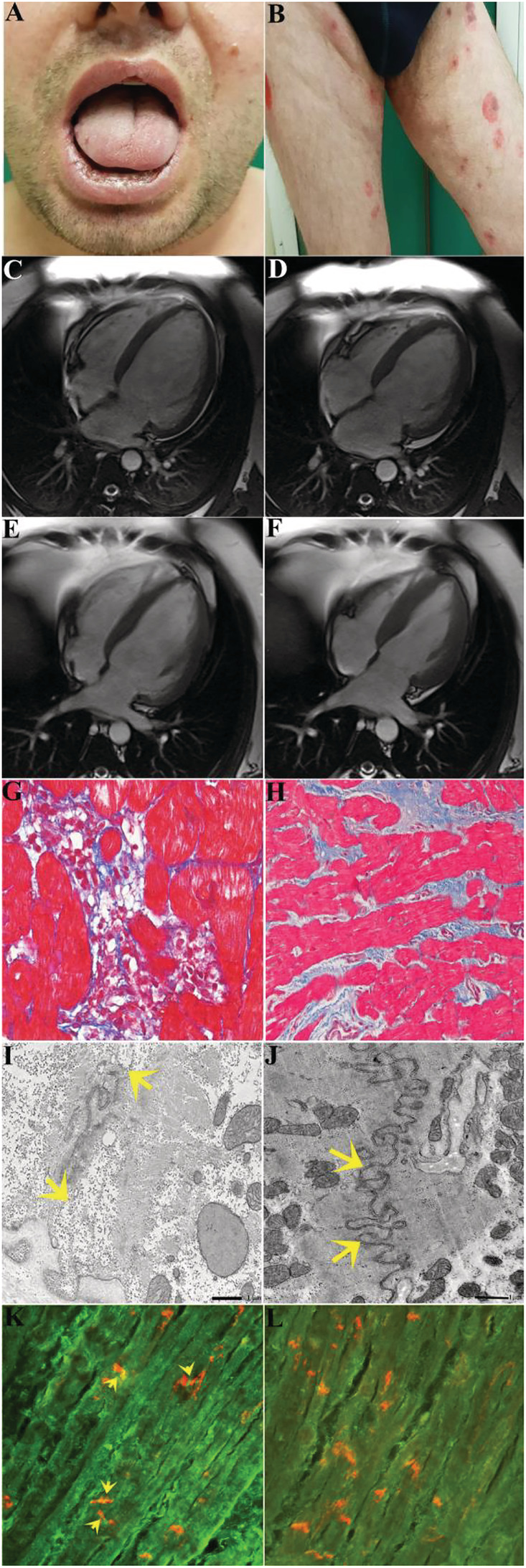
Clinical–histologic findings of pemphigus vulgaris‐associated autoimmune myocarditis before and after immunosuppression therapy. (A and B) Oral and skin lesions caused by pemphigus vulgaris. (C–F) Cardiac magnetic resonance images showing cardiac dilatation [end‐diastolic volume/body surface area (BSA) and end‐systolic volume/BSA: 154 and 119 mL/m2, respectively] and dysfunction (left ventricular ejection fraction: 22%), which recovers at 2 months of follow‐up (end‐diastolic volume/BSA and end‐systolic volume/BSA: 95.18 and 52.65 mL/m2, respectively) with left ventricular ejection fraction of 45% following immunosuppressive therapy. (G and H) Left ventricular endomyocardial biopsy before (G) and after immunosuppression (H) showing active lymphocytic myocarditis progressing to healed phase. (I) Detail of a disorganized intercalated disc. Between the arrows, residual junctional complexes are still visible. The bar represents 1 μm. (J) After therapy, the recovery of the intercalated disc is evident, with all types of junctions well recovered in all regions of the disc. The bar represents 1 μm. (K) shows positive anti‐heart autoantibodies on human heart extended to intercalated disc (in red co‐localization with antibody anti‐n‐cadherin, yellow arrow) (400×). (L) shows negative serum for anti‐heart autoantibodies on human heart and on intercalated disc (400×).
A skin perilesional biopsy showed sub‐epidermal vesicles with associate infiltrate of lymphocytes, histiocytes, and eosinophils. Direct immunofluorescence of perilesional skin showed infiltration of IgG autoantibodies against desmoglein 3 and C3d along the basement membrane of keratinocytes, suggesting the diagnosis of pemphigus vulgaris.
On cardiac side, electrocardiogram showed sinus rhythm (85 b.p.m.) with diffuse and non‐specific ventricular repolarization abnormalities. Two‐dimensional echocardiography showed a dilated and globally hypokinetic left ventricle (LV) [LV end‐diastolic diameter = 62 mm, LV end‐diastolic volume = 132 mL/m2, LV ejection fraction (LVEF) calculated with biplane Simpson method = 24%], moderate mitral regurgitation, and left atrial enlargement. Right ventricle showed mild dilation with preserved systolic function and mild tricuspid regurgitation. No pericardial effusion was present.
Cardiac magnetic resonance (CMR) confirmed a severe LV dilation (end‐diastolic volume/body surface area 153.8 mL/m2, Figure 1 C , with reduced LVEF of 22%, Figure 1 D and Supporting Information, Movie S1 ). No myocardial oedema on T2‐weighted images was detected. Late gadolinium enhancement technique revealed only a nuanced area of non‐specific fibrotic meaning in the inferior junctional site and in the sub‐epicardial area at the level of the interventricular septum.
Invasive studies including coronary and LV angiography and endomyocardial biopsy were performed. Coronary arteries were normal. Left endomyocardial biopsy revealed the presence of lymphomononuclear infiltrates with associated necrosis of adjacent cardiomyocytes, suggesting an active myocarditis (Figure 1 G ). The inflammatory infiltrates were positive for CD45RO+ T lymphocytes. Cardiomyocyte overexpression of TLR‐4 and the absence of viral genomes on myocardial tissue (as PCR for the most common cardiotropic viruses was negative) was indicative of an immune‐mediated myocarditis. Assessment of anti‐heart antibodies obtained on frozen section from normal myocardium of 0 group showed an intense (Figure 1 K ) positivity extended to the intercalated disc. At electron microscopy, damage of intercalated disc was revealed, suggesting a correlation with pemphigus (Figure 1 I ).
Patient was treated with prednisone 1 mg/kg/day for 4 weeks followed by 0.33 mg/kg/day for 5 months and azathioprine 2 mg/kg/day for 6 months according to the TIMIC protocol. 19 At 2 months of follow‐up, the patient presented a remarkable clinical improvement; the NYHA functional class from III became I. The electrocardiogram showed sinus rhythm (70 b.p.m.) with no specific ventricular repolarization abnormalities nor QT prolongation. At 2D echocardiography, the LV appeared of reduced size (LV end‐diastolic diameter = 54 mm) with recovered function. Also, the left atrium returned to normal size, and the mitral regurgitation became mild with no haemodynamic effects. CMR confirmed the recovery of cardiac dimensions (Figure 1 E ) with LV function rising to 45% EF (Figure 1 F and Supporting Information, Movie S2 ). The small nuanced area of non‐specific fibrotic meaning in the inferior junctional site and in the sub‐epicardial area at the level of the interventricular septum was unchanged.
Cardiac improvement was maintained at a 6 month follow‐up CMR. At that time, control endomyocardial biopsy revealed resolution of myocardial inflammation (Figure 1 H ) as well as recovery of intercalated disc (Figure 1 J and 1 L ).
Discussion
Although pemphigus has been associated with various autoimmune disorders as well as haematological, oropharyngeal, gastrointestinal, and lung neoplasias, 3 cardiac involvement in pemphigus has been rarely documented. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15
In the case of pemphigus, the most likely explanation is an antigenic mimicry between cardiomyocytes and the basement membrane zone. For example, collagen XVII, which is an antigen of bullous pemphigus, has been shown to be expressed in mouse heart. 18 Furthermore, collagen XVII has also been reported to be the target of IgA autoantibodies, in a case of IgA‐type bullous dermatosis arising after heart transplantation. 19 , 20 However, beyond the registration of increased cardiac dimension and reduced contractility sometimes associated with pemphigus, cause and mechanisms of cardiac damage have not been clarified so far. On the other hand, definition of histological and molecular pathway is essential for the instauration of a specific and effective treatment.
In our report, a pemphigus‐associated progressive cardiac dilatation and dysfunction has been investigated by LV endomyocardial biopsy and its histologic substrate attributed to an autoimmune myocarditis. Autoimmune nature of myocardial inflammation was based on negative PCR for viral genomes, presence of anti‐heart autoantibodies, and overexpression of TLR4 on myocardiocytes. Pathogenetic correlation of myocarditis to pemphigus was supported by serologic elevation of antibody titre for desmoglein 3 and by the reversible damage of intercalated disc at electron microscopy.
Immunosuppressive therapy based on combination of steroids with azathioprine as from TIMIC trial 21 was able to revert the cardiac disease with the LVEF rising from 22% to 45% and NYHA class from III to I. The patient had no side effects from therapy; no QT prolongation was detected on the electrocardiogram.
The association between steroid and immunosuppressive drugs is useful to limit the possible cardiac side effects of high‐dose steroid therapy alone. 16 , 22
It has been demonstrated that the activation of Foxo transcription factor and subsequent overexpression of atrogin‐1 promotes ubiquitin‐dependent proteasome proteolysis of endogenous contractile elements causing cardiac dysfunction. 23
Presence of myocardial inflammation was not suspected from CMR findings as no specific alteration of T1, T2, and late gadolinium enhancement signals was registered. This is not unusual for cardiomyopathic phenotype of myocarditis where CMR sensitivity for inflammation is around 50% 24 and explained with a slowly progressive disorder.
Contribution of endomyocardial biopsy was crucial as it could demonstrate the presence of autoimmune myocarditis and connect the myocardial to the skin lesion revealing the common damage of intercalated disc.
In conclusion, occurrence of dilated cardiomyopathy in patients with pemphigus should be appropriately screened with CMR and possibly endomyocardial biopsy as it could be the myocardial extension of a potentially reversible autoimmune disorder.
Conflict of interest
None declared.
Funding
This work was supported by European Project ERA‐CVD ‘Transnational Research Projects on Cardiovascular Diseases’ (JTC 2016 IKDT‐IGCM) and by Italian Ministry of Health ‘Ricerca corrente’ IRCCS Spallanzani.
Supporting information
Movie S1. Cardiac dilatation with severe left ventricular dysfunction (EF 22%) (Movie S1) that recover after immunosuppressive therapy (EF 45%) (Movie S2).
Movie S2. Cardiac dilatation with severe left ventricular dysfunction (EF 22%) (Movie S1) that recover after immunosuppressive therapy (EF 45%) (Movie S2).
Frustaci, A. , Francone, M. , Verardo, R. , Scialla, R. , Bagnato, G. , Alfarano, M. , Chimenti, C. , Frustaci, E. , Sansone, L. , and Russo, M. (2021) Pemphigus‐associated cardiomyopathy: report of autoimmune myocarditis and review of literature. ESC Heart Failure, 8: 3690–3695. 10.1002/ehf2.13474.
References
- 1. Porro AM, Seque CA, Ferreira MCC, Enokihara MMSES. Pemphigus vulgaris. An Bras Dermatol 2019; 94: 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pan M, Liu X, Zheng J. The pathogenic role of autoantibodies in pemphigus vulgaris. Clin Exp Dermatol 2011; 36: 703–707. [DOI] [PubMed] [Google Scholar]
- 3. Hsu DY, Brieva J, Sinha AA, Langan SM, Silverberg J. Comorbidities and inpatient mortality for pemphigus in the USA. Br J Dermatol 2016; 174: 1290–1298. [DOI] [PubMed] [Google Scholar]
- 4. Abréu‐Vélez AM, Beutner EH, Montoya F, Bollag WB, Hashimoto T. Analyses of autoantigens in a new form of endemic pemphigus foliaceus in Colombia. J Am Acad Dermatol 2003; 49: 609–614. [DOI] [PubMed] [Google Scholar]
- 5. Huang YH, Kuo CF, Chen YH, Yang YW. Incidence, mortality, and causes of death of patients with pemphigus in Taiwan: a nationwide population‐based study. J Invest Dermatol 2012; 132: 92–97. [DOI] [PubMed] [Google Scholar]
- 6. Abreu‐Velez AM, Howard MS, Jiao Z, Gao W, Yi H, Grossniklaus HE, Duque‐Ramírez M, Dudley SC Jr. Cardiac autoantibodies from patients affected by a new variant of endemic pemphigus foliaceus in Colombia. South America J Clin Immunol 2011; 31: 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abréu‐Vélez AM, Hashimoto T, Bollag WB, Arroyave ST, Abrèu‐Velez CE, Londoño ML, Montoya F, Beutner EH. A unique form of endemic pemphigus in Northern Colombia. J Am Acad Dermatol 2003; 4: 599–608. [DOI] [PubMed] [Google Scholar]
- 8. Hisamatsu Y, Abreu Velez AM, Amagai M, Ogawa MM, Kanzaki T, Hashimoto T. Comparative study of autoantigen profile between Colombian and Brazilian types of endemic pemphigus foliaceus by various biochemical and molecular biological techniques. J Dermatol Sci 2003; 32: 33–41. [DOI] [PubMed] [Google Scholar]
- 9. Sehgal VN, Srivastava G. Paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome. Int J Dermatol 2009; 48: 162–169. [DOI] [PubMed] [Google Scholar]
- 10. Leung CL, Zheng M, Prater SM, Liem RK. The BPAG1 locus: alternative splicing produces multiple isoforms with distinct cytoskeletal linker domains, including predominant isoforms in neurons and muscles. J Cell Biol 2001; 154: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leung CL, Sun D, Zheng M, Knowles DR, Liem RK. The intermediate filament protein peripherin is the specific interaction partner of mouse BPAG1‐n (dystonin) in neurons. J Cell Biol 1999; 144: 435–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abreu‐Velez AM, Howard MS, Velazquez‐Velez JE. Cardiac rhythm and pacemaking abnormalities in patients affected by endemic pemphigus in Colombia may be the result of deposition of autoantibodies, complement, fibrinogen, and other molecules. Heart Rhythm 2018; 15: 725–731. [DOI] [PubMed] [Google Scholar]
- 13. Lee HC, Melduni RM. Autoimmunity and cardiac arrhythmias in endemic pemphigus foliaceus—association, correlation, or causation? Heart Rhythm 2018; 15: 732–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abreu‐Velez AM, Upegui‐Zapata YA, Valencia‐Yepes CA, Upegui‐Quiceno E, Jiménez‐Echavarría AM, Niño‐Pulido CD, Smoller BR, Howard MS. Involvement of the areae compositae of the heart in endemic pemphigus foliaceus. Dermatol Pract Concept 2019; 9: 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bachmeyer C, Seoud J, Carlotti A, Bernard P, Batteux F, Weill B, Roujeau JC. Bullous pemphigoid associated with acute myocarditis. Dermatology 2002; 204: 161–162. [DOI] [PubMed] [Google Scholar]
- 16. Shahidi‐Dadras M, Pishgahi M, Tabary M, Kheradmand Z, Araghi F, Dadkhahfar S, Robati RM. Cardiac function in pemphigus vulgaris patients before and after steroid pulse therapy. J Dermatolog Treat 2020; 3: 1–5. [DOI] [PubMed] [Google Scholar]
- 17. Caforio ALP, Adler Y, Agostini C, Allanore Y, Anastasakis A, Arad M, Böhm M, Charron P, Elliott PM, Eriksson U, Felix SB, Garcia‐Pavia P, Hachulla E, Heymans S, Imazio M, Klingel K, Marcolongo R, Matucci Cerinic M, Pantazis A, Plein S, Poli V, Rigopoulos A, Seferovic P, Shoenfeld Y, Zamorano JL, Linhart A. Diagnosis and management of myocardial involvement in systemic immune‐mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38: 2649–2662. [DOI] [PubMed] [Google Scholar]
- 18. Kondo J, Kusachi S, Ninomiya Y, Yoshioka H, Oohashi T, Doi M, Murakami T, Moritani H, Kumashiro H, Tsuji T. Expression of type XVII collagen alpha 1 chain mRNA in the heart. Jpn Heart J 1998; 39: 211–220. [DOI] [PubMed] [Google Scholar]
- 19. Petit D, Borradori L, Rybojad M, Morel P. Linear IgA bullous dermatosis after heart transplantation. J Am Acad Dermatol 1990; 22: 851. [DOI] [PubMed] [Google Scholar]
- 20. Schumann H, Baetge J, Tasanen K, Wojnarowska F, Schacke H, Zilikens D, Bruckner‐Tuderman L. The shed ectodomain of collagen XVII/BP 180 is targeted by autoantibodies in different blistering skin diseases. Am J Pathol 2000; 156: 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus‐negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J 2009; 30: 1995–2002. [DOI] [PubMed] [Google Scholar]
- 22. Pishgahi M, Dadkhahfar S, Robati RM, Kheradmand Z, Shahidi‐Dadras M, Zargari O, Elpern DJ. Electrocardiographic changes after high‐dose corticosteroid pulse therapy in pemphigus patients. J Dermatolog Treat 2018; 29: 802–805. [DOI] [PubMed] [Google Scholar]
- 23. Frustaci A, Letizia C, Verardo R, Grande C, Calvieri C, Russo MA, Chimenti C. Atrogin‐1 pathway activation in cushing syndrome cardiomyopathy. J Am Coll Cardiol 2016; 67: 116–117. [DOI] [PubMed] [Google Scholar]
- 24. Francone M, Chimenti C, Galea N, Scopelliti F, Verardo R, Galea R, Carbone I, Catalano C, Fedele F, Frustaci A. CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy‐proven acute myocarditis. JACC Cardiovasc Imaging 2014; 7: 254–263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Cardiac dilatation with severe left ventricular dysfunction (EF 22%) (Movie S1) that recover after immunosuppressive therapy (EF 45%) (Movie S2).
Movie S2. Cardiac dilatation with severe left ventricular dysfunction (EF 22%) (Movie S1) that recover after immunosuppressive therapy (EF 45%) (Movie S2).


