Abstract
Despite significant advances in drug‐based and device‐based therapies, heart failure remains a major and growing public health problem associated with substantial disability, frequent hospitalizations, and high economic costs. Keeping patients well and out of the hospital has become a major focus of heart failure disease management. Achieving and maintaining such stability in heart failure patients requires a holistic approach, which includes at least the management of the underlying heart disease, the management of comorbidities and the social and psychological aspects of the disease, and the management of haemodynamic/fluid status. In this regard, accurate assessment of elevated ventricular filling pressures or volume overload, that is, haemodynamic or pulmonary congestion, respectively, before the onset of worsening heart failure symptoms represents an important management strategy. Unfortunately, conventional methods for assessing congestion, such as physical examination and monitoring of symptoms and daily weights, are insensitive markers of worsening heart failure. Assessment tools that directly measure congestion, accurately and in absolute terms, provide more actionable information that enables the application of treatment algorithms designed to restore patient stability, in a variety of clinical settings. Two such assessment tools, implantable haemodynamic monitors and remote dielectric sensing (ReDS), meet the prerequisites for useful heart failure management tools, by providing accurate, absolute, and actionable measures of congestion, to guide patient management. This review focuses on the use of such technologies, across the spectrum of heart failure treatment settings. Clinical data are presented that support the broad use of pulmonary artery pressure‐guided and/or ReDS‐guided heart failure management in heart failure patients with reduced and preserved left ventricular ejection fraction.
Keywords: Heart failure management, Remote monitoring, Pulmonary pressure, Lung fluid
Introduction
Despite significant advances in drug‐based and device‐based therapies, heart failure (HF) remains a major and growing global public health problem. 1 , 2 , 3 , 4 Efforts to develop new approaches that improve functional status, quality of life, exercise tolerance, and morbidity and mortality continue to be a priority. In addition, driven by the need to reduce costs to hospitals, healthcare systems, and payers, there is also an imperative to reduce the number of hospitalizations (including 30 day readmissions), emergency department visits, and unnecessary physician office visits. Consequently, the focus of HF disease management has shifted from managing episodes of decompensation requiring hospitalization, a reactive approach to HF care, to achieving and maintaining stability in order to keep patients out of the hospital, a more proactive approach. To enable this latter strategy, a holistic approach, which includes at least the management of the underlying heart disease, the management of comorbidities and the social and psychological aspects of the disease, and the management of haemodynamic/fluid status, is needed. In this regard, assessment tools focused on the measurement of patient volume status, particularly on haemodynamic and/or pulmonary congestion, are essential. Such tools may help the clinician keep patients well and out of the hospital, by avoiding episodes of volume retention.
Prior attempts to estimate changes in haemodynamic and/or pulmonary congestion, and achieve or maintain stability, were mostly dependent upon identifying worsening HF signs and symptoms, alterations in body weight, or changes in biomarkers. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 Unfortunately, these changes appear late and are relatively insensitive indicators of clinical status in patients with HF. For example, while recommended by HF guidelines, daily measurement of body weight has a sensitivity of only 10% to 20% for the development of worsening HF. 7 Moreover, HF therapy guided by monitoring of signs, symptoms, weight, and biomarkers does not reliably improve clinical outcomes when used at the point‐of‐care or in the home. 14 Only when incorporated into a very well‐resourced and intensive telemonitoring system has their use resulted in reduced unplanned days in hospital and mortality. 11
In this review, we first summarize the ideal criteria for a HF monitoring approach to be successful in improving clinical outcomes. Second, we present the current evidence showing that these criteria may be met by the use of implantable haemodynamic monitoring systems or by a non‐invasive remote dielectric sensing (ReDS) monitoring system.
Principles for successful heart failure monitoring technology
Based on more than 25 years of clinical and research experience and on a prior review of HF monitoring technologies and clinical trials, there seems to be at least five prerequisites for success in the development of HF assessment tools (Figure 1 ).
Figure 1.
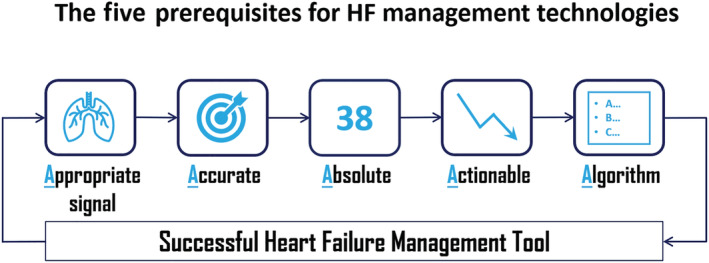
The five prerequisites for success in the development of heart failure assessment tools (central illustration).
First, these tools should measure an appropriate signal. That is, sensors must measure the underlying pathophysiology contributing to worsening HF symptoms and clinical events (e.g. hospitalizations). Based on numerous observations, including those from trials of implantable haemodynamic monitoring systems, increases in intracardiac and pulmonary artery pressures and in lung fluid content are among the earliest physiological changes and the proximate cause of worsening HF. 15 Thus, direct measurement of haemodynamic and/or pulmonary congestion, rather than assessment of their downstream consequences, may provide the best target and greatest opportunity for proactive intervention and avoidance of hospitalizations.
Second, sensors must be accurate, providing measurements that have been validated against gold standards such as the Swan–Ganz catheter in the case of haemodynamic pressure sensors or computed tomography (CT) in the case of lung fluid content assessment devices.
Third, sensors appear to be most effective when they provide absolute values, rather than relative ones. For example, in the case of lung fluid content assessment, it is not sufficient to know when the lungs are relatively wetter or dryer. Rather, it is imperative to know when the lungs are normally dry and, conversely, to know quantitatively the amount of abnormal fluid if they are wet in order to guide therapy. The relativistic nature of impedance‐based technologies measuring lung fluid content may explain the failure to date of most trans‐thoracic and intra‐thoracic impedance assessment in HF management.
Fourth, the information provided must be directly actionable, in that we must understand the meaning of the information to know what an appropriate response is. In the case of implantable haemodynamic monitors, we understand the meaning of intracardiac and pulmonary artery pressures, and we know what to do with this information. In the case of relative changes in thoracic impedance, we do not.
Finally, it is necessary that an algorithm can be provided to guide clinicians on how to use the information provided by the technology, and to adjust medical therapies when abnormal values are recorded. In pressure and lung fluid content‐guided HF management, cut points have been established and treatment algorithms have been developed around these cut points. If the information is not used, it is certain that patient outcomes will not be improved.
Currently, two available HF monitoring systems meet all five of these requirements for successful HF monitoring technologies, the implantable haemodynamic monitor—CardioMEMS HF System (Abbott, Sylmar, California), which is the only FDA approved system for outpatient PAP monitoring, and non‐invasive lung fluid monitor—ReDS System (Sensible Medical Innovations, Ltd., Netanya, Israel), which is FDA cleared for use in the United States, and are discussed below. The CardioMEMS HF System is available worldwide, while the ReDS System is available in the European Union and Israel in addition to the US. Trials with new HF‐monitor systems, which also meet all five proposed requirements [e.g. V‐LAP System for direct measurement of left atrial pressure (Vectorious Medical Technologies, Ltd., Tel Aviv, Israel) and the Cordella System for PAP monitoring (Endotronix, Inc., Lisle, Illinois)] are ongoing.
Overview and validation of the CardioMEMS heart failure system
Increases in ventricular filling pressures, in both diastolic and systolic HF patients, occur weeks before HF hospitalization. 15 , 16 , 17 By targeting day‐to‐day maintenance of normal ventricular filling pressures, an implantable HF management system using ambulatory intracardiac or PAP monitoring may succeed in keeping patients out of the hospital and perhaps in reducing HF mortality. 18 , 19 The success of this approach has been demonstrated in a series of investigations using the CardioMEMS HF System (Table 1 ). 18 , 19 , 20 , 21 , 22
Table 1.
Summary of CardioMEMS clinical studies
| Study | Methodology | Patient population | Sample size | Endpoint | HF hospitalization | Relative risk reduction (RRR) |
|---|---|---|---|---|---|---|
| CHAMPION | Prospective, randomized, double‐blinded |
NYHA III HFpEF or HFrEF |
550 280—control 270—treatment |
HFH 6 months |
0.44—control 0.32—treatment |
28% [0.60–0.85], P = 0.0002 37% (annualized) [0.52–0.77], P < 0.0001 |
| CMS post‐approval | Retrospective cohort | FFS Medicare HFrEF/HFpEF | 1114 | HFH 6 months |
1020 events—pre 381 events—post |
55% [0.49–0.61], P < 0.001 |
| CardioMEMS post‐approval | Prospective, open‐label, single‐arm | NYHA Class III & prior HFH within 12 months | 1200 | HFH 12 months |
1.25—pre 0.54—post |
57% [0.39–0.47], P < 0.0001 |
| MEMS‐HF | Prospective, open‐label, single‐arm | NYHA Class III, ≥1 HFH in the preceding year | 234 | HFH 12 months |
1.55—pre 0.60—post |
62% [0.31–0.48], P < 0.0001 |
| GUIDE‐HF | Prospective, randomized, double‐blinded | NYHA Class II–IV, ≥1 HFH or elevated BNP in the preceding year | 3600 | HFH, IV diuretics visits and all‐cause mortality 12 months | In process | In process |
CI, confidence interval, CMS, Center for Medicare and Medicaid Services; FFS, fee for service, GUIDE‐HF; Hemodynamic‐GUIDEd Management of Heart Failure; HFH, heart failure hospitalization; HFpEF, heart failure preserved ejection fraction; HFrEF, heart failure reduced ejection fraction; NYHA, New York Heart Association
This system employs a novel, wireless, battery‐free, PAP sensor, that is essentially a coil and a pressure‐sensitive capacitor encased in a capsule. It is implanted in a branch of the pulmonary artery using standard right heart catheterization technique and a proprietary delivery system. The coil and capacitor form an electrical circuit that resonates at a specific frequency, and pressure applied to the sensor causes deflections of the pressure‐sensitive surface, resulting in a characteristic shift in the resonant frequency. Electromagnetic coupling is achieved by an external antenna which is held against the patient's body. The antenna provides power to the device, continuously measuring its resonant frequency, which is then converted to a pressure waveform by an external patient home electronics unit, which uploads PAP information to a secure, web‐based patient management system. Pressure measurements derived by this system have been validated during right heart catheterization, using standard fluid‐filled catheters as a ‘gold standard’. 23 , 24
Use of the CardioMEMS heart failure system in the ambulatory setting
The primary use case scenario for CardioMEMS has been in the ambulatory (in‐home outpatient) setting, where daily measurements have been used to guide HF management. 18 , 19 , 20 , 21 , 22 Studies of its use in other HF treatment settings, such as in the emergency department or hospital ward, are lacking. In the ambulatory setting, the use of PAP‐guided HF management has been shown to substantially lower the risk of recurrent HF hospitalization, as first demonstrated 10 years ago by the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial. 18 , 19
In CHAMPION, 550 NYHA Class III patients, regardless of left ventricular ejection fraction, were randomized to either daily measurement of PAP in addition to standard of care (treatment group; n = 270), vs. standard of care alone (control group; n = 280), to manage patients. There were specific pressure targets and treatment algorithms that were mandated by protocol to ensure adequate testing of the hypothesis that PAP‐guided HF management would lower the rate of HF hospitalization. 25 The primary endpoint, the rate of HF hospitalization over 6 months, was significantly reduced from a rate of 0.44 in the control group to 0.32 in the treatment group, a 28% relative risk reduction. 18 Over the entire follow‐up period averaging more than 17 months, there was a 37% annualized relative risk reduction for HF hospitalization. Significant reductions in HF hospitalizations were seen in HF patients with either reduced or preserved LVEF. 26 Pulmonary artery pressure‐guided increases or decreases in diuretic dosing accounted for the majority of these risk reductions, while PAP‐guided addition or titration of vasodilator therapies also contributed.
Real‐world evidence also supports the utility of PAP‐guided HF management, with adherence and complication rates similar to those seen in the CHAMPION trial. Desai et al. 21 performed a retrospective cohort study to evaluate the effectiveness of haemodynamic monitoring in reducing HF hospitalization among Medicare patients during the period after FDA approval. Fee‐for‐service Medicare beneficiaries undergoing PAP sensor implantation between June 1, 2014 and December 31, 2015, with at least 6 months of continuous Medicare enrolment before and after implantation, were included. Analysis of administrative claims data from the Center for Medicare and Medicaid Services standard analytic file demonstrated a 55% reduction in cumulative HF hospitalizations during the 6 months post‐CardioMEMS implantation compared with the 6 months before.
The 1 year results of the CardioMEMS post‐approval study, a multi‐centre, prospective, open‐label, observational, single‐arm trial of 1200 patients enrolled across 104 US sites, provide further support for the effectiveness of PAP‐guided HF management. 20 In these patients with NYHA Class III HF and a prior HF hospitalization within 12 months of enrolment, adherence rates to daily pressure transmission was 76 ± 24% and PAP declined significantly. The rate of HF hospitalization was significantly reduced by 57% at 1 year compared with the year before implantation. The rate of all‐cause hospitalization was also significantly reduced. The results were consistent across multiple pre‐defined subgroups, including by LVEF subgroup.
Similarly, the CardioMEMS European Monitoring Study for Heart Failure (MEMS‐HF) demonstrated a significant 62% reduction in the rate of HF hospitalization, comparing the 12 months post‐implant vs. pre‐implant. 22 This study evaluated the safety, feasibility, and performance of the CardioMEMS device in Germany, The Netherlands, and Ireland to determine if US CardioMEMS findings could be replicated in health systems outside of the United States. Beyond the finding of reduced HF hospitalizations, MEMS‐HF also showed very large improvements in patient‐reported quality of life, using both the Kansas City Cardiomyopathy Questionnaire and the 9‐item Patient Health Questionnaire.
Thus, in routine clinical practice as in clinical trials, PAP‐guided HF management reduces PAP, lowers the rates of HF and all‐cause hospitalizations, and improves patient‐reported quality of life in patients with symptomatic HF and a prior HF hospitalization. Across studies, the magnitude of benefit is best described by the average annualized reduction in HF hospitalization, which ranges from 37% to 62%. The ongoing Hemodynamic‐GUIDEd Management of Heart Failure (GUIDE‐HF) trial will further assess the safety and effectiveness of CardioMEMS‐guided HF management on morbidity and mortality (including all‐cause mortality) in a broader population of HF patients. Finally, a validated non‐invasive HF assessment tool may represent an attractive alternative or adjunct to CardioMEMS, despite its excellent safety profile and proven effectiveness. ReDS represents such a tool.
Overview of remote dielectric sensing technology
Remote dielectric sensing technology is based on a miniature radar system that employs low‐power electromagnetic signals transmitted through the thorax between two externally applied sensors with results displayed via a bedside console. One sensor is positioned anteriorly on the chest and another is positioned posteriorly on the back; the sensors are not required to make contact with the skin, that is, they can be placed on top of clothing. For consistent placement between readings, sensors are embedded either in a vest that is adjusted to fit each patient's body habitus, or in a simple‐to‐apply clip that fits over the shoulder. The bedside console has a signal processor that assesses the electromagnetic signal as it passes through the thorax and calculates the average dielectric coefficient of the lung tissue between the electrodes. Because lung tissue is primarily composed of water and air, with water having a very high dielectric coefficient and air having the lowest dielectric coefficient, the average dielectric coefficient reflects the percentage of lung tissue fluid content.
The system provides an onscreen reading within 90 s and can be transmitted to a cloud platform. Measurements are recorded in units of percent (%), representing the percent of lung tissue volume that is occupied by fluid. The normal value of lung fluid volume, as measured by CT and confirmed by ReDS, ranges between 20% and 35%. 27 The full range of lung fluid volume reported by the ReDS system spans from 15% to 60%.
Validation of remote dielectric sensing technology
Computed tomography has been considered to be the most accurate means for quantifying lung fluid content. 28 , 29 , 30 However, as it is difficult to obtain imaging in an acutely dyspnoeic patient because of the requirement to be in the prone position and, because of its harmful radiation characteristics, it is not desirable for routine testing in humans. Thus, a two‐step approach was used to validate ReDS against the gold standard assessment of CT (Figure 2 ). First, the accuracy of ReDS using serial CT testing was evaluated using a phantom lung model and during acute volume loading and following diuresis in a pig model of HF. Second, cross‐sectional (one‐time) comparisons of ReDS to high‐resolution chest CT were performed in patients with and without acute decompensated heart failure (ADHF). A nearly linear pattern between ReDS and CT fluid concentration values was observed in the lab and animal experiments, with an intraclass correlation of 0.94. Similarly, in 16 ADHF patients and 15 non‐ADHF subjects, the intraclass correlation between ReDS and CT was found to be 0.90. In normal humans, lung fluid content averages (±standard deviations) for CT and ReDS were 28.7 ± 5.9% and 27.3 ± 6.6% for the non‐ADHF group, and 40.7 ± 8.8% and 39.8 ± 6.8% for CT and ReDS for ADHF, respectively (P < 0.0001). This compares favourably with each other and is consistent with normally dry lungs in non‐ADHF and wet lungs in ADHF.
Figure 2.
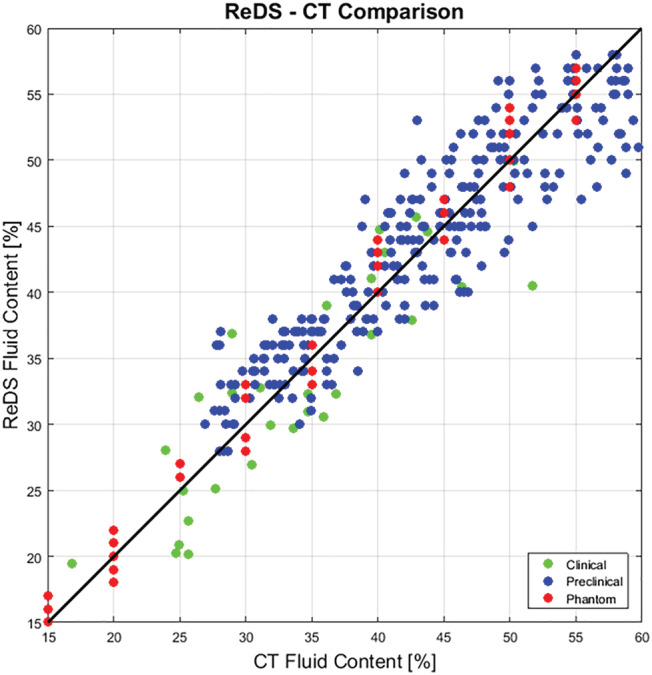
ReDS vs. computed tomography—Comparison of lung fluid content in three settings. CT, computed tomography; ReDS, remote dielectric sensing.
In addition, in a separate study of hospitalized ADHF patients, changes in ReDS values during diuresis were correlated to net fluid balance changes, as another means of validating ReDS assessment. 31 Results from 24 patients demonstrated a reduction in mean (SD) ReDS values by 18 ± 11% during the course of a hospitalization, consistent with a reduction in pulmonary congestion. This finding strongly correlated with changes in net fluid balance.
Finally, the relationship between ReDS assessment of lung fluid content and invasively measured cardiac haemodynamics was investigated. 32 In this prospective single‐centre study, ReDS readings were obtained in the supine position, just before right heart catheterization, in 139 patients with HF. A good correlation was found between ReDS and the pulmonary capillary wedge pressure (PCWP), but as expected, this correlation (r = 0.492, P < 0.001) was weaker than that seen for the ReDS comparison with CT. These findings are consistent with the known dissociation between lung fluid content and PCWP at lower PCWP measurements. However, receiver operating characteristic analysis of the ability to identify a PCWP ≥ 18 mmHg resulted in a ReDS cut‐off value of 34%: C‐statistic = 0.85, sensitivity = 90.7%, and specificity = 77.1%. Overall, a ReDS < 34% carries a negative predictive value of 94.9% for CT measured absence of pulmonary volume overload.
These findings suggest that ReDS technology accurately quantifies lung fluid volume and has great potential for monitoring HF patients through hospitalization and at other points of care, such as outpatient clinics or in the home (Table 2 ).
Table 2.
Summary of ReDS clinical studies
| Study | Methodology | Patient population | Sample size | Endpoint | Results |
|---|---|---|---|---|---|
| ReDS‐CT | Prospective comparison with CT | ADHF and all‐comers | 31 | Correlation | ICC = 0.9 [0.8–0.95] |
| ReDS‐PCWP | Prospective comparison with pulmonary pressure | HF and heart transplant | 139 | AUC, sensitivity, and specificity | AUC = 0.85, Sens = 91% and Spec = 77% for cut‐off of 35% |
| ReDS at RFU clinic | Retrospective, controlled, RWD | HF post‐discharge |
220 140—control 80—treatment |
CVH 30 days | 79% [0.05–0.90], P = 0.04 |
| BEST‐HF (ReDS at discharge) | Prospective, randomized, controlled | Hospitalized ADHF | 108 | Percentage of congested patients at discharge |
43% ReDS of ≥ 35% 32% ReDS of ≥ 39% Congested patients are at higher risk to get readmitted in 30 days—11.8% vs. 1.4%, P = 0.03 |
| ReDS at home visit | Prospective, RWD | ADHF post discharge | 105 | HFH 30 days | Readmission rate of <3% for the programme. Overall hospital readmissions rate dropped from 25% to 15% |
| ReDS‐HF (ReDS at home) | Prospective, open‐label, single‐arm |
NYHA III HFpEF or HFrEF |
50 | HFH 3 months |
87% [0.01–0.54], P = 0.01 79% [0.01–0.88], P = 0.04 |
| ReDS at ED | Prospective comparison with physicians adjudication | Chief complaint of shortness of breath | 57 | AUC, sensitivity, and specificity | AUC = 0.92, Sens = 89% and Spec = 83% for ReDS cut‐off of 37% |
| SMILE | Prospective, randomized, controlled | Hospitalized ADHF | 268 | HFH | In process |
| RADAR‐HF | Prospective, randomized, controlled | Hospitalized ADHF | 100 | Net fluid balance | In process |
| ReDS‐SAFE‐HF | Prospective, randomized, controlled | ADHF post‐discharge | 240 | HF readmission, unplanned IV visit and all‐cause death | In process |
ADHF, acute decompensated heart failure; CI, confidence interval; CT, computed tomography; CVH, cardiovascular hospitalization; ED, emergency department; HFH, heart failure hospitalization; HFpEF, heart failure preserved ejection fraction; HFrEF, heart failure reduced ejection fraction; ICC, Intraclass correlation; IV, intravenous; NYHA, New York Heart Association; ReDS, remote dielectric sensing; RFU, rapid follow up; RWD, real‐world data.
Remote dielectric sensing use cases across the continuum of heart failure care
Remote dielectric sensing readings can be taken and can be potentially helpful across the spectrum of HF states; from stable, to decompensating, to hospitalized, to the post‐discharge state, in a variety of healthcare settings. These settings include the patient's home and skilled nursing facilities, the emergency department (ED), ED‐based or hospital‐based observation units, inpatient hospital wards and outpatient clinics. In all cases, healthcare professionals at any level of experience can be trained to make accurate ReDS measurements. The required techniques are such that even patients can take reliable measurements. Analogous to pressure‐guided HF management, cut points for defining volume status have been established for ReDS, and treatment algorithms have been developed around these cut points (Figure 3 ). Values above 35% define a hypervolemic state, and below 20% indicate dehydration. Such values drive medication changes to restore ReDS values to the normal range and may be helpful in a number of use case scenarios.
Figure 3.
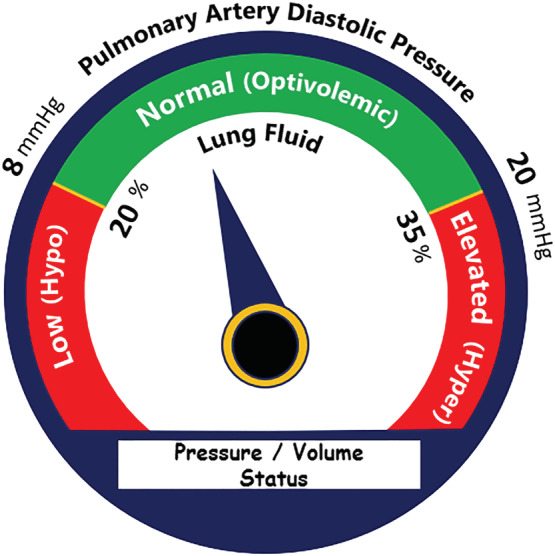
Example patient management algorithm. Green zone indicates normal pulmonary artery diastolic pressure or normal lung fluid values and optivolemic state. Red zones indicate low and elevated values and hypovolemic and hypervolemic states correspondingly. Default cut‐off points are 8–20 mmHg and 20–35% fluid content.
It is important to note that ReDS measures any fluid in the thorax, so thorough clinical assessment remains essential to ReDS‐guided HF management. While the treatment algorithm in Figure 3 focuses on HF‐related fluid/congestion management, other situations and their corresponding treatment should be considered. For example, a large pleural effusion may require pleurocentesis, pneumonia may require antibiotics, and acute decompensated chronic kidney failure may require dialysis.
Post‐discharge clinic remote dielectric sensing measurement to reduce 30 day heart failure readmissions
One strategy being adopted by some healthcare systems to reduce 30 day HF hospital readmissions is to provide rapid outpatient follow‐up in a dedicated post‐discharge clinic within several days of discharge. 33 Advanced practice providers, such as nurse practitioners, frequently staff these clinics. One such clinic at Mount Sinai Hospital (New York, NY), called the rapid follow‐up (RFU) clinic, is run by a nurse practitioner with indirect physician supervision. A retrospective analysis evaluated the use of ReDS in this setting. 34
This analysis included 220 patients discharged from the hospital following an ADHF admission, who showed up for their scheduled RFU clinic visit. Of those patients who presented to the RFU, ReDS measurement was performed in 36% (n = 80). ReDS‐guided medication changes were made in about two‐thirds of these patients. ReDS‐guided management was associated with significantly fewer 30 day cardiovascular readmissions (relative risk reduction of 79%, P = 0.04) and a trend towards a reduction in all‐cause readmissions (relative risk reduction of 57%, P = 0.09) compared with patients without a ReDS assessment.
These findings support the notion that nurse practitioners armed with ReDS technology have the potential to lower the risk of readmission following discharge from an ADHF hospitalization, beyond that which could be accomplished by the RFU clinic approach alone.
Inpatient measurement to assess readiness for hospital discharge
Results from several large registry studies indicate that many patients hospitalized for ADHF are still volume overloaded (inadequately decongested) at the time of discharge. 35 Inadequate decongestion is a significant risk factor for HF readmissions. Patients are frequently discharged with persistent volume overload because fitness for discharge is often based on resolution of symptoms rather than on objective evidence of adequate decongestion. The BEST‐HF study 36 was a single‐centre prospective, randomized, controlled pilot study of 108 patients admitted to Moses Cone Hospital (Greensboro, NC) with ADHF that was designed to objectively measure the amount of residual lung congestion in acute HF patients at discharge. At the time of proposed discharge, all patients underwent a ReDS reading to measure lung fluid, with the results randomized to be blinded or relayed to the treating physicians.
Of 108 HF patients (50% male, age 73.6 ± 12.6 years, BMI 29.3 ± 4.3 kg/m2, EF 38.5 ± 15.1%, BNP 1138 ± 987 pg/mL), 32% demonstrated significant residual lung congestion (defined as a ReDS reading ≥ 39%) and 43% had ReDS reading ≥ 35% at the time of proposed hospital discharge. ReDS‐guided therapy triggered additional diuresis in 30% (18/60) of the patients in the treatment arm (average weight loss 5.6 pounds, P = 0.02). The overall readmission rate was low so 30 day HF readmission rates were similar in the treatment and the control arms (1.7% vs. 4.2%; P = 0.44) but comparing patients with ReDS ≥ 39% who received ReDS‐guided therapy to patients with ReDS ≥ 39% who were discharged home show favorable trends in 30 days (0% vs. 11.8%, P = 0.24) and in 90 days (9.1% vs. 23.5%, P = 0.33). Regardless of group assignment, patients discharged as planned (without additional treatment) with residual lung congestion (ReDS ≥ 39%) had higher 30 day readmission rate compared with patients who were adequately decongested at discharge with ReDS < 39% (11.8% vs. 1.4%, P = 0.03).
Thus, this study confirmed prior findings that a large proportion of patients admitted for ADHF are discharged with residual lung congestion. 35 A ReDS measurement performed at the time of proposed HF discharge can identify patients who may benefit from additional decongestion prior to discharge which may, in the long run, decrease the risk for HF rehospitalization.
Remote dielectric sensing use during visiting nurse home health visits
Remote dielectric sensing has been used as a point‐of‐care measurement in patients' homes, by nurses during post‐hospital‐discharge visits operating under the remote supervision of a HF specialist. In one experience at Randolph Health Hospital (Asheboro, NC), 37 Medicare patients admitted for HF were enrolled into a nurse‐led outpatient transition care programme. In brief, following first planned post‐discharge contact to assess symptoms within 3 days, patients were instructed to make contact with a central office at any time during the follow‐up period for any signs and symptoms of worsening HF like volume overload or increased dyspnoea, which would trigger nurse home visit and ReDS measurement. Patients who received home nurse visits were managed according to a standardized 4 day staged algorithm that incorporated ReDS‐based diuretic adjustments which included supplemental metolazone, or IV furosemide, potassium and blood tests to assess electrolytes and renal function. If the algorithm was unsuccessful in abating symptoms and getting the ReDS reading < 36% by Day 4, physician consultation was obtained.
In the initial experience, the Care Transitions Team evaluated 105 patients over an 18 month period. Age of these patients averaged 81 years, 46% were female and 54% were male. Of 273 ReDS readings, 52 (20%) were >35% and patients were treated using the diuretic adjustment protocol. It took an average of 2.5 (range 1 to 5) visits to titrate diuretics and obtain a goal ReDS reading ≤ 35%. The 30 day all‐cause hospital readmission rate dropped from a historical level of 25% to 15%. This resulted in an immediate benefit to the hospital system by eliminating the Medicare penalty for readmission rates > 20%, which had occurred each of the prior 4 years. In addition, there were no treatment failures, hospitalizations, or ER visits for worsening renal function or adverse events. In summary, this case study showed that a nurse‐led care transition programme employing ReDS technology and a specific diuretic dosing algorithm to assess and treat HF patients, was successful in reducing the 30 day HF readmissions in a community hospital without a dedicated HF programme.
Daily use of remote dielectric sensing in the home
The daily use of ReDS by patients in their homes has been evaluated in an interventional study. 38 Fifty hospitalized patients with ADHF were enrolled at three sites in Israel. Patients performed ReDS readings on a daily basis. Measurements were transmitted to a dedicated cloud enabled patient management and automatic alerts sent to the primary physician for readings above (>35%) or below (<20%) the normal range. Patients were managed based on the principles of the algorithm described above. Each alert prompted a phone call to the patient for evaluation of medical and dietary compliance and, when indicated, modification of medical therapy, primarily adjustment of diuretic dosing. Examples of daily CardioMEMS and ReDS readings and physician responses from single patients are summarized in Figure 4 . Episodes of increased ReDS readings were generally followed by treatment modifications that resulted in decreased ReDS readings over subsequent days.
Figure 4.
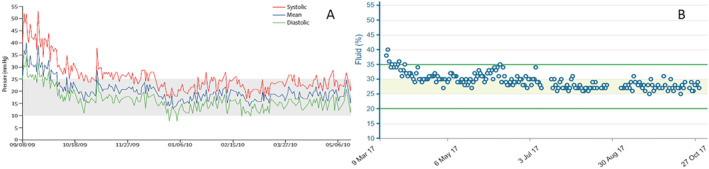
Examples of daily readings transmitted from single patients. Physicians responded to elevated readings with furosemide and stabilized the patients in the normal zone. (A) CardioMEMS (B) ReDS.
For the 50 patients as a whole, the number of HF hospitalizations was compared across three time periods of observation including the 90 days prior to the index admission, the 90 days following discharge from the index admission during active ReDS‐guided HF management, and the subsequent 90 days following withdrawal of ReDS from the patients' homes. Compared with the pre‐REDS and post‐ReDS periods, respectively, there were 87% and 79% reductions in HF hospitalizations during ReDS‐guided HF management. The hazard ratio between the ReDS and the pre‐ReDS period was 0.07 (95% CI [0.01–0.54], P = 0.01) and between the ReDS and the post‐ReDS period was 0.11 (95% CI [0.014–0.88], P = 0.037). These findings demonstrate the feasibility of daily home ReDS measurements and suggest that ReDS‐guided management in this setting has the potential to reduce readmissions in ADHF patients recently discharged from the hospital.
Remote dielectric sensing use in the emergency department
Remote dielectric sensing technology has the potential to be used in the ED setting to help differentiate shortness of breath due to HF from other causes. This was demonstrated in a single‐centre convenience‐sample pilot study of patients presenting with a chief complaint of shortness of breath who were ≥21 years of age. 39 Patients were fitted with the ReDS vest, and data were recorded. After discharge from the ED, a gold standard diagnosis and a volume status were adjudicated by two emergency medicine physicians blinded to ReDS data. ReDS data were evaluated with sensitivity (Sn), specificity (Sp), and receiver operating characteristic for prediction of gold standard diagnosis. Of 57 patients, the mean age was 63.02 (±13.74) years, and 51% were male. ReDS values ranged from 17% to 55%. Adjudicated diagnosis found 35% of patients had volume overload, of which ReDS detected 85%. Using a cut point of 35% (upper limit of physiologic lung fluid), ReDS had a Sn, Sp, NPV, and PPV of 0.85, 0.78, 0.91, and 0.68, respectively. Rule‐out and rule‐in ReDS cut points, providing 100% Sn and Sp were <28% and >41%, respectively. The optimal cut‐off point was determined to be 37% (AUC = 0.92; CI 0.81 to 0.97; P < 0.0001) with a Sn of 0.89, Sp of 0.83, NPV of 0.93, and PPV of 0.74. The interim conclusion of this pilot study is that at a cut point of 37%, the ReDS device has excellent sensitivity and negative predictive value in detecting pathologic levels of lung fluid.
Summary and conclusions
Development of objective means of monitoring fluid status with associated treatment algorithms to reduce hospitalizations has emerged as a priority in the care of HF patients. CardioMEMS has been validated against the gold standard of PCWP assessed by right heart catheterization and several studies now show that hospitalization rates can be reduced using this tool. More recently, ReDS has been validated against the gold standard of CT for direct measurement of lung fluid content, and it compares favourably to invasive assessment of PCWP. CardioMEMS is an implantable device that requires about 90 s for its interrogation and uploading of information to a web‐based patient management system; ReDS is a non‐invasive system and requires up to 90 s to measure lung fluid content uploading measurements to a web‐based patient management system. CardioMEMS measurements are easily taken by patients in their homes; ReDS is used easily by any member of the healthcare team—including physicians, advanced practice providers, nurses, community healthcare associates, and also by patients themselves. Both HF monitoring systems meet five critical prerequisites for an effective HF assessment tool, by measuring the appropriate signal (PAP, lung fluid content) accurately and in absolute terms, thus providing actionable information that can guide management via physiologically and clinically sound treatment algorithms. While CardioMEMS clinical research and clinical use has focused on the ambulatory setting alone, ReDS has been used and evaluated in a variety of clinical settings including in the hospital, in the post‐discharge HF clinic, in the home as a point‐of‐care test administered by visiting nurses, in the home for daily monitoring administered by the patient, and in the emergency department. For both CardioMEMS and for ReDS, substantial clinical trials and real‐world data support the use of these technologies as a means to keep HF patients well and out of the hospital. In this regard, both technologies have been effectively used in HF patients regardless of LVEF, that is, in HF patients with reduced and preserved ejection fraction.
Currently, CardioMEMS is indicated for wirelessly measuring and monitoring pulmonary artery pressure and heart rate in NYHA Class III HF patients who have been hospitalized for HF in the previous year. 40 The ReDS System is intended for use by qualified healthcare practitioners, under the direction of a physician, in hospitals, hospital‐type facilities, and home environments, for the non‐invasive monitoring and management of patients with fluid management problems in a variety of medically accepted clinical applications. The ReDS system is indicated for patients, with fluid management problems, taking diuretic medication, living with HF, and recovering from a coronary artery disease‐related event. 41 Both of these technologies represent valuable additions to our armamentarium for the management of HF patients and should be considered for the routine management of patients with symptomatic HF.
Conflict of interest
WTA: Dr Abraham has received consulting fees from Abbott, Sensible Medical Innovations, Ltd., and Vectorious Medical Technologies. DB: Dr Bensimhon has received consulting fees from Sensible Medical Innovations, Ltd. SPP: Dr Pinney has received consulting fees from Abbott. SCF: Dr Feitell has received consulting fees from Abbott and Sensible Medical Innovations, Ltd. WFP: Dr Peacock has received consulting fees from Abbott. OA: Dr Amir has received consulting fees from Sensible Medical Innovations, Ltd. DB: Dr Burkhoff declares no relevant disclosures.
Abraham, W. T. , Bensimhon, D. , Pinney, S. P. , Feitell, S. C. , Peacock, W. F. , Amir, O. , and Burkhoff, D. (2021) Patient monitoring across the spectrum of heart failure disease management 10 years after the CHAMPION trial. ESC Heart Failure, 8: 3472–3482. 10.1002/ehf2.13550.
References
- 1. Lund LH, Rich MW, Hauptman PJ. Complexities of the global heart failure epidemic. J Card Fail 2018; 24: 813–814. [DOI] [PubMed] [Google Scholar]
- 2. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 3. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017; 3: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, De Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med 2010; 363: 2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Böhm M, Boll H, Baumann G, Honold M, Koehler K, Gelbrich G, Kirwan BA, Anker SD, Telemedical Interventional Monitoring in Heart Failure Investigators . Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation 2011; 123: 1873–1880. [DOI] [PubMed] [Google Scholar]
- 7. Anker SD, Koehler F, Abraham WT. Telemedicine and remote management of patients with heart failure. Lancet 2011; 378: 731–739. [DOI] [PubMed] [Google Scholar]
- 8. van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, Kautzner J, Munoz Aguilera R, Lunati M, Yu CM, Gerritse B. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011; 124: 1719–1726. [DOI] [PubMed] [Google Scholar]
- 9. Adamson PB, Magalski A, Braunschweig F, Böhm M, Reynolds D, Steinhaus D, Luby A, Linde C, Ryden L, Cremers B, Takle T, Bennett T. Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system. J Am Coll Cardiol 2003; 41: 565–571. [DOI] [PubMed] [Google Scholar]
- 10. Alotaibi S, Hernandez‐Montfort J, Ali OE, El‐Chilali K, Perez BA. Remote monitoring of implantable cardiac devices in heart failure patients: a systematic review and meta‐analysis of randomized controlled trials. Heart Fail Rev 2020; 25: 469–479. [DOI] [PubMed] [Google Scholar]
- 11. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Störk S, Butter C, Sechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, Schulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM‐HF2): a randomised, controlled, parallel‐group, unmasked trial. Lancet 2018; 392: 1047–1057. [DOI] [PubMed] [Google Scholar]
- 12. Zhang J, Goode KM, Rigby A, Balk AH, Cleland JG. Identifying patients at risk of death or hospitalisation due to worsening heart failure using decision tree analysis: evidence from the Trans‐European Network‐Home‐Care Management System (TEN‐HMS) study. Int J Cardiol 2013; 163: 149–156. [DOI] [PubMed] [Google Scholar]
- 13. Gardner RS, Singh JP, Stancak B, Nair DG, Cao M, Schulze C, Thakur PH, An Q, Wehrenberg S, Hammill EF, Zhang Y. HeartLogic multisensor algorithm identifies patients during periods of significantly increased risk of heart failure events: results from the MultiSENSE study. Circ Heart Fail 2018; 11: e004669. [DOI] [PubMed] [Google Scholar]
- 14. Chang KW, Fox S, Mojaver S, Maisel AS. Using biomarkers to guide heart failure management. Expert Rev Cardiovasc Ther 2017; 15: 729–741. [DOI] [PubMed] [Google Scholar]
- 15. Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep 2009; 6: 287–292. [DOI] [PubMed] [Google Scholar]
- 16. Abraham WT, Perl L. Implantable hemodynamic monitoring for heart failure patients. J Am Coll Cardiol 2017; 70: 389–398. [DOI] [PubMed] [Google Scholar]
- 17. Zile MR, Bourge RC, Bennett TD, Stevenson LW, Cho YK, Adamson PB, Aaron MF, Aranda JM Jr, Abraham WT, Smart FW, Kueffer FJ. Application of implantable hemodynamic monitoring in the management of patients with diastolic heart failure: a subgroup analysis of the COMPASS‐HF trial. J Card Fail 2008; 14: 816–823. [DOI] [PubMed] [Google Scholar]
- 18. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377: 658–666. [DOI] [PubMed] [Google Scholar]
- 19. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow‐up results from the CHAMPION randomised trial. Lancet 2016; 387: 453–461. [DOI] [PubMed] [Google Scholar]
- 20. Shavelle DM, Desai AS, Abraham WT, Bourge RC, Raval N, Rathman LD, Heywood JT, Jermyn RA, Pelzel J, Jonsson OT, Costanzo MR. Lower rates of heart failure and all‐cause hospitalizations during pulmonary artery pressure‐guided therapy for ambulatory heart failure: one‐year outcomes from the CardioMEMS post‐approval study. Circ Heart Fail 2020; 13: e006863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Desai AS, Bhimaraj A, Bharmi R, Jermyn R, Bhatt K, Shavelle D, Redfield MM, Hull R, Pelzel J, Davis K, Dalal N, Adamson PB, Heywood JT. Ambulatory hemodynamic monitoring reduces heart failure hospitalizations in “real‐world” clinical practice. J Am Coll Cardiol 2017; 69: 2357–2365. [DOI] [PubMed] [Google Scholar]
- 22. Angermann CE, Assmus B, Anker SD, Asselbergs FW, Brachmann J, Brett ME, Brugts JJ, Ertl G, Ginn G, Hilker L, Koehler F, Rosenkranz S, Zhou Q, Adamson PB, Böhm M, for the MEMS‐HF Investigators . Pulmonary artery pressure‐guided therapy in ambulatory patients with symptomatic heart failure: the CardioMEMS European Monitoring Study for Heart Failure (MEMS‐HF). Eur J Heart Fail 2020; 22: 1891–1901. [DOI] [PubMed] [Google Scholar]
- 23. Abraham WT, Adamson PB, Hasan A, Bourge RC, Pamboukian SV, Aaron MF, Raval NY. Safety and accuracy of a wireless pulmonary artery pressure monitoring system in patients with heart failure. Am Heart J 2011; 161: 558–566. [DOI] [PubMed] [Google Scholar]
- 24. Verdejo HE, Castro PF, Concepción R, Ferrada MA, Alfaro MA, Alcaíno ME, Deck CC, Bourge RC. Comparison of a radiofrequency‐based wireless pressure sensor to Swan‐Ganz catheter and echocardiography for ambulatory assessment of pulmonary artery pressure in heart failure. J Am Coll Cardiol 2007; 50: 2375–2382. [DOI] [PubMed] [Google Scholar]
- 25. Adamson PB, Abraham WT, Aaron M, Aranda JM Jr, Bourge RC, Smith A, Stevenson LW, Bauman JG, Yadav JS. CHAMPION trial rationale and design: the long‐term safety and clinical efficacy of a wireless pulmonary artery pressure monitoring system. J Card Fail 2011; 17: 3–10. [DOI] [PubMed] [Google Scholar]
- 26. Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014; 7: 935–944. [DOI] [PubMed] [Google Scholar]
- 27. Amir O, Azzam ZS, Gaspar T, Faranesh‐Abboud S, Andria N, Burkhoff D, Abbo A, Abraham WT. Validation of remote dielectric sensing (ReDS) technology for quantification of lung fluid status: comparison to high resolution chest computed tomography in patients with and without acute heart failure. Int J Cardiol 2016; 221: 841–846. [DOI] [PubMed] [Google Scholar]
- 28. Gattinoni L, Pesenti A, Bombino M, Baglioni S, Rivolta M, Rossi F, Rossi G, Fumagalli R, Marcolin R, Mascheroni D, Torresin A. Relationships between lung computed tomographic density, gas exchange, and PEEP in acute respiratory failure. Anesthesiology 1988; 69: 824–832. [DOI] [PubMed] [Google Scholar]
- 29. Malbouisson LM, Muller JC, Constantin JM, Lu Q, Puybasset L, Rouby JJ. Computed tomography assessment of positive end‐expiratory pressure‐induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001; 163: 1444–1450. [DOI] [PubMed] [Google Scholar]
- 30. Patroniti N, Bellani G, Maggioni E, Manfio A, Marcora B, Pesenti A. Measurement of pulmonary edema in patients with acute respiratory distress syndrome. Crit Care Med 2005; 33: 2547–2554. [DOI] [PubMed] [Google Scholar]
- 31. Amir O, Rappaport D, Zafrir B, Abraham WT. A novel approach to monitoring pulmonary congestion in heart failure: initial animal and clinical experiences using remote dielectric sensing technology. Congest Heart Fail 2013; 19: 149–155. [DOI] [PubMed] [Google Scholar]
- 32. Uriel N, Sayer G, Imamura T, Rodgers D, Kim G, Raikhelkar J, Sarswat N, Kalantari S, Chung B, Nguyen A, Burkhoff D. Relationship between noninvasive assessment of lung fluid volume and invasively measured cardiac hemodynamics. J Am Heart Assoc 2018; 7: e009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McAlister FA, Youngson E, Kaul P, Ezekowitz JA. Early follow‐up after a heart failure exacerbation: the importance of continuity. Circ Heart Fail 2016; 9. [DOI] [PubMed] [Google Scholar]
- 34. Lala A, Barghash MH, Giustino G, Alvarez‐Garcia J, Konje S, Parikh A, Ullman J, Keith B, Donehey J, Mitter SS, Trivieri MG, Contreras JP, Burkhoff D, Moss N, Mancini DM, Pinney SP. Early use of remote dielectric sensing after hospitalization to reduce heart failure readmissions. ESC Heart Failure 2021; 8: 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cooper LB, Mentz RJ, Stevens SR, Felker GM, Lombardi C, Metra M, Stevenson LW, O'Connor CM, Milano CA, Patel CB, Rogers JG. Hemodynamic predictors of heart failure morbidity and mortality: fluid or flow? J Card Fail 2016; 22: 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bensimhon D, Alali SA, Curran L, Gelbart E, Garman DWV, Taylor R, Chase P, Peacock WF. The use of the reds noninvasive lung fluid monitoring system to assess readiness for discharge in patients hospitalized with acute heart failure: a pilot study. Heart & lung: the j crit care 2021; 50: 59–64. [DOI] [PubMed] [Google Scholar]
- 37. Curran L, Peck K, Carter K, McElreath L, Byrd V, Bensimhon D. Use of ReDS technology to treat heart failure in the home health setting. J Card Fail 2018; 24: S48–S49. [Google Scholar]
- 38. Amir O, Ben‐Gal T, Weinstein JM, Schliamser J, Burkhoff D, Abbo A, Abraham WT. Evaluation of remote dielectric sensing (ReDS) technology‐guided therapy for decreasing heart failure re‐hospitalizations. Int J Cardiol 2017; 240: 279–284. [DOI] [PubMed] [Google Scholar]
- 39. Nguyen H, Fisch E, Sekhon N, MaArthur R, Peacock FW, Rafique Z. Remote dielectric sensing in emergency department dyspnea. Acad Emerg Med 2019; 26: S290. [Google Scholar]
- 40. U.S. Food and Drug Adminstration CardioMEMS HF system indications for use. Accessed June 15 2021, 2021. at https://www.accessdata.fda.gov/cdrh_docs/pdf10/P100045A.pdf
- 41. U.S. Food and Drug Adminstration ReDS system indications for use. Accessed June 15 2021, 2021. at https://www.accessdata.fda.gov/cdrh_docs/pdf18/K180479.pdf


