Abstract
Aims
Risk stratification models of sudden cardiac death (SCD) are based on the assumption that risk factors of SCD affect risk to a similar extent in both sexes. The aim of the study is to evaluate differences in clinical outcomes between sexes and evaluate whether risk factors associated with appropriate device therapy (ADT) differ between men and women.
Methods and results
We performed a cohort study of implantable cardioverter defibrillator (ICD) patients referred for primary or secondary prevention of SCD between 2009 and 2018. Multivariable Cox regression models for prediction of ADT were constructed for men and women separately. Of 2300 included patients, 571 (25%) were women. Median follow‐up was 4.6 (inter‐quartile range: 4.4–4.9) years. Time to ADT was shorter for men compared with women [hazard ratio (HR) 1.71, P < 0.001], as was time to mortality (HR 1.37, P = 0.003). In women, only secondary prevention ICD therapy (HR 1.82, P < 0.01) was associated with ADT, whereas higher age (HR 1.20, P < 0.001), absence of left bundle branch block (HR 0.72, P = 0.01), and secondary prevention therapy (HR 1.80, P < 0.001) were independently associated with ADT in men. None of the observed parameters showed a distinctive sex‐specific pattern in ADT.
Conclusions
Male ICD patients were at higher risk of ADT and death compared with female ICD patients, irrespective of an ischaemic or non‐ischaemic underlying cardiomyopathy. Our study highlights the importance to stratify outcomes of ICD trials by sex, as study results differ between men and women. However, none of the available clinical parameters showed a clear sex‐specific relation to ventricular arrhythmias. As a consequence, sex‐specific risk stratification models of SCD using commonly available clinical parameters could not be derived.
Keywords: Implantable cardioverter defibrillator, Sex differences, Sudden cardiac death, Risk stratification
Introduction
The implantable cardioverter defibrillator (ICD) is an established therapy for primary and secondary prevention of sudden cardiac death (SCD) in high‐risk patients. 1 Randomized controlled trials evaluating ICD therapy have predominantly included male patients, ranging from 72% in DANISH up to 92% in MADIT‐I. 2 Despite differences in the number of enrolled men and women in ICD trials, current ICD recommendations are applied without differentiation by sex, based on the assumption that risk factors of SCD affect risk to a similar extent in both sexes.
Presently, the efficacy of ICD implantation in women is debated. In men, prophylactic ICD implantation is associated with a distinct reduction in mortality and a high incidence of appropriate device therapy (ADT), whereas women do not always seem to benefit from ICD implantation. 2 , 3 Moreover, studies focusing on the benefit of ICD therapy have often shown that male sex itself is a risk factor for occurrence of lethal arrhythmias. 3 , 4 , 5 , 6 Data regarding sex‐specific differences in risk stratification of ventricular arrhythmias are scarce. There is some evidence that certain characteristics in women, such as older age, increase the risk of SCD. 6 , 7 , 8 The mechanism of this interaction is not fully understood, but alterations in hormonal status or delayed onset of coronary artery disease (CAD) are suggested to affect susceptibility for SCD in older women. 9 , 10 In addition, studies evaluating cardiac arrest survivors have shown that left ventricular (LV) dysfunction is an important substrate for SCD in men, but less so in women. 11 , 12 However, it is unknown if other clinical parameters associated with SCD show a sex‐specific risk pattern (e.g. protective effect in women compared with significantly higher risk in men or vice versa). Importantly, current practice is to select patients for primary prevention ICD implantation based on a reduced left ventricular ejection fraction (LVEF). It is unclear if the risk of SCD due to an impaired LVEF affects prophylactic ICD male and female patients to a similar extent, and sex‐specific risk factors of SCD may influence studies evaluating the benefit of ICD therapy, for both men and women. The aim of the present study is to evaluate the relationship between sex and outcome in ICD patients. Moreover, we aim to evaluate whether risk factors associated with ADT differ between men and women.
Methods
Study design and study population
This retrospective, observational cohort study was performed by two large and experienced centres and included 2300 consecutive patients who received an ICD between the years 2009 and 2018 in Amsterdam UMC (Vrije Universiteit, Amsterdam, the Netherlands) and Erasmus University Medical Center (Rotterdam, the Netherlands). Patients received an ICD for primary or secondary prevention, with or without resynchronization therapy (CRT‐D), according to the European Society of Cardiology guidelines of SCD. 1 , 13 The local ethics committee approved data collection and management of this study. The need for written informed consent was waived by the local ethics committee. Patients were excluded from the study if (i) they were diagnosed with a hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, systemic infiltrative cardiac disease, congenital cardiomyopathy, or channelopathy or (ii) were lost to follow‐up within 10 days after ICD implantation (e.g. foreign patients or SCA survivors who received follow‐up care in another hospital). The following baseline parameters were collected: demographics, medical history of cardiovascular diseases, medication at discharge, New York Heart Association (NYHA) functional class, laboratory results, electrocardiographic data (QRS width and conduction disorders), LVEF assessed by echocardiography (Simpson's biplane LVEF), cardiovascular magnetic resonance imaging, or nuclear imaging (LVEF measured in resting conditions). Ischaemic heart disease was defined as a history of significant obstructive CAD, myocardial infarction, or coronary revascularization, irrespective of LVEF. Ischaemic cardiomyopathy was defined as ischaemic heart disease with an LVEF ≤ 50%. Non‐ischaemic cardiomyopathy was defined as an LVEF ≤ 50% in the absence of ischaemic heart disease. An LVEF > 50%, without a history of myocardial infarction or revascularization, was classified as preserved LV function.
Follow‐up and outcome
Follow‐up was typically performed with routine device interrogations at 10 days, 2 months, and then every 6 months. Devices of different ICD manufacturers were included. Most patients were connected to remote monitoring, and event transmissions were reviewed by specialized cardiac device technicians and electrophysiologists. Throughout the study period, ICD programming was based on clinical practice routine. Detection rates, detection intervals, and treatment of ventricular tachycardia (VT) or ventricular fibrillation (VF) were based on the PREPARE study (2008), PROVIDE (2011), and the MADIT‐RIT (2012) study. 14 , 15 , 16 The ICD programming was further adapted if clinically required. The primary endpoint was time to first ADT. ADT was defined as anti‐tachycardia pacing and/or ICD shock for the termination of VT or VF. Date, type, and heart rate of ventricular arrhythmia were recorded, as well as the cumulative number of ADT per patient. Secondary endpoints were all‐cause mortality and inappropriate device therapy. Deaths were identified using the National Health and Social Care Information Service and the electronic medical record system. The National Health and Social Care Information Service is a Dutch governmental agency that provides national data concerning mortality status of citizens in the Netherlands.
Statistical analysis
Continuous variables are presented as mean ± standard deviations if normally distributed and median and inter‐quartile range otherwise. Normality was assessed by mean of Q–Q plots. Normally distributed variables were compared between groups using the independent Student's t‐test, whereas the Mann–Whitney U test was used for other continuous variables. Categorical data are summarized by numbers and percentages. Dichotomous and categorical variables were compared between groups using the χ 2 test or Fisher's exact test in case of low cell counts. Primary outcome measures were time to ADT and overall survival time. Kaplan–Meier curves were used for visualization of time‐to‐event outcomes. For analysis of time to ADT, patients without ADT were censored at end of follow‐up or time of death. For comparison of overall survival, patients alive at end of follow‐up were censored at end of follow‐up. The following variables were tested as potential confounder or effect modifier: age, beta‐blocker use, NYHA class, LVEF, CRT‐D, and non‐ischaemic cardiomyopathy. A variable was considered a confounder if the regression coefficient for sex changed ≥10%. Effect modification was tested by including an interaction term in the model. A variable was considered an effect modifier if the interaction term was statistically significant (P < 0.05). Univariable Cox regression was used to find variables associated with time to ADT in the whole group and separately in men and women. The proportional hazard assumption of the Cox regression model was tested by introducing a time‐dependent covariate in the model. Multivariable Cox regression analyses were performed subsequently where variables with a P‐value <0.10 at univariable analysis were included, and a backward elimination procedure was used. Hazard ratios (HRs) and 95% confidence intervals (CIs) were reported as effect sizes. Discriminative ability of the multivariable Cox regression models was quantified by the C‐index. The number of ICD implantations per ADT event was calculated for 1, 2, and 3 years of follow‐up and corrected for the number of patients at risk at each annual. A two‐sided significance level of 5% was used. Statistical analyses were performed using SPSS software package (Version 26.0; IBM Corporation, Armonk, NY, USA). Stata software was used for calculation of the C‐index (StataCorp 2015, Stata Statistical Software: Release 14, College Station, TX, USA: StataCorp LP).
Results
Sex differences in clinical characteristics
A total of 2300 ICD patients were included (see Supporting Information, Figure S1 ). Baseline characteristics were compared between men and women and are described in Table 1 . The majority of the patients were male (75%), and 55% of the patients were diagnosed with ischaemic cardiomyopathy. Compared with men, women were younger (P < 0.001), were more often diagnosed with non‐ischaemic cardiomyopathy (P < 0.001), but less frequently with atrial fibrillation (P < 0.001), used less angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker (P < 0.001), and were more likely to receive resynchronization therapy (P < 0.001) and a primary prevention device (P < 0.001).
Table 1.
Baseline characteristics
| Characteristics | Total population (n = 2300) | Men (n = 1729) | Women (n = 571) | P‐value |
|---|---|---|---|---|
| Age (years) | 62 ± 13 | 63 ± 12 | 61 ± 14 | <0.001 |
| Cardiovascular risk factors | ||||
| Diabetes mellitus | 503 (22%) | 398 (23%) | 105 (18%) | 0.02 |
| Stroke | 249 (11%) | 190 (11%) | 59 (10%) | 0.66 |
| Cardiac history | ||||
| Ischaemic heart disease | 1422 (62%) | 1191 (69%) | 231 (41%) | <0.001 |
| Ischaemic CMP a | 1217 (55%) | 1028 (63%) | 189 (34%) | <0.001 |
| Non‐ischaemic CMP a | 794 (36%) | 487 (30%) | 307 (56%) | <0.001 |
| Preserved LV function a | 109 (5%) | 68 (4%) | 41 (7%) | <0.01 |
| NYHA class ≥II b | 1535 (73%) | 1132 (72%) | 403 (77%) | 0.05 |
| Atrial fibrillation | 615 (27%) | 495 (29%) | 120 (21%) | <0.001 |
| NSVT | 241 (11%) | 178 (10%) | 63 (11%) | 0.62 |
| Creatinine (μmol/L) | 91 (76–112) | 94 (80–116) | 78 (65–97) | <0.001 |
| QRS duration (ms) | 126 ± 32 | 127 ± 32 | 124 ± 33 | 0.04 |
| Left bundle branch block | 575 (25%) | 399 (23%) | 176 (31%) | <0.001 |
| LVEF (%) c | 32 ± 12 | 31 ± 12 | 32 ± 12 | 0.48 |
| Medication at discharge | ||||
| ACEi/ARB | 1885 (82%) | 1448 (84%) | 437 (77%) | <0.001 |
| Beta‐blocker | 1922 (84%) | 1442 (83%) | 480 (84%) | 0.71 |
| MRA | 823 (36%) | 583 (34%) | 240 (42%) | <0.001 |
| Diuretics | 1312 (57%) | 942 (55%) | 370 (65%) | <0.001 |
| Amiodarone | 297 (13%) | 235 (14%) | 62 (11%) | 0.09 |
| Digoxin | 257 (11%) | 177 (10%) | 80 (14%) | 0.01 |
| Device type and device indication | ||||
| ICD | 1648 (72%) | 1270 (74%) | 378 (66%) | 0.001 |
| CRT‐D | 652 (28%) | 459 (27%) | 193 (34%) | |
| Primary prevention | 1494 (65%) | 1089 (63%) | 405 (71%) | 0.001 |
| Secondary prevention | 806 (35%) | 640 (37%) | 166 (29%) | |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CMP, cardiomyopathy; CRT‐D, resynchronization therapy; ICD, implantable cardioverter defibrillator; LV, left ventricular; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NSVT, non‐sustained ventricular tachycardia; NYHA, New York Heart Association.
Based on N = 2195.
Based on N = 2092.
Based on N = 2024.
Arrhythmias and mortality
During a median follow‐up of 4.6 (inter‐quartile range 4.4–4.9) years, 531 (23%) patients received ADT (incidence rate of 5.7% per person‐year), 220 (10%) patients received inappropriate device therapy (incidence rate of 2.2% per person‐year), and 544 (24%) died during follow‐up (incidence rate of 4.9% per person‐year) (Table 2 ). A total of 25% of the men received ADT, compared with 16% of the women. As shown in Figure 1 A , time to ADT was shorter in men compared with women (HR 1.71, 95% CI 1.36–2.14, P < 0.001). We did not identify risk factors that acted as a confounder for the association between sex and time to ADT. Median number of ADT per patient did not differ between men and women receiving ADT during follow‐up (P = 0.87, Table 2 ). In men, time to ADT did not differ between those with an ischaemic cardiomyopathy and those with a non‐ischaemic cardiomyopathy (HR 0.99, 95% CI 0.80–1.22, P = 0.89). Also in women, time to ADT did not differ between those with an ischaemic cardiomyopathy and those with a non‐ischaemic cardiomyopathy (HR 1.27, 95% CI 0.81–1.97, P = 0.30, Figure 2 ). Overall survival was shorter in men compared with women (HR 1.37, 95% CI 1.11–1.69, P < 0.01, Figure 1 B ). No difference was observed in time to inappropriate ICD therapy between both groups (HR 1.01, 95% CI 0.75–1.37, P = 0.94, Figure 1 C ). Figure 3 A compares the number of ICD implantations per ADT event and illustrates that 1 out of 14 male patients received ADT within the first year after ICD implantation for primary prevention male patients, compared with 1 out of 19 for primary prevention female patients, decreasing after 3 years of follow‐up to 1 out of 5 and 1 out of 7, respectively. The number of ICD implantations per ADT event for secondary prevention patients was substantially lower for both sexes. In addition, Figure 3 B compares primary prevention patients stratified by cardiomyopathy aetiology and illustrates that the event rate between men and women with an ischaemic cardiomyopathy is comparable after 3 years of follow‐up, whereas the number of ICD implantations per ADT event between men and women with a non‐ischaemic cardiomyopathy remains different (1 out of 4 and 1 out of 7 for men and women, respectively). As shown in Supporting Information, Figure S3 , men received more ADT compared with women, irrespective of device type (P < 0.001). Supporting Information, Figure S4 demonstrates the cumulative incidence of ADT and mortality after 1, 2, and 3 years of follow‐up (Supporting Information).
Table 2.
Outcomes
| Total (n = 2300) | Complete follow‐up | At 1 year | At 5 years | HR (95% CI) | P‐value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Men (n = 1729) | Women (n = 571) | Men | Women | Men | Women | ||||
| Appropriate device therapy | 531 (23%) | 440 (25%) | 91 (16%) | 187 (11%) | 41 (7%) | 387 (22%) | 82 (14%) | 1.71 (1.36–2.14) | <0.001 |
| ATP only | 308 (13%) | 256 (15%) | 52 (9%) | 113 (7%) | 26 (5%) | 225 (13%) | 49 (9%) | 1.69 (1.26–2.28) | 0.001 |
| Shock | 223 (10%) | 184 (11%) | 39 (7%) | 74 (4%) | 15 (3%) | 164 (9%) | 33 (6%) | 1.61 (1.14–2.28) | 0.01 |
| Cumulative number of appropriate device therapy a | 2 (1–4) | 2 (1–4) | 2 (1–5) | N/A | N/A | N/A | N/A | N/A | 0.87 c |
| Inappropriate device therapy | 220 (10%) | 165 (10%) | 55 (10%) | 77 (4%) | 22 (4%) | 145 (8%) | 44 (8%) | 1.01 (0.75–1.37) | 0.94 |
| Cumulative number of inappropriate device therapy b | 1 (1–2) | 1 (1–2) | 1 (1–2) | N/A | N/A | N/A | N/A | N/A | 0.92 c |
| All‐cause mortality | 544 (24%) | 435 (25%) | 109 (19%) | 80 (4%) | 24 (4%) | 314 (18%) | 72 (13%) | 1.37 (1.11–1.69) | <0.01 |
ATP, anti‐tachycardia pacing; CI, confidence interval; HR, hazard ratio; N/A, not applicable.
The cumulative number of appropriate device therapy per patient is computed in patients who received ≥1 appropriate device therapy.
The cumulative number of inappropriate device therapy per patient is computed in patients who received ≥1 inappropriate device therapy.
Tested using the Mann–Whitney U test.
Figure 1.
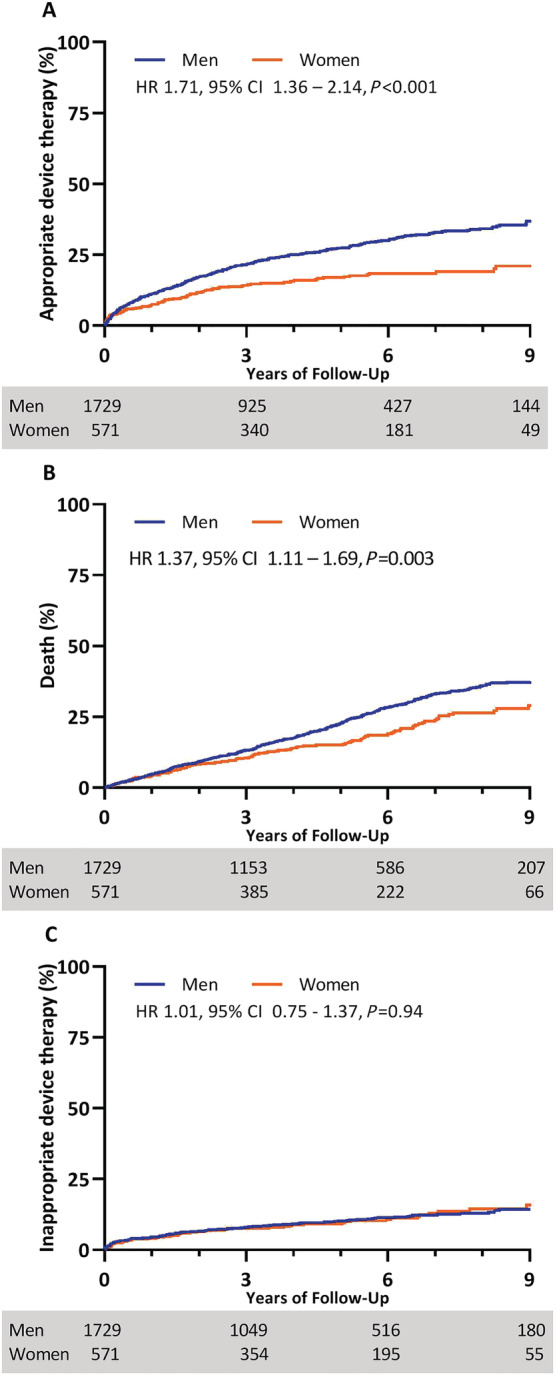
Outcomes: Kaplan–Meier curves depicting differences in (A) appropriate device therapy, (B) all‐cause mortality, and (C) inappropriate device therapy between men and women during 9 years of follow‐up. CI, confidence interval; HR, hazard ratio.
Figure 2.
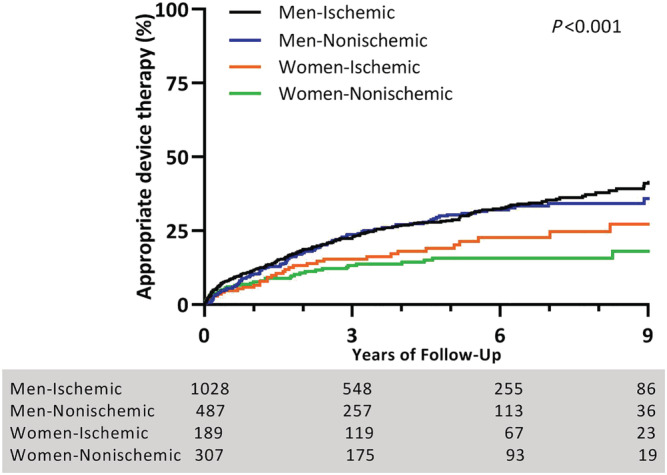
Appropriate device therapy (ADT) and cardiomyopathy: Kaplan–Meier curve depicting differences in ADT between men and women with an ischaemic and non‐ischaemic cardiomyopathy during 9 years of follow‐up. Men received more ADT compared with women, irrespective of aetiology of cardiomyopathy (log‐rank P < 0.001). Comparing men with an ischaemic vs. non‐ischaemic cardiomyopathy showed no significant difference in time to ADT (hazard ratio 0.99, 95% confidence interval 0.80–1.22, P = 0.89). Time to ADT between women with an ischaemic vs. non‐ischaemic cardiomyopathy did also not differ (HR 1.27, 95% CI 0.81–1.97, P = 0.30).
Figure 3.
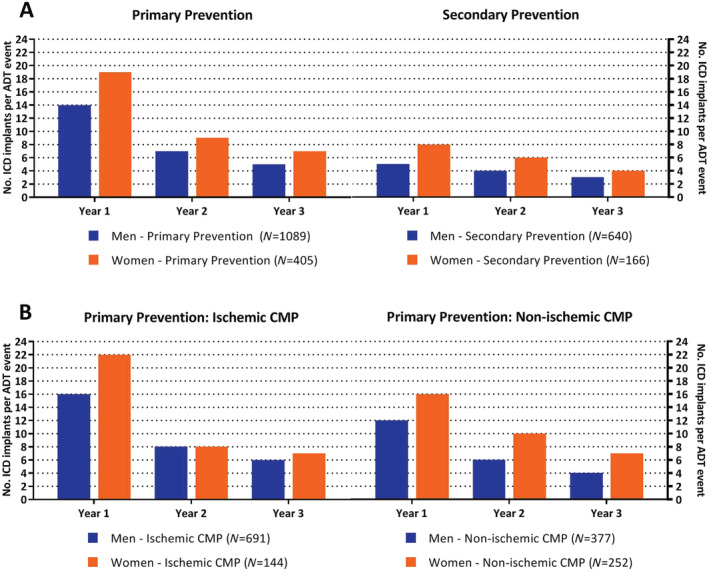
Number of implantable cardioverter defibrillator (ICD) implantations per appropriate device therapy (ADT) event: number of ICD implantations per ADT event calculated for 1, 2, and 3 years of follow‐up. Results are stratified by (A) ICD indication and (B) cardiomyopathy aetiology. CMP, cardiomyopathy.
Risk factors for appropriate device therapy in total cohort
Results for multivariable Cox regression for time to ADT for the total study cohort, primary prevention cohort, and secondary prevention cohort are presented in the Supporting Information. In the total cohort, higher age (HR 1.56, P < 0.001), male sex (HR 1.56, P < 0.001), left bundle branch block (LBBB) (HR 0.74, P < 0.01), and secondary prevention therapy (HR 1.81, P < 0.001) were included as risk factors for shorter time to ADT in the final model, whereas LVEF was not included in the model (Supporting Information, Table S1 ). For the primary prevention cohort (N = 1494), the multivariable Cox regression model included LVEF (HR 0.98 per % increase, P = 0.02), male sex (HR 1.59, P < 0.01), and NYHA Class I (HR 1.76, P < 0.01) as factors associated with time to ADT (Supporting Information, Table S2 ). The multivariable Cox regression model for the secondary prevention cohort (N = 806) included higher age (HR 1.17, P < 0.01) and LVEF (HR 0.99, P < 0.01) (Supporting Information, Table S3 ).
Sex‐specific factors for appropriate device therapy
Figure 4 presents HR for time to ADT for different risk factors separately for men and women, together with a P‐value to indicate whether the HR for the risk factor differs between the sexes. Time to ADT was found to be associated with age in men but not in women (P‐value interaction = 0.04). In male subjects, age (P = 0.001), LBBB (P = 0.001), NYHA Class I (P = 0.03), secondary prevention therapy (P < 0.001), and resynchronization therapy (P = 0.01) were all found to associated with time to ADT in a univariable analysis (Figure 4 ). Multivariable analyses showed that parameters associated with shorter time to ADT in men were higher age (HR 1.20 per 10 years, P < 0.001), absence of LBBB (HR 0.72, P = 0.01), and presence of secondary prevention therapy (HR 1.80, P < 0.001, Table 3 ). The C‐index of the multivariable model for men including age, LBBB, and secondary prevention therapy was 0.62. In contrast, age (P = 0.54) and LBBB (P = 0.29) were not associated with ADT in women. In women, only secondary prevention ICD therapy (HR 1.82, P < 0.01) was found to be associated with time to ADT (Table 3 ). The C‐index of this model for women including only secondary prevention therapy was 0.58.
Figure 4.
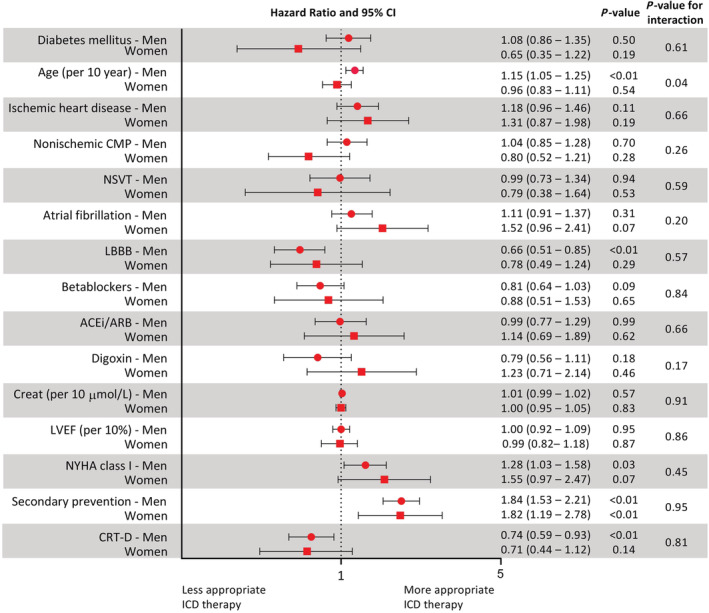
Forest plot evaluating sex‐specific risk stratification: forest plot comparing occurrence of appropriate device therapy in subgroup of patients. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CI, confidence interval; CMP, cardiomyopathy; Creat, creatinine; CRT, resynchronization therapy; ICD, implantable cardioverter defibrillator; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NSVT, non‐sustained ventricular tachycardia; NYHA, New York Heart Association.
Table 3.
Multivariable Cox regression analysis of clinical and imaging parameters for predicting ventricular arrhythmia in men and women
| Parameter (n = 1729) | Multivariable analysis in men | |
|---|---|---|
| HR (95% CI) | P‐value | |
| Age per 10 years | 1.20 (1.11–1.30) | <0.001 |
| Left bundle branch block | 0.72 (0.56–0.92) | 0.01 |
| Secondary prevention | 1.80 (1.49–2.19) | <0.001 |
| Multivariable analysis in women | ||
|---|---|---|
| Parameter (n = 571) | HR (95% CI) | P‐value |
| Secondary prevention | 1.82 (1.19–2.78) | <0.01 |
CI, confidence interval; CRT‐D, resynchronization therapy; HR, hazard ratio; NYHA, New York Heart Association.
The following univariable parameters were included for the multivariable backwards model for male patients: age, CRT‐D, left bundle branch block, NYHA functional class, and secondary prevention. The following univariable parameters were included for the multivariable backwards model for female patients: atrial fibrillation, NYHA functional class, and secondary prevention.
Discussion
The current study evaluated a real‐world ICD population and revealed a significantly higher ADT rate and mortality rate in men compared with women, while there was no difference in inappropriate ICD therapy between both groups. In women, only ICD implantation for secondary prevention was independently associated with a higher risk of ADT. The current study aimed to find sex‐specific patterns related to ventricular arrhythmia. However, none of the tested parameters showed a clear sex‐specific relation to ventricular arrhythmias. As a consequence, we were not able to derive sex‐specific risk stratification models of SCD to identify the high‐risk female (or male) ICD patient (Figure 5 ).
Figure 5.
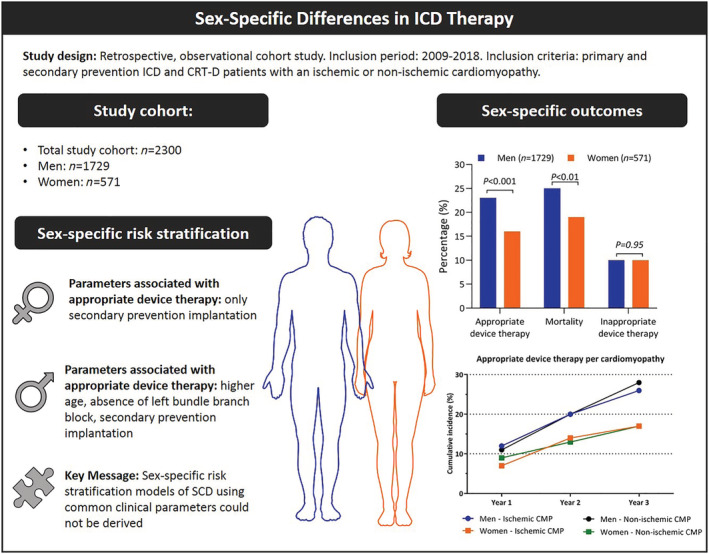
Sex‐specific differences in outcome and risk stratification of sudden cardiac death (SCD) in implantable cardioverter defibrillator (ICD) patients. Parts of the figure are adapted from Servier Medical Art (SMART), Servier: https://smart.servier.com. CMP, cardiomyopathy.
Ventricular arrhythmia
Our data were consistent with previous studies showing that men receive more ADT compared with women. 3 , 5 , 6 , 17 There are studies suggesting that prophylactic ICD implantation may be of smaller benefit in female patients. A meta‐analysis including primary prevention ICD randomized trials showed that ICD implantation in women was not associated with an increase in survival, contrary to men who showed a 25% reduction in mortality. 2 The present study found that 16% of our 571 female patients experienced ADT during a median follow‐up of 4.6 years. Bearing in mind that ADT is not equal to life‐saving therapy, 16 the incidence of lethal arrhythmias in women will be even lower than the numbers reported in the present study. There are multiple hypotheses that may explain the lower incidence of ADT in women. First, women do seem to have a lower susceptibility for SCD, partly due to the influence of sex hormones on cardiac repolarization and depolarization and sex differences in heart rate, autonomic tone, and calcium homeostasis in cardiomyocytes. 10 , 18 , 19 A study using isolated human cardiomyocytes showed that calcium leakage is higher in men compared with women with similar degrees of heart failure, rendering men more prone of SCD caused by delayed after‐depolarizations. 18 Second, the underlying heart disease differs between sexes, as we demonstrated in the present study, with more non‐ischaemic cardiomyopathy and advanced stages of heart failure described in women and more CAD in men. 3 , 5 , 8 , 11 , 12 , 20 This might influence the risk of SCD. However, the present study showed that men with an ischaemic and non‐ischaemic cardiomyopathy were both at higher risk of ventricular arrhythmias compared with women with an ischaemic or non‐ischaemic cardiomyopathy. This finding is consistent with other studies showing that women with CAD are still at lower risk of SCD compared with men with CAD. 11 , 21 Third, at time of cardiac arrest, pulseless electrical activity and asystole are more observed in women, while men are usually presented with VT or VF, rendering an ICD potentially more beneficial in men. 12 , 20 Fourth, women are in general better CRT responders compared with men. Biventricular pacing can cause LV reverse remodelling, further lowering the risk of SCD. 5 , 22 Interestingly, the present study revealed that CRT‐D as well as LBBB were univariably associated with a decrease in ADT, but only LBBB was independently associated with a decrease in ADT in multivariable analyses. This protective effect was only observed in men. A possible explanation for the protective effect of LBBB might be that LV reverse remodelling, and thus risk reduction, is more often seen in CRT patients with a complete LBBB instead of non‐LBBB CRT patients. 23
Identifying women at high risk of SCD is of great importance but remains challenging based on the limited knowledge that is currently available. A European Society of Cardiology consensus document concerning sex differences in cardiac arrhythmia recommends to enrol more women in clinical trials to diminish knowledge gaps concerning sex‐specific cardiac arrhythmia. 24 This study emphasizes the importance of evaluating the benefit of ICD therapy for men and women separately. An adequate power analysis to allow outcomes stratified by sex seems to have incremental value since increased enrolment of women in clinical trials without stratification of outcomes by sex may result in underestimation of the benefit of ICD implantation in men and/or overestimation of the benefit of ICD implantation in women.
Mortality
Previous studies evaluating risk of death between men and women with an ICD are reporting inconsistent results. Several studies are reporting a comparable risk of death between the two groups, whereas others are in line with the current study, reporting a lower risk of death in women. 3 , 5 , 6 , 17 , 25 The lower mortality in women is an interesting finding, as the aforementioned studies are consistently reporting a more severe stage of heart failure in women. A sub‐study of the SCD‐HeFT demonstrated no difference in mortality between ICD‐treated and placebo‐treated women (risk of death 19% vs. 21%, respectively), indicating a low incidence of arrhythmic deaths in women. 17 Other possible explanations for lower mortality in women might be the higher proportion of non‐ischaemic cardiomyopathy in women compared with men, with more than 50% of our female cohort suffering from non‐ischaemic cardiomyopathy. A recent meta‐analysis revealed a higher mortality in patients with ischaemic cardiomyopathy compared with patients with a non‐ischaemic cardiomyopathy. 26
Sex‐specific risk stratification models
Various studies have attempted to enhance risk stratification of SCD, in order to provide ICD recommendations customized to patient characteristics. As mentioned before, male sex is a well‐known risk factor of SCD and is often included as parameter in risk models. 4 However, SCD models evaluating to which extent risk factors affect men and women similarly are lacking. The current study showed that sex‐specific risk stratification based on clinical parameters and the occurrence of ADT is challenging. Although we found differences in parameters associated with ADT between men and women, none of the tested parameters showed a clear sex‐specific risk pattern of SCD (protective effect in women compared with significantly higher risk in men or vice versa). Furthermore, only a secondary prevention device was associated with ADT in women. This latter observation shows, however, that ICD implantation for secondary prevention of SCD is beneficial for both men and women. Our study indicates that the common clinical parameters associated with SCD are not suitable to identify the female patient at high risk for ventricular arrhythmia. It should be noted that the current study evaluated only clinical parameters, while imaging characteristics or advanced electrocardiogram (ECG) parameters were not available for the current study. Studies regarding sex differences in cardiac arrest survivors have shown myocardial fibrosis without an underlying cardiomyopathy, normal ECG tracings and structurally normal hearts were more prevalent in women. 8 , 11 , 12 Future studies focusing on sex differences in ICD patients should focus on ECG characteristics and myocardial tissue differences, for example, assessed using cardiovascular magnetic resonance with late gadolinium enhancement.
Limitations
We must acknowledge several limitations. First, this study has a retrospective design, resulting in different forms of bias, and results should be interpreted with caution. Nevertheless, it provides new insights in order to diminish the knowledge gap concerning in sex‐specific differences in risk stratification of SCD. Second, ADT was used as surrogate of SCD, although previous studies have shown that the incidence of ADT may overestimate the actual occurrence of SCD. 16 It is therefore possible that the value of ICD therapy is overemphasized in this study cohort. Third, this study had an enrolment period of 9 years. This may have impacted results as device programming and optimal medical therapy have changed over time. Last, the C‐index was used to quantify the prognostic performance of our sex‐specific multivariable models. Our result shows that sex‐specific risk stratification models based on currently known clinical risk factors have a low prognostic performance. In a mixed population of men and women, higher C‐indices may be a result of the strong prognostic performance of sex. Further research is needed to identify new, possibly sex‐specific, risk factors that could enhance sex‐specific risk stratification of SCD.
Conclusions
This study showed that women receive less ADT compared with men, irrespective of an ischaemic or non‐ischaemic underlying cardiomyopathy, and that sex‐specific risk stratification of SCD remains challenging. We were not able to derive sex‐specific risk stratification models of SCD to identify the high‐risk female (or male) ICD patient. Secondary prevention ICD implantation seems beneficial for both men and women.
Conflict of interest
None declared.
Funding
None.
Supporting information
Table S1. Univariable and multivariable Cox regression analysis of clinical and imaging parameters for predicting ventricular arrhythmia in the total study cohort.
Table S2. Univariable and multivariable Cox regression analysis of clinical and imaging parameters for predicting ventricular arrhythmia in primary prevention ICD patients.
Table S3. Univariable and multivariable Cox regression analysis of clinical and imaging parameters for predicting ventricular arrhythmia in secondary prevention ICD patients.
Figure S1. Study flowchart.
Figure S2. Effect of implantation year on ADT and included patients.
Figure S3. Appropriate device therapy and device type.
Figure S4. Cumulative incidence of appropriate device therapy and mortality.
van der Lingen, A.‐L. C. J. , Theuns, D. A. M. J. , Rijnierse, M. T. , Becker, M. A. J. , van de Ven, P. M. , van Rossum, A. C. , van Halm, V. P. , Kemme, M. J. B. , Yap, S. C. , and Allaart, C. P. (2021) Sex‐specific differences in outcome and risk stratification of ventricular arrhythmias in implantable cardioverter defibrillator patients. ESC Heart Failure, 8: 3726–3736. 10.1002/ehf2.13444.
References
- 1. Priori SG, Blomstrom‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez‐Madrid A, Nikolaou N, Norekval TM, Spaulding C, Van Veldhuisen DJ, Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology . 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015; 17: 1601–1687. [DOI] [PubMed] [Google Scholar]
- 2. Barra S, Providencia R, Boveda S, Narayanan K, Virdee M, Marijon E, Agarwal S. Do women benefit equally as men from the primary prevention implantable cardioverter‐defibrillator? Europace 2018; 20: 897–901. [DOI] [PubMed] [Google Scholar]
- 3. Sticherling C, Arendacka B, Svendsen JH, Wijers S, Friede T, Stockinger J, Dommasch M, Merkely B, Willems R, Lubinski A, Scharfe M, Braunschweig F, Svetlosak M, Zurn CS, Huikuri H, Flevari P, Lund‐Andersen C, Schaer BA, Tuinenburg AE, Bergau L, Schmidt G, Szeplaki G, Vandenberk B, Kowalczyk E, Eick C, Juntilla J, Conen D, Zabel M, EU‐CERT‐ICD Investigators . Sex differences in outcomes of primary prevention implantable cardioverter‐defibrillator therapy: combined registry data from eleven European countries. Europace 2018; 20: 963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bilchick KC, Wang Y, Cheng A, Curtis JP, Dharmarajan K, Stukenborg GJ, Shadman R, Anand I, Lund LH, Dahlstrom U, Sartipy U, Maggioni A, Swedberg K, O'Conner C, Levy WC. Seattle heart failure and proportional risk models predict benefit from implantable cardioverter‐defibrillators. J Am Coll Cardiol 2017; 69: 2606–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Providencia R, Marijon E, Lambiase PD, Bouzeman A, Defaye P, Klug D, Amet D, Perier MC, Gras D, Algalarrondo V, Deharo JC, Leclercq C, Fauchier L, Babuty D, Bordachar P, Sadoul N, Piot O, Boveda S. Primary prevention implantable cardioverter defibrillator (ICD) therapy in women—data from a multicenter French registry. J Am Heart Assoc 2016; 5: e002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Styles K, Sapp J Jr, Gardner M, Gray C, Abdelwahab A, MacIntyre C, Gao D, Al‐Harbi M, Doucette S, Theriault C, Parkash R. The influence of sex and age on ventricular arrhythmia in a population‐based registry. Int J Cardiol 2017; 244: 169–174. [DOI] [PubMed] [Google Scholar]
- 7. Zeitler EP, Hellkamp AS, Fonarow GC, Hammill SC, Curtis LH, Hernandez AF, Al‐Khalidi HR, Curtis JP, Heidenreich PA, Anstrom KJ, Peterson ED, Mark DB, Hammill BG, Sanders GD, Al‐Khatib SM. Primary prevention implantable cardioverter‐defibrillators and survival in older women. JACC Heart Fail 2015; 3: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haukilahti MAE, Holmstrom L, Vahatalo J, Kentta T, Tikkanen J, Pakanen L, Kortelainen ML, Perkiomaki J, Huikuri H, Myerburg RJ, Junttila MJ. Sudden cardiac death in women. Circulation 2019; 139: 1012–1021. [DOI] [PubMed] [Google Scholar]
- 9. Linde C. Women and arrhythmias. Pacing Clin Electrophysiol 2000; 23: 1550–1560. [DOI] [PubMed] [Google Scholar]
- 10. Gowd BM, Thompson PD. Effect of female sex on cardiac arrhythmias. Cardiol Rev 2012; 20: 297–303. [DOI] [PubMed] [Google Scholar]
- 11. Albert CM, McGovern BA, Newell JB, Ruskin JN. Sex differences in cardiac arrest survivors. Circulation 1996; 93: 1170–1176. [DOI] [PubMed] [Google Scholar]
- 12. Chugh SS, Uy‐Evanado A, Teodorescu C, Reinier K, Mariani R, Gunson K, Jui J. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: the Ore‐SUDS (Oregon Sudden Unexpected Death Study). J Am Coll Cardiol 2009; 54: 2006–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, American College of Cardiology/American Heart Association Task Force, European Society of Cardiology Committee for Practice Guidelines, European Heart Rhythm Association, and the Heart Rhythm Society . ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death—executive summary: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Eur Heart J 2006; 27: 2099–2140. [DOI] [PubMed] [Google Scholar]
- 14. Wilkoff BL, Williamson BD, Stern RS, Moore SL, Lu F, Lee SW, Birgersdotter‐Green UM, Wathen MS, Van Gelder IC, Heubner BM, Brown ML, Holloman KK, Investigators PS. Strategic programming of detection and therapy parameters in implantable cardioverter‐defibrillators reduces shocks in primary prevention patients: results from the PREPARE (Primary Prevention Parameters Evaluation) study. J Am Coll Cardiol 2008; 52: 541–550. [DOI] [PubMed] [Google Scholar]
- 15. Saeed M, Razavi M, Neason CG, Petrutiu S. Rationale and design for programming implantable cardioverter‐defibrillators in patients with primary prevention indication to prolong time to first shock (PROVIDE) study. Europace 2011; 13: 1648–1652. [DOI] [PubMed] [Google Scholar]
- 16. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA 3rd, Greenberg H, Hall WJ, Huang DT, Kautzner J, Klein H, McNitt S, Olshansky B, Shoda M, Wilber D, Zareba W, MADIT‐RIT Trial Investigators . Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012; 367: 2275–2283. [DOI] [PubMed] [Google Scholar]
- 17. Russo AM, Poole JE, Mark DB, Anderson J, Hellkamp AS, Lee KL, Johnson GW, Domanski M, Bardy GH. Primary prevention with defibrillator therapy in women: results from the Sudden Cardiac Death in Heart Failure Trial. J Cardiovasc Electrophysiol 2008; 19: 720–724. [DOI] [PubMed] [Google Scholar]
- 18. Fischer TH, Herting J, Eiringhaus J, Pabel S, Hartmann NH, Ellenberger D, Friedrich M, Renner A, Gummert J, Maier LS, Zabel M, Hasenfuss G, Sossalla S. Sex‐dependent alterations of Ca2+ cycling in human cardiac hypertrophy and heart failure. Europace 2016; 18: 1440–1448. [DOI] [PubMed] [Google Scholar]
- 19. Rosano GM, Leonardo F, Sarrel PM, Beale CM, De Luca F, Collins P. Cyclical variation in paroxysmal supraventricular tachycardia in women. Lancet 1996; 347: 786–788. [DOI] [PubMed] [Google Scholar]
- 20. Wigginton JG, Pepe PE, Bedolla JP, DeTamble LA, Atkins JM. Sex‐related differences in the presentation and outcome of out‐of‐hospital cardiopulmonary arrest: a multiyear, prospective, population‐based study. Crit Care Med 2002; 30: S131–S136. [DOI] [PubMed] [Google Scholar]
- 21. Bogle BM, Ning H, Mehrotra S, Goldberger JJ, Lloyd‐Jones DM. Lifetime risk for sudden cardiac death in the community. J Am Heart Assoc 2016; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barra S, Providencia R, Duehmke R, Boveda S, Marijon E, Reitan C, Borgquist R, Klug D, Defaye P, Sadoul N, Deharo JC, Sadien I, Patel K, Looi KL, Begley D, Chow AW, Le Heuzey JY, Agarwal S, French‐UK‐Sweden CRT Network . Sex‐specific outcomes with addition of defibrillation to resynchronisation therapy in patients with heart failure. Heart 2017; 103: 753–760. [DOI] [PubMed] [Google Scholar]
- 23. van der Bijl P, Khidir M, Leung M, Mertens B, Ajmone Marsan N, Delgado V, Bax JJ. Impact of QRS complex duration and morphology on left ventricular reverse remodelling and left ventricular function improvement after cardiac resynchronization therapy. Eur J Heart Fail 2017; 19: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 24. Linde C, Bongiorni MG, Birgersdotter‐Green U, Curtis AB, Deisenhofer I, Furokawa T, Gillis AM, Haugaa KH, Lip GYH, Van Gelder I, Malik M, Poole J, Potpara T, Savelieva I, Sarkozy A, Group ESCSD . Sex differences in cardiac arrhythmia: a consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace 2018; 20: 1565–1565ao. [DOI] [PubMed] [Google Scholar]
- 25. Burger AL, Schmidinger H, Ristl R, Pezawas T. Sex difference in inappropriate therapy and survival among 1471 implantable cardioverter‐defibrillator recipients. J Cardiovasc Electrophysiol 2019; 30: 1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shun‐Shin MJ, Zheng SL, Cole GD, Howard JP, Whinnett ZI, Francis DP. Implantable cardioverter defibrillators for primary prevention of death in left ventricular dysfunction with and without ischaemic heart disease: a meta‐analysis of 8567 patients in the 11 trials. Eur Heart J 2017; 38: 1738–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariable and multivariable Cox regression analysis of clinical and imaging parameters for predicting ventricular arrhythmia in the total study cohort.
Table S2. Univariable and multivariable Cox regression analysis of clinical and imaging parameters for predicting ventricular arrhythmia in primary prevention ICD patients.
Table S3. Univariable and multivariable Cox regression analysis of clinical and imaging parameters for predicting ventricular arrhythmia in secondary prevention ICD patients.
Figure S1. Study flowchart.
Figure S2. Effect of implantation year on ADT and included patients.
Figure S3. Appropriate device therapy and device type.
Figure S4. Cumulative incidence of appropriate device therapy and mortality.


