Abstract
Aims
Cardio‐oncology is a growing interdisciplinary field which aims to improve cardiological care for cancer patients in order to reduce morbidity and mortality. The impact of cardiac biomarkers, echocardiographic parameters, and cardiological assessment regarding risk stratification is still unclear. We aimed to identify potential parameters that allow an early risk stratification of cancer patients.
Methods and results
In this cohort study, we evaluated 930 patients that were admitted to the cardio‐oncology outpatient clinic of the University Hospital Heidelberg from January 2016 to January 2019. We performed echocardiography, including Global Longitudinal Strain (GLS) analysis and measured cardiac biomarkers including N‐terminal pro brain‐type natriuretic peptide (NT‐proBNP) and high‐sensitivity cardiac troponin T levels (hs‐cTnT). Most patients were suffering from breast cancer (n = 450, 48.4%), upper gastrointestinal carcinoma (n = 99, 10.6%) or multiple myeloma (n = 51, 5.5%). At the initial visit, we observed 86.7% of patients having a preserved left ventricular ejection fraction (LVEF >50%). At the second follow up, still 78.9% of patients showed a preserved LVEF. Echocardiographic parameters or elevation of NT‐proBNP did not significantly correlate with all‐cause mortality (ACM) (logistic regression LVEF <50%: P = 0.46, NT‐proBNP: P = 0.16) and failed to identify high‐risk patients. In contrast, hs‐cTnT above the median (≥7 ng/L) was an independent marker to determine ACM (multivariant logistic regression, OR: 2.21, P = 0.0038) among all included patients. In particular, hs‐cTnT levels before start of a chemotherapy were predictive for ACM.
Conclusions
Based on our non‐selected cohort of cardio‐oncological patients, hs‐cTnT was able to identify patients with high mortality by using a low cutoff of 7 ng/L. We conclude that measurement of hs‐cTnT is an important tool to stratify the risk for mortality of cancer patients before starting chemotherapy.
Keywords: Cardio‐oncology, Cardiac biomarkers, Heart failure, Cardiotoxicity, Cancer survivors, Risk stratification
Introduction
The importance of cardiologic care for cancer patients has emerged over the last years. Three major reasons account for that: improved survival rates of cancer patients due to early detection, personalized treatment options with novel therapies, 1 , 2 and common risk factors for cancer and cardiovascular disease. 3 Moreover, there is evidence that cardiovascular diseases can promote the occurrence of cancer and vice versa. 4 , 5 In general, cardiovascular events such as thrombosis, pulmonary embolism, heart failure, arrhythmia or pericardial effusion have higher incidences in patients suffering from cancer, due to the malignant disease itself or in response to its therapy. 6
So far, there are only a few studies dedicated to oncologic patients and their specific cardiovascular assessment. This is why the role of cardiac biomarkers or cardiac therapies are still not well defined for the care of cancer patients. 3 , 7 , 8 It is further unclear which surveillance algorithms to use in order to fulfil the specific needs of cancer patients treated with certain kinds of chemotherapy/radiotherapy or who are suffering from multiple oncological diseases.
The life expectancy of patients suffering from potentially curable cancer entities such as breast cancer is frequently limited by non‐oncological complications. 9 In fact, cardiovascular events are the main cause for death in breast cancer patients who have survived their malignant disease for over 10 years. 10
These cardiovascular events require special treatment with regard to the medical history of the patients. One critical aim of cardio‐oncological care is to accompany cancer patients throughout their systemic therapy, so that cardiac high‐risk patients can endure potential cardiotoxic drug regimens. Therefore, early detection and subsequent treatment of cardiovascular adverse effects are a cornerstone to improve outcome of cancer patients. 9 , 10
To date, there is a lack of knowledge, how cardiac parameters can predict the mortality of cancer patients. In order to diagnose cardiotoxicity, many studies concentrated on functional alterations in cardiac imaging, especially in echocardiography. 11 , 12 , 13 , 14 Apart from oncological patients, high‐sensitivity cardiac troponin T (hs‐cTnT) has been shown to be a determining factor for all‐cause mortality (ACM) in patient cohorts with stable coronary heart disease. 15 More recently, cardiotoxicity was classified in three categories: higher grades showed a significantly reduced survival. The grading was distinguished by a combination of biomarkers and echocardiographic parameter including the left ventricular ejection fraction (LVEF), the diastolic function (E/E′), and the global longitudinal strain (GLS). The authors did not focus on the role of single factors, whereas the left ventricular function played a predominant role. 16
The primary objective of our study was to determine cardiac factors which help to risk stratify cancer patients in a cardio‐oncological setting. We hypothesize that functional impairments in cardiac function and cardiac biomarkers correlate with the patients' mortality.
In the current cardio‐oncological study cohort, an elevated plasma concentration of hs‐cTnT was associated with increased ACM. This allowed a prediction of the patients' outcome superior to NT‐proBNP or functional cardiac parameters.
Methods
Patients
930 patients attended the cardio‐oncology unit at the University Hospital Heidelberg in the context of systemic therapies at the oncology departments from January 2016 to January 2019. Patient data were collected within the HEidelberg Cardio‐Oncology REgistry (HEartCORE). The study protocol was approved by the Ethics Committee of the Medical Faculty, University Heidelberg (S‐286/2017, 390/2011). The investigation conforms with the principles outlined in the Declaration of Helsinki.
Patients were admitted to the cardio‐oncology unit according to the current guidelines of the American Heart Association (AHA) and European Society of Cardiology (ESC). We did not exclude patients with prior heart failure or reduction in LVEF before the first presentation.
Every patient was examined prospectively by 12‐lead‐ECG, echocardiography including GLS, if technically applicable, and cardiac biomarkers [hs‐cTnT, N‐terminal pro brain‐type natriuretic peptide (NT‐proBNP)] were assessed, if applicable. In 810 patients, hs‐cTnT was measured at the first or second presentation. These patients were included in the following analysis regarding cardiac biomarkers.
If no cardiac alterations were found at the first presentation, we monitored the patients every 12 weeks as long as the oncological therapy was continued. Patients with a terminated regimen presented once. In case of reduction of LVEF, hs‐cTnT or NT‐proBNP‐elevation or symptoms of heart failure or acute coronary syndrome, we assessed the patients more frequently, in general every 4 weeks. Further cardiac assessment via cardiac computer tomography (CT), cardiac magnet resonance tomography (MRI), or cardiac catherization were based on clinical presentation, echocardiographic parameter, and cardiovascular risk factors.
Data acquisition
Patient specific data were extracted from electronic medical records including ECG, laboratory results, echocardiographic measurements, cardiac MRI/CT results, and angiographic results using the cardiac Research Data Warehouse (RWH).
Measurement of hs‐cTnT in plasma samples was performed using the Elecsys® Troponin T high sensitive hs‐cTnT assay (Roche Diagnostics) on a Cobas® e411 immunoassay analyser in the central laboratory at Heidelberg University Hospital. On Cobas e411, limit of blank (LoB), limit of detection (LoD), 10% coefficient of variation (CV), and 99th percentile cutoff values for the hs‐cTnT assay were 3, 5, 13, and 14 ng/L. 17 , 18 N‐terminal pro brain‐type natriuretic peptide was measured by the Stratus® CS Acute Care™ NT‐proBNP assay (Siemens AG, Berlin and Munich, Germany). The glomerular filtration rate (GFR) was measured in the central laboratory at Heidelberg University Hospital according to the Modification of Diet in Renal Disease (MDRD) method. Outcome data, including ACM, the date of the initial cancer diagnosis, and tumour grading, were acquired from the Clinical Cancer Registry of the National Centre for Tumour Diseases (NCT) Heidelberg. Tumour grading was performed via pathological evaluation. If there was no pathological material present, we used the clinical staging data.
Echocardiography was performed on a General Electrics (GE) Vivid E9 machine. Images were acquired ECG‐triggered with at least three beads per image. LVEF was measured by a physician who is experienced in echocardiography using the biplanar calculation (2‐ and 4‐chamber view). GLS was determined by use of a vendor‐dependent analysis software (GE). The endocardial surface was detected automatically with manual adjustments, if necessary.
Statistical analysis
Pie charts and graphs were illustrated in GraphPad Prism, version 6.0. In order to compare continuous variables, we used the Mann–Whitney test. Dichotomic data were compared using the binomial distribution model. A confidence interval of 95% was considered significant.
Logistic regression analyses were performed in R, version 3.6.2. with the use of the packages safeBinaryRegression (version: 0.1–3), MASS (version 7.3) and in‐house‐scripting. The ggplot2 package (version: 3.2.1) was used to illustrate forest plots.
ROC curves and AUC analysis were calculated by the use of the pROC package (version 1.16.2). Kaplan–Meier curves were generated with the survival package (version: 3.1‐8). The observational range was set to 5 years. Patients with shorter follow‐ups were censored. The follow‐up for ACM was defined as the time difference between the initial cancer diagnosis and the date of death or the date of the last reported medical presentation, respectively. Adjustments of hs‐cTnT were performed to left ventricular function, the occurrence of diabetes or arterial hypertension, body mass index (BMI), NT‐proBNP levels, GFR, age, and gender. The log rank test was used to determine differences in survival. A P‐value <0.05 was considered significant.
Elevated hs‐cTnT was determined as levels above the 99th percentile (14 ng/L) 19 and elevated NT‐proBNP as levels above the rule‐out criterion of heart failure (300 ng/L). 20 Median laboratory values in our cohort were used for further analysis in order to predict ACM.
Impairments in the systolic left ventricular function were defined as previously published and recommended in the current guidelines of the European Society of Cardiology (ESC). A drop in LV‐function was defined as a LVEF deterioration of above 5% or below 50%. 21
Results
We examined 930 patients before, during or after their systemic therapies between January 2016 and January 2019. These patients were mainly diagnosed with breast cancer (48.4%), upper gastrointestinal carcinoma (10.6%), multiple myeloma (5.5%), or melanoma (4.1%). Most of the patients were graded T1 or T2 (34.0%) and had no metastasis (M0, 49.8%) or affected lymph nodes (N0, 49.8%).
The majority of patients in the outpatient clinic was seen during an adjuvant or neoadjuvant setting (415 patients, 44.6%). 138 patients (14.8%) attended after terminated oncological therapies or during palliative regimes (228 patients, 24.5%). 318 patients (34.2%) were treated with radiation, 547 patients (58.8%) underwent surgery and 870 patients (93.5%) received chemotherapy (Table 1 ). Among those patients, 413 patients with available data for outcome and hs‐cTnT were seen before the start of their first chemotherapeutic regimen. 123 patients presented initially in the cardio‐oncology unit during chemotherapy and 109 patients after a terminated chemotherapy. Regarding the cardiovascular risk factors (arterial hypertension, diabetes, adiposity, smoking and hyperlipidemia), there were no significant differences between the groups (Table 2 ). Patients were seen again after 98 days at median (IQR: 58, 153 days, n = 392) and after 191 days (IQR: 127, 287 days, n = 191). Follow‐ups of the outcome were obtained for 553 days at median (IQR: 288; 1,213 days, n = 846). The median time between the initial cancer diagnosis and the blood draw of the cardiac biomarkers was 263 days (IQR: 35; 1,758 days, n = 810). All patients received a cardiological assessment including anamnesis, physical examination, echocardiography including GLS and cardiac biomarkers (hs‐cTnT and NT‐proBNP), if applicable. Based on clinical decisions, 15 patients (1.6%) underwent CT scans to rule out any significant coronary heart disease (CHD). In 113 patients (12.1%) a cardiac catheterization and in 129 patients (13.8%) a cardiac MRI was performed. A more detailed description of the patients' characteristics and demographics can be found in Table 1 .
Table 1.
Baseline characteristics
| Total (n = 930) | Total hs‐cTnT (n = 810) | Hs‐cTnT <7 ng/L (n = 374) | Hs‐cTnT ≥7 ng/L (n = 436) | P‐value | |
|---|---|---|---|---|---|
| Age (years) | 61 (52, 70) | 60 (52, 70) | 54 (46, 62) | 67 (58, 75) | <0.001 |
| Male gender | 287 (30.9%) | 240 (29.6%) | 51 (13.6%) | 189 (43.3%) | <0.001 |
| Risk factors | |||||
| Arterial hypertension | 222 (23.9%) | 196 (24.2%) | 56 (15%) | 140 (32.1%) | <0.001 |
| Diabetes | 94 (10.1%) | 85 (10.5%) | 23 (6.1%) | 62 (14.2%) | <0.001 |
| BMI > 35 kg/m2 | 49 (5.3%) | 46 (5.7%) | 21 (5.6%) | 25 (5.7%) | 1.0 |
| Smoking | 272 (30.8%) | 256 (32.7) | 112 (30.7) | 144 (33.0) | 0.522 |
| Hyperlipidemia | 135 (15.3%) | 126 (16.1) | 39 (10.7) | 87 (20.0) | 0.001 |
| Cancer diagnosis | |||||
| Breast cancer | 450 (48.4%) | 404 (49.9%) | 258 (69.0%) | 146 (33.5%) | <0.001 |
| Upper GI tumour | 99 (10.6%) | 90 (11.1%) | 25 (6.7%) | 65 (14.9%) | <0.001 |
| Multiple myeloma | 51 (5.5%) | 41 (5.1%) | 8 (2.1%) | 33 (7.6%) | <0.001 |
| Melanoma | 38 (4.1%) | 36 (4.4%) | 15 (4.0%) | 21 (4.8%) | 0.70 |
| Ovarian cancer | 35 (3.8%) | 31 (3.8%) | 9 (2.4%) | 22 (5.0%) | 0.08 |
| NET | 34 (3.7%) | 28 (3.5%) | 12 (3.2%) | 16 (3.7%) | 0.87 |
| Lymphoma | 31 (3.3%) | 29 (3.6%) | 7 (1.9%) | 22 (5.0%) | 0.03 |
| AML | 29 (3.1%) | 18 (2.2%) | 8 (2.1%) | 10 (2.3%) | 1.0 |
| Sarkoma | 19 (2.0%) | 15 (1.9%) | 2 (0.5%) | 13 (3.0%) | 0.02 |
| Other | 144 (15.5%) | 118 (14.6%) | 30 (8.0%) | 88 (20.2%) | <0.001 |
| Oncologic staging | |||||
| Tis | 2 (0.2%) | 1 (0.1%) | 1 (0.1%) | 0 (0.0%) | 0.93 |
| T1 | 166 (17.8%) | 152 (18.8%) | 104 (27.8%) | 48 (11.0%) | <0.001 |
| T2 | 151 (16.2%) | 134 (16.5%) | 84 (22.5%) | 50 (11.5%) | <0.001 |
| T3 | 125 (13.4%) | 114 (14.1%) | 35 (9.4%) | 79 (18.1%) | <0.001 |
| T4 | 60 (6.5%) | 54 (6.7%) | 24 (6.4%) | 30 (6.9%) | 0.90 |
| N0 | 270 (29.0%) | 252 (31.1%) | 160 (42.8%) | 92 (21.1%) | <0.001 |
| N1 | 212 (22.8%) | 188 (23.2%) | 90 (24.1%) | 98 (22.5%) | 0.65 |
| N2 | 39 (4.2%) | 35 (4.3%) | 8 (2.1%) | 27 (6.2%) | 0.007 |
| Nx | 13 (1.4%) | 31 (3.8%) | 11 (2.9%) | 20 (4.6%) | 0.30 |
| M0 | 463 (49.8%) | 423 (52.2%) | 236 (63.1%) | 187 (42.9%) | <0.001 |
| M1 | 99 (10.6%) | 87 (10.7%) | 28 (7.5%) | 59 (13.5%) | 0.008 |
| Mx | 37 (4.0%) | 34 (4.2%) | 19 (5.1%) | 15 (3.4%) | 0.32 |
| Oncologic therapy | |||||
| Therapy setting | |||||
| Palliative | 228 (24.5%) | 174 (21.5%) | 47 (12.6%) | 127 (29.1%) | <0.001 |
| Adjuvant | 153 (16.5%) | 125 (15.4%) | 58 (15.5%) | 67 (15.4%) | 1.0 |
| Neoadjuvant | 262 (28.2%) | 237 (29.3%) | 141 (37.7%) | 96 (22.0%) | <0.001 |
| Terminated | 138 (14.8%) | 136 (16.8%) | 90 (24.1%) | 46 (10.6%) | <0.001 |
| Other | 149 (16.0%) | 138 (17.0%) | 38 (10.2%) | 100 (22.9%) | <0.001 |
| Chemotherapy | 870 (93.5%) | 752 (92.8%) | 339 (90.6%) | 413 (94.7%) | 0.03 |
| Before CTx | 504 (54.2%) | 445 (54.9%) | 215 (57.5%) | 230 (52.8%) | 0.20 |
| During CTx | 186 (20.0%) | 142 (17.5%) | 42 (11.2%) | 100 (22.9%) | <0.001 |
| After CTx | 130 (14.0%) | 122 (15.1%) | 68 (18.2%) | 54 (12.4%) | 0.028 |
| Anthracyclines | 229 (26.3%) | 195 (25.1%) | 118 (32.4%) | 77 (18.7%) | <0.001 |
| Trastuzumab | 139 (16.0%) | 118 (15.2%) | 67 (18.4%) | 51 (12.4%) | 0.016 |
| TKI | 37 (4.2%) | 25 (3.2%) | 9 (2.5%) | 16 (3.9%) | 0.41 |
| ICI | 76 (8.7%) | 63 (8.1%) | 26 (7.1%) | 37 (9.0%) | 0.50 |
| PI (Carfilzomib) | 11 (1.3%) | 9 (1.2%) | 0 (0.0%) | 9 (2.2%) | 0.014 |
| Other drugs | 242 (26.0%) | 215 (26.5%) | 69 (18.4%) | 146 (33.5%) | <0.001 |
| Radiation | 318 (34.2%) | 280 (36.1%) | 141 (38.7%) | 139 (33.7%) | 0.10 |
| Chest radiation | 241 (25.9%) | 214 (27.6%) | 120 (33.0%) | 94 (22.8%) | 0.001 |
| Surgery | 547 (58.8%) | 489 (63.0%) | 239 (65.7%) | 250 (60.7%) | 0.07 |
| Number of visits | |||||
| 1 visit | 930 (100%) | 810 (100%) | 374 (100%) | 436 (100%) | 1.0 |
| 2 visits | 392 (42.2%) | 341 (42.1%) | 152 (40.6%) | 189 (43.3%) | 0.43 |
| 3 visits | 191 (20.5%) | 167 (20.6%) | 86 (23.0%) | 81 (18.6%) | 0.14 s |
| Clinical chemistry | |||||
| Timepoint (days) | 263 (35, 1758) | 239 (33, 1884) | 137 (30, 1971) | 267 (39, 1490) | 0.738 |
| Hs‐cTnT (ng/L) | 7 (4, 12) | 7 (4, 12) | 4 (3, 5) | 11 (9, 17) | <0.001 |
| NT‐proBNP (ng/L) | 141 (70, 293.5) | 140 (70, 291) | 96 (58, 174) | 210 (101, 533) | <0.001 |
| Creatinine (mg/dL) | 0.75 (0.65, 0.88) | 0.74 (0.65, 0.87) | 0.70 (0.63, 0.77) | 0.82 (0.69, 1.03) | <0.001 |
| GFR (mL/min) | 90.67 (76.4, 105.8) | 90.84 (76.75, 105.7) | 94.14 (84.52, 111.03) | 84.36 (69.68, 99.54) | <0.001 |
| Echocardiography | |||||
| LVEF (%) | 60.0 (55, 60) | 60 (55, 60) | 60 (60, 60) | 60 (55, 60) | <0.001 |
| GLS (−%) | 17.9 (16.0, 20.0) | 18.0 (16.0, 20.1) | 18.5 (16.4, 20.4) | 17.6 (15.2, 19.8) | 0.002 |
| E/E′ | 7.00 (5.00, 9.00) | 7.00 (5.00, 8.95) | 6.00 (5.00, 8.00) | 7.00 (6.00, 9.00) | <0.001 |
| Cardiac catheterization | 110 (11.8%) | 96 (11.9%) | 22 (5.9%) | 74 (17.0%) | <0.001 |
| Stenosis ≥75% | 27 (2.9%) | 27 (3.3%) | 1 (0.2%) | 26 (6.0%) | <0.001 |
| Cardiac CT | 15 (1.6%) | 15 (1.9%) | 4 (1.1%) | 11 (2.5%) | 0.21 |
| Cardiac MRI | 129 (13.9%) | 120 (14.8%) | 40 (10.7%) | 80 (18.3%) | 0.003 |
| All‐cause mortality | 120 (12.9%) | 99 (12.2%) | 24 (6.4%) | 75 (17.2%) | 0.002 |
| Median follow‐up survival (days) | 553 (288, 1213) | 541 (287, 1128) | 518 (283, 1245) | 557 (289, 1079) | 0.13 |
| Causes of death | |||||
| Cancer | 58 (6.2%) | 50 (6.2%) | 14 (3.7%) | 36 (8.3%) | 0.01 |
| Infection | 11 (1.2%) | 9 (1.1%) | 0 (0.0%) | 9 (2.1%) | 0.014 |
| Unknown | 51 (5.5%) | 40 (4.9%) | 10 (2.7%) | 30 (6.9%) | 0.01 |
AML, acute myeloid leukaemia; BMI, body mass index; CT, computer tomography; CTx, chemotherapy; E, early ventricular filling velocity; E′, peak mitral annular velocity during early filling; GFR, glomerular filtration rate; GI, gastrointestinal; GLS, global longitudinal strain; hs‐cTnT, high‐sensitivity cardiac troponin T; ICI, immune checkpoint inhibitor; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; NET, neuroendocrine tumour; NT‐proBNP, N‐terminal pro brain‐type natriuretic peptide; PI, proteasome inhibitor; Timepoint, median time between cancer diagnosis and blood draw (days); TKI, tyrosin kinase inhibitor.
Table 2.
Characteristics of patients with chemotherapy
| Total patients with CTx (n = 707) | Presentation before CTx (n = 413) | Presentation during CTx (n = 123) | Presentation after CTx (n = 109) | Other (n = 62) | P‐value | |
|---|---|---|---|---|---|---|
| Age (year) | 60 (51, 70) | 60 (50, 69) | 59 (48.5, 70) | 60 (53, 67) | 63 (56, 71) | 0.310 |
| Male gender | 214 (30.3%) | 137 (33.2%) | 44 (35.8%) | 14 (12.8%) | 19 (30.6%) | 0.056 |
| Risk factors | ||||||
| Arterial hypertension | 169 (23.9%) | 91 (22.0%) | 40 (32.5%) | 19 (17.4%) | 19 (30.6%) | 0.196 |
| Diabetes | 73 (10.3%) | 46 (11.1%) | 18 (14.6%) | 3 (2.8%) | 6 (9.7%) | 0.471 |
| BMI > 35 kg/m2 | 40 (5.7%) | 27 (6.5%) | 5 (4.0%) | 5 (4.6%) | 3 (4.8%) | 0.301 |
| Smoking | 228 (33.2%) | 134 (33.5%) | 38 (30.4%) | 38 (34.9%) | 20 (33.9%) | 1.0 |
| Hyperlipidemia | 104 (15.1%) | 66 (16.5%) | 19 (15.2%) | 15 (13.8%) | 6 (10.2%) | 0.444 |
| Cancer diagnosis | ||||||
| Breast cancer | 351 (49.6%) | 194 (47.0%) | 43 (35.0%) | 88 (80.7%) | 26 (41.9%) | 0.108 |
| Upper GI tumour | 86 (12.2%) | 74 (17.9%) | 8 (6.5%) | 3 (2.8%) | 1 (1.6%) | <0.001 |
| Multiple myeloma | 31 (4.4%) | 23 (5.6%) | 2 (1.6%) | 0 (0.0%) | 6 (9.7%) | 0.102 |
| Melanoma | 27 (3.8%) | 16 (3.9%) | 7 (5.7%) | 1 (0.9%) | 3 (4.8%) | 1.0 |
| Ovarial cancer | 31 (4.4%) | 18 (4.4%) | 6 (4.9%) | 1 (0.9%) | 6 (9.7%) | 1.0 |
| NET | 25 (3.5%) | 5 (1.2%) | 13 (10.6%) | 3 (2.8%) | 4 (6.5%) | 0.648 |
| Lymphoma | 27 (3.8%) | 17 (4.1%) | 5 (4.1%) | 2 (1.8%) | 3 (4.8%) | 0.772 |
| AML | 17 (2.4%) | 12 (2.9%) | 1 (0.8%) | 3 (2.8%) | 1 (1.6%) | 0.434 |
| Sarkoma | 12 (1.7%) | 6 (1.5%) | 2 (1.6%) | 0 (0.0%) | 4 (6.5%) | 0.763 |
| Other | 100 (14.1%) | 48 (11.6%) | 36 (29.3%) | 8 (7.3%) | 8 (12.9%) | 0.030 |
| Chemotherapy | ||||||
| Anthracyclines | 177 (25.0%) | 76 (18.4%) | 35 (28.5%) | 45 (41.3%) | 21 (33.9%) | <0.001 |
| Trastuzumab | 112 (5.8%) | 66 (16.0%) | 23 (18.7%) | 17 (15.6%) | 6 (9.7%) | 0.336 |
| TKI | 19 (2.7%) | 4 (1.0%) | 12 (9.8%) | 1 (0.9%) | 2 (3.2%) | 0.002 |
| ICI | 55 (7.8%) | 26 (6.3%) | 22 (17.9%) | 2 (1.8%) | 5 (8.1%) | 0.109 |
| PI (Carfilzomib) | 8 (1.1%) | 5 (1.2%) | 2 (1.6%) | 0 (0.0%) | 1 (1.6%) | 1.0 |
| Other | 194 (27.4%) | 105 (25.4%) | 43 (35.0%) | 22 (20.2%) | 24 (38.7%) | 0.181 |
| Clinical chemistry | ||||||
| Hs‐cTnT (ng/L) | 7 (4, 11) | 9 (6, 15.5) | 6 (4, 9) | 8 (4, 12) | 7 (4, 11) | <0.001 |
| Echocardiography | ||||||
| LVEF (%) | 60 (55, 60) | 60 (60, 60) | 60 (55, 60) | 60 (60, 60) | 60 (60, 60) | 0.002 |
| All‐cause mortality | ||||||
| Median follow‐up survival (days) | 541 (284.5, 1151.5) | 391 (235, 610) | 715 (323, 1409) | 1095 (823, 1688) | 1492 (745, 1763.25) | <0.001 |
| Causes of death | ||||||
| Cancer | 50 (7.1%) | 35 (8.4%) | 13 (10.6%) | 0 (0.0%) | 2 (3.2%) | 0.115 |
| Infection | 9 (1.3%) | 8 (1.9%) | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 0.127 |
| Unknown | 40 (5.7%) | 23 (5.6%) | 11 (8.9%) | 3 (2.8%) | 3 (4.8%) | 1.0 |
AML, acute myeloid leukaemia; BMI, body mass index; CTx, chemotherapy; hs‐cTnT, high‐sensitivity cardiac troponin T; ICI, immune checkpoint inhibitor; LVEF, left ventricular ejection fraction; NET, neuroendocrine tumour; PI, proteasome inhibitor; TKI, tyrosin kinase inhibitor.
Functional cardiac assessments
806 patients (86.7%) showed a preserved systolic left ventricular ejection fraction (LVEF >50%) on their first admission to the cardio‐oncology unit. 50.3% of patients (n = 282/561) with data for GLS showed reduced values (GLS > −18%) and 26.7% (n = 174/652) had a reduced diastolic function (E/E′ > 8).
A total of 78.9% of patients still showed a preserved systolic LV function at second follow‐up. However, 17.2% of these patients showed elevated levels of N‐terminal pro brain‐type natriuretic peptide (NT‐proBNP) (above rule‐out, >300 ng/L) and 11.4% of these patients elevated hs‐cTnT (above 99th percentile, >14 pg/mL) (Supporting Information, Figure S1 )
In terms of diastolic function, we saw fewer patients with diastolic dysfunction during follow‐ups (E/E′ > 8: 20.8% of patients at second follow up, n = 192).
However, neither a reduced systolic LV‐function (LVEF <50%, univariate logistic regression, P = 0.46) nor reduced GLS (univariate logistic regression, P = 0.33) did correlate significantly with ACM (Figure 1 ). Consequently, patients with a preserved LVEF >50% did not benefit with regard to survival in Kaplan–Meier analysis (Figure 2 A ).
Figure 1.
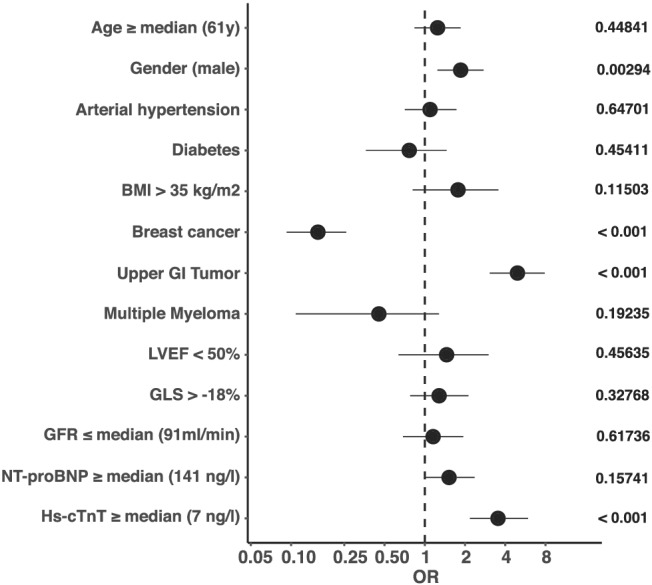
Univariate logistic regression analysis on all‐cause mortality (ACM). Odds ratios (OR) and 95% confidence interval are shown as forest plot. Male gender, upper gastrointestinal (GI) tumours, and hs‐cTnT levels ≥7 ng/L were associated with increased mortality. Breast cancer patients showed reduced mortality rates. P‐values as indicated. BMI, body mass index; GFR, glomerular filtration rate; GI, gastrointestinal; GLS, global longitudinal strain; Hs‐cTnT, high sensitivity cardiac troponin T; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro brain‐type natriuretic peptide.
Figure 2.
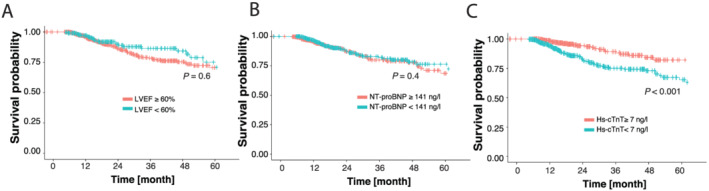
Kaplan–Meier curves on all‐cause mortality (ACM). Patients are divided into two groups according to their (A) left ventricular systolic function (LVEF ≥/< median of 60%), (B) NT‐proBNP levels (≥/< median of 141 ng/L) and (C) hs‐cTnT level (≥/< median of 7 ng/L). Log rank test P‐value as indicated. Hs‐cTnT, high sensitivity cardiac troponin T; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro brain‐type natriuretic peptide.
Cardiac biomarkers and all‐cause mortality
Overall, 120 patients (14.2%) died of any cause, but no deaths due to cardiovascular events were documented (Table 1 ). We included LVEF, GLS, and the cardiac biomarkers NT‐proBNP and hs‐cTnT in addition to demographic parameters and oncological diseases in logistic regression analysis in order to test determination of ACM (Figure 1 ). NT‐proBNP levels above the median (141 ng/L) were not found to be significantly related with the patients' outcome (univariate logistic regression, P = 0.16; log rank test: P = 0.4) (Figure 2 B ).
However, hs‐cTnT above the median of 7 ng/L was associated with ACM (univariate logistic regression, P < 0.001). A multivariate analysis of the significant factors from the univariate approach confirmed hs‐cTnT as an independent marker for the prediction of ACM (P = 0.0038) (Supporting Information, Figure S2B ). Furthermore, in Kaplan–Meier curves, there were significantly fewer deaths of patients with hs‐cTnT levels <7 ng/L (log rank test: P < 0.001) (Figures 1 and 2 C ). Univariate logistic regression analysis of factors which might be correlated with hs‐cTnT ≥7 ng/L is shown in the Supporting Information, Figure S2A . Apart from obesity, breast cancer, and GLS analysis, all tested factors did correlate with hs‐cTnT levels above the median of 7 ng/L.
In clinical use, commonly utilized hs‐cTnT ranges (<5, 5–14, and >14 ng/L) suggest a concentration‐dependent association of plasma hs‐cTnT levels and ACM (log rank test, P = 0.0002). The analysis was adjusted to age, gender, renal function (GFR), NT‐proBNP, the BMI, diabetes, arterial hypertension, and LVEF (Supporting Information, Figure S3 ).
The combined use of NT‐proBNP and LVEF did not improve the prediction of hs‐cTnT for ACM (AUC hs‐cTnT: 0.64, AUC Hs‐cTnT + NT‐proBNP + LVEF: 0.60) (Supporting Information, Figure S4 ). Of note, the proportion of patients with hs‐cTnT levels above 7 ng/L is significantly higher in the group of patients with a LVEF ≤50% (P < 0.001, χ 2 test, Supporting Information, Figure S1 ). In addition, there have been more relevant coronary stenoses (≥75% in any segment) in the patients of the higher hs‐cTnT group (hs‐cTnT <7 ng/L: 4.5%; hs‐cTnT ≥7 ng/L: 35.1%, P < 0.001). Regarding oncological therapy, there are no significant differences in radiations or surgery rates between the hs‐cTnT groups. Interestingly, anthrazyclines and trastuzumab were less frequently used in patients with hs‐cTnT values above 7 ng/L. There is a higher percentage of patients with use of Carfilzomib, and by trend of immune checkpoint inhibitors or tyrosine kinase inhibitors with elevated hs‐cTnT (Table 1 ).
High‐sensitivity cardiac troponin T levels correlate with left ventricular‐dysfunction and all‐cause mortality
We correlated hs‐cTnT with LV‐dysfunction and ACM, as well as with a combined endpoint of both, a drop in LV‐function and ACM. Given the area under the curve (AUC), hs‐cTnT has a good predictive value for a drop in left ventricular function (AUC: 0.82). The correlation with ACM and the combined endpoint is less pronounced (ACM AUC: 0.61, Drop in LV function ACM: 0.66). Nevertheless, we observed high values of the sensitivity at the identified cutoff of hs‐cTnT ≥7 ng/L for the three endpoints. The sensitivity regarding a drop in LV‐function is 92%, regarding ACM 80% and for the combined endpoint 84%, respectively (Figure 3 ).
Figure 3.
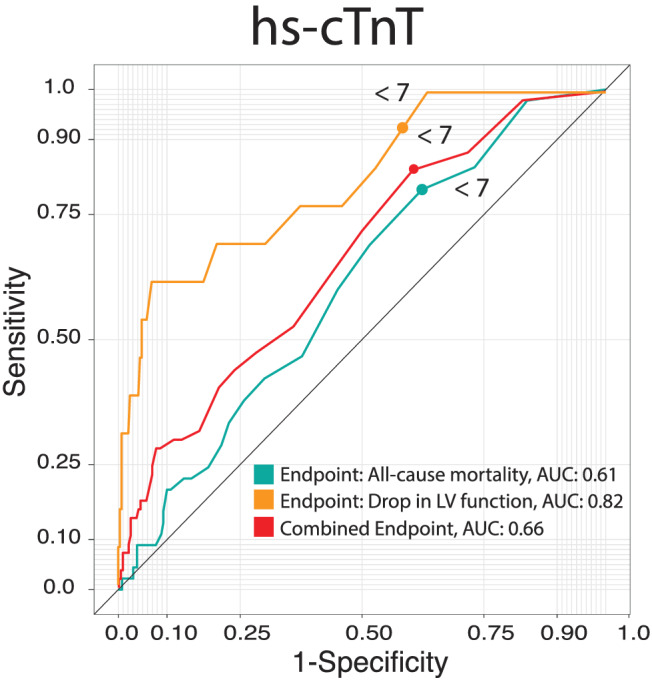
ROC curves showing the predictive value of hs‐cTnT on the endpoints all‐cause mortality (ACM), drop in left ventricular (LV) function and the combined endpoint (ACM and drop in LV function). Area under the curve (AUC) as indicated. Hs‐cTnT, high sensitivity cardiac troponin T.
High‐sensitivity cardiac troponin T levels at the start of chemotherapy are associated with higher all‐cause mortality rates
Further, we compared the predictive value of hs‐cTnT on ACM at different stages in the chemotherapeutic regimen. Patients who presented before the start of their first chemotherapy in the cardio‐oncology unit and whose hs‐cTnT measurement was below our identified cutoff showed a significantly better outcome (log rank test: P‐value: 0.01). The patients of the given cardio‐oncological cohort who presented already during treatment did not differ in survival (log rank test: P‐value: 0.8). Patients who survived their oncological disease showed a lower rate of ACM in general. Hs‐cTnT still distinguishes the survival using the cutoff of 7 ng/L (Figure 4 ). Concentrating on palliative patients, who have a higher mortality than the non‐palliative patients in our cohort, hs‐cTnT with the cutoff of 7 ng/L did not significantly subdivide those patients' ACM (P = 0.1) (Supporting Information, Figure S5 ).
Figure 4.
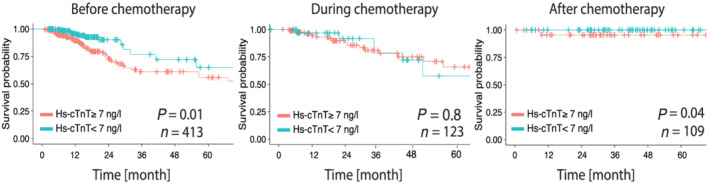
Kaplan–Meier curves on all‐cause mortality (ACM). Patients are divided according to the hs‐cTnT cutoff (7 ng/L), and results are shown for patients before starting chemotherapy, patients with initial presentation during chemotherapy and patients with terminated chemotherapy. Log rank test P‐value as indicated. Hs‐cTnT, high sensitivity cardiac troponin T.
Apart from the baseline hs‐cTnT, we reevaluated the hs‐cTnT of a number of patients during follow‐up. Patients with any increase of hs‐cTnT over time showed a tendency towards an increased ACM (log rank test: P = 0.4; data not shown). Patients with hs‐cTnT levels below the 99th percentile at the first and above the 99th percentile at the second visit showed a significantly increased ACM (log rank test: P = 0.04; Supporting Information, Figure S6 ).
Discussion
Cardiological management of cancer patients
In this single‐centre approach, we report a 3‐year experience of the cardio‐oncology unit of University Hospital Heidelberg. Our primary aim was to determine parameters which might be able to stratify cancer patients regarding their mortality. The cardiological assessment of those patients revealed hs‐cTnT to be the best predictor for mortality next to the oncological parameters.
In recent years, cardio‐oncology focused on cancer therapy‐related side effects and cardiotoxicity of chemotherapies. The detection of cardiotoxicity and cardiological surveillance strategies mainly relied on echocardiographic parameters such as the systolic and diastolic left ventricular function. 11 , 12 , 13 , 14 GLS analysis was further suggested to be a sensitive predictor of early cardiotoxicity. 22 , 23 This notion is underlined by the statements of the European Society of Cardiology (ESC), 24 the German Society of Cardiology (DGK), 25 and practical guidelines of the American Society of Clinical Oncology (ASCO) 1 that highlight the use of functional cardiac parameters. According to the current European Society of Medical Oncology (ESMO) consensus recommendations, cardiac biomarkers may be evaluated before and during chemotherapy for risk stratification in addition to measurements of the systolic LV function. The exact benefit in the daily clinical routine still needs to be determined by prospective clinical trials. 26 There are a few studies that focus on hs‐cTnT and NT‐proBNP and their specific use in cardio‐oncological patients. 27 For example, in breast cancer patients who received trastuzumab, elevated baseline troponin levels were linked to the risk of a drop in LVEF. 28 The authors could not link biomarkers to relevant clinical events or mortality, presumably due to the limited number of patients.
High‐sensitivity cardiac troponin T levels are an independent predictor of all‐cause mortality in cancer patients
A reduced ejection fraction and worsening of left ventricular function predicts the patients' outcome in oncological 16 and non‐oncological patients. 29 In stable 15 and unstable 30 coronary heart disease (CHD), patients whose hs‐cTnT‐levels are above the 99th percentile (14 ng/L) show a significantly higher ACM. In the present cardio‐oncological cohort, increased mortality rates were already seen above 7 ng/L. Considering the clinically used hs‐cTnT‐cutoffs of <5 ng/L (not detectable), 5–14 ng/L (normal), and >14 ng/L (elevated), the oncological patients showed a similar pattern in mortality at generally higher mortality rates in comparison with CHD patients.
These analyses were adjusted to known influencing factors of hs‐cTnT including GFR and cardiac risk factors (age, gender, hypertension, diabetes, and adiposity). Deterioration in renal function, measured via GFR, does not only correlate with elevated levels of hs‐cTnT in our data but was previously depicted as an independent cardiac risk factor as well. 31 Logistic regression analysis to test for co‐appearance of these factors and elevated hs‐cTnT levels (≥7 ng/L) revealed strong correlations, except for adiposity. The cardiac parameters, reduced LVEF and elevated NT‐proBNP, did also correlate with elevated hs‐cTnT. GLS did not correlate with elevated hs‐cTnT.
To our surprise, in our cohort a reduced ejection fraction was not associated with an increase of ACM. This might be explained by the limited number of patients with reductions of LVEF. On the other hand, hs‐cTnT levels correlated well with a drop in LVEF. In ROC‐curves, we found a particularly high sensitivity rate at the identified hs‐cTnT cutoff (≥7 ng/L) to predict worsening of the left ventricular function. Using hs‐cTnT as a risk stratification for cardio‐oncological patients, hardly any patients who experience a drop in LV function are overlooked. Among the patients which were further examined by cardiac catheterization, there has been hardly any stenosis in the low hs‐cTnT group.
Thus, at least in the present study cohort, echocardiography results correlated well with hs‐cTnT. Hs‐cTnT, however, used as a single marker, was superior to identify a high risk patient cohort.
Neither reduced GLS, nor elevated levels of NT‐proBNP were able to predict ACM. Further, neither age, diabetes, arterial hypertension, adiposity, nor reduced GFR correlated significantly with unfavourable outcome.
Identification of patients at risk and follow‐up
According to the current guidelines of the cardiological and oncological societies, the main method to keep cancer patients under cardiac surveillance is echocardiography. As proposed by current results from the CARDIOvascular TOXicity induced by cancer‐related therapies (CARDIOTOX) registry, outcome‐relevant cardiotoxic events include the occurrence of a reduced systolic left ventricular function. 16 These data excluded patients with history of heart failure (HF) or current occurrence of HF. The registry contained a higher number of female patients (84%), higher rates of breast cancer (64%) and lymphoma (20.5%) and lower rates of gastrointestinal tumours (2%) than the present cohort. In our cohort, the diagnosis of breast cancer was associated with a lower mortality whereas gastrointestinal tumours showed higher mortality. This might explain the low predictive value of echocardiographic parameters. Breast cancer and gastrointestinal tumours were depicted as independent predictors for ACM in multivariant logistic regression analysis. Nonetheless, patients with gastrointestinal tumours showed higher levels of hs‐cTnT (66.7% of patients with hs‐cTnT >7 ng/L) than breast cancer patients (28.2% of patients with hs‐cTnT >7 ng/L). A stronger association of baseline hs‐cTnT values to cardiovascular events and mortality has been shown for females and further supports the possible impact of hs‐cTnT in female patients with cancer. 32
ACM was higher in the present study (14.2% compared with 6.2% in the CARDIOTOX registry). The percentage of patients who experienced a drop in left ventricular function (4.3% in the present study, 5% in the CARDIOTOX registry) was comparable between both studies. Due to the differences in the patient characteristics as mentioned above and the observance intervals, we may see differences in the predicting factors.
In contrast to the approach of the CARDIOTOX registry that aimed to identify relevant cardiotoxic events during chemotherapies, we found that hs‐cTnT levels are valuable for a risk stratification before the start of chemotherapy. It might be the case that reductions in left ventricular function appear in advanced cases of cardiotoxicity, whereas elevations in cardiac biomarkers are suitable for early risk stratification. In patients without prior cardiotoxic therapy and without prior cardiac disease, a low cutoff of 5 ng/L was already shown to be associated with ACM. 33
The herewith presented real‐world data with the enrolment of patients with prior cardiovascular diseases highlight the general role of hs‐cTnT in risk‐stratification of cardio‐oncological patients and the potential impact of the plasma concentration of hs‐cTnT as a critical determinant for the prediction of mortality. Particularly, baseline hs‐cTnT values in patients before starting chemotherapy were predictive. The first evaluation of hs‐cTnT during or after chemotherapy played a minor role in HEartCORE patients. Of note, in a subgroup of patients we observed a prognostic value of an elevation of hs‐cTnT >14 ng/L in the follow‐up.
Conclusions
Based on the present data, measurement of cardiac hs‐cTnT can possibly be used for the risk stratification of cancer patients. In particular, hs‐cTnT values which were measured before starting chemotherapy showed a predictive value. Patients with higher hs‐cTnT plasma concentration (≥7 ng/L) should be considered for a more detailed cardiac surveillance strategy. Hs‐cTnT might additionally be implemented in cancer studies to early identify high‐risk patients.
Further prospective, randomized, multi‐centre trials need to confirm these findings in other clinical cohorts. It further needs to be determined, if functional measurements including echocardiography might be dispensable in low‐risk patients characterized by hs‐cTnT levels <7 ng/L.
Study limitations
Patients were evaluated in the context of a cancer‐related therapy. The study cohort describes a real‐world collective without patient selection according to specific oncological or cardiological parameters.
During follow‐up consultations, there is an additional selection bias that needs to be noted, because patients with pathological findings (e.g. elevated cardiac biomarker or reduction of LVEF) were admitted to a more stringent follow‐up regimen. Patients who presented themselves after a terminated therapy can be considered as cancer survivors and showed a considerably lower overall mortality rate.
Data of the all‐cause mortality were retrieved from the Clinical Cancer Registry of the National Centre for Tumour Diseases (NCT) Heidelberg. The causes of death could be linked to the oncological disease in most cases. Nevertheless, 51/120 cases had an unknown cause of death and were not able to be linked to specific, e.g. cardiovascular complications.
Conflict of interest
H.A.K. received honoraria for lecturers from Roche Diagnostics, AstraZeneca, Bayer Vital, Daiichi‐Sankyo, and held a patent on cTnT that has expired. E.G. received honoraria for lectures from Roche Diagnostics, AstraZeneca, Bayer Vital, Daiichi‐Sankyo, Eli Lilly Deutschland. He serves as a consultant for Roche Diagnostics, BRAHMS Thermo Fisher, Boehringer Ingelheim, and has received research funding from BRAHMS Thermo Fisher, Roche Diagnostics, Bayer Vital and Daiichi Sankyo. L.H.L. has served on the advisory board for Daiichi Sankyio, Senaca and Servier and received speakers' honoraria from MSD. The remaining authors have nothing to disclose.
Funding
M.H. is recipient of the rotation grant of the German Centre for Cardiovascular Research (DZHK). L.H.L. is supported by the Deutsche Forschungsgemeinschaft (DFG; LE 3570/2‐1; 3570/3‐1) and the Bundesministerium für Forschung (BMBF; 01KC2006B).
Supporting information
Figure S1. (A) Pie charts of LV‐function, measured via echocardiography at the first three consultations. Patients numbers and distribution of preserved LVEF (LVEF > 50%), mid‐range reduced LVEF (LVEF 40–50%) und reduced LVEF (LVEF < 40%) as indicated. (B) Distribution of elevated NT‐proBNP (> rule out criterion, 300 ng/l) in patients with preserved and reduced LVEF (LVEF >/≤50%). (C) Distribution of elevated hs‐cTnT (> 99 percentile, 14 ng/l) in patients with preserved and reduced LVEF. (D) Distribution of elevated hs‐cTnT with a cutoff of 7 ng/l in patients with preserved and reduced LVEF.
Figure S2. (A) Univariate logistic regression analysis of elevated hs‐cTnT (≥ 7 ng/l). Odds Ratio (OR), confidential interval and p‐value as indicated. (B) Multivariate logistic regression analysis for all‐cause mortality including the significant factors (Gender, Breast cancer, Upper GI Tumour, hs‐cTnT ≥ 7 ng/l) from the univariate analysis. Odds Ratio (OR), confidential interval and p‐value as indicated.
Figure S3. Kaplan Meier curves on all‐cause mortality (ACM). Patients are divided into three groups according to their hs‐cTnT level (< 5 ng/l, 5‐14 ng/l, > 14 ng/l). Adjustments of hs‐cTnT were performed to left ventricular function, the occurrence of diabetes or arterial hypertension, body mass index (BMI), NT‐proBNP levels, GFR, age and gender. Logrank‐Test p‐value as indicated.
Figure S4. ROC curves to test for the prediction of all‐cause mortality. Single curves are shown for hs‐cTnT, NT‐proBNP, LVEF as continuous variables and hs‐cTnT, NT‐proBNP, LVEF together. Area under the curve (AUC) as indicated.
Figure S5. Kaplan Meier curves on all‐cause mortality (ACM). Patients are divided into four groups according to a palliative and non‐palliative treatment and their hs‐cTnT level (</≥ 7 ng/l), respectively. Patient numbers per group as indicated. Logrank‐Test p‐value for the comparison of ACM in non‐palliative and palliative patients as indicated.
Figure S6. Kaplan Meier curves on all‐cause mortality (ACM). Patients are divided into two groups according to the change of hs‐cTnT between the first and the second measurement (n = 213). Patients whose hs‐cTnT was below 14 ng/l at the first visit and above 14 ng/l at the second visit are shown in turquoise. Patients with no increase from below to above 14 ng/l are shown in red. Logrank‐Test p‐value as indicated.
Finke, D. , Romann, S. W. , Heckmann, M. B. , Hund, H. , Bougatf, N. , Kantharajah, A. , Katus, H. A. , Müller, O. J. , Frey, N. , Giannitsis, E. , and Lehmann, L. H. (2021) High‐sensitivity cardiac troponin T determines all‐cause mortality in cancer patients: a single‐centre cohort study. ESC Heart Failure, 8: 3709–3719. 10.1002/ehf2.13515.
References
- 1. Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K, Lenihan D. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2017; 35: 893–911. [DOI] [PubMed] [Google Scholar]
- 2. Witteles RM, Telli M. Underestimating cardiac toxicity in cancer trials: lessons learned? J Clin Oncol 2012; 30: 1916–1918. [DOI] [PubMed] [Google Scholar]
- 3. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Pandini C, Sandri MT, Cipolla CM. Cardio‐oncology: a new medical issue. Ecancermedicalscience. 2008; 2: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hasin T, Gerber Y, Weston SA, Jiang R, Killian JM, Manemann SM, Cerhan JR, Roger VL. Heart Failure After Myocardial Infarction Is Associated With Increased Risk of Cancer. J Am Coll Cardiol 2016; 68: 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abu‐Khalaf MM, Juneja V, Chung GG, DiGiovanna MP, Sipples R, McGurk M, Zelterman D, Haffty B, Reiss M, Wackers FJ, Lee FA, Burtness BA. Long‐term assessment of cardiac function after dose‐dense and ‐intense sequential doxorubicin (A), paclitaxel (T), and cyclophosphamide (C) as adjuvant therapy for high risk breast cancer. Breast Cancer Res Treat 2007; 104: 341–349. [DOI] [PubMed] [Google Scholar]
- 6. Nhola LF, Villarraga HR. Rationale for Cardio‐Oncology Units. Rev Esp Cardiol (Engl Ed) 2017; 70: 583–589. [DOI] [PubMed] [Google Scholar]
- 7. Cardinale D, Sandri MT, Martinoni A, Borghini E, Civelli M, Lamantia G, Cinieri S, Martinelli G, Fiorentini C, Cipolla CM. Myocardial injury revealed by plasma troponin I in breast cancer treated with high‐dose chemotherapy. Ann Oncol 2002; 13: 710–715. [DOI] [PubMed] [Google Scholar]
- 8. Riddell E, Lenihan D. The role of cardiac biomarkers in cardio‐oncology. Curr Probl Cancer 2018; 42: 375–385. [DOI] [PubMed] [Google Scholar]
- 9. Ammon M, Arenja N, Leibundgut G, Buechel RR, Kuster GM, Kaufmann BA, Pfister O. Cardiovascular management of cancer patients with chemotherapy‐associated left ventricular systolic dysfunction in real‐world clinical practice. J Card Fail 2013; 19: 629–634. [DOI] [PubMed] [Google Scholar]
- 10. Darby SC, McGale P, Taylor CW, Peto R. Long‐term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol 2005; 6: 557–565. [DOI] [PubMed] [Google Scholar]
- 11. Ewer MS, Lippman SM. Type II chemotherapy‐related cardiac dysfunction: time to recognize a new entity. J Clin Oncol 2005; 23: 2900–2902. [DOI] [PubMed] [Google Scholar]
- 12. Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J 2013; 34: 1102–1111. [DOI] [PubMed] [Google Scholar]
- 13. Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 2002; 20: 1215–1221. [DOI] [PubMed] [Google Scholar]
- 14. Alexander J, Dainiak N, Berger HJ, Goldman L, Johnstone D, Reduto L, Duffy T, Schwartz P, Gottschalk A, Zaret BL. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N Engl J Med 1979; 300: 278–283. [DOI] [PubMed] [Google Scholar]
- 15. Biener M, Giannitsis E, Lamerz J, Mueller‐Hennessen M, Vafaie M, Katus HA. Prognostic value of elevated high‐sensitivity cardiac troponin T levels in a low risk outpatient population with cardiovascular disease. Eur Heart J Acute Cardiovasc Care 2016; 5: 409–418. [DOI] [PubMed] [Google Scholar]
- 16. Lopez‐Sendon J, Alvarez‐Ortega C, Zamora Aunon P, Buno Soto A, Lyon AR, Farmakis D, Cardinale D, Canales Albendea M, Feliu Batlle J, Rodriguez Rodriguez I, Rodriguez Fraga O, Albaladejo A, Mediavilla G, Gonzalez‐Juanatey JR, Martinez Monzonis A, Gomez Prieto P, Gonzalez‐Costello J, Serrano Antolin JM, Cadenas Chamorro R, Lopez FT. Classification, prevalence, and outcomes of anticancer therapy‐induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J 2020; 41: 1720–1729. [DOI] [PubMed] [Google Scholar]
- 17. Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high‐sensitivity cardiac troponin T assay. Clin Chem 2010; 56: 254–261. [DOI] [PubMed] [Google Scholar]
- 18. Saenger AK, Beyrau R, Braun S, Cooray R, Dolci A, Freidank H, Giannitsis E, Gustafson S, Handy B, Katus H, Melanson SE, Panteghini M, Venge P, Zorn M, Jarolim P, Bruton D, Jarausch J, Jaffe AS. Multicenter analytical evaluation of a high‐sensitivity troponin T assay. Clin Chim Acta 2011; 412: 748–754. [DOI] [PubMed] [Google Scholar]
- 19. Celik S, Giannitsis E, Wollert KC, Schwobel K, Lossnitzer D, Hilbel T, Lehrke S, Zdunek D, Hess A, Januzzi JL, Katus HA. Cardiac troponin T concentrations above the 99th percentile value as measured by a new high‐sensitivity assay predict long‐term prognosis in patients with acute coronary syndromes undergoing routine early invasive strategy. Clin Res Cardiol 2011; 100: 1077–1085. [DOI] [PubMed] [Google Scholar]
- 20. Januzzi JL Jr, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, Tung R, Cameron R, Nagurney JT, Chae CU, Lloyd‐Jones DM, Brown DF, Foran‐Melanson S, Sluss PM, Lee‐Lewandrowski E, Lewandrowski KB. The N‐terminal Pro‐BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 2005; 95: 948–954. [DOI] [PubMed] [Google Scholar]
- 21. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 22. Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab‐induced cardiotoxicity. J Am Soc Echocardiogr 2013; 26: 493–498. [DOI] [PubMed] [Google Scholar]
- 23. Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 2014; 63: 2751–2768. [DOI] [PubMed] [Google Scholar]
- 24. Eschenhagen T, Force T, Ewer MS, de Keulenaer GW, Suter TM, Anker SD, Avkiran M, de Azambuja E, Balligand JL, Brutsaert DL, Condorelli G, Hansen A, Heymans S, Hill JA, Hirsch E, Hilfiker‐Kleiner D, Janssens S, de Jong S, Neubauer G, Pieske B, Ponikowski P, Pirmohamed M, Rauchhaus M, Sawyer D, Sugden PH, Wojta J, Zannad F, Shah AM. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2011; 13: 1–10. [DOI] [PubMed] [Google Scholar]
- 25. Rassaf T, Totzeck M, Backs J, Bokemeyer C, Hallek M, Hilfiker‐Kleiner D, Hochhaus A, Luftner D, Muller OJ, Neudorf U, Pfister R, von Haehling S, Lehmann LH, Bauersachs J, Committee for Clinical Cardiovascular Medicine of the German Cardiac S . Onco‐Cardiology: Consensus Paper of the German Cardiac Society, the German Society for Pediatric Cardiology and Congenital Heart Defects and the German Society for Hematology and Medical Oncology. Clin Res Cardiol. 2020; 109: 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, Herrmann J, Porter C, Lyon AR, Lancellotti P, Patel A, DeCara J, Mitchell J, Harrison E, Moslehi J, Witteles R, Calabro MG, Orecchia R, de Azambuja E, Zamorano JL, Krone R, Iakobishvili Z, Carver J, Armenian S, Ky B, Cardinale D, Cipolla CM, Dent S, Jordan K, clinicalguidelines@esmo.org EGCEa . Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 2020; 31: 171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Michel L, Rassaf T, Totzeck M. Biomarkers for the detection of apparent and subclinical cancer therapy‐related cardiotoxicity. J Thorac Dis 2018; 10: S4282–S4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zardavas D, Suter TM, Van Veldhuisen DJ, Steinseifer J, Noe J, Lauer S, Al‐Sakaff N, Piccart‐Gebhart MJ, de Azambuja E. Role of Troponins I and T and N‐Terminal Prohormone of Brain Natriuretic Peptide in Monitoring Cardiac Safety of Patients With Early‐Stage Human Epidermal Growth Factor Receptor 2‐Positive Breast Cancer Receiving Trastuzumab: A Herceptin Adjuvant Study Cardiac Marker Substudy. J Clin Oncol 2017; 35: 878–884. [DOI] [PubMed] [Google Scholar]
- 29. Steinberg BA, Fang JC. Long‐Term Outcomes of Acute Heart Failure: Where Are We Now? J Am Coll Cardiol 2017; 70: 2487–2489. [DOI] [PubMed] [Google Scholar]
- 30. Giannitsis E, Biener M, Hund H, Mueller‐Hennessen M, Vafaie M, Gandowitz J, Riedle C, Lohr J, Katus HA, Stoyanov KM. Management and outcomes of patients with unstable angina with undetectable, normal, or intermediate hsTnT levels. Clin Res Cardiol 2020; 109: 476–487. [DOI] [PubMed] [Google Scholar]
- 31. Chesnaye NC, Szummer K, Barany P, Heimburger O, Magin H, Almquist T, Uhlin F, Dekker FW, Wanner C, Jager KJ, Evans M, dagger ESI, Investigatorsdagger ES . Association Between Renal Function and Troponin T Over Time in Stable Chronic Kidney Disease Patients. J Am Heart Assoc. 2019; 8: e013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suthahar N, Meems LMG, van Veldhuisen DJ, Walter JE, Gansevoort RT, Heymans S, Schroen B, van der Harst P, Kootstra‐Ros JE, van Empel V, Mueller C, Bakker SJL, de Boer RA. High‐Sensitivity Troponin‐T and Cardiovascular Outcomes in the Community: Differences Between Women and Men. Mayo Clin Proc 2020; 95: 1158–1168. [DOI] [PubMed] [Google Scholar]
- 33. Pavo N, Raderer M, Hulsmann M, Neuhold S, Adlbrecht C, Strunk G, Goliasch G, Gisslinger H, Steger GG, Hejna M, Kostler W, Zochbauer‐Muller S, Marosi C, Kornek G, Auerbach L, Schneider S, Parschalk B, Scheithauer W, Pirker R, Drach J, Zielinski C, Pacher R. Cardiovascular biomarkers in patients with cancer and their association with all‐cause mortality. Heart 2015; 101: 1874–1880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (A) Pie charts of LV‐function, measured via echocardiography at the first three consultations. Patients numbers and distribution of preserved LVEF (LVEF > 50%), mid‐range reduced LVEF (LVEF 40–50%) und reduced LVEF (LVEF < 40%) as indicated. (B) Distribution of elevated NT‐proBNP (> rule out criterion, 300 ng/l) in patients with preserved and reduced LVEF (LVEF >/≤50%). (C) Distribution of elevated hs‐cTnT (> 99 percentile, 14 ng/l) in patients with preserved and reduced LVEF. (D) Distribution of elevated hs‐cTnT with a cutoff of 7 ng/l in patients with preserved and reduced LVEF.
Figure S2. (A) Univariate logistic regression analysis of elevated hs‐cTnT (≥ 7 ng/l). Odds Ratio (OR), confidential interval and p‐value as indicated. (B) Multivariate logistic regression analysis for all‐cause mortality including the significant factors (Gender, Breast cancer, Upper GI Tumour, hs‐cTnT ≥ 7 ng/l) from the univariate analysis. Odds Ratio (OR), confidential interval and p‐value as indicated.
Figure S3. Kaplan Meier curves on all‐cause mortality (ACM). Patients are divided into three groups according to their hs‐cTnT level (< 5 ng/l, 5‐14 ng/l, > 14 ng/l). Adjustments of hs‐cTnT were performed to left ventricular function, the occurrence of diabetes or arterial hypertension, body mass index (BMI), NT‐proBNP levels, GFR, age and gender. Logrank‐Test p‐value as indicated.
Figure S4. ROC curves to test for the prediction of all‐cause mortality. Single curves are shown for hs‐cTnT, NT‐proBNP, LVEF as continuous variables and hs‐cTnT, NT‐proBNP, LVEF together. Area under the curve (AUC) as indicated.
Figure S5. Kaplan Meier curves on all‐cause mortality (ACM). Patients are divided into four groups according to a palliative and non‐palliative treatment and their hs‐cTnT level (</≥ 7 ng/l), respectively. Patient numbers per group as indicated. Logrank‐Test p‐value for the comparison of ACM in non‐palliative and palliative patients as indicated.
Figure S6. Kaplan Meier curves on all‐cause mortality (ACM). Patients are divided into two groups according to the change of hs‐cTnT between the first and the second measurement (n = 213). Patients whose hs‐cTnT was below 14 ng/l at the first visit and above 14 ng/l at the second visit are shown in turquoise. Patients with no increase from below to above 14 ng/l are shown in red. Logrank‐Test p‐value as indicated.


