Abstract
Aims
Little is known about the association of temporal changes in inflammatory biomarkers and the risk of death and cardiovascular diseases. We aimed to evaluate the association between temporal changes in C‐reactive protein (CRP), fibrinogen, and interleukin‐6 (IL‐6) and risk of heart failure (HF), cardiovascular disease (CVD), and all‐cause mortality in individuals without a history of prior CVD.
Methods and results
Participants from the Multi‐Ethnic Study of Atherosclerosis (MESA) cohort with repeated measures of inflammatory biomarkers and no CVD event prior to the second measure were included. Quantitative measures, annual change, and biomarker change categories were used as main predictors in Cox proportional hazard models stratified based on sex and statin use. A total of 2258 subjects (50.6% female, mean age of 62 years) were studied over an average of 8.1 years of follow‐up. The median annual decrease in CRP levels was 0.08 mg/L. Fibrinogen and IL‐6 levels increased by a median of 30 mg/dL and 0.24 pg/mL annually. Temporal changes in CRP were positively associated with HF risk among females (HR: 1.18 per each standard deviation increase, P < 0.001) and other CVD in both female (HR: 1.12, P = 0.004) and male participants (HR: 1.24, P = 0.003). The association of CRP change with HF and other CVD was consistently observed in statin users (HR: 1.23 per SD increase, P = 0.001 for HF and HR: 1.19 per SD increase, P < 0.001 for other CVD). There were no significant associations between temporal changes of fibrinogen or IL‐6 with HF or other CVD. Men with sustained high values of IL‐6 had a 2.3‐fold higher risk of all‐cause mortality (P < 0.001) compared with those with sustained low values.
Conclusions
Temporal change in CRP is associated with HF only in women and statin users, and other CVD in both women and men, and statin users. Annual changes in fibrinogen and IL‐6 were not predictive of cardiovascular outcomes in either sex.
Keywords: C‐reactive protein, Interleukin, Fibrinogen, Heart failure, Longitudinal cohort study
Introduction
Inflammation is a well‐established mainstay of atherosclerosis and has ongoing interaction with the entire process of atherogenesis and even atherosclerosis complications. Therefore, the difference between short‐term and long‐term effects of inflammatory markers on cardiovascular events has been an area of interest in cardiovascular care and is still a matter of debate. 1
The addition of CRP and fibrinogen to predictive models based on traditional risk factors has improved CVD risk prediction. 2 In that respect, according to the Emerging Risk Factors Collaboration, the addition of CRP and fibrinogen to models with conventional risk factors resulted in a 1.52% and 0.83% net reclassification improvement for 10‐year risk of cardiovascular events. 2 The predictive power of CRP for HF events has been consistent in diverse patient populations using models with different cutoffs. 3 Studies on the MESA population reveal independent positive associations of CRP and IL‐6 with the risk of HF. 4 Prospective follow‐up studies spanning more than 10 years in MESA demonstrated that among statin users, CRP predicts CVD modestly but is strongly associated with incident HF and all‐cause mortality. 5 Also, in non‐statin users, CRP levels did not improve the prediction of HF or other CVD events. 6 IL‐6 had independent prognostic value for HF, other CVD, and all‐cause mortality, particularly among baseline statin users. 7 Therefore, significant controversy remains on the utility of inflammatory biomarkers for CVD event prediction based on statin use and sex. 8
While the value of measuring inflammatory markers as risk predictors is widely accepted, such measures are commonly performed as single evaluations, particularly for primary prevention purposes. Prior studies involving serial biomarker determinations demonstrated an increased risk of CVD among patients with acute coronary syndrome and increasing CRP over 16 weeks. 9 Similarly, patients with a history of MI had increased CV mortality associated with increased CRP levels, longitudinally, 10 and patients with various clinical conditions and increased CRP levels had a 6.7‐fold hazard of death than those with sustained normal CRP during a 1‐year interval. 11 There is, therefore, an essential gap in the literature on the potential role of serial changes in biomarker levels for the prediction of HF and other CVD events, particularly among CVD‐free individuals.
This study aims to fill such a knowledge gap by investigating the association of longitudinal changes in inflammatory biomarkers and the risk for incident HF and other CVD, and all‐cause mortality, in a population without baseline CVD.
Method
Study population
This observational study was a nested study within the MESA cohort. The details of the MESA study design have been published previously. 12 Briefly, the baseline MESA cohort consisted of 3601 female and 3213 male participants aged 45 to 84 years, with four race/ethnic categories: non‐Hispanic White, Chinese American, African American, and Hispanic. 12 Upon recruitment, subjects were free of known cardiovascular diseases. Institutional review boards at each of the six field centres in the USA approved the study protocol, and all participants gave written informed consent.
Biomarker measurements
Although serum levels of inflammatory markers were measured in blood samples of almost all participants at baseline exam, CRP and fibrinogen measurements were repeated for 2219 and 2260 participants in the second (2002–2004), third (2004–2005), or fourth exams (2005–2007). IL‐6 concentration was re‐assessed in 1892 subjects in the third exam. All three biomarkers were measured at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT). The laboratory details on biomarker measurements and definition and diagnostic criteria of covariates are described in supporting information. Intra‐assay and inter‐assay analytical coefficient of variation (CV) for CRP measurements ranged from 2.3–4.4% and 2.1–5.7%, respectively. Intra‐assay and inter‐assay analytical CVs for fibrinogen were 2.7% and 2.6%, respectively, and the laboratory analytical CV for IL‐6 assay was 6.3%. Participants with a history of fever within the last two weeks of the baseline or second exam were excluded from the analysis to prevent the potential confounding effect of active or recent inflammation.
Outcome endpoints
Cardiovascular disease events included coronary heart disease (including myocardial infarction, definite angina, probable angina followed by revascularization), stroke, resuscitated cardiac arrest, and cardiovascular death. In this cohort, incident HF was defined as either a definite or a probable diagnosis in symptomatic patients. Definite HF indicated having evidence of either chest X‐ray‐derived pulmonary congestion/oedema, imaging‐based LV dilatation or dysfunction, or diastolic dysfunction. Probable diagnosis referred to physician‐made diagnosis or documented use of HF‐specific medication. The average follow‐up time for the included participants was 14.6 years from baseline.
Statistical analysis
To measure longitudinal changes in inflammatory biomarkers, we categorized participants based on the level of biomarkers into four categories: ‘Sustained Low’, ‘Decreased’, ‘Increased’, and ‘Sustained High’. For CRP, the increased and decreased groups were defined based on the cut‐off level of 3 mg/L. For fibrinogen and IL‐6 levels, the median value was selected as the threshold for categorization.
For survival analysis, subjects with recorded events within the time interval between baseline and the second measure were excluded. The time origin (T0) of the time‐to‐event analysis was the date of the repeated biomarker test for each individual. Missing values in covariates used for adjustment were imputed with logistic regression or mean estimation methods on a variable‐based approach. The change in biomarker values was divided by the interval and expressed as the annual change of the biomarker to address the discrepancy in time intervals between the two measures.
Cox proportional hazard models were used to investigate the time‐to‐event association of each marker with different events. We developed three models to evaluate each pair of biomarker‐event associations, including (i) categories of biomarker change, (ii) second biomarker quantitative value only, and (iii) annual change in biomarker levels with baseline value adjustment. Regarding the possible significant confounding effect of sex and statin use, stratified analyses were performed for each of the two covariates. Based on potential covariates derived from the literature, multivariable Cox‐PH models were adjusted for age, race, BMI, SBP, anti‐hypertensive medication, statin use, smoking status, physical activity, diabetes, HDL, LDL, and estimated GFR values at baseline. For models with a primary quantitative variable, hazard ratios (HR) were reported for each standard deviation (SD) increase.
To evaluate the risk assessment models' overall performance, calculated Harrell's concordance indices were utilized for comparisons. Given the two types of variation (categorized and quantitative) for each biomarker and evaluation for three events, significance for hypothesis testing was set at the 0.0083 (0.05/6) level using Bonferroni correction. All analyses were performed using R programming software (v. 3.6.1).
Results
Population characteristics
A total of 1080 women (median age of 62 years) and 1023 men (median age of 61 years) with a repeated CRP measure were studied (Table 1 ). Median (IQR) CRP at baseline and follow‐up was 2.6 (4.6) and 1.9 (3.5) mg/L in women and 1.4 (2.4) and 1.2 (1.7) mg/L in men, respectively. At baseline, 14.4% of individuals were taking statins (169 women and 134 men). Detailed population characteristics based on CRP level categories are available in Supporting Information, Table S1 . There were 388 deaths (42.8% women), 104 HF (42.3% women), and 298 other CVD events (37.2% female) within a mean follow‐up period of 8.1 years after the second measure.
Table 1.
Population structure in individuals with repeated measures of the three biomarkers
| CRP group (N = 2103) | Fibrinogen group (N = 2143) | IL‐6 group (N = 1799) | ||||
|---|---|---|---|---|---|---|
| Female (1080) | Male (1023) | Female (1092) | Male (1051) | Female (900) | Male (899) | |
| Age at baseline (years) | 62 (16) | 61 (17) | 62 (16) | 61 (17) | 63 (15) | 61 (16) |
| Race/Ethnicity | ||||||
| Caucasian | 414 (38.3) | 443 (43.3) | 422 (38.6) | 455 (43.3) | 344 (38.2) | 383 (42.6) |
| Chinese American | 136 (12.6) | 129 (12.6) | 140 (12.8) | 136 (12.9) | 117 (13.0) | 125 (13.9) |
| African American | 262 (24.3) | 198 (19.4) | 262 (24.0) | 201 (19.1) | 205 (22.8) | 166 (18.5) |
| Hispanic | 268 (24.8) | 253 (24.7) | 268 (24.5) | 259 (24.6) | 234 (26.0) | 225 (25.0) |
| Education | ||||||
| Under high school | 149 (13.8) | 107 (10.5) | 146 (13.4) | 109 (10.4) | 137 (15.2) | 101 (11.2) |
| High school to associate degree | 591 (54.8) | 456 (44.6) | 596 (54.6) | 472 (44.9) | 488 (54.3) | 394 (43.8) |
| Higher degrees | 339 (31.4) | 460 (45.0) | 349 (32.0) | 470 (44.7) | 274 (30.5) | 404 (44.9) |
| Physical activity (Minutes*MET) | 4042 (4965) | 4545 (6735) | 4016 (5010) | 4552 (6722) | 4012 (4792) | 4410 (6675) |
| SBP (mmHg) | 123 (31.5) | 122.5 (27.2) | 123 (31) | 122.5 (27.5) | 124 (31.2) | 123 (27.7) |
| Anti‐hypertensive medication use | 404 (37.4) | 327 (32.0) | 403 (36.9) | 338 (32.2) | 334 (37.1) | 305 (33.9) |
| BMI (kg/m2) | 27.5 (7.8) | 27.5 (5.5) | 27.5 (7.8) | 27.4 (5.5) | 27.2 (7.5) | 27.4 (5.6) |
| Smoking | ||||||
| Former | 309 (28.6) | 459 (44.9) | 315 (28.8) | 474 (45.1) | 257 (28.6) | 399 (44.4) |
| Current | 121 (11.2) | 137 (13.4) | 122 (11.2) | 140 (13.3) | 105 (11.7) | 120 (13.3) |
| Diabetes mellitus | 107 (9.9) | 113 (11.0) | 108 (9.9) | 116 (11.0) | 85 (9.4) | 102 (11.3) |
| HDL (mg/dL) | 54 (19) | 43 (14) | 54 (19) | 43 (14) | 54 (20) | 43 (13) |
| LDL (mg/dL) | 117 (39) | 118 (39) | 117 (39) | 118 (39) | 117 (39) | 118 (37.7) |
| GFR (mL/min/m2) | 77.8 (23.0) | 80.5 (20.7) | 77.8 (23.2) | 80.7 (20.6) | 77.2 (23.2) | 80.2 (20.9) |
| Statin use | 169 (15.6) | 134 (13.1) | 170 (15.6) | 141 (13.4) | 152 (16.9) | 124 (13.8) |
| Aspirin use | 245 (22.7) | 297 (29.0) | 252 (23.1) | 313 (29.8) | 207 (23.0) | 268 (29.8) |
| Baseline biomarker | 2.62 (4.6) | 1.42 (2.4) | 351 (94) | 322 (81) | 1.2 (1.1) | 1.1 (1) |
| Repeated biomarker | 1.9 (3.5) | 1.21 (1.7) | 434 (110.2) | 400 (98.5) | 1.9 (1.7) | 1.8 (1.6) |
| Annual change in biomarker | −0.38 (2.2) | −0.13 (1.2) | 30.0 (40.6) | 29.9 (35.3) | 0.25 (0.5) | 0.23 (0.5) |
| Events | ||||||
| HF | 44 | 60 | 44 | 61 | 38 | 57 |
| CVD | 111 | 187 | 112 | 190 | 99 | 168 |
| Death | 166 | 222 | 168 | 225 | 146 | 200 |
Variables are described as median (Inter‐quartile range [IQR]) or prevalence (%) based on the quantitative or qualitative type.
BMI, body mass index; CVD, cardiovascular disease; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; HF, heart failure; LDL, low‐density lipoprotein; MET, metabolic equivalent; SBP, systolic blood pressure.
For fibrinogen level, a total of 1092 female (median age of 62 years) and 1051 male (median age of 61 years) participants were included (Table 1 ). The prevalence of baseline statin users was 14.5% (170 women and 141 men). Median baseline and second fibrinogen levels were 351 (94) and 434 (110.2) mg/dL in women and 322 (81) and 400 (98.5) in men, respectively. Supporting Information, Table S2 provides a more detailed description of this population. In total, 393 subjects died (42.7% female), 105 had HF (41.9% female), and 302 had other CVD events (37.1% women) after the second measure of fibrinogen.
Individuals with repeated IL‐6 measures consisted of 900 female (median age of 63 years) and 899 male participants (median age of 61 years), with 15.4% using statin (152 women and 124 men) at the baseline. All subjects had normal values of IL‐6 at baseline with a median baseline and repeated IL‐6 of 1.2 and 1.9 pg/mL in women and 1.1 and 1.8 pg/mL in men, respectively. Within the follow‐up period, 346 deaths (42.2% female), 95 incident HF events (40% female), and 267 other CVD events (37% female) occurred in this population. Detailed characteristics of subjects with two recorded IL‐6 measures are shown in the Supporting Information, Table S3 .
Inflammatory biomarkers and incident heart failure
In women, both higher CRP values at the follow‐up exam (HR: 1.19 per each SD increase, P < 0.001) and annual change in CRP were associated with a higher risk of incident HF (HR: 1.18 per SD increase, P < 0.001); whereby in men, neither the recent CRP nor the change in CRP values was associated with the risk of incident HF (Table 2 and Supporting Information, Table S4 ). A descriptive illustration of the percentage of HF in follow‐up in different groups of CRP change is provided in Figure 1 A . There were no significant differences in HF hazard among different categories of CRP change for women in multivariable analysis. (Figure 2 A ) When stratified based on baseline statin use, both the second CRP measure and changes in CRP were associated with higher HF risk in statin users (HR: 1.32 per SD increase, P < 0.001 and HR: 1.23 per SD increase, P = 0.001) but not in individuals with no history of statin use at baseline (Supporting Information, Table S5 ). Models with annual changes in CRP had similar power for HF risk prediction (C‐index for women: 0.825) compared with models without CRP values (C‐index for women: 0.818) (Table 5 ). However, among statin users, models with the annual change in CRP had higher predictive strength (C‐index: 0.821) than models without CRP (C‐index: 0.781) (Supporting Information, Table S8 ).
Table 2.
Gender‐specific association of change in the three inflammatory biomarkers and risk of heart failure (HF)
| Female | Male | ||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | ||
| HR (95% CI) P value | HR (95% CI) P value | HR (95% CI) P value | HR (95% CI) P value | ||
| With categorized CRP | Sustained high | 2.42 (1.25–4.7) 0.009 | 2.56 (1.18–5.56) 0.018 | 1.85 (0.97–3.55) 0.064 | 1.23 (0.60–2.53) 0.569 |
| Increased | 0.86 (0.2–3.78) 0.846 | 1.06 (0.23–4.79) 0.938 | 0.48 (0.12–1.99) 0.312 | 0.47 (0.11–2.03) 0.315 | |
| Decreased | 1.18 (0.46–3.05) 0.727 | 1.01 (0.37–2.78) 0.976 | 1.2 (0.58–2.49) 0.618 | 0.97 (0.46–2.05) 0.933 | |
| Sustained low | Ref. | Ref. | Ref. | Ref. | |
| With change and baseline CRP | Annual change in CRP | 1.15 (1.06–1.25) < 0.001 | 1.18 (1.08–1.29) < 0.001 | 1.25 (1.03–1.52) 0.021 | 1.21 (0.98–1.49) 0.077 |
| With categorized fibrinogen | Sustained high | 1.27 (0.62–2.6) 0.521 | 0.67 (0.30–1.48) 0.323 | 1.84 (1.04–3.26) P = 0.037 | 1.00 (0.53–1.86) P = 0.994 |
| Increased | 1.09 (0.4–2.96) 0.861 | 0.93 (0.34–2.57) 0.893 | 1.94 (0.95–3.95) 0.070 | 1.40 (0.67–2.93) 0.372 | |
| Decreased | 0.81 (0.26–2.55) 0.719 | 0.56 (0.17–1.80) 0.328 | 0.4 (0.12–1.33) 0.135 | 0.26 (0.08–0.90) 0.034 | |
| Sustained low | Ref. | Ref. | Ref. | Ref. | |
| With change and baseline fibrinogen | Annual change in fibrinogen | 1.34 (1.09–1.64) 0.005 | 1.26 (0.98–1.62) 0.067 | 1.39 (1.16–1.65) p < 0.001 | 1.25 (1.01–1.56) 0.038 |
| With categorized IL‐6 | Sustained high | 1.30 (0.60–2.84) 0.502 | 0.54 (0.21–1.35) 0.188 | 4.59 (2.36–10.39) P < 0.001 | 1.84 (0.80–4.25) 0.150 |
| Increased | 2.20 (0.91–5.31) 0.079 | 1.32 (0.52–3.38) 0.559 | 2.11 (0.79–5.67) 0.138 | 1.19 (0.43–3.31) 0.735 | |
| Decreased | 0.80 (0.22–2.88) 0.735 | 0.48 (0.13–1.78) 0.270 | 2.61 (1.04–6.58) 0.042 | 1.48 (0.57–3.85) 0.421 | |
| Sustained low | Ref. | Ref. | Ref. | Ref. | |
| With change and baseline IL‐6 | Annual change in IL‐6 | 0.98 (0.71–1.34) 0.880 | 0.83 (0.55–1.26) 0.393 | 1.26 (1.07–1.48) 0.005 | 1.13 (0.94–1.36) 0.184 |
Model 1 is crude. Model 2 is adjusted for age, body mass index, race, smoking, education level, physical activity, systolic blood pressure, anti‐hypertensive medications, diabetes, statin use, glomerular filtration rate, high‐density lipoprotein, and low‐density lipoprotein. For quantitative measures, hazard ratio (HR) is reported per every standard deviation (SD) increase in the predictor.
CRP, C‐reactive protein.
Figure 1.
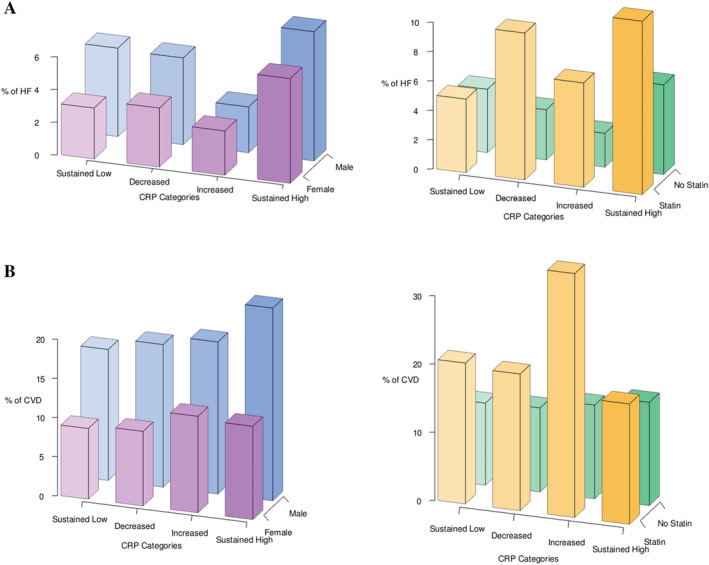
The percentage of participants with events among different categories of CRP change. (A) The percentage of participants with HF in follow‐up in different groups of change in CRP. (B) The percentage of participants with other CVD in follow‐up in different groups of change in CRP. The graphs on the left are further stratified by sex and the graphs on the right are further stratified by statin use. CRP, C‐reactive protein; HF, heart failure; CVD, cardiovascular disease.
Figure 2.
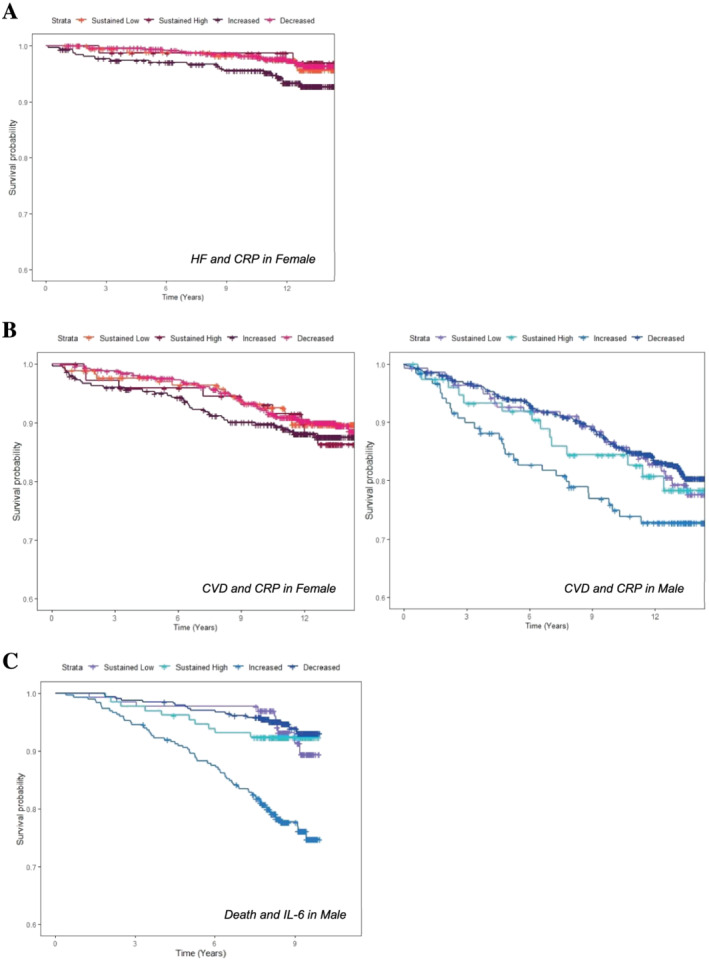
Survival curves for time‐to‐event study. (A) The survival probability in different groups of change in CRP in women and risk of HF. (B) The survival probability of different groups of change in CRP in women (left) and men (right) and risk of other CVD. (C) The survival probability in different groups of change in IL‐6 and risk of all‐cause mortality in men. CRP, C‐reactive protein; HF, heart failure; CVD, cardiovascular disease.
Table 5.
C‐statistics of adjusted models without or with biomarkers
| CRP | Fibrinogen | IL‐6 | |||||
|---|---|---|---|---|---|---|---|
| Event | Models | Female | Male | Female | Male | Female | Male |
| HF | Without biomarker | 0.818 (0.027) | 0.82 (0.023) | 0.823 (0.026) | 0.82 (0.023) | 0.834 (0.027) | 0.823 (0.024) |
| With categorized biomarker | 0.824 (0.028) | 0.821 (0.024) | 0.822 (0.026) | 0.831 (0.022) | 0.848 (0.023) | 0.828 (0.024) | |
| With recent measure only | 0.829 (0.026) | 0.825 (0.024) | 0.826 (0.025) | 0.647 (0.038) | 0.833 (0.026) | 0.835 (0.023) | |
| With annual change and baseline level | 0.825 (0.027) | 0.824 (0.023) | 0.825 (0.026) | 0.625 (0.037) | 0.863 (0.027) | 0.832 (0.023) | |
| CVD | Without biomarker | 0.728 (0.023) | 0.708 (0.018) | 0.729 (0.023) | 0.704 (0.018) | 0.721 (0.025) | 0.717 (0.018) |
| With categorized biomarker | 0.727 (0.023) | 0.715 (0.018) | 0.736 (0.021) | 0.706 (0.018) | 0.723 (0.025) | 0.717 (0.018) | |
| With recent measure only | 0.728 (0.023) | 0.717 (0.017) | 0.729 (0.023) | 0.712 (0.018) | 0.723 (0.025) | 0.717 (0.018) | |
| With annual change and baseline level | 0.728 (0.023) | 0.715 (0.017) | 0.733 (0.022) | 0.711 (0.017) | 0.722 (0.025) | 0.713 (0.018) | |
| Death | Without biomarker | 0.768 (0.018) | 0.766 (0.015) | 0.765 (0.018) | 0.768 (0.015) | 0.765 (0.02) | 0.768 (0.016) |
| With categorized biomarker | 0.769 (0.018) | 0.766 (0.015) | 0.764 (0.018) | 0.77 (0.015) | 0.77 (0.02) | 0.783 (0.015) | |
| With recent measure only | 0.768 (0.018) | 0.768 (0.015) | 0.764 (0.018) | 0.772 (0.015) | 0.765 (0.02) | 0.79 (0.015) | |
| With annual change and baseline level | 0.771 (0.018) | 0.768 (0.015) | 0.769 (0.018) | 0.771 (0.015) | 0.766 (0.02) | 0.788 (0.015) | |
Standard errors are in parentheses. Bold numbers belong to the models with significant association between event and biomarker.
After adjustment for covariates, no significant associations were observed between either single‐measure, temporal change, or median‐based categories of fibrinogen or IL‐6 change and the risk of HF in either sex (Table 2 and Supporting Information, Table S4 ). Also, changes in fibrinogen and IL‐6 were associated with HF in neither statin users nor non‐statin users (Supporting Information, Table S5 ).
To sum up, among inflammatory biomarkers, temporal change in CRP was associated with a higher risk of HF in the female population, and statin users with greater change in CRP had a higher likelihood of developing HF.
Inflammatory biomarkers and other cardiovascular disease events
In addition to single CRP measure (second measure), the annual change in CRP was associated with the risk of other CVD in both female (HR: 1.12 per SD increase, P = 0.004) and male (HR: 1.24 per SD increase, P = 0.003) MESA participants (Table 3 ). A descriptive illustration of the percentage of other CVD in follow‐up in different groups of CRP change is provided in Figure 1 B . When CRP levels were categorized based on the clinically used cut point for normality, there was no difference in the risk of other CVD between different groups in either sex (Figure 2 B ). In contrast to non‐statin users, statin users had a higher risk of other CVD with greater change in CRP values (HR: 1.19 per SD increase, P < 0.001) (Supporting Information, Table S6 ).
Table 3.
Gender‐specific association of change in the three inflammatory biomarkers and risk of other cardiovascular disease (CVD)
| Female | Male | ||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | ||
| HR (95% CI) P value | HR (95% CI) P value | HR (95% CI) P value | HR (95% CI) P value | ||
| With categorized CRP | Sustained high | 1.24 (0.8–1.9) 0.334 | 1.26 (0.77–2.06) 0.354 | 1.93 (1.32–2.82) 0.001 | 1.42 (0.94–2.14) 0.099 |
| Increased | 1.2 (0.59–2.44) 0.618 | 1.36 (0.66–2.81) 0.408 | 1.15 (0.66–2.01) 0.617 | 1.14 (0.65–2.01) 0.643 | |
| Decreased | 0.96 (0.55–1.69) 0.888 | 0.81 (0.45–1.46) 0.481 | 1.1 (0.71–1.69) 0.671 | 0.94 (0.60–1.46) 0.774 | |
| Sustained low | Ref. | Ref. | Ref. | Ref. | |
| With change and baseline CRP | Annual change in CRP | 1.12 (1.03–1.21) 0.006 | 1.12 (1.04–1.21) 0.004 | 1.30 (1.15–1.48) < 0.001 | 1.24 (1.08–1.43) 0.003 |
| With categorized fibrinogen | Sustained high | 2.64 (1.50–4.63) < 0.001 | 1.69 (0.93–3.06) 0.082 | 1.58 (1.12–2.21) 0.009 | 1.10 (0.76–1.58) 0.623 |
| Increased | 2.60 (1.32–5.12) 0.006 | 2.28 (1.14–4.54) 0.019 | 1.52 (0.98–2.36) 0.064 | 1.21 (0.77–1.89) 0.416 | |
| Decreased | 2.12 (1.03–4.40) 0.043 | 1.72 (0.82–3.61) 0.151 | 1.2 (0.78–1.86) 0.402 | 0.96 (0.61–1.50) 0.854 | |
| Sustained low | Ref. | Ref. | Ref. | Ref. | |
| With change and baseline fibrinogen | Annual change in fibrinogen | 1.18 (1.01–1.38) 0.032 | 1.08 (0.91–1.29) 0.375 | 1.28 (1.13–1.45) < 0.001 | 1.15 (1.00–1.32) 0.050 |
| With categorized IL‐6 | Sustained high | 1.49 (0.93–2.37) 0.096 | 0.85 (0.49–1.48) 0.573 | 2.78 (1.91–4.06) < 0.001 | 1.66 (1.08–2.55) 0.022 |
| Increased | 1.42 (0.76–2.64) 0.274 | 1.04 (0.54–1.98) 0.912 | 1.81 (1.11–2.94) 0.017 | 1.42 (0.86–2.35) 0.171 | |
| Decreased | 1.01 (0.49–2.08) 0.975 | 0.75 (0.36–1.57) 0.445 | 1.39 (0.82–2.36) 0.217 | 1.07 (0.62–1.83) 0.810 | |
| Sustained low | Ref. | Ref. | Ref. | Ref. | |
| With change and baseline IL‐6 | Annual change in IL‐6 | 1.09 (0.93–1.29) 0.285 | 1.05 (0.85–1.29) 0.669 | 1.23 (1.11–1.36) < 0.001 | 1.11 (0.99–1.25) 0.076 |
Model 1 is crude. Model 2 is adjusted for age, body mass index, race, smoking, education level, physical activity, systolic blood pressure, anti‐hypertensive medications, diabetes, statin use, glomerular filtration rate, high‐density lipoprotein, and low‐density lipoprotein. For quantitative measures, hazard ratio (HR) is reported per every standard deviation (SD) increase in the predictor.
CRP, C‐reactive protein.
Like HF, neither single measure nor change in fibrinogen was associated with other CVD in either sex (Table 3 and Supporting Information, Table S4 ). The change in fibrinogen was not associated with other CVD in statin‐use strata (Supporting Information, Table S6 ).
The interleukin‐6 level at the follow‐up exam was associated with other CVD (HR: 1.22 per SD increase, P = 0.004) in male participants (Supporting Information, Table S4 ); however, temporal change in IL‐6 was not associated with the risk of other CVD in either sex or strata of statin use (Table 3 and Supporting Information, Table S6 ).
To summarize, among the three inflammatory biomarkers, temporal change in CRP was associated with a higher risk of other CVD in female and male participants, and statin users with greater change in CRP values had a higher risk of HF development.
Inflammatory biomarkers and all‐cause mortality
There were no significant associations between repeated measures of CRP or fibrinogen and the risk of all‐cause mortality in either sex (Table 4 ). Similarly, the hazard of death was not different between categories of CRP or fibrinogen change. Even after stratification for statin use, no associations were detected (Supporting Information, Table S7 ).
Table 4.
Gender‐specific association of change in the three inflammatory biomarkers and risk of all‐cause mortality
| Female | Male | ||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | ||
| HR (95% CI) P value | HR (95% CI) P value | HR (95% CI) P value | HR (95% CI) P value | ||
| With categorized CRP | Sustained high | 1.18 (0.83–1.69) 0.354 | 1.22 (0.80–1.84) 0.353 | 1.62 (1.13–2.33) 0.008 | 1.13 (0.76–1.67) 0.546 |
| Increased | 0.52 (0.23–1.19) 0.121 | 0.61 (0.26–1.41) 0.247 | 0.95 (0.56–1.63) 0.863 | 1.00 (0.58–1.73) 0.990 | |
| Decreased | 1.35 (0.9–2.03) 0.146 | 1.25 (0.81–1.92) 0.319 | 1.26 (0.87–1.83) 0.217 | 1.11 (0.76–1.63) 0.592 | |
| Sustained low | Ref. | Ref. | Ref. | Ref. | |
| With change and baseline CRP | Annual change in CRP | 1.04 (0.95–1.13) 0.397 | 1.04 (0.97–1.12) 0.244 | 1.15 (1.01–1.32) 0.032 | 1.05 (0.91–1.20) 0.510 |
| With categorized fibrinogen | Sustained high | 1.61 (1.08–2.39) 0.018 | 1.05 (0.69–1.60) 0.824 | 1.96 (1.44–2.68) < 0.001 | 1.25 (0.90–1.75) 0.185 |
| Increased | 1.01 (0.57–1.81) 0.967 | 0.78 (0.43–1.41) 0.410 | 1.91 (1.28–2.85) 0.001 | 1.40 (0.93–2.11) 0.106 | |
| Decreased | 1.82 (1.1–3.02) 0.020 | 1.42 (0.84–2.38) 0.186 | 1.24 (0.82–1.9) 0.309 | 0.97 (0.63–1.49) 0.883 | |
| Sustained low | Ref. | Ref. | Ref. | Ref. | |
| With change and baseline fibrinogen | Annual change in fibrinogen | 1.11 (0.98–1.25) 0.114 | 0.94 (0.81–1.09) 0.395 | 1.33 (1.21–1.47) < 0.001 | 1.10 (0.98–1.24) 0.104 |
| With categorized IL‐6 | Sustained high | 1.88 (1.29–2.75) 0.001 | 1.04 (0.66–1.63) 0.872 | 4.13 (2.87–5.93) < 0.001 | 2.33 (1.56–3.48) < 0.001 |
| Increased | 0.94 (0.51–1.73) 0.847 | 0.61 (0.33–1.15) 0.127 | 1.59 (0.95–2.66) 0.077 | 1.18 (0.69–2.00) 0.542 | |
| Decreased | 0.93 (0.50–1.73) 0.813 | 0.66 (0.34–1.25) 0.202 | 1.31 (0.76–2.25) 0.325 | 1.07 (0.62–1.86) 0.805 | |
| Sustained low | Ref. | Ref. | Ref. | Ref. | |
| With change and baseline IL‐6 | Annual change in IL‐6 | 1.05 (0.90–1.21) 0.549 | 0.91 (0.75–1.10) 0.346 | 1.29 (1.16–1.44) < 0.001 | 1.18 (1.04–1.34) 0.010 |
Model 1 is crude. Model 2 is adjusted for age, body mass index, race, smoking, education level, physical activity, systolic blood pressure, anti‐hypertensive medications, diabetes, statin use, glomerular filtration rate, high‐density lipoprotein, and low‐density lipoprotein. For quantitative measures, hazard ratio (HR) is reported per every standard deviation (SD) increase in the predictor.
CRP, C‐reactive protein.
Conversely, IL‐6 level at follow‐up exam was associated with all‐cause mortality (HR: 1.44 per SD increase, P < 0.001) in male but not female MESA participants. Men with repeated measures of IL‐6 that were greater than median levels had a 2.33‐fold risk of death (P < 0.001), compared with men with sustained low levels of IL‐6. However, there was no association between the quantitative change in IL‐6 and mortality (Table 4 ). The C‐statistic of models with categorized IL‐6 for death improved to 0.783 from 0.768 in models without IL‐6 (Table 5 ). Moreover, in non‐statin users, the annual change in IL‐6 values was associated with mortality (HR: 1.17 per SD increase, P = 0.006); wherein subjects with sustained high IL‐6 values had a higher risk of all‐cause mortality (HR: 2.17, P = 0.001) (Supporting Information, Table S7, and Figure 2 C ).
To sum up, male participants and non‐statin users with sustained high IL‐6 levels had a higher likelihood of all‐cause mortality.
Discussion
In this longitudinal study, we demonstrated that (i) the temporal change in CRP is associated with time‐to‐HF in women; (ii) the addition of CRP to prediction models without inflammatory biomarkers increases the predictive adequacy of HF development in women; (iii) the temporal change in CRP predicts the incidence of other CVDs in both sexes; (iv) the association of annual change in CRP with HF and other CVDs was specific to statin users; (v) in non‐statin users, temporal change in IL‐6 was a predictor of all‐cause mortality.
Few studies have investigated the relationship between temporal changes in inflammatory biomarkers and cardiovascular events. A survey of 5811 patients with one year apart CRP measurements (standard cutoff: 3 mg/L) showed that patients with sustained abnormal, increased, or decreased CRP values had an all‐cause mortality HR of 7.73 (95% CI: 2.89–20.69), 6.70 (2.42–18.59), and 3.55 (1.24–10.12) compared with the sustained normal group. 11 However, the latter focused on patients with subacute baseline CRP levels (1–10 mg/L) and did not evaluate the event association with quantitative changes in CRP measures. An ancillary survey of the CHART‐2 (Chronic Heart Failure Registry and Analysis in the Tohoku district‐2) study have reported that within a median follow‐up of 6.4 years, the age and sex‐adjusted risk of CVD and all‐cause mortality were higher in the sustained high group compared to the decreased group (HR: 1.70, 95% CI: 1.28–2.26 for all‐cause and HR: 2.45, 95% CI: 1.23–4.88 for cardiovascular death), and in the increased group compared with the sustained low group ((HR: 1.59, 95% CI: 1.18–2.13 for all‐cause and HR: 1.91, 95% CI: 1.21–3.02 for cardiovascular death). 10 In contrast to that study, we excluded patients with a history of CVD prior to the second CRP measurement. The Atherosclerosis Risk in Communities (ARIC) study used a larger sample size (10 160 individuals) and a wider interval between the repeated measures of CRP (6 years). 13 They reported that with cutoff points of either 3 or 2 mg/L, changes in CRP values are associated with HF, CHD, and mortality. Individuals with repeated high measures had the highest HR among different groups. 13 However, in neither of these studies, the quantitative change in CRP was set as the predictor.
Inflammatory biomarkers not only have been well‐known as predictors of incident HF, especially in older adults, 14 , 15 but also CRP level has been an independent biomarker for morbidity and mortality of HF. 16 , 17 Higher CRP levels being associated with earlier first morbid events (all‐cause death, sudden death with resuscitation, hospitalization for HF, or administration of an intravenous inotropic or vasodilator drug) 16 and higher risk of cardiovascular and non‐cardiovascular death. 17 Consistent with observations of this study, HF prediction by CRP was the most robust among these three markers. 18 Our findings are in agreement with a study evaluating the predictive power of baseline CRP for HF in the same cohort that existed in statin users but not non‐statin users. 5 Moreover, a multi‐cohort study (including MESA) of inflammatory biomarkers and subtypes of HF showed that increasing CRP level is associated with a higher pooled hazard ratio for incident HF with reduced ejection fraction compared to HF with preserved ejection fraction. 18 On the other hand, the predictive effect of IL‐6 and fibrinogen for HF was not significantly different between reduced vs. preserved ejection fraction. 18 The underlying mechanism for increased inflammation in HF is still unclear. It is proposed that the haemodynamic imbalance caused by HF leads to an inflammatory response and increases the concentration of pro‐inflammatory cytokines like IL‐6. 19 Nonetheless, there is still debate on whether the increased level of inflammatory biomarkers is a response to an established HF state, a predecessor to HF development, or a bidirectional association. 20 Previous imaging‐based studies have declared that CRP is independently associated with myocardial function and progressive deterioration of function over time, partly explaining why this biomarker is a predictor of HF. 21 Based on the literature, the association between baseline CRP and the ventricular strain was prominent in men. 22 Our findings showed that the association between single‐measure CRP and HF faded after adjusting cardiovascular risk factors in the male population. Obesity is a well‐known risk factor for HF development, and inflammation is the major pathway through which obesity causes LV remodelling. 23 Based on the latter, we have adjusted our models for BMI, and the reported associations in multivariable analysis were independent of BMI.
In addition to HF, a meta‐analysis of 54 studies showed that CRP concentration is a predictor of cardiovascular diseases and all‐cause mortality, 24 yet the exact pathology is unclear. Prediction models using machine learning applied on the MESA population's deep phenotyping have also identified inflammatory markers as top predictors of HF, CVD, and mortality. 25 The Emerging Risk Factors Collaboration aggregated 52 studies on individuals without a history of CVD and reported that CRP is a preventive tool for 10 year CVD risk. 26 The addition of CRP to traditional risk assessment reclassified 5.2% of intermediate‐risk individuals to a 10‐year CVD risk of 20% or more, which would indicate statin therapy based on ATP (Adult Treatment Plan) III. 2 A prospective study on the MESA cohort also reported that individuals with high CRP values would require statin therapy at an average of 4.5 years. 27 In this study, even among those who were taking statins, longitudinal change in CRP values was associated with other CVD events in the follow‐up period.
Higher fibrinogen values, even within the normal range, were reported to be associated with an increased risk of CVD since more than three decades ago. 28 , 29 Fibrinogen Studies Collaboration pooled the results of 31 prospective studies in healthy adults and revealed a strong association between plasma fibrinogen and the risk of CHD, stroke, and mortality. 30 According to the Emerging Risk Factors Collaboration, the addition of fibrinogen to risk assessment models for cardiovascular event increases the predictive adequacy of models for low, intermediate, or high‐risk individuals and reclassifies 4.7% of intermediate‐risk individuals based on traditional risk factors to individuals who would need to begin statin therapy. 2 As a surrogate for myocardial dysfunction, LV circumferential strain measured by CMR was inversely associated with fibrinogen levels in MESA. 31 Changes in fibrinogen levels over a 13‐year interval were associated with changes in traditional risk factors, including BMI, serum lipid levels, diastolic blood pressure, and physical activity. 32 To our knowledge, this is the first study investigating the association of change in fibrinogen level, as well as IL‐6, with incident HF, CVD, and mortality. Based on our observations, annual changes in fibrinogen levels were not predictive of events in either sex, implying a lack of any added value in repeated measurements of fibrinogen. We only observed a significant association between single fibrinogen measurements and the risk of other CVD and all‐cause mortality in non‐statin users.
MESA‐derived studies also reported that baseline IL‐6 measures had an added value in predicting HF, CVD, and all‐cause mortality, especially in statin users. 7 In line with that, we found that second measures of IL‐6 were predictive of CVD and mortality, especially in the male population. A CMR‐based cross‐sectional study in a subpopulation of the MESA cohort showed an independent negative association between single IL‐6 values and morphological features of both ventricles, regional and global LV systolic function, as well as myocardial fibrosis measured by extracellular volume extent. 33 , 34 , 35 We have reported for the first time that in addition to a single IL‐6 measure, the annual change of IL‐6 is predictive of mortality in male subjects, and this association is more profound in non‐stain users. However, all IL‐6 values in this cohort were within the normal range.
Study limitations
This study's main limitation was that repeated measures of biomarkers were not available for the entire cohort, and we based our analysis on a subgroup of the MESA cohort. Also, the diagnosis of HF was not uniformly defined in all patients, with some having a definite imaging‐based diagnosis and some with probable diagnosis based on patient history and medications. Moreover, the aetiology of HF and ejection fraction at the time of diagnosis were not available for a remarkable number of participants, we could not perform subgroup analysis to compare ischaemic vs. non‐ischaemic cardiomyopathy and HF with preserved ejection fraction vs. HF with reduced ejection fraction. Another limitation of this study was the low number of events in some groups after categorization, which was partly compensated for by adding models with continuous measures. Similar to all longitudinal analyses, the selection of covariates among different time points of measurement was controversial. We chose the baseline measurements to have the lowest number of missing values for each covariate.
Conclusions
In this study, we demonstrated sex‐specific associations of temporal changes in inflammatory biomarkers with the risk of CVD. CRP changes augmented predictive power for incident HF in women and CVD risk assessment in both sexes. The predictive role of change in CRP was stronger in statin users than non‐users. We also observed a non‐statin user‐specific association of IL‐6 change with mortality. Changes in the fibrinogen levels were not associated with cardiovascular events. Further prospective large‐sample studies should be conducted to evaluate the association of temporal changes in different inflammatory biomarkers with cardiovascular events.
Conflict of interest
Authors of this work has nothing to disclose. All authors declare that the submitted work is original and has not been published and is not under consideration for publication elsewhere. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services.
Funding
This research was supported by contracts N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168 and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute and by grants UL1‐TR‐000040 and UL1‐TR‐001079 from National Center for Research Resources.
Supporting information
Table S1. Population structure in individuals with repeated CRP measures.
Table S2. Population structure in individuals with repeated fibrinogen measures.
Table S3. Population structure in individuals with repeated IL‐6 measures.
Table S4. Association of recent measure of inflammatory biomarkers with events, in this population, within the strata of gender.
Table S5. Association of single measure and annual changes in inflammatory biomarkers and risk of heart failure (HF) within strata of statin use.
Table S6. Association of single measure and annual changes in inflammatory biomarkers and risk of other cardiovascular disease (CVD) within strata of statin use.
Table S7. Association of single measure and annual changes in inflammatory biomarkers and risk of all‐cause mortality within strata of statin use.
Table S8. C‐statistics of adjusted models without or with biomarkers in strata of statin use.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa‐nhlbi.org.
Shabani, M. , Bakhshi, H. , Ostovaneh, M. R. , Ma, X. , Wu, C. O. , Ambale‐Venkatesh, B. , Blaha, M. J. , Allison, M. A. , Budoff, M. J. , Cushman, M. , Tracy, R. P. , Herrington, D. M. , Szklo, M. , Cox, C. , Bluemke, D. A. , and Lima, J. A. C. (2021) Temporal change in inflammatory biomarkers and risk of cardiovascular events: the Multi‐ethnic Study of Atherosclerosis. ESC Heart Failure, 8: 3769–3782. 10.1002/ehf2.13445.
References
- 1. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107: 499–511. [DOI] [PubMed] [Google Scholar]
- 2. Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, Butterworth AS, Amouyel P, Assmann G, Bakker SJ, Barr EL, Barrett‐Connor E, Benjamin EJ, Björkelund C, Brenner H, Brunner E, Clarke R, Cooper JA, Cremer P, Cushman M, Dagenais GR, D'Agostino RB, Sr Dankner R, Davey‐Smith G, Deeg D, Dekker JM, Engström G, Folsom AR, Fowkes FG, Gallacher J, Gaziano JM, Giampaoli S, Gillum RF, Hofman A, Howard BV, Ingelsson E, Iso H, Jørgensen T, Kiechl S, Kitamura A, Kiyohara Y, Koenig W, Kromhout D, Kuller LH, Lawlor DA, Meade TW, Nissinen A, Nordestgaard BG, Onat A, Panagiotakos DB, Psaty BM, Rodriguez B, Rosengren A, Salomaa V, Kauhanen J, Salonen JT, Shaffer JA, Shea S, Ford I, Stehouwer CD, Strandberg TE, Tipping RW, Tosetto A, Wassertheil‐Smoller S, Wennberg P, Westendorp RG, Whincup PH, Wilhelmsen L, Woodward M, Lowe GD, Wareham NJ, Khaw KT, Sattar N, Packard CJ, Gudnason V, Ridker PM, Pepys MB, Thompson SG, Danesh J. C‐reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 2012; 367: 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Araujo JP, Lourenco P, Azevedo A, Frioes F, Rocha‐Goncalves F, Ferreira A, Bettencourt P. Prognostic value of high‐sensitivity C‐reactive protein in heart failure: a systematic review. J Card Fail 2009; 15: 256–266. [DOI] [PubMed] [Google Scholar]
- 4. Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd‐Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi‐Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008; 51: 1775–1783. [DOI] [PubMed] [Google Scholar]
- 5. Cainzos‐Achirica M, Miedema MD, McEvoy JW, Cushman M, Dardari Z, Greenland P, Nasir K, Budoff MJ, Al‐Mallah MH, Yeboah J, Blumenthal RS, Comin‐Colet J, Blaha MJ. The prognostic value of high sensitivity C‐reactive protein in a multi‐ethnic population after >10 years of follow‐up: the Multi‐Ethnic Study of Atherosclerosis (MESA). Int J Cardiol 2018; 264: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, Blaha MJ, Miedema MD, Sibley CT, Carr JJ, Burke GL, Goff DC Jr, Psaty BM, Greenland P, Herrington DM. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol 2016; 67: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cainzos‐Achirica M, Enjuanes C, Greenland P, McEvoy JW, Cushman M, Dardari Z, Nasir K, Budoff MJ, Al‐Mallah MH, Yeboah J, Miedema MD, Blumenthal RS, Comin‐Colet J, Blaha MJ. The prognostic value of interleukin 6 in multiple chronic diseases and all‐cause death: the Multi‐Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2018; 278: 217–225. [DOI] [PubMed] [Google Scholar]
- 8. Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, Blumenthal RS, Budoff MJ. High‐sensitivity C‐reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol 2013; 62: 397–408. [DOI] [PubMed] [Google Scholar]
- 9. Mani P, Puri R, Schwartz GG, Nissen SE, Shao M, Kastelein JJP, Menon V, Lincoff AM, Nicholls SJ. Association of initial and serial C‐reactive protein levels with adverse cardiovascular events and death after acute coronary syndrome: a secondary analysis of the VISTA‐16 trial. JAMA Cardiol 2019; 4: 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oikawa T, Sakata Y, Nochioka K, Miura M, Abe R, Kasahara S, Sato M, Aoyanagi H, Saga C, Ikeno Y, Shiroto T, Sugimura K, Takahashi J, Miyata S, Shimokawa H. Association between temporal changes in C‐reactive protein levels and prognosis in patients with previous myocardial infarction—a report from the CHART‐2 Study. Int J Cardiol 2019; 293: 17–24. [DOI] [PubMed] [Google Scholar]
- 11. Currie CJ, Poole CD, Conway P. Evaluation of the association between the first observation and the longitudinal change in C‐reactive protein, and all‐cause mortality. Heart 2008; 94: 457–462. [DOI] [PubMed] [Google Scholar]
- 12. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002; 156: 871–881. [DOI] [PubMed] [Google Scholar]
- 13. Parrinello CM, Lutsey PL, Ballantyne CM, Folsom AR, Pankow JS, Selvin E. Six‐year change in high‐sensitivity C‐reactive protein and risk of diabetes, cardiovascular disease, and mortality. Am Heart J 2015; 170: 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton‐Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation 2003; 108: 2317–2322. [DOI] [PubMed] [Google Scholar]
- 15. Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol 2010; 55: 2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anand IS, Latini R, Florea VG, Kuskowski MA, Rector T, Masson S, Signorini S, Mocarelli P, Hester A, Glazer R, Cohn JN. C‐reactive protein in heart failure. Circulation 2005; 112: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 17. Pellicori P, Zhang J, Cuthbert J, Urbinati A, Shah P, Kazmi S, Clark AL, Cleland JGF. High‐sensitivity C‐reactive protein in chronic heart failure: patient characteristics, phenotypes, and mode of death. Cardiovasc Res 2020; 116: 91–100. [DOI] [PubMed] [Google Scholar]
- 18. de Boer RA, Nayor M, de Filippi CR, Enserro D, Bhambhani V, Kizer JR, Blaha MJ, Brouwers FP, Cushman M, Lima JAC, Bahrami H, van der Harst P, Wang TJ, Gansevoort RT, Fox CS, Gaggin HK, Kop WJ, Liu K, Vasan RS, Psaty BM, Lee DS, Hillege HL, Bartz TM, Benjamin EJ, Chan C, Allison M, Gardin JM, Januzzi JL, Jr Shah SJ, Levy D, Herrington DM, Larson MG, van Gilst WH, Gottdiener JS, Bertoni AG, Ho JE. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiology 2018; 3: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakayama H, Otsu K. Translation of hemodynamic stress to sterile inflammation in the heart. Trends Endocrinol Metab 2013; 24: 546–553. [DOI] [PubMed] [Google Scholar]
- 20. Murphy SP, Kakkar R, McCarthy CP, Januzzi JL. Inflammation in heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol 2020; 75: 1324–1340. [DOI] [PubMed] [Google Scholar]
- 21. Choi EY, Yan RT, Fernandes VR, Opdahl A, Gomes AS, Almeida AL, Wu CO, Liu K, Carr JJ, McClelland RL, Bluemke DA, Lima JA. High‐sensitivity C‐reactive protein as an independent predictor of progressive myocardial functional deterioration: the multiethnic study of atherosclerosis. Am Heart J 2012; 164: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosen BD, Cushman M, Nasir K, Bluemke DA, Edvardsen T, Fernandes V, Lai S, Tracy RP, Lima JA. Relationship between C‐reactive protein levels and regional left ventricular function in asymptomatic individuals: the Multi‐Ethnic Study of Atherosclerosis. J Am Coll Cardiol 2007; 49: 594–600. [DOI] [PubMed] [Google Scholar]
- 23. Triposkiadis F, Xanthopoulos A, Starling RC, Iliodromitis E. Obesity, inflammation, and heart failure: links and misconceptions. Heart Fail Rev 2021. 10.1007/s10741-021-10103-y [DOI] [PubMed] [Google Scholar]
- 24. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C‐reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta‐analysis. Lancet 2010; 375: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ambale‐Venkatesh B, Yang X, Wu CO, Liu K, Hundley WG, McClelland R, Gomes AS, Folsom AR, Shea S, Guallar E, Bluemke DA, Lima JAC. Cardiovascular event prediction by machine learning: the Multi‐Ethnic Study of Atherosclerosis. Circ Res 2017; 121: 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, Butterworth AS, Amouyel P, Assmann G, Bakker SJ, Barr EL, Barrett‐Connor E, Benjamin EJ, Bjorkelund C, Brenner H, Brunner E, Clarke R, Cooper JA, Cremer P, Cushman M, Dagenais GR, D'Agostino RB Sr, Dankner R, Davey‐Smith G, Deeg D, Dekker JM, Engstrom G, Folsom AR, Fowkes FG, Gallacher J, Gaziano JM, Giampaoli S, Gillum RF, Hofman A, Howard BV, Ingelsson E, Iso H, Jorgensen T, Kiechl S, Kitamura A, Kiyohara Y, Koenig W, Kromhout D, Kuller LH, Lawlor DA, Meade TW, Nissinen A, Nordestgaard BG, Onat A, Panagiotakos DB, Psaty BM, Rodriguez B, Rosengren A, Salomaa V, Kauhanen J, Salonen JT, Shaffer JA, Shea S, Ford I, Stehouwer CD, Strandberg TE, Tipping RW, Tosetto A, Wassertheil‐Smoller S, Wennberg P, Westendorp RG, Whincup PH, Wilhelmsen L, Woodward M, Lowe GD, Wareham NJ, Khaw KT, Sattar N, Packard CJ, Gudnason V, Ridker PM, Pepys MB, Thompson SG, Danesh J. C‐reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 2012; 367: 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mann DM, Shimbo D, Cushman M, Lakoski S, Greenland P, Blumenthal RS, Michos ED, Lloyd‐Jones DM, Muntner P. C‐reactive protein level and the incidence of eligibility for statin therapy: the Multi‐Ethnic Study of Atherosclerosis. Clin Cardiol 2013; 36: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kannel WB, D'Agostino RB, Belanger AJ. Update on fibrinogen as a cardiovascular risk factor. Ann Epidemiol 1992; 2: 457–466. [DOI] [PubMed] [Google Scholar]
- 29. Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: a meta‐analysis and review of the literature. Ann Intern Med 1993; 118: 956–963. [DOI] [PubMed] [Google Scholar]
- 30. Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, Goldbourt U, Willeit J, Kiechl S, Yarnell JW, Sweetnam PM, Elwood PC, Cushman M, Psaty BM, Tracy RP, Tybjaerg‐Hansen A, Haverkate F, de Maat MP, Fowkes FG, Lee AJ, Smith FB, Salomaa V, Harald K, Rasi R, Vahtera E, Jousilahti P, Pekkanen J, D'Agostino R, Kannel WB, Wilson PW, Tofler G, Arocha‐Piñango CL, Rodriguez‐Larralde A, Nagy E, Mijares M, Espinosa R, Rodriquez‐Roa E, Ryder E, Diez‐Ewald MP, Campos G, Fernandez V, Torres E, Marchioli R, Valagussa F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Cremer P, Nagel D, Curb JD, Rodriguez B, Yano K, Salonen JT, Nyyssönen K, Tuomainen TP, Hedblad B, Lind P, Loewel H, Koenig W, Meade TW, Cooper JA, De Stavola B, Knottenbelt C, Miller GJ, Cooper JA, Bauer KA, Rosenberg RD, Sato S, Kitamura A, Naito Y, Palosuo T, Ducimetiere P, Amouyel P, Arveiler D, Evans AE, Ferrieres J, Juhan‐Vague I, Bingham A, Schulte H, Assmann G, Cantin B, Lamarche B, Després JP, Dagenais GR, Tunstall‐Pedoe H, Woodward M, Ben‐Shlomo Y, Davey Smith G, Palmieri V, Yeh JL, Rudnicka A, Ridker P, Rodeghiero F, Tosetto A, Shepherd J, Ford I, Robertson M, Brunner E, Shipley M, Feskens EJ, Kromhout D, Dickinson A, Ireland B, Juzwishin K, Kaptoge S, Lewington S, Memon A, Sarwar N, Walker M, Wheeler J, White I, Wood A. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta‐analysis. Jama 2005; 294: 1799–1809. [DOI] [PubMed] [Google Scholar]
- 31. Yan RT, Fernandes V, Yan AT, Cushman M, Redheuil A, Tracy R, Vogel‐Claussen J, Bahrami H, Nasir K, Bluemke DA, Lima JA. Fibrinogen and left ventricular myocardial systolic function: the Multi‐Ethnic Study of Atherosclerosis (MESA). Am Heart J 2010; 160: 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okwuosa TM, Klein O, Chan C, Jenny NS, Schreiner P, Green D, Liu K. 13‐year long‐term associations between changes in traditional cardiovascular risk factors and changes in fibrinogen levels: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Atherosclerosis 2013; 226: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yan AT, Yan RT, Cushman M, Redheuil A, Tracy RP, Arnett DK, Rosen BD, McClelland RL, Bluemke DA, Lima JA. Relationship of interleukin‐6 with regional and global left‐ventricular function in asymptomatic individuals without clinical cardiovascular disease: insights from the Multi‐Ethnic Study of Atherosclerosis. Eur Heart J 2010; 31: 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harhay MO, Tracy RP, Bagiella E, Barr RG, Pinder D, Hundley WG, Bluemke DA, Kronmal RA, Lima JA, Kawut SM. Relationship of CRP, IL‐6, and fibrinogen with right ventricular structure and function: the MESA‐Right Ventricle Study. Int J Cardiol 2013; 168: 3818–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marques MD, Nauffal V, Ambale‐Venkatesh B, Vasconcellos HD, Wu C, Bahrami H, Tracy RP, Cushman M, Bluemke DA, Lima JAC. Association between inflammatory markers and myocardial fibrosis. Hypertension 2018; 72: 902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Population structure in individuals with repeated CRP measures.
Table S2. Population structure in individuals with repeated fibrinogen measures.
Table S3. Population structure in individuals with repeated IL‐6 measures.
Table S4. Association of recent measure of inflammatory biomarkers with events, in this population, within the strata of gender.
Table S5. Association of single measure and annual changes in inflammatory biomarkers and risk of heart failure (HF) within strata of statin use.
Table S6. Association of single measure and annual changes in inflammatory biomarkers and risk of other cardiovascular disease (CVD) within strata of statin use.
Table S7. Association of single measure and annual changes in inflammatory biomarkers and risk of all‐cause mortality within strata of statin use.
Table S8. C‐statistics of adjusted models without or with biomarkers in strata of statin use.


