Abstract
Aims
Pulmonary artery pulsatility index (PAPi), defined as [(pulmonary artery systolic pressure − diastolic pulmonary artery pressure)/mean right atrial pressure], is a novel haemodynamic index that predicts right ventricular failure after myocardial infarction and left ventricular assist device implantation. We analysed if a low PAPi is associated with death in our 14 ‐ year pulmonary arterial hypertension (PAH) registry.
Methods
Consecutive patients with newly diagnosed PAH and complete haemodynamic data were prospectively enrolled into our standing registry between January 2003 and December 2016. PAPi was calculated from baseline invasive right heart catheterization data. A prognostic cut‐off value was determined with a decision tree. Baseline characteristics of ‘high’ and ‘low’ PAPi groups based on this cut‐off were compared, as well as odds of death and time‐to‐death.
Results
One hundred and two patients were included. Mean age was 53 years, and 77% were women. Our multi‐ethnic cohort was 64% Chinese, 23% Malay, and 10% Indian. The aetiologies were idiopathic (33%), connective tissue disease (31%), congenital heart disease (24%), and others (12%). The low PAPi group (<5.3) had a greater age (56 years vs. 49 years), lower pulmonary artery systolic pressure (71 mmHg vs. 85 mmHg), and higher mean right atrial pressure (14 mmHg vs. 6 mmHg). Mortality risk was higher in the low PAPi group (adjusted odds ratio: 2.98 and adjusted hazard ratio: 2.23). Mean right atrial pressure was the strongest predictor (hazard ratio 1.114, P = 0.009) when components of PAPi were analysed.
Conclusions
Pulmonary artery pulsatility index was found to be predictive of mortality in PAH and may be a valuable marker for risk stratification. Its prognostic strength may be driven by mean right atrial pressure.
Keywords: Pulmonary hypertension, PAPI, Survival, Risk factors, Registries
Introduction
Pulmonary arterial hypertension (PAH) is a rapidly progressive disease of the pulmonary vasculature that leads to right ventricular (RV) dysfunction and death. 1 , 2 It is highly morbid and incurable, although survival has improved remarkably in the modern era with advances in PAH therapy. 3 Accurate and comprehensive risk prediction is critical in guiding treatment decision. The search for an ideal predictor of outcome has led to the growing attention on various risk stratification models and metrics that may discriminate prognosis. 4 , 5 We have previously published survival outcomes and validated the use of the REVEAL risk score in our PAH cohort. 6
The pulmonary artery pulsatility index (PAPi) is a novel haemodynamic index that indexes pulmonary artery pulse pressure (PAPP) as a surrogate of cardiac output over right heart filling pressure and is defined as [(pulmonary artery systolic pressure (PASP) − pulmonary artery diastolic pressure (PADP))/mean right atrial pressure (mRAP)]. It has been shown to predict RV failure in patients with acute inferior myocardial infarction 7 and after left ventricular assist device implantation. 8 , 9 , 10 , 11
Pulmonary artery pulsatility index as a risk predictor in PAH has also been investigated in a cohort comprising only idiopathic, familial, and anorexigen‐associated aetiologies and found to be significantly associated with mortality. The main limitations of this study were narrow inclusion criteria, lack of echocardiographic correlates, and lack of contemporaneous data; data utilized for haemodynamic analysis was referenced from the National Institutes of Health Registry for Primary Pulmonary Hypertension, which was published in the 1980s. 12
No studies have generalized these results to a multi‐ethnic Asian PAH cohort, with underlying aetiologies including idiopathic PAH, congenital heart disease associated PAH, connective tissue disease associated PAH, pulmonary veno‐occlusive disease, among others.
Our hypothesis is that this index would be predictive for mortality in a generalized PAH population such as ours. In this study, we analysed if a low PAPi was associated with death over a 14 year follow‐up in our PAH registry and compared it against conventional risk predictors.
Methods
Study design
The enrolment details, patient characteristics, and survival outcomes of our PAH registry have previously been published. 6 We consecutively recruited all adult patients (≥18 years old) with newly diagnosed PAH [World Health Organization (WHO) Group 1 pulmonary hypertension] when they presented to our pulmonary hypertension specialty centre between 1 January 2003 and 29 December 2016. The diagnosis of PAH was defined as mean pulmonary arterial pressure greater than 25 mmHg at rest and pulmonary capillary wedge pressure less than 15 mmHg on right heart catheterization. Pulmonary hypertension specialists allocated the cases to the correct WHO group prospectively through usual clinical algorithms. 13 All cases were reviewed by a second specialist for appropriate allocation and a third adjudicated if there were discrepancies between the former two. Patients with incomplete invasive haemodynamic data or follow‐up were excluded.
Demographics, clinical parameters, WHO functional status, and treatment on recruitment were obtained from hospital electronic medical records. Invasive haemodynamic data were obtained from right heart catheterization performed by pulmonary hypertension specialists without sedation at the point of diagnosis. All invasive parameters were recorded at end‐expiration. PAPi was calculated from baseline invasive right heart catheterisation data on retrospective review of records. Patients were followed up in our centre at regular intervals and progress documented on electronic records. The primary outcome of death was captured through review of institutional health records. Time to death was recorded as the time interval between first diagnosis and date of death.
Statistical methods
The quantitative and qualitative data were expressed in mean ± SD and percentages, respectively. Preliminary analyses were carried out with Kruskal–Wallis and χ 2 tests, and time to death with Kaplan–Meier curves and log‐rank tests. PAPi from invasive haemodynamic data was discretized into ‘low’ and ‘high’ cohorts with the chi‐square automated interactions detector algorithm. 14 This is a multi‐way splitting decision tree useful for (i) identifying the predictors (discrete and continuous) for explaining an outcome based on the adjusted Bonferroni testing and (ii) determining the optimal cut‐off(s) for the predictors. The discretization would enable one to examine the underlying patterns of association between PAPi and the outcomes. Confirmatory analyses were performed with the generalized structural equation model (gSEM), 15 with Binomial and Weibull chosen as the underlying distributions for analysing (i) the occurrence of death (0: no, 1: yes) and (ii) time to death (years), respectively. Analysis was performed with consideration of data censoring from death. Other than the discretised PAPi, the predictors also included baseline demographics, clinical parameters, and invasive haemodynamics. The final results were presented based on a backward elimination procedure in model‐selection, which was in turned based on the unadjusted analyses. The accuracy of the identified PAPi cut‐off was examined with the receiver operating characteristics (ROC) curve. This included the final gSEM model's out‐sample predictive accuracy of occurrence of mortality, with the original sample randomly divided into a training subsample (80%) and a validation subsample (20%). Analysed with Stata MP v14 (Stata Corp. Texas, USA), all statistical tests were performed at 5% level of significance.
Ethics committee approval
The study protocol was approved by the National Healthcare Group Domain Specific Review Board, reference number 2016/01204.
Results
We included 102 out of 148 patients from our PAH registry after excluding 36 patients with incomplete haemodynamic data or follow‐up. The number of deaths was 41 (40.2%), and the median follow‐up time was 3.84 years (range: 0.08–13.87). The baseline characteristics of the cohort are illustrated in Table 1 . The mean age was 52.6 ± 15.1 years and majority were females (77%). Our multi‐ethnic cohort reflects our national population census and comprised Chinese (64%), Malays (23%), Indians (10%), and others (4%). Idiopathic PAH (n = 34; 33%) and connective tissue disease associated PAH (n = 32; 31%) formed the largest aetiology subgroups, followed by congenital heart disease associated PAH (n = 24, 24%) and other forms of PAH (n = 12; 12%). Most patients were in a good WHO functional class (75% in functional class I and II) at presentation. Treatment with monotherapy was most common (66%), and combination therapy was administered in 26% of the cohort.
Table 1.
Baseline patient characteristics
| Characteristic | All patients | Low PAPi (<5.3) | High PAPi (≥5.3) | P value |
|---|---|---|---|---|
| Number (n) | 102 | 51 | 51 | NA |
| Age and gender | ||||
| Age (years) | 52.6 ± 15.1 | 56.1 ± 14.5 | 49.2 ± 15.0 | 0.024 |
| Female (n, %) | 79 (77.5) | 39 (76.5) | 40 (78.4) | 0.813 |
| Ethnicity | ||||
| Chinese (n, %) | 65 (63.7) | 31 (60.7) | 34 (66.7) | 0.527 |
| Malay (n, %) | 23 (22.5) | 14 (27.5) | 9 (17.6) | |
| Indian (n, %) | 10 (9.8) | 5 (9.8) | 5 (9.8) | |
| Others (n, %) | 4 (3.9) | 1 (2.0) | 3 (5.8) | |
| BMI | ||||
| Low < 18.5 (n, %) | 17 (16.7) | 6 (11.8) | 11 (21.6) | 0.487 |
| Normal 18.6 to 22.9 (n, %) | 36 (35.2) | 16 (11.8) | 20 (39.2) | |
| Overweight ≥ 23 (n, %) | 49 (48.0) | 29 (56.9) | 20 (39.2) | |
| Aetiology | ||||
| iPAH (n, %) | 34 (33.3) | 19 (37.3) | 15 (29.4) | 0.396 |
| CHD‐PAH (n, %) | 24 (23.5) | 13 (25.5) | 11 (21.6) | |
| CTD‐PAH (n, %) | 32 (31.4) | 12 (23.5) | 20 (39.2) | |
| Others (n, %) | 12 (11.8) | 7 (13.7) | 5 (9.8) | |
| WHO functional class | ||||
| FC I & II (n, %) | 75 (75.8) | 38 (79.2) | 37 (72.5) | 0.443 |
| FC III & IV (n, %) | 24 (24.2) | 10 (20.8) | 14 (27.5) | |
| Clinical parameters | ||||
| Systolic BP (mmHg) | 124.4 ± 20.2 | 123.7 ± 17.8 | 125.0 ± 22.4 | 0.960 |
| Heart rate (BPM) | 81.3 ± 15.9 | 82.2 ± 17.3 | 80.3 ± 14.4 | 0.698 |
| 6MWD (m) | 346.9 ± 111 | 331.3 ± 129.7 | 358.8 ± 94.6 | 0.438 |
| Creatinine (mmol/L) | 77.4 ± 44.9 | 87.1 ± 60.3 | 68.1 ± 17.9 | 0.138 |
| NT‐proBNP (pg/mL) | 2032.3 ± 3621.1 | 2430.9 ± 3524.3 | 1633.7 ± 3709.9 | 0.052 |
| Haemodynamic parameters | ||||
| PASP (mmHg) | 78.2 ± 25.6 | 71.3 ± 22.7 | 85.1 ± 26.6 | 0.010 |
| PADP (mmHg) | 29.9 ± 13.1 | 30.9 ± 12.2 | 28.9 ± 14.0 | 0.243 |
| mPAP (mmHg) | 49.7 ± 16.3 | 47.3 ± 16.2 | 52.0 ± 16.1 | 0.081 |
| mRAP (mmHg) | 9.9 ± 5.4 | 13.5 ± 4.8 | 6.2 ± 2.9 | <0.001 |
| CI (L/m2) | 2.26 ± 0.8 | 2.2 ± 0.7 | 2.3 ± 0.9 | 0.889 |
| PVR (wood units) | 12.7 ± 9.1 | 11.0 ± 8.8 | 14.6 ± 9.1 | 0.013 |
| SVO2 (%) | 67.8 ± 10.6 | 67.2 ± 11.1 | 68.3 ± 10.1 | 0.538 |
| Treatment | ||||
| None (n, %) | 8 (7.8) | 5 (9.8) | 3 (5.9) | 0.346 |
| Monotherapy (n, %) | 68 (66.7) | 36 (70.6) | 32 (62.7) | |
| Combination (n, %) | 26 (25.5) | 10 (19.6) | 16 (31.4) | |
| REVEAL score | 6.7 ± 2.5 | 7.2 ± 2.7 | 6.2 ± 2.1 | 0.072 |
6MWD, 6 min walk distance; BMI, body mass index; BP, blood pressure; CHD‐PAH, congenital heart disease associated pulmonary arterial; CI, confidence interval; CTD‐PAH, connective tissue disease associated pulmonary arterial hypertension; iPAH, idiopathic pulmonary arterial hypertension; mRAP, mean right atrial pressure; mPAP, mean pulmonary arterial pressure; NT‐proBNP, N‐terminal pro brain natriuretic peptide; PADP, pulmonary artery diastolic pressure; PASP, pulmonary artery systolic pressure; PVR, pulmonary vascular resistance; WHO, World Health Organization.
Low pulmonary artery pulsatility index (high risk) vs. high pulmonary artery pulsatility index (low risk)
The determined prognostic cut‐off for PAPi was 5.3, which coincided with the median value. Distribution of values was positively skewed (Figure 1 ). Patients were analysed based on low (<5.3) and high (≥5.3) PAPi scores, which indicates high risk and low risk groups, respectively (Table 1 ). The low PAPi group was older (56 years vs. 49 years, P = 0.024) but otherwise similar in terms of gender, ethnicity breakdown, body mass index, aetiology breakdown, as well as clinical parameters. Notably, there was no difference in WHO functional class or 6 min walk distance between both groups.
Figure 1.
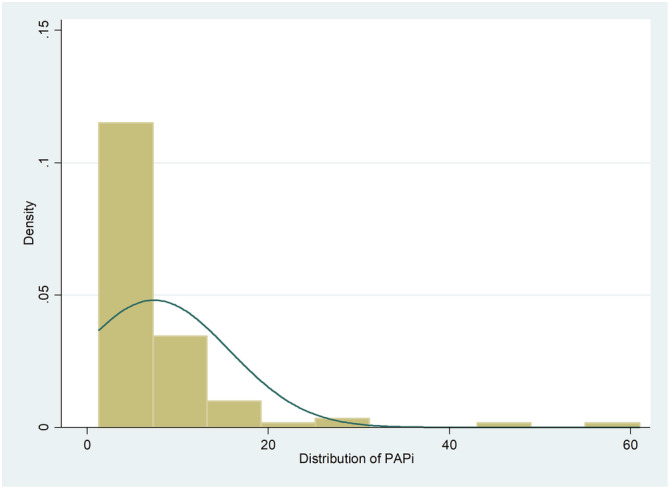
Distribution of PAPi in cohort. PAPi, pulmonary artery pulsatility index.
With reference to invasive haemodynamic parameters, the low PAPi group had higher mean right atrial pressure (14 mmHg vs. 6 mmHg, P < 0.001) as anticipated, but paradoxically also lower PASP (71 mmHg vs. 85 mmHg, P = 0.010) and lower pulmonary vascular resistance (PVR) (11 WU vs. 14.6 WU, P = 0.013). In relation to other severity indices, the low PAPi group also tended to have higher N‐terminal pro brain natriuretic peptide (NT‐proBNP) values and higher REVEAL score, which is clinically congruent; however, this difference was not significant.
Survival analysis
In the low PAPi group, the survival rates were as follows: 77.2% (1 year), 58.6% (3 years), 50.4% (5 years), 39.6% (7 years), and 31.7% (10 years), respectively. On the other hand, the high PAPi group had survival rates of 95.7%, 86.2%, 80.4%, 71.2%, and 53.4%, respectively (Figure 2 ). The survival difference between the two groups remained statistically significant across the follow‐up period shown in Figure 2 (P < 0.001). This effect was retained even when there was further risk stratification by age. Younger patients ≤ 53 years old with high PAPi were found to have the best prognosis while older patients >53 years old with low PAPi had the worst survival outcomes (Figure 3 ).
Figure 2.
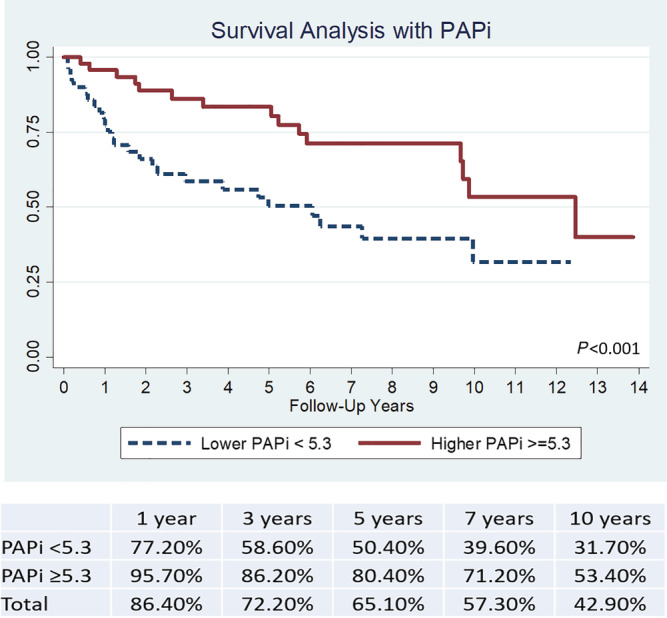
Survival analysis of PAPi cohorts with Kaplan–Meier curve. PAPi, pulmonary artery pulsatility index.
Figure 3.
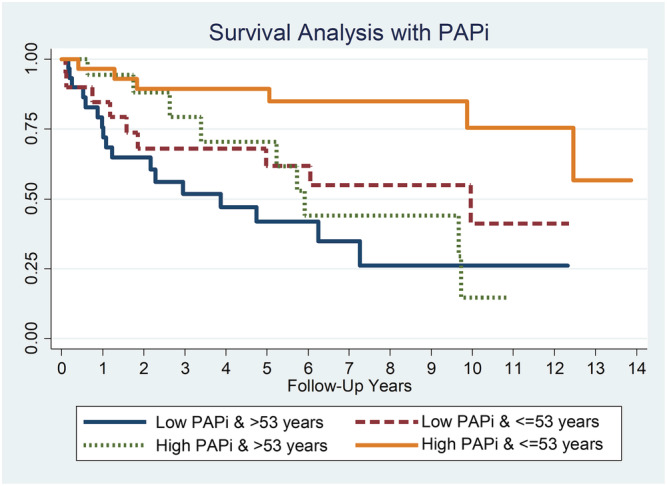
Survival analysis of PAPi cohorts with Kaplan–Meier curve (stratified by age). PAPi, pulmonary artery pulsatility index.
Occurrence of mortality
Receiver operator characteristics for prediction of mortality were analysed (Figure 4 ). PAPi was found to be a useful risk predictor for mortality [area under the curve (AUC) 0.612], similar to age as a risk predictor (AUC 0.647). Consistent with our previously published analysis, the REVEAL score was found to be the most reliable risk predictor for mortality (AUC 0.737). Of the haemodynamic parameters that made up PAPi, PASP and PADP were found to be weak predictors individually (AUCs 0.563 and 0.540, respectively), whereas mRAP confers a greater predictive ability (AUC 0.673). Based on ROC curves, the composite PAPi does not have better discriminatory ability compared with using measured mRAP alone in predicting mortality.
Figure 4.
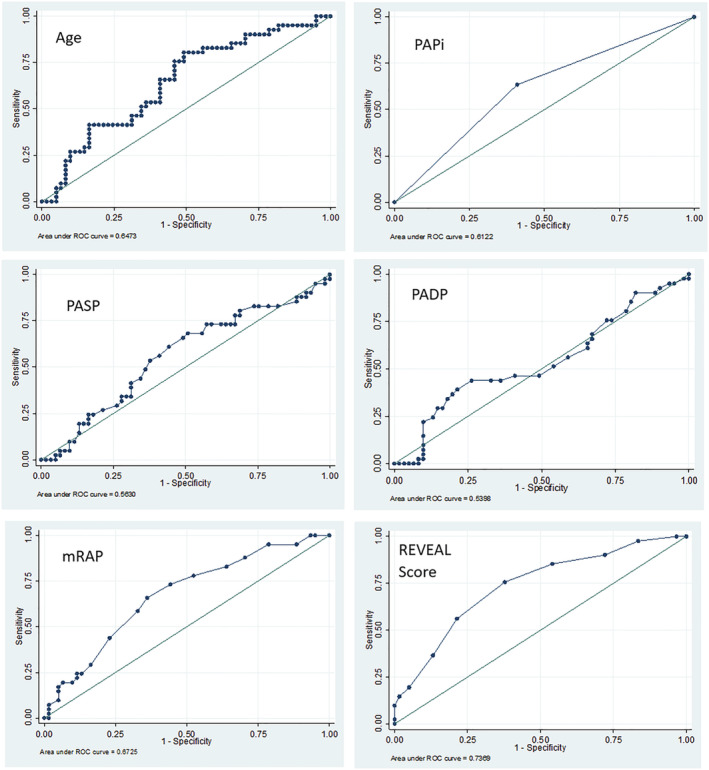
Receiver operating characteristic curves for risk of mortality. mRAP, mean right atrial pressure; PADP, pulmonary artery diastolic pressure; PAPi, pulmonary artery pulsatility index; PASP, pulmonary artery systolic pressure.
The unadjusted odds ratio for mortality for PAPi was 2.496 [95% confidence interval (CI): 1.105–5.639; P = 0.028]. The results of other unadjusted analyses are also shown in Table 2 . Six‐minute walk distance, NT‐proBNP, mRAP, REVEAL score, and PAPi were found to be statistically significant for occurrence of death as well as time to death. Of note, PASP and PADP were not found to be significant.
Table 2.
Unadjusted logistic and Cox regression analyses
| Predictor | Occurrence of death (yes/no) | Time to death (year) |
|---|---|---|
| Unadjusted odds ratio (95% CI) | Unadjusted hazard ratio (95% CI) | |
| WHO FC | ||
| 1: I/II | Reference | Reference |
| 2: III/IV | ||
| 6MWD (m) | 0.995 (0.990–1.000)* | 0.997 (0.993–1.000)* |
| NT‐proBNP (per 1000 ng/mL) | 1.21 (1.032–1.388)* | 1.110 (1.053–1.167)* |
| PASP (mmHg) | 1.004 (0.988–1.019) | 0.997 (0.986–1.009) |
| PADP (mmHg) | 1.000 (0.970–1.031) | 0.991 (0.970–1.012) |
| mRAP (mmHg) | 1.114 (1.028–1.208)* | 1.066 (1.015–1.120)* |
| PVR (wood units) | 1.032 (0.986–1.082) | 1.006 (0.974–1.039) |
| CI (L/min/m2) | 0.649 (0.375–1.124) | 0.773 (0.510–1.172) |
| SVO2 (%) | 0.983 (0.946–1.022) | 0.980 (0.951–1.010) |
| REVEAL score | 1.495 (1.214–1.840)* | 1.281 (1.146–1.432)* |
| PAPi | 0.901 (0.816–0.995)* | 0.909 (0.837–0.987)* |
6MWD, 6 min walk distance; CI, confidence interval; mRAP, mean right atrial pressure; NT‐proBNP, N‐terminal pro brain natriuretic peptide; PADP, pulmonary artery diastolic pressure; PAPi, pulmonary artery pulsatility index; PASP, pulmonary artery systolic pressure; PVR, pulmonary vascular resistance; WHO, World Health Organization.
Statistically significant at 5%.
Confirmatory analyses
After considering the demographics, clinical characteristics, aetiology, WHO functional class, and therapy, the gSEM confirmed that patients with low PAPi had both a significantly higher occurrence of death (area under ROC 2.977, 95% CI: 1.160–7.639) and a significantly higher hazard of time‐to‐death (adjusted hazard ratio 2.229, 95% CI: 1.053–4.719) (Table 3 ). Adjustment with haemodynamic parameters was not considered as PAPi is a derivative of the variables included.
Table 3.
Confirmatory analyses with the generalized structural equation model
| Predictor | Occurrence of death (yes/no) | Time to death (year) | ||
|---|---|---|---|---|
| Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) | Unadjusted hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) | |
| Aetiology subtypes | ||||
| 1: iPAH | Reference | Reference | Reference | Reference |
| 2: CHD‐PAH | 0.714 (0.240–2.123) | 0.579 (0.166–2.022) | 1.271 (0.529–3.055) | 1.660 (0.616–4.474) |
| 3: CTD‐PAH | 0.977 (0.366–2.609) | 0.741 (0.158–3.473) | 0.618 (0.287–1.332) | 0.793 (0.256–2.456) |
| 4: Others | 1.4285 (0.381–5.357) | 1.149 (0.263–5.017) | 1.626 (0.616–4.288) | 1.773 (0.580–5.420) |
| Age | ||||
| 1: < =53 years | Reference | Reference | Reference | Reference |
| 2: >53 years | 2.496 (1.105–5.639)* | 2.342 (0.909–6.034) | 2.973 (1.524–5.799) | 2.242 (1.122–5.218) |
| Incident | ||||
| 1: Incident | Reference | Reference | Reference | Reference |
| 2: Prevalent | 1.365 (0.617–3.018) | 2.177 (0.616–7.685) | 0.760 (0.407–1.420) | 0.899 (0.364–2.221) |
| BMI category | ||||
| 1: Normal | Reference | Reference | Reference | Reference |
| 2: Underweight | 1.990 (0.618–6.415) | 2.412 (0.600–9.696) | 1.833 (0.777–4.327) | 2.670 (0.917–7.772) |
| 3: Overweight/Obese | 1.121 (0.460–2.730) | 0.945 (0.334–2.672) | 1.089 (0.534–2.218) | 1.106 (0.485–2.526) |
| WHO functional class | ||||
| 1: I/II | Reference | Reference | Reference | Reference |
| 2: III/IV | 1.420 (0.561–3.597) | 1.678 (0.597–4.714) | 1.195 (0.593–2.406) | 1.779 (0.792–3.998) |
| Therapy | ||||
| 0: No | Reference | Reference | Reference | Reference |
| 1: Mono | 0.659 (0.152–2.860) | 1.004 (0.176–5.710) | 0.704 (0.242–2.047) | 0.531 (0.141–2.001) |
| 2: Combination | 0.625 (0.127–3.081) | 1.293 (0.201–8.320) | 0.610 (0.189–1.967) | 0.637 (0.162–2.503) |
| PAPi | ||||
| 1: > =5.3 | Reference | Reference | Reference | Reference |
| 2: <5.3 | 2.496 (1.105–5.639)* | 2.976 (1.160–7.634)* | 2.458 (1.280–4.718)* | 2.229 (1.053–4.719)* |
BMI, body mass index; CHD‐PAH, congenital heart disease associated pulmonary arterial; CI, confidence interval; CTD‐PAH, connective tissue disease associated pulmonary arterial hypertension; iPAH, idiopathic pulmonary arterial hypertension; PAPi, pulmonary artery pulsatility index; WHO, World Health Organization.
Statistically significant at 5%.
The model's out‐sample predictive accuracy of occurrence of mortality was examined next, in particular how the optimum PAPi cut‐off of ≥5.3 compared with the heuristic tertiles. The area under ROC with PAPi > 5.3 was 0.52 (95% CI: 0.27–0.77), while that based on the tertiles was 0.45 (95% C.I.: 0.20–0.71). Thus, there was numerical evidence suggesting that the out‐sample predictive accuracy of PAPi was higher than the PAPi tertiles.
Discussion
To our knowledge, this is the first study to evaluate the prognostic utility of PAPi in a multi‐ethnic Asian cohort of patients with PAH (WHO Group 1 pulmonary hypertension). Our key findings are as follows: (i) low PAPi < 5.3 at diagnosis confers higher risk and poorer survival compared with high PAPi, even after age stratification, and (ii) PAPi is a predictor of both occurrence of death and time to death across a long 14 year follow‐up period, with its predictive value driven mainly by mRAP. Together, these findings suggest that haemodynamic risk assessment of PAH patients remained crucial in prognostication. However, while PAPi is useful as a mortality predictor compared with other recommended parameters in published PAH risk prediction models, it does not have an improved predictive ability compared with using mRAP alone. This may limit utility of this index.
The thought of using PAPi as a predictor for RV failure and mortality in PAH is an attractive idea because this index has been proven useful in other disease populations. However, the derived threshold for PAPi and its prognostic accuracy varies considerably in different populations. Following RV myocardial infarction, PAPi ≤ 0.9 identified a higher risk for mortality and/or RV mechanical support. 7 After left ventricular assist device implantation, a PAPi cut‐off of 1.85 to 2.0 identified right heart failure. 8 , 9 , 10 , 11 Additional haemodynamic studies have shown that a lower PAPi portends increased RV filling pressures and impaired RV to pulmonary artery coupling. 16 In our PAH population, the established cut‐off value PAPi < 5.3 is much higher than in other disease populations, and pales in performance as a risk predictor (AUC 0.612 in PAH vs. 0.998 in RV infarction).
The difference in PAPi thresholds and its predictive strengths can be explained by understanding the physiologic basis behind the PAPi formula—which is the ratio of PAPP to right atrial pressure. PAPP is stroke volume indexed against pulmonary arterial capacitance (PAC), and PAC is inversely related to PVR. 17 Therefore, both increased RV stroke volume and increased PVR raises PAPP in the numerator for PAPi. With RV infarction, an acute ischemic insult causes sudden loss of RV contractile function and stroke volume, without changes in PAC or PVR, and PAPP diminishes. Right atrial compliance is low and mRAP rapidly increases. The resultant PAPi in this setting is markedly reduced and predicts RV failure realistically. With PAH, the RV does not lose contractility acutely, at the same time, elevated PVR in this disease state adds to increased PAPP. Hence, PAPP may not reflect disease severity accurately in PAH. Right atrial pressure also elevates less markedly and more gradually than in RV infarction due to chronicity and a resultant compensatory increase in right atrial compliance. 18 The PAPi threshold in PAH is consequently higher, and its accuracy as a predictive marker for RV failure and mortality is more nuanced. Essentially, PAPi can be affected by multiple determinants in the right heart system, ranging from systemic venous return, RV function, and the pulmonary circulation. The pathophysiology of a disease must be carefully considered before applying PAPi.
There are several other notable novel haemodynamic indices that have been studied for mortality prediction in PAH. PAC has been studied and shown reasonable prognostication ability. 19 , 20 , 21 A prior study showed an AUC of 0.61 for discrimination of 1 year mortality; discriminatory ability was similar for PAC, mRAP, mean pulmonary arterial pressure, cardiac index, and PVR. 21 Cardiac index and stroke volume have been acknowledged to be strong predictors of prognosis but were not significant for prediction of mortality in our study (Table 2 ). Unexpectedly, although PAPi is an amalgamation of stroke volume and PAC adjusted against preload (indicated by mRAP), our study showed the prognostic ability of using PAPi is non‐superior to using individual components alone.
Using the REVEAL score for risk prediction surpasses using PAPi solely (AUC 0.737 vs. 0.612, respectively). Interestingly, the risk classification ability in our study cohort was similar to that of the original REVEAL cohort of PAH patients (AUC 0.737 vs. AUC 0.74, respectively). 22 It was notable that when PAPi was used to stratify our PAH patients into high risk and low risk groups, the two groups did not differ significantly in WHO functional status and 6 min walk distance, which are commonly used risk predictors in clinical practice. Yet the odds of death were starkly different—three times higher in the low PAPi group compared with the high PAPi group. This finding implies that haemodynamic variables could carry more prognostic implications than bedside clinical variables at the time of diagnosis. In this long follow‐up study, baseline PAPi also appeared to have a consistent correlation to mortality over time, even when corrected for age (Figure 2 ). Future research should examine strategically, if PAPi performed in a serial fashion across treatment course can identify changes in disease trajectory.
The development of RV dysfunction hastens mortality in PAH 23 , 24 and preservation of RV function is the main therapeutic goal. However, clinical experience tells us that progressive RV dysfunction does not necessarily occur in parallel with worsening pulmonary arterial constriction. Studies suggest that the RV decompensates when it becomes maladapted to a given afterload due to impaired ventricular‐arterial uncoupling. 25 , 26 , 27 Increased PVR is not synonymous with increased RV dysfunction and vice versa. In our study, the low PAPi group also had higher mean NT‐proBNP levels by about 800 pg/mL although statistical significance was missed marginally likely due to small sample size. NT‐proBNP is a well‐characterized biomarker predictive of heart failure, representative of the extent of haemodynamic impairment. This trend supports our finding that PAPi effectively categorizes risk for RV failure and has reasonable prognostic utility.
Despite the different aetiologies associated with PAH, there is a similar pathophysiology of progressive pulmonary vascular narrowing, proliferation, and re‐modelling. 28 These conditions are also classified together because of their likelihood to respond to PAH‐specific therapy and follow a common goal‐directed treatment approach.
Current treatment strategy is based on severity of newly diagnosed PAH and guided by multiparametric risk stratification. The guidelines also recommend continual follow up and to aim to achieve ‘low risk’ status 13 by working towards target levels on the same risk parameters. Multiple registries have come with risk prediction models in recent years: the REVEAL registry, 2 the Swedish Pulmonary Artery Hypertension Register, 29 the Comparative Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension group, 30 and the French Pulmonary Hypertension Registry. 31 All of these risk prediction models contain mRAP as a variable, not pulmonary artery pressures, emphasizing the prognostic value of mRAP in predicting survival. It remains questionable if incorporating PAPi in current risk prediction models adds incremental value compared with using mRAP alone.
Limitations
The lack of a sizeable cohort carried the usual statistical limitations. The utility of PAPi as a risk predictor would require validation with larger studies that could add more precision. Also, this study was a retrospective analysis using a cohort of PAH patients prospectively enrolled into our registry over the last two decades. As such, this population may not reflect contemporary phenotype of patients, and latest advances in PAH therapy would have affected survival differently across the time horizon. From a statistical perspective, the current study is exploratory in nature and therefore susceptible to Type I error.
Conclusions
In our analysis, low PAPi was found to be a significant but modest independent predictor for death and time to death. While PAPi appears promising as a novel risk indicator in PAH, it does not reclassify mortality risk further when compared with using the mRAP alone, which is the denominator in calculating this index. The REVEAL score stands out as the strongest risk prediction tool still. In PAH, accurate clinical prognostication can lead to an actionable outcome, but risk prediction is best improved when a combination of risk markers is considered. PAPi should be examined rigorously in further studies for its possibility of adding incremental value to current models of clinical risk prediction.
Conflict of interest
All authors above take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. There are no conflicts of interest or grant support for this study.
Acknowledgements
We thank our pulmonary hypertension nurses Ms Geying Li, Ms Ai Houng Then, and Ms Margaret Choong for their tireless contribution in maintaining the PAH database registry and supporting our PAH patients across the years, without whom this study will not be possible. The publication cost for this article is supported by the Collaborative Centre Grant Seed Fund.
Lim, Y. , Low, T.‐T. , Chan, S. P. , Lin, W. , Teo, T. W. , Jang, J.‐H. J. , Kuntjoro, I. , Tay, E. L.‐W. , and Yip, J. W.‐L. (2021) Does pulmonary artery pulsatility index predict mortality in pulmonary arterial hypertension?. ESC Heart Failure, 8: 3835–3844. 10.1002/ehf2.13450.
Yinghao Lim and Ting‐Ting Low shared joint first authorship.
References
- 1. Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long‐term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012; 142: 448–456. [DOI] [PubMed] [Google Scholar]
- 2. Farber HW, Miller DP, Poms AD, Badesch DB, Frost AE, Muros‐Le Rouzic E, Romero AJ, Benton WW, Elliott CG, McGoon MD, Benza RL. Five‐year outcomes of patients enrolled in the REVEAL Registry. Chest 2015; 148: 1043–1054. [DOI] [PubMed] [Google Scholar]
- 3. Maron BA, Galiè N. Diagnosis, treatment, and clinical management of pulmonary arterial hypertension in the contemporary era: a review. JAMA Cardiol 2016; 1: 1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benza RL, Gomberg‐Maitland M, Elliott CG, Farber HW, Foreman AJ, Frost AE, McGoon MD, Pasta DJ, Selej M, Burger CD, Frantz RP. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL Risk Score Calculator 2.0 and comparison with ESC/ERS‐based risk assessment strategies. Chest 2019; 156: 323–337. [DOI] [PubMed] [Google Scholar]
- 5. Raina A, Humbert M. Risk assessment in pulmonary arterial hypertension. Eur Respir Rev 2016; 25: 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim Y, Low TT, Chan SP, Teo TW, Jang JJ, Yip N, Kuntjoro I, Tay EL, Yip JW. Pulmonary arterial hypertension in a multi‐ethnic Asian population: Characteristics, survival and mortality predictors from a 14‐year follow‐up study. Respirology 2019; 24: 162–170. [DOI] [PubMed] [Google Scholar]
- 7. Korabathina R, Heffernan KS, Paruchuri V, Patel AR, Mudd JO, Prutkin JM, Orr NM, Weintraub A, Kimmelstiel CD, Kapur NK. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheter Cardiovasc Interv 2012; 80: 593–600. [DOI] [PubMed] [Google Scholar]
- 8. Kang G, Ha R, Banerjee D. Pulmonary artery pulsatility index predicts right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant 2016; 35: 67–73. [DOI] [PubMed] [Google Scholar]
- 9. Morine KJ, Kiernan MS, Pham DT, Paruchuri V, Denofrio D, Kapur NK. Pulmonary artery pulsatility index is associated with right ventricular failure after left ventricular assist device surgery. J Card Fail 2016; 22: 110–116. [DOI] [PubMed] [Google Scholar]
- 10. Raymer DS, Moreno JD, Sintek MA, Nassif ME, Sparrow CT, Adamo L, Novak EL, LaRue SJ, Vader JM. The combination of tricuspid annular plane systolic excursion and HeartMate risk score predicts right ventricular failure after left ventricular assist device implantation. ASAIO J 2019; 65: 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gudejko MD, Gebhardt BR, Zahedi F, Jain A, Breeze JL, Lawrence MR, Shernan SK, Kapur NK, Kiernan MS, Couper G, Cobey FC. Intraoperative hemodynamic and echocardiographic measurements associated with severe right ventricular failure after left ventricular assist device implantation. Anesth Analg 2019; 128: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mazimba S, Welch TS, Mwansa H, Breathett KK, Kennedy JLW, Mihalek AD, Harding WC, Mysore MM, Zhuo DX, Bilchick KC. Haemodynamically derived pulmonary artery pulsatility index predicts mortality in pulmonary arterial hypertension. Heart Lung Circ 2019; 28: 752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 14. Kass GV. An exploratory technique for investigating large quantities of categorical data. Appl Stat 1980; 29: 119–127. [Google Scholar]
- 15. Rabe‐Hesketh S, Skrondal A, Pickles A. Generalized multilevel structural equation modeling. Psychometrika 2004; 69: 167–190. [Google Scholar]
- 16. Bernardo R, Vanderpool R, Rischard F. The pulmonary artery pulsatility index correlates with ventriculo‐vascular coupling and elevated filling pressures in patients with pulmonary arterial hypertension. Eur Respir J 2018; 52: PA3314. [Google Scholar]
- 17. Lim HS, Gustafsson F. Pulmonary artery pulsatility index: physiological basis and clinical application. Eur J Heart Fail 2020; 22: 32–38. [DOI] [PubMed] [Google Scholar]
- 18. Gaynor SL, Maniar HS, Bloch JB, Steendijk P, Moon MR. Right atrial and ventricular adaptation to chronic right ventricular pressure overload. Circulation 2005; 112: I212–I218. [DOI] [PubMed] [Google Scholar]
- 19. Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 2006; 47: 799–803. [DOI] [PubMed] [Google Scholar]
- 20. Gan CT, Lankhaar JW, Westerhof N, Marcus JT, Becker A, Twisk JW, Boonstra A, Postmus PE, Vonk‐Noordegraaf A. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 2007; 132: 1906–1912. [DOI] [PubMed] [Google Scholar]
- 21. Al‐Naamani N, Preston IR, Hill NS, Roberts KE. The prognostic significance of pulmonary arterial capacitance in pulmonary arterial hypertension: single‐center experience. Pulm Circ 2016; 6: 608–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benza RL, Miller DP, Gomberg‐Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension. Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 23. Vonk Noordegraaf A, Galiè N. The role of the right ventricle in pulmonary arterial hypertension. Eur Respir Rev 2011; 20: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF, Ammash NM, McCully RB, Miller FA, Pellikka PA, Oh JK, Kane GC. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest 2011; 139: 1299–1309. [DOI] [PubMed] [Google Scholar]
- 25. Vonk‐Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, Torbicki A, Voelkel NF, Hassoun PM. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013; 62: D22–D33. [DOI] [PubMed] [Google Scholar]
- 26. Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol 2017; 69: 236–243. [DOI] [PubMed] [Google Scholar]
- 27. Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res 2014; 115: 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, Rabinovitch M. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 2019; 53: 1801887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kylhammar D, Kjellström B, Hjalmarsson C, Jansson K, Nisell M, Söderberg S, Wikström G, Rådegran G. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2018; 39: 4175–4181. [DOI] [PubMed] [Google Scholar]
- 30. Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, Olsson KM, Meyer K, Vizza CD, Vonk‐Noordegraaf A, Distler O, Opitz C, Gibbs JSR, Delcroix M, Ghofrani HA, Huscher D, Pittrow D, Rosenkranz S, Grünig E. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: 1700740. [DOI] [PubMed] [Google Scholar]
- 31. Boucly A, Weatherald J, Savale L, Jaïs X, Cottin V, Prevot G, Picard F, de Groote P, Jevnikar M, Bergot E, Chaouat A, Chabanne C, Bourdin A, Parent F, Montani D, Simonneau G, Humbert M, Sitbon O. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700889. [DOI] [PubMed] [Google Scholar]


