Abstract
Background/objectives
Hypertension treatment reduces cardiovascular events. However, uncertainty remains about benefits and harms of deintensification or further intensification of antihypertensive medication when systolic blood pressure (SBP) is tightly controlled in older multimorbid patients, because of their frequent exclusion in trials. We assessed the association of hypertension treatment deintensification or intensification with clinical outcomes in older adults with tightly controlled SBP.
Design
Longitudinal cohort study (2011–2013) with 9‐month follow‐up.
Setting
U.S.‐nationwide primary care Veterans Health Administration healthcare system.
Participants
Veterans aged 65 and older with baseline SBP <130 mmHg and ≥1 antihypertensive medication during ≥2 consecutive visits (N = 228,753).
Exposure
Deintensification or intensification, compared with stable treatment.
Main outcomes and measures
Cardiovascular events, syncope, or fall injury, as composite and distinct outcomes, within 9 months after exposure. Adjusted logistic regression and inverse probability of treatment weighting (IPTW, sensitivity analysis).
Results
Among 228,753 patients (mean age 75 [SD 7.5] years), the composite outcome occurred in 11,982/93,793 (12.8%) patients with stable treatment, 14,768/72,672 (20.3%) with deintensification, and 11,821/62,288 (19.0%) with intensification. Adjusted absolute outcome risk (95% confidence interval) was higher for deintensification (18.3% [18.1%–18.6%]) and intensification (18.7% [18.4%–19.0%]), compared with stable treatment (14.8% [14.6%–15.0%]), p < 0.001 for both effects in the multivariable model). Deintensification was associated with fewer cardiovascular events than intensification. At baseline SBP <95 mmHg, cardiovascular event risk was similar for deintensification and stable treatment, and fall risk lower for deintensification than intensification. IPTW yielded similar results. Mean follow‐up SBP was 124.1 mmHg for stable treatment, 125.1 mmHg after deintensification (p < 0.001), and 124.0 mmHg after intensification (p < 0.001).
Conclusion
Antihypertensive treatment deintensification in older patients with tightly controlled SBP was associated with worse outcomes than continuing same treatment intensity. Given higher mortality among patients with treatment modification, confounding by indication may not have been fully corrected by advanced statistical methods for observational data analysis.
Keywords: cardiovascular event, deintensification, elderly, fall injury, hypertension, intensification, syncope, treatment, Veterans
Key Points
Deintensifying antihypertensive medication in older adults with systolic blood pressure (SBP) <130 mmHg was not associated with better outcomes, except when baseline SBP was <95 mmHg.
It is possible that some patients deintensifying treatment at low SBP were addressing unobservable symptoms related to falling, thus confounding our observational study. Therefore, a trial is needed.
Why Does this Paper Matter?
Benefits and harms of deintensifying antihypertensive medication in older adults with low SBP is uncertain.
INTRODUCTION
Hypertension affects over 70% U.S. adults after the age of 60 years. 1 While there is strong evidence that modest blood pressure (BP) control reduces cardiovascular risk in older adults, intensive control for those patients remains controversial. In the HYVET trial, a target systolic BP (SBP) <150 mmHg reduced cardiovascular event relative risk by 34% in patients aged 80 and older. 2 In the SPRINT trial, a target SBP <120 mmHg reduced cardiovascular disease and mortality, even in patients aged 75 and older, but adverse events were increased. 3 , 4 After SPRINT, U.S. hypertension guidelines were revised to lower BP targets, 5 , 6 although a meta‐analysis (including SPRINT) found no reduction in mortality or major cardiovascular events in trials where patients with a baseline SBP <140 mmHg were treated to lower targets. 7 However, because older multimorbid patients were often excluded from trials (e.g., high‐fall risk patients, such as patients with dementia or living in nursing home, were excluded in HYVET and SPRINT), 2 , 3 little is known about optimal BP goals for a real‐world population that includes individuals at high risk of adverse events. 8
In several observational studies, patients with SBP <130 mmHg had higher rates of cardiovascular events, mortality, and cognitive impairment, supporting a J‐curve hypothesis, i.e., an increased risk of cardiovascular events not only for hypertensive BP values, but also when BP is very low. 9 , 10 Among Medicare patients with hypertension, patients taking antihypertensive medications were also found to have a higher risk of serious fall injuries compared with those not taking medication. 11 A study among 211,667 Veterans reported that almost 40% of treated older multimorbid adults have a SBP <120 mmHg, yet less than 20% of those with SBP <120 mmHg received deintensification. 12 Responding to these observations, some studies have looked for benefits of deintensification. The DANTE trial of 385 participants aged 75 and older found no change in cardiovascular and cognitive outcomes 16 weeks after deintensification, 13 while two small studies reported lower fall risk after deintensification. 14 , 15 The OPTIMISE trial found that there was little difference in SBP after deintensification (569 patients, ≥80 years, baseline SBP <150 mmHg), but long‐term clinical outcomes were not reported. 16
Accordingly, there remains uncertainty about the potential benefits and harms of intensive BP control in older adults with multimorbidity. Given recent evidence, clinicians may fear that deintensification for older patients with low SBP will lead to an increased risk of cardiovascular events. 17 , 18 Yet, some older adults fear serious fall injuries as much as they fear cardiovascular events. 19 Nonetheless, the SPRINT trial, performance measures, and lower BP targets in guidelines will inevitably lead to more aggressive treatment of older patients, potentially including those at high risk for adverse events, to whom the guidelines may not explicitly apply. We therefore examined the association between change in hypertension treatment intensity and cardiovascular events, syncope, and fall injuries, among over 200,000 older patients with treated BP in the Veterans Health Administration (VHA), the largest U.S. integrated health system. We focused on those outcomes because they can significantly impact the functional status of older patients. We hypothesized that hypertension treatment deintensification would be associated with fewer syncope and fall injury events, without increasing cardiovascular event risk in older adults with tightly controlled SBP.
METHODS
Data from the study are not openly available to other researchers due to protected patient information by the VHA. Statistical codes can be provided by the corresponding author upon request. The codes for the hypertension daily dose (HDD, see below) and fall injury have been published in open source format. 20 , 21
Overall approach
We used data from electronic health records from the national Department of Veterans Affairs (VA) healthcare system from July 2011 through June 2013, linked to VA and Medicare pharmacy and outcome data. In all Veterans aged 65 and older with tightly controlled SBP, we first assessed whether modifying the intensity of hypertension treatment was associated with cardiovascular, syncope, and fall injury events. Second, we assessed the effect of treatment intensity modification on subsequent SBP.
Study population
The study included all patients aged 65 and older with ongoing primary care at the VHA, a diagnosis of hypertension, and tightly controlled SBP, defined as SBP <130 mmHg with ≥1 antihypertensive medication on ≥2 consecutive visits (Figure 1). The 130 mmHg threshold was chosen as being 10 mmHg below the recommended SBP target at the study time (2011–2013). 22 To increase precision, we used mean SBP of the two visits to define baseline SBP. This research was conducted under Human Subjects review (VA IRB 2015‐286).
FIGURE 1.
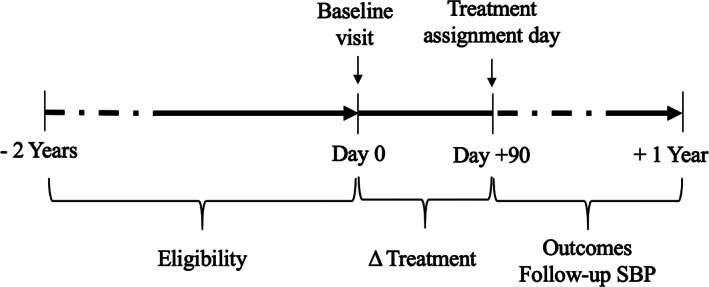
Study design. Eligibility was defined as two consecutive visits with systolic blood pressure <130 mmHg and ≥1 antihypertensive medication within a 2‐year period. Treatment assignment was defined by calculating the difference (Δ) in dose and medication count between day 0 (baseline) and day 90. The follow‐up was between 90 days and 1 year after baseline
Variable construction and definition
Using ICD‐9 codes, we classified comorbidities into chronic conditions, including general medical conditions, cardiovascular risk factors, and geriatric conditions (Table S1), and defined multimorbidity as ≥1 chronic condition in addition to hypertension. 23 We selected all conditions potentially associated with our treatment and/or outcomes of interest, as well as markers of patient sickness or frailty. To capture antihypertensive treatment, we applied an algorithm that we previously validated in older Veterans, which uses VA and Medicare Part D pharmacy fills to determine the most likely antihypertensive medication on any day. 24 We standardized doses to HDDs, allowing comparison across medications. 20 We defined one HDD as half the maximum beneficial dose demonstrated in trials (Table S2), 25 , 26 , 27 and aggregated the standardized doses to obtain total dose. For example, hydrochlorothiazide 50 mg (maximal beneficial dose: 2 HDDs) with candesartan 16 mg (half maximal beneficial dose: 1 HDD) would equal 3 total HDDs.
Our primary exposure of interest was a three‐level treatment strategy based on dose changes between baseline and +90 days (Figure 1): deintensification (any dose decrease), intensification (any dose increase), or stable treatment intensity (no dose change). The 90‐day period was selected because it is the usual refill period. To interpret our results in light of prior studies that have historically reported the results of changes in medication count only, 13 , 14 , 28 we conducted sensitivity analyses that similarly defined treatment strategy by modification in medication count.
The primary outcome was a binary composite outcome of any inpatient (hospitalizations) or outpatient (emergency department visits) encounter for acute cardiovascular events, syncope, or fall injury, within 9 months after treatment strategy assignment (i.e., 90–365 days after baseline, Figure 1). Cardiovascular events included only acute diagnoses of hemorrhagic and ischemic stroke, acute coronary syndrome, and decompensated heart failure. We used ICD‐9 codes for cardiovascular events and syncope (Table S3), and a validated algorithm for capturing comprehensive new fall injury events across inpatient and outpatient care. 21 We assessed cardiovascular events, syncope, and fall injury separately as secondary outcomes to avoid missing potential opposite associations, and because some patients may value avoiding one event more than the other. 19
Statistical analyses
In order to estimate the association between treatment intensity modification and outcome, we used a logistic regression with categorical indicators for treatment strategy, adjusting for baseline covariates, which included age, chronic conditions, baseline SBP, and baseline antihypertensive medication dose. Since close proximity to death could directly result in a general deintensification of preventive medications, we did not include death as outcome, and the analytic cohort included only patients who were survived until end of follow‐up (i.e., 365 days after baseline) (96.4% of all identified patients). To reduce potential bias resulting from deaths during the follow‐up period, we weighted the analyses by the inverse probability of survival. This main model resulted in estimated marginal risk associated with the three treatment strategies as if the patients who died were included in the analysis and survived. Next, in order to reduce bias resulting from patient factors associated with deintensification (vs no deintensification), we conducted a sensitivity analysis using inverse probability of treatment weighting (IPTW) by propensity scores (PS). Models to derive both survival and treatment weights included all baseline covariates. Since patients with cardiovascular disease or heart failure may benefit from a lower SBP target, we conducted a sensitivity analysis assessing patients with and without cardiovascular disease or heart failure separately. All outcome models included two interaction terms, between age and baseline SBP (to account for and nonlinear effect of SBP with increasing age) and between treatment strategy and baseline SBP (to account for effect modification that baseline SBP might have on treatment effect).
To assess the association between treatment strategy and follow‐up SBP, we calculated the mean SBP across all visits during follow‐up period, and compared changes in mean SBP between baseline and follow‐up, according to treatment strategy and adjusting for baseline SBP. We compared proportions with ≥10 mmHg change in mean SBP using chi‐square tests.
Details of the statistical analyses are provided in File S1. All analyses were performed with Stata/MP 15.1 (StataCorp) and SAS Enterprise Guide 7.1 (SAS Institute).
RESULTS
After excluding 8,461 (3.6%) patients with missing outcome data due to death before end of follow‐up, 228,753 patients (mean age 75.2 [SD 7.5] years, 98.2% males) were included in the analytic cohort. Mean baseline SBP was 116.7 (SD 7.6) mmHg, and 183,436 (80.2%) patients had multimorbidity. Baseline characteristics according to treatment strategy are presented in Table 1. Deintensification was observed in 72,672 (31.8%) patients, and intensification in 62,288 (27.2%) patients. Patients with deintensification had the highest mortality rate (4.1%), followed by those with intensification (3.2%), and stable treatment (1.8%, p < 0.001).
TABLE 1.
Baseline characteristics according to treatment strategy
| Characteristic (No. = 228,753) | No dose change (No. = 93,793) | Dose decrease (No. = 72,672) | Dose increase (No. = 62,288) |
|---|---|---|---|
| Age, mean (SD), years | 74.9 (7.4) | 75.6 (7.6) | 75.2 (7.5) |
| Male | 92,051 (98.1) | 71,380 (98.2) | 61,242 (98.3) |
| Baseline SBP, mean (SD), mmHg | 117.5 (7.1) | 115.6 (8.3) | 116.9 (7.5) |
| ≤110.0 mmHg | 14,775 (15.7) | 17,202 (23.7) | 11,329 (18.2) |
| 110.5–120.0 mmHg | 41,238 (44.0) | 31,225 (43.0) | 27,113 (43.5) |
| 120.5–129.0 mmHg | 37,780 (40.3) | 24,245 (33.3) | 23,846 (38.3) |
| Chronic conditions | |||
| Number, mean (SD) | 3.0 (3.1) | 4.3 (3.6) | 3.7 (3.5) |
| Multimorbidity a | 70,850 (75.5) | 62,013 (85.3) | 50,573 (81.2) |
| Vascular disorder b | 25,602 (27.3) | 29,503 (40.6) | 23,091 (37.1) |
| Heart failure or valve disorder | 11,500 (12.3) | 17,295 (23.8) | 13,681 (22.0) |
| Diabetes mellitus, type II | 29,341 (31.3) | 28,859 (39.7) | 23,689 (38.0) |
| Fall risk c | 15,788 (16.8) | 18,089 (24.9) | 13,196 (21.2) |
| Antihypertensive medication | |||
| Total dose, mean (SD), HDD | 2.2 (1.8) | 2.9 (2.2) | 2.3 (1.9) |
| Medication count, mean (SD) | 2.2 (1.1) | 2.8 (1.3) | 2.6 (1.2) |
Note: Dichotomous variables are presented as N (%), and continuous variables as mean with standard deviation.
Abbreviations: HDD, hypertension daily dose; No., number.
Hypertension with ≥1 additional chronic condition.
Cardiac, peripheral, and/or cerebral vascular disorder.
Fall risk included Parkinson's disease, peripheral neuropathy, ataxia, vertigo/dizziness, orthostatic hypotension, walking difficulty/gait abnormality/lack of coordination, muscle weakness, syncope, history of fall (ICD‐9 codes: 340–342.91, 356.XX, 357.XX, 386.XX, 438.2–438.22, 438.40–438.42, 438.84, 438.85, 458.0, 719.7, 728.87, 780.2, 780.4, 781.1, 781.2, 781.3, V15.88).
Association of deintensification and intensification with the composite outcome
The primary composite outcome occurred in 38,571 (16.9%) patients, including 25,601 cardiovascular events, 3,438 syncope episodes, and 20,282 fall injuries (Table S4). The composite outcome occurred in 12.8% (11,982/93,793) of patients with stable treatment, 20.3% (14,768/72,672) with deintensification, and 19.0% (11,821/62,288) with intensification.
Adjusted absolute outcome risk (95% confidence interval [CI]) was higher for deintensification (18.3% [18.1%–18.6%]) and intensification (18.7% [18.4%–19.0%]), compared with stable treatment (14.8% [14.6%–15.0%]), p < 0.001) (Table 2 and Figure 2A). Although the overall composite outcome risk associated with deintensification was higher compared with stable treatment, the risks associated with these two treatment strategies were similar in those with low baseline SBP (<95 mmHg), while the risk associated with deintensification was higher than that associated with stable treatment with higher baseline SBP (Figure 2A). In the sensitivity analysis with treatment strategy defined using medication count change, adjusted outcome risk (95% CI) was 16.2% (16.0%–16.3%; reference) for stable treatment, 19.8% (19.4%–20.3%; p < 0.001) for deintensification, and 22.1% (21.5%–22.7%; p < 0.001) for intensification (Table 2 and Figure S1A).
TABLE 2.
Adjusted outcome risks according to treatment strategy (No. = 228,753 patients)
| Adjusted absolute risks (95% CI), % a | ||
|---|---|---|
| Outcomes | Dose change | Medication count change |
| Composite outcome | ||
| Stable treatment b | 14.8 (14.6 to 15.0) | 16.2 (16.0 to 16.3) |
| Deintensification | 18.3 (18.1 to 18.6) | 19.8 (19.4 to 20.3) |
| Intensification | 18.7 (18.4 to 19.0) | 22.1 (21.5 to 22.7) |
| Cardiovascular event | ||
| Stable treatment b | 9.1 (8.9 to 9.2) | 10.5 (10.4 to 10.6) |
| Deintensification | 12.3 (12.0 to 12.5) | 13.4 (13.1 to 13.8) |
| Intensification | 13.2 (12.9 to 13.4) | 16.7 (16.2 to 17.3) |
| Syncope | ||
| Stable treatment b | 1.3 (1.2 to 1.3) | 1.4 (1.4 to 1.5) |
| Deintensification | 1.7 (1.6 to 1.8) | 1.8 (1.6 to 1.9) |
| Intensification | 1.6 (1.5 to 1.7) | 1.8 (1.6 to 2.0) |
| Fall injury | ||
| Stable treatment b | 8.3 (8.1 to 8.5) | 8.7 (8.6 to 8.8) |
| Deintensification | 9.7 (9.5 to 9.9) | 10.2 (9.9 to 10.5) |
| Intensification | 9.1 (8.9 to 9.4) | 9.9 (9.4 to 10.4) |
Abbreviation: CI, confidence interval.
Based on logistic regression model weighted to account for missing outcome. The model included interaction terms between age and systolic blood pressure and between systolic blood pressure and treatment strategy, and was also adjusted for baseline antihypertensive medication dose and for chronic conditions (Table S1).
Reference group, defined as no dose or medication count change, respectively, is displayed in italic to facilitate reading.
FIGURE 2.

Adjusted absolute risk for (A) composite outcome, (B) cardiovascular event, (C) syncope, and (D) fall injury, according to dose change and baseline systolic blood pressure. Based on logistic regression models weighted to account for missing outcome. The models included interaction terms between age and systolic blood pressure and between systolic blood pressure and treatment strategy, and were also adjusted for baseline antihypertensive medication dose and for chronic conditions (Table S1)
Association of deintensification and intensification with cardiovascular events, syncope, and fall injuries as separate outcomes
The relationship between treatment modification and cardiovascular events was similar to the main analysis of the composite outcome, except for that for patients with baseline SBP <95 mmHg, there was no cardiovascular difference in risk associated with deintensification and stable treatment (Figure 2B, Table 2 and Figure S1B, Table S5). Compared with intensification, cardiovascular event risk was 1.0% (95% CI −1.4% to −0.6%; p < 0.001) lower for dose deintensification, and 3.7% (95% CI −4.5% to −3.0%; p < 0.001) lower for medication count reduction. Both deintensification and intensification were associated with an increased syncope risk across all baseline SBP values, compared with stable treatment. Overall, deintensification was associated with a 1.5% (95% CI 1.1%–1.9%; p < 0.001) greater risk of fall injury compared with stable treatment. However, at low baseline SBP (<95 mmHg), deintensification was associated with the lowest fall risk (Figure 2D).
Sensitivity analysis: inverse probability weights to reduce bias of patient characteristic leading to different treatments
We found that IPTW was appropriate, improving balance in covariates between treatment groups from a maximum standardized difference of 36.9% to 4.2% (Figures S2–S5). After applying the IPTW, we found no differences with the main analyses (Table S5).
Sensitivity analysis: patients with versus without cardiovascular disease or heart failure
Patients with cardiovascular disease or heart failure had an absolute outcome risk (95% CI) of 21.5% (21.1%–22.0%) for stable treatment, 26.1% (25.6%–26.5%) for deintensification, and 27.2% (26.7%–27.7%) for intensification. Patients without cardiovascular disease or heart failure had an absolute outcome risk (95% CI) of 10.3% (10.1%–10.6%) for stable treatment, 13.2 (12.9%–13.5%) for deintensification, and 13.0 (12.7%–13.4%) for intensification.
Follow‐up SBP
Mean SBP at baseline was low and increased over the 1‐year follow‐up in all treatment groups: from 117.5 to 124.1 mmHg for stable treatment, 115.7 to 125.1 mmHg for deintensification, and 117.0 to 124.0 mmHg for intensification (p < 0.001), consistent with regression to the mean (Table S6A). A ≥10 mmHg decrease between mean baseline and follow‐up SBP was less frequent after deintensification (5.7%; p < 0.001), and more frequent after intensification (7.5%; p < 0.001), compared with stable treatment (6.7%; reference); a ≥10 mmHg increase was observed in 45.9% of patients with deintensification, 38.6% with intensification, and 37.0% with stable treatment (p < 0.001; Table S6B). Similar findings were found using medication count instead of dose modification.
DISCUSSION
In this large‐scale study of over 228,000 older adults with tightly controlled SBP at baseline, we hypothesized, but did not find, that deintensification would be associated with a short‐term reduction in injurious falls and syncope. The risk of cardiovascular events and fall injury increased when baseline SBP was low, while syncope risk varied little by baseline SBP. Deintensification was associated with fewer cardiovascular events than intensification, and there was a pattern for a lower risk of cardiovascular events and fall injury when baseline SBP was very low. Changes in SBP relative to baseline were small and did not vary by treatment strategy. Mean SBP remained <130 mmHg during follow‐up for all treatment strategies.
We report our findings in the context of prior literature. Previous studies found that SBP <130 mmHg was associated with higher rates of cardiovascular events, mortality, and cognitive impairment, 9 , 10 and that antihypertensive medications were associated with a higher risk of serious fall injuries. 11 We observed a pattern for fewer fall injuries after deintensification compared with stable treatment only below baseline SBP 95 mmHg, which is consistent with a study reporting 11.4% lower fall risk after deintensification in nursing home patients with baseline 80–100 mmHg, but non‐significant association at 101–120 mmHg. 14 Another study found a 0.35 hazard ratio for recurrent fall after withdrawing fall‐risk increasing medications in 67 patients. 15 The results of those studies, compared with ours, may be explained by their focus on high fall‐risk patients, intervention on several fall‐risk increasing medications, or assessment of falls not leading to medical visit. While we observed an increased fall risk after deintensification at baseline SBP 95–130 mmHg, our findings may be related to unmeasured confounders (e.g., uncaptured higher fall risk in patients with treatment modification, treatment change related to other fall‐risk increasing medications).
In the DANTE trial, there was no difference in cardiovascular outcomes after deintensification, but patients with serious cardiovascular disease were excluded, mean baseline SBP was 148 mmHg, and follow‐up was limited to 16 weeks. 13 In SPRINT, reducing SBP from a mean of 139.7 to 121.5 mmHg decreased cardiovascular events and mortality, even in patients aged 75 and older. 3 , 4 However, none of the patients we studied would have been eligible for SPRINT, because their baseline SBP was already treated to <130 mmHg. Moreover, we included patients who were excluded in SPRINT (diabetes mellitus type 2, stroke, heart failure, dementia, 65–74 years without cardiovascular history). In this population, we found that deintensification was associated with worse outcomes, compared with a strategy of maintaining antihypertensive treatment intensity, except when baseline SBP was very low.
Our hypothesis that deintensification would be associated with fewer syncope and fall injuries, without increasing cardiovascular event risk, was not confirmed. Although we used robust methods for observational data analysis, we could not control for all confounders. It is possible that continued unmeasured confounding and/or confounding by indication may have led to our results, despite these advanced methods. Baseline characteristics and death rates during follow‐up indeed suggest that deintensified and intensified patients were sicker than those with stable treatment. One‐year mortality was indeed more than twice as high among patients who deintensified, compared with those with stable treatment. Furthermore, the association of treatment modification with adverse outcomes was greater in patients with cardiovascular disease or heart failure. This suggests that patients with treatment intensity modification had a higher baseline outcome risk. Greater outcome risk and more severe disease may have driven both treatment intensity modification and outcome occurrence. In addition, unlike a trial where patients would have been assigned to deintensification or intensification based on their baseline SBP in otherwise stable clinical conditions, we could not capture reasons other than SBP that may have led to treatment modification if they were not coded (e.g., change in control of heart failure or atrial fibrillation, end‐of‐life, medication adverse effects). These reasons could also have contributed to treatment intensification despite low baseline SBP. The outcomes may have been different in a randomized controlled trial than in this retrospective study using administrative data. Another possible explanation is that any change to a medication regimen is in itself a significant risk for older patients. Polypharmacy and age‐related changes in medication metabolism and elimination can make multimorbid older patients more sensitive to medication adverse effects and interactions. 29 The relatively small differences in follow‐up SBP across treatment groups themselves argue that the differences in outcomes observed for those with a change in their regimen versus stable treatment was mediated through something else than the BP level alone.
Strengths and limitations
Our study has several strengths. First, we included a far larger number of patients than previous studies. 14 , 16 Second, we used the best‐available real‐world data that included clinical BP measurements, and a near‐universal data source on medication fills from the largest U.S. healthcare system and Medicare. Third, we simulated an experiment by assessing the association between treatment modification and events occurring after treatment modification, using advanced statistical methods to reduce the risk of confounding: in the multivariable regression, we adjusted for a broad range of variables, and we additionally conducted a sensitivity analysis using IPTW to assure balance on average of all of that wide range of measured covariates. Furthermore, since proximity to death may lead to deintensification, we additionally weighted the sample by the inverse probability of survival. Fourth, unlike prior studies, 13 , 14 , 16 , 28 we quantified treatment intensity modification with two measures (standardized dose and medication count), averaged two consecutive SBP measurements to reduce measurement error in a key baseline covariate, and used two consecutive visits to define eligibility, according to both baseline SBP and medication, limiting the risk of including patients without tightly controlled SBP. Finally, we assessed multiple clinical outcomes over 9 months, while previous studies were limited to a few weeks, or have not yet reported clinical outcomes. 13 , 14 , 15 , 16
We must acknowledge some limitations. First, we cannot exclude unmeasured confounding resulting from bias in distributing the treatment approach in observational studies. We addressed this bias with two different methods for causal inference in observational data (regression adjustment in the main analysis and an IPTW sensitivity analysis). The similar results of the IPTW, with good PS and covariate balance, add validity to our results. However, as discussed above, administrative data alone cannot provide enough data for us to control for confounding by indication, and the small difference in SBP suggests that the results were mediated through something else than BP alone. Second, pharmacy fill data do not ascertain medication consumption. However, there is no gold standard for medication consumption in outpatient care, and prior research has proposed fills as a reliable source of data. 30 , 31 Third, the use of administrative data precluded our ability to ascertain the clinical reasoning behind any dose modification event. Fourth, the study time frame (2011–2013) predates the newest guidelines (after the SPRINT trial), which lowered recommended SBP to <130 mmHg for most older adults. 5 However, SBP target used by most healthcare systems for performance measures was identical at the time of the study to current measures (<140 mmHg). 32 , 33 , 34 Fifth, because our overarching interest was on clinical events that can impact older adults' functional status, we focused on cardiovascular events, syncope and fall injuries. We cannot draw conclusions about other types of outcomes such as acute kidney injury or electrolyte abnormalities, events that were also increased by SPRINT intervention. 3 Finally, as all studies in older Veterans, the results may not be generalizable to older women. However, the great strengths for this dataset for studying health systems outweigh this limitation. No other national dataset exists and is compiled in a national repository that includes medications, BP, comorbid conditions, and outcomes.
CONCLUSION
In this large national healthcare sample with robust administrative, medication and vital signs data, deintensifying antihypertensive treatment in older patients with tightly controlled SBP (baseline treated SBP <130 mmHg) was not associated with a lower risk of fall injury or syncope. Given higher mortality and more severe baseline cardiovascular disease severity among patients with deintensification, it is likely that patients' declining clinical state, which was inadequately recognized in the administrative data, may have both prompted attempts at deintensification and led to adverse events. Trial research will be needed to overcome these limitations of observational data, before we can conclude that treatment can be safely deintensified in older multimorbid adults without leading to adverse clinical outcomes.
ACKNOWLEDGMENTS
CONFLICT OF INTEREST
The authors declare that they do not have a conflict of interest.
AUTHOR CONTRIBUTIONS
Dr. Aubert had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the analyses. Study concept and design: Aubert, Hofer, Kerr, Min, Rodondi. Acquisition and analysis of data: Aubert, Ha, Kim, Min. Interpretation of data: Aubert, Hofer, Kerr, Kim, Min. Drafting of the manuscript: Aubert. Critical revision of the manuscript for important intellectual content: all authors.
SPONSOR'S ROLE
This research was funded by R01 from the National Institute on Aging (Min AG047178) and the Veterans Health Administration (Min IIR 14‐083). Dr. Aubert was supported by an Early Postdoc.Mobility grant from the Swiss National Science Foundation (grant P2LAP3_184042). The funders had no roles in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supporting information
File S1. Details on the study methods.
Table S1. Definition of chronic conditions.
Table S2. Blood pressure medication classes, names, and doses, based on American College of Cardiology/American Heart Association (ACC/AHA), Joint National Committee (JNC) 7, and literature reviews.
Table S3. International Classification of Diseases‐9 codes for cardiovascular events and syncope.
Table S4. Outcome rates according to treatment strategy.
Table S5. Adjusted marginal effects of treatment strategy on composite and secondary outcomes.
Table S6A. Mean change in SBP between baseline and follow‐up visits according to treatment strategy and baseline SBP.
Table S6B. Change in mean SBP ≥10 mmHg between baseline and follow‐up visits according to treatment strategy.
Figure S1. Adjusted absolute risk for composite and secondary outcomes according to medication count change and baseline systolic blood pressure.
Figure S2A. Density of the treatment propensity score for dose deintensification vs. no dose change.
Figure S2B. Absolute standardized differences for covariates of the treatment propensity score for dose deintensification vs. no dose change, in the weighted and unweighted samples.
Figure S3A. Density of the treatment propensity score for dose deintensification vs. dose intensification.
Figure S3B. Absolute standardized differences for covariates of the treatment propensity score for dose deintensification vs. dose intensification in the weighted and unweighted samples.
Figure S4A. Density of the treatment propensity score for medication count deintensification vs. no medication count change.
Figure S4B. Absolute standardized differences for covariates of the treatment propensity score for medication count deintensification vs. no medication count change, in the weighted and unweighted samples.
Figure S5A. Density of the treatment propensity score for medication count deintensification vs. medication count intensification.
Figure S5B. Absolute standardized differences for covariates of the treatment propensity score for medication count deintensification vs. medication count intensification in the weighted and unweighted samples.
Aubert CE, Ha J‐K, Kim HM, et al. Clinical outcomes of modifying hypertension treatment intensity in older adults treated to low blood pressure. J Am Geriatr Soc. 2021;69(10):2831–2841. 10.1111/jgs.17295
Funding information National Institute on Aging, Grant/Award Number: Min AG047178; Schweizerischer Nationalfonds zur Forderung der Wissenschaftlichen Forschung, Grant/Award Number: P2LAP3_184042; Veterans Health Administration HSR and D, Grant/Award Number: Min IIR 14‐083
REFERENCES
- 1. Dorans KS, Mills KT, Liu Y, He J. Trends in prevalence and control of hypertension according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline. J Am Heart Assoc. 2018;7:e008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887‐1898. [DOI] [PubMed] [Google Scholar]
- 3. Sprint Research Group , Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673‐2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127‐e248. [DOI] [PubMed] [Google Scholar]
- 6. Department of Veterans Affairs—Department of Defense . VA/DoD clinical practice guidelines for diagnosis and management of hypertension in the primary care setting. Version 4.0. 2020.
- 7. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta‐analysis. JAMA Intern Med. 2018;178:28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta‐analysis. Ann Intern Med. 2017;166:419‐429. [DOI] [PubMed] [Google Scholar]
- 9. Bangalore S, Messerli FH, Wun CC, et al. J‐curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J. 2010;31:2897‐2908. [DOI] [PubMed] [Google Scholar]
- 10. Liu H, Gao S, Hall KS, et al. Optimal blood pressure for cognitive function: findings from an elderly African‐American cohort study. J Am Geriatr Soc. 2013;61:875‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sussman JB, Kerr EA, Saini SD, et al. Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern Med. 2015;175:1942‐1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moonen JE, Foster‐Dingley JC, de Ruijter W, et al. Effect of discontinuation of antihypertensive treatment in elderly people on cognitive functioning – the DANTE Study Leiden: a randomized clinical trial. JAMA Intern Med. 2015;175:1622‐1630. [DOI] [PubMed] [Google Scholar]
- 14. Song W, Intrator O, Lee S, Boockvar K. Antihypertensive drug deintensification and recurrent falls in long‐term care. Health Serv Res. 2018;53:4066‐4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Velde N, Stricker BH, Pols HA, van der Cammen TJ. Risk of falls after withdrawal of fall‐risk‐increasing drugs: a prospective cohort study. Br J Clin Pharmacol. 2007;63:232‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sheppard JP, Burt J, Lown M, et al. Effect of antihypertensive medication reduction vs usual care on short‐term blood pressure control in patients with hypertension aged 80 years and older: the OPTIMISE randomized clinical trial. JAMA. 2020;323:2039‐2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson K, Stowasser D, Freeman C, Scott I. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ Open. 2014;4:e006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luymes CH, Boelhouwer NJ, Poortvliet RK, de Ruijter W, Reis R, Numans ME. Understanding deprescribing of preventive cardiovascular medication: a Q‐methodology study in patients. Patient Prefer Adherence. 2017;11:975‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tinetti ME, McAvay GJ, Fried TR, et al. Health outcome priorities among competing cardiovascular, fall injury, and medication‐related symptom outcomes. J Am Geriatr Soc. 2008;56:1409‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Min L, Ha JK, Aubert CE, et al. A method to quantify mean hypertension treatment daily dose intensity using health care system data. JAMA Netw Open. 2021;4:e2034059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Min L, Tinetti M, Langa KM, Ha J, Alexander N, Hoffman GJ. Measurement of fall injury with health care system data and assessment of inclusiveness and validity of measurement models. JAMA Netw Open. 2019;2:e199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560‐2572. [DOI] [PubMed] [Google Scholar]
- 23. Multimorbidity: Technical Series on Safer Primary Care . Geneva: World Health Organization; 2016. Licence: CC BY‐NC‐SA 3.0 IGO.
- 24. Min L, Ha JK, Hofer TP, et al. Validation of a health system measure to capture intensive medication treatment of hypertension in the Veterans Health Administration. JAMA Netw Open. 2020;3:e205417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206‐1252. [DOI] [PubMed] [Google Scholar]
- 26. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507‐520. [DOI] [PubMed] [Google Scholar]
- 27. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426‐e483. [DOI] [PubMed] [Google Scholar]
- 28. Luymes CH, Poortvliet RKE, van Geloven N, et al. Deprescribing preventive cardiovascular medication in patients with predicted low cardiovascular disease risk in general practice – the ECSTATIC study: a cluster randomised non‐inferiority trial. BMC Med. 2018;16:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mangoni AA, Jackson SH. Age‐related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grymonpre R, Cheang M, Fraser M, Metge C, Sitar DS. Validity of a prescription claims database to estimate medication adherence in older persons. Med Care. 2006;44:471‐477. [DOI] [PubMed] [Google Scholar]
- 31. Anghel LA, Farcas AM, Oprean RN. An overview of the common methods used to measure treatment adherence. Med Pharm Rep. 2019;92:117‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Center for Medicare & Medicaid Services . Physician quality reporting system (physician quality reporting) measure specifications manual for claims and registry reporting of individual measures. 2011. https://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/PQRS/downloads/2011_PhysQualRptg_MeasureSpecificationsManual_033111.pdf. Accessed June 01, 2021.
- 33. National Committee for Quality Assurance (NCQA) . HEDIS measures and technical resources. controlling high blood pressure (CBP). https://www.ncqa.org/hedis/measures/controlling-high-blood-pressure/. Accessed June 01, 2021.
- 34. Center for Medicare and Medicaid Services . Health insurance exchange. 2021 Quality rating system measure technical specifications. 2020. https://www.cms.gov/files/document/2021-qrs-measure-technical-specifications.pdf. Accessed June 01, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Details on the study methods.
Table S1. Definition of chronic conditions.
Table S2. Blood pressure medication classes, names, and doses, based on American College of Cardiology/American Heart Association (ACC/AHA), Joint National Committee (JNC) 7, and literature reviews.
Table S3. International Classification of Diseases‐9 codes for cardiovascular events and syncope.
Table S4. Outcome rates according to treatment strategy.
Table S5. Adjusted marginal effects of treatment strategy on composite and secondary outcomes.
Table S6A. Mean change in SBP between baseline and follow‐up visits according to treatment strategy and baseline SBP.
Table S6B. Change in mean SBP ≥10 mmHg between baseline and follow‐up visits according to treatment strategy.
Figure S1. Adjusted absolute risk for composite and secondary outcomes according to medication count change and baseline systolic blood pressure.
Figure S2A. Density of the treatment propensity score for dose deintensification vs. no dose change.
Figure S2B. Absolute standardized differences for covariates of the treatment propensity score for dose deintensification vs. no dose change, in the weighted and unweighted samples.
Figure S3A. Density of the treatment propensity score for dose deintensification vs. dose intensification.
Figure S3B. Absolute standardized differences for covariates of the treatment propensity score for dose deintensification vs. dose intensification in the weighted and unweighted samples.
Figure S4A. Density of the treatment propensity score for medication count deintensification vs. no medication count change.
Figure S4B. Absolute standardized differences for covariates of the treatment propensity score for medication count deintensification vs. no medication count change, in the weighted and unweighted samples.
Figure S5A. Density of the treatment propensity score for medication count deintensification vs. medication count intensification.
Figure S5B. Absolute standardized differences for covariates of the treatment propensity score for medication count deintensification vs. medication count intensification in the weighted and unweighted samples.


