Abstract
Background/Objective:
Medicare Advantage (MA) and Accountable Care Organizations (ACOs) operate under incentives to reduce burdensome and costly care at the end of life. We compared end-of-life care for persons with dementia who are in MA, ACOs, or traditional Medicare (TM).
Design, Setting, and Participants:
Retrospective study of decedents with dementia enrolled in MA, attributed to an ACO, or in TM. Decedents had a nursing home stay between 91 and 180 days prior to death, two or more functional impairments, and mild to severe cognitive impairment.
Measurements:
Hospitalization, invasive mechanical ventilation (IMV) use, and in-hospital death in the last 30 days of life reported in Medicare billing.
Results:
Among 370,094 persons with dementia, 93,801 (25.4%) were in MA (mean age [SD], 86.9 [7.7], 67.6% female), 39,586 (10.7%) were ACO attributed (mean age [SD], 87.2 [7.6], 67.3% female), and 236,707 (63.9%) were in TM (mean age [SD], 87.0 [7.8], 67.6% female). The proportion hospitalized in the last 30 days of life was higher among TM enrollees (27.9%) and those ACO attributed (28.1%) than among MA enrollees (20.5%, p ≤ 0.001). After adjustment for socio-demographics, cognitive and functional impairments, comorbidities, and Hospital Referral Region, adjusted odds of hospitalization in the 30 days prior to death was 0.72 (95% confidence interval [CI] 0.70–0.74) among MA enrollees and 1.05 (95% CI 1.02–1.09) among those attributed to ACOs relative to TM enrollees. Relative to TM, the adjusted odds of death in the hospital were 0.78 (95% CI 0.75–0.81) among MA enrollees and 1.02 (95% CI 0.96–1.08) for ACO participants. Dementia decedents in MA had a lower likelihood of IMV use (adjusted odds ratio 0.80, 95% CI 0.75–0.85) compared to TM.
Conclusions:
Among decedents with dementia, MA enrollees but not decedents in ACOs experienced less costly and potentially burdensome care compared with those with TM. Policy changes are needed for ACOs.
Keywords: Accountable Care Organizations, dementia, end of life, Medicare Advantage plans
INTRODUCTION
Alzheimer’s disease and related dementias (ADRD) are a group of devastating, progressive neurological disorders that afflicts one-in-nine Americans. Persons with ADRD have high costs and high needs.1 Among persons who die with dementia, average Medicare spending in the last 5 years of life is $287,038, exceeding end-of-life spending for other leading causes of death.2 ADRD patients and their families often experience burdensome and costly interventions at the end of life that may be of limited benefit and not aligned with patients’ preferences. Examples include percutaneous endoscopic gastrostomy tube insertion,3–5 invasive mechanical ventilation (IMV),6 and transfers from nursing homes (NHs) to hospitals.7
Some observers contend that the high use of procedures and hospitalizations at the end of life may be driven by traditional Medicare’s fee-for-services payment incentives, which reward the volume and intensity of care. In contrast, alternative payment models such as Accountable Care Organizations (ACOs) and Medicare Advantage (MA) focus on reducing per-person spending and offer financial rewards for improving healthcare quality. Federal policy has stimulated substantial growth in MA plans and ACOs, with 11.2 million Medicare beneficiaries attributed to an ACO and 24.1 million enrolled in an MA plan in 2020. However, there is limited evidence about whether these alternative payment models impact the care of seriously ill patients at the end of life, and comparisons of care in MA plans with that in ACOs are lacking.
The main objective of this report is to better understand the impact of the varied payment models covering older persons with dementia on the intensity of care at the end of life. To accomplish this objective, we used nationwide data to identify Medicare decedents with a NH stay in the last months of life with ADRD between 2017 and 2019, and categorized them as being covered by TM, attributed to an ACO, or enrolled in MA in the last year of life. We examined the association between each payment model and the following outcomes: hospitalization in the last 30 days of life, IMV in the last 30 days of life, and in-hospital death.
METHODS
Data and study population
Using national Minimum Data Set (MDS) assessments from 2017 and 2018, we identified a cohort of all NH decedents with ADRD who had an MDS assessment between 91 and 180 days prior to death for either skilled nursing facility services or custodial care (see Figure S1). The MDS is a federally mandated assessment that contains demographic and clinical information such as measures of cognition, function, and medical diagnoses for all Medicare beneficiaries treated at Medicare or Medicaid-certified NHs. The study population included decedents who met all three criteria: (1) a diagnosis of ADRD; (2) a Cognitive Function Scale (CFS) score of 2–4,8 indicating mild to severe impairments; and (3) impairments in two or more activities of daily living (ADLs). Persons were eligible if study cohort members were categorized as attributed to an ACO, enrolled in MA plan, or enrolled in TM in the final month of life. For MA plans, we examined enrollment during the 12th month prior to death. The Center for Medicare and Medicaid Services creates measures of ACO attribution based on whether a patient received a majority of evaluation and management visits between January 1 and December 31 from an ACO-affiliated healthcare provider for primary care services. The use of Centers for Medicare and Medicaid (CMS) data was approved by a data use agreement. Brown University Institutional Review Board waived informed consent in this population based on secondary data analysis.
Measures
The primary study outcomes were hospitalizations in the last 30 day of life, use of IMV during hospitalization, and dying in an acute care hospital. Starting in 2008, hospitals began submitting encounter records for hospitalizations of MA beneficiaries, which allowed CMS to calculate disproportionate share payments and indirect and direct medical education adjustments.9 Critical access hospitals and those hospitals that cared for only MA patients were not required to submit hospitalization claims data; however, prior work has suggested that over 90% of MA hospitalizations are captured in these data.10 Validated procedure codes were used to identify use of IMV (ICD-9/10-CM codes: 96.7x/5A1935Z, 5A1945Z, 5A1955Z) while hospitalized.11–15 We also used hospice claims which are reported for enrollees in MA, ACOs, and TM to calculate hospice use and hospice length of stay for descriptive purposes.
Individual characteristics
To characterize differences between decedents enrolled in MA, ACO, or TM, the Medicare Master Beneficiary Summary File and the MDS version 3.0 were used. We classified patients as MA enrollees, ACO participants, or TM beneficiaries. MA was defined by enrollment in an MA plan during the month of death; ACO was defined as being attributed to an ACO based on the final attribution of the year. All other patients not in an MA plan or attributed to an ACO were defined as TM. Sensitivity analyses described below classified MA enrollment using the period 12 months prior to death. Age and sex were based on information collected by the Social Security Administration as a part of the Medicare Master Beneficiary Summary File. We utilized the Research Triangle Institute race/ethnicity code which improves on the basic race/ethnicity variable. Information from the MDS included medical diagnoses, ADL impairments, cognitive function as measured by the CFS, dual eligibility with Medicaid, and other patient characteristics based on the MDS completed between 91 and 180 days prior to death. The decedent’s zip code was used to identify Hospital Referral Region (HRR) based on crosswalk of zip code to HRR from the Dartmouth Atlas of Health Care.
Statistical analysis
Descriptive statistics were used to assess the characteristics of the decedents in the three insurance categories, using means and standard deviations for continuous variables and frequencies for categorical variables. Multivariable logistic regression models examined the association of MA, ACO, and TM with being hospitalized in the last 30 days of life, dying in an acute care hospital, and the use of IMV during the hospitalization closest to death. The multivariable model adjusted for age, gender, race/ethnicity, number of ADL impairments (2–7), cognitive function, whether the NH stay was a skilled NH stay for rehabilitation, dual eligibility with Medicaid, and comorbidities from the MDS assessment and included fixed effects for HRR. Thus, our models compared the difference in outcomes for MA, ACO, and TM enrollees residing in the same HRR. Enrollment in a TM plan was treated as the reference category.
Three sensitivity analyses were performed. First, we classified MA enrollment and ACO attribution based on the year prior to death. Second, analysis was done among those with a CFS score of 4, which indicated severe cognitive impairment. Third, we restricted the analyses to the exclude critical access hospitals and those hospitals that did not take indirect medical education payment. Data analyses were performed in Stata version 16 (Stata Corp LLC) from November 23, 2020 to February 11, 2021. We specified a two-tailed significance threshold of 0.05.
RESULTS
Among 370,094 persons with dementia, 93,801 (25.4%) were in an MA plan (mean age [SD], 86.9 [7.7], 67.6% female), 39,586 (10.7%) were attributed to an ACO (mean age [SD], 87.2 [7.6], 67.3% female), and 236,707 (63.9%) were in TM (mean age [SD], 87.0 [7.8], 67.6% female). Table 1 examines characteristics of decedents with dementia in an MA plan, ACO, or TM. Compared to TM and MA, Medicare beneficiaries in ACOs were more likely to be white and less likely to be dual-eligible, severely cognitively impaired, or have seven ADL impairments. Otherwise, the differences between MA, ACO, and TM were modest.
TABLE 1.
Characteristics of 2017 and 2018 Medicare decedents with dementia in traditional Medicare, Medicare Advantage, and Accountable Care Organizations
| Socio-demographic characteristics | Entire sample (n = 370,094) | Traditional Medicare (n = 236,707) | Medicare Advantage (n = 93,801) | Accountable Care Organizations (n = 39,586) |
|---|---|---|---|---|
| Age, mean (SD) | 86.9 (7.7) | 87.0 (7.8) | 86.9 (7.6) | 87.2 (7.6) |
| Female sex (%) | 67.6 | 67.6 | 67.9 | 67.3 |
| Race/ethnicity, % (95% CI) | ||||
| White | 81.4 (81.3, 81.5) | 81.2 (81.0, 81.4) | 79.9 (79.6, 80.1) | 86.3 (86.0,86.7) |
| Black | 10.6 (10.5, 10.7) | 10.5 (10.4, 10.7) | 11.6 (11.4, 11.8) | 8.4 (8.2, 8.7) |
| Asian | 1.9 (1.8, 1.9) | 2.1 (2.0, 2.1) | 1.9 (1.8, 2.0) | 0.9 (0.8, 1.0) |
| Hispanic | 5.2 (5.1, 5.3) | 5.2 (5.1, 5.3) | 5.9 (5.7, 6.0) | 3.7 (3.5, 3.9) |
| Dual-eligible status, % (95% CI) | 60.0 (59.8, 60.2) | 60.1 (59.9, 60.3) | 61.4 (61.0, 61.7) | 56.3 (55.8, 56.7) |
| Comorbidities, % (95% CI) | ||||
| Heart failure | 22.8 (22.7, 23.0) | 22.8 (22.6, 22.9) | 22.6 (22.3, 22.8) | 23.9 (23.5, 24.3) |
| Pneumonia | 3.8 (3.8, 3.9) | 3.8 (3.7, 3.9) | 3.7 (3.6, 3.9) | 4.2 (4.0, 4.4) |
| Hip fracture | 3.9 (3.8, 4.0) | 3.8 (3.8, 3.9) | 3.8 (3.7, 3.9) | 4.3 (4.1, 4.5) |
| CVA, TIA, or stroke | 10.9 (10.8, 11.0) | 11.0 (10.8, 11.1) | 10.6 (10.4, 10.7) | 11.1 (10.8, 11.4) |
| Asthma, COPD, or chronic lung disease | 20.3 (20.2, 20.5) | 20.5 (20.3, 20.6) | 19.7 (19.5, 19.9) | 20.9 (20.5, 21.2) |
| Cognitive function score, % (95% CI) | ||||
| 2 | 18.3 (18.2, 18.5) | 18.0 (17.8, 18.1) | 18.4 (18.1, 18.6) | 20.4 (20.0, 20.8) |
| 3 | 54.8 (54.7, 55.0) | 54.6 (54.4, 54.8) | 55.1 (54.8, 55.4) | 55.6 (55.1, 56.1) |
| 4 | 26.8 (26.7, 27.0) | 27.4 (27.3, 27.6) | 26.5 (26.2, 26.8) | 23.9 (23.5, 24.4) |
| ADL impairments, % (95% CI) | ||||
| 2 | 2.7 (2.7, 2.8) | 2.7 (2.6, 2.7) | 2.8 (2.7, 2.9) | 3.2 (3.0, 3.4) |
| 3 | 4.0 (3.9, 4.0) | 3.8 (3.7, 3.9) | 4.1 (4.0, 4.2) | 4.3 (4.1, 4.5) |
| 4 | 4.3 (4.2, 4.3) | 4.2 (4.1, 4.2) | 4.3 (4.1, 4.4) | 4.9 (4.7, 5.1) |
| 5 | 11.0 (10.9, 11.1) | 10.9 (10.8, 11.1) | 11.1 (10.9, 11.3) | 11.3 (11.0, 11.6) |
| 6 | 34.5 (34.4, 34.7) | 33.8 (33.6, 34.0) | 35.3 (35.0, 35.7) | 36.6 (36.1, 37.1) |
| 7 | 43.5 (43.3, 43.7) | 44.6 (44.4, 44.8) | 42.4 (42.1, 42.7) | 39.7 (39.2, 40.2) |
| NH stay was for skilled nursing and/or rehabilitation, % (95% CI) | 12.8 (12.7, 13.0) | 12.8 (12.7, 13.0) | 11.8 (11.6, 12.0) | 15.2 (14.8, 15.5) |
Abbreviations: ADL, activities of daily living; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; NH, nursing home; TIA, transient ischemic attack.
End-of-life care
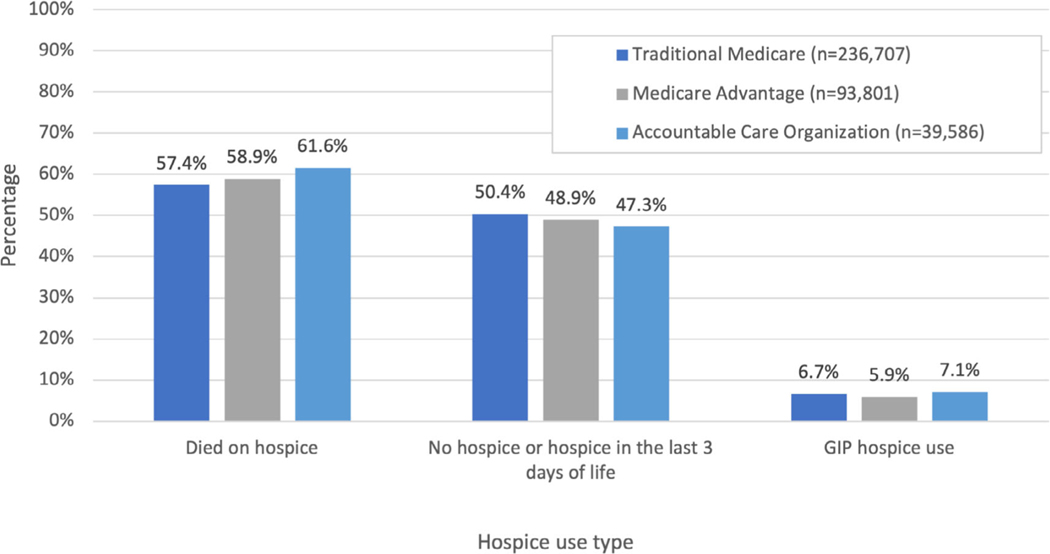
Table 2 presents the univariate and adjusted outcomes for participant enrollment in MA and ACOs compared to TM with the three intensity of care outcomes. Slightly more than one in four (26.0%) persons with dementia were hospitalized in the last 30 days of life, with 8.3% dying in an acute care hospital. TM and ACO decedents were more likely to be hospitalized in the last 30 days of life compared to MA plans (27.9% and 28.1%. respectively, compared to 20.5% in MA, p < 0.001). After adjustment for potential confounders in the HRR fixed effects model, dementia patients in MA experienced a 28% lower proportion of hospitalizations (adjusted odds ratio [AOR] 0.72, 95% confidence interval [CI] 0.70–0.74) while persons attributed to an ACO had slightly higher hospitalizations (AOR 1.05, 95% CI 1.02–1.09) compared to TM. Lower proportions of MA enrollees died in the hospital or received IMV compared to TM or ACO (see Table 2). The lower risk of IMV use in MA patients was largely driven by avoiding hospitalizations in the last 30 days of life. When the risk of IMV use was examined among those hospitalized, the AOR for the risk of IMV among MA patients was 0.96 (95% CI 0.89–1.0), with TM patients as the reference group. Figure 1 documents the use of hospice, finding that both MA plans and ACO had higher use of hospice than TM but the number of days in hospice in the last 30 days of life was similar.
TABLE 2.
Association of Medicare Advantage, attribution to Accountable Care Organization, and traditional Medicare with end-of-life care for persons with dementia
| Traditional Medicare (n = 236,707) |
Medicare Advantage (n = 93,801) |
Accountable Care Organizations (n = 39,586) |
||||
|---|---|---|---|---|---|---|
| Utilization measure | Univariate, % (95% CI) | Multivariate AOR (95% CI) | Univariate, % (95% CI) | Multivariate AOR (95% CI) | Univariate, % (95% CI) | Multivariate AOR (95% CI) |
| Hospital stay in last 30 days | 27.9 (27.7, 28.1) | Ref. | 20.5 (20.3, 20.8) | 0.72 (0.70, 0.74) | 28.1 (27.7, 28.5) | 1.05 (1.02, 1.09) |
| Died in hospital | 9.0 (8.9, 9.2) | Ref. | 6.6 (6.4, 6.8) | 0.78 (0.75, 0.81) | 8.2 (8.0, 8.5) | 1.02 (0.96, 1.08) |
| Invasive mechanical ventilation | 4.5 (4.4, 4.6) | Ref. | 3.5 (3.4, 3.6) | 0.80 (0.75, 0.85) | 3.8 (3.7, 4.0) | 1.04 (0.97, 1.13) |
Abbreviation: AOR, adjusted odds ratio.
FIGURE 1.
Hospice use among traditional Medicare, Medicare Advantage, and Accountable Care Organizations. The figure depicts the proportion of decedents enrolled in a Medicare Advantage plan, attributed to an Accountable Care Organization, and in traditional Medicare use of hospice services
The Supporting Information reports sensitivity analyses. A concern is that the sickest patients may disenroll from MA and that attribution drops off from ACOs in the year of death. Table S1 reports the same multivariate analyses with ACO attribution in year prior to death (e.g., for 2018 deaths, the final ACO attribution of 2017 was used) and MA enrollment 12 months prior to death. A similar direction and magnitude of results were found. We also repeated the analysis among persons with a CFS equal to 4, indicating severe cognitive impairment and found similar results (Table S2). A third sensitivity analyses restricts to hospitals taking Indirect Medical Education funding and excludes critical access hospitals.
DISCUSSION
In 2017 and 2018, more than one in three decedents with dementia, functional impairments, and NH use were enrolled in MA or attributed to an ACO. These two alternative payment models operate under incentives to lower healthcare spending, which could potentially reduce burdensome, low value, and costly care at the end of life for persons with dementia. In this analysis, MA enrollment was associated with reduced hospitalizations, less use of IMV, and lower rates of dying in the hospital among persons with dementia. Contrary to MA, we found no evidence that attribution to an ACO reduced the risk of hospitalizations at the end of life. Under the current alternative payment models, managed care, but not ACOs, are associated with a substantial reduction in acute and invasive care at the end of life among persons with dementia.
As of January 1, 2020, there were 517 ACO organizations serving over 11.2 million Medicare beneficiaries. ACOs are responsible for the total costs of care as long as persons remain attributed to that ACO. The majority of Medicare ACOs use retrospective attribution which assigns a Medicare beneficiary to an ACO based on evaluation and management billing for primary care services of eligible providers affiliated with that ACO. About 24 million persons are enrolled in MA plans which are increasingly providing care to Medicare beneficiaries who die.10 Through enhanced care coordination, MA and ACOs could potentially improve the quality of care for persons with ADRD, especially in the last year of life.
Previous studies have documented lower use of hospital care among MA enrollees compared to TM.9,16–18 Stevenson et al. contrasted MA and TM decedents from 2003 to 2009, finding increased use of hospice that narrowed with time and lower rate of inpatient admissions in MA.19 Prior research suggests that MA achieves this efficiency through increasing the intensity of primary care services in NHs, which prevents hospitalizations.20,21 Since 2009, MA has increasingly provided care for persons with dual Medicare/Medicaid eligibility and for decedents. However, the sickest and most costly patients are more likely to disenroll from MA.22–25 Using Medicare Beneficiary Survey data from 2010 to 2016, Park et al. found only small differences in healthcare utilization of ADRD patients in MA compared to TM.18 No study to date has examined MA and ACO care of persons with dementia, a high-cost and high-need population.
Unlike previous studies finding that MA plans enrolled beneficiaries that are healthier, our population of persons with dementia all had an MDS assessment prior to death that provided information on both functional and cognitive status as well as other covariates, which reduced heterogeneity in the patients in the three payment models. As shown in our sensitivity analysis, the lower use of hospital care in MA is unlikely to result from differential disenrollment in the year prior to death. While prior research has found selective disenrollment in MA plans with sicker Medicare beneficiaries exiting MA,23,24 our sensitivity analysis that examined MA enrollment 1 year prior to death found similar results (see Table S1). One prospective study with Medicare Current Beneficiary Survey data from 2010 to 2016 found a modest difference in inpatient use between MA and TM persons with self-identified dementia or dementia reported by proxies in a prospective cohort.18 We found a 28% reduction in hospitalizations in the last month of life. This avoidance of hospitalizations resulted in a reduction in use of IMV, which may be lifesaving for some, but burdensome to dying persons in the last days of life.
Persons discharged from ACO hospitals to ACO-affiliated skilled nursing facilities were reported as having lower hospital readmission rates, shorter hospital lengths of stay, and decreased Medicare costs relative to non-ACO decedents.26 Cost-saving in ACOs has been found in persons with multiple chronic illnesses,27 and clinically vulnerable older persons report higher quality of care in ACOs compared to TM.28 The lower hospitalization and IMV rates in MA but not ACOs may be explained by differences in financing and organization in these two alternative payment models. MA plans, unlike both ACOs and TM, are paid based on a capitated basis, which means that they bear risk for financing all covered medical care. This liability potentially incentivizes improved care coordination, advance care planning, and referral to hospice. ACO incentives focus on healthcare professionals, including hospitals, forming local healthcare delivery systems that can retain a portion of cost savings if their assigned beneficiaries’ per-capita medical spending is below a prespecified benchmark. Additionally, ACOs are unable to use cost-containment strategies available to MA plans, such as restricting provider networks, requiring prior authorization for the use of health services, or changing cost-sharing or other features of insurance benefits. These strategies may explain the lower use of acute and invasive care among MA enrollees.
At this stage of ACO development, there are concerns with ACOs’ ability to control costs. The majority of ACOs do not have a downside risk of losing money if the ACO does not achieve cost savings. ACOs taking downside risks saved on average $96 dollars per beneficiary compared to $68 dollars per beneficiary in those ACOs without downside risk.29 In our sample of 2018 deaths, only 41 out of 649 ACOs had any potential for financial losses based on their type of ACO model. A 2019 rule entitled, Pathway to Success, aims to shorten the time an ACO can only participate in only upside financial risk.30 Other reforms have been suggested by Medicare Payment Advisory Commission as well as by Antos and Capretta.29 ACOs may need to enhance their ability to coordinate care through tracking, monitoring, and managing the end-of-life care of this high-need, high-cost population.
An important limitation of our research and the use of Medicare administrative data is lack of consumer perceptions of the quality of dying in these three payment models. Using a retrospective family or close friend survey, Ankuda et al. found that decedents enrolled in TM reported higher ratings of the quality of care compared to those enrolled in MA at the time of death.31 A key difference between the research reported in this article is our cohort of decedents with dementia and functional impairment are less likely to benefit from hospitalization, and prior research suggests that the vast majority of families of persons with dementia prefer care focused on comfort.32 A previous study found similar perceptions of the quality of care in MA versus TM among a prospective cohort of persons with dementia.18
Our study has additional limitations. First, while the MDS provides detailed clinical data, there may be unmeasured differences between the three groups in the study, including patient preferences. Because research has found the sickest and costliest persons disenroll from MA,24 we conducted a sensitivity analysis (see Table S1) that examined persons enrolled in an MA plan 12 months prior to death with and found results with a similar direction and effect size. It is still possible that persons could have disenrolled from MA years prior to death. Second, our sample selection required an MDS assessment to ascertain dementia diagnoses as claims-based diagnosis information for persons enrolled in MA is limited. Furthermore, we used assessments between 91 and 180 days prior to death, a time frame that allowed us to observe whether NH patients were hospitalized after their MDS assessment. Based on TM billing data, this MDS-based approach captured 46.8% of persons with a chronic condition indicator of decedents with dementia diagnosis (see Table S2 sensitivity finding similar findings even among those with the most advanced cognitive impairment). This potentially limited the generalizability of our findings, but the use of the MDS allowed the study to adjust for difference in function, cognition, and other comorbidities, and it is likely that a high proportion of decedents with advanced dementia receive NH care during the last 6 months of life.33 Third, the use of MA data from MEDPAR does not capture hospitalizations from critical access hospitals and hospitals that only care for MA patients (see Table S3). It is estimated that MEDPAR MA data cover 92% of the hospitalizations and our sensitivity analyses restricting to hospitalizations that report their MA billing yielded similar findings. Finally, MA enrollees observation stays and ER utilization is not captured in Medicare administrative data.
These results suggest that MA is more efficient than ACOs or TM. As noted, ACO are unable to employ cost containment strategies available to MA plans such as use of prior authorization, changes to cost sharing, or restricting the provider network. Prior research found that MA intensified the provision of primary care services in NH to prevent hospitalizations. Our findings of decrease hospitalizations, but once hospitalized no difference in the use of IMV is consistent with this prior research. Future research is needed to confirm these findings given potential concerns with generalizability as well concerns with understanding the potential mechanisms of MA achieving lower rates of potentially burdensome treatment in this seriously ill population with dementia. It would be important to note whether the lower rates of hospitalizations are based on respect of patient preferences and a shared decision-making process involving healthcare proxies, which respects autonomy and ensures high-quality end-of-life care in the NH setting. If the reduction in hospitalizations is based on preferences voiced by the person in advance or by proxy, these results support policy changes to create incentives that improves care of seriously ill persons in ACOs.
CONCLUSION
Persons with dementia have high costs, substantial and often unmet medical needs, and are at high risk for receiving care not consistent with their goals of care, including burdensome transitions, feeding tube placement, and IMV at the end of life. In this study of Medicare beneficiaries with dementia, cognitive decline, and functional impairment, persons in MA plans, but not those in ACOs, had substantially lower rates of hospitalization and IMV at the end of life compared to those in TM. Future research should compare patient or family perceptions of the quality of care across alternative payment models. If confirmed, these results provide evidence for further modifications of ACOs and continued research regarding the reasons for less intensive care at the end of life among MA enrollees with dementia.
Supplementary Material
Figure S1. Sample flow
Table S1. Utilization outcomes based on different assignment to MA or ACO
Table S2. Sensitivity analysis among those with severe cognitive impairment
Table S3. Analysis restricted to only those institutions that took Indirect Medical Education (IME) funding and the hospital was not classified as a critical access hospital
Key Points.
Alternative payment models such as Medicare Advantage and Accountable Care Organizations change the incentives of traditional Medicare by focusing on value.
Potentially, these new payment models reduce incentives for more intensive care at end of life.
Patients with dementia enrolled in Medicare Advantage plans, but not Accountable Care Organizations, were less likely to be hospitalized, die in a hospital, and receive mechanical ventilation compared to those in traditional Medicare.
Why Does this Paper Matter?
Compared to traditional Medicare, dementia patients enrolled in Medicare Advantage plans, but not Accountable Care Organizations, were less likely to receive burdensome and costly acute care at the end of life.
ACKNOWLEDGMENTS
The authors thank Robert Wolf who prepared the data base for analysis as part of his job.
FINANCIAL DISCLOSURE
Research in this article was supported by the National Institute on Aging of the National Institutes of Health (NIH) (2P01AG027296-11). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The code sharing document used in this study is part of the Brown University Digital Repository (DOI: https://doi.org/10.26300/gvs1-eh83).
Funding information
National Institute on Aging, Grant/Award Number: 2P01AG027296-11
SPONSOR’S ROLE
The sponsor did not have any role in the design, conduct, writing, or review of the submitted version of the manuscript.
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
REFERENCES
- 1.Keeney T, Belanger E, Jones RN, Joyce NR, Meyers DJ, Mor V. High-need phenotypes in Medicare beneficiaries: drivers of variation in utilization and outcomes. J Am Geriatr Soc. 2020;68(1):70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelley AS, McGarry K, Gorges R, Skinner JS. The burden of health care costs for patients with dementia in the last 5 years of life. Ann Intern Med. 2015;163(10):729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teno JM, Mor V, DeSilva D, Kabumoto G, Roy J, Wetle T. Use of feeding tubes in nursing home residents with severe cognitive impairment. JAMA. 2002;287(24):3211–3212. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell SL, Teno JM, Roy J, Kabumoto G, Mor V. Clinical and organizational factors associated with feeding tube use among nursing home residents with advanced cognitive impairment. JAMA. 2003;290(1):73–80. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell SL, Mor V, Gozalo PL, Servadio JL, Teno JM. Tube feeding in US nursing home residents with advanced dementia, 2000–2014. JAMA. 2016;316(7):769–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teno JM, Gozalo P, Khandelwal N, et al. Association of increasing use of mechanical ventilation among nursing home residents with advanced dementia and intensive care unit beds. JAMA Intern Med. 2016;176(12):1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gozalo P, Teno J, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365(13):1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas KS, Dosa D, Wysocki A, Mor V. The minimum data set 3.0 cognitive function scale. Med Care. 2017;55(9):e68–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teno JM, Gozalo P, Trivedi AN, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000–2015. JAMA. 2018;320(3):264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuman P, Jacobson GA. Medicare advantage checkup. N Engl J Med. 2018;379(22):2163–2172. [DOI] [PubMed] [Google Scholar]
- 11.de Miguel-Diez J, Jimenez-Garcia R, Hernandez-Barrera V, et al. National trends in hospital admissions for asthma exacerbations among pediatric and young adult population in Spain (2002–2010). Respir Med. 2014;108(7):983–991. [DOI] [PubMed] [Google Scholar]
- 12.Stefan MS, Priya A, Pekow PS, et al. The comparative effectiveness of noninvasive and invasive ventilation in patients with pneumonia. J Crit Care. 2018;43:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valley TS, Walkey AJ, Lindenauer PK, Wiener RS, Cooke CR. Association between noninvasive ventilation and mortality among older patients with pneumonia. Crit Care Med. 2017;45 (3):e246–e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wunsch H, Kramer A, Gershengorn HB. Validation of intensive care and mechanical ventilation codes in Medicare data. Crit Care Med. 2017;45(7):e711–e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta AB, Douglas IS, Walkey AJ. Evidence-based utilization of noninvasive ventilation and patient outcomes. Ann Am Thorac Soc. 2017;14(11):1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belanger E, Silver B, Meyers DJ, et al. A retrospective study of administrative data to identify high-need Medicare beneficiaries at risk of dying and being hospitalized. J Gen Intern Med. 2019;34(3):405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huckfeldt PJ, Escarce JJ, Rabideau B, Karaca-Mandic P, Sood N. Less intense postacute care, better outcomes for enrollees in Medicare advantage than those in fee-for-service. Health Aff (Millwood). 2017;36(1):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S, White L, Fishman P, Larson EB, Coe NB. Health care utilization, care satisfaction, and health status for Medicare Advantage and traditional Medicare beneficiaries with and without Alzheimer disease and related dementias. JAMA Netw Open. 2020;3(3):e201809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson DG, Ayanian JZ, Zaslavsky AM, Newhouse JP, Landon BE. Service use at the end-of-life in Medicare advantage versus traditional Medicare. Med Care. 2013;51(10):931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane RL, Flood S, Bershadsky B, Keckhafer G. Effect of an innovative medicare managed care program on the quality of care for nursing home residents. Gerontologist. 2004;44(1): 95–103. [DOI] [PubMed] [Google Scholar]
- 21.Goldfeld KS, Grabowski DC, Caudry DJ, Mitchell SL. Health insurance status and the care of nursing home residents with advanced dementia. JAMA Intern Med. 2013;173(22):2047–2053. 10.1001/jamainternmed.2013.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jutkowitz E, Bynum JPW, Mitchell SL, et al. Diagnosed prevalence of Alzheimer’s disease and related dementias in Medicare Advantage plans. Alzheimers Dement (Amst). 2020;12(1): e12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahman M, Keohane L, Trivedi AN, Mor V. High-cost patients had substantial rates of leaving Medicare Advantage and joining traditional Medicare. Health Aff (Millwood). 2015;34(10): 1675–1681. 10.1377/hlthaff.2015.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyers DJ, Belanger E, Joyce N, McHugh J, Rahman M, Mor V. Analysis of drivers of disenrollment and plan switching among Medicare advantage beneficiaries. JAMA Intern Med. 2019;179(4):524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyers DJ, Rahman M, Rivera-Hernandez M, Trivedi AN, Mor V. Plan switching among Medicare Advantage beneficiaries with Alzheimer’s disease and other dementias. Alzheimers Dement (N Y). 2021;7(1):e12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal D, Werner RM. Effect of hospital and post-acute care provider participation in accountable care organizations on patient outcomes and Medicare spending. Health Serv Res. 2018;53(6):5035–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colla CH, Lewis VA, Kao LS, O’Malley AJ, Chang CH, Fisher ES. Association between Medicare accountable care organization implementation and spending among clinically vulnerable beneficiaries. JAMA Intern Med. 2016;176(8):1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare shared savings program. JAMA Intern Med. 2017;177: 518–526. 10.1001/jamainternmed.2016.9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antos JRC, Capretta JC. Treat ACOs and MA plans equally? By all means. Health Affairs Blog. 2019. https://www.healthaffairs.org/do/10.1377/hblog20191112.742234/full/. Accessed December 22, 2020. [Google Scholar]
- 30.Verma S. More ACOs taking accountability under MSSP through “pathways to success”. Health Affairs Blog. 2019. https://www.healthaffairs.org/do/10.1377/hblog20190717.482997/full/ Accessed January 3, 2021. [Google Scholar]
- 31.Ankuda CK, Kelley AS, Morrison RS, Freedman VA, Teno JM. Family and friend perceptions of quality of end-of-life care in Medicare Advantage vs traditional Medicare. JAMA Netw Open. 2020;3(10):e2020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crouch E, Probst JC, Bennett K, Eberth JM. Differences in Medicare utilization and expenditures in the last six months of life among patients with and without Alzheimer’s disease and related disorders. J Palliat Med. 2019;22(2):126–131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Sample flow
Table S1. Utilization outcomes based on different assignment to MA or ACO
Table S2. Sensitivity analysis among those with severe cognitive impairment
Table S3. Analysis restricted to only those institutions that took Indirect Medical Education (IME) funding and the hospital was not classified as a critical access hospital