Abstract
Background/Objectives:
Neurohormonal therapy, which includes beta-blockers and ACEi/ARBs, is the cornerstone of heart failure with reduced ejection fraction (HFrEF) treatment. While neurohormonal therapies have demonstrated efficacy in randomized clinical trials, older patients, which now comprise the majority of HFrEF patients, were underrepresented in those original trials. This study aimed to determine the association between short (30 day) and long-term (1 year) mortality and the use of neurohormonal therapy in HFrEF patients, across the age spectrum.
Design/Setting/Participants:
This is a population-based, retrospective, cohort study between January 2008 and December 2015. We used 100% Medicare Parts A and B and a random 40% sample of Part D to create a cohort of 295,494 fee-for-service beneficiaries with at least one hospitalization for HFrEF between 2008 and 2015. All analyses were performed between May 2019 and July 2020.
Exposure:
We used Part D data to determine exposure to beta-blocker and angiotensin converting enzyme inhibitor (ACEi) and angiotensin receptor blocker (ARB) therapy.
Results:
We found that in 295,494 patients admitted for HFrEF between 2008–2015, the average age was 80 years, 54% were female and 17% were non-white. The baseline mortality rate was higher among those aged ≥85, but the mortality benefits of neurohormonal therapy were preserved across the age spectrum. Among those ≥85 years old, the hazard ratio for death within 30 days was 0.59 (95% CI 0.56,0.62; p<0.001) for beta-blockers and 0.47 (95% CI 0.44,0.49; p<0.001) for ACEi/ARBs. The hazard ratio for death within 1-year was 0.37–0.56 (95% CI 0.35–0.58; p<0.001) for beta-blockers and 0.38–0.53 (95% CI 0.37–0.55; p<0.001) for ACEi/ARB.
Conclusion:
At a population level, neurohormonal therapy was associated with lower short and long-term mortality across the age spectrum.
Keywords: Heart Failure, Pharmacology, Aging, Quality and Outcomes
Introduction
Neurohormonal therapy, which includes beta-blockers and angiotensin converting inhibitors (ACEi)/angiotensin receptor blockers (ARBs), is a cornerstone of therapy for patients with heart failure with reduced ejection fraction (HFrEF).1–3 As a result, use of neurohormonal therapy after an admission for HFrEF and then continued over time is recommended by current guidelines4 and forms the basis for HFrEF performance measures5 and quality metrics.6 An important limitation of the original trials of neurohormonal therapy, upon which current guidelines and quality measures rest however, was that those studies enrolled relatively young patients (average age 50–60 years) with few comorbidities.2, 3, 7–9 Today, over half of Medicare’s heart failure population is ≥75 years old and over two-thirds have multiple chronic conditions.10 Given the rising age of Medicare beneficiaries, the increasing incidence of heart failure in the older adults, and the significant heterogeneity that exists within the older heart failure population, there is a pressing need to understand the role that age plays in a patients’ response to neurohormonal therapy.
As patients age, their potential for the long-term mortality benefits decreases. At the same time, their risk for short-term adverse events increases due to age-related physiologic changes as well as the concurrence of geriatric conditions like frailty and cognitive impairment which increase the risk of adverse drug events.11 Because large-scale clinical trials of neurohormonal therapy, specifically in older adults, are unlikely to ever be done, the aim of this study is to use Medicare claims data to describe the risks and benefit tradeoffs of neurohormonal therapy after HFrEF hospitalization and the variation by age.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files. This study was approved by the Institutional Review Board at Dartmouth College. This study is also compliant with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for retrospective cohort studies.
Study Population
We used the 100% national sample of patients enrolled in both Medicare Parts A and B and a random 40% sample of Part D enrollment to create a cohort of FFS beneficiaries with at least one hospitalization (index admission) for HFrEF between 2008 and 2015. Only the patient’s first hospitalization for HFrEF during the study period was included to avoid over-representing readmitted patients. HFrEF was defined using International Classification of Diseases (ICD) 9 and 10 codes and a methodology based on previously validated studies (Supplemental Table S1).12, 13 We required 1 year of FFS coverage prior to the index HFrEF admission to determine comorbidities and 1 year of FFS coverage after discharge (through 2016) to determine drug exposure and outcomes.
Patients who died during admission were excluded as were those that underwent cardiac transplant, or placement of a durable mechanical circulatory support (MCS) device during their index admission and those admitted from or discharged to hospice or with home intravenous inotropes (not including digoxin). Patients with previously placed, durable MCS devices or a prior cardiac transplant were also excluded.
For the study population, we determined patient age, sex, race/ethnicity, dual-enrollment status, and disability status directly from the enrollment file. Zip-code level estimates for socioeconomic variables were obtained using the patient’s zip-code and 5-year estimates from the 2012 American Community Survey data.14 Patient geography was ascertained using the patient’s zip-code and the Centers for Disease Control’s census regions.15 Comorbidities were determined using the Elixhauser comorbidity algorithm.16 Prior hospitalizations and procedures were determined using Part A data and other drug exposure was determined using Part D data.
Exposure
We used Part D data to determine exposure to neurohormonal therapy. Neurohormonal therapy included beta blockers, ACE inhibitors and ARBs. Beta blockers included: metoprolol succinate, bisoprolol, carvedilol. ACE inhibitors included: benazepril, captopril, enalapril, fosinopril, lisinopril, perindopril, quinapril, ramipril and trandolapril. ARBs included: valsartan, trandolapril candesartan eprosartan, Irbesartan, losartan, olmesartan and telmisartan. Sacubitril/Valsartan was included after its approval by the Food and Drug Administration in July 2015.
For the short-term, 30-day analysis, we defined drug exposure as binary based on whether or not there was a fill for the drug within 30 days of discharge. Over 50% of fills were made in the first 5 days after discharge (Supplemental Figure S1). For the long-term, 1-year, analysis, we measured drug exposure over time using the proportion of days covered (PDC).17 We separated patients into three exposure groups: “No” exposure (0 PDC), “moderate” exposure (1–80 PDC) and “high” exposure (80+ PDC) based on the threshold for adherence being 80 PDC.17
Outcomes
The short-term outcome of interest is all-cause death within 30 days of hospital discharge. Long-term outcomes included time to all-cause death within 365 days, conditional on survival to 30 days post discharge. Death was determined using the master beneficiary summary file.
Statistical Methods
Inverse probability weighting was used to balance the observed characteristics (Table 1) across the exposure groups at baseline (prior to follow-up) in each of the analyses.18 After weighting, there were no clinically significant differences between exposed and unexposed patients on any of the measurable covariates (Supplemental Table S2). For the short-term analysis, we used inverse probability weighted logistic regression to compare death rates between patients who either did or did not fill a prescription for beta-blocker or ACEi/ARB within 30 days of discharge. For the long-term analysis, we used an inverse probability weighted time-to-event analysis to compare the time-to-death between days 30 and 365 post discharge between patients in three PDC groups. Errors were clustered by the hospital referral region. A p-value of <0.05 was considered significant. All hypotheses were tested using two-sided tests. All analyses were performed between May 2019 and July 2020 using SAS version 9.4, Cary, North Carolina.
Table 1:
Baseline Characteristics of Patients based on Beta-Blocker and ACEi/ARB Exposure after HFrEF Hospitalization, 2008–2015
| Beta-Blocker Exposure N / Mean (%/SD) |
ACEi/ARB/ARNI Exposure N / Mean (%/SD) |
|||||
|---|---|---|---|---|---|---|
| No Exposure (0 PDC) | Some Exposure (1–80 PDC) | High Exposure (80+ PDC) | No Exposure (0 PDC) | Some Exposure (1–80 PDC) | High Exposure (80+ PDC) | |
| N | 82183 (27.8) | 104898 (35.5) | 108413 (36.7) | 113102 (38.3) | 101844 (34.5) | 80548 (27.3) |
| Demographic Characteristics | ||||||
| Mean Age | 82.0 (8.3) | 79.6 (8.0) | 79.5 (8.0) | 81.8 (8.2) | 79.3 (8.0) | 79.2 (8.0) |
| Age Category | ||||||
| 66–74 | 17718 (21.6) | 32042 (30.5) | 33627 (31.0) | 25133 (22.2) | 32372 (31.8) | 25882 (32.1) |
| 75–84 | 30031 (36.5) | 41546 (39.6) | 42976 (39.6) | 42175 (37.3) | 40499 (39.8) | 31879 (39.6) |
| 85+ | 34434 (41.9) | 31310 (29.8) | 31810 (29.3) | 45794 (40.5) | 28973 (28.4) | 22787 (28.3) |
| Sex | ||||||
| Male | 36256 (44.1) | 50269 (47.9) | 50556 (46.6) | 53678 (47.5) | 47661 (46.8) | 35742 (44.4) |
| Female | 45927 (55.9) | 54629 (52.1) | 57857 (53.4) | 59424 (52.5) | 54183 (53.2) | 44806 (55.6) |
| Race/Ethnicity | ||||||
| White | 70102 (85.3) | 84804 (80.8) | 90537 (83.5) | 97235 (86.0) | 82351 (80.9) | 65857 (81.8) |
| Black | 6384 (7.8) | 11383 (10.9) | 9230 (8.5) | 8730 (7.7) | 10707 (10.5) | 7560 (9.4) |
| Hispanic | 3650 (4.4) | 5833 (5.6) | 5532 (5.1) | 4457 (3.9) | 5817 (5.7) | 4741 (5.9) |
| Other | 2047 (2.5) | 2878 (2.7) | 3114 (2.9) | 2680 (2.4) | 2969 (2.9) | 2390 (3.0) |
| Socioeconomic Characteristics | ||||||
| Dual Eligibility | 29115 (35.4) | 35894 (34.2) | 36406 (33.6) | 37550 (33.2) | 35314 (34.7) | 28551 (35.4) |
| % Bachelor’s Degree* | 26.8 (15.7) | 26.7 (15.6) | 27.2 (15.7) | 27.3 (15.7) | 26.5 (15.5) | 27.0 (15.7) |
| % below Federal Poverty Line* | 15.7 (9.1) | 16.0 (9.5) | 15.5 (9.2) | 15.4 (9.1) | 16.1 (9.4) | 15.8 (9.4) |
| Geography | ||||||
| Midwest | 20776 (25.3) | 26668 (25.4) | 30007 (27.7) | 29499 (26.1) | 26157 (25.7) | 21795 (27.1) |
| Northeast | 17218 (21.0) | 21267 (20.3) | 24174 (22.3) | 25311 (22.4) | 19859 (19.5) | 17489 (21.7) |
| South | 33103 (40.3) | 43194 (41.2) | 40469 (37.3) | 44229 (39.1) | 41973 (41.2) | 30564 (37.9) |
| West | 11086 (13.5) | 13769 (13.1) | 13763 (12.7) | 14063 (12.4) | 13855 (13.6) | 10700 (13.3) |
| Medical Comorbidities † | ||||||
| ≤ 3 Elixhauser Comorbidities | 35647 (43.4) | 48978 (46.7) | 55781 (51.5) | 45526 (40.3) | 50124 (49.2) | 44756 (55.6) |
| ≥ 4 Elixhauser Comorbidities | 46536 (56.6) | 55920 (53.3) | 52632 (48.5) | 67576 (59.7) | 51720 (50.8) | 35792 (44.4) |
| Frailty Score | 0.22 (0.07) | 0.21 (0.06) | 0.20 (0.06) | 0.22 (0.07) | 0.21 (0.06) | 0.20 (0.06) |
| Implantable Cardiac Defibrillator | 8163 (9.9) | 15914 (15.2) | 15404 (14.2) | 14234 (12.6) | 14801 (14.5) | 10446 (13.0) |
| Index Admission Comorbidities ‡ | ||||||
| Hypotension | 6265 (7.6) | 7150 (6.8) | 7462 (6.9) | 9546 (8.4) | 6556 (6.4) | 4775 (5.9) |
| Bradycardia | 4394 (5.3) | 5265 (5.0) | 5848 (5.4) | 5727 (5.1) | 5362 (5.3) | 4418 (5.5) |
| Acute Kidney Injury | 16523 (20.1) | 20513 (19.6) | 20696 (19.1) | 29225 (25.8) | 17570 (17.3) | 10937 (13.6) |
| Medication Use Prior to Admission § | ||||||
| 0 PDC | 62825 (76.4) | 41765 (39.8) | 37210 (34.3) | 83582(73.9) | 41232 (40.5) | 24240 (30.1) |
| 1–79 PDC | 15077 (18.3) | 48813 (46.5) | 47527 (43.8) | 27084 (23.9) | 55881 (54.9) | 51246 (63.6) |
| 80+ PDC | 4281 (5.2) | 14320 (13.7) | 23676 (21.8) | 2436 (2.2) | 4731 (4.6) | 5062 (6.3) |
| Total number of Drugs at Discharge | 4.0 (3.6) | 5.5 (3.4) | 6.9 (2.9) | 4.5 (3.6) | 5.8 (3.3) | 6.9 (2.8) |
| All-Cause Hospitalizations in Year Prior | ||||||
| 0 Hospitalizations | 36145 (44.0) | 48573 (46.3) | 56402 (52.0) | 48079 (42.5) | 49432 (48.5) | 43609 (54.1) |
| 1–2 Hospitalizations | 21562 (26.2) | 26872 (25.6) | 26715 (24.6) | 29832 (26.4) | 25765 (25.3) | 19552 (24.3) |
| 3+ Hospitalizations | 24476 (29.8) | 29453 (28.1) | 25296 (23.3) | 35191 (31.1) | 26647 (26.2) | 17387 (21.6) |
ACEi is angiotensin-converting enzyme inhibitors; ARB is angiotensin II receptor blockers; ARNI is angiotensin receptor-neprilysin inhibitor; MRA is mineralocorticoid receptor antagonists; PDC is percentage of days covered
ZCTA-level characteristics derived from linking beneficiary zip codes with US Census Data
Individual Elixhauser comorbidities are included in inverse probability weighting
Relevant comorbidities that might impact use of neurohormonal therapy
Beta-blocker use prior to admission for beta blocker exposure group, ACEi/ARB/ARNI use prior to admission for ACEi/ARB/ARNI exposure group.
Results
Baseline Characteristics
Baseline characteristics of the 295,494 patients in the HFrEF cohort, stratified by neurohormonal therapy exposure after HFrEF hospitalization, are displayed in Table 1. The mean age across all groups was 80 years. Across all exposure groups, the majority of patients were female (52–56%) and white (81–85%). Thirty-four percent of patients were dually eligible for Medicaid and this did not vary across exposure groups. Over 50% of patients had at least one hospitalization in the year prior and over 53% had ≥4 concurrent comorbidities. The average Frailty Score was 0.20–0.22 ± 0.06–0.07.19 Approximately 7% of patients had hypotension during their index admission; 5% had bradycardia and 20% had acute kidney injury. The average number of drugs prescribed at discharge was 5.5 ± 3.3.
Use rates of both beta-blockers and ACEi/ARBs after hospital discharge were low. Forty-nine percent of patients filled a beta-blocker within 30 days of discharge and 39% filled and ACEi/ARB. Among those age 85+, only 42% received a beta-blocker and 32% received and ACEi/ARB. During the year following discharge, 42% of patients aged 85+ had no beta-blocker exposure and 41% had no ACEi/ARB exposure.
The 30-day mortality rates among those who filled a beta-blocker within 30 days of discharge was 1.9–4.3% (Table 2), with the oldest patients having the highest mortality rate. Among those without a beta-blocker fill, the 30-day mortality rate was ranged from 6.1–12.7% (p<0.001 across all 3 age strata). The number needed to treat (NNT) to prevent 1 death at 30-days with beta-blockers ranged from 12–24. The 30-day mortality rate among those who filled an ACEi/ARB was 1.6–3.7%, compared to 5.7–11.9% among those who did not. The NNT to prevent 1 death at 30-days with ACEi/ARB was 12–24. The 1-year mortality rates ranged from 16.2–32.9% among those with any beta-blocker exposure and from 26.3–39.2% among those with no exposure. The NNT to prevent 1 death at 1 year (conditional on survival to 30 days) was 10–16. The 1-year mortality rates ranged from 14.2–29.8% among those with any ACEi/ARB exposure and from 27.9% to 41% for those without no ACEi/ARB exposure. The NNT ranged from 7–9.
Table 2:
Unadjusted 30-day and 1-year* Mortality Rates after First Hospitalization for HFrEF based on Age and Neurohormonal Therapy Use after Discharge, 2008–2015
| Age 66–74 | Age 75–84 | Age 85+ | |
| 30-Day Mortality | |||
| Beta-Blockers | |||
| No Fill within 30d | 2289 (6.1) | 4739 (8.2) | 7147 (12.7) |
| Fill within 30d | 885 (1.9) | 1440 (2.5) | 1795 (4.3) |
| Number Needed to Treat (30-day mortality) | 24 | 18 | 12 |
| ACEi/ARB/ARNI | |||
| No Fill within 30d | 2581 (5.7) | 5288 (7.7) | 7739 (11.9) |
| Fill within 30d | 593 (1.6) | 891 (1.9) | 1203 (3.7) |
| Number Needed to Treat (30-day mortality) | 24 | 17 | 12 |
| 1-Year Mortality * | Age 66–74 | Age 75–84 | Age 85+ |
| Beta-Blocker Exposure | |||
| No Exposure (0 PDC) | 4667 (26.3) | 9513 (31.7) | 13484 (39.2) |
| Any Exposure (1+ PDC) | 10601 (16.2) | 18318 (21.7) | 20714 (32.9) |
| Number Needed to Treat (1-year mortality) | 10 | 10 | 16 |
| ACEi/ARB/ARNI Exposure | |||
| No Exposure (0 PDC) | 7005 (27.9) | 14129 (33.5) | 18756 (41.0) |
| Any Exposure (1+ PDC) | 8263 (14.0) | 13702 (18.7) | 15442 (29.4) |
| Number Needed to Treat (1-year mortality) | 7 | 7 | 9 |
Conditional on survival to 30-days after discharge
Short Term Analysis (30-day mortality)
There was a significantly decreased odds of death within 30 days after HFrEF discharge among patients who filled a prescription for neurohormonal therapy, across all age strata (Table 3). In the 66–74 age stratum, the odds ratio (OR) for death within 30 days was OR=0.68 (95% confidence interval [CI] 0.63, 0.74; p<0.001) among those patients that filled a prescription for beta-blockers and OR=0.53 (95% CI 0.48, 0.58; p<0.001) among those patients that filled a prescription for ACEi/ARBs, compared to patients who do not fill a prescription for the respective drug classes. In the 85+ age stratum, there was a significantly decreased odds of death among those who filled prescriptions for beta-blockers (OR=0.59; 95% CI 0.56, 0.58; p<0.001) and ACEi/ARBs (OR=0.47; 95% CI 0.44, 0.49; p<0.001) compared to those who did not. To guard against immortal time bias, we also ran the short-term analysis as a “target trial” with a narrow exposure window of 7 days after discharge and found a similar benefit, preserved in magnitude across the age spectrum (Supplemental Table S3).
Table 3:
Odds of Death within 30 Days of Hospital Discharge based on Neurohormonal Drug Fill Status, Stratified by Age, 2008–2015
| Drug Fill within 30-days of Discharge | Odds Ratio | 95% Confidence Interval | P-Value |
|---|---|---|---|
| Beta Blocker Fill | |||
| Age 66–74 | 0.68 | 0.63, 0.74 | <0.001 |
| Age 75–84 | 0.65 | 0.61, 0.69 | <0.001 |
| Age 85+ | 0.59 | 0.56, 0.62 | <0.001 |
| ACEi/ARB/ARNI Fill | |||
| Age 66–74 | 0.53 | 0.48, 0.58 | <0.001 |
| Age 75–84 | 0.46 | 0.43, 0.49 | <0.001 |
| Age 85+ | 0.47 | 0.44, 0.49 | <0.001 |
Long-Term Analysis (1-year mortality)
There was also a significantly decreased risk of death within 1 year among patients exposed to at least some neurohormonal therapy, across the age spectrum (Table 4). In the 66–74 age stratum, there was a similar decrease in the risk of death among those with moderate beta-blocker exposure (HR=0.46, 95% CI 0.44, 049; p<0.001) and ACEi/ARB exposure (HR=0.43, 95% CI 0.42, 0.45; p<0.001) and a slightly larger benefit among those with high beta-blocker exposure (HR=0.37, 95% CI 0.35, 0.39; p<0.001) and high ACEi/ARB exposure (HR=0.39, 95% CI 0.37, 0.41; p<0.001), compared to those without beta-blocker or ACEi/ARB exposure. Among those 85+, there was also a decreased risk of death among those with moderate beta-blocker exposure (HR=0.56, 95% CI 0.54, 0.58, p<0.001) and moderate ACEi/ARB exposure (HR=0.53, 95% CI 0.52, 0.55, p<0.001) and a slightly larger benefit among those with high beta-blocker exposure (HR=0.43, 95% CI 0.42, 0.45, p<0.001) and high ACEi/ARB exposure (HR=0.45, 95% CI 0.43, 0.47, p<0.001). Again, to test the robustness of these findings, we ran a sensitivity analysis among beneficiaries that survived to 6 months. We determined their PDC during the first 6 months and examined time to mortality in the second 6 months. We found a similar benefit for neurohormonal therapy, again with efficacy preserved across the age spectrum (Supplemental Table S3).
Table 4:
Hazard Ratios for Death within 1 Year* after Hospital Discharge based on Neurohormonal Drug Exposure, Stratified by Age, 2008–2015†
| Hazard Ratio | 95% Confidence Interval | P-Value | |
|---|---|---|---|
| Beta Blocker Exposure | |||
| Age 66–74 | |||
| 1–80 PDC | 0.46 | 0.44, 0.49 | <0.001 |
| 80+ PDC | 0.37 | 0.35, 0.39 | <0.001 |
| Age 75–84 | |||
| 1–80 PDC | 0.49 | 0.47, 0.51 | <0.001 |
| 80+ PDC | 0.39 | 0.37, 0.40 | <0.001 |
| Age 85+ | |||
| 1–80 PDC | 0.56 | 0.54, 0.58 | <0.001 |
| 80+ PDC | 0.43 | 0.42, 0.45 | <0.001 |
| ACEi/ARB/ARNI Exposure | |||
| Age 66–74 | |||
| 1–80 PDC | 0.43 | 0.42, 0.45 | <0.001 |
| 80+ PDC | 0.39 | 0.37, 0.41 | <0.001 |
| Age 75–84 | |||
| 1–80 PDC | 0.45 | 0.44, 0.47 | <0.001 |
| 80+ PDC | 0.38 | 0.37, 0.40 | <0.001 |
| Age 85+ | |||
| 1–80 PDC | 0.53 | 0.52, 0.55 | <0.001 |
| 80+ PDC | 0.45 | 0.43, 0.47 | <0.001 |
PDC is proportion of days covered which is the number of days “covered by drug” based on Part D prescription fills over the number of days in the follow-up period
No beta-blocker exposure and no ACEi/ARB exposure is the reference group.
Conditional on survival to 30 days after discharge
Errors are clustered at the hospital referral region level.
The cumulative incidence of mortality through 1-year of follow-up is displayed in Figure 1 (additional detail in Supplemental Figure S2). There is a clear association between neurohormonal therapy exposure and decreased mortality across all levels of the age spectrum. Similar to the 30-day analysis, while the overall death rate is higher among those in the 85+ age stratum, the magnitude of mortality benefit appears preserved for both beta-blockers and ACEi/ARBs.
Figure 1: Survival Curves for Death within 1 Year* after Hospital Discharge based on Beta-Blocker and ACEi/ARB/ARNI Exposure, Stratified by Age, 2008–2015.
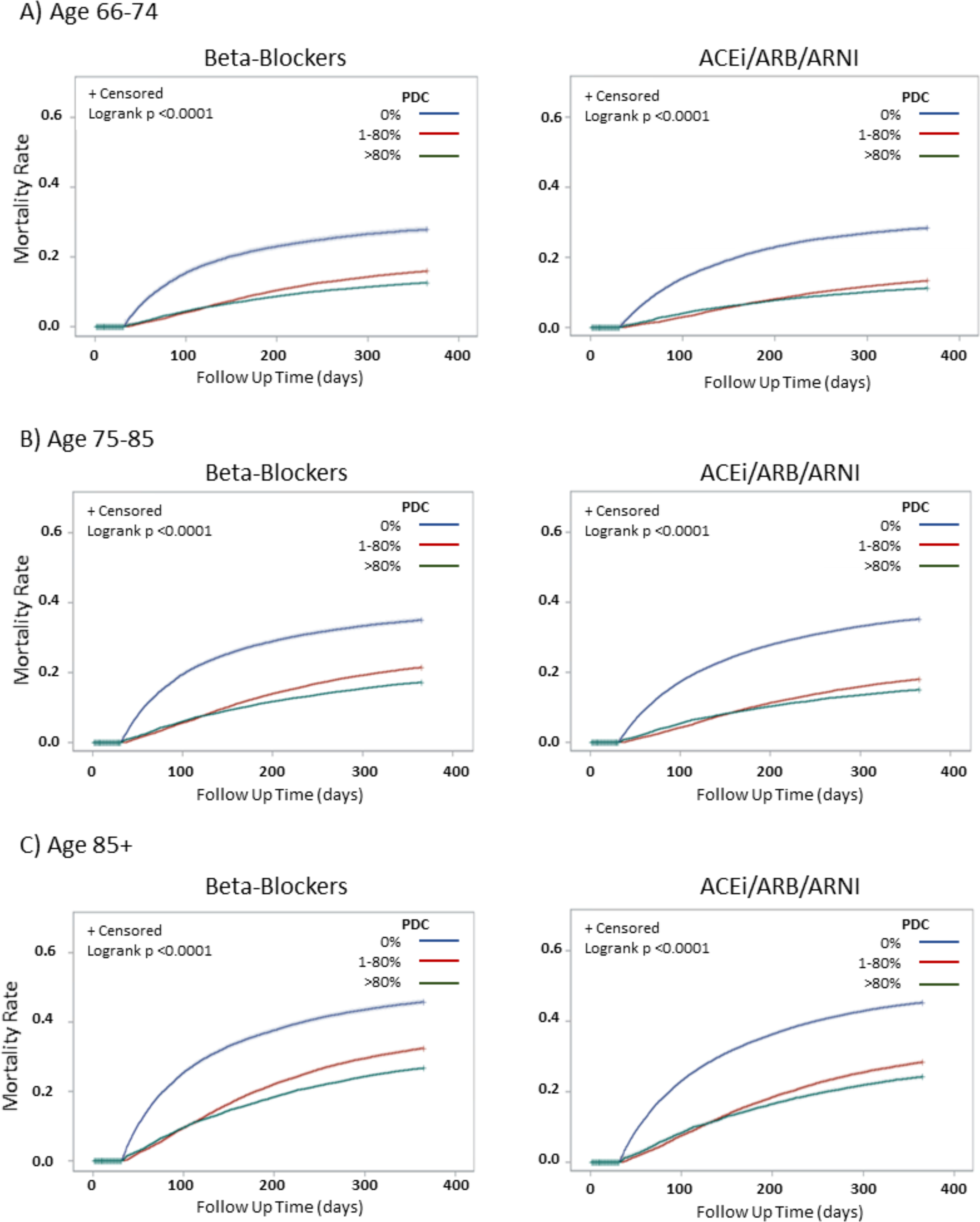
* Conditional on survival to 30 days after discharge.
This figure shows the Kaplan Meier curves for 1-year time to death, by age, based on exposure to beta-blockers (left side) and ACEi/ARB/ARNI (right side). Row 1 (A) shows the survival curves for beneficiaries aged 66–74. Row 2 (B) shows the survival curves for beneficiaries aged 75–85 and Row 3 (C) shows the survival curves for beneficiaries aged 85+. In each panel, patients are stratified by the proportion of days covered (PDC) by relevant drug: “no exposure” 0 PDC, “moderate” exposure 1–80 PDC and “high exposure” 80+ PDC.
Discussion
The landmark clinical trials which demonstrated the mortality benefits of neurohormonal therapy in HFrEF were conducted decades ago. Then, as today, clinical trial populations were not always representative of the “real-world” patient populations.20 Time has further compounded this problem because today’s HFrEF population is older and more medically complex than even 20 years ago. While neurohormonal therapy is commonly used in older patients, there is uncertainty about whether older patients benefit as much as their younger counterparts and whether it should even be attempted in those of advanced age (>85 years). In this study, we sought to determine if the increasing age alters the risk/benefit ratio of neurohormonal therapy and, if so, how. These results will inform both clinical care and the refinement of quality metrics.21, 22
To date, there has been little empirical data to inform clinical practice and guide the development of nuanced quality metrics in this area. Subgroup analyses of original trial data from MERIT-HF, CIBIS-II and the early Carvedilol studies, suggested a benefit in “elderly” populations, but in these cases, “elderly” was defined as ≥65 years old and all post hoc analyses were limited by power and sample size.3, 8, 9 In 2005, Flather et al. investigated the utility of nebivolol, notably not a HF-specific beta-blocker, in 2,128 patients aged ≥70 in the SENIORS trial and found a HR= 0.86 (95% CI 0.74–0.99, p=0.039) for the composite outcome of all-cause mortality or cardiovascular hospitalization among those exposed to nebivolol.23 In 2006, Cleland et al. randomized 850 patients ≥70 years old to perindopril (also not a HF-specific beta-blocker) or placebo in the PEP-CHF study. With 1-year follow up data for 207 of those patients, the authors found a trend toward a reduction in the composite endpoint of all-cause mortality and HF hospitalization and a significant reduction in HF hospitalization (HR=0.63: 95% CI 0.41–0.97; p=0.033).24 In both of these studies however, populations ≥80 were underpowered. In 2018, Savarese et al. published a propensity matched, observational study of 2,416 Swedish octogenarians with HFrEF and found a HR=0.78 (95% CI 0.72, 0.86) for all-cause mortality and a HR=0.86 (95% CI 0.79, 0.94) for all-cause mortality or heart failure hospitalization among those exposed to ACEi/ARBs.25
Our study confirms and extends this prior work and fills a gap in empirical evidence. Specifically, some have questioned whether neurohormonal therapy immediately after HFrEF hospitalization is safe in older patients.26 Among those aged 85+, we found that the mortality benefits of neurohormonal therapy outweigh the mortality risk from short-term adverse events, within 30 days of HF discharge. Similarly, there have been questions about efficacy of continuing long-term neurohormonal therapy in older patients26,27–29 As the 1-year mortality data from this study demonstrates, even in the oldest patients, the longer-term benefits of neurohormonal therapy appear well preserved over time.
Limitations
This study has important limitations. First, this study was performed using fee-for-service Medicare beneficiaries and care should be used when extrapolating the results to other populations, including those with managed care plans. Next, it uses Medicare claims which afford a robust sample size but limit data granularity. Despite sophisticated weighting techniques, residual confounding due to the lack of vital signs and lab measurements remains a significant limitation. Importantly however, the number needed to treat observed in this paper for beta-blockers and ACEi/ARB in the youngest age stratum (age 66–74) is similar to composite estimates from prior randomized, controlled trials.4 This provides evidence that our observational estimates align closely with prior clinical trial findings, somewhat mitigating this concern.30 In addition, we calculated E-values, which describe how large an unmeasured confounder would have to be in order to alter the results.31 For the short-term analysis, the e-values ranged from 1.72–2.32. For the long-term analysis, the e-values ranged from 2.26–3.38. To compare, we found that the HR for well known risk factors for 30-day mortality such as renal failure (HR 1.3, 95% CI 1.28–1.32), chronic lung disease (HR 1.16, 95% CI 1.14–1.17) and cancer (HR 1.10, 95% CI 1.07–1.13) were markedly lower. Therefore, it is unlikely that an unmeasured or unknown confounder would have a substantially greater association with mortality than these known risk factors by having a relative risk exceeding 2.26–3.38 (Supplemental Table S4). Second, the short-term analysis in this project may suffer from residual immortal time bias but since our primary analysis and sensitivity checks yielded similar results, we contend that any residual bias is small. Thirdly, in the long-term analysis, the use of the PDC as the exposure may introduce bias. However, again, since our main analysis and sensitivity check yielded similar results, any residual bias is likely small. Finally, these results reflect population-based estimates and should not supersede clinical judgement.
Conclusion
At a population level, after accounting for individual patient differences, patients who receive neurohormonal therapy, namely beta-blocker and ACEi/ARB, after HFrEF hospitalization and then longitudinally over time have significantly lower short and long-term mortality than those who do not, across the age spectrum. The benefit of neurohormonal therapy does not appear to wane with age. Therefore, it is reasonable for clinical guidelines and quality metrics to continue recommending neurohormonal therapy at and after hospital discharge for all HFrEF patients, regardless of age.
Supplementary Material
Supplementary Table S1: HFrEF Identification
Supplementary Table S2: Pre/Post Weighting for Short- and Long-Term Analyses
Supplementary Table S3: Sensitivity Analysis for Short- and Long-Term Analyses
Supplementary Table S4: E-Values for Short and Long Analyses
Supplementary Figure S1: Distribution of Days to Drug fill for those Exposed to Neurohormonal Therapy after Hospital Discharge
Supplementary Figure S2: Survival Curves for Death within 1 Year after Hospital Discharge based on Beta-Blocker and ACEi/ARB/ARNI Exposure, Stratified by Age, 2008-2015
Key Points.
Neurohormonal therapy, which includes beta-blockers and ACEi/ARB/ARNI, are prescribed at lower rates among older adults with HFrEF as compared to similar, younger patients.
Despite concern that a higher risk of side effects might alter the net risk/benefit ratio for these therapies in older HFrEF patients, the benefits of neurohormonal therapy do not decrease with increasing age.
Why does this paper matter?
Absent a strong clinical contraindication, beta-blockers and ACEi/ARBs should at least be trialed in all HFrEF patients, regardless of age and efforts should be made to increase their use among eligible, older adults with HFrEF.
Acknowledgements:
Sponsor’s Role: This study was funded by the National Heart Lung and Blood Institute (K23-HL142832). The sponsor had no role in the design and conduct of this study.
Funding Statement: This work is supported by the National Heart Lung and Blood Institute (NHLBI) [K23HL142835 to LG]; the National Institute on Aging (NIA) [P01AG019783 to AMA, AB and JOM]; American Heart Association (AHA) [18IPA34170185 to PG]; and the National Center for Advancing Translational Science (NCATS) [UL1TR001086 to ANAT]. None of the funding sources (NHLBI, NIA, AHA or NCATS) had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit for publication.
Footnotes
Conflicts of Interest: Dr. Skinner is a consultant for Sutter Health, an investor in Dorsata, Inc., and was a consultant for the Government of Singapore. The remaining authors have no relationships with industry to report
References
- [1].Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. N Engl J Med. 1992;327: 685–691. [DOI] [PubMed] [Google Scholar]
- [2].Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334: 1349–1355. [DOI] [PubMed] [Google Scholar]
- [3].Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. The New England journal of medicine. 2001;344: 1651–1658. [DOI] [PubMed] [Google Scholar]
- [4].Writing Committee M, Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128: e240–327. [DOI] [PubMed] [Google Scholar]
- [5].American Academy of Family P, American Academy of H, Palliative M, et al. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association-Physician Consortium for Performance Improvement. J Am Coll Cardiol. 2012;59: 1812–1832. [DOI] [PubMed] [Google Scholar]
- [6].Measures Quality. MACRA/MIPS: Quality Payment Program: Center for Medicare and Medicaid Services, 2017. [Google Scholar]
- [7].Investigators S, Yusuf S, Pitt B, Davis CE, Hood WB, Jr., Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327: 685–691. [DOI] [PubMed] [Google Scholar]
- [8].Erdmann E, Lechat P, Verkenne P, Wiemann H. Results from post-hoc analyses of the CIBIS II trial: effect of bisoprolol in high-risk patient groups with chronic heart failure. Eur J Heart Fail. 2001;3: 469–479. [DOI] [PubMed] [Google Scholar]
- [9].Deedwania PC, Gottlieb S, Ghali JK, Waagstein F, Wikstrand JC, Group M-HS. Efficacy, safety and tolerability of beta-adrenergic blockade with metoprolol CR/XL in elderly patients with heart failure. Eur Heart J. 2004;25: 1300–1309. [DOI] [PubMed] [Google Scholar]
- [10].Chronic Conditions Among Medicare Beneficiaries. Centers for Medicare and Medicaid Services; 2012. [Google Scholar]
- [11].Goyal P, Bryan J, Kneifati-Hayek J, et al. Association Between Functional Impairment and Medication Burden in Adults with Heart Failure. J Am Geriatr Soc. 2019;67: 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li Q, Glynn RJ, Dreyer NA, Liu J, Mogun H, Setoguchi S. Validity of claims-based definitions of left ventricular systolic dysfunction in Medicare patients. Pharmacoepidemiol Drug Saf. 2011;20: 700–708. [DOI] [PubMed] [Google Scholar]
- [13].Loop MS, Van Dyke MK, Chen L, et al. Comparison of Length of Stay, 30-Day Mortality, and 30-Day Readmission Rates in Medicare Patients With Heart Failure and With Reduced Versus Preserved Ejection Fraction. Am J Cardiol. 2016;118: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].ACS. American Community Survey. US Census Bureau, 2018. [Google Scholar]
- [15].CDC. Census Regions and Divisions in the United States. Centers for Disease Control; 2017. [Google Scholar]
- [16].Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36: 8–27. [DOI] [PubMed] [Google Scholar]
- [17].Preiss D, Campbell RT, Murray HM, et al. The effect of statin therapy on heart failure events: a collaborative meta-analysis of unpublished data from major randomized trials. Eur Heart J. 2015;36: 1536–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Medicaid Enrollment by Race/Ethnicity. Kaiser Family Foundation; Volume 2020, 2013. [Google Scholar]
- [19].Kim DH, Patorno E, Pawar A, Lee H, Schneeweiss S, Glynn RJ. Measuring Frailty in Administrative Claims Data: Comparative Performance of Four Claims-Based Frailty Measures in the U.S. Medicare Data. J Gerontol A Biol Sci Med Sci. 2020;75: 1120–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Masoudi FA, Havranek EP, Wolfe P, et al. Most hospitalized older persons do not meet the enrollment criteria for clinical trials in heart failure. Am Heart J. 2003;146: 250–257. [DOI] [PubMed] [Google Scholar]
- [21].Bonow RO, Bennett S, Casey DE Jr., et al. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures) endorsed by the Heart Failure Society of America. J Am Coll Cardiol. 2005;46: 1144–1178. [DOI] [PubMed] [Google Scholar]
- [22].Bonow RO, Bennett S, Casey DE Jr., et al. ACC/AHA Clinical Performance Measures for Adults with Chronic Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures): endorsed by the Heart Failure Society of America. Circulation. 2005;112: 1853–1887. [DOI] [PubMed] [Google Scholar]
- [23].Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). European heart journal. 2005;26: 215–225. [DOI] [PubMed] [Google Scholar]
- [24].Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27: 2338–2345. [DOI] [PubMed] [Google Scholar]
- [25].Savarese G, Dahlstrom U, Vasko P, Pitt B, Lund LH. Association between renin-angiotensin system inhibitor use and mortality/morbidity in elderly patients with heart failure with reduced ejection fraction: a prospective propensity score-matched cohort study. Eur Heart J. 2018;39: 4257–4265. [DOI] [PubMed] [Google Scholar]
- [26].Gilstrap LG, Snipelisky D, AbouEzzeddine O, et al. Unanswered Questions in Contemporary Heart Failure. J Card Fail. 2017;23: 770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rossello X, Pocock SJ, Julian DG. Long-Term Use of Cardiovascular Drugs: Challenges for Research and for Patient Care. J Am Coll Cardiol. 2015;66: 1273–1285. [DOI] [PubMed] [Google Scholar]
- [28].Julian DG, Pocock SJ. Effects of long-term use of cardiovascular drugs. Lancet. 2015;385: 325. [DOI] [PubMed] [Google Scholar]
- [29].Grossman E, Messerli FH. Why beta-blockers are not cardioprotective in elderly patients with hypertension. Curr Cardiol Rep. 2002;4: 468–473. [DOI] [PubMed] [Google Scholar]
- [30].Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA. 2013;309: 241–242. [DOI] [PubMed] [Google Scholar]
- [31].Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to Assess the Potential Effect of Unmeasured Confounding in Observational Studies. JAMA. 2019;321: 602–603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: HFrEF Identification
Supplementary Table S2: Pre/Post Weighting for Short- and Long-Term Analyses
Supplementary Table S3: Sensitivity Analysis for Short- and Long-Term Analyses
Supplementary Table S4: E-Values for Short and Long Analyses
Supplementary Figure S1: Distribution of Days to Drug fill for those Exposed to Neurohormonal Therapy after Hospital Discharge
Supplementary Figure S2: Survival Curves for Death within 1 Year after Hospital Discharge based on Beta-Blocker and ACEi/ARB/ARNI Exposure, Stratified by Age, 2008-2015


