Abstract
Background:
Studies comparing dipeptidyl peptidase-4 inhibitors (DPP4Is) to sulfonylureas (SUs) are unavailable for frail older adults, especially nursing home (NH) residents. We examined the effects of DPP4Is versus SUs on severe adverse glycemic events, cardiovascular events, and death among NH residents.
Methods:
We conducted a national retrospective cohort study of long-stay NH residents aged ≥ 65 years using 2008 to 2010 national US Minimum Data Set clinical assessment data and linked Medicare claims. Exposure was new DPP4I versus new SU use assessed via Medicare Part D drug claims. One-year outcomes were severe hypoglycemia, severe hyperglycemia, acute myocardial infarction (AMI), heart failure (HF), major adverse cardiovascular events plus HF (MACE+HF), and death. We compared outcomes after propensity score matching using Cox proportional hazards regression models.
Results:
The cohort (N=2,016) had a mean (SD) age of 81 (8.1) years and was 72% female. Compared to SU users, DPP4I users had a lower one-year rate of severe hypoglycemic events (HR=0.57, 95%CI 0.34-0.94), but statistically-similar rates of severe hyperglycemic events (HR=0.94, 95%CI 0.52-1.72), AMI (HR=0.76, 95%CI 0.44-1.30), HF (HR=1.01, 95%CI 0.79-1.30), MACE+HF (HR=0.90, 95%CI 0.72-1.12), and death (HR=0.97, 95%CI 0.86-1.10).
Conclusions:
DPP4Is should be a preferred treatment option over SUs for NH residents and other frail older adults given the importance of avoiding hypoglycemia.
Keywords: Sulfonylurea Compounds, Dipeptidyl-Peptidase IV Inhibitors, Nursing Homes, Diabetes Mellitus, Frailty
INTRODUCTION
The prevalence of type 2 diabetes (T2DM) exceeds 30% in nursing home residents, yet little data exist to support the safest and most effective glucose-lowering treatment regimens. Avoiding hypoglycemia rather than achieving strict glycemic targets is generally the primary goal for most NH residents with T2DM.1 While metformin remains the first-line recommended treatment for older adults with T2DM, it is infrequently used in nursing homes (NHs) given the high burden of chronic renal failure. In fact, as few as 6% of NH residents with T2DM use metformin monotherapy and just 30% use metformin with other glucose-lowering treatments.2, 3 Sulfonylureas (SUs) are often used as monotherapy instead2, 3, but are potentially harmful for NH residents given their high risk of hypoglycemia. Fortunately, several newer agents are available as alternatives to SUs, in particular, dipeptidyl peptidase-4 inhibitors (DPP4Is).
The use of DPP4Is has been increasing among NH residents in recent years, though little is known about the comparative safety and efficacy of DPP4Is and SUs in this unique population.2, 3 Vulnerable older adults may be particularly susceptible to adverse effects of glucose-lowering medications and may also be less likely to live long enough to benefit from their preventative effects.4 A large randomized controlled trial of community-dwelling adults with T2DM, the Cardiovascular Outcome Study of Linagliptin vs Glimepiride in Type 2 Diabetes (CAROLINA) trial, suggested no difference in efficacy of DDP4Is and SUs as the investigators found similar rates of cardiovascular events among subjects randomized to glimepiride versus linagliptin.5, 6 Overall rates of adverse events were similar between the two drug classes, but hypoglycemia was more common with SU than DDP4I use. However, the mean age of subjects in this trial was just 64 years with the vast majority (83%) concomitant metformin users. Thus, uncertainty remains as to the whether these drug classes are similarly effective and safe in a frail older population, such as NH residents. This knowledge gap, and the vulnerability of older NH residents, warrants a comparison of DPP4Is versus SUs.
We compared the effects of DPP4Is versus SUs on adverse glycemic events, adverse cardiovascular events, and death among frail, older adults in NHs. We hypothesized that prescribing DPP4Is would result in fewer severe hypoglycemic events, but no difference in other outcomes compared to SUs.
METHODS
Study Design and Data Source
This was a retrospective new-user cohort study that used the following linked national datasets for 2007-2010: Medicare fee-for-service enrollment information, Part A inpatient claims, Part B outpatient claims, and Part D prescription drug claims; Minimum Data Set (MDS) version 2.0 assessments; and Online Survey Certification and Reporting System (OSCAR) data. The MDS is a comprehensive, clinical assessment instrument used to document health status of NH residents, including functional status, cognitive status, and psychological information. OSCAR data provided NH-level information. Our observational study was designed to emulate a hypothetical pragmatic trial that could have been conducted had it been feasible (Table S1).7, 8 This study was approved by the Brown University Institutional Review Board.
Study Population
The study population was adults aged ≥65 years who were long-stay NH residents (>100 days in the NH) on January 1, 2008, or who became a long-stay resident between January 1, 2008 and September 30, 2010. The index date was the date of the first eligible dispensing of a DPP4I or SU between January 1, 2008 and September 30, 2010 after becoming a long-stay resident. Individuals were required to have continuous enrollment in fee-for-service (i.e., traditional) Medicare Parts A, B, and D and no enrollment in Medicare Advantage in the twelve months prior to the index date. Individuals who were in hospice, had cancer, who were comatose or paralyzed, or who had missing data on any covariate used in the analyses were excluded from the study cohort (Supplementary Figure S1).
Exposures and Causal Contrast of Interest
Exposures of interest were new use of DPP4Is (saxagliptin, sitagliptin) or SUs (glimepiride, glipizide, glyburide) in the NH.9 New use was defined as the first dispensing of a DPP4I or SU after 6 months without a dispensing of either class or another glucose-lowering treatment other than metformin. The causal contrasts of interest were defined as the effects of initiating DPP4Is versus SUs regardless of subsequent treatment discontinuation or switching (i.e., the observational study analog of the intention-to-treat [ITT] estimand).7, 8
Outcomes
The outcomes were all-cause death, and hospitalizations or emergency department (ED) visits for adverse glycemic events (severe hypoglycemia and hyperglycemia), heart failure (HF), and major adverse cardiovascular events (MACE) plus HF. MACE included acute myocardial infarction (AMI), stroke, intracerebral hemorrhage, and subarachnoid hemorrhage. All of the non-death outcomes were defined using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes on Part A hospital claims and Part B ED claims in any coding position (Supplementary Methods 1).
Follow-Up
Follow-up started on the day after the first DPP4I or SU dispensing and continued until Medicare disenrollment (from Parts A, B, or D) or enrollment in Medicare Advantage, death, an outcome (each evaluated separately), one year of follow-up, or study end (December 31, 2010).
Baseline characteristics
One hundred ninety eight characteristics that were correlated with receiving DPP4Is versus SUs and the outcomes were pre-specified and measured before the index date (Supplementary Table S2).2, 3, 10 The 198 characteristics that we selected a priori were all expected to be variables (or proxies of variables) that would either influence 1) both the probability of exposure to DPP4Is versus SUs and experiencing an outcome, or 2) just the probability of experiencing an outcome.11
Statistical Analyses
We adjusted for potential confounding by baseline covariates by estimating propensity scores using a logistic regression model that included the 198 baseline characteristics to predict the initiation of DPP4Is versus SUs. We matched DPP4I to SU users using a 1:1 greedy (nearest neighbor) 5-to-1 digit matching algorithm without replacement.12 Cox proportional hazards regression models with robust standard errors were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) comparing DPP4I versus SU users.
Stability and Sensitivity Analyses
We conducted several stability analyses to test the robustness of the treatment effect estimates to study design and analytic decisions. First, to assess the impact of missing data, we performed multiple imputation with chained equations to impute missing covariate data for 582 residents excluded from the primary analysis.13 Second, because the risk for misclassification of treatment is higher over longer follow-up periods with the ITT estimand, we examined 3-month and 6-month outcomes. Third, we estimated the propensity score using generalized boosted regression models to evaluate possible misspecification of the parametric propensity score model. Fourth, we used Fine and Gray competing risks regression models to account for the potential competing risk of death. Finally, we conducted a sensitivity analysis using the E-value (see Supplementary Methods 2).14
RESULTS
Study Cohort
The study cohort (N=7,885) included 1,064 new DPP4I and 6,821 new SU users before propensity score matching (Table 1 and Table S2). Before matching, DPP4I users were more likely than US users to use metformin (37.7% versus 29.2%) and received a greater number of medications (average 14.1 versus 12.7) (Table 1 and Table S2). Propensity score matching yielded 1,008 DPP4I users and 1,008 SU users (Table 1). The mean (SD) age was 81 (8) years, 72% were female, and 37% had received metformin. Matching balanced covariates well. All but two characteristics (bladder incontinence and percent of private pay clients in the NH) were within an absolute standardized mean difference of 0.06 or less (Table S2). After matching, across both treatment groups, death was the most common outcome (49.1%), followed by MACE+HF (15.0%), HF (12.8%), severe hypoglycemia (3.1%), AMI (2.6%), and severe hyperglycemia (2.1%).
Table 1.
Characteristics of Nursing Home Residents Initiating Dipeptidyl Peptidase-4 Inhibitors or Sulfonylureas Before and After Propensity Score Matching.
| Resident Characteristics | n (%) | |||
|---|---|---|---|---|
| Before Matching | After Matching | |||
| DPP4I users (N=1,064) |
SU users (N=6,821) |
DPP4I users (N=1,008) |
SU users (N=1,008) |
|
| Age, mean (SD) years | 80.4 (8.1) | 81.4 (8.2) | 80.5 (8.1) | 80.6 (8.1) |
| Female sex | 766 (72.0) | 4,816 (70.6) | 725 (71.9) | 732 (72.6) |
| Race | ||||
| White | 773 (72.7) | 5,164 (75.7) | 742 (73.6) | 726 (72.0) |
| Black | 165 (15.5) | 1,066 (15.6) | 159 (15.8) | 167 (16.6) |
| Other | 126 (11.8) | 591 (8.7) | 107 (10.6) | 115 (11.4) |
| Conditions | ||||
| Renal disease | 115 (10.8) | 639 (9.4) | 103 (10.2) | 108 (10.7) |
| Peripheral vascular disease | 195 (18.3) | 1,150 (16.9) | 180 (17.9) | 171 (17.0) |
| Diabetic retinopathy | 19 (1.8) | 132 (1.9) | 18 (1.8) | 20 (2.0) |
| Heart failure | 338 (31.8) | 1,934 (28.4) | 315 (31.3) | 331 (32.8) |
| Ischemic heart disease | 198 (18.6) | 1,039 (15.2) | 177 (17.6) | 204 (20.2) |
| Depression | 596 (56.0) | 3,818 (56.0) | 566 (56.2) | 575 (57.0) |
| Chronic Obstructive Pulmonary Disease | 230 (21.6) | 1,365 (20.0) | 215 (21.3) | 217 (21.5) |
| Hypertension | 812 (76.3) | 5,261 (77.1) | 774 (76.8) | 783 (77.7) |
| Hip fracture | 17 (1.6) | 121 (1.8) | 17 (1.7) | 18 (1.8) |
| Number of conditions, median, (IQR) | 6 (4-8) | 5 (4-7) | 6 (4-7) | 6 (4-8) |
| ADL score, mean (SD)† | 15.8 (7.6) | 15.8 (7.7) | 15.8 (7.6) | 16.1 (7.4) |
| CPS score, mean (SD)‡ | 2.4 (1.6) | 2.6 (1.6) | 2.5 (1.6) | 2.5 (1.6) |
| CHESS score, mean (SD)§ | 0.5 (0.8) | 0.5 (0.8) | 0.5 (0.8) | 0.5 (0.8) |
| Number of medications, mean (SD) | 14.1 (5.2) | 12.7 (4.8) | 13.9 (5.0) | 13.8 (5.0) |
| Medication use | ||||
| Metformin | 401 (37.7) | 1,992 (29.2) | 370 (36.7) | 379 (37.6) |
| Statins | 487 (45.8) | 2,680 (39.3) | 450 (44.6) | 448 (44.4) |
| Clopidogrel | 214 (20.1) | 959 (14.1) | 194 (19.3) | 215 (21.3) |
| Warfarin | 169 (15.9) | 1,015 (14.9) | 161 (16.0) | 168 (16.7) |
| Antipsychotics | 330 (31.0) | 2,000 (29.3) | 313 (31.1) | 279 (27.7) |
| Steroids (oral) | 138 (13.0) | 857 (12.6) | 128 (12.7) | 122 (12.1) |
| Angiotensin-converting enzyme inhibitors | 455 (42.8) | 2,578 (37.8) | 423 (42.0) | 437 (43.4) |
| Angiotensin receptor blockers | 169 (15.9) | 758 (11.1) | 146 (14.5) | 151 (15.0) |
| Beta blockers | 543 (51.0) | 3,063 (44.9) | 507 (50.3) | 528 (52.4) |
| Length of nursing home stay before treatment initiation, median (IQR) days | 647 (296-1,305) | 589 (273-1,181) | 649 (295-1,296) | 640 (294-1,225) |
| Any physician visits in prior two weeks | 639 (60.0) | 3,948 (57.9) | 595 (59.0) | 589 (58.4) |
| Number of physician order changes in prior two weeks, mean (SD) | 2.1 (2.0) | 1.9 (1.8) | 2.0 (1.9) | 2.1 (1.9) |
| Any overnight hospitalizations in prior 90 days | 335 (31.5) | 2,011 (29.5) | 316 (31.4) | 319 (31.7) |
| Any ED visits in prior 90 days | 80 (7.5) | 541 (7.9) | 76 (7.5) | 73 (7.2) |
| Nursing Home Facility Characteristics | ||||
| Ownership | ||||
| For profit | 771 (72.5) | 4,835 (70.9) | 735 (72.9) | 758 (75.2) |
| Non-profit | 231 (21.7) | 1,514 (22.2) | 215 (21.3) | 189 (18.8) |
| Government | 62 (5.8) | 472 (6.9) | 58 (5.8) | 61 (6.1) |
| Quality indicators | ||||
| % of residents restrained, median (IQR) | 1.9 (0.0-5.1) | 1.8 (0.0-4.7) | 2.0 (0.0-5.1) | 1.9 (0.0-5.1) |
| No. of quality-of-life deficiencies, mean (SD) | 4.1 (6.1) | 3.8 (6.9) | 4.0 (6.1) | 4.2 (10.0) |
| % of residents with pressure sores, mean (SD) | 6.9 (4.3) | 6.6 (4.2) | 6.8 (4.3) | 6.8 (4.5) |
| Staffing | ||||
| Direct care hours/resident/day, mean (SD) | 2.9 (1.2) | 2.9 (1.3) | 2.8 (1.2) | 2.9 (1.2) |
Physical function was measured using activities of daily living (ADL) via the Minimum Data Set Morris 28-point ADL score. The ADL scores range from 0 to 28, with 0 indicating total independence and 28 indicating total dependence in all ADLs.
Cognitive status was measured using the Minimum Data Set Cognitive Performance Scale (CPS), where higher values indicate greater cognitive impairment. The CPS scores range from 0 to 6, with 0 indicating intact cognitive function and 6 indicating very severe cognitive impairment.
The Minimum Data Set Changes in Health, End-stage disease and Symptoms and Signs (CHESS) score is a composite measure addressing changes in health, end-stage disease, and symptoms and signs of medical problems. The CHESS scores range from 0 to 5, with 0 indicating no health instability and 5 indicating very high health instability.
Treatment Effects
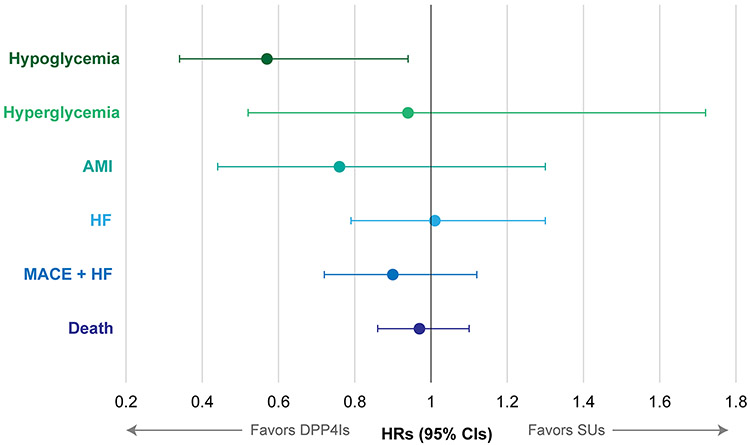
Through one year of follow-up after propensity score matching, DPP4I users had a lower rate of severe hypoglycemic events than SU users (HR: 0.57, 95%CI 0.34-0.94)(Figure 1, Table 2, and Supplementary Figure S2). The rates of severe hyperglycemic events were statistically similar between the two groups (HR: 0.94, 95%CI 0.52-1.72), as were the rates of AMI (HR 0.76, 95%CI 0.44-1.30), HF (HR 1.01, 95%CI 0.79-1.30), MACE plus HF (HR 0.90, 95%CI 0.72-1.12), and death (HR 0.97, 95% CI: 0.86-1.10)(Supplementary Figures S3-S6).
Figure 1.
Effects of Dipeptidyl Peptidase-4 Inhibitors versus Sulfonylureas on One-year Outcomes After Propensity Score Matching among Nursing Home Residents. Abbreviations: DPP4I, dipeptidyl peptidase-4 inhibitor; SU, sulfonylurea; AMI, acute myocardial infarction; MACE, major adverse cardiovascular events; HF, heart failure.
Table 2:
Effects of Dipeptidyl Peptidase-4 Inhibitors versus Sulfonylureas on One-year Outcomes Before and After Propensity Score Matching.
| Before Matching | After Matching | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Treatment | Events | PY | Rate* | HR (95% CI) |
Events | PY | Rate* | HR (95% CI) |
| Hypoglycemia | SU | 207 | 5,052.3 | 41.0 | Ref | 40 | 739.0 | 54.1 | Ref |
| DPP4I | 24 | 756.7 | 31.7 | 0.78 (0.51-1.18) | 23 | 747.6 | 30.8 | 0.57 (0.34-0.94) | |
| Hyperglycemia | SU | 142 | 5,067.4 | 28.0 | Ref | 22 | 743.6 | 29.6 | Ref |
| DPP4I | 21 | 758.7 | 27.7 | 0.99 (0.63-1.57) | 21 | 749.6 | 28.0 | 0.94 (0.52-1.72) | |
| AMI | SU | 155 | 5,078.4 | 30.5 | Ref | 30 | 744.9 | 40.3 | Ref |
| DPP4I | 23 | 760.9 | 30.2 | 0.99 (0.64-1.53) | 23 | 751.8 | 30.6 | 0.76 (0.44-1.30) | |
| HF | SU | 726 | 4,946.1 | 146.8 | Ref | 127 | 723.3 | 175.6 | Ref |
| DPP4I | 131 | 736.7 | 177.8 | 1.21 (1.01-1.46) | 130 | 727.8 | 178.6 | 1.01 (0.79-1.30) | |
| MACE + HF | SU | 862 | 4,924.8 | 176.4 | Ref | 158 | 717.9 | 220.0 | Ref |
| DPP4I | 147 | 735.4 | 203.5 | 1.14 (0.96-1.36) | 144 | 726.7 | 198.2 | 0.90 (0.72-1.12) | |
| Death | SU | 3,124 | 5,186.3 | 602.4 | Ref | 501 | 749.2 | 668.7 | Ref |
| DPP4I | 523 | 789.6 | 662.4 | 1.10 (1.00-1.21) | 488 | 753.7 | 647.5 | 0.97 (0.86-1.10) | |
Per 1,000 person-years of follow-up
Abbreviations: PY, person-years; SU, sulfonylurea; DPP4I, dipeptidyl peptidase-4 inhibitor; AMI, acute myocardial infarction; MACE, major adverse cardiovascular events; HF, heart failure.
Note: In the initial design of the study, we had planned to examine the 365-day outcome for stroke, but preliminary analyses of the study data demonstrated that there were too few outcome events and reporting them would have violated the CMS’s Cell Size Suppression Policy governing our use of the data.
Stability and Sensitivity Analyses
The main results were generally consistent when implementing multiple imputation of missing baseline covariate information (Table S3), examining 3-month and 6-month outcomes (Table S4), estimating the propensity score using generalized boosted regression (Table S5), and employing Fine and Gray models to account for the competing risk of death (Table S6). Of note, the estimates from the generalized boosted regression propensity score-matched cohort suggested a relative reduction in death for DPP4I versus SU use at 6 months (HR 0.83, 95%CI 0.70-0.99) and 1 year (HR 0.83, 95%CI 0.73-0.95). For the main severe hypoglycemia estimate, the E-value was 2.9 for the point estimate and 1.32 for the confidence interval, suggesting results were moderately robust to confounding given the large number of measured covariates addressed in the analyses.
DISCUSSION
In this large national cohort study of NH residents, we observed a decreased rate of severe hypoglycemic events among new users of DPP4Is compared to new users of SUs through 365-days of follow-up. We did not observe any differences in the risk of severe hyperglycemia, AMI, heart failure, MACE plus heart failure, or death between the two treatment groups. There are no known prior observational or interventional studies of the comparative effects of DPP4Is and SUs for any outcomes among NH residents. However, it is notable that the 2019 results of the CAROLINA trial comparing linagliptin and glimepiride are consistent with our findings despite arising from a younger, less complex population.5, 6 The CAROLINA trial demonstrated no differences in major adverse cardiovascular outcomes or death, but showed a markedly lower rate of moderate or severe hypoglycemia for linagliptin users versus SU users (HR 0.18, 95%CI 0.15-0.21).5, 6 Our results may also complement forthcoming results from the “Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study” (GRADE) pragmatic trial, which will compare outcomes for DPP4I and SU users and may have included some NH residents despite its exclusion of clinically or medically unstable individuals with an expected survival of less than one year.15 Since minimizing hypoglycemia is typically the most important goal in NH residents, and there is no difference in cardiovascular outcomes, our study supports the selection of DPP4Is over SUs whenever possible for this population.
Limitations
Our results must be interpreted in light of at least two key limitations. First, because this study was observational, we cannot rule out the possibility of residual confounding (e.g., by HbA1c or other measures of glycemic control unmeasured in our data). Second, there may be under-ascertainment of outcomes in claims data for NH residents. Many occurrences of hypoglycemia and hyperglycemia will not require a hospitalization or ED visit because the NH staff are capable of managing all but the most severe occurrences. If misclassification is non-differential by treatment group and the specificity of claims-based measures is high, relative effect measures are unlikely to be biased. Finally, we did not quantify the as-treated estimand as a causal contrast because we did not have medication administration record data to classify person-time spent “on drug” with very high accuracy.
Summary
Use of DPP4Is for T2DM among older NH residents may result in a lower risk of severe hypoglycemia compared to use of SUs but is not associated with a differential risk of severe hyperglycemia, AMI, heart failure, MACE plus heart failure, or death. Additional studies are needed to better understand the comparative safety and effectiveness of other newer glucose-lowering treatments in the NH setting, especially sodium-glucose co-transporter-2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) agonists. However, our study provides new evidence to guide treatment decisions for NH residents and other frail older adults with T2DM, supporting the use of DPP4Is as a preferred treatment over SUs to decrease severe hypoglycemia risk.
Supplementary Material
Supplementary Table S1. Summary of the Protocol of the Hypothetical Target Trial Emulated to Compare Dipeptidyl Peptidase-4 Inhibitors and Sulfonylureas among Older Nursing Home Residents.
Supplementary Table S2. Covariates Included in the Propensity Score Estimation and Standardized Differences Before and After Propensity Score Matching.
Supplementary Table S3. Effects of Dipeptidyl Peptidase-4 Inhibitors versus Sulfonylureas on Mortality, Adverse Glycemic, and Adverse Cardiovascular Outcomes Using Multiple Imputation of Missing Pretreatment Covariate Information.
Supplementary Table S4. Effects of Dipeptidyl Peptidase-4 Inhibitors versus Sulfonylureas on Mortality, Adverse Glycemic, and Adverse Cardiovascular Outcomes at 3 months and 6 months.
Supplementary Table S5. Effects of Dipeptidyl Peptidase-4 Inhibitors versus Sulfonylureas on Mortality, Adverse Glycemic, and Adverse Cardiovascular Outcomes Using Generalized Boosted Regression to Estimate the Propensity Score (N=1,790).
Supplementary Table S6. Effects of Dipeptidyl Peptidase-4 Inhibitors versus Sulfonylureas on Adverse Glycemic and Cardiovascular Outcomes Using Fine and Gray Models to Address the Competing Risk of Death.
Supplementary Figure S1. Study Cohort Flow Diagram.
Supplementary Figure S2. Kaplan Meier Plot of Hypoglycemia over 365 Days of Follow-Up Stratified by Dipeptidyl Peptidase-4 inhibitor Versus Sulfonylurea Use after Propensity Score Matching.
Supplementary Figure S3. Kaplan Meier Plot of Hyperglycemia over 365 Days of Follow-Up Stratified by Dipeptidyl Peptidase-4 inhibitor Versus Sulfonylurea Use after Propensity Score Matching.
Supplementary Figure S4. Kaplan Meier Plot of Acute Myocardial Infarction over 365 Days of Follow-Up Stratified by Dipeptidyl Peptidase-4 inhibitor Versus Sulfonylurea Use after Propensity Score Matching.
Supplementary Figure S5. Kaplan Meier Plot of Heart Failure over 365 Days of Follow-Up Stratified by Dipeptidyl Peptidase-4 inhibitor Versus Sulfonylurea Use after Propensity Score Matching.
Supplementary Figure S6. Kaplan Meier Plot of Major Adverse Cardiovascular Events plus Heart Failure over 365 Days of Follow-Up Stratified by Dipeptidyl Peptidase-4 inhibitor Versus Sulfonylurea Use after Propensity Score Matching.
Supplementary Methods 1. Measurement of Outcomes.
Supplementary Methods 2. Sensitivity Analysis using the E-value.
Key Points:
Dipeptidyl peptidase-4 inhibitors (DPP4Is) reduced the risk of severe hypoglycemia by 43% compared to sulfonylureas (SUs) among older nursing home (NH) residents.
No differences were observed in hyperglycemia, cardiovascular, or death outcomes.
Why does this matter? DPP4Is should be a preferred treatment over SUs for long-stay NH residents.
ACKNOWLEDGEMENTS
Funding Sources:
This study was supported by grants R01AG045441, RF1AG061221, R01AG065722, and R21AG061632 from the National Institute on Aging (NIA) and by grant U54GM1156775 from the National Institute of General Medical Sciences (NIGMS), which funds Dr. Zullo and Advance Clinical and Translational Research (Advance-CTR). Dr. Zullo was also supported by a Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship in Health Services Research and Development. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Other Acknowledgements:
Dr. Zullo is a U.S. Government employee; the views expressed in this article are those of the author and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Footnotes
Conflicts of Interest: Dr. Berry previously received grant money from Amgen unrelated to the current project. All other authors have no relevant conflicts of interest to report.
Sponsors’ Role: The sponsors had no role in the design, methods, subject recruitment, analysis, and preparation of the paper or any other aspect of the work.
REFERENCES
- 1.Munshi MN, Florez H, Huang ES, et al. Management of Diabetes in Long-term Care and Skilled Nursing Facilities: A Position Statement of the American Diabetes Association. Diabetes Care. February 2016;39(2):308–18. doi: 10.2337/dc15-2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zullo AR, Dore DD, Gutman R, Mor V, Smith RJ. National Glucose-Lowering Treatment Complexity Is Greater in Nursing Home Residents than Community-Dwelling Adults. Journal of the American Geriatrics Society. November 2016;64(11):e233–e235. doi: 10.1111/jgs.14485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zullo AR, Dore DD, Daiello L, et al. National Trends in Treatment Initiation for Nursing Home Residents With Diabetes Mellitus, 2008 to 2010. Journal of the American Medical Directors Association. July 1 2016;17(7):602–8. doi: 10.1016/j.jamda.2016.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Routledge PA, O'Mahony MS, Woodhouse KW. Adverse drug reactions in elderly patients. British Journal of Clinical Pharmacology. February 2004;57(2):121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheen AJ. Cardiovascular safety of DPP-4 inhibitors compared with sulphonylureas: Results of randomized controlled trials and observational studies. Diabetes & Metabolism. November 2018;44(5):386–392. doi: 10.1016/j.diabet.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 6.Rosenstock J, Kahn SE, Johansen OE, et al. Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial. JAMA : the journal of the American Medical Association. September 19 2019;doi: 10.1001/jama.2019.13772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huitfeldt A, Hernan MA, Kalager M, Robins JM. Comparative Effectiveness Research Using Observational Data: Active Comparators to Emulate Target Trials with Inactive Comparators. EGEMS (Wash DC). 2016;4(1):1234. doi: 10.13063/2327-9214.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernan MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. American Journal of Epidemiology. April 15 2016;183(8):758–64. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. American Journal of Epidemiology. November 1 2003;158(9):915–20. [DOI] [PubMed] [Google Scholar]
- 10.Zullo AR, Dore DD, Gutman R, Mor V, Alvarez CA, Smith RJ. Metformin Safety Warnings and Diabetes Drug Prescribing Patterns for Older Nursing Home Residents. Journal of the American Medical Directors Association. October 1 2017;18(10):879–884 e7. doi: 10.1016/j.jamda.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. American Journal of Epidemiology. June 15 2006;163(12):1149–56. doi:kwj149 [pii] 10.1093/aje/kwj149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsons L Reducing Bias in a Propensity Score Matched-Pair Sample Using Greedy Matching Techniques. 2001: [Google Scholar]
- 13.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Statistics in Medicine. March 30 1999;18(6):681–94. doi: [DOI] [PubMed] [Google Scholar]
- 14.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Annals of Internal Medicine. August 15 2017;167(4):268–274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 15.Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. August 2013;36(8):2254–61. doi: 10.2337/dc13-0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Summary of the Protocol of the Hypothetical Target Trial Emulated to Compare Dipeptidyl Peptidase-4 Inhibitors and Sulfonylureas among Older Nursing Home Residents.
Supplementary Table S2. Covariates Included in the Propensity Score Estimation and Standardized Differences Before and After Propensity Score Matching.
Supplementary Table S3. Effects of Dipeptidyl Peptidase-4 Inhibitors versus Sulfonylureas on Mortality, Adverse Glycemic, and Adverse Cardiovascular Outcomes Using Multiple Imputation of Missing Pretreatment Covariate Information.
Supplementary Table S4. Effects of Dipeptidyl Peptidase-4 Inhibitors versus Sulfonylureas on Mortality, Adverse Glycemic, and Adverse Cardiovascular Outcomes at 3 months and 6 months.
Supplementary Table S5. Effects of Dipeptidyl Peptidase-4 Inhibitors versus Sulfonylureas on Mortality, Adverse Glycemic, and Adverse Cardiovascular Outcomes Using Generalized Boosted Regression to Estimate the Propensity Score (N=1,790).
Supplementary Table S6. Effects of Dipeptidyl Peptidase-4 Inhibitors versus Sulfonylureas on Adverse Glycemic and Cardiovascular Outcomes Using Fine and Gray Models to Address the Competing Risk of Death.
Supplementary Figure S1. Study Cohort Flow Diagram.
Supplementary Figure S2. Kaplan Meier Plot of Hypoglycemia over 365 Days of Follow-Up Stratified by Dipeptidyl Peptidase-4 inhibitor Versus Sulfonylurea Use after Propensity Score Matching.
Supplementary Figure S3. Kaplan Meier Plot of Hyperglycemia over 365 Days of Follow-Up Stratified by Dipeptidyl Peptidase-4 inhibitor Versus Sulfonylurea Use after Propensity Score Matching.
Supplementary Figure S4. Kaplan Meier Plot of Acute Myocardial Infarction over 365 Days of Follow-Up Stratified by Dipeptidyl Peptidase-4 inhibitor Versus Sulfonylurea Use after Propensity Score Matching.
Supplementary Figure S5. Kaplan Meier Plot of Heart Failure over 365 Days of Follow-Up Stratified by Dipeptidyl Peptidase-4 inhibitor Versus Sulfonylurea Use after Propensity Score Matching.
Supplementary Figure S6. Kaplan Meier Plot of Major Adverse Cardiovascular Events plus Heart Failure over 365 Days of Follow-Up Stratified by Dipeptidyl Peptidase-4 inhibitor Versus Sulfonylurea Use after Propensity Score Matching.
Supplementary Methods 1. Measurement of Outcomes.
Supplementary Methods 2. Sensitivity Analysis using the E-value.