Abstract
Although colorectal cancer (CRC) screening is highly effective, screening rates lag far below recommended levels, particularly for low-income people. The Colorectal Cancer Control Program (CRCCP) funded $100 million in competitively awarded grants to 25 states from 2009–2015 to increase CRC screening rates among low-income, uninsured populations, in part by directly providing and paying for screening services. Using data from the 2001–2015 Behavioral Risk Factor Surveillance System (BRFSS) and a difference-in-differences strategy, we find no effects of CRCCP on the use of relatively cheap fecal occult blood tests (FOBT). We do, however, find that the CRCCP significantly increased the likelihood that uninsured 50–64-year-olds report ever having a relatively expensive endoscopic CRC screening (sigmoidoscopy or colonoscopy) by 2.9 percentage points, or 10.7 percent. These effects are larger for women, minorities, and individuals who did not undertake other types of preventive care. We do not find that the CRCCP led to significant changes in CRC cancer detection. Our results indicate that the CRCCP was effective at increasing CRC screening rates among the most vulnerable.
Keywords: colorectal cancer screenings, CRCCP
1. Introduction
Colorectal cancer (CRC) is the third leading cause of cancer-related death in the United States, responsible for over 50,000 deaths per year. According to the American Cancer Society, regular screening for CRC through fecal occult blood stool tests (FOBT) and through relatively more invasive colonoscopy or sigmoidoscopy is highly effective: the five-year survival rate for colorectal cancers that are detected early is around 90 percent. CRC screening for asymptomatic individuals age 50–74 has thus earned a grade of ‘A’ from the United States Preventive Services Task Force (USPSTF), indicating strong scientific consensus on the medical efficacy and appropriateness of CRC screening (Bibbins-Domingo et al. 2016). This is in noted contrast to the associated grade for screening for asymptomatic individuals for breast cancer (earning a grade of B for 50–74-year-olds), lung cancer (earning a grade of B for older adults with 30 years of smoking history), and skin cancer (earning a grade of I, or inconclusive).
The unusually strong scientific consensus surrounding the efficacy of CRC screening for asymptomatic adults age 50–74 is also notable because population rates of such screenings lag well below recommended levels. For example, while Healthy People 2020 set a target of 70.5 percent of the population receiving CRC screenings, data from the 2018 National Health Interview Surveys indicate that only 65.2 percent of the age-appropriate population had received one (U.S. Department of Health and Human Services 2020). Importantly, public health research shows that there is a large socioeconomic status gradient in receipt of CRC screening: individuals with health insurance are much more likely to be up to date with CRC screening recommendations than are individuals without health insurance (Joseph et al. 2012), and these gradients are especially pronounced among racial and ethnic minorities (Trivers et al. 2008).
In this paper we provide new evidence on the effects of one of the largest federal efforts to increase CRC screenings among low-income uninsured individuals: the federally-supported Colorectal Cancer Control Program (CRCCP). From 2009 to 2015, the CRCCP awarded $100 million in grants to 25 states to provide direct funding provision for CRC screenings to 50–74-year-old individuals without insurance. Multiple studies have examined how state public health departments and local clinics implemented the CRCCP (see, for example, Hannon et al. 2013, Maxwell et al. 2014, and others), but to our knowledge there has been no research that has evaluated whether CRCCP was effective at achieving its intended goal: increasing population-wide CRC screening rates among low-income uninsured people.
To evaluate the effects of the CRCCP we exploit the fact that although all states can and do apply for CRCCP funds, limited resources mean that CRCCP effectively treats some states but not others.1 This variation gives rise to our difference-in-differences research design: we compare over-time differences in CRC screening rates and colorectal cancer detection outcomes for individuals without insurance in states that received CRCCP grants and guidance to the associated changes for individuals in all the other states that applied for but did not receive CRCCP support. This geographic heterogeneity is important because of other national, secular policy changes that were adopted around the same time period such as the 2010 preventive services mandate of the Affordable Care Act (ACA) which should have affected some individuals in all states. Our data (described below) allow us to consider the two main types of CRC screening: blood stool tests and more invasive endoscopy (sigmoidoscopy or colonoscopy). We also observe demographic characteristics which permit us to examine heterogeneity across gender, race/ethnicity, education, marital status, age, and other characteristics.
To preview, our two-way fixed effects models using data from the 2000–2015 waves of the Centers for Disease Control and Prevention’s (CDC’s) Behavioral Risk Factor Surveillance System (BRFSS) indicate that the CRCCP had no effects on population rates of lifetime FOBT use for uninsured adults age 50–64 who were targeted by the program.2 However, we do find that the CRCCP was associated with statistically significant increases in the likelihood that uninsured adults age 50–64 reported that they ever had had an endoscopy (i.e., a sigmoidoscopy or colonoscopy). The effect size we estimate – 2.9 percentage points – is statistically significant at the one-percent level.
When we examine heterogeneity in the effects, we find larger effects of the CRCCP program at increasing lifetime endoscopy among women (compared to men), nonwhites (compared to non-Hispanic whites), and individuals who did not use recent preventive care (compared to individuals who did use recent preventive care), which suggests the program was effective at targeting the most vulnerable populations. Finally, we also examine the effects of the CRCCP on CRC cancer detections and do not find evidence of significant changes, though these estimates are impacted by data and policy timing constraints. Overall, our results indicate that the CRCCP was successful at increasing CRC screening rates among the most vulnerable.
The paper proceeds as follows: Section 2 outlines institutional details regarding colorectal cancer, CRC screenings, and the CRCCP. Section 3 provides a literature review. We describe the research design, data, and empirical approach in Section 4, and Section 5 presents the results. Section 6 concludes.
2. Colorectal Cancer, CRC Screening, and CRCCP Institutional Details
2.1. Colorectal Cancer and CRC Screening
Colorectal cancer refers to cancers of the colon and rectum, the final part of the gastrointestinal system which processes food and rids the body of solid waste. The colon has four sections: the ascending colon, the transverse colon, the descending colon, and the sigmoid colon. The sigmoid colon is the final/lowest portion of the colon and joins the rectum. Most colorectal cancers begin as noncancerous polyps that grow slowly on the inner lining of the colon or rectum. Early colorectal cancers often show no symptoms, thus increasing the importance of CRC screening. As CRC tumors grow, they can result in bleeding and black stools.
Nearly 150,000 colorectal cancer cases will be diagnosed in the United States in 2020, and over 50,000 will die from the disease in 2020 (Siegel et al. 2020). Approximately 4.6 percent of men and 4.2 percent of women will be diagnosed with colorectal cancer in their lifetime. The most important risk factor for colorectal cancer is advanced age although men, African Americans, and individuals with a family history of CRC also have higher risk. Behavioral factors such as smoking, sedentary lifestyle, and others are also related to CRC risk. Colorectal cancer survival rates are very high if the cancer is caught at an early stage: the five-year survival rate for localized-stage CRC is 90 percent. If the cancer is not caught until the regional stage, the five-year survival rate falls to 71 percent, and for patients diagnosed with distant-stage CRC the five-year survival rate is just 14 percent.
CRC screening has historically been recommended to begin at age 50 for most asymptomatic adults, though recently the American Cancer Society reduced their recommendation for the starting age to age 45. Individuals with identifiable risk factors (including African American race) are recommended to start screening earlier. Methods of CRC screening include: visual examination, stool-based tests such as Cologuard that can be performed at home, and relatively more invasive endoscopic exams that include flexible sigmoidoscopy and colonoscopy. Stool tests work by testing for intestinal bleeding caused by large polyps. Annual stool tests are recommended, with follow-up diagnostic colonoscopy in the event of a positive test.
The most common CRC screening modalities today are flexible sigmoidoscopy and colonoscopy; the former can be done reasonably quickly with few complications and does not require sedation. The latter is more successful at detecting cancer but requires sedation due to the additional complexity of the procedure (the flexible sigmoidoscopy only views the rectum and the bottom third of the colon; the colonoscopy examines the entire colon). Small polyps can be removed in both procedures. Adults of average risk with a negative colonoscopy (flexible sigmoidoscopy) are not recommended to get another one for ten (five) years.
Over most of our sample period, blood stool tests were reasonably cheap, on the order of $20 or $30 out of pocket.3 More invasive endoscopy, however, was much more expensive – approximately $600 – over most of our sample period.4 For patients without insurance, these costs all are paid out of pocket, though more recently the preventive services mandate of the Affordable Care Act required that for insured individuals these CRC screenings must be offered at no cost to individuals aged 50–74.5 Again, our primary focus in this paper is on uninsured individuals, and it is clear for this population the costs of endoscopy are much larger than for blood stool tests.
2.2. The Colorectal Cancer Control Program
The Colorectal Cancer Control Program (CRCCP) is supported by federal funds to the Centers for Disease Control and Prevention. Unlike the companion programs for breast and cervical cancer (the National Breast and Cervical Cancer Early Detection Program, or NBCCEDP), the CRCCP is not mandated by law. All states apply for CRCCP funding to the CDC, and the applications are independently reviewed and scored by an internal CDC review committee. The top scoring applications receive CRCCP funds in each five-year phase.
Our primary focus is on CRCCP grants made to 25 states in fiscal years 2009 and 2010, shown in Figure 1.6 These grants were primarily made to state health departments to directly pay for CRC screenings and to implement a range of process guidelines to improve population screening rates for uninsured 50–74-year-olds in the state. In FY 2010, the grants to states ranged from $600,000 (Nevada) to $2.2 million (Washington state). More recent phases of the CRCCP (funded since 2015) changed the focus of the program and no longer focused on paying directly for CRC screenings (although some states continued to receive direct funding for screenings). Instead, states now partner with local health systems and federally qualified health centers (FQHCs) to increase population CRC screening rates in their area. We restrict attention to evaluating the effects of the grants made in fiscal years 2009 and 2010 that focused on a combination of directly paying for CRC screenings and implementing process improvements, with our sample ending in July 2015.7
Figure 1. States Receiving CRCCP Grants in FY 2009/2010.
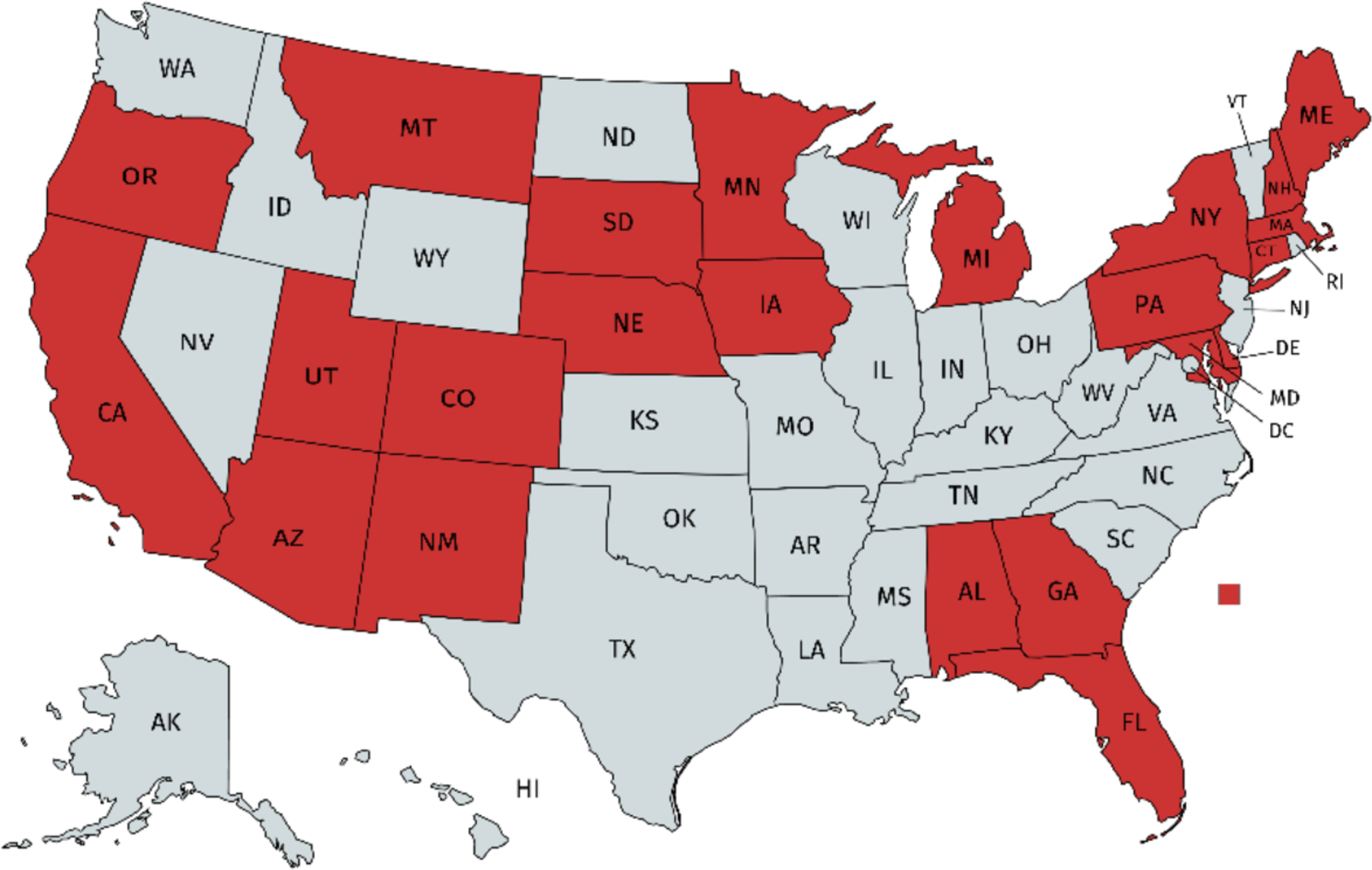
Notes: Figure presents states receiving CRCCP grants starting in FY 2009 (7/2008–6/2009) or FY 2010 (7/2009–6/2010).
3. Literature Review
Several studies by CDC researchers and academic partners document the implementation of the CRCCP (Joseph et al. 2011), the use of evidence-based interventions within state programs (Hannon et al. 2013, Maxwell et al. 2014), and program-related expenditures (Tangka et al. 2017, Subramanian et al. 2017). We are not aware, however, of any comprehensive population-based evaluations of the effects of CRCCP state awards on CRC screening rates over our period of study.8 DeGroff et al. (2017) reference CDC-supported evaluations of the effects of CRCCP on ‘changes in state-wide screening rates using data from the BRFSS’, noting that ‘[e]valuators found that program reach was insufficient to detect impact at the state level’. In contrast, our results below suggest that state CRCCP grants were significantly related to increased endoscopy rates among uninsured 50–64-year-old adults.
Other research in economics and public health has evaluated the effects of other policy initiatives to increase CRC screening and reduce CRC cancer burden. For example, Cram et al. (2003) find that colonoscopy rates increased significantly following Katie Couric’s on-air colonoscopy in March 2000 on the Today Show, suggesting the importance of celebrity endorsements. Pantel et al. (2020) study the designation of March as National Colorectal Cancer Awareness Month and find that Google Trends searches for colorectal cancer increase significantly in March despite that endoscopy screening rates do not. Kadiyala and Strumpf (2016) study age-based screening recommendations from major medical and public health organizations. Using regression discontinuity methods, they find that CRC screening rates increase significantly at age 50 by about 78 percent, while CRC detection rates increase by 49 percent.
Several studies focus on insurance-based interventions related to CRC screening. For example, Hamman and Kapinos (2016) study the effects of state mandates requiring private insurance plans to cover or offer CRC screenings. Using data from the 1997–2008 BRFSS and a triple differences strategy leveraging variation across states, years, and whether the individual is under age 65 (since Medicare-eligible individuals should be far less responsive to private insurance benefit mandates), they find that these state insurance laws significantly increased endoscopy among men by 2–3 percentage points. Cokkinides et al. (2011) find a similar but smaller result using BRFSS data from 2002–2008 and focusing on 50–64-year-olds in a difference-in-differences framework.
More recently, in 2010 the Preventive Services Mandate of the Affordable Care Act required insurance plans to cover CRC screening for 50–74-year-olds without cost-sharing (i.e., co-pays or deductibles) because of its USPSTF grade of ‘A’. Medicare similarly eliminated cost-sharing for initial colonoscopy in 2011. Results on the effects of these provisions on CRC screening rates and cancer detection outcomes have been mixed. Examining trends in CRC screening over time, Hamman and Kapinos (2015) find that colonoscopy rates among men age 66–75 increased by a statistically significant 4 percentage points, with no effects for women. In contrast, Richman et al. (2016) use data from the Medical Expenditure Panel Survey and do not find evidence that the ACA preventive services mandate was associated with statistically significant increases in colonoscopy, sigmoidoscopy, or FOBT rates for either 50–64-year-olds or 65–75-year-olds. Mehta et al. (2015) compared changes in screening rates of 50–64-year-olds in grandfathered plans (that were not subject to the ACA preventive services mandate) to those for same-age individuals in non-grandfathered plans who were directly treated by the 2010 law. They found no significant changes before and after 2010 in colonoscopy rates across the two groups, suggesting minimal effects of the cost-sharing removal.9 Finally, Whaley (2018) uses data from the 2009–2013 Health Care Cost Institute which includes information on which insurance plans were previously covering colonoscopies in a generous fashion (i.e., without cost-sharing) and thus were plausibly unaffected by the 2010 preventive services mandate. Using these plans as a control group, Whaley (2018) estimates that the 2010 ACA provision increased colonoscopies by 1.7 percent.10
Finally, studies have also examined the effects of general insurance-based interventions that are not directly CRC-related or preventive-care related on CRC screening rates. For example, Myerson et al. (2020, forthcoming) study the age-65 Medicare eligibility discontinuity in the United States using a regression discontinuity design. Using BRFSS data, they find that past year CRC screening rates increase significantly by 2.4–3.3 percentage points. Using cancer registry data, they find that at age 65, colorectal cancer detection increases significantly, while there is no significant change in colorectal cancer mortality. As the newly insured individuals at age 65 are disproportionately from low socioeconomic status groups (Card et al. 2008, 2009), these results are particularly relevant for our study of uninsured 50–64-year-olds. Okoro et al. (2014) study the Massachusetts health reform and find that colonoscopy rates significantly increased in Massachusetts coincident with its insurance mandate relative to the associated changes in colonoscopy rates for other New England states. Related to this, Sabik et al. (2020) use cancer registry data and difference-in-differences models to show that the Massachusetts insurance mandate was associated with reductions in the likelihood that CRC cancers were detected at advanced stages, suggestive of improvements in CRC screening. A recent analysis of the 2008 Oregon Medicaid Lottery Experiment did not find that individuals who were randomly assigned access to Medicaid in the state had significantly higher colonoscopy rates than individuals who were not randomly assigned such access (Marino et al. 2016), while a study of subsequent randomized Oregon Medicaid invitations in 2012 and 2013 found large and statistically significant increases in colonoscopy rates (but not FOBT rates) associated with a randomized offer of Medicaid in the state (Wright et al. 2016). Finally, two recent studies of the ACA Medicaid expansions have used BRFSS data and found evidence that low-income adults in expansion states age 50–64 had significant increases in CRC screening rates compared to otherwise similar low-income adults in states that did not adopt the Medicaid expansion (Zerhouni et al. 2019, Hendryx and Luo 2018).11
Thus, the literature examining the effects of insurance-based interventions has reached mixed conclusions on the effects of these policies on CRC screening and CRC detection. Unlike these prior studies, we are examining programs which directly fund CRC screening. Our study is related to the literature on insurance mandates which expand coverage to specific procedures, although unlike the vast majority of this literature the intervention we study targets uninsured individuals as opposed to insured individuals. As such, our results are more relevant for thinking about relatively direct service provision to vulnerable populations by local, state, and federal governments.12
4. Data Description and Empirical Approach
Our main data on CRC screening come from the Center for Disease Control’s Behavioral Risk Factor Surveillance System (BRFSS). The BRFSS has included questions about CRC screenings for a large share of the time the survey has been fielded, though these questions do not always appear on the core questionnaire. Surveys are conducted by telephone by the individual states and then sent to CDC to be compiled into a public-use dataset. We focus our attention on data from 2001-June 2015; we end in June 2015 as this is when CRCCP was transformed from a program paying for screenings to one that provided outreach and case management funding. CRC screening questions appeared on the core questionnaire in 2001 and in every subsequent even-numbered BRFSS year during this period; in odd-numbered years from 2003–2015 a very small number of states also asked a module of CRC-related questions, and we include those in our main sample. We explore robustness to which states and years are in the data below.
The BRFSS questions on CRC screening allow us to create consistent measures of blood stool tests and endoscopy for adults age 50 and older; adults under age 50 were not asked the CRC questions. Specifically, individuals are asked: “A blood stool test is a test that may use a special kit at home to determine whether the stool contains blood. Have you ever had this test using a home kit?” Individuals who say ‘yes’ are then asked: “How long has it been since you had your last blood stool test using a home kit?”. Response options are: within the past year, within the past two years, within the past five years, and five or more years ago. Individuals are then asked “Sigmoidoscopy and colonoscopy are exams in which a tube is inserted in the rectum to view the bowel for signs of cancer and other health problems. Have you ever had either of these exams?” Individuals who say ‘yes’ are then asked: “How long has it been since you had your last sigmoidoscopy or colonoscopy?”. Response options are: within the past year, within the past two years, with the past five years, within the past ten years, and ten or more years ago. The BRFSS does not ask whether the most recent screening was for screening or diagnostic purposes, so we cannot further examine these outcomes.
We create three main outcome variables. First, we identify Ever Had a Blood Stool Test as equal to one if the individual reports ever having had a blood stool test and zero otherwise. Second, we create Blood Stool Test in the Past Year as equal to one if the individual reports that she had a blood stool test within the past year and zero otherwise.13 Third, we create Ever Had a Sigmoidoscopy or Colonoscopy (Endoscopy) as equal to one if the individual reports ever having had a sigmoidoscopy or colonoscopy and zero otherwise.14 We also observe (and control for) standard demographic characteristics in the BRFSS, including age (in five-year groups), race/ethnicity, education, and marital status. The BRFSS also includes a very basic measure of health insurance coverage which is sufficient to identify our treated group: we can identify whether the individual is covered by ‘any health plan.’15 Of course, this is current coverage, and may not fully describe the past year or further back, but it is likely conservative.
To estimate the effects of the CRCCP program on outcomes we use a two-way fixed effects model (which controls for unrestricted state and year dummies) in which we rely on variation in the timing of participation in the federal CRCCP program across states to identify the effects. Note that state CRCCP grants were awarded in fiscal years 2009 or 2010 (July of these years, with 3 states adding to the others in 2010), around the same time as the preventive services mandate of the Affordable Care Act which required insurance plans to cover screening endoscopy without cost-sharing as of September 23, 2010. Because the ACA affected everyone on the United States, this state-specific participation in the CRCCP program is critical for isolating the causal effects of the CRCCP from other national policies or secular trends (which will be accounted for in our empirical models with time dummies).
We write the two-way fixed effects model as:
| (1) |
where Yist are the various dichotomous screening outcomes for individual i in state s at time t. Xist is a vector of individual-level demographic controls that includes dummies for sex, 5-year age groups, race/Hispanic ethnicity, education, and marital status. CRCCP is an indicator variable equal to one if the state participates in the federal CRCCP program (joined during 2009/2010). While we shorten the variable names for brevity in writing out equation (1), strictly speaking each treatment variable equals the share of the relevant reference window the individual is treated by its state CRCCP program.16 For the ‘Ever Had A Screening’ outcomes we use the contemporaneous policy in place in the individual’s state.
Zst is a vector of covariates that vary at the state and year level that are associated with insurance coverage and use of preventive care. These include: the unemployment rate; the HMO penetration rate; the share of individuals who work (or whose spouses work) at private firms of various sizes (<100, 100+); the fraction Black; and the fraction Hispanic. Additionally, Zst captures any state-specific laws regarding health insurance mandates that require insurance firms to offer and/or cover colorectal and prostate cancer screenings. It also includes state responses to expanding Medicaid related to the ACA – early expansion, 2014 expansion, or private option. Dummy variables for each state are captured by Ss and in the two-way fixed effects models control for time-invariant state-specific factors. Dummy variables for each fiscal year of the program are captured by Tt and in the two-way fixed effects specifications control for period-specific shocks common to all states in any given year.17 Throughout, we cluster the standard errors at the state level (Bertrand, Duflo, and Mullainathan 2004) both because the bulk of the policy variation is at the state by year level and because BRFSS is not an independent and identically distributed sample of persons, but samples disproportionately within states in certain geographic areas. Regressions are weighted to be population representative, and the main sample is all adults age 50–64 interviewed by the BRFSS in survey years 2001 through June 2015 in state-year cells when the CRC screening questions were asked.18
5. Results
5.1. Descriptive Statistics
In Figures 2 and 3 we show the trend from 2002–2014 for the likelihood that respondents age 50–64 report ever having had a fecal occult blood stool test (Figure 2) or a more invasive sigmoidoscopy or colonoscopy (Figure 3). We show these rates separately by insurance status and whether a state participated in the CRCCP program.19 A few patterns are notable. First, CRC screening rates are substantially higher for insured individuals compared to uninsured individuals. In Figure 3, for example, we see that the likelihood of having a sigmoidoscopy or colonoscopy is about 20 to 30 percentage points higher for insured 50–64-year-olds than for uninsured 50–64-year-olds, consistent with prior descriptive research in public health. Second, FOBT rates fell markedly over this time period, while endoscopy rates increased. Figure 2 shows that lifetime rates of FOBT use fell by 10–15 percentage points, while Figure 3 shows that lifetime rates of sigmoidoscopy or colonoscopy increased by about 20–25 percentage points from 2002 to 2014. Third, CRC screening rate improvements were larger for insured adults than for uninsured adults in the 2000s, regardless of CRCCP participation. This could be attributable to national health reforms such as the ACA preventive services mandate.
Figure 2. Trends in the Probability an Individual Ever had a Fecal Occult Blood Stool Test, by Insurance Status and Whether State Participated in CRCCP.
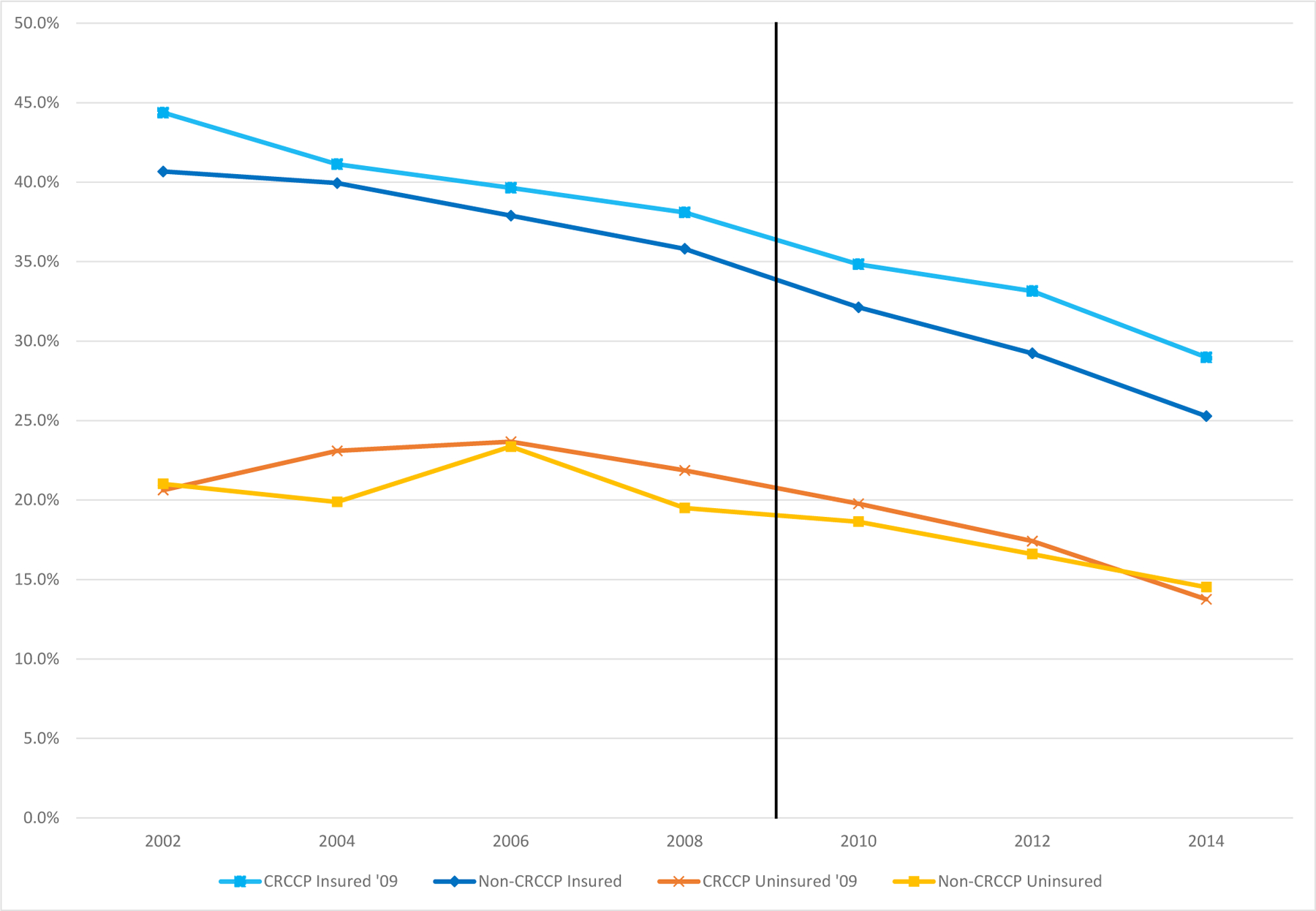
50–64-year-old men and women, 2002–2014 BRFSS (even years only)
Notes: The figure shows means for ever having had FOBT in even years by whether or not the state adopted CRCCP in 2009/2010 and by insurance status, using BRFSS data for 2002–2014 for 50–64 year olds. The orange line with cross hatches represents means for the uninsured 50–64-year olds in states who adopted CRCCP in 2009 or 2010, the yellow line with square markers represents means for the uninsured in states which never adopt CRCCP during 2002–2014. The light blue line with square markers represents means for those 50–64-year olds with insurance who adopted CRCCP in 2009/2010 and the darker blue line with diamond markers represents means for the insured in states which never adopt CRCCP during 2002–2014. Means are weighted to be population representative.
Figure 3. Trends in the Probabily an Individual Ever had a Sigmoidoscopy or Colonoscopy, by Insurance Status and Whether State Participated in CRCCP.
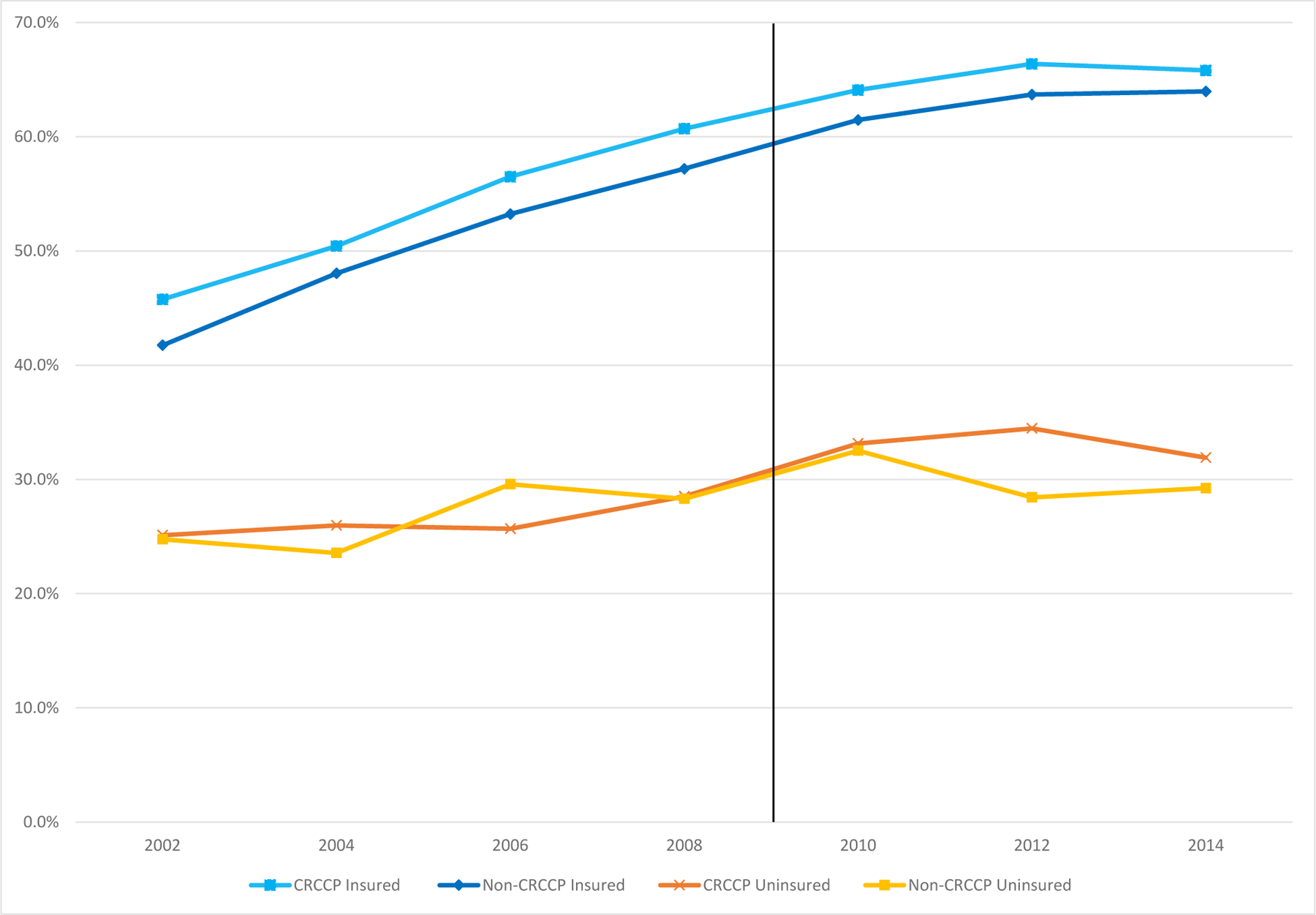
50–64-year-old men and women, 2002–2014 BRFSS (even years only)
Notes: The figure shows means for ever having had a sigmoidoscopy or colonoscopy in even years by whether or not the state adopted CRCCP in 2009/2010 and by insurance status, using BRFSS data for 2002–2014 for 50–64 year olds. The orange line with cross hatches represents means for the uninsured 50–64-year olds in states who adopted CRCCP in 2009 or 2010, the yellow line with square markers represents means for the uninsured in states which never adopt CRCCP during 2002–2014. The light blue line with square markers represents means for those 50–64-year olds with insurance who adopted CRCCP in 2009/2010 and the darker blue line with diamond markers represents means for the insured in states which never adopt CRCCP during 2002–2014. Means are weighted to be population representative.
Fourth, the visual evidence for a CRCCP program effect on lifetime rates of FOBT screening is weak. Although lifetime FOBT rates appear to fall more after 2010 for uninsured individuals in CRCCP states compared to uninsured individuals in non-CRCCP states (suggesting that if anything the CRCCP program reduced FOBT use), the same pattern is observed for insured individuals in CRCCP states compared to insured individuals in non-CRCCP states. Given that CRCCP was not targeted at insured people, the similarity in the patterns for insured and uninsured people suggests some other confounding pattern. In contrast, however, the visual evidence for a CRCCP program effect on lifetime rates of sigmoidoscopy or colonoscopy is stronger. Specifically, the lifetime use of these relatively more invasive CRC screening modalities is similar for uninsured 50–64-year-olds in participating and non-participating states until 2012 at which time a clear difference emerges whereby uninsured adults in CRCCP states have increased rates of sigmoidoscopy or colonoscopy, on the order of 3 percentage points. Moreover, the associated differences for insured individuals in CRCCP participating and non-participating states do not change over the period: those two trends are parallel from 2002–2014. This difference may not be surprising given that, for those without insurance, during our sample period, colonoscopies were about 20 times as expensive as a FOBT.
Table 1 presents descriptive statistics for the key demographic variables used in this analysis for adults in the BRFSS. Column 1 presents means for 50–64-year-old women and column 2 presents means for 50–64-year-old men. We present statistics for basic demographic characteristics (e.g., age, race/ethnicity, education, marital status), cancer screening outcomes, and CRCCP rollout variation. The pattern of demographic characteristics across groups indicates that most of the sample for each age group is white non-Hispanic, while at most 26 percent of the sample is black non-Hispanic, other race non-Hispanic, or Hispanic. Over 30 percent of adults have a bachelor’s degree or more. The majority of the sample is married, and 87 percent of the sample has a health plan. Regarding the cancer-screening outcomes, only 35.1 percent of women and 33 percent of men in this age group report ever having had a blood stool test, and 13 percent report having had one in the past year. 54.3 percent of women and 52.9 percent of men in this age group report ever having had a sigmoidoscopy or colonoscopy. Regarding CRCCP exposure, 25.7 percent of women and 25.8 percent of men are observed in a state and time period with an active CRCCP award from the CDC.
Table 1.
Descriptive Statistics, BRFSS Adults Age 50–64, 2001–2015
| Variable | Women | Men | ||
|---|---|---|---|---|
| White non-Hispanic | .742 | (.438) | .744 | (.437) |
| Black non-Hispanic | .108 | (.311) | .094 | (.292) |
| Other race non-Hispanic | .044 | (.205) | .051 | (.220) |
| Hispanic | .093 | (.291) | .095 | (.293) |
| Less than high school degree | .104 | (.305) | .110 | (.313) |
| HS degree | .291 | (.454) | .271 | (.445) |
| Some college | .294 | (.455) | .261 | (.439) |
| Bachelor’s degree or more | .307 | (.461) | .353 | (.478) |
| Married | .641 | (.480) | .718 | (.450) |
| Widowed/Divorced/Separated | .270 | (.444) | .179 | (.384) |
| Has any health insurance | .874 | (.332) | .870 | (.337) |
| Ever had a blood stool test | .351 | (.477) | .330 | (.470) |
| Had a blood stool test within the past year | .129 | (.335) | .132 | (.339) |
| Ever had a sigmoidoscopy or colonoscopy | .543 | (.498) | .529 | (.499) |
| CRCCP in respondent’s state | .257 | (.437) | .258 | (.438) |
| N | 1,025,719 | 685,904 | ||
Author calculations, 2001–2015 BRFSS adults age 50–64. Weighted means (standard deviations). Sample size for each variable varies slightly due to certain questions not being asked in each year. Reported sample size is the sample size for the demographic characteristics (race/ethnicity, education, and marital status) which were asked in each wave.
5.2. Results on Colorectal Cancer Screenings
We present the first set of evaluative results on the effects of the CRCCP program in Table 2. Column 1 presents results for lifetime FOBT use; column 2 presents results for past year FOBT use, and column 3 presents results for lifetime endoscopy screening. We present results for the full sample in the top panel, results for adults with a health plan in the middle panel, and results for adults without a health plan (i.e., the targeted group) in the bottom panel.20 Columns 1 and 2 of Table 2 provide little support for the idea that CRCCP played an important role at increasing use of FOBT blood stool tests. We find no meaningful relationship between state CRCCP participation and lifetime or past year rates of this CRC screening modality, and this null finding does not differ by insurance status in the middle and bottom panels.
Table 2.
CRCCP Is Not Related to FOBT Rates But Significantly Increased Lifetime Endoscopy Rates among the Uninsured BRFSS 2001–2015, 50–64-year-olds
| (1) | (2) | (3) | |
|---|---|---|---|
| 50–64-year-olds | 50–64-year-olds | 50–64-year-olds | |
| Ever had a blood stool test (FOBT) | Had a blood stool test in past year | Ever had a sigmoidoscopy or colonoscopy | |
| Full Sample | |||
| Pre-reform mean, 2001–2008 | .381 | .159 | .482 |
| CRCCP | .004 | .003 | .005 |
| (.015) | (.012) | (.005) | |
| Adjusted R squared | .06 | .03 | .10 |
| N | 926,915 | 920,059 | 927,454 |
| Adults with a health plan | |||
| Pre-reform mean, 2001–2008 | .402 | .169 | .510 |
| CRCCP | .008 | .006 | .001 |
| (.015) | (.012) | (.005) | |
| Adjusted R squared | .06 | .03 | .09 |
| N | 818,449 | 812,153 | 819,292 |
| Adults without a health plan | |||
| Pre-reform mean, 2001–2008 | .228 | .088 | .270 |
| CRCCP | −.013 | −.004 | .029*** |
| (.013) | (.010) | (.009) | |
| Adjusted R squared | .05 | .02 | .06 |
| N | 106,517 | 105,995 | 106,227 |
Notes: Each panel of each column shows the results from a separate regression model. The reported estimate is the coefficient on an indicator for living in a state with an active CRCCP program. The CRCCP exposure variable for the models in column 2 is adjusted to equal the share of the year prior to the interview that the individual’s state had an active CRCCP program. Additional controls in all models include: sex; five-year age group dummies; insurance mandates for colon cancer screenings; ACA expansion, Medicaid private option expansion, race/ethnicity; education; marital status; the unemployment rate; the level of HMO penetration (as a share of the population); share black; share Hispanic; and state, year, and month of interview fixed effects.
significant at 10%;
significant at 5%;
significant at 1%. Standard errors throughout are clustered at the state level and show in parentheses. Estimates are weighted.
In contrast, column 3 of Table 2 returns evidence that state CRCCP participation significantly increased lifetime rates of sigmoidoscopy or colonoscopy. Specifically, in the bottom panel of column 3 of Table 2 we estimate that the CRCCP program was associated with a 2.9 percentage point increase in lifetime use of these CRC screening modalities, and this estimate is statistically significant at the one percent level.21 Relative to the pre-program mean, this is a 10.7 percent effect. Importantly, we find no evidence in the middle panel that state CRCCP participation was meaningfully related to these outcomes for insured 50–64-year-olds who were not directly targeted by the CRCCP program.
We considered but decided against estimating event study models of the effects of CRCCP on CRC screening. This is because of the nature of the rollout of the program, whereby all treated states received treatment in either 2009 or 2010, at a single point in time. Moreover, only a few states asked the colonoscopy questions in the odd-numbered years. So, any event study cannot use single event years and simultaneously be a balanced panel, and we would have to pool event years into 2-year bins. Thus, there is very little variation in the treatment variable with which to estimate a standard event study among only the treated states, and even with the control states in the model, we do not think this provides much information beyond simple time trends. Put differently, given the data constraints, our preferred specification does not allow for examination of meaningful staggered adoption.
In Table 3, we examine the robustness of the main finding that CRCCP increased lifetime endoscopy for uninsured adults age 50–64. Column 1 reprints our baseline estimate from the bottom panel of column 3 of Table 2, a 2.9 percentage point increase. Column 2 of Table 3 shows that if we drop all odd-numbered years (since the CRC screening questions were only asked during odd years by a small number of states beginning in 2003) our core finding is unchanged. Doing so effectively yields a balanced panel of all 50 states and DC from 2002–2014 and returns estimates that are slightly smaller than the baseline but still statistically significant at the five percent level. In column 3 of Table 3, we show that the core finding is robust to additionally controlling for linear state-specific time trends. Column 4 of Table 3 shows that if we restrict attention to individuals in states that did not expand Medicaid under the Affordable Care act, we continue to find evidence that CRCCP participation increased lifetime endoscopy among the uninsured. This addresses concerns that the composition of who is uninsured may be changing over our time period due to other policies (notwithstanding the null relationship between CRCCP participation and the likelihood the individual has a health plan referenced above). Columns 5 to 7 of Table 3 show that our main results are robust to a range of concerns dealing with sample weighting and the 2011 BRFSS redesign in which the survey added a cellphone sample. Excluding weights (column 5), dropping all cellphone respondents (column 6), and implementing the Simon et al (2017) weighting adjustment (column 7) all return evidence that CRCCP participation was associated with significant increases in lifetime endoscopy rates for uninsured 50–64-year-olds.22
Table 3.
Effects of CRCCP on Lifetime Endoscopy Rates Are Robust BRFSS 2001–2015, 50–64-year-olds without a Health Plan Outcome is: Ever had a Sigmoidoscopy or Colonoscopy
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | |
|---|---|---|---|---|---|---|---|
| Baseline | 1, but drop all odd numbered years | 1, with linear state trends | 1, but drop any state that expanded Medicaid under the ACA | 1, but drop weights | 1, but drop any cellphone respondent | 1, but use Simon et al. (2017) adjustment | |
| Any CRCCP | .029*** | .026*** | .028** | .023* | .021*** | .035*** | .028*** |
| (.009) | (.010) | (.014) | (.012) | (.008) | (.010) | (.009) | |
| Adjusted R squared | .06 | .06 | .06 | .05 | .05 | .06 | .06 |
| N | 106,227 | 90,989 | 106,227 | 53,533 | 106,227 | 96,091 | 106,227 |
See notes to Table 2. Each column represents a single regression, with the main results in column (1) and other changes in columns 2–7.
In Table 4, we report results on effect heterogeneity where we separately estimate the baseline model on uninsured 50–64-year-olds in different demographic groups. For each group (in rows) we report the pre-reform mean of the lifetime CRC screening rate and the associated point estimate on the CRCCP dummy variable as well as the sample size on which the regression estimate is based. The patterns in Table 4 reveal a striking gender difference in the effects of the CRCCP program: it increased the likelihood that uninsured 50–64-year-old women reported ever having had a sigmoidoscopy, colonoscopy, or proctoscopy by a statistically significant 4.1 percentage points, while the associated estimate for men in the same group is less than half the size of the estimate for women and is not statistically significant. In the next panel of Table 4, we further explore whether the gender difference in the CRCCP effect varies by marital status, and we do not see much evidence that marital status explains the heterogeneity in the relationship between CRCCP exposure and lifetime endoscopy by gender. These gender differences accord with the general pattern of preventive-care use generally: women are more likely to obtain care (Vaidya, Partha, and Karmakar, 2012). To the extent that the sites where the screening was offered are FHQC or look-alikes, it could also reflect that the population using these sites in these age groups is disproportionately female.
Table 4.
CRCCP Effects on Lifetime Endoscopy Are Larger for Women, Minorities, and Those Who Did Not Recently Use Other Forms of Preventive Care BRFSS 2001–2015, 50–64-year-olds Without a Health Plan Outcome is: Ever Had a Sigmoidoscopy or Colonoscopy
| (1) | (2) | (3) | |
|---|---|---|---|
| 2001–2008 mean | CRCCP estimate (SEs) | N | |
| Men | .246 | .016 (.014) | 43,365 |
| Women | .292 | .041 (.011)*** | 62,862 |
| Married men | .264 | .026 (.024) | 19,018 |
| Unmarried men | .221 | .012 (.016) | 24,347 |
| Married women | .303 | .048 (.020)** | 27,077 |
| Unmarried women | .281 | .040 (.017)** | 35,785 |
| White, non-Hispanic | .288 | −.002 (.014) | 76,658 |
| Black, non-Hispanic | .316 | .058 (.016)*** | 12,685 |
| Other race, non-Hispanic | .250 | .046 (.047) | 5,096 |
| Hispanic | .189 | .098 (.033)*** | 10,058 |
| HS or less | .247 | .035 (.010)*** | 58,486 |
| Some college | .307 | .025 (.016) | 28,445 |
| BA or more | .306 | .001 (.023) | 19,025 |
| Individuals who had a flu shot in past yr | .389 | .012 (.022) | 25,525 |
| Individuals who did not have a flu shot in past yr | .236 | .034 (.009)*** | 80,702 |
See notes to Table 2. Each row represents the results for the subgroup identified by the row label.
When we examine differences by race and ethnicity, another clear pattern emerges: effects of the CRCCP at increasing lifetime endoscopy are much larger among non-whites (e.g., Hispanics or other non-Hispanic non-Whites) than among white non-Hispanics. The large effects for uninsured African Americans are particularly notable because they are at increased risk for colorectal cancer and are recommended to be screened more aggressively than asymptomatic individuals of other races. Effects by education confirm that CRCCP effects on lifetime endoscopy are largest among those with a high school degree or less, which is not surprising given the targeting of the program at uninsured 50–64-year-olds. These estimates suggest that the CRCCP was effective at targeting the most vulnerable and at-risk populations and contrasts with recent work documenting selection among those more advantaged into cancer screening in other contexts (Kowalski 2019, Einav et al. 2019).23
5.3. Results on Cancer Diagnoses
In Table 5, we examine the effects of the CRCCP on CRC cancer diagnoses. The results above suggested that CRCCP rollout as associated with significant increases in the likelihood of ever having had an endoscopic screening, particularly for women, minorities, and those who did not take up other forms of preventive care. This suggests that the CRCCP-induced endoscopy may plausibly have affected cancer diagnoses, since the individuals screened could be expected to have had polyps and pre-cancerous growths go unnoticed for a longer period of time.24
Table 5.
CRCCP Did Not Affect Detection of Colorectal Cancers Among Uninsured People SEER 2007–2015, 50–64-year-olds
| (1) | (2) | (3) | (4) | (5) | |
|---|---|---|---|---|---|
| In-situ pre-cancers | Localized | Regional | Distant | Total Incidence – including in situ | |
| All Adults | |||||
| CRCCP | .013 | −.023 | −.002 | .005 | .003 |
| (.013) | (.030) | (.014) | (.031) | (.013) | |
| Adjusted R-squared | .06 | .28 | .24 | .24 | .18 |
| N | 16,200 | 16,200 | 16,200 | 16,200 | 81,000 |
| Men | |||||
| CRCCP | .017 | −.035 | .006 | .024 | .006 |
| (.021) | (.029) | (.020) | (.028) | (.014) | |
| Adjusted R-squared | .07 | .30 | .27 | .27 | .20 |
| N | 8,100 | 8,100 | 8,100 | 8,100 | 40,500 |
| Women | |||||
| CRCCP | .010 | −.007 | −.011 | −.013 | .0001 |
| (.009) | (.037) | (.018) | (.040) | (.018) | |
| Adjusted R-squared | .05 | .26 | .22 | .20 | .16 |
| N | 8,100 | 8,100 | 8,100 | 8,100 | 40,500 |
Notes: Each entry shows the coefficient from a separate regression model. The dependent variable is one plus the log of the number of colon cancer diagnoses to adults who are uninsured age 50–64 using SEER-18 data. Though not shown, all models include state and year fixed effects and dummies for 5-year age groups and race. All models also include one plus the log of the relevant populations in each age-race group. All models also include all the state-level Xs discussed in the text.
significant at 10%;
significant at 5%;
significant at 1%. Standard errors throughout are clustered at the state level and are shown in parentheses.
To test this, we examine total cancer incidence as well as diagnoses at the earliest stage (in-situ) versus malignant stages using data from the Surveillance Epidemiology and End Results (SEER) system, which are registry data on the universe of cancer diagnoses (and also on in-situ pre-cancers) in a subset of geographic areas in the US. We use the SEER 18 data which include areas/states that have been collected since 2000 (SEER Research Data 2000–2016).25 These are the standard cancer diagnosis data used in the field. We use SEER 18 data as opposed the more recent SEER 21 data which cover a larger geographic area because the former data include information on health insurance status (since 2007), and the CRCCP should primarily affect diagnoses among the uninsured.
We examine the effects of the CRCCP on colorectal cancer detections by estimating models where the outcome is the natural log of 1 plus the count of the number of cancers at each stage detected for uninsured individuals age 50–64 in each state and year, and we include the same right hand side variables as in equation (1).26 We assume a 1-month delay between initial screening and diagnosis, and we control for the natural log of 1 plus the population of individuals as an additional independent variable. The format of Table 5 is as follows: each panel reports the coefficient on the CRCCP rollout variable from a model estimated separately for all uninsured adults (top panel), uninsured men (middle panel) and uninsured women (bottom panel).
The results in Table 5 do not indicate that the CRCCP-induced increases in CRC screenings had statistically significant effects on detections of colorectal cancers or pre-cancers. The point estimates for women are suggestive that the CRCCP may have reduced distant stage colorectal cancers and increased detection of in-situ colorectal cancers (i.e., causing cancers to be detected earlier), but none of the estimates are statistically significant. A challenge here is that the timing of the follow-up period in our analysis is unlikely to be sufficient to observe meaningful changes in CRC detections given the slow growing nature of most colorectal cancers; the United States Preventive Services Task Force, for example, indicates that “the benefit of screening is not seen in trials until at least 7 years later” (USPSTF 2008). We therefore view the results on cancer detections as suggestive and exploratory.
6. Discussion and Conclusion
The results above suggest that the federal Colorectal Cancer Control Program (CRCCP) – which directly paid for CRC screenings alongside incentivizing process improvements in state health departments and related health clinics in 25 states from 2009/10 to 2015 – played an important role at increasing preventive cancer-related health utilization for adults without a health plan in the early 21st century, a period of unprecedented increases in cancer screening (Cutler 2008). Specifically, we estimate that the CRCCP program significantly increased lifetime rates of sigmoidoscopy or colonoscopy by 2.9 percentage points among 50–64-year-old adults without a health plan. This represents a ten percent increase over the baseline screening rate for this group. We did not find any relationship between CRCCP and lifetime CRC screening rates for insured 50–64-year-olds, consistent with the idea that the program targeted uninsured and underinsured adults. A variety of other robustness tests support our interpretation that CRCCP was responsible for significant improvements in CRC screening rates among the uninsured. We do not find any evidence that the CRCCP program affected fecal occult blood stool tests, however. FOBT screening is much cheaper (around $20-$30) than endoscopy; it could be that the direct funding model is less relevant for the less expensive test.
Is the magnitude of our estimated effect plausible? The Centers for Disease Control and Prevention reported in 2015 that since 2009 it had provided almost 55,000 CRC screenings, with 13,425 people screened in program year 2014 alone (CDC 2015). According to the 2010 Census there were approximately 33 million adults age 50–64 in states that received a CRCCP grant; approximately 12.8 percent are uninsured according to the BRFSS data. This translates to 4,224,000 uninsured 50–64-year-olds. Our BRFSS data also report that 27.0 percent of those individuals, or 1,140,480 people, ever had an endoscopic screening. We estimate that the CRCCP increased lifetime endoscopy rates by 2.9 percentage points (e.g., increasing the rate from 27.0 percent to 29.9 percent), suggesting about 122,496 additional people being screened due to CRCCP. Thus, our estimate is about twice the size of the CDC estimate, which may partially be explained by across-person spillover effects.27
Another useful set of calculations is whether the size of the CRCCP funding was large enough to cover the costs of CRC screening given what we know about the size of the uninsured population in CRCCP grantee states. Since the screening costs vary significantly across the various CRC screening modalities, this exercise yields a range of estimates. Consider that there were approximately 2.5 million uninsured 50–64-year-olds in CRCCP-funded states in 2010 based on age-specific population estimates from SEER and uninsurance rates from the March Current Population Survey. Would the $26.9 million in CRCCP funding to these states have been sufficient to provide screenings to all uninsured 50–64-year-olds in those states, ignoring outreach concerns (i.e., supposing that all uninsured adults could be identified and offered a screening)? For the cheapest modality (FOBT, approximately $25), the funding would be insufficient but is on the same order of magnitude (2.5 million uninsured adults * $25 = $62.5 million, or about 2.3 times the CRCCP funding level).28 For the most expensive modality (endoscopy, approximately $600), the relevant calculation would imply worse insufficiency: even though colonoscopies (sigmoidoscopies) are recommended only once every ten (five) years for otherwise healthy asymptomatic adults in this age range, this corresponds to 250,000 uninsured 50–64-year-olds in CRCCP funded states who were targeted by the program, suggesting that $150 million would be required to cover the costs of endoscopy, or about 5.6 times the CRCCP funding level.29
Would the additional CRCCP-induced screenings pass standard cost-benefit tests? There are many tradeoffs to consider, each characterized by significant uncertainty. For example, it is not obvious that the CRCCP-induced screenings that led to detection of polyps and/or colorectal cancer also led to effective treatment. Unlike its predecessor program in the context of breast and cervical cancer which generated a companion program to pay for cancer treatment for tumors found through program-induced screenings for uninsured adults, there was no similar funding provided to pay for treatment of the colorectal cancers found by the CRCCP. Even setting this issue aside, however, the costs and benefits of CRCCP-induced screenings are uncertain. Regarding costs, in addition to the dollar costs of the grants to the states, there are additional costs for CRCCP patients associated with time and anxiety in getting screened, risk of false-positive results, and risk of complications from the screening itself (more substantial for endoscopy than for FOBT). Regarding the benefits of CRCCP-induced screenings, there is also great uncertainty, including the fact that we do not know how many polyps were removed and whether those polyps would have been slow-growing, though the fact that the CRCCP recipients were uninsured makes it less likely that they would have been found and removed in the absence of the program. Taking account of these uncertainties, reasonable assumptions about the value of CRCCP-induced screenings based on off-the-shelf estimates of the life years saved by CRC screening from the epidemiologic literature yield a wide range of estimated benefits from $61.6 million to over $125.9 million (relative to the total program cost of $100 million).30
Although we are not aware of prior research that has comprehensively examined the effects of the CRCCP program, it is notable that our results differ from prior studies on policy determinants of CRC screenings with respect to gender. Specifically, prior research examining insurance-related public policies such as mandates that private plans cover CRC screening (Hamman and Kapinos 2014) and elimination of cost-sharing for Medicare recipients (Hamman and Kapinos 2015) have found effects for men but not women. In contrast, our setting examining the effects of direct funding provision for uninsured people finds clear effects for women with more limited effects for men. Differential employment rates by gender may partly explain why state CRC screening mandates – which should differentially affect individuals with employer-provided health insurance – have larger effects at increasing CRC screening rates among men than among women, though more research is needed to understand gender differences in responses to CRC screening programs.
Our results are not without limitations, many owing to limitations of the data. For example, all of our BRFSS outcomes are self-reported, and there is evidence that social disadvantage is positively related to over-reporting of preventive service use (e.g., Lofters et al. 2013). We think it unlikely that such reporting bias would be systematically correlated with the variation in the timing of CRCCP participation across states, but it is not something we can directly test. Moreover, the fact that we find effects of CRCCP on some but not all types of CRC screening is broadly inconsistent with reporting biases fully explaining the findings. Another data limitation is that we do not observe the sequencing of various outcomes or the ordering of when a person received an FOBT versus a more invasive CRC screening. This information would be helpful for more credibly measuring spillover effects of direct funding provision, for example. Finally, we do not know what goes on inside the ‘black box’ at each CRCCP site. Later phases of the program were focused increasingly on process improvements (e.g., electronic reminders), and future research should examine whether this variation helps explain program effectiveness.
Despite these limitations, our results significantly advance our understanding of the policy determinants of highly effective CRC screening and suggest that federal programs with direct funding provision like the CRCCP can be very effective at increasing population rates of preventive cancer screenings among vulnerable populations.
Acknowledgments
We are grateful to the National Institute on Aging (Grant # R21AG053029-02) for generous funding. We thank Amy DeGroff and Tim Geiger for helpful discussions, and we thank seminar participants at San Diego State University for useful feedback. All errors are our own.
Appendix Table 1
CRCCP Effects on Endoscopy, Different Time Windows BRFSS 2001–2015, 50–64-year-olds
| (1) | (2) | (3) | |
|---|---|---|---|
| Had a sigmoidoscopy or colonoscopy in past year | Had a sigmoidoscopy or colonoscopy in past five years | Had a sigmoidoscopy or colonoscopy in past ten years | |
| Full Sample | |||
| Pre-reform mean, 2001–2008 | .154 | .407 | .452 |
| CRCCP | −.003 | −.014* | −.005 |
| (.004) | (.007) | (.011) | |
| Adjusted R squared | .01 | .06 | .09 |
| N | 923,104 | 923,104 | 923,104 |
| Adults with a health plan | |||
| Pre-reform mean, 2001–2008 | .166 | .435 | .482 |
| CRCCP | −.003 | −.020** | −.017 |
| (.004) | (.008) | (.011) | |
| Adjusted R squared | .01 | .05 | .09 |
| N | 815,457 | 815,457 | 815,457 |
| Adults without a health plan | |||
| Pre-reform mean, 2001–2008 | .072 | .197 | .233 |
| CRCCP | .003 | .030* | .077*** |
| (.006) | (.017) | (.028) | |
| Adjusted R squared | .01 | .04 | .05 |
| N | 105,760 | 105,760 | 105,760 |
See notes to Table 2. The CRCCP exposure variable for the models in column 1 is adjusted to equal the share of the year prior to the interview that the individual’s state had an active CRCCP program. The CRCCP exposure variable for the models in column 2 is adjusted to equal the share of the five years prior to the interview that the individual’s state had an active CRCCP program. The CRCCP exposure variable for the models in column 3 is adjusted to equal the share of the ten years prior to the interview that the individual’s state had an active CRCCP program.
Footnotes
Applications are reviewed by an independent committee; the top scoring applications receive CRCCP funds. We do not have information on the actual review scores each state’s application received.
Although CRCCP focused its services on 50–74-year-olds, we focus on uninsured 50–64-year-olds because of near universal eligibility for Medicare at age 65. Sample sizes of uninsured 65–74-year-olds are so small as to be uninformative in our setting.
More recent DNA-based CRC stool tests are much more expensive (Cologuard was listed at $649 in 2017).
Whaley (2018) reports an average price for a colonoscopy of $1,395 based on 2009–2013 HCCI data.
According to the American Cancer Society, if during a screening colonoscopy a polyp is found and removed, some insurers consider that procedure a diagnostic test instead of a screening test and thus the patient would be responsible for relevant co-pays and deductibles. The United States Department of Health and Human Services clarified that all screening colonoscopies – including those that involve finding and removing polyps – should be covered by private insurance without cost-sharing, but there may still be a co-pay for Medicare patients. Moreover, colonoscopies that may be considered preventive ex ante can sometimes be charged as diagnostic ex post, even when no polyps are found (Rosato 2019).
A very small pilot program from 2005–2009 was implemented in one state, 2 large cities, and three university and tribal sites. We separately control for this pre-existing pilot program throughout our empirical work, but we do not include these sites in our definition of state CRCCP programs. Our main findings are robust to excluding the control for the pilot program and to excluding the states with pilot CRCCP grants.
One might worry that states are awarded grants because they have a more robust public health infrastructure and this leads to better screening outcomes. We have explored at the state level the probability of getting a grant during by 2009/2010 as a function of state characteristics, such as CRC mortality, insurance rules about CRC screening, region dummies, the number of FHQCs at the state level, HMO penetration, and the percent Black and Hispanic. We find only CRC mortality is significant, and it is negatively associated with getting a grant by 2009/2010.
Notably, Bitler and Carpenter (2019) evaluate a related precursor program for breast and cervical cancer screenings for uninsured women. They find that state participation in the National Breast and Cervical Cancer Early Detection Program significantly increased breast and cervical cancer screenings for uninsured 40–64 year old women.
Two studies using Medicare claims data also find no effects of the cost-sharing elimination for Medicare beneficiaries age 70 and older (Cooper et al. 2016, 2017). Interestingly, Lissenden and Yao (2017) use cancer registry data and find that ACA-related generosity increases for Medicare recipients were associated with significant increases in diagnoses of early stage colorectal cancers among those 65 and older, with no associated change for those 50–64.
The effects from studies exploiting cost-sharing changes among a sample of insured people are likely to be smaller than the effects of the direct provision targeted at uninsured people we study here if cost is a decisive factor for not obtaining screenings. This is because even without the prohibitions on cost-sharing, insured people were likely facing a far lower effective cost of screening than uninsured people (who face the full cost).
For other recent examples of insurance-related policy changes and CRC outcomes, see Lich et al. (2019) and Davis et al. (2019).
A growing public health literature has also examined racial/ethnic disparities in CRC screening rates (see, for example, Burnett-Hartman 2016, May et al. 2019, and Powell et al. 2020).
Item non-response is fairly low for these questions. We omit observations with a “don’t know” or “refused” response to the blood stool test or endoscopy questions.
Unfortunately, the frequency recommendations differ between flexible sigmoidoscopy and colonoscopy, and we cannot distinguish between the two types of endoscopy throughout our entire sample period. For completeness we report results for ‘endoscopy in the past year’, ‘endoscopy in the past five years’, and ‘endoscopy in the past ten years’ in the appendix, but we cannot create a direct measure of whether the individual is ‘up to date’ with current endoscopic screening recommendations. Moreover, we are also concerned about recall bias and telescoping effects for events that happened in the distant past. For these reasons, we prefer the lifetime measure for endoscopy.
We refer to individuals without any health plan as uninsured. Over our sample period the BRFSS does not contain information on the source or type of the health plan or who pays for the health plan except for 1996–2000.
We know the interview date in the BRFSS, and we assign treatment timing based on the CRCCP grant cycle and our understanding of when states had the funds. The CRCCP program year t runs from July 1 of year t to June 30 of year t+1.
We also include month of interview dummies throughout (though not shown in the equation) to account for idiosyncratic month effects (e.g., March is Colon Cancer Awareness Month).
Below we explore robustness of the results to alternative weighting approaches. In particular, the BRFSS introduced a cellphone-only sample in 2011. We find that our results are robust to dropping weights entirely, to excluding all cellphone respondents, to additionally adjusting non-cellphone weights to sum to the same state year totals by race and age group, and to adopting the weighting adjustment proposed in Simon et al. (2017). This robustness is perhaps not surprising given that we are focusing on 50–64-year-old uninsured people who are unlikely to be exclusively cellphone-dependent.
In these figures, we restrict attention to even-numbered years when the CRC screening questions were included on the BRFSS core questionnaire. For visual clarity we exclude the early adopting ‘pilot’ program states. Results including all states look very similar.
In results not reported but available upon request, we examined the relationship between state CRCCP participation and the likelihood of having a health plan. In a similarly specified difference-in-differences model as equation (1), we found that state CRCCP participation was unrelated to insurance coverage: the coefficient on CRCCP was −.0001 with a standard error of .003. This suggests that our models stratified by the presence of a health plan should provide meaningful tests of the effects of CRCCP. To address concerns about other contemporaneous programs that may be shifting the composition of the uninsured, below we also explore robustness to excluding individuals in states that expanded Medicaid under the ACA.
Appendix Table 1 presents estimates for other screening windows for endoscopy. Recall that we cannot distinguish between sigmoidoscopy and colonoscopy throughout the entire sample period, and the guidelines differ across the two modalities (because colonoscopy is more invasive than flexible sigmoidoscopy, an individual with a clean colonoscopy can wait ten years for another screening, while an individual with a clean flexible sigmoidoscopy should get another screening in five years). We estimate that the CRCCP was associated with significant increases in past five-year and past ten-year endoscopy rates for uninsured individuals.
If we adjust weights within each year to be population representative after dropping cellphone observations, we estimate that state CRCCP participation was associated with increased lifetime endoscopy rates for the target sample, though the estimate is not statistically significant.
We also investigated whether there was evidence of dose-response effects of CRCCP grant funding by replacing the Any CRCCP indicator with a continuous measure of dollars of CRCCP funding per uninsured 50–64-year-old in the state. We obtained estimates of uninsured 50–64-year-olds from the March Current Population Surveys, and we obtained estimates of the population of 50–64-year-olds in each state from SEER. Although we acknowledge that this exercise is mainly descriptive given that we do not have plausibly exogenous variation in the size of each state’s CRCCP award, we found mixed evidence for a dose-response effect. While we did find that more CRCCP grant dollars were significantly associated with higher CRC screening rates, this pattern was sensitive to how we controlled for the distribution of CRCCP grant dollars. For example, dividing states into quartiles of the distribution of CRCCP grant dollars received did not indicate a monotonic relationship between the quartile of CRCCP dollars and the magnitude of increased CRC screening, although all quartiles showed significant improvements. Thus, there was mixed evidence for a dose response relationship between CRCCP grant dollars awarded to a state and CRC screening rates.
Below, we estimate that CRCCP increased endoscopy for 122,496 uninsured people over the duration of the program. USPSTF (2002) indicates that first time sigmoidoscopic screening detects approximately 7 cancers per 1,000 examinations; this would correspond to approximately 860 cancers detected (recall that we cannot separately distinguish sigmoidoscopy from colonoscopy in the BRFSS).
We study 2007–2015. The 18 sites/states in SEER are: Alaska (Alaska Native), Connecticut, Detroit, Atlanta, Greater Georgia, Rural Georgia, San Francisco/Oakland, San Jose/Monterey, Greater California, Hawaii, Iowa, Kentucky, Los Angeles, Louisiana, New Mexico, New Jersey, Seattle/Puget Sound, and Utah. Note that when the National Cancer Institute (NCI) refers to total cancer incidence, it generally excludes the earliest stage in-situ cancers but includes a very small number of unstaged cancers (National Cancer Institute, 2013).
In order not to drop the small number of cells with zero cancer detections, we add one because the log of zero is not defined. Note that we combine diagnoses within 5-year age bands and have estimated similar models combining Black, white, and other race individuals together for this analysis because there are some SEER sites with very small populations of black and other race individuals and thus the ‘zero cancer detections’ problem is substantially worse if we consider race groups separately. We present the log count models for ease of interpretation. We control for age group and race dummies and the natural log of 1 plus the population in the cell, but no other demographics are available in SEER.
Of course, the CDC estimate may contain both FOBT and endoscopy, and we find no effects on FOBT. On the other hand, the CDC estimate will not include informational or other types of spillovers such as from CRCCP advertising or word of mouth from CRCCP recipients. There may be other types of spillovers as well; for example, an individual induced to get a first CRC screening due to CRCCP may increase adherence to CRC screening over the course of their lifetime, resulting in a within-person spillover. There may also be a within-provider spillover, whereby providers learn about effective ways to provide CRC screening to uninsured 50–64 year olds.
Moreover, some subset of these FOBT tests would have come back positive, requiring more invasive and more expensive endoscopic screening.
Of course, these calculations ignore the other ways that states spent CRCCP grant dollars, including outreach, case management, personnel, and other activities to increase CRC screening.
We calculate the low end of this range as follows: we estimate that 122,496 additional endoscopies were performed due to CRCCP over the sample period. We estimate from the BRFSS data that 13.1 percent of the uninsured 50–64-year-olds do not receive screening within a 10-year time frame, so the lower bound on screenings is 106,449 (86.9 percent of 122,496). We divide this number by 220 based on Atkin et al. (2017) who performed an RCT for sigmoidoscopy and found that in a 17 year follow-up period one death was prevented for every 220 screenings. This suggests that 484 deaths were prevented. Maciosek et al. (2006) estimate that CRC screening increases life expectancy at death by 10.7 years and that the value of each life year saved is $11,900. Thus, 484 * 10.7 * $11,900 = $61,627,720. We calculate the high end of this range as follows: Of the 122,496 additional screenings due to CRCCP, 557 deaths would have been prevented using the Atkin et al. (2017) estimate. Adjusting the life expectancy estimate for the age range in our younger sample (the Maciosek et al. 2006 is for persons up to age 84) and using the 2010 Social Security actuarial tables returns a higher life expectancy, 19 years. Thus, the high end of the range of benefits is 557 * 19 * $11,900 = $125,937,700.
We have no conflicts of interest to disclose.
Contributor Information
Marianne P. Bitler, Department of Economics, UC Davis, NBER, & IZA
Christopher S. Carpenter, Department of Economics, Vanderbilt University, NBER, & IZA.
Danea Horn, Department of Agricultural and Resource Economics, UC Davis.
BIBLIOGRAPHY
- American Cancer Society (2017). “Colorectal Cancer Fact & Figures 2017–2019,” Atlanta: American Cancer Society. [Google Scholar]
- Bertrand Marianne, Duflo Esther, and Mullainathan Sendhil (2004). “How Much Should We Trust Difference-In-Differences Estimates?” Quarterly Journal of Economics, 119(1): 249–275. [Google Scholar]
- Bibbins-Domingo Kirsten, Grossman David C., Curry Susan J., Davidson Karina W., Epling John W. Jr., Garcia Francisco A. R., Gillman Matthew W., Harper Diane M., Kemper Alex R., Krist Alex H., Kurth Ann E., Landefeld C. Seth, Mangione Carol M., Owens Douglas K., Phillips William R., Phipps Maureen G., Pignone Michael P., and Siu Albert L. (2016). “Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement,” JAMA, 315(23): 2564–2575. [DOI] [PubMed] [Google Scholar]
- Bitler Marianne P. and Carpenter Christopher S. (2019). “Effects of Direct Care Provision to the Uninsured: Evidence from Federal Breast and Cervical Cancer Programs,” NBER Working Paper #26140. [Google Scholar]
- Burnett-Hartman Andrea N., Mehta Shivan J., Zheng Yinge, Ghai Nirupa R., McLerran Dale, Chubak Jessica, Quinn Virginia P., Skinner Celette Sugg, Corley Douglas A., Inadomi John, and Doubeni Chyke A., on behalf of the PROSP Consortium (2016). “Racial/Ethnic Disparities in Colorectal Cancer Screening Across Healthcare Systems,” American Journal of Preventive Medicine, 51(4): e107–e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card David, Dobkin Carlos, and Maestas Nicole (2009). “Does Medicare Save Lives,” Quarterly Journal of Economics, 124(2): 597–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ----- (2008). “The Impact of Nearly Universal Insurance Coverage on Health Care Utilization: Evidence from Medicare,” American Economic Review, 98(5): 2242–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2015). “CDC Awards $22,800,000 to Increase Colorectal Cancer Screening,” Press Release available at: https://www.cdc.gov/media/releases/2015/p0930-cancer-screening.html.
- Cokkinides Vilma, Bandi Priti, Shah Mona, Virgo Katherine, and Ward Elizabeth (2011). “The association between state mandates of colorectal cancer screening coverage and colorectal cancer screening utilization among US adults age 50–64 years with health insurance,” BMC Health Services Research, 11(19): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper Gregory S., Kou Tzuyung Doug, Dor Avi, Koroukian Siran M., and Schluchter Mark D. (2017). “Cancer Preventive Services, Socioeconomic Status, and the Affordable Care Act,” Cancer, 123: 1585–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper Gregory S., Kou Tzuyung D., Schluchter Mark D., Dor Avi, and Koroukian Siran M. (2016). “Changes in Receipt of Cancer Screening in Medicare Beneficiaries Following the Affordable Care Act,” Journal of the National Cancer Institute, 108(5): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram Peter, Fendrick A. Mark, Inadomi John, Cowen Mark E., Carpenter Daniel, and Vijan Sandeep (2003). “The Impact of a Celebrity Promotional Campaign on the Use of Colon Cancer Screening,” Archives of Internal Medicine, 163(13): 1601–1605. [DOI] [PubMed] [Google Scholar]
- Cutler David (2008). “Are We Finally Winning the War on Cancer?” Journal of Economic Perspectives, 22(4): 3–26. [DOI] [PubMed] [Google Scholar]
- Davis Melinda M., Shafer Paul, Renfro Stephanie, Lich Kristen Hassmiller, Shannon Jackilen, Coronado Gloria D., McConnell K. John, and Wheeler Stephanie B. (2019). “Does a transition to accountable care in Medicaid shift the modality of colorectal cancer testing,” BMC Health Services Research, 19(1): Article 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroff Amy, Sharma Krishna, Satsangi Anamika, Kenney Kristy, Joseph Djenaba, Ross Katherine, Leadbetter Steven, Helsel William, Kammerer William, Firth Rick, Rockwell Tanner, Short William, Tangka Florence, Wong Faye, and Richardson Lisa (2018). “Increasing Colorectal Cancer Screening in Health Care Systems Using Evidence-Based Interventions,” Preventing Chronic Disease, 15(E100): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroff Amy, Boehm Jennifer, Seeff Laura C., Green Sonya Goode, and Holden Debra (2008). “Facilitators and Challenges to Start-Up of the Colorectal Cancer Screening Demonstration Program,” Preventing Chronic Disease, 5(2): 1–8. [PMC free article] [PubMed] [Google Scholar]
- DeGroff Amy, Holden Debra, Green Sonya Goode, Boehm Jennifer, Seeff Laura C., and Tangka Florence (2008). “Start-Up of the Colorectal Cancer Screening Demonstration Program,” Preventing Chronic Disease, 5(2): 1–9. [PMC free article] [PubMed] [Google Scholar]
- Einav Liran, Finkelstein Amy, Oostrom Tamar, Ostriker Abigail J., and Williams Heidi L. (Forthcoming). “Screening and Selection: The Case of Mammograms,” American Economic Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamman Mary and Kapinos Kandace (2016). “Colorectal Cancer Screening and State Health Insurance Mandates,” Health Economics, 25: 178–191. [DOI] [PubMed] [Google Scholar]
- ----- (2015). “Affordable Care Act Provision Lowered Out-of-Pocket Cost and Increased Colonoscopy Rates Among Men in Medicare,” Health Affairs, 34(12): 2069–2076. [DOI] [PubMed] [Google Scholar]
- Hannon Peggy A., Maxwell Annette E., Escoffery Cam, Vu Thuy, Kohn Marlana, Leeman Jennifer, Carvalho Michelle L., Pfeiffer Debbie J., Dwyer Andrea, Fernandez Maria E., Vernon Sally W., Liang Lily, and DeGroff Amy (2013). “Colorectal Cancer Control Program Grantees’ Use of Evidence-Based Interventions,” American Journal of Preventive Medicine, 45(5): 644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendryx Michael and Luo Juhua (2018). “Increased Cancer Screening for Low-income Adults Under the Affordable Care Act Medicaid Expansion,” Medical Care, 56(11): 944–949. [DOI] [PubMed] [Google Scholar]
- Joseph Djenaba A., DeGroff Amy S., Hayes Nikki S., Wong Faye L., and Plescia Marcus (2011). “The Colorectal Cancer Control Program: partnering to increase population level screening,” Gastrointestinal Endoscopy, 73(3): 429–434. [DOI] [PubMed] [Google Scholar]
- Joseph Djenaba A., King Jessica B., Miller Jacqueline W., and Richardson Lisa C. (2012). “Prevalence of Colorectal Cancer Screening Among Adults – Behavioral Risk Factor Surveillance System, United States, 2010,” Morbidity and Mortality Weekly Report, 61(2 Supplement): 51–56. [PubMed] [Google Scholar]
- Kadiyala Srikanth and Strumpf Erin (2016). “How Effective is Population-Based Cancer Screening? Regression Discontinuity Estimates from the U.S. Guideline Screening Initiation Ages,” Forum for Health Economics & Policy, 19(1): 87–139. [DOI] [PubMed] [Google Scholar]
- Kowalski Amanda (2019). “Behavior within a Clinical Trial and Implications for Mammography Guidelines,” NBER Working Paper 25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lich Kristen Hassmiller, O’Leary Meghan C., Nambiar Siddhartha, Townsley Rachel M., Mayorga Maria E., Hicklin Karen, Frerichs Leah, Shafer Paul R., Davis Melinda M., and Wheeler Stephanie B.(2019). “Estimating the impact of insurance expansion on colorectal cancer and related costs in North Carolina: A population-level simulation analysis,” Preventive Medicine, 129 Supplement: Article 105847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissenden Brett and Yao Aaron (2017). “Affordable Care Act Changes to Medicare Led to Increased Diagnosis of Early-Stage Colorectal Cancer Among Seniors,” Health Affairs, 36(1): 101–107. [DOI] [PubMed] [Google Scholar]
- Marino Miguel, Bailey Steffani R., Gold Rachel, Hoopes Megan J., O’Malley Jean P., Huguet Nathalie, Heintzman John, Gallia Charles, McConnell K. John, and DeVoe Jennifer E. (2016). “Receipt of Preventive Services After Oregon’s Randomized Medicaid Experiment,” American Journal of Preventive Medicine, 50(2): 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell Annettte E., Hannon Peggy A., Escoffery Cam, Vu Thuy, Kohn Marlana, Vernon Sally W., and DeGroff Amy S. (2014). “Promotion and Provision of Colorectal Cancer Screening: A Comparison of Colorectal Cancer Program Grantees and Nongrantees 2011–2012,” Preventing Chronic Disease, 11: E170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May Folasade P., Yang Liu, Corona Edgar, Glenn Beth A., and Bastani Roshan (2020). “Disparities in Colorectal Cancer Screening in the United States Before and After Implementation of the Affordable Care Act,” Clinical Gastroenterology and Hepatology, 18(8): 1796–1804. [DOI] [PubMed] [Google Scholar]
- Mehta Shivan J., Polsky Daniel, Zhu Jingsan, Lewis James D., Kolstad Jonathan T., Loewenstein George, and Volpp Kevin (2015). “ACA-Mandated Elimination of Cost Sharing for Preventive Screening Has Had Limited Early Effect,” American Journal of Managed Care, 21(7): 511–517. [PMC free article] [PubMed] [Google Scholar]
- Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. (2006). “Colorectal cancer screening: health impact and cost effectiveness.” American Journal of Preventive Medicine, 31:80–9. [DOI] [PubMed] [Google Scholar]
- Myerson Rebecca M., Tucker-Seeley Reginald D., Godman Dana P., and Lakdawalla Darius N. (2020, forthcoming). “Does Medicare Coverage Improve Cancer Detection and Mortality Outcomes?” Journal of Policy Analysis and Management, forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoro Catherine A., Dhingra Satvinder S., Coates Ralph J., Zack Matthew, and Simoes Eduardo J. (2014). “Effects of Massachusetts Health Reform on the Use of Clinical Preventive Services,” Journal of General Internal Medicine, 29(9): 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantel Haddon J., Kleinman David A., Kuhnen Angela H., Marcello Peter W., Stafford Caitlin, and Ricciardi Rocco (2020, forthcoming). “Has National Colorectal Cancer Awareness Month Increased Endoscopy Screening Rates and Public Interest in Colorectal Cancer?” Surgical Endoscopy, forthcoming. [DOI] [PubMed] [Google Scholar]
- Powell Wizdom, Frerichs Leah, Townsley Rachel, Mayorga Maria, Richmond Jennifer, Corbie-Smith Giselle, Wheeler Stephanie, and Lich Kristen Hassmiller (2020). “The potential impact of the Affordable Care Act and Medicaid Expansion on reducing colorectal cancer screening disparities in African American males,” PLoS One, 15(1): e0226942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman Ilana, Asch Steven M., Bhattacharya Jay, and Owens Douglas K. (2015). “Colorectal Cancer Screening in the Era of the Affordable Care Act,” Journal of General Internal Medicine, 31(3): 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato Donna (2019). “What to Do When Your Insurer Won’t Cover Free Preventive Care,” Consumer Reports article, January 17, 2019. Accessed at https://www.consumerreports.org/health-insurance/what-to-do-when-your-insurer-wont-cover-free-preventive-care/ on January 5, 2020. [Google Scholar]
- Sabik Lindsay M., Eom Kirsten Y., Dahman Bassam, Li Jie, Yao Nengliang, van Londen GJ, and Bradley Cathy J. (2020). “The Impact of Massachusetts Health Reform on Colorectal and Breast Cancer Stage at Diagnosis,” Medical Care, 58(2): 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel Rebecca L., Miller Kimberly D., Sauer Ann G., Fedewa Stacey A., Butterly Lynn F., Anderson Joseph C., Cercek Andrea, Smith Robert A., and Jemal Ahmedin (2020). “Colorectal Cancer Statistics, 2020,” CA: A Cancer Journal for Clinicians, 70(3): 145–164. [DOI] [PubMed] [Google Scholar]
- Simon Kosali, Soni Aparna, and Cawley John (2017). “The Impact of Health Insurance on Preventive Care and Health Behaviors: Evidence from the First Two Years of the ACA Medicaid Expansions,” Journal of Policy Analysis and Management, 36(2): 390–417. [DOI] [PubMed] [Google Scholar]
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1975–2016), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. [Google Scholar]
- Trivers Katrina F., Shaw Kate M., Sabatino Susan A., Shapiro Jean A., and Coates Ralph J. (2008). “Trends in Colorectal Cancer Screening Disparities in People Aged 50–64 Years, 2000–2005,” American Journal of Preventive Medicine, 35(3): 185–193. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2020). Healthy People 2020. Washington, DC: Office of Disease Prevention and Health Promotion; [accessed January 5, 2021]. Available at: https://www.healthypeople.gov/2020/. [Google Scholar]
- United States Preventive Services Task Force (2008). “Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement,” Annals of Internal Medicine, 149(9): 627–637. [DOI] [PubMed] [Google Scholar]
- Vaidya Varun, Partha Gautam, and Karmakar Monita (2012). “Gender Differences in Utilization of Preventive Care Services in the United States,” Journal of Women’s Health, 21(2): 140–145. [DOI] [PubMed] [Google Scholar]
- Whaley Christopher (2018). “The Effects of Cost Sharing on Cancer Screening and Price-Shopping: Evidence from the Affordable Care Act,” SSRN Working Paper #3264607. [Google Scholar]
- Wright Bill J., Conlin Alison K., Allen Heidi L., Tsui Jennifer, Carlson Matthew J., and Li Hsin Fang (2016). “What Does Medicaid Expansion Mean for Cancer Screening and Prevention? Results from a Randomized Trial on the Impacts of Acquiring Medicaid Coverage,” Cancer, 122: 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerhouni Yasmin A., Trinh Quoc-Dien, Lipsitz Stuart, Goldberg Joel, Irani Jennifer, Bleday Ronald, Haider Adil, and Melnitchouk Nelya (2019). “Effete of Medicaid Expansion on Colorectal Cancer Screening Rates,” Diseases of the Colon & Rectum, 62(1): 97–103. [DOI] [PubMed] [Google Scholar]


