SUMMARY
Itch is a discrete and irritating sensation tightly coupled to a drive to scratch. Acute scratching developed evolutionarily as an adaptive defense against skin irritants, pathogens, or parasites. In contrast, the itch-scratch cycle in chronic itch is harmful, inducing escalating itch and skin damage. Clinically and preclinically, scratching incidence is currently evaluated as a unidimensional motor parameter and believed to reflect itch severity. We propose that scratching, when appreciated as a complex, multidimensional motor behavior, will yield greater insight into the nature of itch and the organization of neural circuits driving repetitive motor patterns. We outline the limitations of standard measurements of scratching in rodent models and present new approaches to observe and quantify itch-evoked scratching. We argue that accurate quantitative measurements of scratching are critical for dissecting the molecular, cellular, and circuit mechanisms underlying itch and for preclinical development of therapeutic interventions for acute and chronic itch disorders.
In brief
Scratching is a complex, multidimensional motor behavior reflective of itch sensation. Wimalasena et al. discuss methods to assess scratching behavior in humans and animal models. Using high-speed recording, they outline novel approaches for detailed observation and quantification of scratching behavior, going beyond incidence to identify new scratching-related parameters.
ASSESSING ITCH IN HUMANS IN A CLINICAL SETTING
In humans, itch sensation varies widely within and between individuals. Indeed, the language needed to describe the nature of itch and its associated sensations borders on the philosophical. For example, the Eppendorf Itch Questionnaire, designed in 1997 as a modification of the McGill Pain Questionnaire (Darsow et al., 1997,2001), asks users to rate their itchiness on traditional scales such as “painful,” “stinging,” or “burning” but also “cruel,” “merciless,” and “no room for other feelings.” In total, the questionnaire features 80 potential descriptors for the sensation of itch alone. A 2008 survey of individuals with atopic dermatitis (AD), conducted using a similar questionnaire, showed that 31 of 32 such descriptors were correlated significantly with itch intensity (Dawn et al., 2009).
Unfortunately, many standard questionnaires used by clinicians often omit or do not inquire in depth about the behavioral responses to itch. In 2012, the International Forum for the Study of Itch (IFSI) published a consensus paper defining a total of 14 dimensions of itch, including scratch response (Weisshaar et al., 2012), but found that it was only included in 37% of questionnaires (Dominick et al., 2019). Furthermore, when included, questions about scratching are often qualitative, asking for descriptions of the behavior. The Eppendorf Questionnaire asks individuals to evaluate their scratching behavior with descriptors such as “compulsive,” “permanent urge to scratch,” “satisfaction,” and “ecstasy” (Darsow et al., 2001). More severe degrees of behavior are also included, such as “scratching until it bleeds” and “digging fingernails in.” Unfortunately, there is a limited ability to understand the quality and severity of the behavior through these types of self-assessments, which limits comparisons and prevents a deeper understanding of scratching variation in different dermatoses.
It is known that itch quality and the degree of scratching vary between different chronic dermatologic conditions. A 2011 study found that individuals with AD reported more frequent and more intense itch than those with psoriasis and that scratching pleasurability was correlated weakly with the intensity of itch in these people (O’Neill et al., 2011). Brenaut et al. (2013) carried out a comparative study of itch sensation among individuals suffering from AD, non-atopic eczema, urticaria, psoriasis, and scabies. They found that these dermatoses varied along several dimensions. For example, individuals with AD complained of stinging, stabbing, or pinching sensations significantly more than those with the other conditions. On the other hand, a tickling sensation was associated significantly with scabies. Interestingly, scratching also had variable effects on individuals with these disorders. Scratching was described as pleasurable by a majority of individuals with AD (69%), non-atopic eczema (76%), and psoriasis (65%) but less so in those with urticaria (46%) and scabies (47%) (Brenaut et al., 2013). In such studies, pleasurability in response to scratching is often the only scratching-related parameter included, likely because of the inherent subjectivity in self-reports of scratching behavior. However, unlike the sensation of itch itself, which is inaccessible to an observer, scratching behavior can be observed, recorded, and quantified—an approach that is likely to be more reliable in a clinical setting (Smith et al., 2019).
To this end, Lam Hoai et al. (2021) took a different approach to look at scratching in individuals with scabies compared with those with other pruritic conditions. Rather than a survey-based method, they looked at the likelihood of individuals to scratch in the course of a clinical consultation as a proxy for the need to scratch. They found that those with scabies were significantly more likely to scratch in this time period than those with another dermatological condition (Lam Hoai et al., 2021). Advancements have also been made in observer-independent object methods to quantify scratching behavior in individuals with dermatologic conditions, particularly during sleep. Ebata et al. (2001) tested the use of a wearable wrist activity monitor equipped for an accelerometer to detect scratching, a technology that has since been shown to be effective in detecting scratching in individuals with AD and correlated with other metrics of disease severity in clinical trials (Ikoma et al., 2019). More recently, this technology has also been integrated with pipelines for automated analysis using machine learning algorithms (Mahadevan et al., 2021; Moreau et al., 2018). In addition to wearable wrist monitors, others have also tested a whole-body vibrometer and use of acoustic detection of scratching, which has also been used successfully in animal models (Kogure and Ebata, 2018; Noro et al., 2014).
These assessments of scratching behavior overcome several significant limitations of survey-based methods, including, but not limited to, subjective recollections by affected individuals; lack of reliable scales for self-reporting scratching frequency, urgency, and intensity; and unconscious or automatic scratching behavior, particularly during sleep. We suggest that scratching behavior is an important consideration for diagnosis and management of a variety of dermatoses that cause itch; therefore, careful and detailed objective observations of scratching behavior of individuals could generate substantially more clinically relevant information than survey-based methods.
The drive to scratch
Scratching behavior has a long and intimate history with itch sensation and perception. Through much of evolutionary time, scratching has been tied specifically and robustly to itch and is therefore uniquely suited to yield insight into its nature. Acute itch sensations arise in response to a variety of external stimuli, such as insects, parasites, allergens, and chemical irritants. For itch associated with parasite infiltration, scratching is induced as a tightly linked reflex-like response to remove harmful substances/organisms embedded in the skin. On the other hand, in the case of a mosquito bite, where itch-evoked scratching often occurs after the mosquito has left the skin, itch may serve a protective function to promote avoidance behaviors, and scratching may prime the immune system to diminish future infection by mosquito-transmitted diseases (Donovan et al., 2007). This raises questions as to whether the intensity, frequency, and duration of the scratching response to itch are tuned to carry out these protective functions. For example, do parasites or other infiltrating agents trigger a different degree of itch intensity compared with other irritants, and does this determine the ferocity of the scratch response fine-tuned to remove them?
Itch-evoked scratching is also a symptom of chronic inflammatory skin conditions, such as AD and psoriasis, liver and kidney disease, and peripheral neuropathy. The consequences of scratching are likely distinct in these different settings, but no functional benefit has yet been uncovered. Rather than warning of the presence of an external, potentially dangerous agent as in acute itch, chronic itch disorders are thought to be caused by sensory neurons reacting to endogenous factors triggered by local disease states. This kind of itch is likely a maladaptive adaptation of a process that is protective under other circumstances and initiates a damaging itch-scratch cycle. In response to acute adaptive itch, scratching can occur on a range of seconds to days in response to the offending stimulus. However, without a recurring stimulus, the itch then subsides along with the urge to scratch. On the other hand, in chronic itch, the itch and scratching persist, often for a duration sufficient to cause damage to the skin (Figure 1). In animal models and humans, an itch-scratch cycle is initiated where cycles of skin damage and healing result in increased itch and further scratching. Breaking this cycle requires treatment of the itch, typically via topical steroids, antihistamines or other drugs that address the underlying causes of the itch.
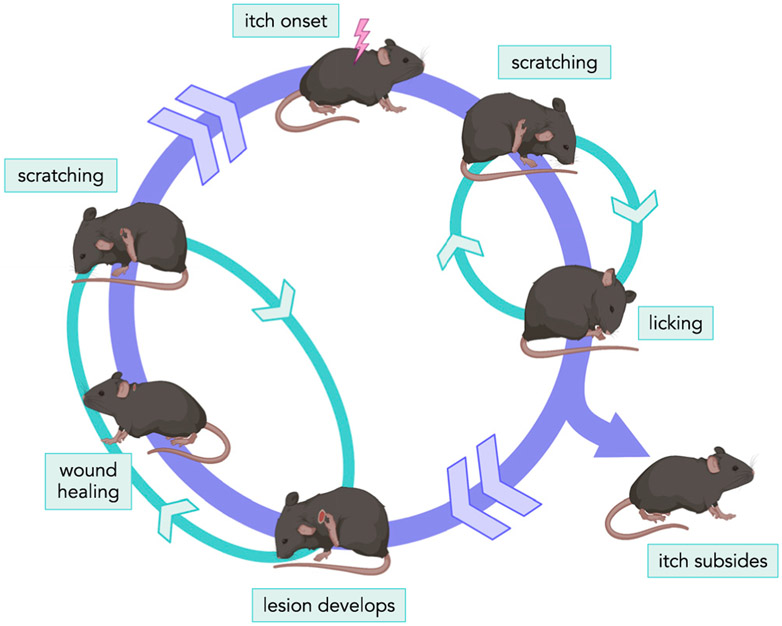
Figure 1. The cycle of itch-evoked scratching.
Itch onset triggers repetitive scratching and licking behavior in an animal, which is terminated when the immediate itch sensation subsides. If an animal experiences chronic itch, sporadic scratching-licking cycles continue. Depending on the frequency and intensity of this behavior, the animal can develop a lesion at the site of scratching, which may start with loss of fur, followed by damage to the skin. Eventually the animal may even scratch through the epidermis to the dermis. As the frequency of scratching episodes fluctuates, a lesion often heals and is then re-wounded in a cyclical fashion. This cycle can end if the itch subsides for a sufficient duration for the skin to fully heal but can begin again if the itch sensation persists or returns.
Questions remain as to how scratching in the context of a persistent itch-scratch cycle, where scratching is a pathological and maladaptive process, differs from adaptive scratching, which is finely tuned to acute pathogen removal from the skin, as this behavior diverges from its original evolutionarily driven function.
Scratching as a motor output for itch
Careful observations of the scratching motor response were first made by Sherrington (1906), who demonstrated that mechanical or electrical (unipolar faradization) stimulation in dogs with spinal cord transections could elicit a scratch response. He found that scratching frequency was highly consistent and notably independent of the stimulus frequency or stimulus type. However, importantly, Sherrington observed: “under gradation of intensity of stimulation, the scratch-reflex markedly exhibits correspondent grading of intensity of motor response.” He found that this increased intensity manifested in the amplitude of the movement as well as in the number of beats (scratches) and the duration of the reflex in seconds, saying: “The increase in intensity of the reflex shows itself in increase of the amplitude of beat of the movement with usually little or no acceleration of the rhythm.” In line with human psychophysical studies, these observations suggest that the intensity of input stimulation determines the magnitude and duration of the behavioral output of scratching.
Extensive work has also been done to characterize scratch motor responses in the turtle (Trachemys scripta elegans). These studies suggest that there is a central pattern generator (CPG) in the spinal cord for scratching (Berkowitz and Hao, 2011; Currie and Stein, 1988; Field and Stein, 1997; Mui et al., 2012) with overlapping networks of spinal interneurons forming CPGs for scratching and locomotion. A more recent study also suggests that, in addition to specialized scratching-tuned interneurons in the spinal cord, there are motor neurons that are activated preferentially during scratching compared with other behaviors, such as swimming (Bannatyne et al., 2020). In rodent models, Inagaki et al. (2003) developed MicroAct, an embedded magnet-based system for recording scratching by measuring perturbations in an applied electrical field. They found that the incidence of events, total scratching time, and total number of beats detected by the system increased depending on the intensity of the itch stimulus (Inagaki et al., 2003), which is consistent with Sherrington’s initial observations in the dog. However, to date, the field has focused on quantifying scratching incidence as a proxy for the degree of itch and has not prioritized investigation into differences in scratching along other dimensions, such as intensity, in the study of various acute and chronic itch models.
Itch neurobiology underlying common pruritogens
In contemporary itch research, scratching is often the sole window into itch sensation and is therefore used as a critical, objective behavioral indicator of increased or decreased itch, generally based only on scratching incidence or the number of scratching bouts. Whether there is any variation in scratching frequency/intensity/quality in commonly used itch models, such as histamine, chloroquine (CQ), or serotonin (5-hydroxytryptamine [5-HT]) is simply unknown, although these compounds act via independent mechanisms and, in some cases, on different subsets of sensory neurons.
Histamine receptors
Histamine, perhaps the best-studied pruritogen, has been shown to cause itch in humans and animal models (Akiyama and Carstens, 2013). It is most commonly thought to promote itch through activation of the histamine receptors H1 and H4 (Bell et al., 2004; Rossbach et al., 2009). Interestingly, several studies have also suggested that antagonism of the H3 receptor promotes itch, as evidenced by increased scratching in mice (Hossen et al., 2003; Rossbach et al., 2011; Sugimoto et al., 2004). The histamine receptors H1 and H4 are expressed primarily in non-peptidergic primary mouse sensory neurons, particularly in subsets defined by mas-related G protein-coupled receptor A3 (MrgprA3) expression (termed NP2 by Usoskin et al., 2015) and interleukin 31 receptor A (IL-31RA) expression (termed NP3 by Usoskin et al., 2015) (Meixiong and Dong, 2017), and histaminergic itch signaling in these neurons is thought to be dependent on downstream activation of TRPV1 and phospholipase-C β3 (Imamachi et al., 2009; Shim et al., 2007). It is unknown whether different histaminergic itch sensations are elicited by different histamine receptor subtypes or through independent subsets of pruriceptors (for example, MrgprA3+ versus IL-31RA+ neurons, which are physiologically or evolutionarily distinct) and, furthermore, whether such mechanistic differences cause distinct scratching responses.
Mas-related G protein-coupled receptors (Mrgprs)
In addition to histamine, MrgprA3-expressing pruriceptors are also responsive to CQ, the ligand for the MrgprA3 receptor (Liu et al., 2009). As with histaminergic itch, downstream signaling in response to MrgprA3 activation is thought to be dependent on transient receptor potential (TRP) channels (Lay and Dong, 2020). In addition to MrgprA3 itself, this subset of pruriceptors also expresses several other Mrgprs involved in itch signaling, including MrgprC11, MrgprB2, and MrgprA1 (Meixiong and Dong, 2017).
The population of non-peptidergic neurons characterized by MrgprD expression (termed NP1 by Usoskin et al., 2015), although not associated canonically with itch, nevertheless contains a subset of neurons that respond to β-alanine and produce itch in animal models in an MrgprD-dependent manner (Liu et al., 2012; Shinohara et al., 2004). Unlike most MrgprA3+ neurons, this population of MrgprD+ neurons is unresponsive to histamine.
In addition to these canonically used pruritogens, novel compounds are being identified continuously that cause itch via Mrgprs, and the action of these compounds is not limited to sensory neurons. For example, recent work has shown that a range of antidepressants (clomipramine, paroxetine, and desipramine), act in a dose-dependent manner to cause itch through the MrgprB2 receptor (MrgprX2 in humans), which is expressed by mast cells (Wolf et al., 2021). Although itch signaling via Mrgprs proceeds via common downstream effectors in some cases, it is unknown whether and how the cell type, receptor, and degree of activation affect the subsequent motor response.
Serotonin (5-HT) receptors
Serotonin acts on several 5-HT receptors to cause itch, most commonly 5-HT2 (Yamaguchi et al., 1999), which is expressed in several subtypes of peptidergic and non-peptidergic C-fiber sensory neurons (Usoskin et al., 2015) in a phospholipase-C β3-dependent manner (Imamachi et al., 2009). Certain selective serotonin reuptake inhibitors (SSRIs) also act via the same pathway (Lee et al., 2018) but cause itch in a TRPC4-dependent manner. In addition to 5-HT2, there is also evidence that serotonin acts to produce itch via 5-HT7 (Morita et al., 2015), which is expressed in an overlapping population of C-fiber neurons.
These distinct pruritogens cause itch by acting on a variety of cell types and signaling through a range of mechanisms to ultimately evoke scratching. However, although it is clear from clinical reports that itch sensation caused by different conditions varies in severity and quality and that scratching varies in incidence and quality as a result, whether there are qualitative differences in the scratching behavior induced by acute or chronic itch models has not been studied.
Current standard for quantifying scratching in mouse models
The standard in the field for quantifying preclinical scratching behavior is to use low-frame-rate video cameras to record and score mouse scratching behaviors. However, what constitutes a bout is poorly defined. Shimada and LaMotte (2008) state: “The number of scratches during a bout could be one or many. A bout of scratching could last several seconds and was initiated by lifting of the hind paw to the region of the body to be scratched.”. Others in the field have used number of scratches and number of bouts interchangeably.
Not only does the field suffer from a lack of consensus on this topic, but we also note several limitations in the current overall approach of quantifying scratching bouts. First, by counting total bouts, a large amount of temporal information about the latency to first bout and grouping and spacing of bouts is lost. Second, counting bouts does not take into account differences in scratching quality or intensity, including parameters like the force exerted on the skin, the duration of bouts, and the frequency and duration of individual scratches, which all ultimately affect the damage done to the skin and may more accurately reflect the degree of itch. Third, individual scratches are exceptionally difficult, if not impossible, to count accurately using a standard video camera. Fourth, the itch field has long been focused on stimulus-evoked scratching caused by acute local delivery of a pruritogen rather than spontaneous or chronic scratching. Counting localized scratching bouts is likely not sufficient to adequately characterize long-term, multifocal scratching such as that caused by genetic models of itch or chronic skin inflammatory disorders. Finally, the process of counting scratching bouts is tedious, labor-intensive, and subject to human error and bias.
Alternative methods for quantifying scratching in mouse models
Although standard video recording combined with manual counting of scratching remains the standard in the field, several groups have developed automated methods of detecting scratching behavior. These include use of a surgically implanted magnet (Inagaki et al., 2003) or an externally placed removable metal band (Marino et al., 2012) on the hindpaw and subsequent measurement of perturbations in an applied electrical field. This approach does not require video recording and has the advantage of capturing individual scratches rather than bouts. This method has been used successfully by several groups as an alternative to standard video-based methods and has been integrated successfully with recordings of neuronal activity (Gao et al., 2019; Langedijk et al., 2021; Mu et al., 2017). In an orthogonal approach, other groups have developed acoustic detection methods for scratching that rely on the sound made by the animal as it scratches (Elliott et al., 2017; Umeda et al., 2006) and have been translated into human studies. Similar to the magnet-based system, this approach is able to detect individual scratches and has the advantage of being functional in darkness, when mice are most active; however, the accuracy of detection is somewhat limited. Still others have used standard video recording methods and focused instead on automated detection of scratching behavior in post hoc analyses (Nie et al., 2009; Orito et al., 2004). More recently, with advances in machine vision algorithms and neural networks for analysis of video data, several others have developed pipelines to automatically detect and quantify scratching behavior in standard low-frame rate video (Bohnslav et al., 2020; Kobayashi et al., 2021; Park et al., 2019).
Importantly, these existing methods address several limitations of quantifying scratching behavior in rodent models using manual counting from low-frame-rate video recordings, which remains the most commonly used approach in the field. These approaches are designed to streamline and automate the process of quantifying the incidence of scratching behavior exhibited by mouse models under various conditions. Data regarding the number of scratching bouts or other parameters reflective of the incidence of scratching behavior are and will remain critical in the field, and advancements that simplify or expedite their quantification will be invaluable. However, these novel methods have not yet shifted the focus in the field toward a more detailed, granular analysis of scratching. Simply stated, we suggest that parameters of scratching behavior exist that are orthogonal to scratching incidence and that, if the goal is to better understand scratching as a behavioral output that provides insight into the circuits underlying itch, we as a field must attempt to extract the complex set of parameters embedded in this intricate motor pattern. This is what we set out to achieve here.
Analysis
Because current methodologies limit our understanding of the complexities of itch-evoked behaviors, we propose a two-fold approach for detailed evaluation of scratching behavior. First, we propose that multiple behavioral parameters should be reported in itch studies. For current commonly reported parameters (bout number, time spent scratching, and bout duration), a detailed comparison has not been done between different itch models, and most often results are reported using only one of the above three parameters. These parameters should be reported in conjunction to provide a more complete picture of scratching behavior. Second, we propose that a detailed analysis of scratching as a motor output will help to identify novel parameters relevant to understanding scratching as a response to, and indicator of, itch. We utilized high-speed recordings to begin to identify such parameters, which can be used to complement existing magnet-based and video methods and has similar advantages, such as the ability to be automated, and greatly reduce subjectivity compared with manual analyses.
Scratching behaviors vary across acute and chronic itch models
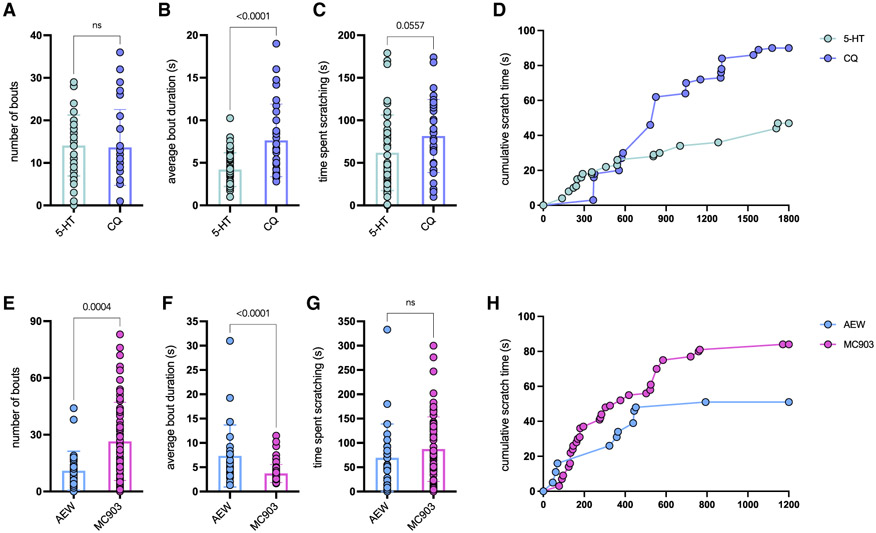
To illustrate the importance of measuring multiple itch-scratch parameters, we re-analyzed data from previously published experiments that examined scratching behaviors in acute and chronic models of itch. Quantitative and qualitative differences in scratching behavior are observed between different models. First, the total number of scratch bouts did not vary significantly between CQ- or 5-HT-injected animals in the cheek model of acute itch (Figure 2A). However, the average bout duration was significantly longer in response to CQ than 5-HT, resulting in a longer total scratch time in response to CQ (Figures 2B and 2C). In addition, although both pruritogens trigger the same number of bouts, the temporal pattern of bouts is quite distinct. For example, 5-HT induced many short bouts during the first 10 min post-injection that diminished over the next 20 min, whereas CQ induced longer bouts spread throughout the 30-min recording period (Figure 2D). Furthermore, increased paw licking and biting were observed in response to CQ in comparison to 5-HT or vehicle. Interestingly, both pruritogens caused scratching ipsilateral and contralateral to the injection site, whereas vehicle-injected animals displayed only a few ipsilateral scratching bouts.
Figure 2. Scratch bout number does not fully capture the complex behaviors observed in acute and chronic itch.
(A–C) Chloroquine (CQ) triggers the same number of itch bouts as 5-hydroxytryptamine (5-HT), but each bout is significantly longer in duration, increasing the total time the mice scratch. The number of bouts, average bout duration, and time spent scratching was quantified by re-scoring data published previously (Wilson et al., 2013a, Figure 4; Morita, et al., 2015, Figures 1 and 5; Walsh et al., 2019, Figure 4). n = 30 animals across 8 experimental cohorts (CQ) and 50 animals across 12 experimental cohorts (5HT). Although the graphs display data from individual animals, similar results were obtained when comparing across cohorts (bout number: 14.28 ± 2.46 CQ versus 14.45 ± 1.05 5-HT, p = 0.9457; bout duration: 7.45 ± 0.81 s CQ versus 4.32 ± 0.40 s 5-HT, p = 0.0012; time spent scratching: 84.98 ± 0.88 s CQ versus 64.53 ± 9.3 s 5-HT, p = 0.14958). p values were calculated by two-tailed, unpaired t test in each case.
(D) Graph displaying the cumulative scratch time of bouts measured over 30 min following injection of CQ or 5-HT.
(E–G) 50% ether/50% acetone (AEW) treatment triggers fewer scratch bouts than MC903 treatment, but each bout is significantly longer in duration. The time spent scratching, number of bouts, and average bout duration were quantified by re-scoring data published previously (Wilson et al., 2013b, Figures 1, 3, and 4; Walsh et al., 2019, Figures 1 and 2). n = 26 animals across 7 cohorts (AEW) and 69 animals across 16 experimental cohorts (MC903). Although the graphs display data from individual animals, similar results were obtained when comparing across cohorts (bout number: 9.95 ± 1.87 AEW versus 27.96 ± 3.04 MC903, p = 0.0011; bout duration: 7.1 ± 1.47 s AEW versus 3.6 ± 0.41 s MC903, p = 0.0055; time spent scratching: 59.21 ± 15.68 s AEW versus 88.5 ± 8.26 s MC903, p = 0.0841). p values were calculated by two-tailed, unpaired t test in each case.
(H) Graph displaying the cumulative scratch time of spontaneous scratch bouts measured over 20 min.
We next compared itch behaviors between the acetone/ether/water (AEW) dry skin itch model and the MC903 model of AD. Although mice in both models showed robust scratching behaviors, MC903 induced significantly more bouts (Figure 2E). However, AEW induced significantly longer bouts so that the total scratching time was not significantly different between models (Figures 2F and 2G). As described for CQ and 5-HT, the temporal pattern of bouts is quite distinct among AEW and MC903-treated animals (Figure 2H). Finally, individual mice can display dramatically different scratching behaviors even when the number of bouts or time spent scratching to the same pruritogen is similar. These results suggest that scratching bout incidence and bout duration are weakly correlated— raising questions about how these two parameters are regulated independently and which parameter may be better correlated with “itchiness” as well as with risk of damage to the skin. In addition, this new analysis indicates that the current standard in the field for quantifying scratching, bout number, does not fully capture the complex nature of the behavior in acute and chronic itch models. We suggest that multiple parameters are necessary to more accurately compare scratching behavior in diverse models of itch and that bout number and duration appear to vary independently. We also hypothesize that there are potentially other orthogonal parameters of scratching behavior that could provide novel insight into itch neurobiology.
Observing CQ-evoked scratching using high-speed recording
To identify additional scratching-associated behavioral parameters, we performed high-speed video recordings of mice injected with 1 mM or 1 mM CQ in the nape of the neck. Animals were recorded from the side at 500 frames per second (fps) as they scratched. At this high frame rate, qualities that are not obvious when viewing a standard (25–30 fps) video, such as the rapid and highly stereotyped pattern of the behavior, are readily observable. Single scratches within a bout (defined here as a series of scratches ending with hindpaw licking or placing the hindpaw on the floor) can be resolved easily, and other aspects of scratching behavior, such as licking between bouts and the shifting of body weight for balance before scratching is commenced, are also readily apparent.
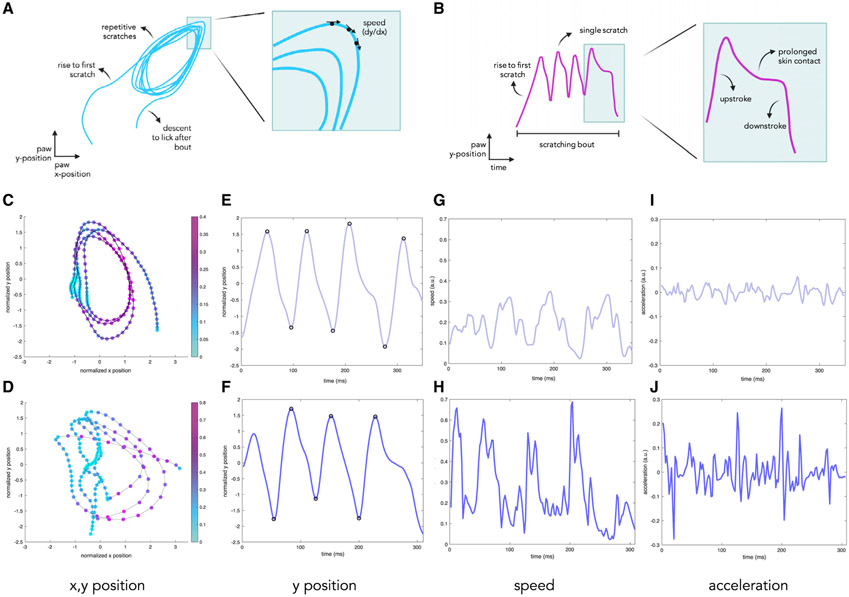
To analyze these high-speed recordings, we used DeepLab-Cut (Mathis et al., 2018; Nath et al., 2019) to automatically track the x, y position of the relevant hindpaw in each frame of the video, allowing us to trace the location of the paw over time. We can then plot the x and y coordinates to generate a trajectory that shows the rise and fall of the paw before and after each bout as well as the stereotyped motion of the hindpaw over individual scratches. We also used the derivative of the position to calculate speed at each point (Figure 3A) and automatically quantitated individual scratches by plotting the y position over time and identifying peaks. By looking at the y position over time, the duration of each scratch and instances where the paw makes prolonged skin contact are readily identifiable based on the shape of each peak (Figure 3B).
Figure 3. Hindpaw motion tracking during itch-evoked scratching allows extraction of multiple scratching-related parameters.
(A) A schematic of the hindpaw x, y position for visualization of the scratching motion, including the rise of the paw to make contact with the skin, repetitive scratching, and the descent of the paw to lick upon completion of a bout (left). From this trajectory, we can use the slope of the graph at each time point to calculate the speed (√((dx/dt)2 + (dy/dt)2)) (right).
(B) A schematic of the hindpaw y position plotted over time. Clusters of peaks in this graph allow identification of scratching bouts (left), whereas single peaks correspond to individual scratches. The rising phase of each peak corresponds to the upstroke of each stroke, whereas the falling phase represents the downstroke. Distortions of the peak in the falling phase represent prolonged contact with the skin during scratching (right).
(C–J) Scratching parameters extracted from high-speed videos (500 fps) of mice treated with 1 μM (top) or 1 mM (bottom) CQ.
(C and D) The normalized and x and y position of the paw; the color bar corresponds to speed (√((dx/dt)2 + (dy/dt)2)) in arbitrary units (a.u.) of the paw in each frame. Under the 1 mM condition, the motion of the paw appears larger, and scratching proceeds at a greater speed.
(E and F) Hindpaw y position plotted over time (milliseconds). Peaks and troughs were identified automatically and correspond to individual scratches.
(G and H) Hindpaw speed (√((dx/dt)2 + (dy/dt)2)) plotted over time (milliseconds) is faster and shows larger fluctuations under the 1 mM condition compared with 1 μM.
(I and J) Hindpaw acceleration plotted over time (milliseconds) shows that peak acceleration and fluctuations in acceleration are greater under the 1 mM condition compared with 1 μM.
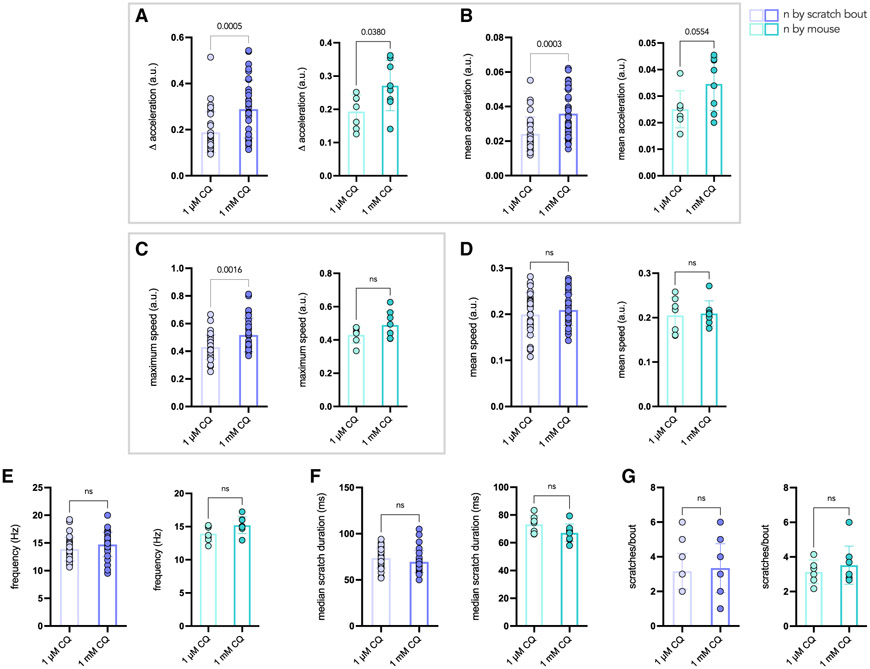
To explore differences in scratching parameters, we analyzed bouts of scratching in response to 1 μM CQ or 1 mM CQ (Videos S1 and S2, respectively). By calculating the speed at each time and overlaying this with each x, y position, the general motion and speed of the paw during each bout can be assessed. The motion of the paw is larger in the 1 mM than the 1 μM CQ case, and the paw moves at a faster rate in response to the higher concentration of CQ (Figures 3C and 3D). We also observed that the speed varies in a highly stereotyped manner during each individual scratch so that it is at a minimum when the paw makes contact with skin (blue) and at a maximum upon release from the skin (pink). From changes in the y position over time, the number of scratches per bout is apparent and appears similar for the two conditions (Figures 3E and 3F). However, when looking at speed (dy/dx) over time, it is clear that the paw reaches greater speeds under the 1 mM CQ condition compared with 1 μM (Figures 3G and 3H) and that there are much larger variations in acceleration, calculated as the derivative of speed over time (Figures 3I and 3J).
Scratching speed and acceleration as predictors of scratching intensity
To identify parameters that might capture overall differences between the two datasets, we looked at the difference between maximum and minimum acceleration (Δ acceleration) and mean acceleration for each trajectory in the 1 μM and 1 mM groups (n = 32 videos and 8 mice in each group) and found that both were increased significantly in response to 1 mM compared with 1 μM CQ (Figures 5A and 5B). Accordingly, the maximum speed, but not the mean speed, was increased slightly in the 1 mM group compared with 1 μM, but this difference was only significant when analyzing the data in aggregate (Figures 5C and 5D). This is consistent with the observation that, during more forceful contact with the skin, the hindpaw slows significantly and then is released at heightened speed, which would be reflected in the maximum but not necessarily the mean speed. Finally, there was no change in the overall frequency, scratch duration, or number of scratches per bout between the two conditions, which also suggests that the scratching motion is similar overall between groups, although it varies in intensity. Therefore, maximum speed, Δ acceleration, and mean acceleration may be useful predictors of scratching intensity.
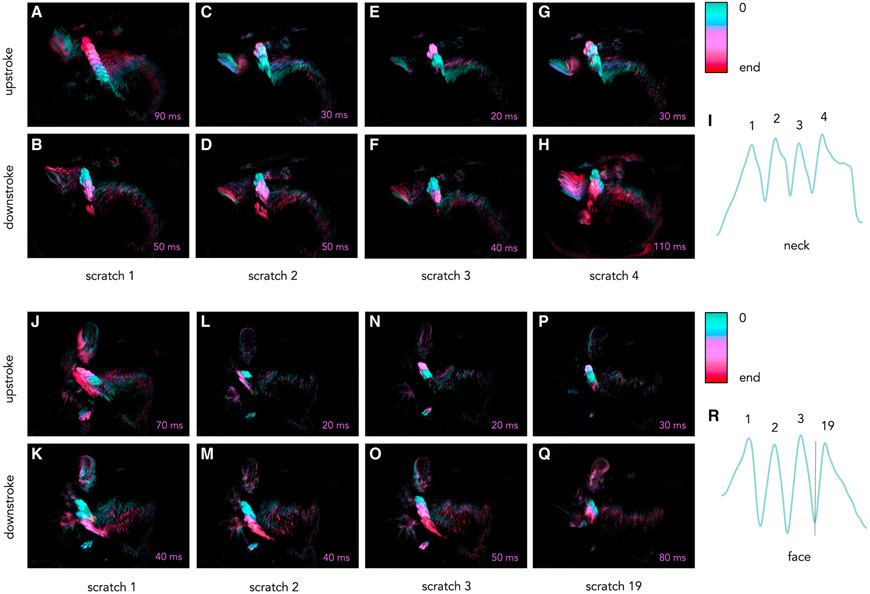
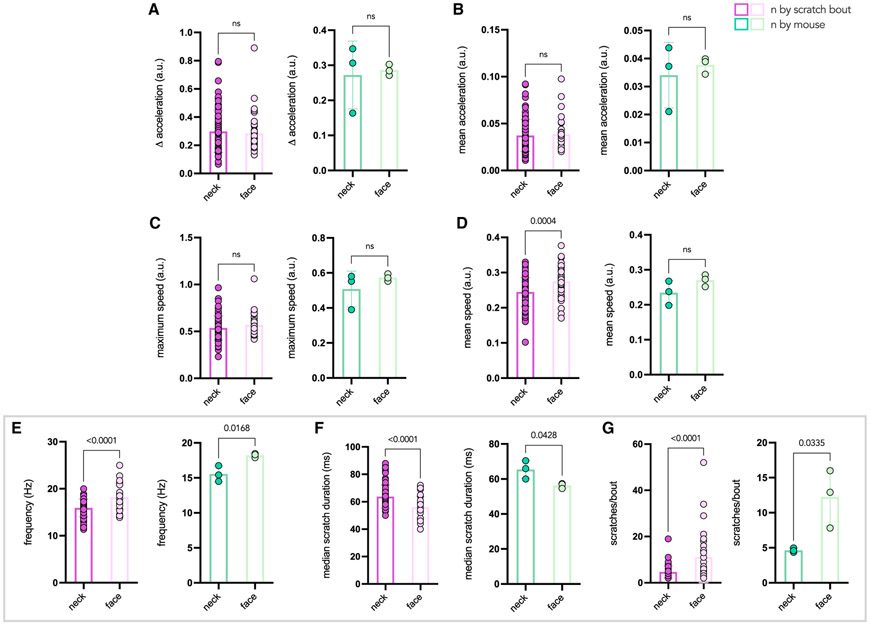
Figure 5. High-speed video allows for dissection of individual scratches within bouts of neck and face scratching.
Still frames at 10-millisecond increments were extracted from a high-speed video, and consecutive images were subtracted in ImageJ. The resulting subtracted images were overlayed, and a color scale was applied to represent time (first frame in green, middle frames in purple, and last in red; see color bar). Each image segment represents 10 milliseconds, and the total time represented in each image is shown in the bottom right corner of each image.
(A–H) A single bout of neck scratching. Shown are the upstroke (top row) and downstroke (bottom row) of all (four) individual neck scratches within a single bout.
(J–Q) A single bout of face scratching. Shown are the upstroke (top row) and downstroke (bottom row) of four individual face scratches within a single bout. Images show the first three and last (19th) scratches of the bout. A trace of the y position of the paw over time extracted from neck (I) and face (R) scratching bouts is shown on the right, with each labeled peak corresponding to the images of the same scratch.
(A and J) First scratches of a given bout have unique trajectories. In neck and face scratching, the upstroke of the first scratch is larger and more prolonged than subsequent upstrokes.
(H and Q) The downstroke of the final scratch is longer in duration in neck and face scratching, as the animal adjusts its posture to bring the paw to the mouth to lick.
(B–F and K–P) Scratches between the first and last scratch are highly stereotyped and follow a similar trajectory during a given bout.
(B, H, and I) Scratches making prolonged contact with the skin show humped peaks in the y position trace (I); this is also reflected in the rapid paw movement away from the skin (B and H).
Dissecting individual scratches within single bouts using high-speed recording
In addition to capturing aggregate parameters over given scratching bouts, high-speed recording highlights differences between individual scratches within bouts. To explore this further, we performed high-speed video recordings of mice presenting with spontaneous, chronic scratching secondary to a genetic mutation in a sodium channel. Because this scratching is not localized to a particular body region, we were able to compare the precise nature of scratching of the neck and face in the same mice (n = 3). To visualize individual scratches, we captured still frames at 10-millisecond (ms) increments from representative videos of neck and face scratching (Videos S3 and S4, respectively). The images were subtracted incrementally, and we overlaid them to generate color-mapped motion images of the motion of the paw during the upstroke and downstroke of neck (Figures 5A-5H) or face (Figures 5J-5Q) scratching. From this analysis, it became clear that the first and last scratches of a given bout are unique in terms of the range of the motion. For neck and face scratching, the upstroke of the first scratch is larger and more prolonged than subsequent upstrokes (Figures 5A and 5J, respectively). Second, the downstroke of the final scratch is also longer in duration as the animal adjusts its posture to bring the paw to the mouth to lick (Figures 5H and 5Q for neck and face, respectively).
However, the scratches between the first and last scratch are highly stereotyped and follow a similar trajectory during a given bout, although individual scratches may vary in intensity. It is apparent from the trace of the y-position over time for neck scratching (Figure 5I) that the peaks corresponding to the second and fourth scratch display a characteristic hump that captures prolonged contact with the skin (Video S1). In the images of the downstroke for the second and fourth scratch (Figures 5D and 5H, respectively), there is evidence of rapid movement away from the skin, with the paw in the final frame (in red) at a greater distance from its previous location than in earlier frames. For comparison, in the third scratch, the movement of the paw is consistent over time, as represented by equally spaced frames. In the face-scratching bout, there was no variation in intensity in the y-position trace (Figure 5R) or images, where the downstroke of each of the first three scratches is very similar (Figures 5J, 5L, and 5N). The variation in individual scratches arises, we conclude, not from a change in the trajectory of the hindpaw but from the slowing and accelerating that occur before and after skin contact. Careful quantitation of the duration of skin contact during individual scratches could be a reliable indication of scratching intensity. It is clear that there is a wealth of information that can be extracted from high-speed video recordings of scratching or other methods with a similar spatial and temporal resolution.
Frequency, duration, and scratches per bout vary in neck versus face scratching
We were also interested in which parameters of scratching varied between neck and face scratching (n = 3 mice with 15, 28, and 40 recordings of neck scratching and 6, 13, and 17 recordings of face scratching). Based on the examples in Figure 5, we hypothesized that differences in posture and in the size of the movements generated by neck and face scratching might be reflected in spatially but not intensity-related parameters. Intrinsic models of chronic scratch, where animals are prone to neck and face scratching independent of local stimuli, are uncommon. Therefore, this mouse model provided an opportunity to explore differences in scratching location because the same mice scratched the neck and face independent of any localized external manipulation.
No changes in maximum speed, Δ acceleration, and mean acceleration were observed between neck and face scratching, consistent with these parameters reflecting scratching intensity (Figures 6A-6C). A modest increase in mean speed (Figure 6D) and significant increases in scratching frequency and median scratch duration were present in face compared with neck scratching (Figures 6E and 6F). Overall, face scratches proceeded more rapidly than neck scratching, likely because of smaller and more spatially restricted movements. There were also significantly more scratches per bout in face scratching than neck scratching (Figure 6G). Further comparisons between these parameters in chronic neck scratching and CQ-evoked neck scratching are shown in Figure S1.
Figure 6. Frequency, scratch duration, and scratches per bout vary between neck and face scratching.
(A–G) Comparison of scratching parameters between neck and face in mice with chronic scratching behavior (n = 3 mice; n = 83 bouts for neck and 36 bouts for face). In each case, the data are plotted so that each data point corresponds to an individual scratching bout (left, pink) or an individual mouse (right, green). Parameters of interest are outlined by the gray box. p values were calculated by two-tailed, unpaired t test in each case, and ns indicates values that are not significant.
(A) Delta acceleration, representing the difference between the maximum and minimum acceleration, is similar between groups.
(B) Mean acceleration is similar between groups.
(C) Maximum speed is similar between neck and face scratching.
(D) Mean speed is slightly higher in face scratching compared with neck scratching (p = 0.0004 by bout, ns by mouse).
(E) Scratching frequency in Hertz is higher in face scratching compared with neck scratching (p < 0.0001 by bout, p = 0.0168 by mouse).
(F) Median scratch duration in milliseconds, calculated using the interpeak interval, is shorter in face scratching compared with neck scratching (p < 0.0001 by bout, 0.0428 by mouse).
(G) There are more scratches per bout in face scratching compared with neck scratching (p < 0.0001 by bout, p = 0.0335 by mouse).
Complexity and consistency of scratching as a motor output
Insight into the highly organized motor response to the sensation of itch are gained readily through the observations enabled by high-speed scratching recordings in mice. Scratching requires coordination of dozens of muscles, most importantly in the ipsilateral hindlimb and digits of the animal but also in the contralateral limbs and often in the back to maintain balance. A bout of scratching is initiated by sensory input from the skin signaling the presence of an itch. Individual scratches then occur at remarkable speed, with durations on the order of ~50–70 ms. Given the simplest hypothetical reflex circuit involving synaptic transmission at three synapses—a sensory afferent activated by a pruritogen and a dorsal horn spinal cord interneuron, this interneuron, and an efferent motor neuron that activates the muscle—the total synaptic delay alone would be expected to be about ~10–30 ms. Therefore, although sensory input is transduced via primary afferents in the skin and transmitted to the spinal cord during scratching, the timing suggests that sensory feedback is not required to initiate an individual scratch but instead may trigger a scratch bout. This model is consistent with the existence of a CPG for scratching. Our data reveal that each scratch within a bout is made in a highly stereotyped manner, where the trajectory of each movement is nearly superimposable and differs only based on skin contact. That this highly complex motor program proceeds rapidly, consistently, and repetitively without interruption or diminishment suggests a highly specialized underlying circuitry that drives an internally consistent program of activity across multiple motor neuron outputs. Whether sensory feedback from the skin or proprioceptive input from muscles and joints feeds into this program needs to be established, but the rapid and uniform scratching within bouts suggests that it is largely autonomous of sensory input.
The mechanisms responsible for termination of a bout of scratching are unknown. One possibility is that the sensory signal from the site of scratching is integrated over time and that scratching ceases at a point when itch-related activity is reduced or when nociceptive signals that trigger pain build up beyond some threshold level. Alternatively, it may be that the CPG has a fixed number of cycles before some internal inhibitory switch terminates the cycle. The application of live neuronal recordings from afferent neurons, interneurons, and motor neurons during scratching will aid in defining the scratch circuit and its regulation.
Conclusions
Scratching is an evolutionarily conserved, critical, complex, and information-rich motor behavior that provides insight into itch neurobiology beyond its current use as merely an indicator of increased or decreased itch. We argue that the current standard in the field of reporting scratch bouts alone does not capture the complexity of scratching behavior. This premise is based on data analysis showing that scratching elicited by distinct pruritogens can vary in scratching bout duration or number of bouts. Although it is unknown why distinct pruritogens result in scratch durations and/or patterns, there are several possibilities. First, the binding affinity/binding kinetics or signaling pathways engaged by different pruritogens may trigger distinct patterns of excitability in primary afferent neurons. Second, the activation of distinct primary afferent subpopulations may engage different central circuits that drive motor output. Third, distinct pruritogens may activate distinct brain regions involved in execution of itch-induced scratching behavior or motivational aspects of itch. Based on our observations that these parameters can vary independently upon treatment with various pruritogens, we suggest that scratching bout incidence and bout duration are relevant and should be reported in tandem when analyzing scratching behavior.
Our high-speed video recording data also demonstrate that other aspects of scratching behavior exist that are relevant for understanding underlying itch circuitry and scratch motor control and are not captured by currently reported metrics. Specifically, we suggest that the speed and acceleration of the hindpaw during scratching are correlated with scratching intensity, which we define as the forcefulness of the scratching while the paw is in contact with the skin, an important factor in development of lesions. This is likely only one of several relevant parameters that can be uncovered from a more careful analysis of scratching behavior.
Additionally, by tracking the position of the hindpaw in high-speed recordings of mouse scratching under acute and chronic itch conditions, we found itch behavior to be strikingly stereotyped and rapid (tens of milliseconds in duration), suggesting that it may be controlled entirely locally at the spinal cord level by fixed movement pattern generator circuits, triggered and sustained by itch-related sensory input in pruriceptors. We also find that the duration and frequency of these movements depend on the site of the body where scratching is directed, and this may reflect different pattern generators for the particular target area of scratching. It will be interesting to determine whether there are differences in nerve fiber subtypes, density of innervation, or changes in receptors or excitability genes within subtypes that drive distinct itch behaviors in the neck versus back. The field stands to benefit considerably from further research of this area, which will require uncovering the link between pruriceptor sensory inflow into the spinal cord, the scratching motor output it generates, and the associated sensation of itch as well as the modifying effects of low- and high-threshold mechanoreceptor activation produced by the scratch in the skin and of proprioceptive inputs resulting from limb movements.
The challenges in quantifying and analyzing animal behavior outlined here are not unique to scratching. Itch-triggering inputs also elicit a range of behaviors such as biting and licking, which can also be triggered by nociceptive inputs that produce pain. For example, the calf model of itch elicits biting behavior rather than hindpaw scratching, which further complicates analyses of itch behavior. Conversely, scratching may reflect the presence of pain in some circumstances. For example, although injection of capsaicin or histamine into the mouse cheek yields wiping or scratching, respectively, which are considered by most to reflect pain- or itch-related behaviors, injection of either compound into the nape of the neck only causes scratching (Shimada and LaMotte, 2008). Although these models may crudely differentiate itch and pain behaviors, more detailed analyses of evoked behaviors are required to differentiate whether and when scratching is caused by pruriceptor or nociceptor input and whether it is associated with itch and pain. The high-speed, detailed motor behavioral analysis of scratching we performed here could be applied to many different disease-related motor behaviors, capturing their full complexity at a temporal scale similar to the neuronal activity that drives them and providing a platform to begin to define the activity patterns of circuits that produce particular behaviors.
This approach can also be extended to quantifying scratching behavior in humans. Clinical methods for recording scratching behavior are now being used successfully as an objective alternative to survey-based self-reports. Accelerometer-based or video recording data could be used in these settings to uncover patterns of scratching behavior that are better diagnostic or prognostic indicators of dermatologic and neurologic disease in individuals with itch. Parameters similar to those assessed here, such as speed and acceleration of the hand, number of scratches, frequency, and scratching duration could be calculated readily using these types of data. Furthermore, parameters related to skin compliance and force on the skin during scratching may be useful indications of scratching intensity. Based on existing clinical observations, it is likely that differences in these features can be found between various dermatoses and, therefore, could be instrumental in generating more specific and selective diagnostic criteria for these conditions. This approach could also be useful in determining the effectiveness of treatments in affected individuals. Instead of behavior being the end of the analysis of nervous system function or dysfunction, it could equally be the beginning.
STAR ★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by Lead Contact, Clifford J. Woolf (clifford.woolf@childrens.harvard.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Raw trajectories for high-speed scratching videos are available at Mendeley Data: https://doi.org/10.17632/bws2s9t8nx.1. All other data reported in this paper will be shared by the lead contact upon request.
All original code is available in this paper’s supplemental information. This study utilized existing code for DeepLabCut, available here: https://github.com/DeepLabCut/DeepLabCut.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
In vivo animal studies
For re-analysis of standard video recordings: 8-10-week-old C57/B6J mice of both sexes were used, and no sex-ralted differences were found in assessments of scratching behavior. Mice were housed with 12 hr light-dark cycle at 21°C. Data re-analyzed for this study were generated in Wilson et al. (2011), Morita et al. (2015), and Walsh et al. (2019) according to the methods reported in those studies. All experiments were performed under the policies and recommendations of the International Association for the Study of Pain and approved by the University of California, Berkeley Animal Care and Use Committee.
For high-speed recordings: For CQ experiments, 8-week-old C57/B6J mice of both sexes were purchased from Jackson Laboratories (Bar Harbor, ME). Because these experiments were done as a proof of concept for this methodology, differences between sexes were not explored in this study, but future studies could apply these measures to the study of gender differences in the scratching behavior evoked by itch. The animals were group housed separated by sex and handled in accordance with IACUC protocol 20-05-4208R (CJW). For chronic scratching experiments, an Nav1.7 gain-of-function mutant model with demonstrated scratching behavior was used, which has been previously described (Chen et al., 2021). Experiments were similarly performed in accordance with IACUC protocol 20-05-4208R (CJW).
METHOD DETAILS
Manual quantification of itch behavior
Quantification of Chloroquine-, 5-hydroxytryptamine-, Acetone/Ether/Water- and MC903-evoked scratching (Figure 2) was done by manually re-scoring movies and data that were previously recorded and published (Hill and Bautista, 2018,2020; Morita et al., 2015; Walsh et al., 2019; Wilson et al., 2011,2013a,b). Broadly, the amount of time each mouse spent scratching, and the number of scratch bouts, were quantified over a 20 or 30-minute period. One bout of scratching was defined as an episode in which a mouse lifted its paw and scratched continuously for any length of time, until the paw was returned to the floor. Behavioral scoring was performed while blind to genotype and to the solution injected. Here, analysis was extended to examine the number of scratch bouts, bout duration and total time spent scratching by a blinded scorer.
High-speed recordings
Animals were placed in a clear plexiglass chamber atop a glass floor. One animal was recorded at a time. Videos were recorded at 500 fps at a resolution of 1264 × 1024 pixels using the Edgertronic SC1 high-speed camera. The camera was placed on a track lateral to the chamber containing the mouse, which was fixed at the previously assessed focal distance for the camera. When scratching was observed and the animal was oriented such that the relevant hind paw was visible to the camera, the camera was manually triggered with a button trigger. The camera was set with a 1.75 s pre-trigger recording window in order capture the onset of the behavior, accounting for human reaction time. Each recording was a total of 5 s in length.
QUANTIFICATION AND STATISTICAL ANALYSIS
High-speed video analysis
Videos were saved in MOV file format by the camera and were subsequently converted to AVI files and cropped into individual scratching bouts. For each video, the location of the hind paw was manually identified in ~20 still images. These images were then used to train DeepLabCut to automatically identify the location of the paw in each frame of the video. Each video was then treated independently, and downstream analysis based on the x, y coordinates of the paw was done using basic functions in MATLAB. x,y coordinates were initially plotted against time and each trajectory was trimmed to include only the motion at the site of scratching to avoid distance artifacts caused by the large movements between the floor and site of scratching. In rare instances in which DeepLabCut had misidentified the paw, aberrant points were identified and replaced with the immediately preceding coordinate to preserve the time structure of each trajectory. Following this, the x,y coordinates were z-scored (zscore, MATLAB) to normalize distances and allow for comparisons of coordinates across videos.
Trajectory analysis
After obtaining normalized trajectories for each video, single scratches were identified using peaks and troughs in the y position of the paw over time (findpeaks, MATLAB). The speed was calculated by taking the derivative of the normalized position of the paw over time (diff, MATLAB). The acceleration was calculated by taking the derivative of the speed over time. The maximum speed refers to the absolute maximum speed over time, whereas because the acceleration contained both positive and negative values, the Δ acceleration, or difference between the maximum (positive) and minimum (negative) acceleration was used. Median scratch duration and frequency were calculated using the median distance between automatically identified troughs in the y-position over time. Statistical details of analysis can be found in the figure legends.
Supplementary Material
Figure 4. Speed- and acceleration-related scratch parameters vary based on CQ dose.
(A–G) Comparison of neck scratching parameters in mice treated acutely with 1 μM or 1 mM CQ (n = 32 bouts per group, n = 8 mice in each group). In each case, the data are plotted so that each data point corresponds to an individual scratching bout (left, purple) or an individual mouse (right, green). Parameters of interest are outlined by the gray box. p values were calculated by two-tailed, unpaired t test in each case, and ns indicates values that are not significant.
(A) Delta acceleration, representing the difference between maximum and minimum acceleration, is higher in the 1 mM group compared with 1 μM CQ (p = 0.0005 by bout, p = 0.0380 by mouse).
(B) Mean acceleration is higher in the 1 mM CQ group compared with 1 μM by scratching bouts (p = 0.0003) and trends higher by mouse (p = 0.0554).
(C) Maximum speed is higher in mice treated with 1 mM CQ compared with 1 μM when looking at scratching bouts (p = 0.0016), and trends higher by mouse (p = 0.1131).
(D) Mean speed is not different between doses of CQ.
(E) Scratching frequency in Hertz does not vary between groups.
(F) Median scratch duration in milliseconds, calculated using the interpeak interval, is similar between groups.
(G) The number of scratches per bout does not vary between groups.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Chloroquine diphosphate salt | Sigma Aldrich | C6628-25G |
| MC903 | R&D Systems | 2700/10 |
| Serotonin | Sigma Aldrich | 153-98-0 |
| Deposited data | ||
| Re-analyzed acute and chronic itch data | Wilson et al., 2011 | n/a |
| Re-analyzed acute and chronic itch data | Morita et al., 2015 | n/a |
| Re-analyzed acute and chronic itch data | Walsh et al., 2019 | n/a |
| Raw trajectories from high-speed video of CQ-evoked and chronic scratching | This paper | Mendeley Data: https://doi.org/10.17632/bws2s9t8nx.1 |
| Experimental models: Organisms/strains | ||
| C57/B6J mice | Jackson Laboratories | 000664 |
| Nav1.7- I228M mutant mice | Chen et al., 2021 | n/a |
| Software and algorithms | ||
| MATLAB version 2019b | MathWorks, Inc. | https://www.mathworks.com/products/matlab.html |
| GraphPad PRISM 9 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| Fiji ImageJ 1.52p | ImageJ | https://imagej.net/software/fiji/ |
| DeepLabCut | Mathis et al., 2018 | https://github.com/DeepLabCut/DeepLabCut |
Highlights.
Common scratching parameters such as bout number and duration can vary independently
Itch severity should be probed beyond measures of scratching incidence
High-speed video of scratching reveals potential metrics of scratching intensity
ACKNOWLEDGMENTS
This work was funded in part by NIH awards R35NS105076 and R01AT011447 and the Bertarelli Foundation (to C.J.W.), an HHMI Faculty Scholars Award (to D.M.B.), and an NSF Graduate Research Fellowship Program award (to N.K.W.). Figures 1,3A, and 3B were made using BioRender.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.neuron.2021.07.020.
DECLARATION OF INTERESTS
C.J.W. is a founding member of Nocion Therapeutics. D.M.B. is on the scientific advisory board of Escient Pharmaceuticals.
REFERENCES
- Akiyama T, and Carstens E (2013). Neural processing of itch. Neuroscience 250, 697–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Hao ZZ, Dyer GMC, Watanabe M, Maxwell DJ, and Berkowitz A (2020). Neurotransmitters and motoneuron contacts of multi-functional and behaviorally specialized turtle spinal cord interneurons. J. Neurosci 40, 2680–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JK, McQueen DS, and Rees JL (2004). Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br. J. Pharmacol 142, 374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz A, and Hao ZZ (2011). Partly shared spinal cord networks for locomotion and scratching. Integr. Comp. Biol 51, 890–902. [DOI] [PubMed] [Google Scholar]
- Bohnslav JP, Wimalasena NK, Clausing KJ, Yarmolinksy D, Cruz T, Chiappe E, Orefice LL, Woolf CJ, and Harvey CD (2020). DeepEthogram: A machine learning pipeline for supervised behavior classification from raw pixels. bioRxiv. 10.1101/2020.09.24.312504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenaut E, Garlantezec R, Talour K, and Misery L (2013). Itch characteristics in five dermatoses: non-atopic eczema, atopic dermatitis, urticaria, psoriasis and scabies. Acta Derm. Venereol 93, 573–574. [DOI] [PubMed] [Google Scholar]
- Chen L, Wimalasena NK, Shim J, Han C, Lee S-I, Gonzalez-Cano R, Estacion M, Faber CG, Lauria G, Dib-Hajj SD, et al. (2021). Two independent mouse lines carrying the Nav1.7 I228M gain-of-function variant display dorsal root ganglion neuron hyperexcitability but a minimal pain phenotype. Pain 162, 1758–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SN, and Stein PSG (1988). Electrical activation of the pocket scratch central pattern generator in the turtle. J. Neurophysiol 60, 2122–2137. [DOI] [PubMed] [Google Scholar]
- Darsow U, Mautner VF, Bromm B, Scharein E, and Ring J (1997). Der Eppendorfer Juckreizfragebogen. Hautarzt 48, 730–733. [DOI] [PubMed] [Google Scholar]
- Darsow U, Scharein E, Simon D, Walter G, Bromm B, and Ring J (2001). Skin Diseases New Aspects of Itch Pathophysiology: Component Analysis of Atopic Itch Using the “Eppendorf Itch Questionnaire.”. Int. Arch. Allergy Immunol 124, 326–331. [DOI] [PubMed] [Google Scholar]
- Dawn A, Papoiu ADP, Chan YH, Rapp SR, Rassette N, and Yosipovitch G (2009). Itch characteristics in atopic dermatitis: results of a web-based questionnaire. Br. J. Dermatol 160, 642–644. [DOI] [PubMed] [Google Scholar]
- Dominick F, van Laarhoven AIM, Evers AWM, and Weisshaar E (2019). A systematic review of questionnaires on itch by the Special Interest Group “Questionnaires” of the International Forum for the Study of Itch (IFSI). Itch (Phila.) 4, e26. [Google Scholar]
- Donovan MJ, Messmore AS, Scrafford DA, Sacks DL, Kamhawi S, and McDowell MA (2007). Uninfected mosquito bites confer protection against infection with malaria parasites. Infect. Immun 75, 2523–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebata T, Iwasaki S, Kamide R, and Niimura M (2001). Use of a wrist activity monitor for the measurement of nocturnal scratching in patients with atopic dermatitis. Br. J. Dermatol 144, 305–309. [DOI] [PubMed] [Google Scholar]
- Elliott P, G’Sell M, Snyder LM, Ross SE, and Ventura V (2017). Automated acoustic detection of mouse scratching. PLoS ONE 12, e0179662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field EC, and Stein PSG (1997). Spinal cord coordination of hindlimb movements in the turtle: intralimb temporal relationships during scratching and swimming. J. Neurophysiol 78, 1394–1403. [DOI] [PubMed] [Google Scholar]
- Gao ZR, Chen WZ, Liu MZ, Chen XJ, Wan L, Zhang XY, Yuan L, Lin JK, Wang M, Zhou L, et al. (2019). Tac1-Expressing Neurons in the Periaqueductal Gray Facilitate the Itch-Scratching Cycle via Descending Regulation. Neuron 101, 45–59.e9. [DOI] [PubMed] [Google Scholar]
- Hill RZ, and Bautista DM (2018). A trio of ion channels takes the heat. Nature 555, 591–592. [DOI] [PubMed] [Google Scholar]
- Hill RZ, and Bautista DM (2020). Getting in Touch with Mechanical Pain Mechanisms. Trends Neurosci. 43, 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossen MA, Sugimoto Y, Kayasuga R, and Kamei C (2003). Involvement of histamine H3 receptors in scratching behaviour in mast cell-deficient mice. Br. J. Dermatol 149, 17–22. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Ebata T, Chantalat L, Takemura K, Mizzi F, Poncet M, and LeClercq D (2019). Measurement of nocturnal scratching in patients with pruritus using a smartwatch: Initial clinical studies with the itch tracker app. Acta Derm. Venereol 99, 268–273. [DOI] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, and Han SK (2009). TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. USA 106, 11330–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Igeta K, Shiraishi N, Kim JF, Nagao M, Nakamura N, and Nagai H (2003). Evaluation and characterization of mouse scratching behavior by a new apparatus, MicroAct. Skin Pharmacol. Appl. Skin Physiol 16, 165–175. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Matsushita S, Shimizu N, Masuko S, Yamamoto M, and Murata T (2021). Automated detection of mouse scratching behaviour using convolutional recurrent neural network. Sci. Rep 11, 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure T, and Ebata T (2018). Activity During Sleep Measured by a Sheet-Shaped Body Vibrometer and the Severity of Atopic Dermatitis in Adults: A Comparison With Wrist Actigraphy. J. Clin. Sleep Med 14, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam Hoai XL, De Maertelaer V, and Simonart T (2021). Prevalence of scratching during examination among patients with scabies and among patients with other pruritic dermatoses. Int. J. Dermatol 60, 70–72. [DOI] [PubMed] [Google Scholar]
- Langedijk J, Bolier R, Tolenaars D, Ten Bloemendaal L, Duijst S, de Waart D, Beuers U, Bosma P, and Elferink RO (2021). Reduced spontaneous itch in mouse models of cholestasis. Sci. Rep 11, 6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay M, and Dong X (2020). Neural Mechanisms of Itch. Annu. Rev. Neurosci 43, 187–205. [DOI] [PubMed] [Google Scholar]
- Lee SH, Cho PS, Tonello R, Lee HK, Jang JH, Park GY, Hwang SW, Park CK, Jung SJ, and Berta T (2018). Peripheral serotonin receptor 2B and transient receptor potential channel 4 mediate pruritus to serotonergic antidepressants in mice. J. Allergy Clin. Immunol 142, 1349–1352.e16. [DOI] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. (2009). Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139, 1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, and Dong X (2012). Mechanisms of itch evoked by β-alanine. J. Neurosci 32, 14532–14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan N, Christakis Y, Di J, Bruno J, Zhang Y, Dorsey ER, Pigeon WR, Beck LA, Thomas K, Liu Y, et al. (2021). Development ofdigital measures for nighttime scratch and sleep using wrist-worn wearable devices. NPJ Digit. Med 4, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M, Huang P, Malkmus S, Robertshaw E, Mac EA, Shatterman Y, and Yaksh TL (2012). Development and validation of an automated system for detection and assessment of scratching in the rodent. J. Neurosci. Methods 211, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, and Bethge M (2018). DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci 21, 1281–1289. [DOI] [PubMed] [Google Scholar]
- Meixiong J, and Dong X (2017). Mas-Related G Protein-Coupled Receptors and the Biology of Itch Sensation. Annu. Rev. Genet 51, 103–121. [DOI] [PubMed] [Google Scholar]
- Moreau A, Anderer P, Ross M, Cerny A, Almazan TH, Peterson B, Moreau A, Anderer P, Ross M, Cerny A, et al. (2018). Detection of Nocturnal Scratching Movements in Patients with Atopic Dermatitis Using Accelerometers and Recurrent Neural Networks. IEEE J. Biomed. Health Inform 22, 1011–1018. [DOI] [PubMed] [Google Scholar]
- Morita T, McClain SP, Batia LM, Pellegrino M, Wilson SR, Kienzler MA, Lyman K, Olsen ASB, Wong JF, Stucky CL, et al. (2015). HTR7 Mediates Serotonergic Acute and Chronic Itch. Neuron 87, 124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Deng J, Liu KF, Wu ZY, Shi YF, Guo WM, Mao QQ, Liu XJ, Li H, and Sun YG (2017). A central neural circuit for itch sensation. Science 357, 695–699. [DOI] [PubMed] [Google Scholar]
- Mui JW, Willis KL, Hao ZZ, and Berkowitz A (2012). Distributions of active spinal cord neurons during swimming and scratching motor patterns. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol 198, 877–889. [DOI] [PubMed] [Google Scholar]
- Nath T, Mathis A, Chen AC, Patel A, Bethge M, and Mathis MW (2019). Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc 14, 2152–2176. [DOI] [PubMed] [Google Scholar]
- Nie Y, Ishii I, Yamamoto K, Orito K, and Matsuda H (2009). Real-time scratching behavior quantification system for laboratory mice using high-speed vision. J. Real-Time Image Process 4, 181–190. [Google Scholar]
- Noro Y, Omoto Y, Umeda K, Tanaka F, Shiratsuka Y, Yamada T, Isoda K, Matsubara K, Yamanaka K, Gabazza EC, et al. (2014). Novel acoustic evaluation system for scratching behavior in itching dermatitis: rapid and accurate analysis for nocturnal scratching of atopic dermatitis patients. J. Dermatol 41, 233–238. [DOI] [PubMed] [Google Scholar]
- O’Neill JL, Chan YH, Rapp SR, and Yosipovitch G (2011). Differences in itch characteristics between psoriasis and atopic dermatitis patients: results of a web-based questionnaire. Acta Derm. Venereol 91, 537–540. [DOI] [PubMed] [Google Scholar]
- Orito K, Chida Y, Fujisawa C, Arkwright PD, and Matsuda H (2004). A new analytical system for quantification scratching behaviour in mice. Br. J. Dermatol 150, 33–38. [DOI] [PubMed] [Google Scholar]
- Park I, Lee K, Bishayee K, Jeon HJ, Lee H, and Lee U (2019). Machine-learning based automatic and real-time detection of mouse scratching behaviors. Exp. Neurobiol 28, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossbach K, Wendorff S, Sander K, Stark H, Gutzmer R, Werfel T, Kietzmann M, and Bäumer W (2009). Histamine H4 receptor antagonism reduces hapten-induced scratching behaviour but not inflammation. Exp. Dermatol 18, 57–63. [DOI] [PubMed] [Google Scholar]
- Rossbach K, Nassenstein C, Gschwandtner M, Schnell D, Sander K, Seifert R, Stark H, Kietzmann M, and Bäumer W (2011). Histamine H1, H3 and H4 receptors are involved in pruritus. Neuroscience 190, 89–102. [DOI] [PubMed] [Google Scholar]
- Sherrington CS (1906). Observations on the scratch-reflex in the spinal dog. J. Physiol 34, 1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, and Oh U (2007). TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J. Neurosci 27, 2331–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, and LaMotte RH (2008). Behavioral differentiation between itch and pain in mouse. Pain 139, 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Harada M, Ogi K, Maruyama M, Fujii R, Tanaka H, Fukusumi S, Komatsu H, Hosoya M, Noguchi Y, et al. (2004). Identification of a G protein-coupled receptor specifically responsive to β-alanine. J. Biol. Chem 279, 23559–23564. [DOI] [PubMed] [Google Scholar]
- Smith MP, Ly K, Thibodeaux Q, Weerasinghe T, Wu JJ, Yosipovitch G, Bhutani T, and Liao W (2019). Emerging Methods to Objectively Assess Pruritus in Atopic Dermatitis. Dermatol. Ther. (Heidelb.) 9, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Iba Y, Nakamura Y, Kayasuga R, and Kamei C (2004). Pruritus-associated response mediated by cutaneous histamine H3 receptors. Clin. Exp. Allergy 34, 456–459. [DOI] [PubMed] [Google Scholar]
- Umeda K, Noro Y, Murakami T, Tokime K, Sugisaki H, Yamanaka K, Kurokawa I, Kuno K, Tsutsui H, Nakanishi K, and Mizutani H (2006). A novel acoustic evaluation system of scratching in mouse dermatitis: rapid and specific detection of invisibly rapid scratch in an atopic dermatitis model mouse. Life Sci. 79, 2144–2150. [DOI] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, et al. (2015). Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci 18, 145–153. [DOI] [PubMed] [Google Scholar]
- Walsh CM, Hill RZ, Schwendinger-Schreck J, Deguine J, Brock EC, Kucirek N, Rifi Z, Wei J, Gronert K, Brem RB, et al. (2019). Neutrophils promote CXCR3-dependent itch in the development of atopic dermatitis. eLife 8, e48448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar E, Gieler U, Kupfer J, Furue M, Saeki H, and Yosipovitch G; International Forum on the Study of Itch (2012). Questionnaires to assess chronic itch: a consensus paper of the special interest group of the International Forum on the Study of Itch. Acta Derm. Venereol 92, 493–496. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, and Bautista DM (2013a). The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 155, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, and Bautista DM (2011). TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci 14 (5), 595–602. 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, Lumpkin EA, and Bautista DM (2013b). The ion channel TRPA1 is required for chronic itch. J. Neurosci 33, 9283–9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Kühn H, Boehm F, Gebhardt L, Glaudo M, Agelopoulos K, Ständer S, Ectors P, Zahn D, Riedel YK, et al. (2021). A group of cationic amphiphilic drugs activates MRGPRX2 and induces scratching behavior in mice. J. Allergy Clin. Immunol, S0091-6749(21)00229-3. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Nagasawa T, Satoh M, and Kuraishi Y (1999). Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci. Res 35, 77–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw trajectories for high-speed scratching videos are available at Mendeley Data: https://doi.org/10.17632/bws2s9t8nx.1. All other data reported in this paper will be shared by the lead contact upon request.
All original code is available in this paper’s supplemental information. This study utilized existing code for DeepLabCut, available here: https://github.com/DeepLabCut/DeepLabCut.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.