Abstract
From January 1995 to May 1998, 57 episodes of bacteremia due to viridans group streptococci were identified in 50 febrile neutropenic patients with hematologic malignancies. Four patients experienced two separate episodes of streptococcal bacteremia, and one patient had four separate episodes of streptococcal bacteremia. Strains were identified to species level as Streptococcus mitis (n = 37), Streptococcus oralis (n = 19), and Streptococcus salivarius (n = 1). Epidemiologic relatedness of these strains was studied by using PCR-based fingerprinting with M13 and ERIC-2 primers and pulsed-field gel electrophoresis with restriction enzyme SmaI. All strains that were isolated from different patients exhibited unique fingerprint patterns, thus suggesting that viridans group streptococcal bacteremia usually derives from an endogenous source. Cross-transmission of strains between patients could not be established. Four S. mitis isolates recovered during four separate bacteremic episodes in a single patient had identical fingerprint patterns. Susceptibility testing was carried out by broth microdilution technique according to National Committee for Clinical Laboratory Standards guidelines. The MICs at which 90% of the isolates are inhibited were (in milligrams per liter) as follows: 0.5 (penicillin), 0.5 (amoxicillin), 0.25 (cefotaxime), 2 (chloramphenicol), 4 (erythromycin), 0.5 (clindamycin), ≥32 (tetracycline), ≥32 (trimethoprim-sulfamethoxazole), 4 (ciprofloxacin), 0.5 (sparfloxacin), 0.5 (vancomycin), 0.25 (teicoplanin), and 1 (quinupristin-dalfopristin). High-level penicillin resistance (MIC, ≥4 mg/liter) was found in one isolate only, but intermediate penicillin resistance was noted in 11 isolates (19%). Resistance rates to other drugs were as follows: 7% (amoxicillin), 4% (cefotaxime), 4% (chloramphenicol), 32% (erythromycin), 9% (clindamycin), 39% (tetracycline), 68% (trimethoprim-sulfamethoxazole), 23% (ciprofloxacin), 0% (sparfloxacin), 0% (vancomycin), 0% (teicoplanin), and 0% (quinupristin-dalfopristin).
Viridans group streptococci, part of the normal human commensal flora, have emerged as important pathogens causing bacteremia and sepsis in neutropenic cancer patients (3–5, 7, 9, 12, 14). Clinical manifestations of viridans group streptococcal bacteremia may include fever, hypotension, shock, pneumonia, and adult respiratory distress syndrome, with a mortality rate ranging between 6 and 30% (6). The species most frequently isolated from blood cultures of neutropenic patients are Streptococcus mitis, Streptococcus oralis and Streptococcus sanguis (5, 8, 12, 28). The increased frequency of these infections has been attributed to several factors, including high-dose chemotherapy with cytosine arabinoside, oropharyngeal mucositis, and prophylactic therapy with cotrimoxazole or a fluoroquinolone (5, 11, 12, 17, 28, 30). Protective factors have included the early administration of parenteral antibiotics during febrile neutropenia and the prophylactic administration of penicillin (6). Oral and gastrointestinal mucosal lesions and intravascular catheters have been suggested as the most frequent portals of entry (5, 12, 14, 28).
In the past, viridans group streptococci were considered to be uniformly susceptible to β-lactam antimicrobial agents, macrolides, and tetracyclines. However, the emergence of strains resistant to β-lactams and other antibiotics is a cause of concern and could compromise currently used prophylactic and therapeutic antibiotic regimens (8, 10, 22, 25). In one recent study, after the introduction of penicillin prophylaxis, resistance to penicillin increased from 0% in 1989 to more than 80% in 1994 (19).
Viridans group streptococcal bacteremia in both immunocompetent and immunocompromised patients is usually considered to be of endogenous origin. There are, however, limited data based on modern molecular typing methods to support this hypothesis. The purpose of this study was to investigate the molecular epidemiology and antimicrobial susceptibilities of viridans group streptococci isolated from the blood of neutropenic patients with hematologic malignancies. The possible impact of patient-to-patient transmission of these organisms was studied by randomly amplified polymorphic DNA (RAPD) analysis and analysis of genomic DNA by pulsed-field gel electrophoresis (PFGE).
(This work was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998 [31].)
MATERIALS AND METHODS
Facility description.
The Cologne University Hospital is a 1,380-bed, tertiary-care teaching hospital which houses a 68-bed adult hematology-oncology unit as well as a 15-bed pediatric oncology unit. Annually, about 1,200 patients are admitted for treatment of hematologic malignancies. Trimethoprim-sulfamethoxazole plus colistin given orally is the routinely used prophylactic regimen in these patients. All microbiologic support for the hospital is managed at the Institute of Medical Microbiology and Hygiene.
Bacterial isolates.
A total of 57 viridans group streptococci isolated consecutively from blood cultures of neutropenic cancer patients from 1 January 1995 through 31 May 1998 were studied. Isolates were included if patients had one or more blood cultures positive for viridans group streptococci and met the Centers for Disease Control and Prevention definition for nosocomial bloodstream infection (13). Data from patients, which were retrospectively abstracted from the clinical records, included age, gender, and underlying malignancy.
Viridans group streptococci were identified to species level by using the Rapid ID 32 Strep system (Biomérieux, Marcy-L’Etoile, France), following the instructions of the manufacturer. The antimicrobial susceptibilities of the strains were initially determined by the disc diffusion technique according to National Committee for Clinical Laboratory Standards (NCCLS) recommendations (23). Isolates were stored at −70°C on porous beads (Microbank; Mast Diagnostics, Reinfeld, Germany) until further use.
Typing methods.
Epidemiologic relatedness of these strains was studied by using two molecular typing methods. RAPD-PCR analysis was performed with two different primers (M13 and ERIC-2) and Ready-to-Go RAPD Analysis Beads (Pharmacia Biotech, Freiburg, Germany) (15). These beads contain thermostable polymerases (AmpliTaq and Stoffel fragment), lyophilized buffer, deoxynucleoside triphosphates, and bovine serum albumin. An internal S. mitis control strain was run in four lanes per gel. Amplified fragments were separated by agarose gel electrophoresis and visualized under UV illumination after staining with ethidium bromide. Analysis of genomic DNA by PFGE was performed with the restriction enzyme SmaI as described previously (21). Fingerprint patterns were photographed and compared visually and by computer-aided analysis with Molecular Analyst Software (Bio-Rad Laboratories, Munich, Germany).
Susceptibility testing.
MICs were determined by the microbroth dilution method with a commercially manufactured plate (Micronaut-S; Merlin Diagnostics, Bornheim, Germany) and cation-adjusted Mueller-Hinton broth supplemented with lysed horse blood as recommended by the NCCLS (24). The following antimicrobial agents were included: penicillin, amoxicillin, cefotaxime, erythromycin, clindamycin, tetracycline, ciprofloxacin, sparfloxacin, vancomycin, teicoplanin, and quinupristin-dalfopristin. In addition, high-level gentamicin resistance was tested for at a concentration of 500 mg/liter. Plates were read after incubation at 35°C for 20 to 24 h in ambient atmosphere. The MIC was defined as the lowest concentration of drug that prevented visible growth. Susceptibilities to trimethoprim-sulfamethoxazole and chloramphenicol were tested with Etest (AB Biodisk, Solna, Sweden) on Mueller-Hinton agar supplemented with 5% sheep blood according to the recommendations of the manufacturer. Resistance rates were determined by using NCCLS breakpoints if applicable (24). Laboratory reference strains Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, and Streptococcus pneumoniae ATCC 49619 were used as controls.
RESULTS
Study population and patient characteristics.
From January 1995 through May 1998, 4,154 patients with hematologic malignancies were admitted to the hematology-oncology units of the Cologne University Hospital. Among 379 patients with documented bloodstream infection, 57 clinically significant episodes of bacteremia due to viridans group streptococci were identified in 47 adult and 3 pediatric patients, accounting for an attack rate of 13.7 per 1,000 admissions. Nineteen patients were female (38%). Patients had a mean age of 44 ± 18.5 years (range, 3 to 69 years). Underlying malignancies included acute myelogenous leukemia (57%), non-Hodgkin’s lymphoma (18%), acute lymphoblastic leukemia (15%), Hodgkin’s disease (5%), and multiple myeloma (5%). Four patients experienced two separate episodes of streptococcal bacteremia, and one patient had four separate episodes of streptococcal bacteremia during separate hospital stays. The possibility of an intravascular source of infection was ruled out in the latter patient, suggesting true recurrent bacteremia.
Species identification and epidemiological typing.
Among microorganisms isolated from blood cultures from neutropenic cancer patients during the study period, viridans group streptococci (accounting for 13.2% of all isolates) ranked third after coagulase-negative staphylococci (24.5% of isolates) and Escherichia coli (22.3% of isolates). Viridans group streptococci were further identified as S. mitis (n = 37), S. oralis (n = 19), and S. salivarius (n = 1).
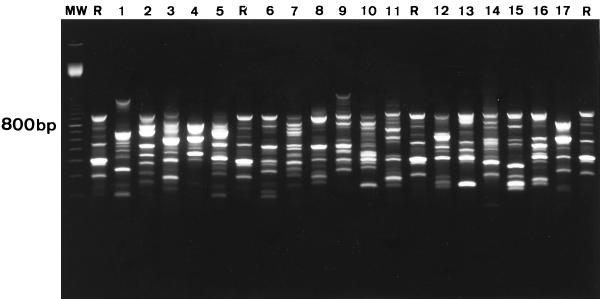
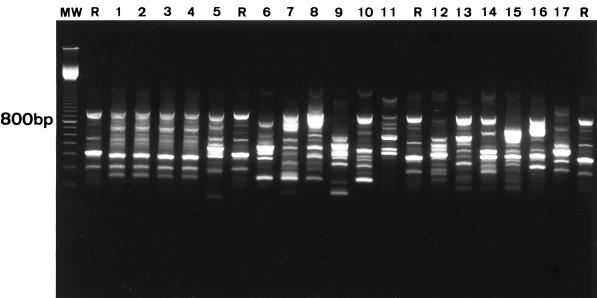
RAPD-PCR typing of the 19 S. oralis strains with both M13 and ERIC-2 primers resulted in 19 unique fingerprint patterns (Fig. 1). These included two S. oralis strains recovered from a patient who experienced two bacteremic episodes that occurred 3 months apart. All S. mitis strains that were isolated from different patients exhibited unique fingerprint patterns (Fig. 2). Different fingerprints were also found in two S. mitis strains isolated from a patient who experienced two bacteremic episodes that occurred 19 months apart. RAPD-PCR typing revealed identical fingerprint patterns in four S. mitis isolates recovered during four separate bacteremic episodes in a single patient (Fig. 2). Among two other patients with recurrent episodes of viridans group streptococcal bacteremia, the strains isolated were shown to represent different species (for both cases, one isolate was S. mitis, and the other was S. oralis; episodes occurred 2 and 7 months apart, respectively). DNA fingerprinting did not provide evidence of cross-transmission of strains between patients (Fig. 1 and 2). Typing results obtained by RAPD-PCR fingerprinting were all confirmed by PFGE, i.e., every strain that had a distinct RAPD-PCR profile also produced a unique PFGE pattern (data not shown).
FIG. 1.
Fingerprint patterns obtained for S. oralis blood culture isolates following PCR amplification with M13 primer. Lanes 1 and 2, isolates from patient A obtained from two separate bacteremic episodes showing different strain types; lanes 3 to 17, isolates obtained from different patients showing unique strain types; R, S. mitis internal reference strain; MW, 100-bp ladder.
FIG. 2.
Fingerprint patterns obtained for S. mitis blood culture isolates following PCR amplification with M13 primer. Lanes 1 to 4, isolates from patient B obtained from four separate bacteremic episodes showing identical strain types; lanes 5 and 6, isolates from patient C obtained from two separate bacteremic episodes showing different strain types; lanes 7 to 17, isolates obtained from different patients showing unique strain types; R, S. mitis internal reference strain; MW, 100-bp ladder.
Susceptibility testing.
The activities of the various antimicrobial agents are summarized in Table 1. Thirty of the 37 S. mitis strains (81%) were susceptible to penicillin, with a mean MIC of 0.25 mg/liter, as were 14 of the 19 S. oralis strains (74%; mean MIC, 0.32 mg/liter). High-level penicillin resistance (MIC of ≥4 mg/liter) was found in one isolate only (2%), an S. mitis strain, but intermediate penicillin resistance was noted in 11 isolates (19%; S. mitis, n = 6; S. oralis, n = 5). Similarly, low resistance rates to amoxicillin (7%) and cefotaxime (4%) were detected. Resistance rates were high, however, for erythromycin (32%), tetracycline (39%), ciprofloxacin (23%), and trimethoprim-sulfamethoxazole (68%). High-level gentamicin resistance was not detected. With the exception of tetracycline, with 26% of S. oralis versus 46% of S. mitis isolates being resistant, no significant differences in susceptibilities to antimicrobial agents were observed in the two predominant streptococcal species.
TABLE 1.
In vitro activities of selected antimicrobial agents against viridans group streptococci isolated from blood cultures of neutropenic cancer patients
| Antimicrobial agent | MIC range (mg/liter) | MIC50 (mg/liter)a | MIC90 (mg/liter)b | % Resistant (breakpoint)c |
|---|---|---|---|---|
| Penicillin | ≤0.016–4 | 0.03125 | 0.5 | 2 (≥4) |
| Amoxicillin | ≤0.016–≥4 | 0.03125 | 0.5 | 7 (≥2) |
| Cefotaxime | ≤0.016–2 | 0.03125 | 0.25 | 4 (≥2) |
| Chloramphenicold | 0.25–16 | 1.5 | 2 | 4 (≥16) |
| Erythromycin | ≤0.06–≥32 | ≤0.06 | 4 | 32 (≥1) |
| Clindamycin | ≤0.06–≥32 | ≤0.06 | 0.5 | 9 (≥1) |
| Tetracycline | 0.25–≥32 | 0.5 | ≥32 | 39 (≥8) |
| Cotrimoxazoled | ≤0.002–≥32 | 8 | ≥32 | 68 (≥4) |
| Ciprofloxacin | 0.25–4 | 2 | 4 | 23 (≥4) |
| Sparfloxacin | 0.125–1 | 0.25 | 0.5 | 0 (≥8) |
| Vancomycin | 0.125–0.5 | 0.5 | 0.5 | 0 (≥2) |
| Teicoplanin | 0.06–0.5 | 0.125 | 0.25 | |
| Quinupristin-dalfopristin | 0.5–2 | 1 | 1 | 0 (≥4) |
MIC50, MIC at which 50% of the isolates are inhibited.
MIC90, MIC at which 90% of the isolates are inhibited.
MIC breakpoints for resistance are those recently defined by NCCLS for susceptibility testing of viridans group streptococci to all agents except those for which no breakpoints have been established. For amoxicillin, cotrimoxazole, and sparfloxacin, the NCCLS resistance breakpoints for S. pneumoniae were applied. For ciprofloxacin, the NCCLS resistance breakpoint for nonfastidious organisms was applied; for quinupristin-dalfopristin, the resistance breakpoint suggested by the manufacturer for S. pneumoniae was used. No NCCLS-approved breakpoints are currently available for teicoplanin.
Susceptibility to antimicrobial agents was tested by Etest.
DISCUSSION
Over the past decades, viridans group streptococci have emerged as important nosocomial pathogens in neutropenic patients with hematologic malignancies. Recent studies revealed that these organisms were responsible for 9 to 30% of bacteremias in these highly compromised patients and may contribute substantially to morbidity and mortality (3, 5, 7, 8, 11, 12, 14, 17, 27). In the present study, viridans group streptococci were the third-most-common organisms isolated from 13% of positive blood cultures. The overall incidence of bloodstream infections due to viridans group streptococci (13.7 per 1,000 admissions) was comparable to those in previous reports, with incidences ranging from 1 to 33 per 1,000 admissions (8, 12, 14, 19, 28). S. mitis was recovered most frequently and accounted for 65% of the isolates, followed by S. oralis (33%). S. mitis represented the most frequently isolated species among viridans streptococci recovered from neutropenic patients in several studies (4, 8, 10, 12, 14, 28); however, other investigators detected S. oralis less frequently or not at all. Only Richard et al. reported similar findings, with S. mitis accounting for 60% and S. oralis accounting for 32% of viridans group streptococcal isolates (28). One possible explanation for this apparent discrepancy is that some commercial identification systems are less reliable in the identification of S. oralis, as pointed out by Jacobs et al. (16).
Viridans group streptococcal bacteremia is usually considered to derive from patients’ own gastrointestinal flora. Richard et al. recently showed that viridans streptococci with the same ribotype as the strain responsible for the bacteremia had been recovered before from the mouths of seven patients (28). These researchers concluded that the oral mucosa was the portal of entry for viridans group streptococci causing bacteremia in their neutropenic patients. Considering the increasing rates of resistance to antimicrobial agents among viridans group streptococci isolated from cancer patients (8, 10, 19, 22), nosocomial spread of these organisms could be a cause of concern. It is currently unknown, however, if person-to-person transmission of viridans streptococci does occur. We therefore compared the fingerprint patterns of viridans streptococcal blood isolates recovered consecutively over a period of 41 months from neutropenic patients in the hematology-oncology ward of Cologne University Hospital. PCR-based fingerprinting of viridans streptococci with RAPD produced reliable, discriminatory, and reproducible typing results; in fact, isolates recovered from different patients were all assigned different strain types. This finding supports the concept that bacteremia due to these organisms usually derives from an endogenous source. It also indicates that nosocomial transmission of viridans group streptococci in neutropenic cancer patients is probably rare. To adequately assess the impact of cross-transmission, a prospective study based on a comparison of viridans streptococcal isolates recovered from throat specimens or oral washings of patients at risk is required. Such a study, however, would require the epidemiologic typing of hundreds of isolates.
Recurring bloodstream infection with viridans group streptococci during separate neutropenic episodes was observed in five patients (10%). Not surprisingly, these bacteremias were usually caused by different streptococcal strains, even in a given patient. However, reinfection with the same strain may occur, as illustrated by a case in which one patient experienced four bacteremic episodes due to the same S. mitis strain during four consecutive episodes of neutropenia, each separated by a 4-week interval. To our knowledge, true reinfection with the same viridans streptococcal strain in neutropenic cancer patients had not been documented before.
The increasing resistance of viridans group streptococci to penicillin and other β-lactam antibiotics has been documented in neutropenic cancer patients (6, 10, 19, 22, 25). The incidence of penicillin-resistant viridans streptococcal bacteremia has been associated with previous administration of β-lactams and varies greatly between different institutions (3, 8, 19). In a recent study from Spain, Carratalá et al. reported that 57% of cases of viridans group streptococcal bacteremia in neutropenic cancer patients were caused by penicillin-resistant strains and that these were associated with a less-favorable outcome (8). Of these strains, 77% were highly resistant to penicillin (MIC of ≥4 mg/liter). Krcmery and Trupl observed a decrease in the incidence of viridans streptococcal bacteremia from 11.5 to 2.2% after penicillin was introduced into the prophylactic regimen in patients with acute leukemia (19). However, resistance to penicillin in these organisms increased from 0 to 83%.
In our study, high-level resistance to penicillin was observed in only one isolate, but 19% of viridans streptococcal isolates showed intermediate resistance (MIC, 0.25 to 2 mg/liter). Penicillin-resistant oral streptococci constitute the genetic reservoir for beta-lactam resistance in S. pneumoniae (20). Strains of S. mitis for which beta-lactam antibiotic MICs were unusually high (penicillin MIC, 64 mg/liter; cefotaxime MIC, 128 mg/liter) have recently been isolated in Germany (18). With chromosomal S. mitis DNA, the laboratory strain S. pneumoniae R6 could be transformed to cefotaxime and benzylpenicillin resistance of 64 mg/liter. However, whereas viridans group streptococci with reduced sensitivity to penicillin are common, penicillin resistance in S. pneumoniae is still extremely rare in Germany (27).
The resistance rates to erythromycin (32%), tetracycline (39%), and ciprofloxacin (23%) observed in our study are comparable to those in previous reports (2, 8, 10, 25, 26). Resistance to trimethoprim-sulfamethoxazole was 68% in our study and is probably related to the prophylactic use of trimethoprim-sulfamethoxazole plus colistin in a majority of our neutropenic patients. The prophylactic administration of trimethoprim-sulfamethoxazole has been identified as a risk factor predisposing neutropenic cancer patients to viridans group streptococcal bacteremia (9, 11, 12, 30). In the few studies reporting susceptibility data for this drug, resistance rates ranged between 16 and 76% (9, 10, 30). Excellent antimicrobial activity was shown for the glycopeptides vancomycin and teicoplanin as well as for quinupristin-dalfopristin, a new streptogramin antimicrobial agent. In contrast to data from Alcaide et al. (2), no difference in the activity of quinupristin-dalfopristin against strains that were susceptible or resistant to penicillin or erythromycin was observed.
Unlike other researchers (1, 10, 29), we found similar rates of antimicrobial resistance among S. mitis isolates and other viridans group streptococci. However, these data may be difficult to compare, since the majority of the remaining viridans streptococcal isolates in our study were S. oralis, whereas S. oralis strains were not included in other studies.
In summary, this study shows that patients with hematologic malignancies are at high risk for the development of nosocomial bloodstream infections due to viridans group streptococci, with S. mitis and S. oralis being recovered most frequently. Epidemiologic typing of these isolates substantiates the concept that bloodstream infection due to viridans group streptococci usually derives from an endogenous source rather than from patient-to-patient transmission. True reinfection with the same strain during repeated episodes of neutropenia may occur. High-level penicillin resistance among viridans streptococcal isolates recovered from blood cultures of neutropenic patients was still rare. However, a high incidence of isolates with intermediate resistance to penicillin was observed. Resistance was also high to erythromycin, tetracycline, ciprofloxacin and, in particular, trimethoprim-sulfamethoxazole.
ACKNOWLEDGMENTS
This work was supported by a grant from Köln Fortune Program, Faculty of Medicine, University of Cologne.
We thank M. Edmond for critical review of the manuscript and D. Stefanik for excellent technical assistance.
REFERENCES
- 1.Alcaide F, Linares J, Pallares R, Carratalá J, Benitez M A, Gudiol F, Martin R. In vitro activities of 22 β-lactam antibiotics against penicillin-resistant and penicillin-susceptible viridans group streptococci isolated from blood. Antimicrob Agents Chemother. 1995;39:2243–2247. doi: 10.1128/aac.39.10.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcaide F, Carratalá J, Linares J, Gudiol F, Martin R. In vitro activities of eight macrolide antibiotics and RP-59500 (quinupristin-dalfopristin) against viridans group streptococci isolated from blood of neutropenic cancer patients. Antimicrob Agents Chemother. 1996;40:2117–2120. doi: 10.1128/aac.40.9.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awada G, van der Auwera P, Meunier F, Daneau D, Klastersky J. Streptococcal and enterococcal bacteremia in patients with cancer. Clin Infect Dis. 1992;15:33–48. doi: 10.1093/clinids/15.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Bilgrami S, Feingold J M, Dorsky D, Edwards R L, Clive J, Tutschka P J. Streptococcus viridans bacteremia following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1998;21:591–595. doi: 10.1038/sj.bmt.1701140. [DOI] [PubMed] [Google Scholar]
- 5.Bochud P-Y, Eggiman P, Calandra T, Van Melle G, Saghafi L, Francioli P. Bacteremia due to viridans Streptococcus in neutropenic patients with cancer: clinical spectrum and risk factors. Clin Infect Dis. 1994;18:25–31. doi: 10.1093/clinids/18.1.25. [DOI] [PubMed] [Google Scholar]
- 6.Bochud P-Y, Cometta A, Francioli P. Virulent infections caused by alpha-haemolytic streptococci in cancer patients and their management. Curr Opin Infect Dis. 1997;10:422–430. [Google Scholar]
- 7.Burden A D, Oppenheim B A, Crowther D, Howell A, Morgenstern G R, Scarffe J H, Thatcher N. Viridans streptococcal bacteremia in patients with haematological and solid malignancies. Eur J Cancer. 1991;27:409–411. doi: 10.1016/0277-5379(91)90373-l. [DOI] [PubMed] [Google Scholar]
- 8.Carratalá J, Alcaide F, Fernández-Sevilla A, Corbella X, Linares J, Gudiol F. Bacteremia due to viridans streptococci that are highly resistant to penicillin: increase among neutropenic patients with cancer. Clin Infect Dis. 1995;20:1169–1173. doi: 10.1093/clinids/20.5.1169. [DOI] [PubMed] [Google Scholar]
- 9.Cohen J, Donnelly J P, Warsley A M, Catovsky D, Goldman J M, Galton D A. Septicemia caused by viridans streptococci in neutropenic patients with leukaemia. Lancet. 1983;ii:1452–1454. doi: 10.1016/s0140-6736(83)90799-7. [DOI] [PubMed] [Google Scholar]
- 10.Doern G V, Ferraro M J, Brueggemann A B, Ruoff K L. Emergence of high rates of antimicrobial resistance among viridans group streptococci in the United States. Antimicrob Agents Chemother. 1996;40:891–894. doi: 10.1128/aac.40.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donelly J P, Dompeling E C, Meis J F, De Pauw B E. Bacteremia due to oral viridans streptococci in neutropenic patients with cancer: cytostatics are a more important risk factor than antibacterial prophylaxis. Clin Infect Dis. 1995;20:469–470. doi: 10.1093/clinids/20.2.469. [DOI] [PubMed] [Google Scholar]
- 12.Elting L S, Bodey G P, Keefe B H. Septicemia and shock syndrome due to viridans streptococci: a case-control study of predisposing factors. Clin Infect Dis. 1992;14:1201–1207. doi: 10.1093/clinids/14.6.1201. [DOI] [PubMed] [Google Scholar]
- 13.Garner J S, Jarvis W R, Emori T G, Horan T C, Hughes J M. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 14.González-Barca E, Fernández-Sevilla A, Carratalá J, Granena A, Gudiol F. Prospective study of 288 episodes of bacteremia in neutropenic cancer patients in a single institution. Eur J Clin Microbiol Infect Dis. 1996;15:291–296. doi: 10.1007/BF01695660. [DOI] [PubMed] [Google Scholar]
- 15.Grundmann H J, Towner K J, Dijkshoorn L, Gerner-Smidt P, Maher M, Seifert H, Vaneechoutte M. Multicenter study using standardized protocols and reagents for evaluation of PCR-fingerprinting reproducibility with Acinetobacter spp. J Clin Microbiol. 1997;35:3071–3077. doi: 10.1128/jcm.35.12.3071-3077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs J A, Schouten H C, Stobberingh E E, Soeters P B. Viridans streptococci isolated from the bloodstream. Diagn Microbiol Infect Dis. 1995;22:267–273. doi: 10.1016/0732-8893(95)00137-y. [DOI] [PubMed] [Google Scholar]
- 17.Kern W, Kurrle E, Schmeiser T. Streptococcal bacteremia in adult patients with leukemia undergoing aggressive chemotherapy: a review of 55 cases. Infection. 1990;18:138–145. doi: 10.1007/BF01642101. [DOI] [PubMed] [Google Scholar]
- 18.König A, Reinert R R, Hakenbeck R. Streptococcus mitis with unusually high level resistance to beta-lactam antibiotics. Microb Drug Resist. 1998;4:45–49. doi: 10.1089/mdr.1998.4.45. [DOI] [PubMed] [Google Scholar]
- 19.Krcmery V, Trupl J. Bacteremia due to penicillin-resistant Streptococcus viridans in cancer patients, before and after the prophylaxis with penicillin. Lancet. 1995;346:1362–1363. doi: 10.1016/s0140-6736(95)92374-8. [DOI] [PubMed] [Google Scholar]
- 20.Laible G, Spratt B G, Hakenbeck R. Interspecies recombinational events during the evolution of altered PBP 2x genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol Microbiol. 1991;5:1993–2002. doi: 10.1111/j.1365-2958.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 21.Maslow J N, Slutsky A M, Arbeit R D. Application of pulsed-field gel electrophoresis to molecular epidemiology. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology. Washington, D.C: American Society for Microbiology; 1993. pp. 563–572. [Google Scholar]
- 22.McWhinney P H, Patel S, Whiley R A, Herdie J M, Gillespie S H, Kibbler C C. Activities of potential therapeutic and prophylactic antibiotics against blood culture isolates of viridans streptococci from neutropenic patients receiving ciprofloxacin. Antimicrob Agents Chemother. 1993;37:2493–2495. doi: 10.1128/aac.37.11.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Performance standard for antimicrobial susceptibility disk susceptibility tests, 6th ed. Approved standard. NCCLS publication no. M2-A6. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard. NCCLS publication no. M7-A4. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 25.Pfaller M A, Jones R A, Marshall S A, Edmond M B, Wenzel R P. Nosocomial streptococcal blood stream infections in the SCOPE Program: species occurrence and antimicrobial resistance. The Scope Hospital Study Group. Diagn Microbiol Infect Dis. 1997;4:259–263. doi: 10.1016/s0732-8893(97)00159-4. [DOI] [PubMed] [Google Scholar]
- 26.Potgieter E, Carmichael M, Koornhof H J, Chalkley L J. In vitro antimicrobial susceptibility of viridans streptococci isolated from blood cultures. Eur J Clin Microbiol Infect Dis. 1992;11:543–546. doi: 10.1007/BF01960811. [DOI] [PubMed] [Google Scholar]
- 27.Reinert R R, Queck A, Kaufhold A, Kresken M, Lütticken R. Antimicrobial resistance and type distribution of Streptococcus pneumoniae in Germany, 1992–1994. Clin Infect Dis. 1995;21:1398–1401. doi: 10.1093/clinids/21.6.1398. [DOI] [PubMed] [Google Scholar]
- 28.Richard R, Del Valle G A, Moreau P, Milpied N, Felice M-P, Daeschler T, Harousseau J L, Richet H. Viridans streptococcal bacteremia in patients with neutropenia. Lancet. 1995;345:1607–1609. doi: 10.1016/s0140-6736(95)90117-5. [DOI] [PubMed] [Google Scholar]
- 29.Venditti M, Baiocchi P, Santini C, Brandimarte C, Serra P, Gentile G, Girmenia C, Martino P. Antimicrobial susceptibilities of Streptococcus species that cause septicemia in neutropenic patients. Antimicrob Agents Chemother. 1989;33:580–582. doi: 10.1128/aac.33.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisman S J, Scoopo F J, Johnson G M, Altman A J, Quinn J J. Septicemia in pediatric oncology patients: the significance of viridans streptococcal infections. J Clin Oncol. 1990;8:453–459. doi: 10.1200/JCO.1990.8.3.453. [DOI] [PubMed] [Google Scholar]
- 31.Wisplinghoff H, Reinert R R, Seifert H. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Molecular epidemiology and antimicrobial susceptibility of viridans group streptococci isolated from neutropenic cancer patients, abstr. K-102; p. 531. [Google Scholar]