Abstract
An estimated 25 million tons of animal manure is produced globally every year, causing considerable impact to the environment. These impacts can be managed through the use of anaerobic digestion (AD) This process achieves waste degradation through enzymatic activity, the efficiency of the AD process is directly related to microorganisms that produce these enzymes. Biomethane potential (BMP) assays remain the standard theoretical framework to pre-determine biogas yield and have been used to determine the feasibility of substrates or their combination for biogas production. However, an integrated approach that combines substrate choice and co-digestion would provide an improvement to the current predictive models. PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) addresses the limitations of assays in this regard. In this paper, the biochemical functions of horse, cow, and pig manures are predicted. A total of 135 predicted KEGG Orthologies (KOs) showed amino acids, carbohydrate, energy, lipid, and xenobiotic metabolisms in all the samples. Linear discriminant analysis (LDA) combined with the effect size measurements (LEfSe), showed that fructose, mannose, amino acid and nucleotide sugar, phosphotransferase (PST) as well as starch and sucrose metabolisms were significantly higher in horse manure samples. 36 of the KOs were related to the acidogenesis and/or acetogenesis AD stages. Extended bar plots showed that 11 significant predictions were observed for horse-cow, while 5 were predicted for horse-pig and for cow-pig manures. Based on these predictions, the AD process can be enhanced through co-digestion strategies that takes into account the predicted metabolic contributions of the manure samples. The results supported the BMP calculations for the samples in this study. Biogas yields can be improved if this combined approach is employed in routine analysis before co-digesting different substrates.
Subject terms: Biochemistry, Computational biology and bioinformatics
Introduction
Agricultural processes as well as other industrial activities are synonymous with the generation of significant waste. The uncontrolled degradation of these wastes is harmful to the local ecological system1. The agricultural animal husbandry sector further generates considerable quantities of animal waste2. There is evidence that the uncontrolled emission of biomethane and other greenhouse gases3 contribute significantly to the environmental impact of agricultural activities. Moreover, activities in this sector also have considerable demand for potable water4 and require vast areas of land for grazing and waste disposal5. These wastes may sometimes become repositories for zoonotic infections6. Additionally, the high concentrations of nitrogen and phosphorus in the wastes tend to leach into waterbodies exacerbating the environmental consequences through eutrophication and algal blooms, rendering the surrounding waterbodies unfit for domestic use7, 8. Yet the demand for meat products and the economic viability of the meat industries implies that commercial livestock rearing, and meat production will remain aspects of our day-to-day living. Thus, realistic and feasible solutions to waste management should integrate environmental mitigation strategies that routinely utilise the organic wastes generated from the processes to address the waste-to-energy conundrum9–14. This is especially essential considering that globally, pollution from animal manure is estimated at 25 million tons, annually15. The degradation products of animal waste have potential in the energy market, a potential which is currently not being fully harnessed.
The anaerobic digestion (AD) of animal manure to produce biogas has the added advantage of not only reducing organic load but it also decreases microbial load and infectious aerobic organisms as well as associated odors16, 17. These outcomes are achieved through the sequential enzymatic degradation facilitated by rumen derived microorganisms autochthonous to animal manure. These organisms produce enzymes capable of cellulolytic, amylolytic, proteolytic and methanogenic activities. However, in the broad categorization of anaerobic successional processes towards the production of methane biogas, the following sets of biochemical reactions occur, commencing with hydrolysis, which is the degradation of complex macromolecules (carbohydrates, fats and proteins), through the breaking of chemical bonds present to produce monomeric units that are degradable by microorganisms18, 19. The microorganisms that have been identified to be responsible for hydrolytic reactions include Firmicutes sp., Fibrobacter sp. and Spirochaetes sp.20. The essence of these biochemical reactions is to produce monomers thereby making them biologically available for use as substrate in the second set of biochemical reactions achieved through oxidation and referred to as acidogenesis, which is performed by organisms such as Clostridium sp. and Butyribacterium sp.21 with the intermediate products being volatile fatty acids22. This is followed by acetogenesis and the responsible organisms include Syntrophomonas sp. and Syntrophobacter sp.23. This process converts the acids to acetate and H2, which are the requisite metabolites (substrates) for the last step, methanogenesis, where organisms such as Methanoculleus sp., Methanobacterium sp., Methanobrevibacter sp. and Methanosarcina sp.24, produce methane and CO225.
It should be noted that each step within these sequential degradation processes is dependent on the presence of the responsible microorganisms and the consequential production of the enzymes at concentrations adequate to achieve the intermediate products for the forward chemical reactions and metabolite production. If the ratios of these enzymes (microorganisms) are reduced within the microbial consortia owing to various exogenous factors, such as, feed nutrient composition (sometimes due to seasonal variations), environmental temperature and pH changes, organic load derived toxicity within the digester, etc., such changes will affect the outcome of the anaerobic digestion process and may reduce the final methane yield26. Moreover, other factors such as, species of the livestock and the variable concentrations of nutrients in the livestock feed are known to influence the gut microorganisms and the final consortia composition within the animal manure27. Even with the same animal, slight changes in environmental conditions, may routinely vary the microbial consortia found in the animal manure. As such, a clear understanding of evolving microbial diversity provided for in functional metagenomic analysis is important towards elucidating the critical biochemical roles played by the various digesting organisms when the intention of fermentation is to maximize biogas yield from a given substrate28.
The overarching objective for commercial fermentation processes is to develop strategies that ensure increased product yield. Some of the critical aspects in biogas production optimisation metrics are process stability, substrate degradation efficiency and gas production kinetics29. To improve biodigester performance, co-digestion is a widely accepted optimisation strategy. Typically, co-digestion improves anaerobic digestion optimisation metrics by dilution of inhibitors to levels below minimum inhibitory concentrations (MICs) and/or improves nutritional balances within the substrate, such as the important carbon:nitrogen:phosphorous (C:N:P) ratio necessary to increase microbial proliferation30, 31. Pertinent to the fermentation strategy is the prior determination of maximum biogas yield possible from a given amount of mono substrate or mixture (co-digestion) in a near ideal batch fermentation, which can be estimated using the biomethane potential (BMP) laboratory test/assay32. Although, BMP assay may not often translate similar outcomes in scaled up semi-continuous (fed-batch) industrial operations where conditions may not be optimal, it remains the standard theoretical framework used in decision making in biogas production33, 34. There are other trial and error approaches that have been employed to estimate potential yield, which often rely only on information primarily related to substrates’ nutritional contents and presence of inhibitors for deducing initial experimental ratios31. Several of these prediction approaches are considered cheap and provide quick but inaccurate forecasts as compared to BMP. Current theoretical methods for predicting biogas yield do not generally consider stability, degradability, and kinetics, despite the strong influence of microbial activity as well as the associated inhibitory or stimulatory effects to the process. Furthermore, these methods tend not to consider the presence of recalcitrant organics (lignin)35.
Understanding that the anaerobic digestion process is facilitated by microbial consortia should therefore direct predictive methods and be integrated into decision making on substrate choices and co-digestion strategies. For example, Matheri et al.36 through their experiments showed that chicken manure may assist in stabilizing the abiotics of co-digestion with organic matter from municipal sewage. During co-digestion of swine and cow manures at 28.5 ± 2.5 °C and pH 6.80 ± 0.55, Ogunwande et al.37 showed that a ratio of 3:7 respectively had increased biogas yield by 61.5% after 32 days of reaction. These degradation reactions involving microorganisms are executed through a series of interconnected biochemical pathways. Thus, using biochemical pathway predictions, and by reviewing the contributions made by enzymes in the different phases of anaerobic digestion, it is possible to estimate the potential biochemical function exerted by individual groups of microorganisms within consortia38, such information is provided by the qualitative and quantitative analysis of functional and active genes in the metagenome of microbial consortia.
The application of bioinformatics tools such as PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) is very promising as an aid to metagenomic analysis of functional genes from various environmental microbiota39. Recently, Wilkinson et al.40 used PICRUSt to analyze the biodiversity of the rumen microbiome. In a review by Denman et al.41, the use of PICRUSt in the study of rumen microbiome was discussed as a possible strategy for maximizing feeding efficiencies and even lowering methane emissions. Moreover, Lourenco et al.42 used PICRUSt for a comparison of the microbiota of the beef cattle both ruminal and fecal. Several authors43–46 have specifically focused on its application in biogas processes.
This investigation especially chose the 16S targeted approach for sequencing and obtaining the metagenomic data of bacteria consortia present in the different manure samples to reduce cost with the intention of proffering this approach as routine application for predictive analysis of manure for the purpose of biogas yield potential predictions. It is also noteworthy that the range of bacteria biodiversity is adequately covered using the 16S targeted approach. Although, this approach does not address the archaea population, sufficient information provided from the metagenomic data generated can be used to infer end-product (methane) production, since the metabolites derive in the three preceding stages of anaerobic digestion is what predicts methane production by the slow growing archaea population in AD processes. The present study takes a step further by comparing actual metagenomic data from various animal manures using PICRUSt to predict the possibility of co-digesting substrates for biogas production towards increasing yield. Although, metagenomic analysis provides a comprehensive presentation of microbial biodiversity, it is important to further analyze this information using other bioinformatics statistical tools and a knowledge of the anaerobic process involved in biogas production to make credible inferences. This study intends to present the potential application of PICRUSt in the routine analysis of varied samples of animal manure and to highlight the possibilities of using similar methods for other substrates for increased biogas yield. Such information can be incorporated into BMP models or be included as ancillary correlation to improve predictions. It is hoped that the routine integration of PICRUSt and other genomic functional prediction tools, into yield predictions and consequently process optimization will not only address the short comings of both traditional theoretical BMP prediction models but also provide credible and more accurate information necessary for sound decision making in commercial AD processes. Moreover, this approach may be extended in its application for AD process prediction for sterilized or pre-conditioned manures where the addition of manure is solely for the purpose of providing nutrients rather than as inoculum, where the likelihood of biochemical reactions from one of the manures is considered a possible interference. Overall, this study motivates for the use of bioinformatics prediction tools in routine commercial fermentation processes and its optimization where consortia organisms, functional genes and their enzymes are involved.
Methodology
Feedstock characterization and biomethane potentials
The three different types of manure samples were characterised for dry matter (TS) and volatile solids (VS) according to the American Public Health Association (APHA) standard methods for water and wastewater analysis47. An ultimate analysis of these manures was also carried out according to the EN15104:2011 standard. Characterisation results were then used to estimate biomethane potentials (BMP) of these substrates according to the methods cited and applied by Rashama et al.48. These theoretical BMPs were then compared to experimental values reported by other researchers in literature and discussed in the context of manure codigestion strategies.
Molecular characterisation and sequencing of microbiome from manure samples
Horse, cow, and pig manure samples were collected from different farms as shown in Table 1. All samples were collected in the summer season (February 2020) within an interval of three days and processed for analysis as a batch. The study ensured biological replicates by collecting samples from varied locations. Collected samples were put in sterile plastics and stored in a portable icebox in which they were then transferred to the laboratory for physico-chemical and microbiome analyses. Samples were maintained under refrigeration at 4 °C, not longer than 3 days before analysis.
Table 1.
Animal manure samples and sources.
| Sample | Sources | Sample ID | Location | Description of location and handling of livestock |
|---|---|---|---|---|
| Cow dung | Boerdery farm produce | C1 | − 26.21779, 27.6414784 | A farm producing animal feeds as well as rearing cattle fed on grains and concentrates in Randfontein, Gauteng |
| Kates farm | C2 | − 26.0990952, 27.9103166 | A bed and breakfast farm facility in the outskirts of Johannesburg, Gauteng serving beef and chicken from livestock locally reared in a free-range feeding scheme | |
| Bosheuvel country estates | C3 | − 26.0249182, 27.8929324 | A farm in the Muldersdrift area of Johannesburg with an on-site vintage hotel, restaurant, event venues and conference rooms serving locally reared Pinzgauer cattle, pigs, chickens, and sheep fed mostly on mixed vegetables and tubbers | |
| Horse manure | Earth centre | H1 | − 26.080926, 27.8747353 | A non-profit company situated in Ruimsig, Gauteng specialising in Equine Assisted Programmes for children with disabilities.The horses are fed with a wide range of feed concentrates and probiotics |
| Harveston stables | H2 | − 26.0990952, 27.9103167 | A yard in Honeydew, Gauteng offering horse riding lessons, stabling, kids pony parties, picnics and pony rides. The horses are fed mainly on feed concentrates | |
| Barent horse stables | H3 | − 26.21779, 27.6414783 | A plot rearing horses for family leisure in Randfontein, Gauteng. The horses are fed mostly on lucerne | |
| Pig manure | Bosheuvel country estates | P1 | − 26.0249182, 27.8929324 | A farm in the Muldersdrift area of Johannesburg with an on-site vintage hotel, restaurant, event venues and conference rooms serving locally reared Pinzgauer cattle, pigs, chickens, and sheep fed mostly on mixed vegetables and tubbers |
| Bosheuvel country estates | P2 | − 26.0249182, 27.8929324 | ||
| Country portion farm | P3 | − 26.224517, 27.6325603 | A farm in Randfontein producing vegetables, poultry, and pork products to supply local restaurants and consumers. The poultry and pigs are fed on corn and sorghum and concentrates |
Genomic DNA was extracted from the manure samples using the DNeasy PowerSoil kit (QIAGEN, Germany), following the manufacturer’s instructions. These samples were analysed in triplicate. Amplification of genomic DNA extracts employed polymerase chain reaction (PCR) using the universal bacterial primers, 27 F (5′-AGAGTTTGATCMTGGC-3′) and 518 R (5′-GTATTACCGCGGCTGCTGG-3′) targeting the conserved bacterial 16S rRNA gene as described by Selvarajan et al.49.
PCR products purification was achieved using DNA Clean & Concentrator Kit (ZYMO RESEARCH, Irvin, USA). Prior to the library preparation and sequencing process, the triplicate samples from individual locations were pooled together before Illumina sequencing adapters and dual-index barcodes were added to the amplicon targets using full complement of Nextera XT indices (Illumina, Inc. San Diego, CA, USA) followed by an 8-cycle PCR run (95 °C for 3 min, 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, with a final extension at 72 °C for 5 min, then cooling at 4 °C). Further cleaning of PCR products utilised AMPure XP beads. A bioanalyzer DNA 1000 chip (Agilent, Santa Clara, CA, USA) was then used to validate the fragments size (~ 630 bp) before quantifying them in a fluorometric quantification method (Qubit, USA) integrating dsDNA binding dyes for visualization. Dilutions were achieved on the quantified DNA using 10 mM Tris Buffer (pH 8.5). 5 μl of diluted DNA was aliquoted from each library and mixed for pooling libraries. The pooled final DNA library (4 nM) was denatured and sequenced on an Illumina Miseq System. Sequencing employed paired 300-bp reads to generate high-quality, full-length reads of the V3 and V4 regions. Raw fastq sequence files were then obtained after trimming the adapters and primer sequences. Sequences derived were subjected to bioinformatics analyses.
Bioinformatics analyses
The fastq sequence files from the Miseq were inspected for quality using the FastQC software (v 0.11.7, Babraham Bioinformatics, United Kingdom). Using PANDAseq.37 on the QIIME2 platform, the forward and reverse reads were merged and then clustered into operational taxonomic units (OTUs) at a sequence similarity of 97% for species level identification using the ‘pick_open.reference_otus.py’ script in QIIME, while aligning against the SILVA rRNA (release 132) database38 by using usearch61 and PyNAST aligner. The OTU table generated was exported to the R-studio for further statistical analyses. R packages: vegan, ape, labdsv and ggplot were installed and used for statistical analyses and plotting50.
Taxonomic identification and prediction of functional gene content
To assess the metabolic potential of microbial communities found in the three different animal manures, 16S rRNA sequence reads were clustered into operational taxonomic units (OTUs) using the closed-reference method in QIIME2 software. The generated OTU table was imported into the PICRUSt and the Kyoto Encyclopaedia of Genes and Genomes (KEGG) database was used to predict the functional gene content of the various microbial communities represented in the Greengenes database of 16S rRNA gene sequences51.
The output from KEGG database containing the predicted function was further analysed using Linear discriminant analysis (LDA) combined with linear discriminant analysis effect size (LEfSe). The purpose of this was to identify the most differentially abundant predictions among three different animal manure samples. LEfSe was used online in the Galaxy workflow framework. The predicted functions output from KEGG was also exported to the R-studio for further statistical analysis. The R packages vegan, ape, heatmap.plus, ggplot2 and readxl were installed and used for statistical analysis as well as plotting. STAMP version 2.1.3 was also used as a graphical tool.
Results
Manure characterisation and biomethane potential calculations
The characterisation and BMP results for the three (3) types of manures considered in this study are reported in Table 2 with calculations described in Supplementary Data Table S1. For each manure, three theoretical BMP calculation approaches were used, and two results based on previous laboratory assays were also reported for each substrate.
Table 2.
Substrate physicochemical properties and biomethane potentials.
| Parameter (units) | Cow dung | Pig manure | Horse manure |
|---|---|---|---|
| TS (% of wet mass) | 14.5 (1.7) | 20.4 (2.4) | 21.8 (0.8) |
| VS (% of TS) | 88.7 (2.1) | 82.8 (2.8) | 86.8 (2.4) |
| C (% of TS) | 25.7 (8.3) | 27.2 (6) | 30.6 (5.7) |
| H (% of TS) | 3.8 (0.8) | 4.1 (0.4) | 4.2 (0.4) |
| N (% of TS) | 4.9 (1.6) | 2.1 (1) | 1.5 (0.4) |
| S (% of TS) | 0.2 (0.1) | 0.2 (0.0) | 0.2 (0.1) |
| O (% of TS) | 54.0 (7.8) | 49.2 (8.4) | 50.3 (6.7) |
| Empirical formula | C307H547O404N50S | C330H590N22O447S | C396H649N17O488S |
| ThOD (gO2/gVS) | 0.41 | 0.63 | 0.72 |
| HHV (MJ/t) | 4626.8 | 6287.4 | 7393.0 |
| BMPF (ml CH4/gVS) | 144.8 | 221.6 | 251.4 |
| BMPB (ml CH4/gVS) | 144.0 | 220.6 | 250.4 |
| BMPD (ml CH4/gVS) | 122.5 | 166.4 | 195.7 |
| BMPL1 (ml CH4/gVS) | 204 (12) | 155 (2) | 323 (13) |
| BMPL2 (NL CH4/kgVS) | 10.44 | 14.5 | 17.09 |
Taxonomic identification
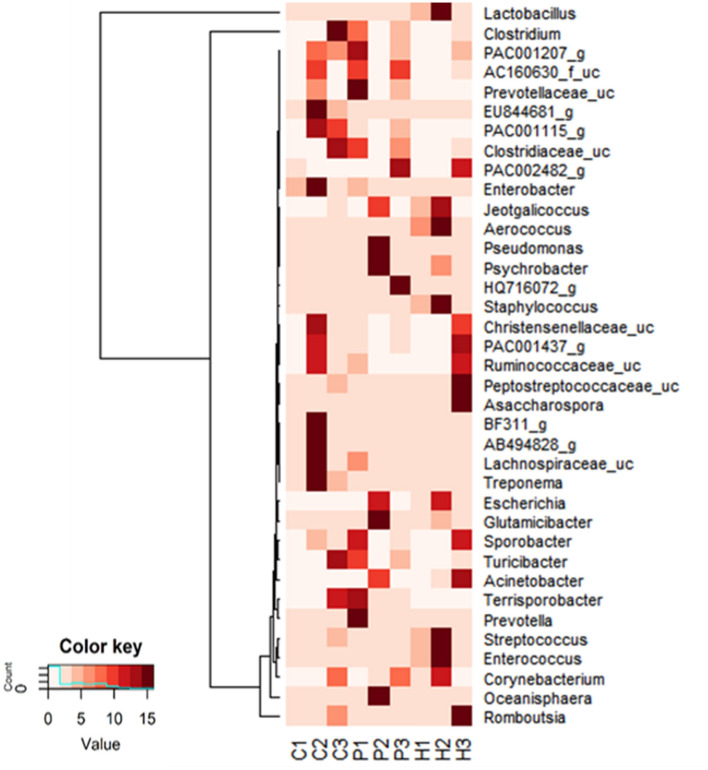
Genus level diversity analyses in manure samples were performed with quality checks implemented on the Silva rRNA gene database at the cut-off level of 97% similarities. The metagenomic qualitative and quantitative analyses of samples have been included in the supplementary data Table S2. The prevalence and relative abundance of the genera in all 9 samples of manure is shown in Fig. 1 as represented with a heat map of bacterial genus distribution and prevalence. Bacteria population distribution showed similarities for some of the different samples, however at varied abundance, it is also showed the presence of genera that were unique to only one manure type but not found in other samples, the specific distribution and predicted metabolic contributions of each of the relevant genera to biogas production are described in Table 3.
Figure 1.
Heat map showing the relative abundance of microorganisms in different animal manures.
Table 3.
Identified genera in animal manure samples and their predicted metabolic contributions to biogas production.
| Genus | Relative abundance in animal manure | Predicted metabolic contribution | References |
|---|---|---|---|
| Bacteria genera in consortia likely playing roles in pathways related to growth and the synthesis and degradation of growth metabolites in manure | |||
| Escherichia | Horse < cow < pig | Facultatively anaerobic with wide spectrum capability for several organic carbon sources necessary for heterotrophic growth, also known to be involved in acidogenesis. Mostly harmless but some species and strains are pathogenic | 57, 58 |
| Glutamicibacter | Horse < cow < pig | Members of the genus are involved in lignocellulosic material saccharification, amino acid metabolism | 59, 60 |
| Psychrobacter | Pig < Horse < cow |
Psychrobacter spp. are capable of producing cold-active enzymes with involvement in the physiological strategies that off-set low temperature effects on cellular ATP and ADP generation, a key requirement in metabolic and energy conservation reactions The KEGG pathway shows involvement in riboflavin and tryptophan metabolism (see P. cryohalolentis and P. articus) |
61 |
| Aerococcus | Horse < cow < pig |
Members of the genus produce acids from a variety of carbohydrates and are directly involved in acidogenesis The KEGG pathway shows involvement of A. urinae in anaerobic energy and selenocompound metabolism. Some species are considered pathogenic |
62–64 |
| Oceanisphaera | Pig | The KEGG pathway shows involvement of Oceanisphaera profunda in selenocompounds, arachidonic and 2-oxocarboxylic acid metabolism | 65 |
| Turicibacter | Horse = pig = cow |
Members of the genus have been identified as being involved in fat metabolism e.g. T. sanguinis, might be important for host lipid and steroid metabolism The KEGG pathway shows the involvement of Turicibacter sp. H121 in Tryptophan metabolism and T. sanguinis involvement in carbohydrate, amino acid, lipid and nucleic acid metabolism |
66–68 |
| Romboutsia | Pig < cow = horse | Members of the genus have a broad range of capabilities in carbohydrate utilisation but not necessarily cellulose and xylose, fermentation of single amino acids, anaerobic respiration and metabolic end products. Although, there are variations in these abilities with different strains in the manner in which they utilise carbohydrates to synthesize vitamins and nitrogen as well as nitrogen assimilation capabilities | 69, 70 |
| Bacteria genera in consortia likely playing roles in pathways related to complex lignocellulose degradation and represented within other hydrocarbon pathways | |||
| Glutamicibacter | Horse < cow < pig | Members of the genus are involved in lignocellulosic material saccharification, amino acid metabolism | 59, 71 |
| Jaetgalicoccus | Horse < pig < cow | Members of the genus are capable of producing terminal alkenes inferring its production of functional enzymes in complex hydrocarbon degradation. It produces enzymes involved the one-step fatty acid decarboxylation reaction employing OleTJE cytochrome P450. KEGG pathway describes thiamine metabolism in Jeotgalicoccus sp. ATCC 8456 | 72, 73 |
| Bacteria genera in consortia likely playing roles in pathways with direct relation to acidogenesis and acetogenesis | |||
| Escherichia | Horse < cow < pig | Facultatively anaerobic with wide spectrum capability for several organic carbon sources necessary for heterotrophic growth, also known to be involved in acidogenesis. Mostly harmless but some species and strains are pathogenic | 57, 74 |
| Jaetgalicoccus | Horse < pig < cow | Members of the genus are capable of producing terminal alkenes inferring its production of functional enzymes in complex hydrocarbon degradation. It produces enzymes involved the one-step fatty acid decarboxylation reaction employing OleTJE cytochrome P450. KEGG pathway describes thiamine metabolism in Jeotgalicoccus sp. ATCC 8456 | 72, 73 |
| Aerococcus | Horse < cow < pig |
Members of the genus produce acids from a variety of carbohydrates and are directly involved in acidogenesis The KEGG pathway shows involvement of A. urinae in anaerobic energy and selenocompound metabolism. Some species are considered pathogenic |
62–64 |
| Enterococcus | Horse < pig < cow | Members of the genus employ fermentative metabolism for the conversion of a variety of carbohydrates to lactic acid. They are strict anaerobes as they lack apparatus for implementing Kreb’s cycle reactions. However, they utilise each of the three possible routes of intermediary carbohydrate metabolism – the Embden-Meyerhof-Parnas (glycolysis), Entner-Doudoroff, and pentose phosphate (phosphogluconate) pathways | 75, 76 |
| Staphylococcus | Horse < pig < cow | Some members of the genus Staphylococcus are facultatively aerobe and in aerobic conditions can synthesize enzymes such as lactate dehydrogenases and alcohol dehydrogenases with an accumulation of lactic acid and acetic acid. In hypoxic conditions they associate and form biofilms for protection | 77, 78 |
| Lactobacillus | Horse = pig < cow | Lactobacilli ferment hexose sugars to produce lactic acid using the phosphoketolase pathway to produce lactate, CO2 and acetate or ethanol as major end products. They are also capable of acidogenesis biosyntheses of amino acids, purine/pyrimidines, and cofactors | 79, 80 |
| Corynebacterium | Horse < cow = pig | Corynebacteria demonstrate fermentative metabolism of various carbohydrates to lactic acid under certain conditions. They are fastidious slow-growing organisms that are also able to produce glutamic acid, lysine and threonine. The KEGG pathway describes nitrogen metabolism in C. glutamicum R | 81 |
| Prevotella | Pig = cow | Members of this genus utilise glucose in anaerobic growth using the Embden-Meyerhof-Parnas pathway and the usual enzymes involved except that phosphofructokinase was pyrophosphate-dependent. The cells use available glucose to produce acetate, formate and succinate | 82, 83 |
| Terrisporobacter | Pig = cow | Members of the genus are able to ferment glucose to produce acetates | 84, 85 |
| Streptococcus | Horse < cow = pig | Species of Streptococcus utilise carbohydrate metabolism to generate energy for growth generating acids in the process. They are mostly pathogenic | 86, 87 |
| Clostridium | Cow < Pig < horse | Most members of these species are pathogenic to animals. They are capable of converting various carbohydrates to succinate and acetate | 70, 88 |
| Sporobacter | Horse = pig | Members of the genus are capable of using organic compounds in metabolism yielding acetates | 89, 90 |
| Bacteria genera in consortia that are likely pathogenic | |||
| Asaccharospora | Horse | One identified species Asaccharospora irregularis resembling in characteristics Clostridium irregularis described as pathogenic | 70 |
| Aerococcus | Horse < cow < pig |
Members of the genus produce acids from a variety of carbohydrates and are directly involved in acidogenesis The KEGG pathway shows involvement of A. urinae in anaerobic energy and selenocompound metabolism. Some species are considered pathogenic |
62–64 |
| Acinetobacter | Horse = pig < cow | Most members of this genus are pathogenic and possess virulence factors but enzymes produced are also involved in amino acid, carbohydrate and lipid transport and metabolism | 91 |
| Pseudomonas | Horse = pig < cow | Pseudomonas spp. perform anaerobic energy metabolism, carbon-sources versatility observed in the free-living bacteria allowing it to selectively assimilate a preferred carbon-source from mixtures in a process known as carbon catabolite repression using regulatory mechanisms. Some species have multiple virulence factors | 92, 93 |
| Streptococcus | Horse < cow = pig | Species of Streptococcus utilise carbohydrate metabolism to generate energy for growth generating acids in the process. They are mostly pathogenic | 86, 87 |
| Clostridium | Cow < Pig < horse | Most members of these species are pathogenic to animals. They are capable of converting various carbohydrates to succinate and acetate | 70, 88 |
| Treponema | Cow | Members of the genus are pathogenic | 94, 95 |
Prediction of functional gene content
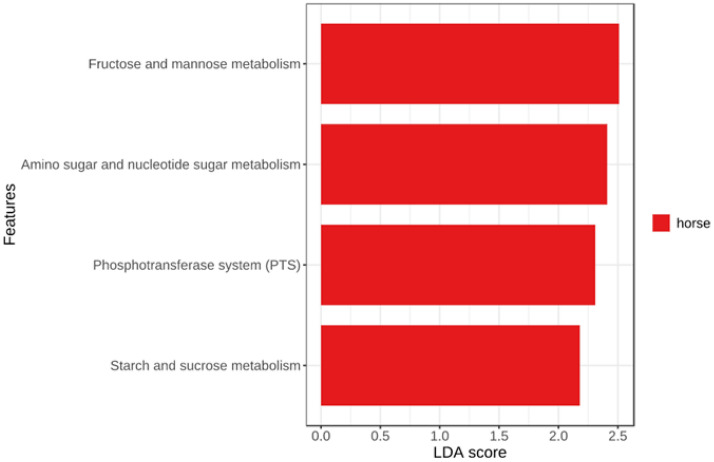
The BIOM format OTU table from QIIME2 was processed on PICRUSt using the KEGG database. A total of 135 predicted KOs (KEGG Orthologies) were grouped into level 2 of categorization. Comparison of metabolism showed four major categories of metabolic activities see Fig. 2. Other classes of metabolism such as carbohydrate, energy, lipid and xenobiotic biodegradation and metabolisms were well represented in each manure sample, with horse manure showing high representation (Supplementary Table S3).
Figure 2.
Linear discriminant analysis (LDA) combined with effect size measurements (LEfSe). Histogram of the LDA scores computed for differentially abundant predicted functions among the three animal manure samples. A p-value of < 0.05 and 2.0 or higher LDA score were considered significant in Kruskal–Wallis.
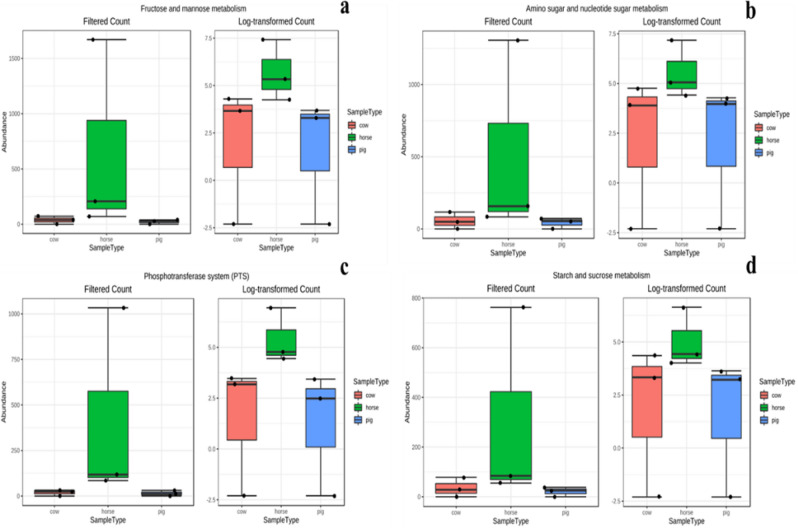
Results obtained from linear discriminant analysis (LDA) combined with the effect size measurements (LEfSe), where the comparison and identification of the predicted functions that were significantly different among the three different animal manures, showed four significant features in horse manure samples. These significant features include KEGG pathways for fructose and mannose metabolism, amino acid and nucleotide sugar metabolism, phosphotransferase PST as well as starch and sucrose metabolism (Fig. 2). The abundance of each significant feature in horse manure, compared to cow and pig manure, was represented using boxplot (Figs. 3a–d).
Figure 3.
Significant features highlighted in LDA (linear discriminant analysis) LefSe = LDA effect size. (a) Fructose and mannose metabolism (KO 00051), (b) amino acid and nucleotide sugar metabolism (KO 00520), (c) phosphotransferase PST pathway (map02060) and (d) starch and sucrose metabolism (KO 00500).
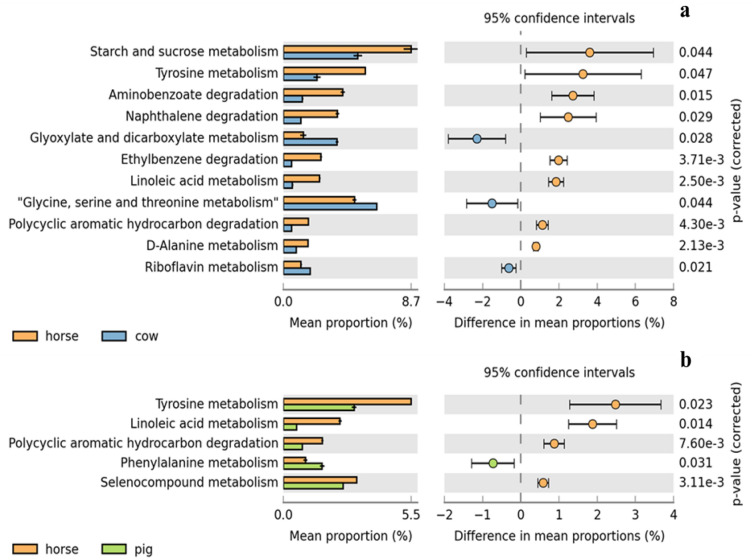
The PICRUSt approach was used to evaluate the functional potential of microbial communities with a particular focus on biogas production. The obtained predicted functions were therefore selected based on their relevance to anaerobic digestion. Out of the 135 predictions, 36 were related to the second or third stage of anaerobic digestion, acidogenesis or acetogenesis. The differences in the number of predictions among the biological replicates of the manure samples obtained from various locations as previously described in Table 1 and represented by the extended bar plot (Figs. 4a,b). The latter compared horse-cow manure, KEGG pathway predictions showed a total of 11 significant predictions (Fig. 4a). While comparison based on horse-pig showed 5 significant predictions (Fig. 4b). There were no significant predictions in the comparison of cow-pig manure. The relative proportions of all the 36 KEGG pathway predictions is shown in S2.
Figure 4.
Extended error bar plot for two-group analysis module comparison of PICRUSt predicted KEGG function data based on horse-cow (a) and horse-pig (b) using Welch’s t-test for two groups. An extended error bar plot was used for the comparison between 2 manure samples and only predicted functions with p < 0.05 are shown. Bar plots on the left side display the mean proportion of each KEGG pathway while the dot plots on the right show the differences in mean proportions between 2 manure samples using p-values.
Correlation analysis of the genus distribution of bacteria in different animal manures
Table 3 is derived from the combined analysis of Fig. 1 in relation to the PICRUSt analysis of predicted functions shown in Figs. 2 and 4 and Supplementary Data Tables S3. Bacteria genus identified were searched from previous literature that identified related functions relevant to metabolic functions of biogas production in focus for this study. Applying KEGG pathway predictions (Fig. 4a), showed that a total of 11 significant predictions were observed compared with horse-cow manure. While comparison based on horse-pig showed 5 significant predictions (Fig. 4b). There was no significant prediction in the comparison of cow-pig manure. Therefore, the correlation analysis presented in Table 3 may be loosely classified as follows:
pathways related to growth and the synthesis and degradation of growth metabolites. Such as that provided by linoleic acid and amino acids including, tyrosine, glycine, serine, threonine, d-Alanine metabolism and phenylalanine, as well as in the metabolism of riboflavin and selenocompounds54, 55;
pathways related to complex lignocellulose degradation as represented within other hydrocarbon pathways of Aminobenzoate, Naphthalene, Ethyl benzene and PAH degradations54, 55;
pathways with direct relationships to acidogenesis and acetogenesis such as those represented with starch and sucrose metabolism54, 55. This is related to the transformation of by-products to short-chain (C1–C5) organic acids, alcohols, hydrogen, and carbon dioxide28. Glyoxylate and dicarboxylate metabolism pathways are also present, which are major reactions involved for acetogenesis56.
These functions were predominantly observed in manure samples obtained from the horse, with remarkable functions of riboflavin metabolism observed in cow to be high, while phenylalanine and selenocompound metabolism was prevalent in pig manure samples. Although, not related directly to biogas production a fourth classification incorporated all pathogenic bacteria genus that did not necessarily contribute to metabolic function but were significantly present in the different manure samples.
Discussion
The mono-digestion biomethane potential of the manures increased in the order of cow, pig and horse based on three predictive models that use the ultimate analyses of the substrates studied. This implies that co-digesting with the highest BMP producing manure, the horse manure, can result in the highest BMP for the resulting mixture using the particular samples from this study. However, this hypothesis may fail if the relevant microbial diversity in the digesters is not balanced to drive the anaerobic digestion processes that result in generation of methane. The current microbial based inference is in agreement with the high BMP of horse manure among the three manure candidates when used as co-substrates. The mono-digestion data also corroborate with our findings where horse manure gave the highest BMP among the three manures.
While investigating the mono-substrate BMPs, Castro-Molano et al.52 studied the co-digestion of these manures in a laboratory BMP assay and established that all mixtures (co-digestions) using different ratios had synergies above the value of 1 with the highest synergy being reported for cow-horse mixture. All co-digestions involving cow-pig-horse manures gave higher BMP values than any single manure, thus indicating the synergistic benefits of co-digestions and selection of the best co-substrate. In an independent co-digestion study, Alfa et al.96 showed that a mixture of cow and horse manure had a higher BMP than any of the two reacted separately. A mixture of pig and horse manure also gave a higher BMP than for the two substrates separately97. The researchers in this investigation attributed the high BMP registered on co-digestion to the synergistic effects of both a richer microbial diversity and balanced nutritional ratios.
In this study, the microbial community structure was analysed using Illumina amplicon sequencing of the 16S rRNA gene. This limited the scope of investigation to bacteria and their roles in the stepwise processes of anaerobic digestion, thus focusing the study on biochemical pathways that are predominantly the domain of bacteria, hydrolysis, acidogenesis and acetogenesis. Acidogenesis and acetogenesis provide the substrates (metabolites) utilised by the predominantly archaea methanogen communities that are pivotal to the last step of bio methanation. It is known that the archaea methanogens transform the products of acetogenesis (acetate, carbon dioxide and hydrogen) into methane98, 99, via interspecies hydrogen and acetate transfer. This is considered the determining factor controlling the completion of anaerobic digestion100, 101. Ensuring the adequate availability of metabolites, and an enabling anoxic environment that are requisites for the growth of methanogens and ensure high biogas yield within the biodigester. This implies that the three stages (hydrolysis, acidogenesis and acetogenesis) essentially direct yield for the slow growing methanogens. As such, this study motivates that the 16S rRNA functional characterisation is more than adequate from a cost-saving perspective for correlation analysis and trouble-shooting protocols to improve biogas yields during anaerobic digestion.
The rather extensive feature of the KEGG database indicates a requirement for selective analysis using the combination of LDA and LEfSe to reduce the scope of analysis and ease the identification of the most differentially abundant predictions (Figs. 2, 3). Moreover, the high variations observed in Figs. 3 and 4 are expected in analysis dealing with biological replicates and microorganisms with their varied functions, features and adaptations102–104 which are the consequences of the different environments from which they originated as described in Table 1.
However, the significant features identified from the KEGG pathways were fructose and mannose metabolism, amino acid and nucleotide sugar metabolism, phosphotransferase PST as well as starch and sucrose metabolism (Fig. 2) and all of which are linked to hydrolysis and initial break down of complex compounds and substrates. All these pathways utilise hydrolytic processes but pertinently provide the ATP and biomolecules required in anaerobic digestion and biogas production. Remarkably, the abundance of these significant pathways was observed to be the most in prevalence in horse manures in comparison to cow and pig manure samples (Fig. 3a–d).
A study on the comparative daily feed intake of horses versus cows by Menard et al.105 highlighted that on average, horses consume 144 g DM kg W−0.75 day−1 while cows consume 88 g DM kg W−0.75 day−1. This implies that horses consume 63% more forage than cows. The same study also discovered that the daily intake of digestible dry matter (nutrient extraction) in all seasons was considerably higher in horses (78 g DM kg W−0.75 day−1) than in cattle (51 g DM kg W −0.75 day−1) indicating a higher functional response in horses than in cattle. It may thus be inferred that the horse GIT has a higher enzymatic capacity to degrade feed faster than the cow and pig GITs. This could explain the prediction of higher fructose, mannose, amino, nucleotide sugar, phosphotransferase PST as well as starch and sucrose metabolism in horses than in cows and pigs. Therefore, the use of horse manure showing this feature can be employed to achieve the initial degradation of complex structured sugars within a biodigester to monomeric biomolecules for other microorganisms to process further.
Table 3 provides a review of the predominantly identified genera of microorganisms found in each manure (derived from Fig. 1), but further provides a brief description of the major functions reported in various literature and the KEGG pathways for previously identified species54, 55. This table buttresses the relationship between the PICRUSt functional predictions, and the identified microorganisms present within manure samples. In Table 3 organisms are placed in this identified classification obtained from the derived PICRUSt functions. Table 3 allows for a panoramic overview making it possible to infer likely functions and contributions by enzymes from the genera in the consortia present in respective animal manures. The emphasis, however, was on the likely roles within the biodigester and anaerobic digestion to produce biogas, therefore a necessary sifting of available literature is necessary when conducting such an analysis.
The analysis made it possible to identify which functions are present or lacking within each manure and provides the possibility of increasing the quantities of a particular type of manure in a co-digestion strategy to compensate for any inadequate microbial genus representations needed for the anaerobic digestion process. For example, it was observed in Table 3 that there was a predominance of most of the relevant microorganisms for all related functions of AD in horse manure, the implication to co-digestion ratios is that it needs to consider increasing the quantities of manure obtained from the horse against the quantities of the other two (cow and pig).
Similarly, should the biodigester be operated in winter conditions, it might benefit from an increase in pig manure to increase the bacterial load of Psychrobacter with its propensity to produce cold active enzymes that off-set low temperature effects on cellular ATP and ADP generation61. Low temperatures have been identified as a challenge in operating biodigesters106, 107. Although, this might be considered small changes, but BMP would not provide such nuanced information, and this makes a significant difference to the process kinetics in this particular scenario. It should also be noted that the microbial profile composition for this study in this context only lends to these two particular changes, but different profiles will provide, after analysis their unique set of changes necessary to make for better process kinetics.
Conversely, the study identified a significant presence of several pathogenic genera including, Asaccharospora, Aerococcus, Acinetobacter, Pseudomonas, Streptococcus, Clostridium, Treponema. This is not surprising but also brings to cognizance the need for an anaerobic digestion treatment of manure before use so as to reduce the majority of the likely zoonotic pathogens present in the different samples of animal manure, preventing its possible transmission to humans108. Thus, reaffirming anaerobic digestion as a useful pre-treatment of manure before use as soil amendment or as fertilisers.
Conclusion
While this study focused on three different types of animal manures, it highlighted the complex variations of bacterial diversity and the likely functional contributions possible from the bacterial communities. However, this diversity is subject to constant changes due to several factors including seasonal variations, temperature, pH and nutrition. Viable commercial process will require routine evaluation and analysis to determine optimal strategies of co-digestion to maximise the yield of biogas. Moreover, such information can be useful in trouble shooting exercises to determine system failures as it relates to abundance or deficiencies in bacteria population and their effects in an operating biodigester for biogas production.
Supplementary Information
Acknowledgements
The authors wish to acknowledge the research contributions on PICRUSt made by Mr Weiz Nurmahomed. We are indebted to Dr James Fox for scientific content analysis of the research paper and English language editing.
Author contributions
G.N.I. developed the methodology and conceptualized the study. A.M. carried out the experiments and collected the data. G.N.I. wrote the first draft of manuscript. R.N. did all formal bioinformatics analysis and prepared all the figures in the manuscript. C.R. carried out the biogas BMP calculations and the related analysis and interpretation. T.M. did the fund acquisition and supervised the project. All authors commented and contributed inputs to previous versions of the manuscript and approved the final manuscript for submission.
Funding
This work was financially supported by the Technology Innovation Agency (TIA): Grant number 2018/FUN/0166 and the South African Department of Science and Innovation (DSI): Grant number DST/CON 0197/2017.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. The raw genomic sequence data used was generated at UNISA in CAES and has been uploaded to an NCBI repository and with other pipelines used during bioinformatics analysis, also all data are available from the corresponding author on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-99389-4.
References
- 1.Zetterström R. Industrial and agricultural pollution: A threat to the health of children living in the Arctic region. Acta Paediatr. 2003;92:1238–1240. doi: 10.1111/j.1651-2227.2003.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 2.Sakadevan K, Nguyen ML. Advances in agronomy. In: Sparks DL, editor. Becтник Pocздpaвнaдзopa. Academic Press; 2017. pp. 147–184. [Google Scholar]
- 3.Grossi G, Goglio P, Vitali A, Williams AG. Livestock and climate change: Impact of livestock on climate and mitigation strategies. Anim. Front. 2019;9:69–76. doi: 10.1093/af/vfy034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doreau M, Corson MS, Wiedemann SG. Water use by livestock: A global perspective for a regional issue? Anim. Front. 2012;2:9–16. doi: 10.2527/af.2012-0036. [DOI] [Google Scholar]
- 5.Runge MC, Sanders-Reed CA, Langtimm CA, Fonnesbeck CJ. Quantitative threats analysis for the Florida manatee (Trichechus manatus latirostris) US Geol. Surv. 2007;14:34. [Google Scholar]
- 6.Manyi-Loh CE, et al. An overview of the control of bacterial pathogens in cattle manure. Int. J. Environ. Res. Public Health. 2016;13:843. doi: 10.3390/ijerph13090843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodds WK, et al. Nitrogen cycling and metabolism in the thalweg of a prairie river. J. Geophys. Res. Biogeosci. 2008;113:1–11. doi: 10.1029/2008JG000696. [DOI] [Google Scholar]
- 8.Yang S, et al. Functional topology of evolving urban drainage networks. Water Resour. Res. 2017;53:8966–8979. doi: 10.1002/2017WR021555. [DOI] [Google Scholar]
- 9.Mohamed NS, Kandeil A, Al-Zubaidy IAH, Kayali G, Ali MA. Genetic and antigenic characterization of avian influenza H9N2 viruses during 2016 in Iraq. Open Vet. J. 2019;9:164–171. doi: 10.4314/ovj.v9i2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Díaz-Vázquez D, et al. Evaluation of biogas potential from livestock manures and multicriteria site selection for centralized anaerobic digester systems: The case of Jalisco, Mexico. Sustainability. 2020;12:3527. doi: 10.3390/su12093527. [DOI] [Google Scholar]
- 11.Muhammad AI, Adamu SB, Alade NK, Amin AB. Studies on haematology and serum biochemistry of broiler chickens finished on an unprocessed and processed velvet bean (Mucuna pruriens (L.)) as dietary protein sources. Biokemistri. 2016;27:68–75. [Google Scholar]
- 12.Mushtaq M, et al. Harnessing genome editing techniques to engineer disease resistance in plants. Front. Plant Sci. 2019;10:1–16. doi: 10.3389/fpls.2019.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obi F, Ugwuishiwu B, Nwakaire J. Agricultural waste concept, generation, utilization and management. Niger. J. Technol. 2016;35:957. doi: 10.4314/njt.v35i4.34. [DOI] [Google Scholar]
- 14.Makgae M. Key areas in waste management: A South African perspective. In: Kumar S, editor. Key Areas in Waste Management: A South African Perspective, Integrated Waste Management. IntechOpen; 2011. pp. 71–82. [Google Scholar]
- 15.FAO . Fao Publications Catalogue 2018 Fao Office for Corporate Communication. FAO; 2018. p. 110. [Google Scholar]
- 16.Ortiz I, Duran F, Pal K, Behrendt C, Acuua-Ulate A. Universal social protection floors: Costing estimates and affordability in 57 lower income countries. SSRN Electron. J. 2018 doi: 10.2139/ssrn.3121542. [DOI] [Google Scholar]
- 17.Batzias FA, Sidiras DK, Spyrou EK. Evaluating livestock manures for biogas production: A GIS based method. Renew. Energy. 2005;30:1161–1176. doi: 10.1016/j.renene.2004.10.001. [DOI] [Google Scholar]
- 18.Niwińska B. Digestion in ruminants. In: Chang C, editor. Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology. IntechOpen; 2013. pp. 137–144. [Google Scholar]
- 19.Henderson G, et al. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015;5:1–15. doi: 10.1038/srep14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cirne DG, Delgado OD, Marichamy S, Mattiasson B. Clostridium lundense sp. nov., a novel anaerobic lipolytic bacterium isolated from bovine rumen. Int. J. Syst. Evol. Microbiol. 2006;56:625–628. doi: 10.1099/ijs.0.63730-0. [DOI] [PubMed] [Google Scholar]
- 21.Wainaina P, Tongruksawattana S, Qaim M. Synergies between different types of agricultural technologies in the Kenyan small farm sector. J. Dev. Stud. 2018;54:1974–1990. doi: 10.1080/00220388.2017.1342818. [DOI] [Google Scholar]
- 22.Sikora M, Benítez-Burraco A. Commentary: Ancient genomes show social and reproductive behavior of early upper paleolithic foragers. Front. Psychol. 2017;8:1–15. doi: 10.3389/fpsyg.2017.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Świątek M, Lewicki A, Szymanowska D, Kubiak P. The effect of introduction of chicken manure on the biodiversity and performance of an anaerobic digester. Electron. J. Biotechnol. 2019;37:25–33. doi: 10.1016/j.ejbt.2018.11.002. [DOI] [Google Scholar]
- 24.Sun Y, et al. The OsSec18 complex interacts with P0(P1–P2)2 to regulate vacuolar morphology in rice endosperm cell. BMC Plant Biol. 2015;15:1–9. doi: 10.1186/s12870-014-0410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laiq Ur Rehman M, Iqbal A, Chang CC, Li W, Ju M. Anaerobic digestion. Water Environ. Res. 2019;91:1253–1271. doi: 10.1002/wer.1219. [DOI] [PubMed] [Google Scholar]
- 26.Rajput AA, Sheikh Z. Effect of inoculum type and organic loading on biogas production of sunflower meal and wheat straw. Sustain. Environ. Res. 2019;1:1–10. [Google Scholar]
- 27.Van Epps A, Blaney L. Antibiotic residues in animal waste: Occurrence and degradation in conventional agricultural waste management practices. Curr. Pollut. Rep. 2016;2:135–155. doi: 10.1007/s40726-016-0037-1. [DOI] [Google Scholar]
- 28.Mutungwazi A, Ijoma GN, Matambo TS. The significance of microbial community functions and symbiosis in enhancing methane production during anaerobic digestion: A review. Symbiosis. 2020;1:1–24. [Google Scholar]
- 29.Cooney MJ, Rong K, Lamichhane KM. Cross comparative analysis of liquid phase anaerobic digestion. J. Water Process. Eng. 2019;29:100765. doi: 10.1016/j.jwpe.2019.02.005. [DOI] [Google Scholar]
- 30.Ma G, Ndegwa P, Harrison JH, Chen Y. Methane yields during anaerobic co-digestion of animal manure with other feedstocks: A meta-analysis. Sci. Total Environ. 2020;728:138224. doi: 10.1016/j.scitotenv.2020.138224. [DOI] [PubMed] [Google Scholar]
- 31.Negro V, et al. Co-digestion of orange peels and marine seaweed with cattle manure to suppress inhibition from toxicants. Biomass Convers. Biorefinery. 2020 doi: 10.1007/s13399-020-00912-w. [DOI] [Google Scholar]
- 32.Weinrich S, Schäfer F, Bochmann G, Liebetrau J. Value of batch tests for biogas potential analysis; method comparison and challenges of substrate and efficiency evaluation of biogas plants. In: Murphy JD, editor. IEA Bioenergy Task. IEA Bioenergy Task; 2018. [Google Scholar]
- 33.Liebetrau J, Weinrich S, Strauber H, Kretzschmar J. Anaerobic fermentation of organic material: Biological processes and their control parameters. In: Meyers RA, editor. Encyclopedia of Sustainability Science and Technology. New York: Springer; 2017. [Google Scholar]
- 34.Koch K, Hafner SD, Weinrich S, Astals S, Holliger C. Power and limitations of biochemical methane potential (BMP) tests. Front. Energy Res. 2020;8:1–4. doi: 10.3389/fenrg.2020.00063. [DOI] [Google Scholar]
- 35.Rashama C, Ijoma GN, Matambo TS. Appraising different models for predicting biomethane potential: The case of avocado oil processing by-products. J. Mater. Cycles Waste Manage. 2020 doi: 10.1007/s10163-020-01116-0. [DOI] [Google Scholar]
- 36.Matheri AN, Ndiweni SN, Belaid M, Muzenda E, Hubert R. Optimising biogas production from anaerobic co-digestion of chicken manure and organic fraction of municipal solid waste. Renew. Sustain. Energy Rev. 2017;80:756–764. doi: 10.1016/j.rser.2017.05.068. [DOI] [Google Scholar]
- 37.Ogunwande GA, Osunade JA, Adeagbo AO, Fakuyi OF. Effects of co-digesting swine manure with chicken Manure on biogas production. Ife J. Sci. 2013;15:1–8. [Google Scholar]
- 38.Dale VH, Kline KL, Wiens J, Fargione J. Biofuels: Implications for land use and biodiversity. Ecological Society of America; 2010. p. 13. [Google Scholar]
- 39.Douglas KM, et al. Understanding conspiracy theories. Polit. Psychol. 2019;40:3–35. doi: 10.1111/pops.12568. [DOI] [Google Scholar]
- 40.Wilkinson DP, Golding N, Guillera-Arroita G, Tingley R, McCarthy MA. A comparison of joint species distribution models for presence–absence data. Methods Ecol. Evol. 2019;10:198–211. doi: 10.1111/2041-210X.13106. [DOI] [Google Scholar]
- 41.Denman SE, Morgavi DP, Mcsweeney CS. Review: The application of omics to rumen microbiota function. Animal. 2018;12:S233–S245. doi: 10.1017/S175173111800229X. [DOI] [PubMed] [Google Scholar]
- 42.Lourenco JM, et al. Comparison of the ruminal and fecal microbiotas in beef calves supplemented or not with concentrate. PLoS ONE. 2020;15:1–17. doi: 10.1371/journal.pone.0231533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin Q, De Vrieze J, Li J, Li X. Temperature affects microbial abundance, activity and interactions in anaerobic digestion. Bioresour. Technol. 2016;209:228–236. doi: 10.1016/j.biortech.2016.02.132. [DOI] [PubMed] [Google Scholar]
- 44.Ren Z, Gao H, Elser JJ, Zhao Q. Microbial functional genes elucidate environmental drivers of biofilm metabolism in glacier-fed streams. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pampillón-González L, Ortiz-Cornejo NL, Luna-Guido M, Dendooven L, Navarro-Noya YE. Archaeal and bacterial community structure in an anaerobic digestion reactor (Lagoon Type) used for biogas production at a pig farm. J. Mol. Microbiol. Biotechnol. 2017;27:306–317. doi: 10.1159/000479108. [DOI] [PubMed] [Google Scholar]
- 46.García-Lozano M, Hernández-De Lira IO, Huber DH, Balagurusamy N. Spatial variations of bacterial communities of an anaerobic lagoon-type biodigester fed with dairy manure. Processes. 2019;7:408. doi: 10.3390/pr7070408. [DOI] [Google Scholar]
- 47.APHA, AWWA, WEF . Standard Methods for the Examination of Water and Wastewater. APHA/AWWA/WEF; 2012. pp. 1–541. [Google Scholar]
- 48.Rashama C, Ijoma GN, Matambo TS. Appraising different models for predicting biomethane potential: The case of avocado oil processing by-products. J. Mater. Cycles Waste Manage. 2021;23:409–415. doi: 10.1007/s10163-020-01116-0. [DOI] [Google Scholar]
- 49.Selvarajan R, et al. Distribution, interaction and functional profiles of epiphytic bacterial communities from the rocky intertidal seaweeds, South Africa. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-56269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nkuna R, Roopnarain A, Adeleke R. Effects of organic loading rates on microbial communities and biogas production from water hyacinth: A case of mono- and co-digestion. J. Chem. Technol. Biotechnol. 2019;94:1294–1304. doi: 10.1002/jctb.5886. [DOI] [Google Scholar]
- 51.Langille MGI, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kafle GK, Chen L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manage. 2016;48:492. doi: 10.1016/j.wasman.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 53.del Castro-Molano LP, Parrales-Ramirez YA, Escalante-Hernandez H. Anaerobic codigestion from cattle, pig manure and horse manure as an alternative to improve the energetical potential in household digesters. Rev. Ion. 2019;32:29–39. doi: 10.18273/revion.v32n2-2019003. [DOI] [Google Scholar]
- 54.Minoru K, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28:1947–1951. doi: 10.1002/pro.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li S, Yang X. Biofuel production from food wastes. In: Luque R, Lin CSK, Wilson JCK, editors. Handbook of Biofuels Production. 2. Woodhead Publishing; 2016. pp. 617–653. [Google Scholar]
- 57.Echeverría-Londoño S, et al. Modelling and projecting the response of local assemblage composition to land use change across Colombia. Divers. Distrib. 2016;22:1099–1111. doi: 10.1111/ddi.12478. [DOI] [Google Scholar]
- 58.Alkhalidi A, Khawaja MK, Amer KA, Nawafleh AS, Al-Safadi MA. Portable biogas digesters for domestic use in Jordanian villages. Recycling. 2019;4:21. doi: 10.3390/recycling4020021. [DOI] [Google Scholar]
- 59.Aarti C, Khusro A, Agastian P. Lignocellulosic biomass as potent feedstock resource for bioethanol production: Recent updates. World News Nat. Sci. 2021;37:164–181. [Google Scholar]
- 60.Das N, Jena PK, Padhi D, Mohanty MK, Sahoo G. A comprehensive review of characterization, pretreatment and its applications on different lignocellulosic biomass for bioethanol production. Biomass Convers. Biorefinery. 2021;3:6. [Google Scholar]
- 61.Amato P, Christner BC. Energy metabolism response to low-temperature and frozen conditions in Psychrobacter cryohalolentis. Appl. Environ. Microbiol. 2009;75:711–718. doi: 10.1128/AEM.02193-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colombo SA, Silva BA, Gómez SYM, Santos R, Tinoco HP, Coelho CM, Teixera JP, Leal CAG. Genital infection by aerococcus viridans in a captive african elephant (Loxodonta africana) Microbiology. 2020;51:1–5. [Google Scholar]
- 63.Martín V, et al. Characterization of Aerococcus viridans isolates from swine clinical specimens. J. Clin. Microbiol. 2007;45:3053–3057. doi: 10.1128/JCM.00156-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu Y, Tsitrin T, Bekele S, Thovarai V, Torralba MG, Singh H, Wolcott R, Doerfert SN, Sizova MV, Epstein SS, Pieper R. Aerococcus urinae and Globicatella sanguinis persist in polymicrobial urethral catheter biofilms examined in longitudinal profiles at the proteomic level. Adv. Tumor Virol. 2019;12:1–15. doi: 10.1177/1178626419875089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu J, Xu C, Chen X, Cai X, Yang S, Sheng Y, Wang T. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition. 2014;3:584–589. doi: 10.1016/j.nut.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 66.Dimova LG, Zlatkov N, Verkade HJ, Uhlin BE, Tietge UJF. High-cholesterol diet does not alter gut microbiota composition in mice. Nutr. Metab. 2017;14:1–7. doi: 10.1186/s12986-017-0170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoffman JM, Margolis KG. Building community in the gut: A role for mucosal serotonin. Nat. Rev. Gastroenterol. Hepatol. 2020;17:6–8. doi: 10.1038/s41575-019-0227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou W, Xu H, Zhan L, Lu X, Zhang L. Dynamic development of fecal microbiome during the progression of diabetes mellitus in Zucker diabetic fatty rats. Front. Microbiol. 2019;10:1–17. doi: 10.3389/fmicb.2019.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y, et al. Romboutsia sedimentorum sp. nov., isolated from an alkaline-saline lake sediment and emended description of the genus Romboutsia. Int. J. Syst. Evol. Microbiol. 2015;65:1193–1198. doi: 10.1099/ijs.0.000079. [DOI] [PubMed] [Google Scholar]
- 70.Gerritsen J, et al. Characterization of Romboutsia ilealis gen. nov., sp. nov., isolated from the gastro-intestinal tract of a rat, and proposal for the reclassification of five closely related members of the genus Clostridium into the genera Romboutsia gen. nov., Intestinib. Int. J. Syst. Evol. Microbiol. 2014;64:1600–1616. doi: 10.1099/ijs.0.059543-0. [DOI] [PubMed] [Google Scholar]
- 71.Ghatak, M., Mahanta, P. Effect of temperature on biogas production from lignocellulosic biomasses. In Proc. 2014 1st Int. Conf. Non Conv. Energy Search Clean Safe Energy, ICONCE 2014, 117–121 (2014).
- 72.Kourist R, Schweiger A, Büchsenschütz H. Enzymatic decarboxylation as a tool for the enzymatic defunctionalization of hydrophobic bio-based organic acids. In Uwe T. Bornscheuer lipid modification by enzymes and engineered microbes. N. Engl. J. Med. 2018;372:89–118. [Google Scholar]
- 73.Putra FJN, Putri SP, Fukusaki E. Metabolomics-based profiling of three terminal alkene-producing Jeotgalicoccus spp. during different growth phase. J. Biosci. Bioeng. 2019;127:52–58. doi: 10.1016/j.jbiosc.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 74.Ammar EM, Wang X, Rao CV. Regulation of metabolism in Escherichia coli during growth on mixtures of the non-glucose sugars: Arabinose, lactose, and xylose. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-017-18704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Portela CAF, Smart KF, Tumanov S, Cook GM, Villas-Bôas SG. Global metabolic response of Enterococcus faecalis to oxygen. J. Bacteriol. 2014;196:2012–2022. doi: 10.1128/JB.01354-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramsey, M., Hartke, A. & Huycke, M. The physiology and metabolism of enterococci. In Enterococci From Commensals to Lead. Causes Drug Resist. Infect., 1–55 (2014).
- 77.Onyango LA, Alreshidi MM. Adaptive metabolism in staphylococci: Survival and persistence in environmental and clinical settings. J. Pathog. 2018;2018:1–11. doi: 10.1155/2018/1092632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fuchs TA, et al. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuratsu M, Hamano Y, Dairi T. Analysis of the lactobacillus metabolic pathway. Appl. Environ. Microbiol. 2010;76:7299–7301. doi: 10.1128/AEM.01514-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Mesquita ARC, et al. Metabolism and physiology of Lactobacilli: A review. J. Environ. Anal. Prog. 2017;2:115–136. doi: 10.24221/jeap.2.2.2017.1202.115-124. [DOI] [Google Scholar]
- 81.Blombach B, Seibold GM. Carbohydrate metabolism in Corynebacterium glutamicum and applications for the metabolic engineering of l-lysine production strains. Appl. Microbiol. Biotechnol. 2010;86:1313–1322. doi: 10.1007/s00253-010-2537-z. [DOI] [PubMed] [Google Scholar]
- 82.Amat S, Lantz H, Munyaka PM, Willing BP. Prevotella in pigs: The positive and negative associations with production and health. Microorganisms. 2020;8:1–27. doi: 10.3390/microorganisms8101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takahashi N, Yamada T. Glucose metabolism by Prevotella intermedia and Prevotella nigrescens. Oral Microbiol. Immunol. 2000;15:188–195. doi: 10.1034/j.1399-302x.2000.150307.x. [DOI] [PubMed] [Google Scholar]
- 84.Deng Y, et al. Terrisporobacter petrolearius sp. nov., isolated from an oilfield petroleum reservoir. Int. J. Syst. Evol. Microbiol. 2015;65:3522–3526. doi: 10.1099/ijsem.0.000450. [DOI] [PubMed] [Google Scholar]
- 85.Xiang Z, et al. A glance at the gut microbiota of five experimental animal species through fecal samples. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saccenti E, Nieuwenhuijse D, Koehorst JJ, Dos Santos VAPM, Schaap PJ. Assessing the metabolic diversity of streptococcus from a protein domain point of view. PLoS ONE. 2015;10:1–20. doi: 10.1371/journal.pone.0137908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pelkonen S, et al. Transmission of Streptococcus equi subspecies zooepidemicus infection from horses to humans. Emerg. Infect. Dis. 2013;19:1041–1048. doi: 10.3201/eid1907.121365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koendjbiharie JG, Wiersma K, van Kranenburg R. Investigating the central metabolism of Clostridium thermosuccinogenes. Appl. Environ. Microbiol. 2018;84:e00363. doi: 10.1128/AEM.00363-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Donnell MM, et al. The core faecal bacterial microbiome of Irish thoroughbred racehorses. Lett. Appl. Microbiol. 2013;57:492–501. doi: 10.1111/lam.12137. [DOI] [PubMed] [Google Scholar]
- 90.Kondo K, Negishi A, Fukuyama M. Isolation and characterization of episulfoxides. Tetrahedron Lett. 1969;10:2461–2464. doi: 10.1016/S0040-4039(01)88190-9. [DOI] [Google Scholar]
- 91.Peleg AY, et al. The success of acinetobacter species; genetic, metabolic and virulence attributes. PLoS ONE. 2012;7:e46984. doi: 10.1371/journal.pone.0046984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Panayidou S, et al. Pseudomonas aeruginosa core metabolism exerts a widespread growth-independent control on virulence. Sci. Rep. 2020;10:1–20. doi: 10.1038/s41598-020-66194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rojo F. Carbon catabolite repression in Pseudomonas: Optimizing metabolic versatility and interactions with the environment. FEMS Microbiol. Rev. 2010;34:658–684. doi: 10.1111/j.1574-6976.2010.00218.x. [DOI] [PubMed] [Google Scholar]
- 94.Mamuad LL, et al. Treponema spp., the dominant pathogen in the lesion of bovine digital dermatitis and its characterization in dairy cattle. Vet. Microbiol. 2020;245:108696. doi: 10.1016/j.vetmic.2020.108696. [DOI] [PubMed] [Google Scholar]
- 95.Pringle M, Bergsten C, Fernström LL, Höök H, Johansson KE. Isolation and characterization of Treponema phagedenis-like spirochetes from digital dermatitis lesions in Swedish dairy cattle. Acta Vet. Scand. 2008;50:1–8. doi: 10.1186/1751-0147-50-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alfa MI, et al. Evaluation of biogas yield and kinetics from the anaerobic codigestion of cow dung and horse dung: A strategy for sustainable management of livestock manure. Energy Ecol. Environ. 2021;6:425–434. doi: 10.1007/s40974-020-00203-0. [DOI] [Google Scholar]
- 97.Lopes M, Baptista P, Duarte E, Moreira ALN. Enhanced biogas production from anaerobic codigestion of pig slurry and horse manure with mechanical pretreatment. Environ. Technol. 2019;40:1289–1297. doi: 10.1080/09593330.2017.1420698. [DOI] [PubMed] [Google Scholar]
- 98.Meegoda JN, Li B, Patel K, Wang LB. A review of the processes, parameters, and optimization of anaerobic digestion. Adv. Biosci. Biotechnol. 2018;2015(6):205. doi: 10.3390/ijerph15102224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Divya D, Gopinath LR, Merlin Christy P. A review on current aspects and diverse prospects for enhancing biogas production in sustainable means. Renew. Sustain. Energy Rev. 2015;42:690–699. doi: 10.1016/j.rser.2014.10.055. [DOI] [Google Scholar]
- 100.Angelidaki I, Mogensen AS, Ahring BK. Degradation of organic contaminants found in organic waste. Biodegradation. 2000;11:377–383. doi: 10.1023/A:1011643014990. [DOI] [PubMed] [Google Scholar]
- 101.Sterling MC, Lacey RE, Engler CR, Ricke SC. Effects of ammonia nitrogen on H2 and CH4 production during anaerobic digestion of dairy cattle manure. Bioresour. Technol. 2001;77:9–18. doi: 10.1016/S0960-8524(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 102.Bell G. Replicates and repeats. BMC Biol. 2016;14:4–5. doi: 10.1186/s12915-016-0227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vaux DL, Fidler F, Cumming G. Replicates and repeats-what is the difference and is it significant? A brief discussion of statistics and experimental design. EMBO Rep. 2012;13:291–296. doi: 10.1038/embor.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bryant PA, Smyth GK, Robins-Browne R, Curtis N. Technical variability is greater than biological variability in a microarray experiment but both are outweighed by changes induced by stimulation. PLoS ONE. 2011;6:1–8. doi: 10.1371/journal.pone.0019556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Menard C, Duncan P, Fleurance G, Georges JY, Lila M. Comparative foraging and nutrition of horses and cattle in European wetlands. J. Appl. Ecol. 2002;39:120–133. doi: 10.1046/j.1365-2664.2002.00693.x. [DOI] [Google Scholar]
- 106.Wang S, et al. Influence of temperature on biogas production efficiency and microbial community in a two-phase anaerobic digestion system. Water (Switzerland) 2019;11:133. [Google Scholar]
- 107.Pham CH, Vu CC, Sommer SG, Bruun S. Factors affecting process temperature and biogas production in small-scale rural biogas digesters in winter in northern vietnam. Asian-Australas. J. Anim. Sci. 2014;27:1050–1056. doi: 10.5713/ajas.2013.13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seruga P, et al. Anaerobic digestion performance: Separate collected vs. mechanical segregated organic fractions of municipal solid waste as feedstock. Energies. 2020;13:3768. doi: 10.3390/en13153768. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article. The raw genomic sequence data used was generated at UNISA in CAES and has been uploaded to an NCBI repository and with other pipelines used during bioinformatics analysis, also all data are available from the corresponding author on request.