Abstract
Videofluoroscopy swallow studies (VFSS) and high-resolution manometry (HRM) methods complement to ascertain mechanisms of infant feeding difficulties. We hypothesized that: (a) an integrated approach (study: parent-preferred feeding therapy based on VFSS and HRM) is superior to the standard-of-care (control: provider-prescribed feeding therapy based on VFSS), and (b) motility characteristics are distinct in infants with penetration or aspiration defined as penetration-aspiration scale (PAS) score ≥ 2. Feeding therapies were nipple flow, fluid thickness, or no modification. Clinical outcomes were oral-feeding success (primary), length of hospital stay and growth velocity. Basal and adaptive HRM motility characteristics were analyzed for study infants. Oral feeding success was 85% [76–94%] in study (N = 60) vs. 63% [50–77%] in control (N = 49), p = 0.008. Hospital-stay and growth velocity did not differ between approaches or PAS ≥ 2 (all P > 0.05). In study infants with PAS ≥ 2, motility metrics differed for increased deglutition apnea during interphase (p = 0.02), symptoms with pharyngeal stimulation (p = 0.02) and decreased distal esophageal contractility (p = 0.004) with barium. In conclusion, an integrated approach with parent-preferred therapy based on mechanistic understanding of VFSS and HRM metrics improves oral feeding outcomes despite the evidence of penetration or aspiration. Implementation of new knowledge of physiology of swallowing and airway protection may be contributory to our findings.
Subject terms: Paediatric research, Gastroenterology
Introduction
Infants with feeding difficulties can be described as having frequent aerodigestive symptoms such as coughing, apnea, bradycardia, or desaturation with oral feeding, or the inability to achieve exclusive oral feeding. Overall, prevalence of infant feeding difficulties is increased and rising in medically complex infants1,2. Establishment of safe feeding is required prior to discharge3,4. In infants with feeding difficulties or frequent symptoms, it is common practice to assess the infants’ eating skills via dynamic x-ray imaging or videofluoroscopy swallowing study (VFSS) to provide structural and functional insight5,6. If penetration or aspiration is observed during the VFSS, feeding modifications are trialed and typically include nipple flow rate or fluid thickness changes further increasing the infant’s radiation exposure and associated risks7–9. Additionally, standardization is still being developed for infants10,11, and evaluation is typically limited to observation of the oral cavity and upper aerodigestive tract as well as only those swallows as captured by the radiologist. High resolution manometry (HRM) is an emerging technology in infants that permits prolonged evaluation of swallowing function without radiation exposure12–20. HRM allows evaluation of swallowing function by examining dynamic and kinetic relationships between the airway (glottal closure and respiratory changes) and the entire foregut (pharynx, upper esophageal sphincter- UES, esophagus, lower esophageal sphincter- LES).
As HRM may be complementary to VFSS, the aim of the current study was to test the main hypothesis that clinical outcomes of an integrated feeding approach (parental preference informed by VFSS and HRM testing) are superior to the standard-of-care approach (control) based on VFSS information alone. A sub-aim was to test the hypothesis that infants with penetration or aspiration have distinct clinical and motility outcomes.
Participants, study design and methods
Study design, setting, participants
This is an observational cohort study conducted between 2015 and 2020 at a single tertiary all-referral center at the Nationwide Children’s Hospital, Columbus, OH, in infants referred for feeding difficulties and VFSS evaluation. In accordance to institutional guidelines and regulations involving human subjects, the protocol was approved by the Institutional Review Board at Nationwide Children’s Hospital, Columbus, OH (Supplement). The study was registered on clinical trials.gov: NCT02583360. Originally, this study was designed as a randomized clinical trial to compare the effects of thickened formula vs nipple flow change feeding modifications in infants undergoing VFSS evaluation. Initially, recruitment was difficult owing to the institution of family-centered care policy, compounded by provider variability with feeding therapeutic strategies, and lack of provider and parental support for randomization. Therefore, based on the advice of the data safety monitoring board and study sponsor, alternative strategies were employed (parent preferred feeding therapies and inclusion of outpatient populations), and study design was modified to the current observational cohort design (study: prospectively collected integrated feeding approach vs control: retrospectively collected standard-of-care approach) to address the original study goals of identifying potential mechanisms and management strategies for infant dysphagia. The integrated feeding (study) approach included parent chosen feeding therapies based on information provided from VFSS and HRM assessments, while the standard-of-care (control) approach included provider-driven therapies based on VFSS only. Feeding therapies for both groups included nipple flow, fluid thickness, or no modification. For the study cohort: Subjects were screened and recruited by the Neonatal and Infant Feeding Disorders Research Program. Written, signed, informed parental consent was obtained prior to the study. Parents were encouraged to attend HRM evaluations, participate in feeding sessions with nipple and fluid thickness changes, and ask questions. Regardless of attendance, parents were educated about their infant’s swallowing limitations and capabilities via explanation of pharyngo-esophageal motility, airway protection, volume intake, and vital sign observations. For the control group: parental consent was not needed (as determined by the Institutional Review Board) as this was a medical record chart review of data from infants receiving the standard-of-care during the concurrent study time period. Inclusion criteria for study and controls were: (a) infants with feeding-related aerodigestive symptoms undergoing a diagnostic VFSS, (b) < 60 weeks postmenstrual age (both pre-term and full-term born), (c) on full enteral feeds with at least partial oral feeds, and (d) on ≤ 1 L per minute oxygen via nasal cannula for respiratory support. Exclusion criteria for study and controls were known genetic, metabolic or syndromic diagnoses: severe neuropathology (≥ grade III intraventricular hemorrhage, neurosurgery, moderate to severe perinatal hypoxic ischemic encephalopathy), gastrointestinal malformations and/or surgery, craniofacial malformations or ear/nose/throat surgeries, and exclusively breastfeeding infants.
Videofluoroscopy swallow study protocol
Using the established evaluation procedures21 and institutional interdisciplinary guidelines of quality and safety11, VFSS was performed with 3 standardized metrics: field of view, magnification, and pulse repetition rate. Briefly, standardized collimation (lips anteriorly, inferior bony orbits superiorly, spinous processes posteriorly, cervical 5 and 6 vertebrae inferiorly) during a VFSS provided a focused view of the oropharyngeal tract and at least one bolus into the esophagus following it through while avoiding unnecessary radiation exposure to radiosensitive organs22. Magnification was ensured to provide anatomic detail but was balanced with the As Low as Reasonably Achievable principle to avoid unnecessary radiation exposure11. A magnification of not exceeding twice magnifications on a standard-three scale was used, but the majority of studies performed without any magnification22. Imaging was carried out in real time of 30 frames per second23. Infant was studied in their typical feeding position, which included side-lying or semi-reclined via a seat (Tumble Forms Feeder Seat Positioner, Patterson Medical, Illinois, USA). Imaging was performed by the radiologist in lateral view during bottle feeding. The infant was bottle fed premixed liquid barium sulfate (Varibar®, Bracco Diagnostics Inc, New Jersey, USA) by the caregiver or occupational therapist. Nipple flow rates and testing liquid thickness (thin, nectar, and thin honey) were determined based on the patient’s clinical needs. As per institutional standard of care, the VFSS team (an occupational therapist, speech language pathologist, and radiologist) performed assessment of oral, pharyngeal, laryngeal, and esophageal regions in real-time which were also verified through post-review for agreement.
Pharyngo-esophageal motility testing protocol
Infants underwent motility testing via HRM as previously published19,24,25. Briefly, a 6 Fr probe with 25 pressure sensors (UniTip High-Resolution Catheter, Unisensor USA) attached to a portable HRM System (Solar GI, Laborie Medical Technologies, Mississauga, ON, Canada and Duracell PowerSource 1800, Duracell Incorporated, Connecticut, US) was zeroed prior to placement, passed nasally, and secured by the study physician at the patient’s bedside. The infant was given adequate time for catheter adaptation (≥ 15 min) to ensure quiescence before recording basal manometry and spontaneous swallows. Nasal airflow thermistor (Integra Life Sciences, Plainsboro, NJ) was utilized to detect respiratory changes and deglutition apnea26–29. VFSS testing as described above was performed concurrently when feasible (N = 54 infants) or sequentially within 7 days (N = 6 infants). If concurrent VFSS and HRM studies occurred, the infant was transported by the study team (physician, registered nurse, two technicians) to the VFSS suite for testing with vitals constantly monitored. Upon VFSS completion, the infant was transported back to the patient room where pharyngeal infusion protocol and oral feeding challenge were performed19,30,31. To perform pharyngeal infusions, a silicone catheter with pharyngeal infusion port (Dentsleeve International, Mui Scientific, Mississauga, ON, Canada) was juxtaposed to the HRM catheter and positioned so that the pharyngeal infusion port was at the level of the pharynx as confirmed by esophago-pressure topography plots in HRM. Sterile water was infused at volumes of 0.1, 0.3 and 0.5 mL in triplicate to evaluate pharyngeal swallowing reflexes. The oral feeding challenge consisted of a 1-min milk bottle feed using the infant’s current bottle and nipple system along with their current formula or human milk. Trial start time begun upon infant latch.
VFSS and HRM data analytical methods
Analytical definitions for VFSS and HRM methods have been previously published18,24,25,29,32–49, are summarized in Table 1, and further explained below.
Table 1.
Videofluoroscopy swallow study and high resolution motility metrics and analysis definitions.
| Anatomic region | Variable name | Unit of measure | Measure type | Testing state | Definition | |||
|---|---|---|---|---|---|---|---|---|
| BS | Px | Milk feed | Barium feed | |||||
| Videofluoroscopy swallow study (based on the infant’s thinnest trial with worst PAS score) | ||||||||
| Oral Cavity | Oral phase | % | Categorical | ✓ |
functional: adequate lip closure, sucking strength, bolus formation, and transit time prior to initiation of pharyngeal swallow delayed but functional: delayed lip seal, bolus formation, transit time impaired: absent/reduced lip seal, sucking strength, bolus formation, transit time43 |
|||
| Pharynx | Pharyngeal phase | % | Categorical | ✓ |
functional: transport of the bolus through the pharynx initiated by hyo-laryngeal elevation delayed but functional: entry of the bolus head into the pharyngeal cavity prior to hyo-laryngeal elevation/decreased laryngeal vestibule closure resulting in inconsistent shallow penetration impaired: reduced hyo-laryngeal elevation, incomplete closure of the laryngeal vestibule, reduced glottal closure resulting in consistent deep laryngeal penetration and/or aspiration before, during or after the swallow43–45 |
|||
| Larynx | Laryngeal phase | # | Categorical | ✓ |
Penetration-Aspiration Scale (PAS) 1- material does not enter the airway 2- material enters the larynx but stays above the vocal folds 3- material enters the larynx to the level of the vocal folds 4- material passes below the vocal folds 5- material enters the airway, contacts the vocal folds, and is not ejected from the airway, 6- material enters the airway, passes below the vocal folds and is ejected into the larynx or out of the airway 7- material enters the airway, passes below the vocal folds, and is not ejected from the trachea despite effort, and 8- material enters the airway, passes below the vocal folds, and no effort is made to eject49 |
|||
| Larynx | Laryngeal phase | % | Categorical | ✓ |
PAS Group No Penetration or Aspiration: PAS = 1 Penetration: PAS = 2–5 Aspiration: PAS = 6–849 |
|||
| High resolution manometry | ||||||||
| Pharynx to Stomach | Peristaltic response occurrence | % | Categorical | ✓ | Presence of pharyngeal reflexive swallow or pharyngo-UES-contractile reflex38,40–42 | |||
| Pharynx to Stomach | Peristaltic response latency | sec | Continuous | ✓ | Time interval between pharyngeal infusion onset and peristaltic response onset25,39 | |||
| Pharynx to Stomach | Peristaltic response duration | sec | Continuous | ✓ | Time interval between peristaltic response onset and offset25,39 | |||
| Pharynx to Stomach | Terminal swallow occurrence | % | Continuous | ✓ | Presence of final clearing pharyngo-esophageal swallow resulting in aerodigestive quiescence25,39 | |||
| Pharynx | Pharyngeal contractions | # | Continuous | ✓ | Total number of pharyngeal contractions induced by pharyngeal infusion stimulus29,38,39 | |||
| Pharynx | Pharyngeal contractile activity | % | Continuous | ✓ | ✓ | Sum of pharyngeal contractile durations/oral feeding duration*10018,34 | ||
| Oro-Pharynx | Pharyngeal contractile vigor | mmHg.cm.s | Continuous | ✓ | ✓ | ✓ | Contractile integral calculated as pharyngeal region amplitude*pharyngeal length*contractile duration for proximal, distal, and overall pharyngeal regions. Proximal contractile integral reflects oro-pharyngeal functional competency18,34,46. | |
| UES and LES | Basal tone | mmHg.cm.s | Continuous | ✓ | Contractile integral (amplitude*length* duration) calculated during a 2 s window at rest prior to basal swallow36,37 | |||
| UES and LES | Relaxation reflex occurrence | % | Categorical | ✓ | Relaxation defined as > 50% decrease from basal tone34,35 | |||
| UES | Contractile reflex | % | Categorical | ✓ | Contraction defined as > 50% increase from basal tone- definition adapted from previous works 32,33 | |||
| Esophagus | Esophageal contractile vigor | mmHg.cm.s | Continuous | ✓ | ✓ | ✓ | Contractile integral (amplitude*length* duration) of esophageal regions. Proximal esophagus: lower UES border to transition zone. Distal esophagus: transition zone to upper LES border24,25 | |
| Esophagus | Peristaltic break during terminal swallow occurrence | % | Categorical | ✓ | Presence of any esophageal gaps in the 20-mmHg isobar contour of the peristaltic contraction associated with the terminal swallow24,48 | |||
| Nasal airflow | DA occurrence | % | Categorical | ✓ | Presence of a pause in breathing associated with pharyngeal contraction38,40 | |||
| Nasal airflow | DA latency | sec | Continuous | ✓ | Time interval between pharyngeal stimulus onset to DA onset38,40 | |||
| Nasal airflow | DA duration | sec | Continuous | ✓ | ✓ | Time interval between respiratory pause onset to offset38,40 | ||
| Nasal airflow | DA during interphase occurrence | % | Categorical | ✓ | Phase of deglutition apnea onset: Inspiration- upstroke in nasal airflow. Expiration- defined as downstroke in nasal airflow thermistor. Interphase- between inspiratory or expiratory phases40 | |||
| Global | Symptom occurrence | % | Categorical | ✓ | Defined as the presence of any symptom during pharyngeal infusion 47 | |||
BS-Basal Swallow, Px-Pharyngeal Infusion, DA- deglutition apnea, Milk Feed- Oral Feeding with Milk, Barium Feed- Oral Feeding with Barium-Sulfate, ✓: variable was analyzed for marked state.
VFSS metrics were based on the infant’s thinnest trial received with the worst PAS score. The oral phase of swallow refers to structural and functional observations of the oral preparatory and oral transit stages of swallow prior to initiation of the pharyngeal phase43, the pharyngeal phase of swallow refers to observations of swallowing as the bolus enters the pharyngeal cavity, bypassing the closed laryngeal region, and exiting the pharyngeal cavity at the level of the UES43,44,50, and laryngeal phase and airway protection was assessed by the Penetration Aspiration Scale (PAS)49. Infants were also categorized as PAS = 1 (no penetration or aspiration) or PAS ≥ 2 (penetration or aspiration).
HRM metrics
HRM metrics were analyzed during resting and adaptive states as follows: (A) Basal Swallowing (resting state): For each study a maximum of ten basal or spontaneous (absence of stimulus) swallows were selected for analysis37,51. Pharyngeal, esophageal, and respiratory characteristics were analyzed as previously published18,19,24,25,38. (B) Pharyngeal infusion (adaptive state): aerodigestive responses to pharyngeal stimulus were analyzed as previously published and included global (peristalsis and symptoms) and regional (pharynx, UES, esophagus, respiratory) characteristics24,25,29,38,39,41,42,47,52. (C) Oral feeding with milk and barium sulfate (adaptive state): Pharyngeal and esophageal characteristics were measured during oral feeding sessions24,25. Volume intake was calculated as volume consumed (mL)/feeding session duration (min).
Statistical methods and outcome measures
Demographic and clinical outcomes were managed using research electronic data capture tools (REDCap)53. The primary clinical outcome was oral feeding success (defined as full oral feeding without aerodigestive feeding-related symptoms) at discharge or 4 weeks (whichever was sooner) for inpatients and 4 weeks for outpatients for study and control cohorts. Sample size of the study group was determined apriori as follows: based on historical data where oral feeding success rate was 60% with VFSS dependent treatment. With 60 patients enrolled, we had at least 90% power to detect an absolute increase of 20% from 60% for those who did not have treatment informed by manometry (historical control) to 80% in those in those informed by manometry with two-sided type I error of 0.05. Secondary clinical outcomes were growth velocities (weight, length, and head circumference) and length of hospital stay. Analysis of secondary outcomes was based on available data with number of subjects as stated in the tables and flow diagram (Fig. 1).
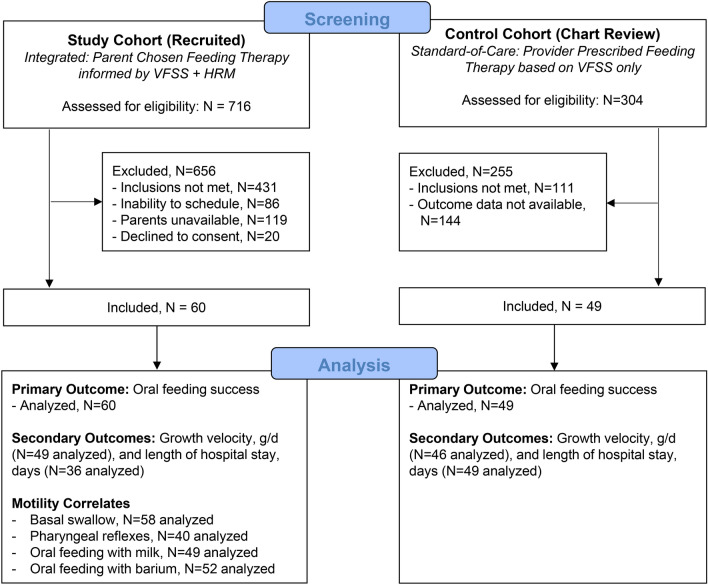
Figure 1.
Study Enrollment. Depicted is the study flow diagram for analysis of study (prospectively collected) and control (retrospectively collected) cohort data for infants referred for VFSS testing.
Statistical Analysis Software (v. 9.4, SAS Institute Inc., Cary, NC, USA) was utilized for analysis. P-values of < 0.05 were considered statistically significant. Descriptive statistics were reported as median [IQR], mean ± SD or total number and percentage for demographics and clinical characteristics. Oral feeding success rate was estimated with 95% Confidence Interval and compared between cohorts using Chi-squared test. Odds ratio with 95% confidence interval was also provided for oral feeding success. Logistic regression was used to calculate the adjusted odds ratio for oral feeding success while controlling for gastroesophageal reflux disease and prematurity to account for potential confounders, as these subject morbidities have been shown to delay feeding milestones. Two-sample t-test or Wilcoxon signed rank test for the continuous variables and chi-squared or Fischer’s exact tests for the categorical variables, whichever was appropriate, were used to compare the clinical and VFSS characteristics between study and control cohorts, and clinical outcomes between PAS = 1 and PAS ≥ 2 groups within and between study and control cohorts. Normality was assessed using Shapiro-Wilks test and visually inspection of the Q-Q plot (normality) and residual plots. For comparison of HRM motility characteristics between PAS = 1 and PAS ≥ 2 groups, linear mixed effect model for continuous measured variables and generalized estimating equation for categorical variables, to predict the likelihood of the specific response, were used. Both models controlled for presence of gastroesophageal reflux disease and prematurity. Pharyngeal infusion data was also controlled for infusion volume. Compound symmetry was specified for the covariance structure of the repeated data. Bonferroni correction was used for multiplicity adjustment to conserve the overall type I error at α = 0.05.
Study oversight
Compliance to protocol and data integrity were maintained. Patient care data was stored and secured. Study recruitment criteria were reported to the data safety monitoring board quarterly (see composition in acknowledgment) and their recommendations complied. Clinical study progress and adverse events were reported to the institutional review board annually. In addition, voluntary audits were conducted at the request of the principal investigator by the institutional audit team (see acknowledgement) and recommendations complied. Outcome variables were documented as route of intake oral or tube, growth metrics, administration of acid suppressive therapies, supplemental oxygen use, adverse events and the data were confirmed using electronic medical records (Epic, Epic Systems Corporation, Verona, WI, USA) as well as parental validation.
Results
Comparison of outcomes in study cohort vs. control cohort
Study enrollment, and approaches for primary outcome analysis are described in the study flow diagram (Fig. 1). Subject characteristics at birth and time of evaluation did not significantly differ between study and control cohorts (Table 2). Additionally, for study vs control groups respectively: Birth APGAR score, median [IQR], at 1 min was 63 – 8 vs 74 – 8, p = 0.53 and at 5 min was 87 – 9 vs 8.57 – 9, p = 0.46. Feeding therapies (nipple flow modification: fluid thickness modification: no modification, %) were 21: 22: 57 for study vs 23: 33: 44 for control, p = 0.35. Parental attendance was 46/60 (77%) in the study group and 27/48 (56%) in the control group, P = 0.30.
Table 2.
Clinical and VFSS characteristics between Study vs. Control Cohorts.
| Characteristic | Study | Control | P-value |
|---|---|---|---|
| (N = 60) | (N = 49) | ||
| Demographics at birth | |||
| Gender [male] (%) | 34 (57%) | 19 (39%) | 0.06 |
| Race (%) | 0.1 | ||
| African American | 9 (15%) | 15 (31%) | |
| Asian | 1 (2%) | 2 (4%) | |
| Bi-racial | 0 (0%) | 1 (2%) | |
| White | 50 (83%) | 31 (63%) | |
| Gestational age (wks) | 34.8 ± 4.8 | 35.7 ± 4.2 | 0.29 |
| Birth weight (kg) | 2.5 ± 1.1, n = 58 | 2.7 ± 1.0, n = 47 | 0.51 |
| Clinical characteristics at evaluation | |||
| Chronologic age (wks) | 10.9 ± 6.0 | 10.0 ± 6.5 | 0.41 |
| Postmenstrual age (wks) | 45.7 ± 5.5 | 45.7 ± 5.1 | 0.95 |
| Weight (kg) | 4.4 ± 1.1 | 4.1 ± 1.0 | 0.14 |
| Infant feeding milk type (%) | 0.88 | ||
| Breast milk | 7 (12%) | 5 (10%) | |
| Breast milk + formula | 17 (28%) | 16 (33%) | |
| Formula | 36 (60%) | 28 (57%) | |
| Morbidity (%) | |||
| Preterm birth | 34 (57%) | 23 (47%) | 0.31 |
| Chronic lung disease of infancy | 14 (23%) | 8 (16%) | 0.36 |
| Intraventricular hemorrhage (grade I or II) | 5 (8%) | 4 (8%) | 0.97 |
| Hypoxic ischemic encephalopathy (mild) | 1 (2%) | 0 (0%) | 0.36 |
| Gastroesophageal reflux disease | 20 (33%) | 25 (51%) | 0.06 |
| VFSS characteristics at evaluation | |||
| Feeding position [semi-reclined] (%) | 44/57 (77%) | 36/48 (75%) | 0.79 |
| Oral phase (%) | 0.98 | ||
| Functional | 29/57 (51%) | 24/48 (50%) | |
| Delayed but functional | 25/57 (44%) | 21/48 (44%) | |
| Impaired | 3/57 (5%) | 3/48 (6%) | |
| Pharyngeal phase (%) | 0.64 | ||
| Functional | 32/57 (56%) | 23 (47%) | |
| Delayed but functional | 17/57 (30%) | 18 (37%) | |
| Impaired | 8/57 (14%) | 8 (16%) | |
| Penetration aspiration scale (PAS) # | 2 [1–7], n = 58 | 2 [2–8] | 0.6 |
| PAS category (%) | 0.93 | ||
| No penetration/aspiration (PAS: 1) | 15/58 (26%) | 12 (24%) | |
| Penetration (PAS: 2–5) | 26/58 (45%) | 21 (43%) | |
| Aspiration (PAS: 6–8) | 17/58 (29%) | 16 (33%) | |
Data presented as n (%), mean ± SD, or median [IQR]. Chronic lung disease of infancy was defined as oxygen use at 36 weeks for infants born ≤ 36 weeks gestational age and oxygen need at discharge for infants born > 36 weeks gestational age. Gastroesophageal reflux disease diagnosis was presumed if treated with acid suppression.
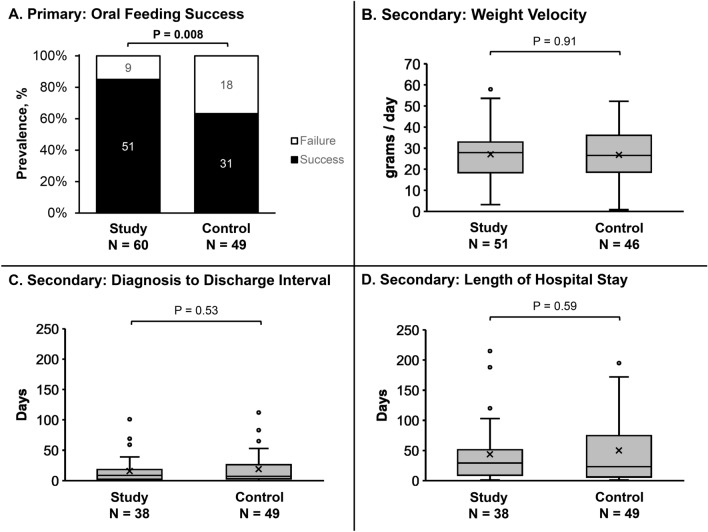
Primary and secondary clinical outcomes are shown in Fig. 2 with primary outcome 85% [76–94%] in study vs 63% [50–77%] in control [unadjusted OR: 3.29 (1.32–8.23), p = 0.008]. After adjusting for gastroesophageal reflux disease and preterm birth, study group was still more likely to achieve oral feeding success [adjusted odds ratio: 4.05 (1.49–10.95), p = 0.005]. Secondary clinical outcome growth velocities (cm/day) were 0.11 ± 0.07 vs 0.11 ± 0.06, p = 0.83 for length, and 0.07 ± 0.04 vs 0.06 ± 0.03, p = 0.17 for head circumference for study vs control, respectively.
Figure 2.
Clinical Outcomes of Infants referred for VFSS (Study Approach: VFSS + HRIM + Parent Preference) and Control (standard-of-care: VFSS informed). On the boxplots, X 's represents the mean while dots represent outliers. Primary clinical outcome success was greater in the study group (A). Secondary outcomes did not significantly differ (B–D). In figures (C,D), there were 22 infants in the study group studied as outpatients and discharged the same day, hence not included in the N value.
Effects of penetration or aspiration on clinical and HRM motility correlates
Clinical outcomes
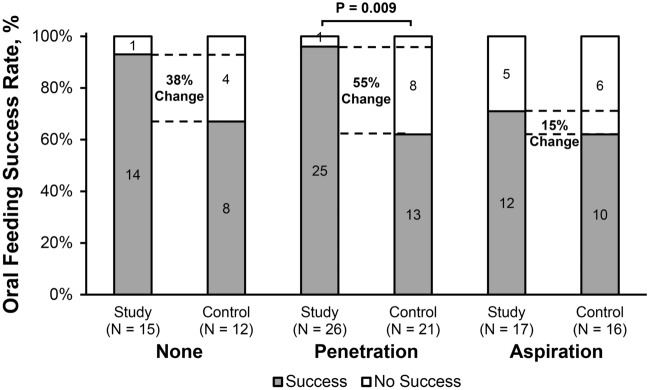
Primary and secondary clinical outcomes did not differ between (a) infants PAS ≥ 2 (vs PAS = 1) in both study and control cohorts, or (b) in study infants with PAS = 1 vs control infants with PAS = 1 (Table 3). However, in infants with PAS ≥ 2, oral feeding success was greater in the study group (Table 3), specifically driven by those infants with penetration (Fig. 3). Feeding therapies in infants with PAS = 1 did not differ between study and control groups (P = 0.30), as well as infants with PAS ≥ 2 between study and control groups (P = 0.29).
Table 3.
Comparison of Clinical Outcomes within and between Study vs. Control Cohorts with and without Penetration or Aspiration.
| Characteristic | Study | Control | PAS = 1: Study vs control P-value | PAS ≥ 2: Study vs control P-value | ||||
|---|---|---|---|---|---|---|---|---|
| PAS = 1 (None) | PAS ≥ 2 (Penetration or Aspiration) | Adjusted P-value | PAS = 1 (None) | PAS ≥ 2 (Penetration or Aspiration) | Adjusted P-value | |||
| N-value | 15 | 43 | 12 | 37 | ||||
| Oral feeding success rate (%) | 14 (93%) | 37 (86%) | 0.99 | 8 (67%) | 23 (62%) | 0.99 | 0.3 | 0.04 |
| Growth velocity | ||||||||
| Weight (g/day) | 27.6 ± 9.7, n = 9 | 27.3 ± 11.6, n = 40 | 0.99 | 27.3 ± 14.0, n = 11 | 26.6 ± 10.8, n = 35 | 0.99 | 0.99 | 0.99 |
| Length (cm/day) | 0.1 ± 0.1, n = 9 | 0.1 ± 0.1, n = 37 | 0.99 | 0.1 ± 0.0, n = 10 | 0.1 ± 0.1, n = 34 | 0.43 | 0.45 | 0.99 |
| Head circumference (cm/day) | 0.1 ± 0.0, n = 9 | 0.1 ± 0.0, n = 35 | 0.99 | 0.1 ± 0.0, n = 9 | 0.1 ± 0.0, n = 33 | 0.99 | 0.99 | 0.9 |
| Nutrition | ||||||||
| Milk type (%) | 0.83 | 0.99 | 0.34 | 0.99 | ||||
| Breast milk | 2/15 (18%) | 3/42 (7%) | 1 (8%) | 3 (8%) | ||||
| Breast milk + Formula | 1/15 (9%) | 12/42 (29%) | 4 (33%) | 8 (22%) | ||||
| Formula | 8/15 (73%) | 27/42 (64%) | 7 (59%) | 26 (70%) | ||||
| Oxygen at discharge* (%) | 0/5 (0%) | 11/31 (35%) | 0.33 | 2 (17%) | 5 (14%) | 0.99 | 0.99 | 0.09 |
| VFSS to discharge interval* (days) | 3 [1–4], n = 5 (1–59) | 9 [2–17], n = 31 (0–101) | 0.99 | 9 [3–18.5] (0–114) | 7 [3–27] (0–112) | 0.99 | 0.99 | 0.99 |
| Length of hospital stay* (days) | 26 [20–38], n = 5 (2–123) | 27 [9–63], n = 31 (1–215) | 0.99 | 19 [7–99] (2–196) | 26 [6–66] (1–198) | 0.99 | 0.99 | 0.99 |
Data presented as n (%), mean ± SE, median [IQR], and (min, max). VFSS: videofluoroscopy swallow study, HRM: high resolution manometry. *Rates calculated for hospital inpatients only.
Figure 3.
No penetration or aspiration: PAS = 1, Penetration: PAS = 2 to 5, Aspiration: PAS = 6 to 8. In the figure legend, success is defined as independent oral feeding, and no success as any tube feeding. Numbers within bars represent n-values of infants. Note in those infants with laryngeal penetration, feeding success was greater in the study group. Although not statistically significant, infants without penetration or aspiration may also clinically benefit from the study approach as indicated by 38% higher oral feeding success.
HRM motility
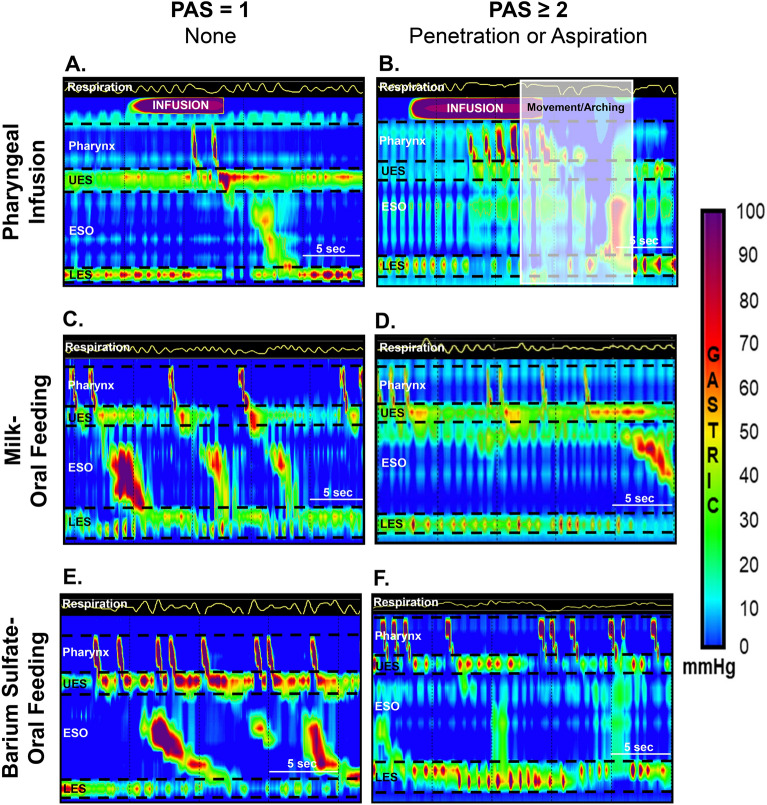
In study infants with PAS ≥ 2 (vs PAS = 1): (a) PAS score was 42 – 8 vs 11 – 1, p < 0.0001; (b) motility outcomes did not significantly differ for basal swallow or oral milk feeding (Table 4); (c) DA during interphase and symptoms were increased with pharyngeal infusions (Table 4); and (d) during oral feeding with barium sulfate, VFSS feeding duration was 85.6 ± 10.3 vs 124.5 ± 16.2 s, p = 0.048, and distal esophageal contractile vigor was decreased (Table 4). Also note during oral feeding, pharyngeal contractile vigor was greater with barium sulfate (vs milk) (Table 4). A representative HRM figure comparing infants with and without penetration-aspiration during pharyngeal infusion and oral feeding is shown (Fig. 4).
Table 4.
Comparison of HRM motility characteristics in study infants with and without penetration or aspiration.
| Characteristic | PAS = 1 | PAS ≥ 2 | P-value |
|---|---|---|---|
| None | Penetration or Aspiration | ||
| Basal Swallow | N = 15 | N = 43 | |
| Pharyngeal vigor (mmHg.cm.s) | 121 ± 12 | 98 ± 8 | 0.11 |
| Proximal vigor (mmHg.cm.s) | 74 ± 10 | 55 ± 6 | 0.12 |
| Distal vigor (mmHg.cm.s) | 47 ± 7 | 42 ± 4 | 0.57 |
| UES: basal tone (mmHg.cm.s) | 28 ± 6 | 20 ± 3 | 0.2 |
| Esophagus | |||
| Proximal vigor (mmHg.cm.s) | 80 ± 15 | 65 ± 9 | 0.41 |
| Distal vigor (mmHg.cm.s) | 337 ± 48 | 360 ± 28 | 0.68 |
| LES: basal tone (mmHg.cm.s) | 68 ± 10 | 62 ± 6 | 0.63 |
| Respiratory: DA duration (s) | 0.8 ± 0.1 | 1.1 ± 0.1 | 0.08 |
| Pharyngeal Reflexes | N = 6 | N = 34 | |
| Peristaltic response occurrence | 0.9 [95% CI 0.5–1.7] | 0.7 | |
| Peristaltic response latency (s) | 4.3 ± 0.7 | 4.8 ± 0.3 | 0.52 |
| Peristaltic response duration (s) | 16.7 ± 2.6 | 19.0 ± 1.1 | 0.42 |
| Pharynx: total contractions (#) | 4 ± 1 | 4 ± 0 | 0.47 |
| UES | |||
| Relaxation reflex occurrence | 1.1 [95% CI 0.4–2.5] | 0.89 | |
| Contraction reflex occurrence | 0.8 [95% CI 0.3–2.2] | 0.71 | |
| LES | |||
| Relaxation reflex occurrence | 2.4 [95% CI 1.0–5.9] | 0.05 | |
| Respiratory | |||
| DA occurrence | 0.9 [95% CI 0.4–2.0] | 0.71 | |
| DA latency, (s) | 4.9 ± 0.8 | 4.9 ± 0.3 | 0.97 |
| DA duration, (s) | 1.1 ± 0.7 | 2.4 ± 0.3 | 0.1 |
| DA during interphase occurrence | 1.9 [95% CI 1.1–3.4] | 0.02 | |
| Terminal swallow occurrence | 0.7 [95% CI 0.4–1.2] | 0.15 | |
| Esophagus: peristaltic break occurrence | 3.4 [95% CI 0.9–13.6] | 0.08 | |
| Symptom occurrence | 2.5 [95% CI 1.2–5.3] | 0.02 | |
| Oral Feeding with Milk | N = 14 | N = 35 | |
| Volume intake rate (mL/min) | 6.1 ± 0.8 | 4.9 ± 0.6 | 0.25 |
| Oral feeding duration (s) | 70.6 ± 11.0 | 95.6 ± 7.0 | 0.06 |
| Pharynx | |||
| Contractile activity (%) | 54.4 ± 14.9 | 52.0 ± 10.6 | 0.9 |
| Vigor (mmHg.cm.s) | 78 ± 10 | 76 ± 7 | 0.85 |
| Proximal vigor (mmHg.cm.s) | 44 ± 8 | 39 ± 5 | 0.65 |
| Distal vigor (mmHg.cm.s) | 33 ± 7 | 36 ± 5 | 0.69 |
| Esophagus: Distal vigor (mmHg.cm.s) | 432 ± 83 | 337 ± 49 | 0.33 |
| Oral Feeding with Barium Sulfate | N = 15 | N = 37 | |
| Volume intake rate (mL/min) | 10.9 ± 3.5 | 14.9 ± 1.9* | 0.32 |
| Oral feeding duration (s) | 124.5 ± 16.2* | 85.6 ± 10.3 | 0.048 |
| Pharynx | |||
| Contractile activity (%) | 65.5 ± 7.2 | 60.4 ± 5.0 | 0.56 |
| Vigor (mmHg.cm.s) | 95 ± 10* | 83 ± 8* | 0.38 |
| Proximal vigor (mmHg.cm.s) | 51 ± 8* | 42 ± 6* | 0.35 |
| Distal vigor (mmHg.cm.s) | 44 ± 8* | 42 ± 6* | 0.82 |
| Esophagus: Distal vigor (mmHg.cm.s) | 460 ± 67 | 217 ± 45 | 0.004 |
Data presented as Mean ± SE or Odds Ratio [95% CI] with PAS = 1 used as reference group for Odds Ratios. Interpretation example: infants with penetration or aspiration are 2.5 times more likely to have symptoms than those without penetration or aspiration. UES- upper esophageal sphincter, LES- lower esophageal sphincter, DA- deglutition apnea.
*p < 0.05 versus oral feeding with milk.
Figure 4.
Motility correlates during pharyngeal infusion, oral feeding with milk, and oral feeding with barium sulfate of infants with and without penetration or aspiration. UES- upper esophageal sphincter, ESO- esophagus, LES- lower esophageal sphincter. Shown are representative esophago-pressure topography plots during HRM. Significantly, note in infants with penetration or aspiration symptoms are increased during pharyngeal infusion (A,B) and esophageal contractions are weaker during oral feeding with barium sulfate (E,F). Also note, stronger pharyngeal vigor during barium-sulfate oral feeding (E,F) vs milk oral feeding (C,D).
Discussion
Overarching purpose, rationale, and goals
Delays with acquisition of safe oral feeding milestones often lead to non-objective “kitchen-sink” approaches, which result in increased length of hospitalization and therefore, health care costs. VFSS is widely available as a ‘gold standard’ radiological procedure to evaluate swallowing functions, but it can be highly subjective in the absence of standardization of testing process, analysis and recommendations. Prolonged provocative physiological testing like crib-side feeding methods while monitoring for symptoms is not possible with VFSS owing to the risks of radiation exposure. Presence or absence of aspiration or penetration during VFSS alone may not be adequate in developing feeding therapies. HRM permits prolonged provocative evaluation in the absence of radiation exposure under physiological conditions at crib-side while monitoring for pathophysiological changes. Furthermore, HRM is emerging as a safer method to assess not only swallowing pathophysiology but also feeding methods and aerodigestive protective reflexes regardless of underlying primary diagnosis. During HRM, oral feeding challenges can be permissible with various feeding systems including breastfeeding, when able. Some empiric approaches to manage feeding difficulties may include evaluation of aerodigestive apparatus for structural details, modifying nutrition or changing nipples, adding thickening agents, changing to breast milk substitutes, beginning gastric acid suppression, or adopting postural modifications54. Any of these methods may not improve outcomes, as feeding is a complex skill and involves understanding of the process, physiology, patient’s skills and airway protective mechanisms. Commonly, when there is failure with empiric approaches, discharge tube-feeding decisions (gastrostomy and or fundoplication, chronic nasogastric or transpyloric feeds) are made, and gastrostomy rates at discharge are increasing55. It is unknown how these diagnostic decisions and management strategies impact oral feeding success and hospital utilization in infants with and without penetration or aspiration, and how pharyngo-esophageal motility differs in infants with penetration or aspiration. Therefore, this study was undertaken to evaluate the effects of (a) an integrated study approach (VFSS and HRM guided decision making for therapy) vs the standard of care approaches (VFSS alone) on oral feeding outcomes and hospital utilization, and (b) penetration or aspiration on oral feeding outcomes, hospital utilization, and pharyngo-esophageal physiology.
Salient findings of our study
Salient findings of the current study are as follows: (1) In study vs control: The study cohort was superior to the control cohort in achieving oral feeding success (primary outcome). Secondary clinical outcomes (growth velocities, nutrition, oxygen requirement at discharge, and length of hospitalization) did not significantly differ. Feeding therapies (nipple flow, fluid thickness, or no modification) did not significantly differ. (2) In infants with penetration or aspiration (vs none): (a) clinical outcomes did not significantly differ, (b) sensory-motor motility characteristics (pharynx, UES, esophageal, and LES) did not significantly differ during basal swallowing or oral milk feeding, (c) DA during interphase and symptoms were more likely to occur during pharyngeal infusion, and d) distal esophageal contractile vigor was lesser during feeding with radiological contrast. (3) Media effects: Barium sulfate (vs milk) resulted in greater pharyngeal contractile vigor.
Clinically important reasons for the study outcomes underlie in study approaches
Providing the additional mechanistic data with HRM at crib-side for a prolonged period, and having parents provide therapy based on their understanding of the combined results of VFSS and HRM findings may have improved the oral feeding outcomes in these complex infants. Empowering parents to make decisions for their infant’s feeding based on the expanded objective data likely led to better outcomes. On a different note, diagnostics can only improve treatments through the selection of several components of the therapeutic bundle, as eating is a complex process. We believe, our study findings address this.
Parent participation/attendance can be variable. However, parents see what is going on during the testing process and ask relevant questions related to feeding and cofounders that are impeding discharge planning. When they see the factual findings as they are happening with regards to swallowing, reflexes, airway protection, volume intake, and vital signs, they then see the capabilities and limitations of their infant. This approach may have improved their confidence to feed, and in some situations, parents could feed their infant during HRM. All these approaches improve parent competency with infant feeding.
Finally, several feeding positions are commonly attempted by mother (as in breast-feeding positions) or by parents and providers in bottle feeding positions. One important thing this study addresses is airway safety, regardless of the feeding position. Unfortunately, we are not powered enough to directly answer this question and additional mechanistic study designs are needed with larger patient numbers. There were no differences in positions between Study and Control (Table 2) infants at VFSS evaluation.
Cross-systems physiology of glottal closure amidst esophageal clearance
In infants with penetration or aspiration, DA (a surrogate marker of glottal closure) was twice as likely to occur during interphase and was approximately twice as long (Table 4), which also translated to 2.5 times more symptoms. It is likely that in those infants with penetration or aspiration, the pause in breathing in between the respiratory phases is extended. With penetration, glottal closure is coordinated and effective in preventing bolus from entering below the vocal cords. With aspiration, coordination of glottal closure fails, where symptoms may occur, or may not occur and is termed “silent aspiration”. Glottal closure and pharyngo-esophageal clearance mechanisms have been described by us and others in infants with and without swallowing abnormalities26–28,33,56,57.
Central swallowing mechanisms are hierarchical, and are adaptational; for example, when these mechanisms are dysfunctional (as in the absence of swallowing, poor propagation, or poor coordination) other cascading reflexes are triggered such as coughing or apnea/bradycardia/desaturation events26–28,33,57. On the other hand, swallowing is also an important restorative mechanism for cardiorespiratory and aerodigestive homeostasis via effective terminal swallowing28,29,33,38,39. Thus, what construes as a troublesome symptom (problem) is actually a sign of adaptive skill in ensuring aerodigestive clearance. In the current study, terminal swallowing was present indicating that the capability exists in infants with penetration or aspiration. However, the presence of esophageal peristaltic breaks during pharyngeal infusion is trending towards significance and distal esophageal contractile vigor is significantly lesser in patients with penetration/aspiration, which are markers of esophageal dysmotility. Therefore, underlying issues maybe associated with dysfunctional esophageal motility and clearance mechanisms or peristaltic coordination, all of which are important components of pharyngo-esophageal propulsion, esophageal clearance and aerodigestive protection. Hence, potential therapeutic targets may be to strengthen esophageal motility mechanisms and cross-system interactions by prescribing oral feeding therapies cautiously. Additionally, as the current study evaluates swallowing function at the patient level (gross abnormalities), evaluation of individual swallows (acute abnormalities) resulting in penetration or aspiration may provide insight into sensory-motor physiologic vs pathophysiologic mechanisms of glottal closure and swallowing coordination.
Implications for standardization of diagnostic and management approaches
Suggested modifications to VFSS evaluation methods are as follows: (a) Shortening Testing Duration: In infants with penetration or aspiration, VFSS trial duration was 85.6 ± 10.3 s indicating that if penetration or aspiration were to occur, it would likely be within this timeframe. In infants without penetration/aspiration, testing was prolonged by more than 30 s, thus increasing radiation exposure (Table 4: Oral Feeding with Barium Sulfate). Therefore, we suggest standardizing and limiting individual VFSS trials to less than 90 s because if aspiration were to happen, it would have in that time frame. (b) Consider changes to testing media: Oral feeding with barium sulfate may not be a physiologic comparator to milk feeding for evaluation of pharyngeal function as pharyngeal contractile vigor was greater with barium sulfate (vs milk) (Table 4). (c) Modified protocol to evaluate protective mechanisms in aspirators: True silent aspiration may result when symptoms do not occur and may be a marker of sensory dysfunction and gross failure of protective mechanisms. Studies have been controversial whether aspiration is truly detrimental. This may be due to operational testing conditions. Normally when the parent or feeding provider sees aerodigestive symptoms during feeding, nipple is immediately removed from the infant’s mouth, which likely triggers a terminal swallow and facilitates aerodigestive clearance, as has happened in our HRM study. Therefore, it is plausible that true silent aspiration is overestimated, and infant may not have met the sensory threshold to activate potential compensatory mechanisms.
Addition of HRM to complement VFSS and improve mechanistic understanding and outcomes: HRM testing includes prolonged and detailed evaluation of kinetic and dynamic swallowing-, breathing-, functional-, and aerodigestive protective mechanisms without the need for neonatal ICU patient transport or exposure to radiation. It also enables assessment of neurologic, cardio-respiratory, and swallowing rhythms in the presence or absence of symptoms. Thus, this approach can add value in improving the feeding and discharge outcomes without the risk of adverse events, even in those with laryngeal penetration and aspiration. Advanced research protocols and quality improvement initiatives can also emerge from this work in future in refining diagnostic and therapeutic strategies in the context of deglutition disorders and aerodigestive complications.
Limitations/Future Directions
This study has limitations as follows: (1) Although randomized allocation of feeding modification therapies would have added scientific rigor (eliminating bias), it was not pragmatic owing to parent-provider hesitancy; hence, study design was modified. (2) The addition of HRM and the parent preferred therapy with concurrent controls provided important clinical outcome data, but control cohort did not have the benefit of HRM. (3) The current study evaluates gross swallowing function abnormalities at the patient level. Detailed evaluation of individual swallows resulting in penetration or aspiration is needed to detect acute swallowing abnormalities in real time. This would likely provide insight into sensory-motor physiologic vs pathophysiologic mechanisms of glottal closure and swallowing coordination. (4) While VFSS is frequently considered by physicians/therapists, there is a variability with the conduct of VFSS studies among neonatal ICU infants with regards to indications, timing, approach, analysis, and recommendations. (5) This study was conducted in a tertiary care referral center where we see complex feeding difficulties, and VFSS is frequently done for infant’s with dysphagia. However, given the superior outcomes using our innovative study approaches, the reliability of VFSS alone in developing long-term feeding strategies is questionable.
Conclusions
The study cohort was superior to the control cohort in achieving oral feeding success in infants referred for VFSS evaluation. This indicates that comprehensive evaluation and individualized management strategies including parental education with feeding engagement practices may be more beneficial than prescribed feeding modifications based on VFSS alone. With the addition of HRM, establishment of compensatory mechanisms, modification of esophageal motility and airway interactions are potential therapeutic targets in infants with or without penetration or aspiration. Diagnostic and mechanistic evidence-based feeding management bundles can then be developed for the most appropriate and pragmatic care, thus resulting in superior clinical outcomes. These approaches may also provide confidence to parents with post-discharge feeding management among neonatal intensive care unit graduates.
Acknowledgements
Study Design Advice and Support: Reza Shaker, MD, and Ivan M. Lang, PhD. Clinical Support: D Gregory Bates, MD, and Benjamin Thompson, MD, for Radiology support, Rebecca K. Moore, MACPR, BSN, RN and Casey Fritter, BSN, RN for nursing coordination and support. Data Safety Monitoring Board: We are appreciative of the Data Safety Monitoring Board at Nationwide Children’s Hospital, Columbus, OH for their oversight, risk-benefit assessment, monitoring of recruitment and safety, and providing guidance throughout the life of this study. Members included: Richard E. McClead, MD (Chief Quality and Safety Officer, Former Chairman of institutional review board at Nationwide Children’s Hospital); Erinn Hade, PhD (Research Assistant Professor of Biostatistics at The Ohio State University College of Medicine); Adriane Bayless, PhD, CCC-SLP (Director of the Velo-Pharyngeal Dysphagia program and co-director of the q22 center at Nationwide Children’s Hospital); Leslie Thomas, MS, RN, APN, (Manager, Advanced Practice Nurse Program at Nationwide Children’s Hospital); Jennifer Hofherr, MS, OTR/L, C/NDT, (Neonatal Clinical Therapies Program Manager at Nationwide Children’s Hospital); Rebecca Romero, MS, RD, LD, CLC, (Clinical Leader Neonatal Nutrition at Nationwide Children’s Hospital); Renee Gardikes Gingery, MS, RN, MSN, (Director of Neonatal Services at Nationwide Children’s Hospital). The committee met on a quarterly basis. Institutional Review Board: We would like to acknowledge the Institutional Review Board at Nationwide Children’s Hospital Columbus, OH for their oversight, guidance and monitoring during this study. The study protocol and amendments during the study were approved by the institutional review board. Audit: The Principal Investigator, Dr. Sudarshan Jadcherla, voluntarily requested an audit to be conducted by the Office of Research Compliance and Integrity during the study to seek guidance on the process of quality improvement. We are thankful to John Psurny, BS, CCRP, Senior Quality Assurance GCP Auditor, and Beth Roley, MBA, MSN, RN, Quality Assurance Medical Auditor for their guidance and oversight.
Abbreviations
- VFSS
Videofluoroscopy swallowing study
- HRM
High resolution manometry
- PAS
Penetration aspiration scale
- UES
Upper esophageal sphincter
- LES
Lower esophageal sphincter
- DA
Deglutition apnea
Author contributions
S.R.J. and L.W. designed the study. S.R.J., K.A.H., E.K.O., H.I., R.H., Z.S., and N.L. performed HRM studies. H.B. and S.S. performed VFSS studies. K.A.H., H.I., R.H., and Z.S. acquired and analyzed HRM data. D.S.L., H.B., and S.S. analyzed VFSS data. S.R.J., K.A.H., E.K.O., H.I., R.H., Z.S., N.L., V.O.Y., and L.W. verified and validated data. V.O.Y. and L.W. performed statistical analysis. S.R.J. and E.K.O. obtained institutional review board approvals. S.R.J. secured funding. S.R.J., K.A.H., and E.K.O. wrote the first draft of the manuscript. S.R.J., K.A.H., E.K.O., D.S.L., H.I., R.H., Z.S., N.L., V.O.Y., H.B., S.S., and L.W. were involved with manuscript editing, writing, and approval of final version.
Funding
Supported by the National Institutes of Health, P01 DK 068051 [to Jadcherla, Lang, Shaker], the National Center for Advancing Translational Sciences (UL1TR002733 [to The Ohio State University Center for Clinical and Translational Science for REDCap support]), and Masters Award in Gastroenterology grant by the American Gastroenterology Association [to Jadcherla].
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to information that may compromise privacy of research participants, and further development of manuscripts are in process to address other project goals. These data may be available upon reasonable request to corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kovacic K, Rein LE, Szabo A, Kommareddy S, Bhagavatula P, Goday PS. Pediatric feeding disorder: A nationwide prevalence study. J. Pediatr. 2021;228:126–131. doi: 10.1016/j.jpeds.2020.07.047. [DOI] [PubMed] [Google Scholar]
- 2.Rommel N, De Meyer AM, Feenstra L, Veereman-Wauters G. The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. J. Pediatr. Gastroenterol. Nutr. 2003;37:75–84. doi: 10.1097/00005176-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 3.A Academy of Pediatrics Committee on Fetus and Newborn Hospital discharge of the high-risk neonate. Pediatrics. 2008;122:1119–1126. doi: 10.1542/peds.2008-2174. [DOI] [PubMed] [Google Scholar]
- 4.Lau C. Development of infant oral feeding skills: What do we know? Am. J. Clin. Nutr. 2016;103:616S–621S. doi: 10.3945/ajcn.115.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin-Harris B, Canon CL, Bonilha HS, Murray J, Davidson K, Lefton-Greif MA. Best practices in modified barium swallow studies. Am. J. Speech Lang Pathol. 2020;29:1078–1093. doi: 10.1044/2020_AJSLP-19-00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGrattan KE, McGhee HC, McKelvey KL, Clemmens CS, Hill EG, DeToma A, Hill JG, Simmons CE, Martin-Harris B. Capturing infant swallow impairment on videofluoroscopy: Timing matters. Pediatr. Radiol. 2020;50:199–206. doi: 10.1007/s00247-019-04527-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogan AH, Bellin E, Douglas L, Levin TL, Esteban-Cruciani N. Radiation exposure of premature infants beyond the perinatal period. Hosp. Pediatr. 2018;8:672–678. doi: 10.1542/hpeds.2017-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko EJ, Sung IY, Choi KH, Kwon YG, Yoon J, Kim T. Radiation exposure during videofluoroscopic swallowing studies in young children. Int. J. Pediatr. Otorhinolaryngol. 2019;121:1–5. doi: 10.1016/j.ijporl.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 9.Bonilha HS, Wilmskoetter J, Tipnis SV, Martin-Harris B, Huda W. Estimating thyroid doses from modified barium swallow studies. Health Phys. 2018;115:360–368. doi: 10.1097/HP.0000000000000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin-Harris B, Carson KA, Pinto JM, Lefton-Greif MA. BaByVFSSImP((c)) a novel measurement tool for videofluoroscopic assessment of swallowing impairment in bottle-fed babies: Establishing a standard. Dysphagia. 2020;35:90–98. doi: 10.1007/s00455-019-10008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson B, Lundine JP, Madhoun L, Hu H, Holliman-Wade D, Bates DG. Standardization of Radiologic Procedures for Pediatric Videofluoroscopic Swallow Studies: A Service-based Quality Improvement Initiative. Pediatr Qual Saf. 2018;3:123. doi: 10.1097/pq9.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staiano A, Boccia G, Miele E, Clouse RE. Segmental characteristics of oesophageal peristalsis in paediatric patients. Neurogastroenterol. Motil. 2008;20:19–26. doi: 10.1111/j.1365-2982.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 13.Goldani HA, Staiano A, Borrelli O, Thapar N, Lindley KJ. Pediatric esophageal high-resolution manometry: Utility of a standardized protocol and size-adjusted pressure topography parameters. Am. J. Gastroenterol. 2010;105:460–467. doi: 10.1038/ajg.2009.656. [DOI] [PubMed] [Google Scholar]
- 14.Rommel N, van Wijk M, Boets B, Hebbard G, Haslam R, Davidson G, Omari T. Development of pharyngo-esophageal physiology during swallowing in the preterm infant. Neurogastroenterol. Motil. 2011;23:e401–408. doi: 10.1111/j.1365-2982.2011.01763.x. [DOI] [PubMed] [Google Scholar]
- 15.Edeani F, Malik A, Kaul A. Characterization of esophageal motility disorders in children presenting with dysphagia using high-resolution manometry. Curr. Gastroenterol. Rep. 2017;19:13. doi: 10.1007/s11894-017-0549-x. [DOI] [PubMed] [Google Scholar]
- 16.Ferris L, King S, McCall L, Rommel N, Scholten I, Teague W, Doeltgen S, Omari T. Piecemeal deglutition and the implications for pressure impedance dysphagia assessment in pediatrics. J. Pediatr. Gastroenterol. Nutr. 2018;67:713–719. doi: 10.1097/MPG.0000000000002080. [DOI] [PubMed] [Google Scholar]
- 17.Rayyan M, Omari T, Naulaers G, Aerts R, Allegaert K, Rommel N. Maturation of esophageal motility and esophagogastric junction in preterm infants. Neonatology. 2020;117:495–503. doi: 10.1159/000506481. [DOI] [PubMed] [Google Scholar]
- 18.Jadcherla SR, Prabhakar V, Hasenstab KA, Nawaz S, Das J, Kern M, Balasubramanian G, Shaker R. Defining pharyngeal contractile integral during high-resolution manometry in neonates: A neuromotor marker of pharyngeal vigor. Pediatr. Res. 2018;84:341–347. doi: 10.1038/s41390-018-0097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prabhakar V, Hasenstab KA, Osborn E, Wei L, Jadcherla SR. Pharyngeal contractile and regulatory characteristics are distinct during nutritive oral stimulus in preterm-born infants: Implications for clinical and research applications. Neurogastroenterol. Motil. 2019;31:1–7. doi: 10.1111/nmo.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson K, O'Rourke A, Fortunato JE, Jadcherla S. The emerging importance of high-resolution manometry in the evaluation and treatment of deglutition in infants, children, and adults: new opportunities for speech-language pathologists. Am. J. Speech Lang. Pathol. 2020;29:945–955. doi: 10.1044/2019_AJSLP-19-00067. [DOI] [PubMed] [Google Scholar]
- 21.Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: Clinical and instrumental approaches. Dev. Disabil. Res. Rev. 2008;14:118–127. doi: 10.1002/ddrr.17. [DOI] [PubMed] [Google Scholar]
- 22.Hiorns MP, Ryan MM. Current practice in paediatric videofluoroscopy. Pediatr. Radiol. 2006;36:911–919. doi: 10.1007/s00247-006-0124-3. [DOI] [PubMed] [Google Scholar]
- 23.Henderson M, Miles A, Holgate V, Peryman S, Allen J. Application and verification of quantitative objective videofluoroscopic swallowing measures in a pediatric population with dysphagia. J. Pediatr. 2016;178:200–205. doi: 10.1016/j.jpeds.2016.07.050. [DOI] [PubMed] [Google Scholar]
- 24.Shubert TR, Sitaram S, Jadcherla SR. Effects of pacifier and taste on swallowing, esophageal motility, transit, and respiratory rhythm in human neonates. Neurogastroenterol. Motil. 2016;28:532–542. doi: 10.1111/nmo.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen PS, Gulati IK, Shubert TR, Sitaram S, Sivalingam M, Hasenstab KA, El-Mahdy MA, Jadcherla SR. Pharyngeal stimulus-induced reflexes are impaired in infants with perinatal asphyxia: Does maturation modify? Neurogastroenterol. Motil. 2017;29:1–16. doi: 10.1111/nmo.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jadcherla SR, Gupta A, Coley BD, Fernandez S, Shaker R. Esophago-glottal closure reflex in human infants: A novel reflex elicited with concurrent manometry and ultrasonography. Am. J. Gastroenterol. 2007;102:2286–2293. doi: 10.1111/j.1572-0241.2007.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jadcherla SR, Gupta A, Wang M, Coley BD, Fernandez S, Shaker R. Definition and implications of novel pharyngo-glottal reflex in human infants using concurrent manometry ultrasonography. Am. J. Gastroenterol. 2009;104:2572–2582. doi: 10.1038/ajg.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasenstab KA, Jadcherla SR. Respiratory events in infants presenting with apparent life threatening events: Is there an explanation from esophageal motility? J. Pediatr. 2014;165:250–255. doi: 10.1016/j.jpeds.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasenstab KA, Nawaz S, Lang IM, Shaker R, Jadcherla SR. Pharyngoesophageal and cardiorespiratory interactions: Potential implications for premature infants at risk of clinically significant cardiorespiratory events. Am. J. Physiol. Gastrointest. Liver Physiol. 2019;316:G304–G312. doi: 10.1152/ajpgi.00303.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jadcherla SR, Peng J, Moore R, Saavedra J, Shepherd E, Fernandez S, Erdman SH, DiLorenzo C. Impact of personalized feeding program in 100 NICU infants: Pathophysiology-based approach for better outcomes. J. Pediatr. Gastroenterol. Nutr. 2012;54:62–70. doi: 10.1097/MPG.0b013e3182288766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jadcherla SR, Stoner E, Gupta A, Bates DG, Fernandez S, Di Lorenzo C, Linscheid T. Evaluation and management of neonatal dysphagia: Impact of pharyngoesophageal motility studies and multidisciplinary feeding strategy. J. Pediatr. Gastroenterol. Nutr. 2009;48:186–192. doi: 10.1097/MPG.0b013e3181752ce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jadcherla SR, Hoffmann RG, Shaker R. Effect of maturation of the magnitude of mechanosensitive and chemosensitive reflexes in the premature human esophagus. J. Pediatr. 2006;149:77–82. doi: 10.1016/j.jpeds.2006.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jadcherla SR, Hasenstab KA, Shaker R, Castile RG. Mechanisms of cough provocation and cough resolution in neonates with bronchopulmonary dysplasia. Pediatr. Res. 2015;78:462–469. doi: 10.1038/pr.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prabhakar V, Hasenstab KA, Osborn E, Wei L, Jadcherla SR. Pharyngeal contractile and regulatory characteristics are distinct during nutritive oral stimulus in preterm-born infants: Implications for clinical and research applications. Neurogastroenterol. Motil. 2019;31:e13650. doi: 10.1111/nmo.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park CH, Lee YT, Yi Y, Lee JS, Park JH, Yoon KJ. Ability of high-resolution manometry to determine feeding method and to predict aspiration pneumonia in patients with dysphagia. Am. J. Gastroenterol. 2017;112:1074–1083. doi: 10.1038/ajg.2017.81. [DOI] [PubMed] [Google Scholar]
- 36.Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol. Motil. 2015;27:160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Triantafyllou T, Theodoropoulos C, Mantides A, Chrysikos D, Smparounis S, Filis K, Zografos G, Theodorou D. Can the upper esophageal sphincter contractile integral help classify achalasia? Ann. Gastroenterol. 2018;31:456–461. doi: 10.20524/aog.2018.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasenstab KA, Sitaram S, Lang IM, Shaker R, Jadcherla SR. Maturation modulates pharyngeal-stimulus provoked pharyngeal and respiratory rhythms in human infants. Dysphagia. 2018;33:63–75. doi: 10.1007/s00455-017-9833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasenstab-Kenney KA, Bellodas Sanchez J, Prabhakar V, Lang IM, Shaker R, Jadcherla SR. Mechanisms of bradycardia in premature infants: Aerodigestive-cardiac regulatory-rhythm interactions. Physiol. Rep. 2020;8:e14495. doi: 10.14814/phy2.14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jadcherla SR, Hasenstab KA, Sitaram S, Clouse BJ, Slaughter JL, Shaker R. Effect of nasal noninvasive respiratory support methods on pharyngeal provocation-induced aerodigestive reflexes in infants. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;310:G1006–1014. doi: 10.1152/ajpgi.00307.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jadcherla SR, Gupta A, Stoner E, Fernandez S, Shaker R. Pharyngeal swallowing: defining pharyngeal and upper esophageal sphincter relationships in human neonates. J. Pediatr. 2007;151:597–603. doi: 10.1016/j.jpeds.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jadcherla SR, Shubert TR, Gulati IK, Jensen PS, Wei L, Shaker R. Upper and lower esophageal sphincter kinetics are modified during maturation: Effect of pharyngeal stimulus in premature infants. Pediatr. Res. 2015;77:99–106. doi: 10.1038/pr.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: Normal and abnormal. Phys. Med. Rehabil. Clin. N Am. 2008;19(691–707):vii. doi: 10.1016/j.pmr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin-Harris B, Brodsky MB, Michel Y, Lee FS, Walters B. Delayed initiation of the pharyngeal swallow: Normal variability in adult swallows. J. Speech Lang Hear Res. 2007;50:585–594. doi: 10.1044/1092-4388(2007/041). [DOI] [PubMed] [Google Scholar]
- 45.Eisenhuber E, Schima W, Schober E, Pokieser P, Stadler A, Scharitzer M, Oschatz E. Videofluoroscopic assessment of patients with dysphagia. Am. J. Roentgenol. 2002;178:393–398. doi: 10.2214/ajr.178.2.1780393. [DOI] [PubMed] [Google Scholar]
- 46.McCulloch TM, Hoffman MR, Ciucci MR. High-resolution manometry of pharyngeal swallow pressure events associated with head turn and Chin Tuc. Ann. Otol. Rhinol. Laryngol. 2010;119:369–376. doi: 10.1177/000348941011900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins CR, Hasenstab KA, Nawaz S, Jadcherla SR. Mechanisms of aerodigestive symptoms in infants with varying acid reflux index determined by esophageal manometry. J. Pediatr. 2019;206:240–247. doi: 10.1016/j.jpeds.2018.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol. Motil. 2012;24(Suppl 1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 50.Eisenhuber E, Schima W, Schober E, Pokieser P, Stadler A, Scharitzer M, Oschatz E. Videofluoroscopic assessment of patients with dysphagia: Pharyngeal retention is a predictive factor for aspiration. AJR Am. J. Roentgenol. 2002;178:393–398. doi: 10.2214/ajr.178.2.1780393. [DOI] [PubMed] [Google Scholar]
- 51.Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE, International High Resolution Manometry Working The Chicago classification of esophageal motility disorders v3.0. Neurogastroenterol. Motil. 2015;27:160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bokisa AE, Bonachea EM, Jadcherla SR. Death by neurologic criteria in a neonate: Implications for organ donation. J. Neonatal. Perinatal. Med. 2015;8:263–267. doi: 10.3233/NPM-15814074. [DOI] [PubMed] [Google Scholar]
- 53.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coon ER, Srivastava R, Stoddard GJ, Reilly S, Maloney CG, Bratton SL. Infant videofluoroscopic swallow study testing, swallowing interventions, and future acute respiratory illness. Hosp. Pediatr. 2016;6:707–713. doi: 10.1542/hpeds.2016-0049. [DOI] [PubMed] [Google Scholar]
- 55.Fox D, Campagna EJ, Friedlander J, Partrick DA, Rees DI, Kempe A. National trends and outcomes of pediatric gastrostomy tube placement. J. Pediatr. Gastroenterol. Nutr. 2014;59:582–588. doi: 10.1097/MPG.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 56.Rommel N, Selleslagh M, Hoffman I, Smet MH, Davidson G, Tack J, Omari TI. Objective assessment of swallow function in children with suspected aspiration using pharyngeal automated impedance manometry. J. Pediatr. Gastroenterol. Nutr. 2014;58:789–794. doi: 10.1097/MPG.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 57.Pickens DL, Schefft GL. Thach BT 1989 Pharyngeal fluid clearance and aspiration preventive mechanisms in sleeping infants. J. Appl. Physiol. 1985;66:1164–1171. doi: 10.1152/jappl.1989.66.3.1164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to information that may compromise privacy of research participants, and further development of manuscripts are in process to address other project goals. These data may be available upon reasonable request to corresponding author.