Abstract
Purpose:
To report an anatomic change observed in a series of patients who underwent subretinal injection of voretigene neparvovec-rzyl (VN) for RPE65-mediated Leber congenital amaurosis.
Design:
Multi-center retrospective chart review.
Participants:
Patients who underwent subretinal VN injection at each of four participating institutions.
Methods:
Patients were identified as having perifoveal chorioretinal atrophy if: i) the areas of atrophy were not directly related to the touch-down site of the subretinal cannula; and ii) the area of atrophy progressively enlarged over time. Demographic data, visual acuity, refractive error, fundus photos, optical coherence tomography, visual fields, and full-field stimulus threshold (FST) were analyzed.
Main Outcome Measures:
Outcome measures included change in visual acuity, FST, visual fields, and location of atrophy relative to subretinal bleb position.
Results:
18 eyes of 10 patients who underwent subretinal injection of VN were identified as having developed perifoveal chorioretinal atrophy. 8/10 patients (80%) developed bilateral atrophy. The mean age was 11.6 years (range: 5–20), and 6 patients (60%) were male. Baseline mean logMAR visual acuity and FST were 0.82 (standard deviation (SD): 0.51) and −1.3 log cd.s/m2(SD: 0.44), respectively. The mean spherical equivalent was −5.7 diopters (range: 11.50 to +1.75). Atrophy was identifiable at an average of 4.7 months (SD: 4.3) following surgery, and progressively enlarged in all cases up to a mean follow-up period of 11.3 months (range: 4–18). Atrophy developed within and outside the area of the subretinal bleb in 10 (55.5%) eyes, exclusively within the area of the bleb in 7 (38.9%) eyes, and exclusively outside the bleb in 1 (5.5%) eye. There was no significant change in visual acuity (p=0.45). There was a consistent improvement in FST with a mean improvement of −3.21 log cd.s/m2(p<0.0001). Additionally, all 13 eyes with reliable pre- and post-operative Goldmann visual fields demonstrated improvement (expansion and/or gain of isopters) following surgery, but 3 (23.1%) eyes demonstrated paracentral scotomas related to the atrophy.
Conclusions:
A subset of patients undergoing subretinal VN injection developed progressive perifoveal chorioretinal atrophy following surgery. Further study is necessary to determine what ocular, surgical delivery, and vector-related factors predispose to this complication.
Précis
We report perifoveal chorioretinal atrophy in 18 eyes of 10 patients who underwent subretinal voretigene injection for RPE65-mediated Leber congenital amaurosis. Anatomic and functional outcomes are described and possible causes are discussed.
Introduction
Leber congenital amaurosis (LCA) represents a group of rare inherited retinal diseases (IRDs), affecting 1 in 50–100,000 people worldwide.1 Patients with LCA have poor vision from birth, which progressively declines to near complete blindness in early to mid-adulthood. To date, over 25 genes have been associated with LCA, including RPE65 (retinal pigment epithelium-specific protein, 65 kDa).2 RPE65 encodes an isomerohydrolase that catalyzes the conversion of all-trans-retinyl esters to 11-cis-retinol in the retinoid cycle.3 For patients lacking RPE65 function, photoreceptors are unable to regenerate visual pigments after exposure to light, leading to profound nyctalopia.
While there isnotreatment for themajority ofgenotypescausingLCA, voretigene neparvovec-rzyl (VN, Luxturna, Spark Therapeutics, Philadelphia, PA) was FDA-approved in 2017 for patients with LCA caused by biallelic RPE65 mutations.4 VN is an adeno-associated virus 2 (AAV2) vector containing a functional copy of the RPE65 gene,whichis injected into the subretinal space via pars plana vitrectomy.4 The phase 3 trial that led to the approval of VN demonstrated substantial improvements in visual function as measured by multi-luminance mobility testing (MLMT) and full-field light sensitivity threshold (FST) testing, as compared to controls.4 Additionally, subjects experienced a mean doubling of their III4e Goldmann visual field isopter.
Although 68% of subjects experienced one or more treatment-associated adverse events, most were minor. The most common adverse events were transient intraocular pressure elevations (18%), cataract (18%), ocular inflammation (8%), retinal tears (8%), dellen (8%), and retinal deposits (8%).4,5 To date, there are no reports of perifoveal chorioretinal atrophy following subretinal VN. In this study, we describe 18 eyes that developed perifoveal chorioretinal atrophy following subretinal VN at 4 of the 10 sites in the US currently treating patients with VN. The characteristics of the atrophy, patient factors, outcomes, and possible causes are discussed.
Methods
Patient Selection and Surgical Delivery
A retrospective chart review was performed on all patients who received subretinal VN injection at each of four participating institutions. The study was approved by the Institutional Review Boards at Children’s Hospital Los Angeles, University of Miami, University of Cincinnati, and University of Michigan. All patients provided consent or parental permission and assent was obtained, as applicable. This study complied with the Health Insurance Portability and Accountability Act and adhered to the tenets of the Declaration of Helsinki. All procedures were performed according to the recommended protocol described by Russell, et al4with the following key points and exceptions: 1) Vector was delivered using surgeon foot-pedal control with the Viscous Fluid Control tubing (Alcon, Fort Worth, TX) connected to the MedOne Microdose syringe (MedOne Surgical, Inc, Sarasota, FL) with maximum injection pressure of 16 PSI; 2) Vector was injected directly into the subretinal space (no saline “pre-bleb”); and 3) Subretinal delivery of vector was confirmed using live intraoperative optical coherence tomography (OCT; ReSCAN 700, Carl Zeiss Meditec, Dublin, CA or Leica EnFocus HD OCT Leica Microsystems, Buffalo Grove, IL).
Data and Outcome Measures
Patients were identified as having perifoveal chorioretinal atrophy if: i) the areas of atrophy were not directly related to the touch-down site of the subretinal cannula; and ii) the area of atrophy progressively enlarged over time. Demographic data, visual acuity, refractive error, fundus photos, optical coherence tomography, visual fields, and full-field stimulus threshold (FST) were analyzed for all patients when available. Main outcome measures included change in visual acuity, FST, visual fields, and location of atrophy relative to subretinal bleb position. Intraoperative photos were used to compare areas of atrophy to the original subretinal bleb position. Descriptive statistics were used to report the data from this retrospective case series. A paired t-test was used to compare pre- and post-operative logMAR visual acuity for each eye in this series.
Results
18 eyes of 10 patients were identified as having developed perifoveal chorioretinal atrophy following subretinal injection of VN (Table 1). 8/10 patients (80%) had bilateral atrophy. Mean age was 11.6 years old (range: 5–20), and 6 patients (60%) were male. Baseline mean logMAR visual acuity and FST were 0.82 (SD: 0.51) and −1.3 log cd.s/m2 (SD: 0.44), respectively. The mean spherical equivalent was −5.7 diopters (range: −11.50 to +1.75). Atrophy was identifiable at an average of 4.7 months (SD: 4.3) following surgery, and progressively enlarged in all cases up to last follow-up examination (mean follow-up 11.3 months; range: 4–18).
Table 1.
Baseline Characteristics for Patients with Perifoveal Chorioretinal Atrophy Following Subretinal Injection of Voretigene Neparvovec-rzyl for RPE65-mediated Leber Congenital Amaurosis
| Baseline Features | ||
|---|---|---|
| Mean or N | SD or % | |
| Age, mean years (SD) | 11.6 | 4.7 |
| Male, n (%) | 6 | 60% |
| Baseline Visual Acuity, mean logMAR (SD) | 0.82 | 0.51 |
| Baseline FST, mean log cd.s/m2 (SD) | −1.3 | 0.44 |
| Bilateral, n (%) | 8 | 80% |
| Spherical Equivalent, mean diopters (SD) | −5.7 | 3.7 |
| Length of Follow-Up, mean months (SD) | 11.3 | 3.7 |
| Time to noticeable atrophy, mean months (SD) | 4.7 | 4.3 |
Abbreviations: FST=Full-field stimulus testing
Figure 1 illustrates a severe example of bilateral progressive chorioretinal atrophy following subretinal VN. This was a 15-year-old patient with a low myopic (−3.00 OD and −2.00 OS) refractive error with no visible areas of atrophy at baseline. A subretinal bleb involving the fovea was created in both eyes intraoperatively. Within the first month of surgery, atrophy was noted along the arcades, and this atrophy progressed. By post-operative year 1.5, these atrophic areas had enlarged and become more confluent with associated outer retinal loss and increased signal transmission from the choroid on OCT.
Figure 1.
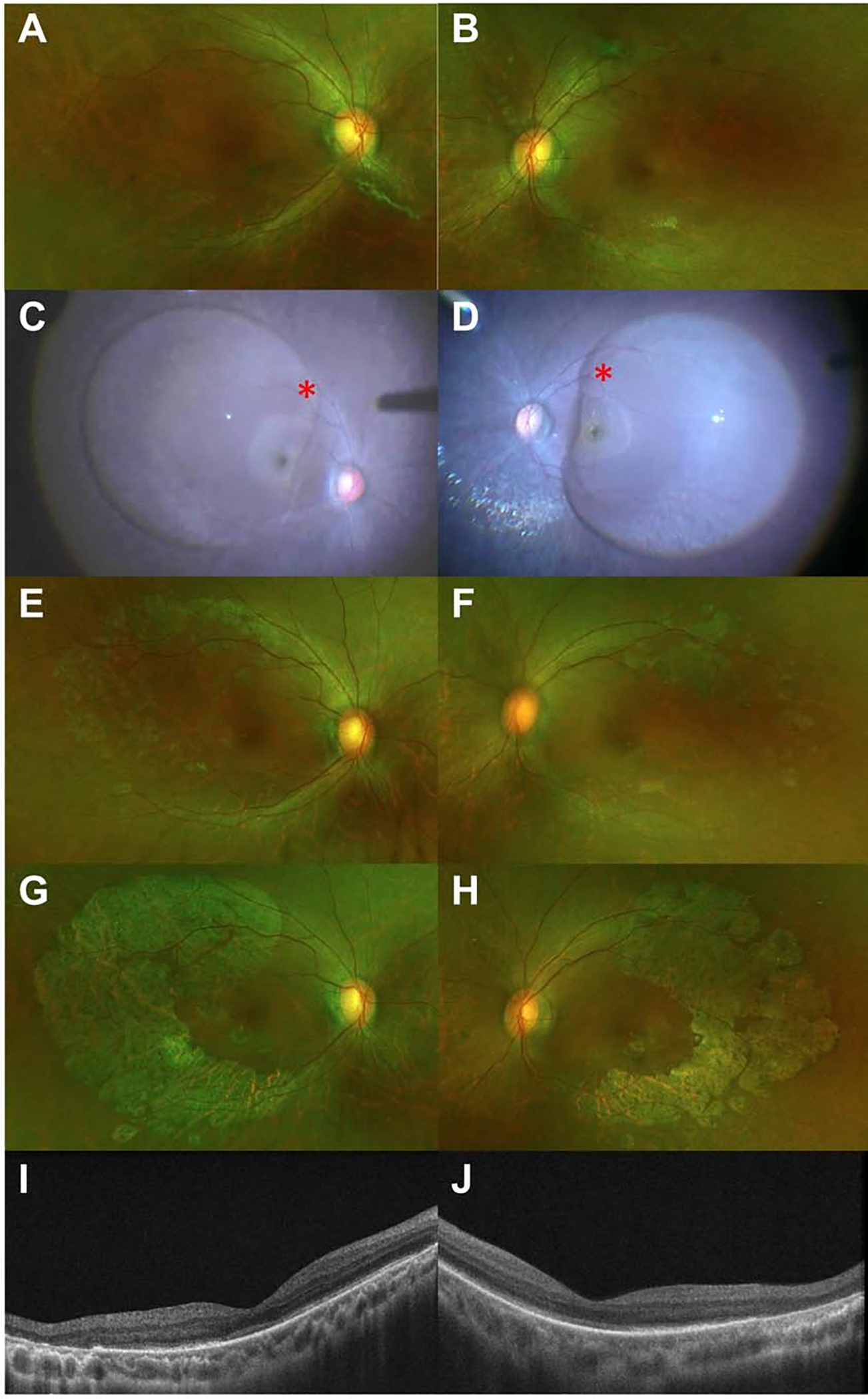
Bilateral progressive chorioretinal atrophy following subretinal voretigene neparvovec-rzyl injection. Preoperative baseline ultra-widefield pseudocolor images of the right (1A) and left (1B) eyes. Intraoperative photographs demonstrating the extent of the subretinal bleb (1C-D). Note the red asterisks indicating the bleb initiation site. Fundus photos from post-operative month 1, with early signs of chorioretinal atrophy in both eyes (1E-F). Fundus photos from postoperative year 1.5, with progressive, more confluent areas of chorioretinal atrophy (1G-H). Optical coherence tomography at post-op year 1.5, with areas of outer retinal loss and increased signal transmission from the choroid noted temporally in both eyes, correlating with areas of atrophy noted on the previously described fundus photos (1I-J).
For the eyes in this series, the atrophy developed within and outside the area of the subretinal bleb in 10 (55.5%) eyes, exclusively within the area of the bleb in 7 (38.9%) eyes, and exclusively outside the bleb in 1 (5.5%) eye (Table 2). Figure 2 highlights a 6-year-old patient with a highly myopic (−8.50 D OU) refractive error showing unilateral progressive chorioretinal atrophy outside the area of the bleb. At post-operative month 1, a small area of atrophy outside the superior arcade is noted, corresponding to the touchdown site of the cannula. At this time, the macula appeared uninvolved. However, at post-operative year 1, a curvilinear area of atrophy was noted in the inferior macula which spared the fovea and was clearly distinct from the cannula touch-down site. Corresponding outer retinal atrophy and increased signal transmission from the choroid is noted on OCT. Comparing the intraoperative bleb site and the resultant atrophy, the atrophic strip is outside the area of the initial subretinal bleb. Of note, the fellow eye did not develop atrophy following subretinal VN.
Table 2.
Clinical Outcomes for Patients with Perifoveal Chorioretinal Atrophy Following Subretinal Injection of Voretigene Neparvovec-rzyl for RPE65-mediated Leber Congenital Amaurosis
| Eyes with Perifoveal Chorioretinal Atrophy | |
|---|---|
| Change in LogMAR Visual Acuity, mean (P-value) | −0.09 (0.45) |
| Change in FST, mean log cd.s/m2* (P-value) | −3.21 (<0.0001) |
| Atrophy relative to Subretinal Bleb Position, n (%) | |
| Within | 7 (38.9%) |
| Within and Outside | 10 (55.5%) |
| Outside | 1 (5.5%) |
| Visual Field Improvement†, n (%) | 13 (100%) |
| Visual Field Findings, n (%) | |
| Paracentral Scotoma Related to Atrophy | 3 (23.1%) |
| Paracentral Scotoma Unrelated to Atrophy | 3 (23.1%) |
| Presence of Inflammation | 2 (11.1%) |
| Growth in Atrophy Over Time, n (%) | 18 (100%) |
Abbreviations: FST=Full-field stimulus testing
n=9 eyes with FST pre-op and post-op
Improvement defined as expansion or gain of isopters on Goldmann visual fields
Figure 2.
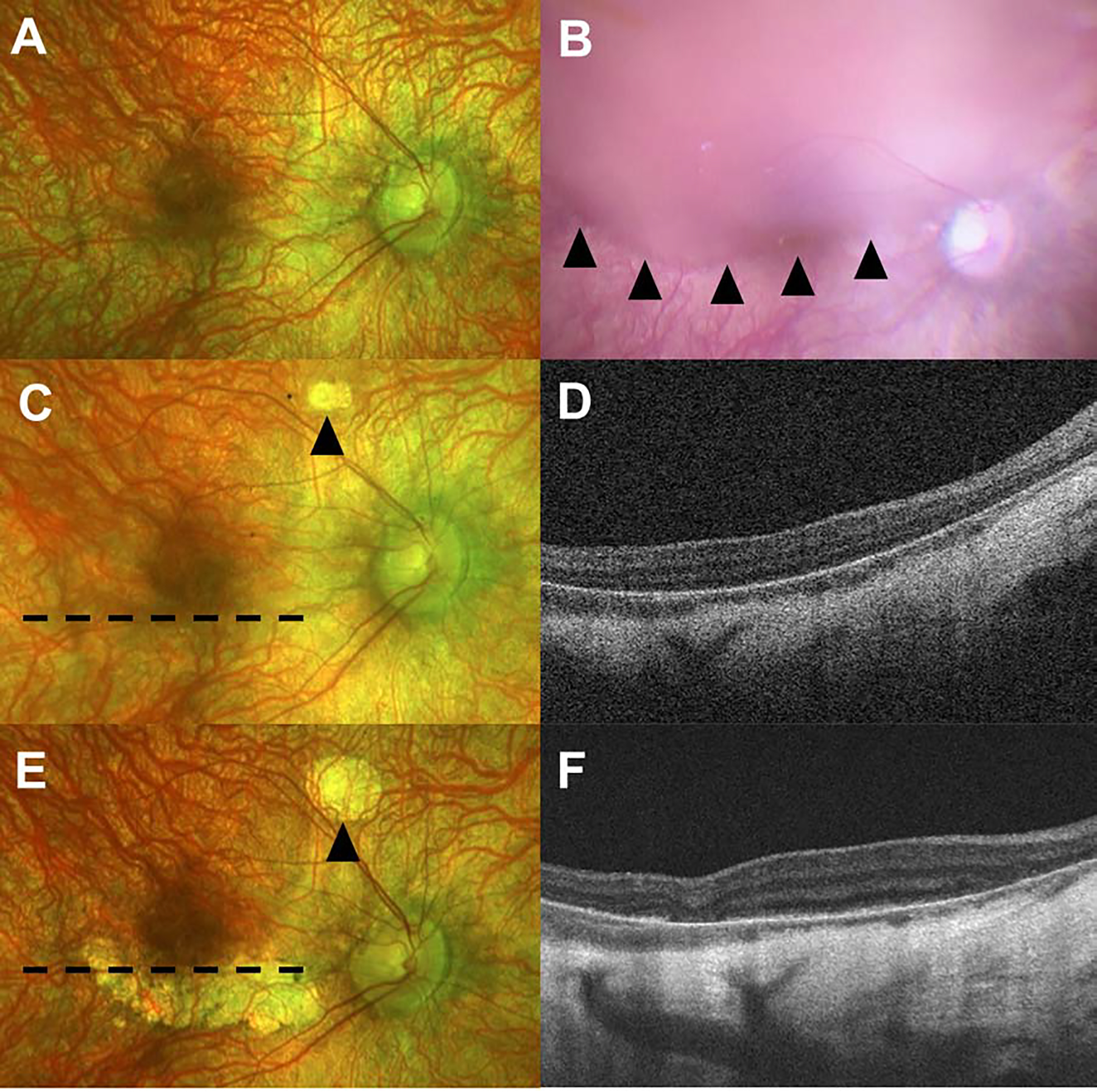
Unilateral progressive chorioretinal atrophy Poutsidethe area of subretinal voretigene neparvovec-rzyl bleb creation. Preoperative baseline pseudocolor image of the right eye (2A) demonstrating a blonde myopic fundus. Intraoperative photograph demonstrating the extent of the subretinal bleb (2B) with arrowheads highlighting the inferior edge. The fundus image from post-operative month 1 shows a small area of atrophy outside the superior arcade corresponding to the touchdown site of the subretinal cannula (2C, arrowhead). Optical coherence tomography at post-operative month 1 through the inferior macula (Fig 2D). Fundus photos from post-operative year 1 are notable for a curvilinear area of atrophy in the inferior macula, outside the subretinal bleb location shown in 2B (2E). The area of atrophy associated with the cannula touchdown site is highlighted by the arrowhead. Optical coherence tomography at post-operative year 1 is notable for outer retinal atrophy with increased signal transmission from the choroid (2F). Dotted lines in 2C and 2E demonstrate the position of the corresponding OCT image.
Visual acuity improved in 12 (66.7%) eyes, did not change in 3 (16.7%) eyes, and worsened in 3 (16.7%) eyes. There was no significant change in mean visual acuity before and after treatment (p=0.45). There was a consistent improvement in FST with a mean change of −3.21 log cd.s/m2 (SD: 0.48, p<0.0001). Additionally, all 13 eyes with reliable pre- and post-operative visual fields demonstrated improvement (expansion or gain of isopters) following surgery, but 3 (23.1%) eyes demonstrated paracentral scotomas likely related to the atrophy. An additional 3 (23.1%) eyes demonstrated paracentral scotomas unrelated to the atrophy. Lastly, 2/18 (11.1%) eyes from 1 patient were noted to have recurrent intraocular inflammation (anterior chamber and vitreous cells and keratic precipitates). The inflammation in both eyes subsided with topical prednisolone acetate tapered over several weeks.
Discussion
The present study reports a previously undescribed complication observed in 18 eyes of 10 patients who underwent post-market VN treatment by subretinal injection. The perifoveal chorioretinal atrophy was progressive and first noticeable anywhere from 1 week to 1 year postoperatively, at a mean of 4.7 months after surgery. Despite this atrophy, the visual acuity improved or remained stable in 83% of patients, likely due to the atrophy sparing the fovea. There was an average of a 3-log unit improvement in FST, which is consistent with results obtained in the clinical trial setting and indicates a successful response to treatment.5 Additionally, all eyes had improvements in perimetry with just 23% of patients noted to have paracentral scotomas related to the atrophy.
Although the mechanism for chorioretinal atrophy is not known at this time, there are several potential factors that must be considered alone or in combination. One possibility is direct toxicity of the AAV2 vector to the photoreceptors and RPE. Xiong et al. studied the ocular toxicity of various AAV vectors in mice and found dose-dependent RPE loss and outer nuclear layer thinning.6 This effect was more pronounced with RPE-specific and broadly active promoters, a category that includes the CAG promoter used in VN. Though Xiong et al. did not test the AAV2 vector specifically, several AAV serotypes showed similar effects, raising the possibility that vector toxicity could at least partially explain this phenomenon. Chorioretinal atrophy as seen in our cases was not reported in the VN phase 1 or phase 3 trial. However, one subject who received a higher-dose (1.5 × 1012 vg) of another AAV2 vector in a phase 1 trial of rAAV2/2.hRPE65p.hRPE65 (ClinicalTrials.gov NCT00643747) did develop focal progressive chorioretinal atrophy first noted at 6 months following treatment, enlarging at post-op year 3.7 Another potential factor is that all 4 surgeons represented in this study injected the vector directly into the subretinal space without using a saline pre-bleb. Depending on the amount of saline used to create the bleb, pre-bleb creation could reduce the concentration of vector, thus lowering the risk of toxicity. Taken together, it is possible that vector concentration and the CAG promoter are associated with the development of atrophy.
Inflammation or immune response to the vector is another possible contributing factor. The FDA-approved, lower-dose (1.5 × 1011 vg) VN formulation was generally safe and well-tolerated in the phase 3 trial, with intraocular inflammation occurring in just 8% of patients.5 In the gene therapy trial of AAV8-coRPGR for X-linked retinitis pigmentosa, the authors demonstrated a more subtle form of presumed inflammation characterized by subretinal deposits in higher dosed patients that was responsive to steroids.8 Though inflammation may contribute to atrophy in some cases, clinically significant inflammation was found in 11.1% of eyes in the current study and there were no instances of subretinal deposits, suggesting postoperative inflammation is unlikely to be the sole cause of the atrophy.
Surgical delivery is another possible contributing factor. Scruggs et al. examined various delivery parameters for subretinal cell therapy in Yucatan mini-pigs.9 The authors found that use of a 41-gauge cannula (as used in this study) was associated with 33.3% rate of “pseudo-geographic atrophy (GA)” as noted by RPE depigmentation and loss of RPE65 expression. Furthermore, there was a significant increase in pseudo-GA with fast subretinal injection rate versus a slow injection rate (1.8 ml/min versus 0.18 ml/min). Delivery rate is difficult to measure and depends on injection pressure, cannula/tubing size and length, intraocular pressure, and photoreceptor-RPE adhesivity. In a large study of 108 patients treated with subretinal gene therapy, higher injection pressures were necessary in children compared to adults.10 Because 9/10 of patients described here were less than 18 years old, this may have played a role. Although we do not have data on injection pressure, the use of foot-pedal control by all surgeons likely resulted in lower and more stable delivery pressures compared to manual delivery by an assistant. However, other factors may be at play since the phase 3 trial only used manual injection and no patients were reported to have experienced atrophy.4 Importantly, although incorrect sub-RPE or intraretinal delivery of vector could theoretically cause atrophy, intraoperative OCT confirmed subretinal bleb delivery in all eyes.
Ocular factors may also predispose to the development of atrophy. This is supported by the fact that 8/10 patients experiencing chorioretinal atrophy developed similar atrophy in the fellow eye. Furthermore, 9/10 patients were myopic with a mean −6.1 D refractive error. Thinning of the RPE/choriocapillaris complex in the setting of high myopia may predispose eyes to vector toxicity. A definitive assessment of ocular factors will require careful comparison to the entire cohort of treated patients.
A final consideration is whether the atrophy described here is consistent with the natural history of the disease—independent of the treatment. While progressive atrophy can be seen in patients with LCA, the stereotypical location of the atrophy, the timing of the onset of the atrophy, and in most cases, the clear relationship to the bleb site (as demonstrated in Figure 1) suggests that this phenomenon is treatment-related. Whereas a minority of patients experienced atrophy only in the area of the subretinal bleb, most patients had at least some areas of atrophy outside the treated areas. This may be due to changes in bleb position immediately following the procedure, especially since all eyes underwent an air-fluid exchange. For example, one eye of a 6-year-old (Figure 2) experienced atrophy inferior to the bleb, which may be explained by bleb spread or non-compliance with supine positioning in the setting of a partial air fill.
Among the limitations of this study, the most notable is that we have exclusively reported on eyes that experienced this phenomenon. Therefore, this precludes an analysis of overall incidence or a statistical comparison of myriad ocular and non-ocular factors between those that did and did not demonstrate atrophy. A comprehensive reporting of VN outcomes and complications is outside the scope of this limited report on perifoveal chorioretinal atrophy, and will be addressed separately in a forthcoming multi-center, post-approval 1-year outcomes study (NCT03597399). Another limitation of this study is that we have solely included sites and surgeons who have volunteered to collaborate, which could have introduced selection bias. Finally, given the relatively recent treatment of the first patients with VN in 2018, our follow-up is inherently limited and therefore we cannot speculate on how this complication will evolve over longer periods of time.
In summary, we present the results of 18 eyes of 10 patients who developed perifoveal chorioretinal atrophy following subretinal voretigene injection. This case series represents the first reported description of perifoveal chorioretinal atrophy following subretinal VN. Despite progressive atrophy, most patients did well on visual function measures, as evidenced by improvement in visual acuity, FST, and visual field in the majority of patients. In light of the growing number of retinal gene therapy trials, including for indications outside of IRDs such as neovascular age-related macular degeneration and diabetic retinopathy, study sponsors and investigators should closely monitor for this complication as it may not be exclusive to VN. Further study is necessary to determine what ocular, surgical delivery, and vector-related parameters predispose patients to this complication.
Acknowledgements:
The authors would like to acknowledge CHLA study coordinators Quy Huynh-Tran and Dilshad Contractor; University of Michigan study coordinators Lindsay Godsey and Callie Gordon.
Financial Support:
This study was supported in part by an unrestricted grant to the Department of Ophthalmology at the USC Keck School of Medicine from Research to Prevent Blindness (TCL, AN), the Las Madrinas Endowment in Experimental Therapeutics for Ophthalmology (TCL, AN), NIH/NEI Career Development Award K08EY030924 (AN), and a Research To Prevent Blindness Career Development Award (AN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: WSG: No financial disclosures; PRAS:Consultant for Allergan, AGTC, EyePoint, Gyroscope, Leica, RegenXBio; CB: Consultant for Janssen, research support from MeiraGTx, 4DMT, Spark Therapeutics; TCL: No financial disclosures; MB: No financial disclosures; JS: No financial disclosures; CM: No financial disclosures; AMB: Consultant for DORC, Allergan, AGTC, ProQR, Occulus; AN: Consultant for Biogen, REGENXBIO, and Allergan Retina.
References
- 1.Allikmets R. Leber congenital amaurosis: a genetic paradigm. Ophthalmic Genetics. 2004;25:67–79. [DOI] [PubMed] [Google Scholar]
- 2.RetNet: Summaries. https://sph.uth.edu/retnet/sum-dis.htm#B-diseases; Accessed 11.12.20. [Google Scholar]
- 3.Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105:15112–15117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maguire AM, Russell S, Wellman JA, et al. Efficacy, Safety, and Durability of Voretigene Neparvovec-rzyl in RPE65 Mutation–Associated Inherited Retinal Dystrophy: Results of Phase 1 and 3Trials. Ophthalmology. 2019;126:1273–1285. [DOI] [PubMed] [Google Scholar]
- 6.Xiong W, Wu DM, Xue Y, et al. AAV cis-regulatory sequences are correlated with ocular toxicity. Proc Natl Acad Sci U S A. 2019;116:5785–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bainbridge JWB, Mehat MS, Sundaram V, et al. Long-Term Effect of Gene Therapy on Leber’s Congenital Amaurosis. N Engl J Med. 2015;372:1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cehajic-Kapetanovic J, Xue K, de la Camara CM-F, et al. Retinal gene therapy in X-linked retinitis pigmentosa caused by mutations in RPGR: Results at 6 months in a first in human clinical trial. Nat Med. 2020;26:354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scruggs BA, Jiao C, Cranston CM, et al. Optimizing Donor Cellular Dissociation and Subretinal Injection Parameters for Stem Cell-Based Treatments. Stem Cells Transl Med. 2019;8:797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scruggs BA, Junior HMV, Pennesi ME, et al. The age of patients with retinal degenerative diseases affects the injection pressure required for subretinal gene therapy delivery. Invest Ophthalmol Vis Sci. 2020;61:4501–4501. [Google Scholar]


