Abstract
Background:
Transcranial direct current stimulation (tDCS) has mixed effects on walking performance in individuals post-stroke. This is likely the result of variations in tDCS electrode montages and individualized responses. The purpose of this study was to quantify the effects of a single session of tDCS using various electrode montages on post-stroke walking performance.
Methods:
Individuals with chronic stroke (n=16) participated in a double-blind, randomized cross-over study with sham stimulation and three tDCS electrode montages. Gait speed, paretic step ratio and paretic propulsion were assessed pre- and post-stimulation at self-selected and fastest comfortable speeds. Changes in muscle activation patterns with self-selected walking were quantified by the number of modules derived from nonnegative matrix factorization of EMG signals for hypothesis generation.
Results:
There was no significant effect of active stimulation montages compared to sham. Comparisons between each participant’s best response to tDCS and sham show personalized tDCS may have a positive effect on fastest comfortable overground gait speed (p=0.084), paretic step ratio (p=0.095) and paretic propulsion (p=0.090), and self-selected paretic step ratio (p=0.012). Participants with 2 or 3 modules at baseline increased module number in response to the all experimental montages and sham, but responses were highly variable.
Conclusions:
A single session of tDCS may affect clinical and biomechanical walking performance, but effects appear to be dependent on individual response variability to different electrode montages. Our findings are consistent with responses to various tDCS electrode montages being the result of underlying neuropathology and we recommend examining how individual factors affect responses to tDCS.
Keywords: Brain stimulation, electromyography, stroke, walking, biomechanics, rehabilitation
Individuals post-stroke commonly report impaired walking performance that is associated with the severity of central nervous system damage.(1) Transcranial direct current stimulation (tDCS) holds promise as a potential therapeutic adjuvant capable of modifying or modulating the central nervous system and may be able to augment standard rehabilitation strategies for recovery of walking function.(2, 3) tDCS is a non-invasive brain stimulation technique that uses a small electrical current to modulate cortical activity and is simple to administer, low-cost, and low-risk.(4, 5) tDCS neuromodulation has been shown to up or down regulate cortical excitability and effect interhemispheric imbalances that can result from a stroke(6, 7) including deeper structures like the leg area of the motor cortex in healthy individuals(2, 8, 9) and persons post-stroke.(3) Regulating cortical excitability or interhemispheric imbalance has been hypothesized as a mechanism for improving motor function(6, 10) (primarily in the upper extremity(7, 11-13)) and aphasia(14, 15). Treating interhemispheric imbalance may have a larger influence on walking performance given the bilateral task requires coordination between hemispheres(16) although this model has not been conclusively translated to the lower extremity.
Despite the promise of tDCS acting on impaired cortical activity to improve motor function the effects are often small bringing into question the therapeutic utility of tDCS in clinical practice especially for walking rehabilitation (i.e. gait speed and endurance).(16-18) One hypothesized reason for this is the limited evidence to guide personalization of tDCS prescription to combat the inherent heterogeneity of the pathology.(18) Few experiments examine the effect of varying available parameters such as; treatment frequency, duration and length, current strength, and electrode montage.(14, 17-19)
Electrode montage is of specific therapeutic interest in post-stroke rehabilitation for walking because electrode placement informed by neuroanatomical and physiological pathology has had a positive effect on clinical measures of upper extremity and hand function.(7, 11-13) Improvements are seen with either excitatory (anode applied over ipsilesional M1)(7) or inhibitory stimulation (cathode is applied over contralesional M1)(11) when compared to sham.(12, 13) Studies examining the effects of dual montages (anode over ipsilesional M1 and cathode over contralesional M1 simultaneously) had positive findings on measures of walking function (decreased Timed Up and Go times(20), increased 6-minute walk test distance(21), and increased paretic power(22)) in response to a single session of tDCS. Yet, it remains unknown how dual montages compare to single anode or cathode configurations against sham stimulation on walking performance.(17)
The purpose of this study was to quantify the effects of a single treatment of tDCS delivered during treadmill walking on gait speed, paretic step ratio and paretic propulsion in individuals post-stroke and to compare three electrode montages to sham stimulation: 1.) Excitatory (anode over ipsilesional M1), 2.) Inhibitory (cathode over contralesional M1, and 3.) Dual (an anode over ipsilesional M1, and a cathode over contralesional M1). We hypothesized the dual electrode montage would have the largest effect on measures of walking performance as walking is a bilateral, coordinated activity resulting from restored interhemispheric balance. In an exploratory analysis, we examined the effect of tDCS stimulation and electrode montage on muscle activation patterns in individuals post-stroke during treadmill walking.
Materials and Methods:
Participants:
Eighteen individuals with chronic stroke were enrolled and sixteen completed all study procedures. One participant dropped-out and one did not meet the inclusion criteria. Demographic data are presented in Table 1.
Table 1:
Participant Demographics
| n | Descriptive Statistics | |
|---|---|---|
|
Age (years) mean (SD) [range] |
16 | 59 (11.5) [30-77] |
|
Sex (male) frequency (%) |
16 | 11/16 (68.8%) |
|
Hemiparetic side (right) frequency (%) |
16 | 9/16 (56.3%) |
|
Chronicity (months) mean (SD) [range] |
16 | 54.56 (76.5) [10-325] |
|
FM-Total LE mean (SD) [range] |
16 | 23.94 (5.78) [13-32] |
|
FM-Synergy mean (SD) [range] |
16 | 15.56 (4.15) [8-21] |
|
Dynamic Gait Index mean (SD) [range] |
15 | 16 (4.55) [7-22] |
|
Berg Balance Scale mean (SD) [range] |
11 | 46.91 (8.94) [25-55] |
|
Gait Speed
(self-selected) (m/s) mean (SD) [range] |
16 | 0.82 (0.33) [0.23-1.43] |
|
Paretic Step Ratio mean (SD) [range] |
16 | 0.51(0.06) [0.37-0.63] |
|
Modules mean (SD) [range] |
16 | 2.94 (0.85) [2-4] |
Inclusion criteria were:
1) age 18 to 85 years old; 2) at least six-months post-stroke; 3) residual lower extremity paresis (Fugl-Meyer Lower Extremity motor score <34); 4) ability to walk independently at least 10 feet; 5) self-selected 10-meter gait speed < 0.8 m/s (at time of consent); and 6) provision of informed consent. Participants were excluded for: 1) significant musculoskeletal problems limiting hip and knee extension or ankle plantarflexion to neutral joint positions; 2) self-reported history of unstable cardiovascular disease or severe osteoporosis, or 3) pregnancy. Screening, testing and tDCS interventions were completed by a team of licensed physical therapists and associated study staff. All participants signed a written informed consent form approved by the Institutional Review Board at the Medical University of South Carolina and conformed to the Declaration of Helsinki.
Experimental Procedure:
We used a double-blind, randomized, cross-over experimental design. A timeline of procedures is presented in Figure 1. Participants were screened and completed a clinical assessment, which included the lower extremity motor portion of the Fugl-Meyer(23), Berg Balance Test(24), and Dynamic Gait Index(25). Participants then completed three single sessions of tDCS and one sham stimulation session. Sessions were blocked randomized to control for order effects and separated by a minimum 48-hour washout period. Participants completed pre- and post-stimulation testing for each session.
Figure 1: Timeline of Experimental Procedures.
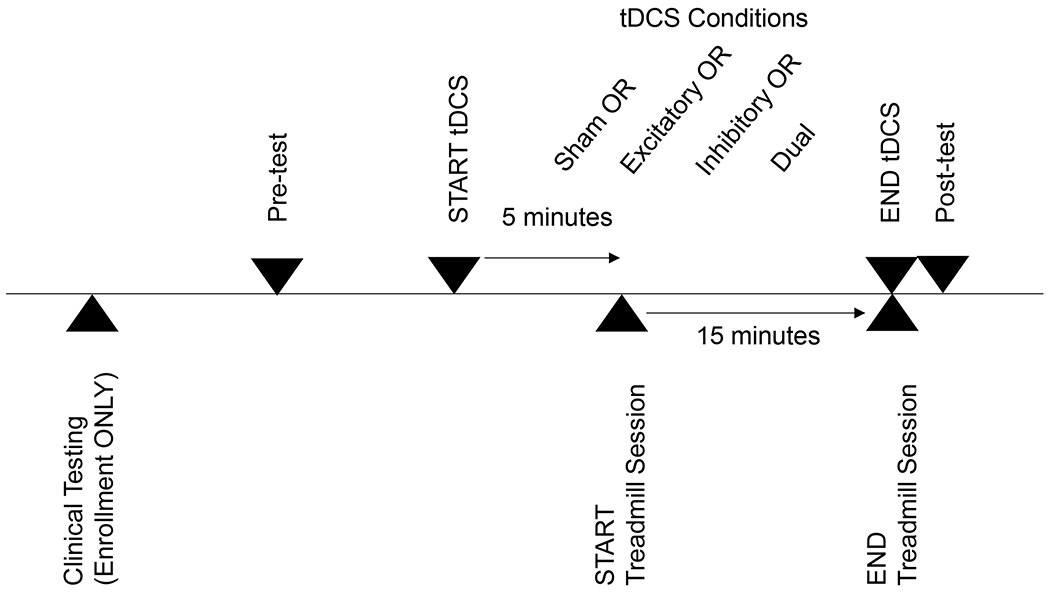
Participants completed clinical testing during enrollment. For each of the four experimental sessions (3 active and one sham), participants completed pre-testing followed by active tDCS or sham stimulation with treadmill walking and concluded with post-testing.
Transcranial direct current stimulation:
tDCS was delivered using an EMPI unit (Chattanooga; Hixson, TN) and 1.75 cm2 sponges prepped with 0.9% saline solution. This created a current density of 0.1 mA/cm2 consistent with recommendations.(26) We informed participants they may feel a slight tingling sensation that should subside within approximately 60 seconds. Stimulation was ramped up to 2mA × at a dose rate of 40mA/min for a total of 20 minutes. Experimental conditions administered tDCS with one of the following electrode montages illustrated in Figure 2: 1) excitatory (anode over target ipsilesional leg M1 area), 2) inhibitory (cathode over target contralesional leg M1 area) or 3) dual (both excitatory and inhibitory montages applied simultaneously to target both leg M1 areas). Reference pads for the excitatory and inhibitory montages were placed on the ipsilateral shoulder. Modeling work has shown that this type of extracephalic pad placement creates a more focal concentration of current under the electrode, increasing penetration depth.(27) Studies with healthy individuals suggest that extracephalic pad placement has a greater effect on cortical excitability (9) and neuromotor output (28) compared to cephalic placement for deeper M1 areas of the leg. The dual montage used two EMPI units to deliver simultaneous active anodal stimulation over ipsilesional M1 and cathodal stimulation over contralesional M1. Reference pads for both units were placed on the respective ipsilateral shoulder. In each montage, M1 location was determined in a forward seated position approximately 1cm lateral to the vertex and 1cm posterior to a hypothetical line between the tragi creating a 2cm gap between the cephalic pads in the dual montage configuration.
Figure 2: Diagram of experimental tDCS montages.
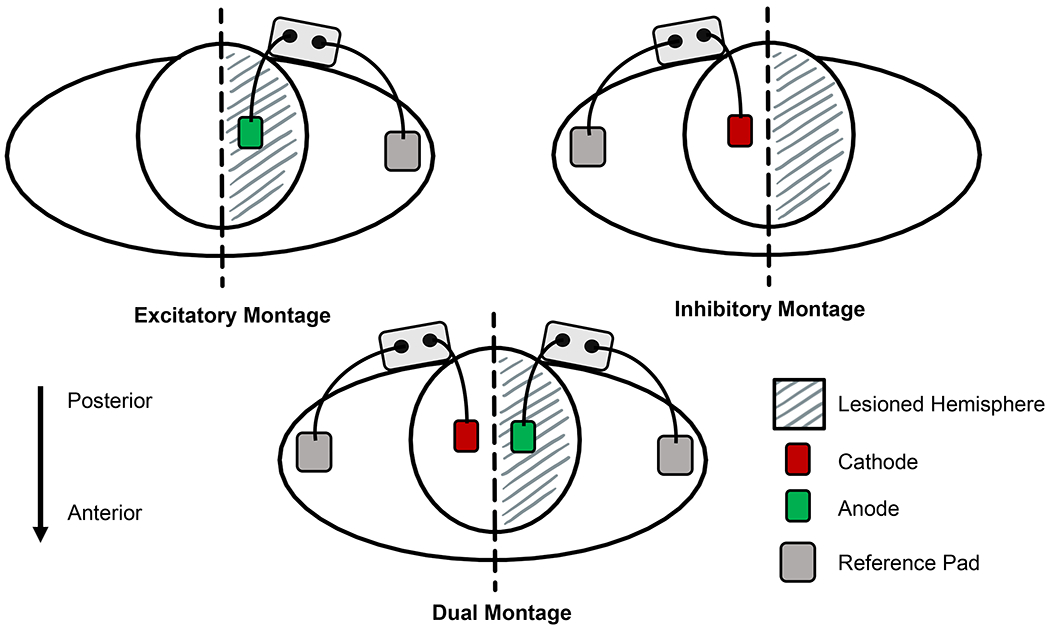
Excitatory Montage: Anode pad was placed over the target ipsilesional M1 leg area, reference pad was placed on the ipsilateral shoulder
Inhibitory Montage: Cathode pad was placed over the target contralesional M1 leg area, reference pad was placed on the ipsilateral shoulder.
Dual Montage: Combination of the Excitatory and Inhibitory montages using 2 tDCS units with one delivering the excitatory (anode placed over the target ipsilesional M1 leg area) and the other inhibitory (cathode pad was placed over the target contralesional M1 leg area) currents.
In each montage the cephalic pad was placed 1cm lateral to the vertex and 1 cm posterior to an imaginary line between the tragi. This created a 2cm gap between cephalic electrodes in the Dual montage.
Stimulation parameters were set prior to each session by an unblinded investigator and participants were fitted with two EMPI units using pad placement described in the dual experimental setup to maintain participant and staff blinding to active stimulation parameters. Sham stimulation was done by turning on the EMPI units to apply 30 seconds of stimulation before manually turning the units off by an unblinded investigator (per published guidelines).(29) Participants received tDCS or sham stimulation for 5 minutes in a seated position and then continued receiving stimulation while walking for 15-minutes on a treadmill. Participants walked at their fastest comfortable speed on the treadmill to provide an adequate training stimulus. Faster walking speeds are commonly used in rehabilitation programs having been shown to have immediate and long-term effects on walking performance for persons post-stroke.(30, 31) Walking was paused every 5-minutes to assess cardiovascular response to exercise (i.e. blood pressure, heart rate, and activity tolerance). Walking immediately resumed unless continuation was contraindicated for safety. A ceiling harness system without body-weight support was used to prevent falls or injury. Verbal cues to alter gait pattern were not provided. Minimal physical assistance was provided to prevent tripping or interruptions in walking and not given during data collection trials.
Data analysis:
GaitRite (CIR Systems, Inc.; Franklin, NJ) data was used to calculate self-selected and fastest comfortable overground gait speeds. Participants walked over a 24-foot GaitRite for one trial at their self-selected speed and three trials at their fastest comfortable speed during pre- and post-testing. Participants also walked for three, 30-second trials on a split belt instrumented treadmill (Bertec; Columbus, OH) at self-selected and fastest comfortable speeds, which did not have to match overground speeds. Ground reaction force (GRF) data was sampled at 1000 Hz to derive paretic step ratio and paretic propulsion using methods previously described by our lab.(32) Paretic propulsion was calculated by dividing the positive anterior impulse of the paretic leg by the anterior impulse of both legs combined.(32) Paretic step ratio was calculated from the percentage of stride length performed by the paretic step.(33) Paretic step ratio and paretic propulsion were expressed as the absolute value of deviation from symmetry (0.5).
Muscle coordination patterns were quantified for each participant during self-selected treadmill walking by extracting modules using a nonnegative matrix factorization (NNMF) algorithm.(34, 35) Surface EMG was recorded at 2000 Hz with bipolar pre-amplified electrodes (Motion Lab Systems; Baton Rouge, LN, USA) at the following eight muscle locations bilaterally: tibialis anterior, soleus, medial gastrocnemius, vastus medialis, rectus femoris, medial hamstrings, lateral hamstrings and gluteus medius.(36) Specific post-processing of EMG signals and selection of modules for the paretic leg can be found in Clark et. al., 2010.(37)
Statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc. Cary, NC) on change scores for gait speed, paretic step ratio and paretic propulsion at self-selected and fastest comfortable speeds. A one-way ANOVA (or Kruskal-Wallis Test for non-parametric data) examined the main effect of active stimulation across all three montages compared to sham for each variable of walking performance. Post-hoc testing with multiple t-tests (or Wilcoxon Rank-Sum tests) were performed to compare the effects of each electrode montage. Corrections for multiple comparisons were not performed for this preliminary study. Changes in module number are reported observationally to generate hypotheses for future studies.
Results:
Walking performance:
No significant main effect of active stimulation montage was observed for overground walking speed at self-selected (ANOVA; F=0.44, p=0.723, df=3) or fastest comfortable speed (Kruskal-Wallis; Χ2=2.419, p=0.490, df=3). Additionally, no significant effect was found for paretic step ratio at self-selected (Kruskal-Wallis; Χ2=3.013, p=0.389, df=3) or fastest comfortable speeds (Kruskal-Wallis; Χ2=1.357, p=0.716, df=3) or paretic propulsion at self-selected (ANOVA; F=0.31, p=0.819, df=3) or fastest comfortable speeds (Kruskal-Wallis; Χ2=0.749, p=0.862, df=3). Group descriptive statistics for each variable are presented in Table 2.
Table 2:
Descriptive statistics for change scores as a result of each tDCS electrode montage and sham stimulation
| tDCS Experimental Condition | |||||
|---|---|---|---|---|---|
| Sham | Excitatory | Inhibitory | Dual | ||
|
Self-
Selected |
Gait Speed
m/s |
0.086 (0.132) | 0.043 (0.128) | 0.068 (0.092) | 0.085 (0.109) |
|
Paretic
Step Ratio |
0.005 (0.012) | 0.009 (0.022) | 0.003 (0.032) ‡ | 0.002 (0.032) | |
|
Paretic
Propulsion |
0.015 (0.061) | 0.006 (0.069) | 0.027 (0.043) | 0.023 (0.074) | |
|
Fastest
Comfortable |
Gait Speed
m/s |
0.059 (0.051) | 0.042 (0.134) | 0.020 (0.081) | 0.036 (0.100) ‡ |
|
Paretic
Step Ratio |
−0.003 (0.012) | −0.000 (0.017) | −0.005 (0.019) | −0.020 (0.0.28) ‡ | |
|
Paretic
Propulsion |
0.012 (0.056) | −0.003 (0.041) ‡ | −0.007 (0.048) ‡ | 0.005 (0.040) | |
Statistics are presented as mean (standard deviation)
Indicates median (interquartile range)
Gait speed was calculated from overground walking trials.
Paretic Step Ratio and Paretic Propulsion were calculated from treadmill walking trials.
Visual inspection of the data showed a high degree of overall response variability to each electrode montage for each measured variable. Table 3 shows each participant’s best response to an electrode montage for each variable.
Table 3:
Individual variability in response to tDCS stimulation based on electrode montage
| Self-Selected Speed | Fastest Comfortable Speed | |||||
|---|---|---|---|---|---|---|
| Participant Number |
Gait Speed |
Paretic Step Ratio |
Paretic Propulsion |
Gait Speed |
Paretic Step Ratio |
Paretic Propulsion |
| 1 | 2 | 1 | 3 | 1 | 2 | 2 |
| 2 | 2 | 3 | 1 | 2 | 1 | 3 |
| 3 | 2 | 1 | 2 | 1 | 1 | 3 |
| 4 | 3 | 1 | 2 | 3 | 2 | 1 |
| 6 | 3 | 2 | 1 | 3 | 3 | 1 |
| 7 | 3 | 3 | 3 | 1 | 1 | 1 |
| 8 | 2 | 2 | 2 | 2 | 2 | 1 |
| 9 | 3 | 3 | 2 | 3 | 3 | 3 |
| 10 | 3 | 3 | 2 | 3 | 1 | 1 |
| 11 | 3 | 1 | × | 1 | 3 | × |
| 12 | 3 | 2 | 2 | 1 | 1 | 3 |
| 13 | 1 | 1 | 3 | 2 | 3 | 1 |
| 15 | 1 | 1 | 1 | 3 | 1 | 3 |
| 16 | 3 | 2 | 1 | 2 | 1 | 1 |
| 17 | 1 | 1 | 2 | 1 | 2 | 2 |
| 18 | 3 | 1 | 3 | 1 | 1 | 2 |
Numeric codes for each variable indicate the montage (electrode placement and stimulation parameters) that elicited the best response for each participant.
1 = Excitatory montage (color = light orange)
2 = Inhibitory montage (color = light blue)
3 = Dual montage (color = white)
× Missing data due to poor GRF quality during data collection.
To examine the possibility participants may exhibit preferential responses to specific montages, we compared each participant’s best response to stimulation with sham for each variable using t-tests (or Wilcoxon Rank-Sum test for non-parametric data). We also accepted a higher false positive rate of 10% (alpha=0.1) for generating exploratory hypotheses. We observed a significant difference and improved fastest comfortable overground gait speed (mean difference=0.06 m/s, 95% CI [-0.008 – 0.12], t-test p=0.084) but not self-selected (mean difference=0.05, 95% CI [-0.036 – 0.137], t-test p=0.242). There was a significant effect for improvement in paretic step ratio (median=0.017, IQR [0.023], Wilcoxon; p=0.012) and paretic propulsion (mean difference=0.035, 95% CI [-0.006 – 0.077], t-test p=0.090) at self-selected speeds and paretic step ratio (mean difference=0.01, 95% CI [-0.002 – 0.02], t-test p=0.095) at fastest comfortable speeds. No differences were found for paretic propulsion (median=0.002, IQR [0.068], Wilcoxon; p=0.645) at fastest comfortable speeds.
Muscle activation patterns:
In our sample, 6 participants used 2 modules, 6 participants used 3 modules and 4 participants used 4 modules for self-selected comfortable treadmill walking at baseline. The change in module number for the participants in response to tDCS with each electrode montage and sham are presented in Table 5.
Table 5:
Individual variability in muscle activation pattern response to tDCS stimulation
| Change in Module Number | |||||
|---|---|---|---|---|---|
| Participant Number |
Baseline Module Number |
Excitatory | Inhibitory | Dual | Sham |
| 1 | 2 | 0 | 1 | 2 | 2 |
| 7 | 2 | 1 | 1 | 0 | 2 |
| 11 | 2 | 0 | 0 | 0 | 0 |
| 12 | 2 | 0 | 2 | 1 | 2 |
| 16 | 2 | 1 | 0 | 1 | 0 |
| 17 | 2 | 1 | 1 | 1 | 1 |
| 3 | 3 | 0 | 1 | 0 | 1 |
| 4 | 3 | 1 | 0 | 1 | 1 |
| 8 | 3 | 0 | 0 | 0 | 1 |
| 10 | 3 | 0 | 0 | 0 | −1 |
| 15 | 3 | 0 | 1 | 1 | 1 |
| 2 | 4 | 0 | 0 | 0 | 0 |
| 6 | 4 | 0 | 0 | 0 | × |
| 9 | 4 | 0 | × | −1 | −1 |
| 13 | 4 | 0 | 0 | 0 | 0 |
| 18 | 4 | 0 | −1 | 0 | 0 |
Missing data due to poor EMG quality during data collection.
Only one of the 6 participants with 2 modules did not change their module number after tDCS or sham stimulation. Four of the remaining 5 participants increased module number with the excitatory, inhibitory and/or dual electrode montage and 4 of the 6 participants increased module number with sham stimulation. One 2 module participant increased module number with all electrode montages and sham stimulation.
One of the 6 participants with 3 modules did not change their module number after tDCS or sham stimulation. Two participants did not change their module number in response to any tDCS condition but did to sham stimulation. One of these individuals improved module number and the other decreased. One participant increased module number after the inhibitory montage, one in response to the excitatory montage and dual montage and one in response to the inhibitory and dual montage. However, all three of these participants also increased module number in response to sham stimulation.
Although module number cannot increase from 4, we observed two instances where module number decreased in those with 4 modules at baseline. One participant reduced module number to 3 in response to the dual montage and sham stimulation, the other reduced module number to 3 in response to the inhibitory montage.
A small positive association was found between a change in module number and change in paretic propulsion symmetry (r=0.29; p=0.0251) across all conditions; including sham stimulation. However, this association was not present within experimental and sham conditions separately. There were no associations between changes in module number and changes in gait speed, paretic step ratio or paretic propulsion at self-selected walking speeds when examining all tDCS conditions or within each experimental montage group.
Discussion:
Our aim was to compare the immediate effects of three tDCS electrode montages and sham stimulation on post-stroke walking performance. We used a double blind, placebo controlled, randomized cross-over design to evaluate changes in gait speed, paretic step ratio and paretic propulsion. We found no group main effects for any of the electrode montages compared to sham stimulation on walking performance immediately following one session of tDCS, which was inconsistent with our hypothesis.
Our lack of a single session effect on post-stroke walking performance is comparable with findings from other experiments.(20–22) The immediate effect of tDCS with an excitatory or dual electrode montage has not had a significant effect on walking performance with the exception of the 6-minute walk and Timed-up and Go tests, compared to sham.(20–22) The authors hypothesized the large variation in participant response to tDCS likely caused the negative findings(20–22) and this heterogeneity continues to be a key challenge in post-stroke tDCS neuromodulation research.(18) In an exploratory attempt to address variation in our sample, we tested the main effect of tDCS by comparing each participant’s best response of the electrode montages to sham stimulation. This is based on the assumption that individuals may respond to different montages based on unknown characteristics likely arising from the variety of motor network impairments result from lesion location, size and cortical reorganization.(22, 38, 39) A higher false positive rate, alpha=0.1, was accepted to generate hypotheses for future research. We found tDCS stimulation had a positive effect on fastest comfortable gait speed, paretic step ratio during self-selected and fastest comfortable speeds, and paretic propulsion at self-selected speeds. We also observed the “best montage” often varied for each measure of walking performance (Table 3) within individuals. The lack of a specific pattern lends support to the idea that “one-size does not fit all” in tDCS prescription.(40) Investigators should consider that different electrode montages may impact different features of walking performance on an individual level based on gait speed, clinical features like stroke chronicity(40), or presence, type and degree of interhemispheric imbalance. It is important to note we did not investigate the reproducibility and robustness of our observed effects (i.e., would the electrode configuration that shows the best result be the same under a second test). Nor did we have a large enough sample to examine whether clinical performance or baseline walking performance could predict tDCS response. Previous investigations have demonstrated the reproducibility and benefit of personalized tDCS electrode montage in language rehabilitation.(41, 42) Our results suggest this effect should also be tested in walking rehabilitation and whether markers of clinical or baseline walking performance predict participant response. Investigators should screen individuals for interhemispheric imbalances using TMS in addition to prioritizing assessment of neurophysiological effects from tDCS to establish associations between stimulation and neural pathophysiological changes.
Our exploratory comparison of the effect of tDCS on muscle activation patterns offers one potential window into the mechanisms by which different tDCS electrode configurations may influence walking. Many participants with more severe impairment (ie, 2 or 3 modules) were able to move away from mass flexion and extension muscle activation patterns in response to tDCS. We hypothesize that tDCS can modulate the cortex to enhance voluntary muscle activity during walking for some individuals. This is supported by evidence that tDCS can increase force production in lower extremity muscles and helps explain the association we found between paretic propulsion and improved muscle activation patterns.(22, 29, 43) The individual variability we observed could be explained by the fact that walking is also influenced by subcortical structures. Thus, individuals with more severe cortical impairments may have a greater response to tDCS. However, like our findings related to the best response condition, we cannot be certain that we have captured a true effect since we did not test the reproducibility of our findings and we have a very small sample for each module number at baseline limiting our statistical power. We recommend that more research examine the effects of tDCS on muscle activation patterns since they can provide a mechanistic understanding for biomechanical changes in task performance.(44)
Finally, there are a few methodological choices in our design that may have impacted the results. First, we are unable to know whether current was shunted during the dual montage experimental condition. It is possible that current may have crossed between the two cephalic pads creating a different stimulation environment than hypothesized. Second, our washout period of 48 hours may not have been sufficiently long enough. There is precedence for a 48-hour washout in post-stroke tDCS literature(3, 11) but no formal investigation into the optimal length of time has been done and recent recommendations call for a minimum 1-week period(26). Lastly, the robust response to sham for many subjects suggests that twenty minutes of walking may provide a neuromodulating effect as potent as a single tDCS session and appears to have been an active ingredient in our experiment. Ojardias et. al.(21) saw a similar response to walking on walking performance in individuals post-stroke after examining a single session of tDCS and there is recent evidence to support that moderate intensity aerobic activity can increase neurophysiological markers of corticospinal excitability.(45–47) An intriguing question is whether the increase in corticospinal excitability that accompanies tDCS has a similar mechanism to the increase with walking practice, and whether the effects are additive when the two stimuli are combined. Future research should be designed to further investigate the effects of walking practice on excitability, with and without concomitant tDCS.
In summary, our observation that individuals may have an optimal tDCS electrode montage to elicit improvements in walking performance is perhaps the most important finding of this study. The possibility that individuals post-stroke likely need personalized stimulation parameters has important implications for hypothesis generation and future tDCS studies attempting to optimize tDCS prescription. It is imperative for investigators to employ research methods to best understand how electrode placement will impact walking performance considering clinical presentation corticomotor response, neuroanatomy and tractography to address heterogeneity in participant response to tDCS.
Table 4:
Descriptive statistics for change scores comparing the effect of tDCS compared to sham stimulation using a participant’s best response to each of the three electrode montages
| Sham | Pooled Best Response to tDCS |
||
|---|---|---|---|
| Self-Selected |
Gait Speed
m/s |
0.086 (0.132) | 0.136 (0.106) |
|
Paretic Step
Ratio |
0.005 (0.019) ‡ | 0.017 (0.023) ‡ ** | |
|
Paretic
Propulsion |
0.015 (0.061) | 0.051 (0.049) * | |
|
Fastest
Comfortable |
Gait Speed m/s | 0.059 (0.051) | 0.114 (0.111) * |
|
Paretic Step
Ratio |
−0.003 (0.012) | 0.007 (0.019) * | |
|
Paretic
Propulsion |
0.002 (0.068) ‡ | 0.026 (0.053) ‡ | |
Indicates statistical significance at p<0.05.
Indicates statistical significance at p<0.10.
Statistics are presented as mean (standard deviation)
Indicates median (interquartile range)
Pooled Best Response was created by pooling each participant’s best response to any of the tDCS electrode montages for a given variable
Gait speed was calculated from overground walking trials.
Paretic Step Ratio and Paretic Propulsion were calculated from treadmill walking trials.
Funding:
Funding for this project was provided by the American Heart Association (12IRG9430057), the VA Office of Research and Development (ORD), with additional support from the VA/ORD Rehabilitation R&D Service (1IK6RX003075 and 1I01RX001935) and from the National Institutes of Health (NIH P20 GM109040 and NIH P2C HD086844). This publication was partially supported by a Promotion of Doctoral Studies Level I Scholarship from the Foundation for Physical Therapy Research.
Any opinions expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Veteran Affairs or the NIH.
Footnotes
Conflict of Interest:
The authors declare no competing financial interests.
References:
- 1.Mayo NE, Wood-Dauphinee S, Cote R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Archives of physical medicine and rehabilitation. 2002. Aug;83(8):1035–42. [DOI] [PubMed] [Google Scholar]
- 2.Madhavan S, Stinear JW. Focal and bi-directional modulation of lower limb motor cortex using anodal transcranial direct current stimulation. Brain stimulation. 2010. Jan;3(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhavan S, Weber KA 2nd, Stinear JW. Non-invasive brain stimulation enhances fine motor control of the hemiparetic ankle: implications for rehabilitation. Exp Brain Res. 2011. Mar;209(1):9–17. [DOI] [PubMed] [Google Scholar]
- 4.Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Arch Neurol. 2008. Dec;65(12):1571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016. Feb;127(2):1031–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009. Sep;23(7):641–56. [DOI] [PubMed] [Google Scholar]
- 7.Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabilitation and neural repair. 2005. Mar;19(1):14–9. [DOI] [PubMed] [Google Scholar]
- 8.Jeffery DT, Norton JA, Roy FD, Gorassini MA. Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Experimental Brain Research. 2007. 2007/09/01;182(2):281–7. [DOI] [PubMed] [Google Scholar]
- 9.Tatemoto T, Yamaguchi T, Otaka Y, Kondo K, Tanaka S, editors. Anodal Transcranial Direct Current Stimulation over the Lower Limb Motor Cortex Increases the Cortical Excitability with Extracephalic Reference Electrodes. 2013; Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- 10.Reis J, Fritsch B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Current opinion in neurology. 2011. Dec;24(6):590–6. [DOI] [PubMed] [Google Scholar]
- 11.Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005. Sep 28;16(14):1551–5. [DOI] [PubMed] [Google Scholar]
- 12.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restorative neurology and neuroscience. 2007;25(2):123–9. [PubMed] [Google Scholar]
- 13.Kim DY, Lim JY, Kang EK, You DS, Oh MK, Oh BM, et al. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2010. Nov;89(11):879–86. [DOI] [PubMed] [Google Scholar]
- 14.Fridriksson J, Basilakos A, Stark BC, Rorden C, Elm J, Gottfried M, et al. Transcranial direct current stimulation to treat aphasia: Longitudinal analysis of a randomized controlled trial. Brain stimulation. 2019. Jan - Feb;12(1):190–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fridriksson J, Rorden C, Elm J, Sen S, George MS, Bonilha L. Transcranial Direct Current Stimulation vs Sham Stimulation to Treat Aphasia After Stroke: A Randomized Clinical Trial. JAMA neurology. 2018. Dec 1;75(12):1470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Fan J, Yang J, He C, Li S. Effects of transcranial direct current stimulation on walking ability after stroke: A systematic review and meta-analysis. Restorative neurology and neuroscience. 2018;36(1):59–71. [DOI] [PubMed] [Google Scholar]
- 17.Lefaucheur J-P, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology. 2017. 2017/01/01/;128(1):56–92. [DOI] [PubMed] [Google Scholar]
- 18.Vaz PG, Salazar A, Stein C, Marchese RR, Lukrafka JL, Plentz RDM, et al. Noninvasive brain stimulation combined with other therapies improves gait speed after stroke: a systematic review and meta-analysis. Top Stroke Rehabil. 2019. Apr;26(3):201–13. [DOI] [PubMed] [Google Scholar]
- 19.Lefebvre S, Liew SL. Anatomical Parameters of tDCS to Modulate the Motor System after Stroke: A Review. Frontiers in neurology. 2017;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tahtis V, Kaski D, Seemungal BM. The effect of single session bi-cephalic transcranial direct current stimulation on gait performance in sub-acute stroke: A pilot study. Restorative neurology and neuroscience. 2014;32(4):527–32. [DOI] [PubMed] [Google Scholar]
- 21.Ojardias E, Aze OD, Luneau D, Mednieks J, Condemine A, Rimaud D, et al. The Effects of Anodal Transcranial Direct Current Stimulation on the Walking Performance of Chronic Hemiplegic Patients. Neuromodulation : journal of the International Neuromodulation Society. 2019. May 23. [DOI] [PubMed] [Google Scholar]
- 22.van Asseldonk EH, Boonstra TA. Transcranial Direct Current Stimulation of the Leg Motor Cortex Enhances Coordinated Motor Output During Walking With a Large Inter-Individual Variability. Brain stimulation. 2016. Mar-Apr;9(2):182–90. [DOI] [PubMed] [Google Scholar]
- 23.Fugl-Meyer A, Jaasko L, Leyman I, Olsson S, Seglind S. The post-stroke hemiplegic patient: a method of evaluation of physical performance. Scandinavian journal of rehabilitation medicine. 1975;7:13–31. [PubMed] [Google Scholar]
- 24.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Canadian journal of public health Revue canadienne de sante publique. 1992. Jul-Aug;83 Suppl 2:S7–11. [PubMed] [Google Scholar]
- 25.Shumway-Cook A, Woollacott M. Motor Control: Theory and Practical Applications. Philadelphia: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 26.Thair H, Holloway AL, Newport R, Smith AD. Transcranial Direct Current Stimulation (tDCS): A Beginner's Guide for Design and Implementation. Frontiers in neuroscience. 2017;11:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noetscher GM, Yanamadala J, Makarov SN, Pascual-Leone A. Comparison of cephalic and extracephalic montages for transcranial direct current stimulation--a numerical study. IEEE transactions on bio-medical engineering. 2014. Sep;61(9):2488–98. [DOI] [PubMed] [Google Scholar]
- 28.Angius L, Pageaux B, Hopker J, Marcora SM, Mauger AR. Transcranial direct current stimulation improves isometric time to exhaustion of the knee extensors. Neuroscience. 2016. Dec 17;339:363–75. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka S, Takeda K, Otaka Y, Kita K, Osu R, Honda M, et al. Single session of transcranial direct current stimulation transiently increases knee extensor force in patients with hemiparetic stroke. Neurorehabilitation and neural repair. 2011. Jul-Aug;25(6):565–9. [DOI] [PubMed] [Google Scholar]
- 30.Lamontagne A, Fung J. Faster is better: implications for speed-intensive gait training after stroke. Stroke. 2004. Nov;35(11):2543–8. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Archives of physical medicine and rehabilitation. 2002. May;83(5):683–91. [DOI] [PubMed] [Google Scholar]
- 32.Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006. Mar;37(3):872–6. [DOI] [PubMed] [Google Scholar]
- 33.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Archives of physical medicine and rehabilitation. 2007. Jan;88(1):43–9. [DOI] [PubMed] [Google Scholar]
- 34.Lee DD, Seung HS. Learning the parts of objects by non-negative matrix factorization. Nature. 1999. Oct 21;401(6755):788–91. [DOI] [PubMed] [Google Scholar]
- 35.Ting LH, Macpherson JM. A limited set of muscle synergies for force control during a postural task. J Neurophysiol. 2005. Jan;93(1):609–13. [DOI] [PubMed] [Google Scholar]
- 36.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000. Oct;10(5):361–74. [DOI] [PubMed] [Google Scholar]
- 37.Clark DJ, Subramanian S, Neptune RR, SA K, editors. Fewer basic activation patterns account for lower extremity EMG during walking in adults post-stroke compared to healthy controls. Neural Control of Movement Annual Meeting; 2008; Naples, FL. [Google Scholar]
- 38.Jones TA. Motor compensation and its effects on neural reorganization after stroke. Nature reviews Neuroscience. 2017. May;18(5):267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lefebvre S, Dricot L, Laloux P, Gradkowski W, Desfontaines P, Evrard F, et al. Neural substrates underlying stimulation-enhanced motor skill learning after stroke. Brain : a journal of neurology. 2015. Jan;138(Pt 1):149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCambridge AB, Stinear JW, Byblow WD. Revisiting interhemispheric imbalance in chronic stroke: A tDCS study. Clin Neurophysiol. 2018. Jan;129(1):42–50. [DOI] [PubMed] [Google Scholar]
- 41.Shah-Basak PP, Norise C, Garcia G, Torres J, Faseyitan O, Hamilton RH. Individualized treatment with transcranial direct current stimulation in patients with chronic non-fluent aphasia due to stroke. Front Hum Neurosci. 2015;9:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sebastian R, Tsapkini K, Tippett DC. Transcranial direct current stimulation in post stroke aphasia and primary progressive aphasia: Current knowledge and future clinical applications. NeuroRehabilitation. 2016;39(1):141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sohn MK, Jee SJ, Kim YW. Effect of transcranial direct current stimulation on postural stability and lower extremity strength in hemiplegic stroke patients. Annals of rehabilitation medicine. 2013. Dec;37(6):759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bowden MG, Clark DJ, Kautz SA. Evaluation of abnormal synergy patterns poststroke: relationship of the Fugl-Meyer Assessment to hemiparetic locomotion. Neurorehabil Neural Repair. 2010. May;24(4):328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garnier YM, Lepers R, Stapley PJ, Papaxanthis C, Paizis C. Changes in corticospinal excitability following uphill versus downhill treadmill exercise. Behavioural brain research. 2017. Jan 15;317:242–50. [DOI] [PubMed] [Google Scholar]
- 46.Bonnard M, Camus M, Coyle T, Pailhous J. Task-induced modulation of motor evoked potentials in upper-leg muscles during human gait: a TMS study. The European journal of neuroscience. 2002. Dec;16(11):2225–30. [DOI] [PubMed] [Google Scholar]
- 47.Yamazaki Y, Sato D, Yamashiro K, Nakano S, Onishi H, Maruyama A. Acute Low-Intensity Aerobic Exercise Modulates Intracortical Inhibitory and Excitatory Circuits in an Exercised and a Non-exercised Muscle in the Primary Motor Cortex. Frontiers in physiology. 2019;10:1361. [DOI] [PMC free article] [PubMed] [Google Scholar]


