Abstract
OBJECTIVES
The purpose of this study was to develop a risk prediction model for patients with nonobstructive CAD.
BACKGROUND
Among stable chest pain patients, most cardiovascular (CV) events occur in those with nonobstructive coronary artery disease (CAD). Thus, developing tailored risk prediction approaches in this group of patients, including CV risk factors and CAD characteristics, is needed.
METHODS
In Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) computed tomographic angiography patients, a core laboratory assessed prevalence of CAD (nonobstructive 1% to 49% left main or 1% to 69% stenosis any coronary artery), degree of stenosis (minimal: 1% to 29%; mild: 30% to 49%; or moderate: 50% to 69%), high-risk plaque (HRP) features (positive remodeling, low-attenuation plaque, and napkin-ring sign), segment involvement score (SIS), and coronary artery calcium (CAC). The primary end point was an adjudicated composite of unstable angina pectoris, nonfatal myocardial infarction, and death. Cox regression analysis determined independent predictors in nonobstructive CAD.
RESULTS
Of 2,890 patients (age 61.7 years, 46% women) with any CAD, 90.4% (n = 2,614) had nonobstructive CAD (mean age 61.6 yrs, 46% women, atherosclerotic cardiovascular disease [ASCVD] risk 16.2%). Composite events were independently predicted by ASCVD risk (hazard ratio [HR]: 1.03; p = 0.001), degree of stenosis (30% to 69%; HR: 1.91; p = 0.011), and presence of ≥2 HRP features (HR: 2.40; p = 0.008). Addition of ≥2 HRP features to: 1) ASCVD and CAC; 2) ASCVD and SIS; or 3) ASCVD and degree of stenosis resulted in a statistically significant improvement in model fit (p = 0.0036; p = 0.0176; and p = 0.0318; respectively). Patients with ASCVD ≥7.5%, any HRP, and mild/moderate stenosis had significantly higher event rates than those who did not meet those criteria (3.0% vs. 6.2%; p = 0.007).
CONCLUSIONS
Advanced coronary plaque features have incremental value over total plaque burden for the discrimination of clinical events in low-risk stable chest pain patients with nonobstructive CAD. This may be a first step to improve prevention in this cohort with the highest absolute risk for CV events.
Keywords: computed tomographic angiography, nonobstructive coronary artery disease, risk stratification
For patients with low- to intermediate-risk stable chest pain, coronary computed tomographic angiography (CTA) has emerged as a diagnostic test with the unique ability to noninvasively rule out obstructive disease (1,2). Obstructive CAD presents the highest relative risk for future adverse cardiovascular (CV) events and is the cornerstone for current invasive and medical treatment recommendations (3–5) although it is found in fewer than 15% of symptomatic patients (6). In fact, the majority of low-risk chest pain patients present with nonobstructive CAD (<50% stenosis) (6,7), which despite its less severe appearance, bears a 3-fold higher risk for adverse CV events compared with those without CAD (8). Moreover, because of the high prevalence of nonobstructive CAD, this group harbors the highest absolute number of events, more than twice as many as individuals with obstructive CAD (9). Data from previous multinational and Korean studies demonstrate that the number of involved segments (10) and the number of involved segments plus coronary artery calcium (CAC) score (11) permit risk stratification within the group of patients with nonobstructive CAD. However, the use of more advanced CTA-based CAD assessment to provide further risk stratification has not been evaluated. It is desirable to provide these data in the setting of a generalizable US cohort of patients with stable chest pain.
We therefore aimed to determine independent predictors for CV events in patients with stable chest pain and nonobstructive CAD with the use of data from the PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) trial. Our primary goal was to determine whether information on advanced characteristics of coronary atherosclerosis can improve risk prediction beyond traditional CV risk factors in this understudied at-risk population.
METHODS
STUDY COHORT.
The PROMISE trial was a pragmatic comparative effectiveness trial that enrolled patients with stable chest pain and without known CAD to determine the presence of obstructive disease or myocardial ischemia with the use of either anatomic (i.e., coronary CTA) or functional testing. Patients with acute/unstable angina or contraindications to coronary CTA were excluded from the trial. Trial details have been described previously (12).
For this analysis, we included all patients from the anatomic testing arm with diagnostic coronary CTA as initial test who also had noncontrast calcium scoring. Patients with incomplete imaging datasets or non-diagnostic images were excluded. Furthermore, subjects with no CAD (defined as no coronary plaque on coronary CTA) or with obstructive CAD (≥50% diameter stenosis in left main or ≥70% diameter stenosis in any coronary artery) on coronary CTA images were excluded. The selection of patients for this analysis is detailed in Figure 1. The primary end point was a composite of all-cause mortality, myocardial infarction (MI), and hospitalization for unstable angina pectoris (UAP), as in the PROMISE trial. The secondary end point was a composite of MI and CV death.
FIGURE 1. CONSORT Diagram.
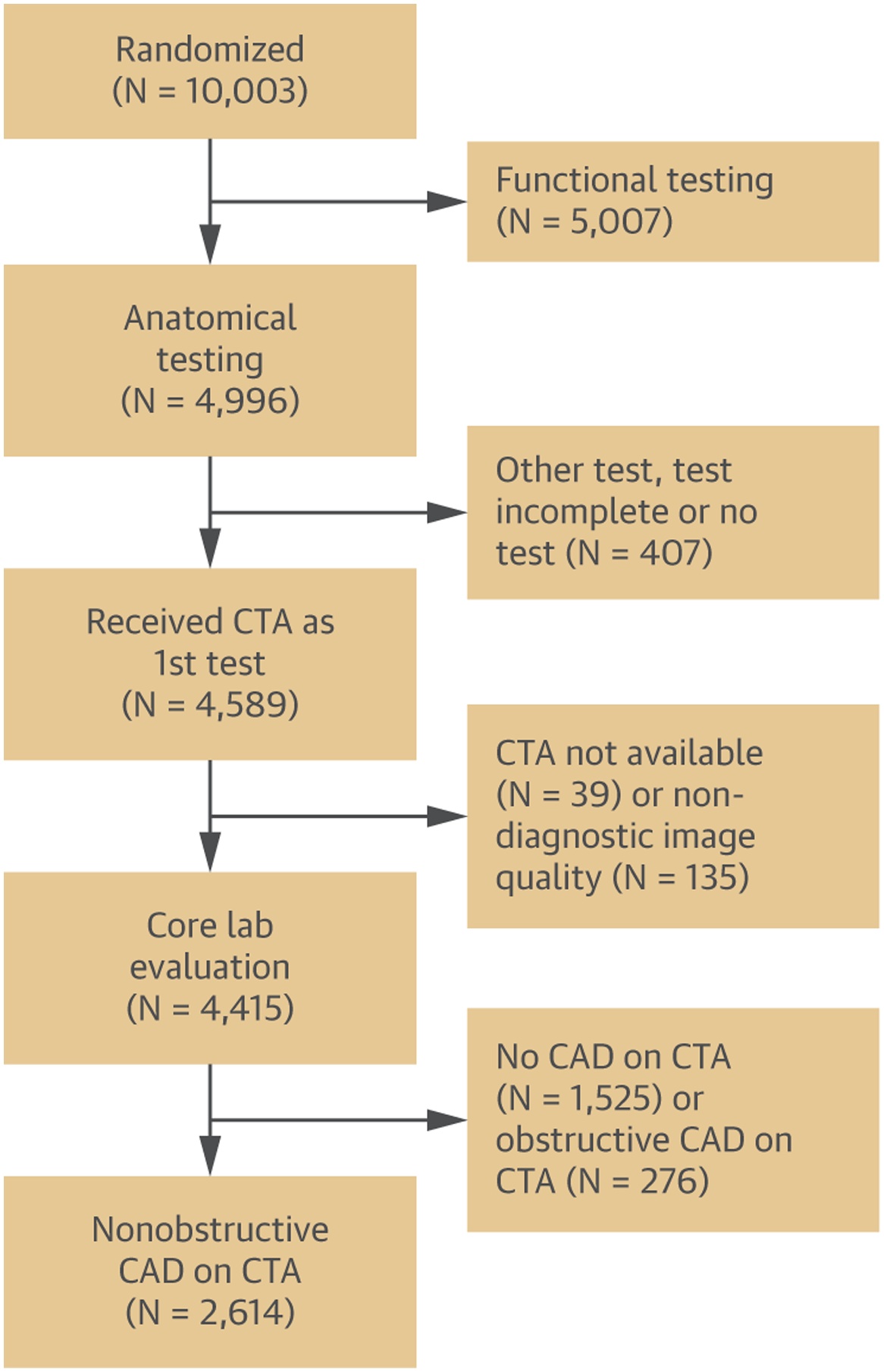
Flowchart of patient enrollment from the anatomic testing arm of the PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) trial. CAD = coronary artery disease; CTA = coronary computed tomographic angiography.
Local and central institutional review boards approved the study, and all participants provided written informed consent.
CORONARY CTA AND IMAGE ANALYSIS.
Coronary CTA was performed with ≥64-slice multidetector CT scanner. Images were interpreted on-site and confirmed by a dedicated core laboratory. Confirmed core laboratory results were used for analysis. Each coronary segment was evaluated for the presence of CAD, segment involvement score (SIS; defined as the total number of diseased segments) (13), degree of stenosis (minimal: 1% to 29%; mild: 30% to 49%; moderate: 50% to 69%; severe: 70% to 99%; obstruction: 100% diameter stenosis), the presence of high risk plaque features (HRP; previously defined as positive remodeling [remodeling index >1.1], low CT attenuation (mean CT number <30 Houndsfield units [HU]) or napkin-ring sign (central low attenuation with a ring-like periphery of high attenuation) (14), and CAC score (using the Agatston method) (15). The presence of at least 2 HRP was used to define a high burden of HRP features. Nonobstructive CAD was defined as 1% to 49% stenosis in the left main coronary artery or 1% to 69% stenosis in any coronary artery.
STATISTICAL ANALYSIS.
Continuous variables are described with the use of mean SD or median (interquartile range). Categoric variables are given in absolute and relative frequencies. For comparison between groups, a 2-sample Student’s t-test or Wilcoxon rank sum test was performed for continuous variables, Fisher’s exact test for categoric variables, and the log-rank test for equality of survivor functions regarding events. McNemar’s test was used for paired dichotomous variables. To determine the relationship of primary end points with traditional CV risk factors and CAD characteristics in patients with nonobstructive CAD, we estimated hazard ratios (HRs) with 95% confidence intervals (CIs) by constructing Cox proportional hazard models. We started with 2 separate models (model 1 including traditional CV risk factors, model 2 including CAD characteristics). To determine the final model, we included all clinically relevant covariates (defined as p < 0.20) from both prior models. The discriminatory ability of adding CAD characteristics to traditional CV risk was assessed by the likelihood-ratio test for nested models. The calibration of the model containing: 1) atherosclerotic cardiovascular disease (ASCVD), CAC, and ≥2 HRP features; 2) ASCVD, SIS, and ≥2 HRP features; or 3) ASCVD, degree of stenosis, and ≥2 HRP features was tested by means of the Grønnesby-Borgan test for goodness of fit.
Reclassification into low- versus high-risk groups was performed using statistically significant predictors (p < 0.05) from the final model. For the reclassification, we dichotomized ASCVD risk into ASCVD <7.5% and ASCVD ≥7.5% categories. Patients with ASCVD <7.5%, no HRP features, and minimal stenosis (1% to 29%) were considered low-risk, those with ASCVD ≥7.5%, the presence of ≥2 HRP features, and mild/moderate stenosis (30% to 69%) were considered high-risk. Cumulative event rates were computed by means of the Kaplan-Meier method.
All statistical calculations were done with the use of Stata, version 14.2 (StataCorp, College Station, Texas). The p values <0.05 were considered to be statistically significant.
RESULTS
STUDY COHORT AND PATIENT CHARACTERISTICS.
Out of the 10,003 patients enrolled in the PROMISE trial, 4,996 were randomized into the anatomic testing arm, and 4,686 underwent coronary CTA. Of those, 4,415 were available for image analysis. A total of 2,614 or those 4,415 (59.2%) had nonobstructive CAD and were included in this study.
CV RISK FACTORS.
Mean age was 61.6 years and 1,203 of the 2,614 (46.0%) were female. Mean ASCVD risk score was 16.2 with high prevalence of obesity (1,223 of 2,614, 47.2%), metabolic syndrome (1,030 of 2,614, 39.4%), and diabetes mellitus (601 of 2,614, 23.0%). In addition, 71.3% had dyslipidemia or were on statin therapy at study entry, 69.9% had a diagnosis of hypertension or were on antihypertensive treatment, and 54.6% were former or current smokers.
Among all patients with nonobstructive CAD, 49.5% (1,246 of 2,519) were receiving statin treatment at baseline and 63.3% (1,595 of 2,519) at 60 days (p < 0.001). Similar results were seen in those with ASCVD risk ≥7.5%.
CAD FEATURES.
Minimal-degree stenosis was observed in 53.2% of patients, while degree of stenosis 30% to 49% and 50% to 69% were observed in 33.8% and 13.0%, respectively. At least one HRP feature was present in 19.3% and ≥2 HRP features were found in 5.6% of patients. All descriptive baseline characteristics and CAD measures are presented in Table 1.
TABLE 1.
Descriptive Baseline Data in Patients With Nonobstructive CAD (1% to 49% Stenosis Left Main or 1% to 69% Stenosis Any Coronary Artery)
| Women | 1,203/2,614 (46) |
| Age (yrs) | 61.6 ± 8.4 |
| BMI (kg/m2) | 30.4 ± 5.9 |
| CV risk factors | |
| Obesity (BMI ≥30 kg/m2) | 1,223/2,614 (47.2) |
| Hypertension/antihypertensive medication (beta-blocker, ACE inhibitor) | 1,828/2,614 (69.9) |
| Dyslipidemia/statin | 1,863/2,614 (71.3) |
| Diabetes mellitus | 601/2,614 (23.0) |
| Metabolic syndrome | 1,030/2,614 (39.4) |
| Family history of premature CAD (at <55 yrs of age) | 885/2,605 (34.0) |
| Former/current smoker | 1,427/2,614 (54.6) |
| History of depression | 484/2,614 (18.5%) |
| CV risk | |
| ASCVD | 16.2 ± 11.7 |
| Events | |
| Composite clinical events (UAP, MI, death) | 86/2,614 (3.3) |
| Event type | |
| Unstable angina pectoris | 27/2,614 (1.0) |
| Myocardial infarction | 17/2,614 (0.7) |
| Death | 42/2,614 (1.6) |
| CV death | 22/2,614 (0.8) |
| Statin treatment | |
| At baseline | 1,246/2,519 (49.5) |
| At 60 days follow-up | 1,595/2,519 (63.3) |
| In ASCVD ≥7.5% at baseline | 934/1,895 (49.3) |
| In ASCVD ≥7.5% at 60 days follow-up | 1,198/1,895 (63.2) |
| CAD characteristics | |
| Coronary calcium score | |
| 0 | 184/2,297 (8.0) |
| 1–100 | 1,115/2,297 (48.5) |
| 101–300 | 500/2,297 (21.8) |
| >300 | 498/2,297 (21.7) |
| High-risk plaque features (defined as positive remodeling, low-attenuation plaque, napkin-ring sign) | |
| Any HRP | 505/2,614 (19.3) |
| ≥2 HRP | 147/2,614 (5.6) |
| Positive remodeling | 470/2,614 (18.0) |
| Low attenuation | 144/2,614 (5.5) |
| Napkin-ring sign | 100/2,614 (3.8) |
| Degree of stenosis | |
| Minimal (1%−29%) | 1,391/2,614 (53.2) |
| Mild (30%−49%) | 884/2,614 (33.8) |
| Moderate (50%−69%) | 339/2,614 (13.0) |
| SIS | 3 (2–6) |
Values are n/N (%), mean SD, or median (interquartile range).
ACE = Angiotensin-converting enzyme; ASCVD = atherosclerotic cardiovascular disease; BMI = body mass index; CAD = coronary artery disease; CV = cardiovascular; HRP = high-risk plaque; MI = myocardial infarction; SIS = segment involvement score; UAP = unstable angina pectoris.
During a median follow-up of 26 months, there were a total of 86 (3.3%) primary end point events, including 42 deaths, 17 MIs, and 27 UAP presentations.
RISK FACTORS AND EVENT RATES IN PATIENTS WITH NONOBSTRUCTIVE CAD.
In univariate analysis, there was a significantly higher incidence of primary end point events in smokers versus non-smokers (p = 0.022) and in patients ≥60 versus <60 years of age (p = 0.020). No significant differences in incident events were observed in other risk factor strata, including sex (Table 2).
TABLE 2.
Incident Primary End Point Events Stratified by Risk Factors in Patients With Nonobstructive CAD (1% to 49% Stenosis Left Main or 1% to 69% Stenosis Any Coronary Artery)
| Nonobstructive CAD | p Value (Log-Rank Test) | |
|---|---|---|
| All | 86/2,614 (3.3) | |
| CV risk factors | ||
| Women | 41/1,203 (3.4) | 0.677 |
| Men | 45/1,411 (3.2) | |
| Age <60 yrs | 28/1,191 (2.4) | 0.020* |
| Age ≥60 yrs | 58/1,423 (4.1) | |
| No diabetes mellitus | 64/2,013 (3.2) | 0.532 |
| Diabetes mellitus | 22/601 (3.7) | |
| Nonobese | 53/1,366 (3.9) | 0.084 |
| Obese | 33/1,223 (2.7) | |
| No HTN/no medication for HTN | 18/786 (2.3) | 0.052 |
| HTN/antihypertensive medication (beta-blocker, ACE inhibitor) | 68/1,828 (3.7) | |
| No family history | 60/1,720 (3.5) | 0.504 |
| Family history of premature CAD | 26/885 (2.9) | |
| No dyslipidemia/no statin | 31/751 (4.1) | 0.110 |
| Dyslipidemia/statin | 55/1,863 (3.0) | |
| Nonsmoking | 29/1,187 (2.4) | 0.022* |
| Smoking | 57/1,427 (4.0) | |
| No depression | 71/2,130 (3.3) | 0.723 |
| Depression | 15/484 (3.1) | |
| No metabolic syndrome | 52/1,584 (3.3) | 0.971 |
| Metabolic syndrome | 34/1,030 (3.0) | |
| ASCVD <7.5% | 15/630 (2.4) | 0.139 |
| ASCVD ≥7.5% | 71/1,956 (3.6) | |
| CAD features | ||
| <2 HRP | 75/2,467 (3.0) | 0.006* |
| ≥2 HRP | 11/147 (7.5) | |
| Minimal stenosis (1%−29%) | 27/1,391 (1.9) | <0.001* |
| Mild/moderate stenosis (30%−69%) | 59/1,223 (4.8) | |
| No CAC | 8/184 (4.4) | 0.443 |
| Any CAC | 68/2,113 (3.2) | |
| SIS (≤median) | 31/1,387 (2.2) | 0.001* |
| SIS (>median) | 55/1,227 (4.5) |
Values are n/N (%).
Significant difference (p < 0.05).
CAC = coronary artery calcium; HTN = hypertension; other abbreviations as in Table 1.
Among CAD characteristics as assessed by coronary CTA, incident primary end point events were significantly greater in patients with mild/moderate versus minimal stenosis (p < 0.001), ≥2 HRP vs. <2 or no HRP (p = 0.006), and SIS > median vs. SIS ≤ median (p = 0.001) (Figure 2).
FIGURE 2. Incident Events by CAD Features.
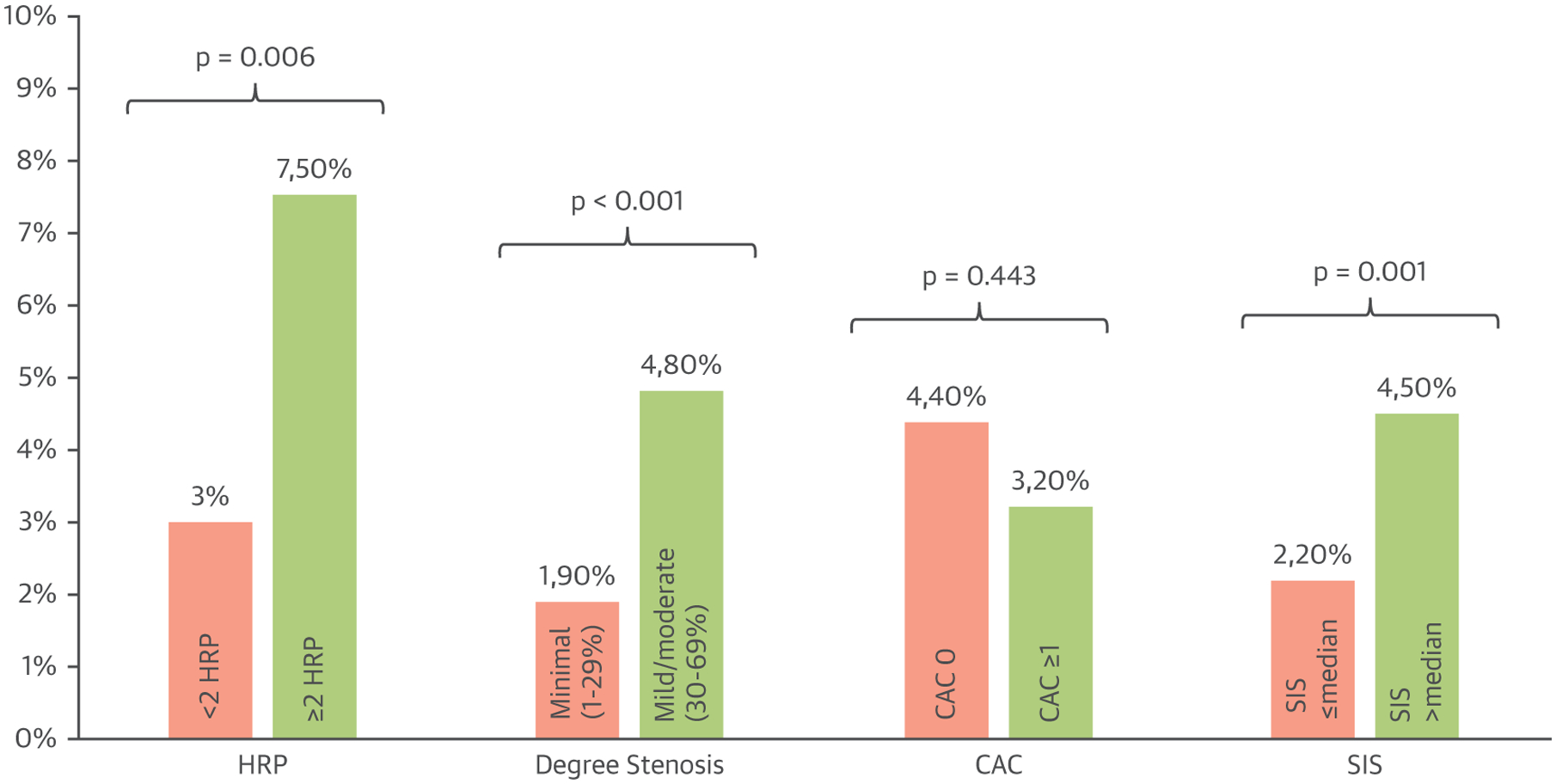
Bar graph of incident primary end point events stratified by CTA-detected coronary artery disease featured in patients with nonobstructive CAD. CAC = coronary artery calcium; HRP = high-risk plaque; SIS = segment involvement score; other abbreviations as in Figure 1.
INDEPENDENT PREDICTORS OF CLINICAL EVENTS IN NONOBSTRUCTIVE CAD.
In a multivariate Cox proportional hazard model of traditional CV risk factors (age, sex, and ASCVD risk score), only ASCVD risk score (HR 1.03; p = 0.007) was an independent predictor of incident events.
In a second multivariate Cox proportional hazard model of CAD features as assessed with the use of coronary CTA (HRP features, CAC, SIS, and degree of stenosis), mild/moderate stenosis (HR 1.87; p = 0.021) and the presence of ≥2 HRP features (HR 2.24; p = 0.015) remained independent predictors of events.
In a third model including both risk factors and CAD variables with p < 0.20 from models 1 and 2 (ASCVD risk, HRP features, degree of stenosis, and CAC), ASCVD risk (HR: 1.03; p = 0.001), mild/moderate stenosis (HR: 1.91; p = 0.011), and the presence of ≥2 HRP features (HR: 2.40; p = 0.008) independently predicted events, but CAC score did not (p = 0.096) (Table 3). When limiting the events to MI/CV death in this third model, all associations except ASCVD risk were attenuated.
TABLE 3.
Multivariate Analysis for Events in Nonobstructive CAD (1% to 49% Stenosis Left Main or 1% to 69% Stenosis Any Coronary Artery)
| HR (95% CI) | p Value | |
|---|---|---|
| Model 1: Traditional CV risk (n = 2,586) | ||
| Age (continuous) | 1.00 (0.97–1.04) | 0.813 |
| Male sex (vs. female sex) | 0.87 (0.55–1.38) | 0.561 |
| ASCVD risk (continuous) | 1.03 (1.01–1.05) | 0.007* |
| Model 2: CAD characteristics (n = 2,297) | ||
| ≥2 HRP (vs. <2 HRP) | 2. 24 (1.17–4.30) | 0.015* |
| CAC (continuous)† | 1.00 (1.00–1.00) | 0.101 |
| SIS (continuous) | 1.05 (0.95–1.15) | 0.343 |
| Mild/moderate stenosis (vs. minimal stenosis) | 1.87 (1.10–3.16) | 0.021* |
| Model 3: Traditional CV risk plus | ||
| CAD characteristics (using covariates with p < 0.20 from models 1 and 2) (n = 2,271)‡ | ||
| ASCVD risk (continuous) | 1.03 (1.01–1.04) | 0.001* |
| ≥2 HRP (vs. <2 HRP) | 2.40 (1.25–4.58) | 0.008* |
| Mild/moderate stenosis (vs. minimal stenosis) | 1.91 (1.16–3.16) | 0.011* |
| CAC (continuous)† | 1.00 (0.9999–1.0006) | 0.096 |
Significant (p < 0.05).
Similar results are found when using log CAC instead of CAC.
Results are similar when using a full specified model that includes all variables (Supplemental Table 1).
Furthermore, adding the presence of ≥2 HRP features to 3 different baseline models that included: 1) ASCVD and CAC; 2) ASCVD and SIS; or 3) ASCVD and degree of stenosis resulted in a statistically significant improvement in model fit (p = 0.0036, p = 0.0176, and p = 0.0318, respectively). A Grønnesby-Borgan test for goodness of fit of models 1, 2, and 3 with ≥2 HRP features was also nonsignificant, suggesting no gross model violations.
INDEPENDENT PREDICTORS OF CLINICAL EVENTS IN NONOBSTRUCTIVE CAD (1% TO 49% STENOSIS).
In a subanalysis including only patients with 1%–49% stenosis with a total of 58 (2.9%) primary end point events, we were able to confirm the results of the combined model using risk factors and CAD variables. ASCVD risk (HR: 1.03; p < 0.001), mild/moderate stenosis (HR: 1.79; p = 0.035), and the presence of ≥2 HRP features (HR: 2.25; p = 0.046) independently predicted events, whereas CAC score did not show a significant association (p = 0.235) (Table 4).
TABLE 4.
Multivariate Analysis for Events in Nonobstructive CAD (1% to 49% Stenosis Left Main or 1% to 69% Stenosis Any Coronary Artery)
| HR (95% CI) | p Value | |
|---|---|---|
| Traditional CV risk plus CAD characteristics (using covariates with p < 0.20 from models 1 and 2) (n = 1,982) | ||
| ASCVD risk (continuous) | 1.03 (1.02–1.05) | <0.001* |
| ≥2 HRP (vs. <2 HRP) | 2.25 (1.01–5.01) | 0.046* |
| Mild/moderate stenosis (vs. minimal stenosis) | 1.79 (1.04–3.08) | 0.035* |
| CAC (continuous)† | 1.00 (0.9998–1.0006) | 0.235 |
Results are similar when using a full specified model that includes all variables (Supplemental Table 1).
Significant (p < 0.05).
Similar results are found when using log CAC instead of CAC.
ASCVD = atherosclerotic cardiovascular disease; CAC = coronary Artery Calcium; CAD = coronary artery disease; CI = confidence interval; CV = cardiovascular; HR = hazard ratio; HRP = high risk plaque; SIS = segment involvement score
When limiting the events to MI/CV death, all associations except ASCVD risk were attenuated.
When adding ≥2 HRP features to the 3 different baseline models as mentioned above, we found a significant improvement of model fit for the model including ASCVD plus CAC (p = 0.0250). However, no significant improvement was seen when the models including ASCVD plus SIS or degree of stenosis had HRP added (p = 0.0541 and p = 0.0887, respectively).
IDENTIFICATION OF A HIGHER-RISK PATIENT POPULATION WITH NONOBSTRUCTIVE CAD.
To enable more pragmatic identification of a higher-risk patient population among those with nonobstructive CAD, we dichotomized significant predictors from the third prediction model as follows: ASCVD <7.5% versus ≥7.5%, no HRP features versus any HRP features, and minimal versus mild/moderate stenosis.
In patients with 1% to 69% stenosis, those with ASCVD ≥7.5%, any HRP features, and mild/moderate stenosis had a significantly higher event rate than those that did not meet these higher risk criteria (6.2% [16 of 257] vs. 3.0% [70 of 2,357]; p = 0.007). Patients that met 2 of these higher risk criteria similarly demonstrated a significantly higher event rate than those that did not (4.8% [56 of 1,159] vs. 2.1% [30 of 1,455]; p < 0.001). These results were confirmed in patients with 1% to 49% stenosis with a p value of 0.002 for those that met 3 higher risk criteria and p < 0.001 for those with 2 higher risk criteria (Central Illustration).
CENTRAL ILLUSTRATION. Kaplan-Meier Curves of Higher- Versus Lower-Risk Patients With Nonobstructive CAD.
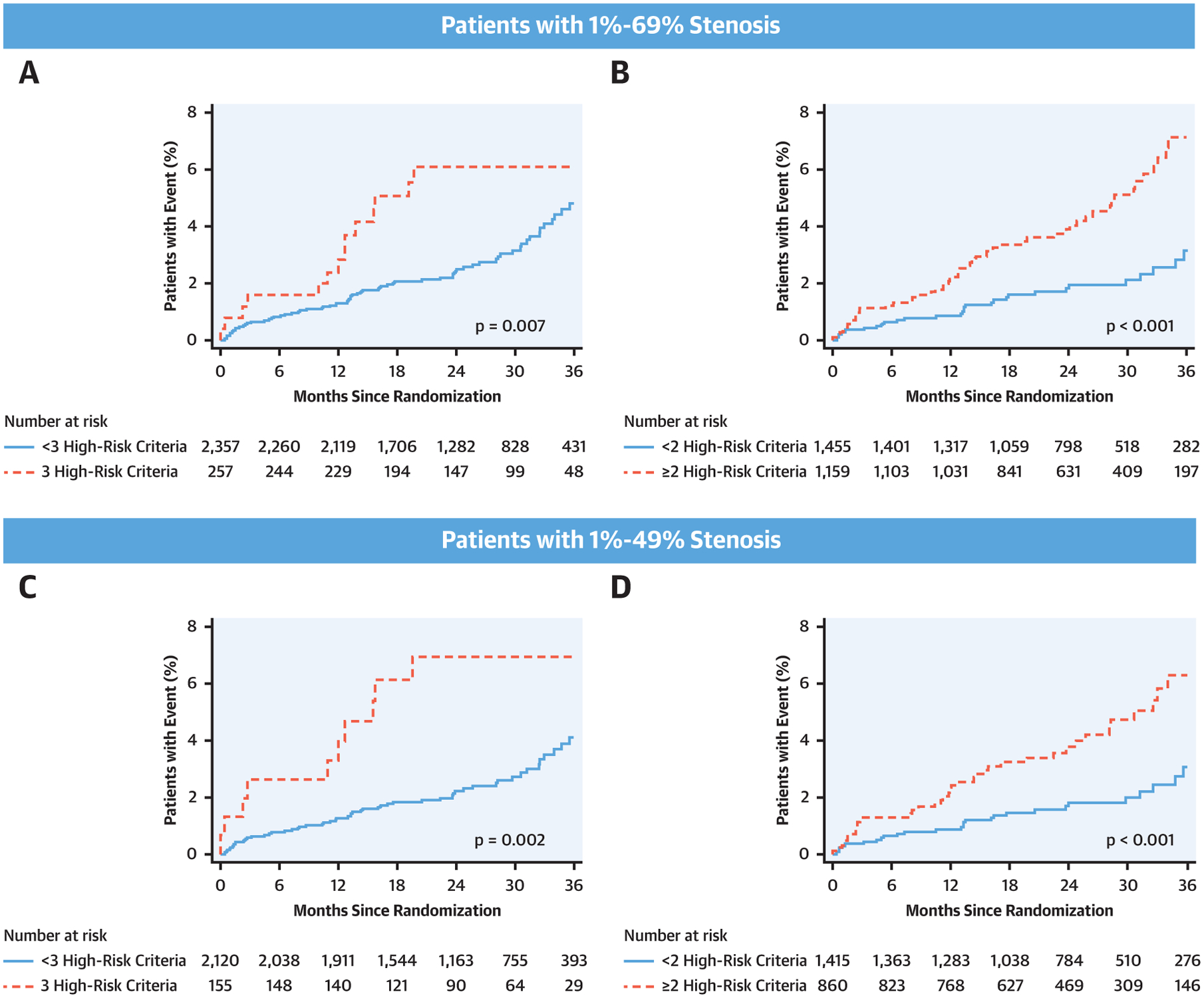
Kaplan-Meier curves of higher (ASCVD ≥7.5%, any HRP features, and mild/moderate stenosis) versus lower risk in patients with 1% to 69% stenosis (A and B) and those with 1% to 49% stenosis (C and D). Patients that met 3 criteria versus those that did not are displayed in A and C, respectively. Patients that met 2 of these higher risk criteria versus those that did not are shown in B and D, respectively. Event rates were significantly higher in patients classified as higher-risk nonobstructive CAD: p = 0.007 and p = 0.002 for A and C and p < 0.001 for (B) and (D). ASCVD = atherosclerotic cardiovascular disease; other abbreviations as in Figures 1 and 2.
DISCUSSION
This study demonstrates, in the PROMISE randomized trial participants, that CAD characteristics as determined with the use of coronary CTA can improve risk stratification in patients with stable chest pain and nonobstructive CAD. We were able to demonstrate that ASCVD risk (HR: 1.03; p = <0.001), mild/moderate stenosis (HR: 1.91; p = 0.011), and the presence of ≥2 HRP features (HR: 2.40; p = 0.008) independently predicted the occurrence of adverse events in this population. We furthermore showed that the addition of adverse plaque characteristics (≥2 HRP) significantly improves discriminatory ability when added to a baseline model including ASCVD risk score and information in plaque burden (CAC score, SIS, or degree of stenosis). This allowed recognition of a subgroup among the heterogeneous cohort of patients with nonobstructive CAD with significantly higher event rates than others in this population. Thus, patients with at least 2 at-risk criteria (ASCVD ≥7.5%, any HRP, and mild or moderate stenosis) had more than 2-fold higher event rates than those who did not (p < 0.001 and p = 0.007 for patients with 2 and 3 higher-risk criteria, respectively).
In a subgroup of patients with minimal/mild stenosis (1% to 49%), we were able to confirm the results of our prediction model for the occurrence of adverse events. Similarly, ASCVD, mild stenosis, and the presence of ≥2 HRP independently predicted events. We think that this is relevant for clinical practice, because guidelines do not yet specify the value of CAD characteristics in those with <50% luminal narrowing for medical therapy. Puchner et al. previously reported that all patients with mild stenosis (1% to 49%) that experienced an acute coronary syndrome had at least one high-risk plaque (including spotty calcifications) suggesting that these patients cannot be safely discharged from the ED (16). This is in line with our findings, showing that degree of stenosis and plaque composition (i.e., HRP) are independent predictors of events and that as a result, all of these are important for risk stratification.
RISK PREDICTION SCORES IN PATIENTS WITH NONOBSTRUCTIVE CAD.
Although nonobstructive CAD has historically been considered to be less important than obstructive CAD, recent reports have identified these patients as a large at-risk group (9,17–19) carrying a significant socioeconomic burden given the high prevalence and the higher absolute number of events (20). Furthermore, patients with more advanced nonobstructive CAD and adverse CAD characteristics exhibit a higher risk compared with those with minimal disease (9–11,17,18).
Ferencik et al. (14) demonstrated in a subanalysis of their study on the predictive value of HRP in patients with stable chest pain that especially in the group of nonobstructive CAD patients, the presence of HRP increased the risk of major adverse cardiac events compared with those without any plaque (adjusted HR with HRP: 4.31 vs. without HRP: 2.64; 95% CI: 2.25 to 8.26 vs. 1.49 to 4.69). Nevertheless, there is little data to provide further risk stratification based on CAD characteristics in addition to clinical information in this heterogeneous cohort and to inform treatment recommendations to those benefiting the most. Thus, the novel element of our analysis is that we were able to show that plaque characteristics have incremental value over total plaque burden (i.e., degree of stenosis, CAC score, and SIS) by adding HRP features to 3 different baseline models (ASCVD risk score plus CAC, SIS, or degree stenosis). Discriminatory ability was significantly increased by adding HRP to the 3 different baseline models including traditional risk factors and information on plaque burden. In a subanalysis of patients with minimal to mild stenosis (1% to 49%), we saw an incremental value of HRP in the model including ASCVD plus CAC (p = 0.0250), but not in those including ASCVD plus SIS or ASCVD plus degree stenosis. This likely reflects the nature of this subpopulation (i.e., stable chest pain patients with minimal disease) but also the low sample size.
Two recent studies have approached risk prediction of events with the use of clinical characteristics and CAD features in patients with nonobstructive CAD in Western and Korean populations (10,11). Both reported that CTA-based CAD findings provide additive value beyond traditional risk factors. For example, in the international CONFIRM registry, the number of involved coronary segments (SIS score) was of stronger prognostic information than traditional risk factors (i.e., age, sex, body mass index, diabetes, hypertension, hypercholesterolemia, smoking, and positive family history) in patients with nonobstructive CAD (<50% stenosis). The addition of SIS to a model of clinical risk factors led to a significant increase of the C-statistic (0.70 vs. 0.67; p = 0.001) (10).
In the present study of a cohort of U.S. patients with similar demographics, we were similarly able to improve the discriminatory ability to predict events by adding CTA-based CAD characteristics to ASCVD risk (p = 0.0036, p = 0.0176, and p = 0.0318, respectively, for addition of ≥2 HRP features to: 1) ASCVD and CAC; 2) ASCVD and SIS; or 3) ASCVD and degree stenosis). Conversely, in our cohort, SIS was no independent predictor of events. Moreover, in our model using CAD characteristics only, degree of stenosis and HRP were found to be of stronger prognostic information than SIS. Nevertheless, it needs to be mentioned that there is likely a high collinearity between CTA-based CAD characteristics. However, the predictive value for degree of stenosis and HRP improved in our third model of traditional risk factors plus CAD characteristics when not using SIS, demonstrating that ASCVD risk remains the best summary measure to predict events and is complemented by information on degree of stenosis and the presence of HRP, both known strong predictors of events (14,21). Interestingly, CAC did not predict events, most likely because of exclusion of those without CAD and those with significant stenosis resulting in crowding of CAC scores between 1 and 100 (48.5%). We believe that excluding the extreme phenotypes caused this result.
IDENTIFICATION OF HIGHER-RISK PATIENTS WITH NONOBSTRUCTIVE CAD.
Using information on CAD from coronary CTA in stable chest pain patients with nonobstructive disease could potentially have a large impact on further risk stratification and future patient management. It has been shown that incorporating the information of nonobstructive CAD into the pooled cohort equation can lead to reclassification of 14% of patients with acute chest pain without acute coronary syndrome toward statin eligibility, allowing risk stratification and more precise allocation of statin treatment to those at higher clinical and anatomic risk (8). Because patients with chest pain who undergo coronary CTA for diagnostic reasons would have information on CAD features readily available at no additional cost, a strategy incorporating this information into therapeutic decision making is attractive.
Using additional CTA information to identify a higher-risk group of patients with nonobstructive CAD may enable a more individualized approach for prevention through both lifestyle and medical interventions. Hwang et al. (11) used a model-based scoring system with clinical risk factors, involved vessels, and CAC score to stratify a cohort of Korean patients with nonobstructive CAD (<50% stenosis) into 4 risk groups (low to very high). Patients classified as low risk had similar outcomes to those without any CAD, whereas those classified as very high risk had event rates similar to patients with obstructive disease after 5 years of follow-up (11). Similarly, we were able to identify a higher-risk subpopulation, with the use of intermediate ASCVD risk (≥7.5%) and CAD characteristics (presence of ≥2 HRP features and/or mild/moderate stenosis), with a more than 2-fold higher risk for events compared with patients that did not meet these criteria (for example, patients with 1% to 69% stenosis meeting all 3 criteria vs. not: 6.2% vs. 3.0%; p = 0.007). This elevated event rate is similar to patients with single-vessel obstructive (>70% stenosis) disease (6.2% vs. 7.8%) (9).
A 2-fold increase in events is especially relevant considering the relatively low event rates of the whole cohort (3.3% after a follow-up of 26 months). Similarly to CAC data, which demonstrated the most benefit of statin treatment in patients with a CAC >100 (22), the presence of ≥2 higher-risk criteria may be a threshold at which more intense secondary prevention should be considered. More supporting evidence for the use of risk stratification in patients with nonobstructive CAD to optimize treatment allocation comes from Hwang et al. (11), who were able to demonstrate better outcomes in their high- and very-high-risk patients with nonobstructive disease on statin treatment (HR: 0.62; 95% CI: 0.39 to 0.96; p = 0.033) during 5 years of follow-up. However, such strategies need to be tested prospectively.
INCIDENT EVENTS IN RISK STRATA.
In our univariate analysis, there was another noteworthy finding. Namely, outcomes stratified by a number of well-known risk factors (e.g., sex, diabetes mellitus, family history of premature CAD, and so on) were not significantly different. One explanation may again be our cohort itself, a group at low to intermediate risk for the occurrence of events, with limited differentiation power. Results would likely be different in a population also including patients with no CAD or obstructive disease (i.e., with very low or very high risk for future events). Nevertheless, the similar event rate in women and men with nonobstructive CAD is remarkable and an interesting approach for further evaluation given the emphasis of nonobstructive CAD in women.
STUDY LIMITATIONS.
First, trial participants had a relatively small number of events and a short median follow-up of 26 months. Therefore, confirmation of our results and further development of prediction models in other cohort studies are warranted. Second, trial data was acquired before management recommendations using pooled cohort equations to calculate 10-year ASCVD risk were published (23,24). Based on current guidelines (23), we would expect all symptomatic patients with CAD to start lipid-lowering therapy. Nevertheless, use of statin treatment at 60 days of follow-up was 63.3% in this study, which may have affected outcomes.
CONCLUSIONS
Advanced coronary plaque analysis using coronary CTA can improve risk prediction for future events in stable chest pain patients with nonobstructive CAD beyond traditional ASCVD risk scoring and have incremental value over total plaque burden for the discrimination of clinical events. Careful evaluation of plaque features from coronary CTA in this population may be a first step to improve risk stratification. Better risk stratification may not only allow for more directed medication use but also for more targeted approaches using intensive lifestyle interventions in this cohort with the highest absolute risk for CV events.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
Patients with chest pain who undergo coronary CTA for diagnostic reasons have information on CAD features readily available at no additional cost, so a strategy incorporating this information into therapeutic decision making is attractive. Using information on CAD from coronary CTA in stable chest pain patients with nonobstructive disease could potentially have a large impact on further risk stratification and future patient management, allowing for more directed medication use but also for more targeted approaches using intensive lifestyle interventions in this cohort with the highest absolute risk for CV events.
TRANSLATIONAL OUTLOOK:
Further clinical studies are necessary to validate the impact of CT-based CAD characteristics to further risk stratification and optimize management in patients with nonobstructive CAD.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
The PROMISE trial was supported by grants from the National Heart Lung and Blood Institute, Bethesda, Maryland: R01HL098237, R01HL098236, R01HL098305, and R01HL098235. Dr. Taron is funded by the Deutsche Forschungsgemeinschaft (German Research Foundation; TA 1438/1-2); and is on the Speakers Bureau for Siemens Healthcare, unrelated to this work. Dr. Osborne has received grant support from the National Institutes of Health (KL2TR002542) and Intrinsic Imaging, for unrelated work. Dr. Meyersohn has received support from the National Institutes of Health/National Heart, Lung, and Blood Institute (T32 HL076136). Dr. Lu has received consulting fees with PQBypass, a research grant from the Nvidia Corporation Academic Program, work as a co-investigator on research funded by Astrazeneca and Kowa, and grant support from the American Heart Association Precision Medicine Institute (18UNPG34030172) and the Harvard University Center For AIDS Research (NIH/NIAID 5P30AI060354-14). Dr. Ferencik has received grant support from the American Heart Association (13FTF16450001). Dr. Pagidipati has received research grants from Amgen, AstraZeneca, Baseline Study, Boehringer Ingleheim, Duke Clinical Research Institute, Eli Lilly & Company, Novo Nordisk Pharmaceutical Company, Regeneron Pharmaceuticals, Sanofi, and Verily Sciences Research Company; and consulting fees from AstraZeneca, Boehringer Ingleheim, Esperion Therapeutics, Eli Lilly & Company, and Novo Nordisk Pharmaceutical Company. Dr. Douglas has received a research grant from HeartFlow. Dr. Hoffmann has received a grant from the National Institutes of Health (K24HL113128), research support on behalf of the Massachusetts General Hospital from Duke University, HeartFlow, Kowa Company, and MedImmune/AstraZeneca; and consulting fees from Duke University and Recor Medical unrelated to this research. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- ASCVD
atherosclerotic cardiovascular disease
- CAC
coronary artery calcium
- CTA
coronary computed tomographic angiography
- HRP
high-risk plaque
- HU
Houndsfield units
- MI
myocardial infarction
- SIS
segment involvement score
- UAP
unstable angina pectoris
Footnotes
APPENDIX For a supplemental table, please see the online version of this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation 2004;109: 14–7. [DOI] [PubMed] [Google Scholar]
- 2.Hamon M, Biondi-Zoccai GGL, Malagutti P, et al. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: a meta-analysis. J Am Coll Cardiol 2006;48:1896–910. [DOI] [PubMed] [Google Scholar]
- 3.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cordial 2012;60:e44–164. [DOI] [PubMed] [Google Scholar]
- 4.Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. J Am Coll Cardiol 2014;64:1929–49. [DOI] [PubMed] [Google Scholar]
- 5.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41: 407–77. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann U, Bamberg F, Chae CU, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain. J Am Coll Cardiol 2009;53:1642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho I, Chang H-J, Ó Hartaigh B, et al. Incremental prognostic utility of coronary CT angiography for asymptomatic patients based upon extent and severity of coronary artery calcium: results from the Coronary CT Angiography Evaluation for Clinical Outcomes International Multicenter (CONFIRM) study. Eur Heart J 2015;36:501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emami H, Takx RAP, Mayrhofer T, et al. Nonobstructive coronary artery disease by coronary ct angiography improves risk stratification and allocation of statin therapy. J Am Coll Cardiol Img 2017;10:1031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann U, Ferencik M, Udelson JE, et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;135:2320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Rosendael AR, Bax AM, Smit JM, et al. Clinical risk factors and atherosclerotic plaque extent to define risk for major events in patients without obstructive coronary artery disease: the long-term coronary computed tomography angiography CONFIRM registry. Eur Heart J Cardiovasc Imaging 2020;21:479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang I-C, Lee H, Yoon YE, et al. Risk stratification of nonobstructive coronary artery disease for guidance of preventive medical therapy. Atherosclerosis 2019;290:66–73. [DOI] [PubMed] [Google Scholar]
- 12.Douglas PS, Hoffmann U, Lee KL, et al. Prospective Multicenter Imaging Study for Evaluation of Chest Pain: rationale and design of the PROMISE trial. Am Heart J 2014;167:796–803.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayoub C, Erthal F, Abdelsalam MA, et al. Prognostic value of segment involvement score compared to other measures of coronary atherosclerosis by computed tomography: a systematic review and meta-analysis. J Cardiovasc Comput Tomogr 2017;11:258–67. [DOI] [PubMed] [Google Scholar]
- 14.Ferencik M, Mayrhofer T, Bittner DO, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain. JAMA Cardiol 2018;3:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15: 827–32. [DOI] [PubMed] [Google Scholar]
- 16.Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary computed tomography angiography predicts acute coronary syndrome independent of significant stenosis in patients with acute chest pain—results from ROMICAT II trial. J Am Coll Cardiol 2014;64:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin FY, Shaw LJ, Dunning AM, et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease. J Am Coll Cardiol 2011;58: 510–9. [DOI] [PubMed] [Google Scholar]
- 18.Chow Benjamin JW, Small Gary, Yam Yeung, et al. Prognostic and therapeutic implications of statin and aspirin therapy in individuals with nonobstructive coronary artery disease. Arterioscler Thromb Vasc Biol 2015;35: 981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho Y-K, Nam C-W, Koo B-K, et al. Usefulness of baseline statin therapy in nonobstructive coronary artery disease by coronary computed tomographic angiography: from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) study. PLoS One 2018;13:e0207194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bittencourt MS, Hulten E, Ghoshhajra B, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging 2014;7:282–91. [DOI] [PubMed] [Google Scholar]
- 21.Nam K, Hur J, Han K, et al. Prognostic value of Coronary Artery Disease-Reporting and Data System (CAD-RADS) score for cardiovascular events in ischemic stroke. Atherosclerosis 2019; 287:1–7. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell JD, Fergestrom N, Gage BF, et al. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol 2018;72:3233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285–350. [DOI] [PubMed] [Google Scholar]
- 24.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. J Am Coll Cardiol 2014; 63:2889–934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


