Abstract
Background:
Downregulation of claudin-5 in the heart is associated with the end-stage heart failure. However, the underlying mechanism of claudin-5 is unclear. Here we investigated the molecular actions of claudin-5 in perspective of mitochondria in cardiomyocytes to better understand the role of claudin-5 in cardioprotection during ischemia.
Methods and Results:
Claudin-5 was detected in the murine heart tissue and the neonatal rat cardiomyocytes (NRCM). Its protein level was severely decreased after myocardial ischemia/reperfusion (I/R; 30 min/24 h) or hypoxia/reoxygenation (H/R; 24 h/4 h). Claudin-5 was present in the mitochondria of NRCM as determined by confocal microscopy. H/R-induced downregulation of claudin-5 was accompanied by mitochondrial fragmentation. The protein level of mitofusin 2 (Mfn2) was dramatically decreased while the expression of dynamin-related protein (Drp) 1 was significantly increased after H/R. H/R-induced mitochondrial swelling and fission were observed by transmission electron microscope (TEM). Overexpression of claudin-5 by adenoviral infection reversed these structural disintegration of mitochondria. The mitochondria-centered intrinsic pathway of apoptosis triggered by H/R and indicated by the expression of cytochrome c and cleaved caspase 3 in the cytoplasm of NRCMs was also reduced by overexpressing claudin-5. Overexpression of claudin-5 in mouse heart also significantly decreased cleaved caspase 3 expression and the infarct size in ischemic heart with improved systolic function.
Conclusion:
We demonstrated for the first time the presence of claudin-5 in the mitochondria in cardiomyocytes and provided the firm evidence for the cardioprotective role of claudin-5 in the preservation of mitochondrial dynamics and cell fate against hypoxia- or ischemia-induced stress.
Keywords: Claudin-5, Mirochondrial fragmentation, Mitochondrial dynamics, Myocardial ischemia/reperfusion, Hypoxia/reoxygenation
Brief summary
Claudin-5 is known as a tight junction protein controlling the endothelial permeability. It was found expressed in cardiomyocyte’s mitochondria and its expression was dramatically decreased along with mitochondiral fission after ischemia/reperfusion (I/R) injury. Overexpression of claudin-5 in cardiomyocytes prevented mitochondial fission and apoptosis and improved heart function after myocardial I/R. The present findings may improve our current understanding of the role of claudin-5 in the cell and provide new theraputic target for ischemic heart disease.
Introduction
Claudin-5 is a transmembrane cell junction protein that controls paracellular permeability on endothelial cell layers 1, 2. The disruption of blood-brain barrier is usually associated with the degraded claudin-5 levels caused by ischemic injury 3. Increasing claudin-5 in brain microvascular endothelial cells has already been recognized as a novel target that contributes to the stabilization of the integrity in the blood-nerve barrier, and to the decrease in the endothelial hyper-permeability 4, 5. In addition, increasing claudin-5 in microvascular myocardial endothelial cell line also leads to an improvement in vascular structural integrity and barrier function of the vascular endothelium 6.
Interestingly, the recent studies reported that claudin-5 genes (claudin-5a and 5b) were required for Xenopus heart tube formation, indicating that claudin-5 may be involved in cardiogenesis 7. Later findings from animal experiments and clinical research showed that claudin-5 was mainly localized to the lateral membranes of murine cardiomyocytes, and was severely downregulated in cardiomyocytes from the human failing heart at its end-stage 8–10. Sustaining cardiac claudin-5 levels in the murine model of muscular dystrophy and cardiomyopathy by recombinant claudin-5 adeno-associated virus prevented the development of cardiomyopathy and attenuated cardiac damage 11. This evidences suggest that claudin-5 is a cardioprotective factor, but the underlying mechanism and the exact role of claudin-5 on the cardiomyocyte is unclear.
Recently, attention has focused on the concept of mitochondrial dynamics that may plays a pivotal role in determining cell fate in the heart under ischemic stress or in cardiomyopathy 12–14. Mitochondrial fusion/fission dynamics is essential to normal mitochondrial homeostasis and controls many aspects of mitochondrial biology, including but not limited to energy production, metabolism, and apoptosis that are imperative in cardiac function 15, 16. Genetic inhibition of mitochondrial fusion causes mitochondrial fragmentation and heart failure whereas inhibiting mitochondrial fission protects against cardiomyocyte apoptosis and cardiac injury 17. Nevertheless, the molecular machinery that regulates mitochondrial dynamics in the heart was still not fully understood.
The role of claudin-5 on the surface of epithelial/endothelial cells as a tight junction protein has been somewhat characterized 18. However, its role in mitochondria dynamics in the context of cardioprotection is unknown. The aim of the present study is to investigate the role of claudin-5 in mitochondrial dynamics in cardiomyocytes and to provide the molecular mechanisms of the protective actions by claudin-5 on the heart from the perspective of mitochondrial fusion/fission dynamics. Our results indicate that claudin-5 is required for preventing mitochondrial swelling and fission in cardiomyocytes. This in turn leading to the attenuation of hypoxic/ischemic-induced cardiomyocyte apoptosis, thereby protecting the heart from ischemic-induced cardiac injury and improving the heart function.
Materials and Methods
The investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). All study was approved by the ethical committee for animal experiments at University of California Irvine (Protocol #2007–2755). For full details of the methods, please see the Supplemental Methods. Here, we provide a condensed description.
Human cardiomyocyte culture and heart sample isolation
AC16 human cardiomyocyte cell line (Cat#SCC109) was purchased from Millipore Corporation. Then cardiomyocytes were cultured according to the manufacturer’s protocol. With approval from local human research ethics review, cardiac tissue from the left atrial appendage was obtained from a patient undergoing radiofrequency ablation maze procedure.
Myocardial ischemia/reperfusion injury
The myocardial ischemia/reperfusion (I/R) injury model was developed as described previously 19.
Neonatal rat cardiomyocyte isolation and culture
NRCM were isolated from 1–2 day old Sprague-Dawley rats according to the protocol previously described 20. Hypoxia for 24 h and reoxygenation for 4 h used to stimulate I/R injury in cellular level was based on our previous studies 20, 21.
Silencing RNA knockdown and adenovirus infection
Claudin-5 specific small-interfering RNA (siRNA) and nontargeted control siRNA (Life Technologies) were transfected into NRCM with Lipofectamine 3000 transfection kit (Invitrogen) for 24 h. The adenovirus were made in our lab then injected into left ventricle below the LCA ligation point in three positions before I/R to overexpress the claudin-5 for 24 h then followed by I/R surgery.
Western blotting
Claudin-5, Mfn 2, Drp-1, Cytochrome c, Cleaved caspase 3, Caspase 3 expression was characterized by means of Western blot analysis.
Mitochondrial isolation
The mitochondrial protein and cytoplasmic protein were separated by a commercially available mitochondria isolation kit (Cat# 89874, Thermo Scientific).
Immunofluorescence staining
The fluorescence of endogenous claudin-5 (green) and mitochondria (red) were observed in cardiomyocytes under confocal microscope (Zeiss).
Transmission electron microscopy (TEM)
The mitochondrial morphology in NRCM was observed by an experienced TME technician in the UCI Medical Center.
Echocardiographic observation
The systolic function of the heart was observed and measured by a high-frequency small animal ultrasound system as previously described 22.
2,3,5-Triphenyl-2H-tetrazolium chloride (TTC) staining
TTC staining was used to access the myocardial infarction as previously described23.
Statistical analysis
All experiments were expressed as the mean ± standard error of the mean and p < 0.05 was considered to indicate statistical significance. The sample number indicates the number of biological replicates. The paired t-test was used for comparisons between two groups. One-way ANOVA with post hoc analysis by the Fisher exact probability test was employed for multiple comparisons. All analyses were performed using SPSS 13.0 software (SPSS Inc., Chicago, IL).
Results
Claudin-5 is co-localized with mitochondria in human cardiomyocyte
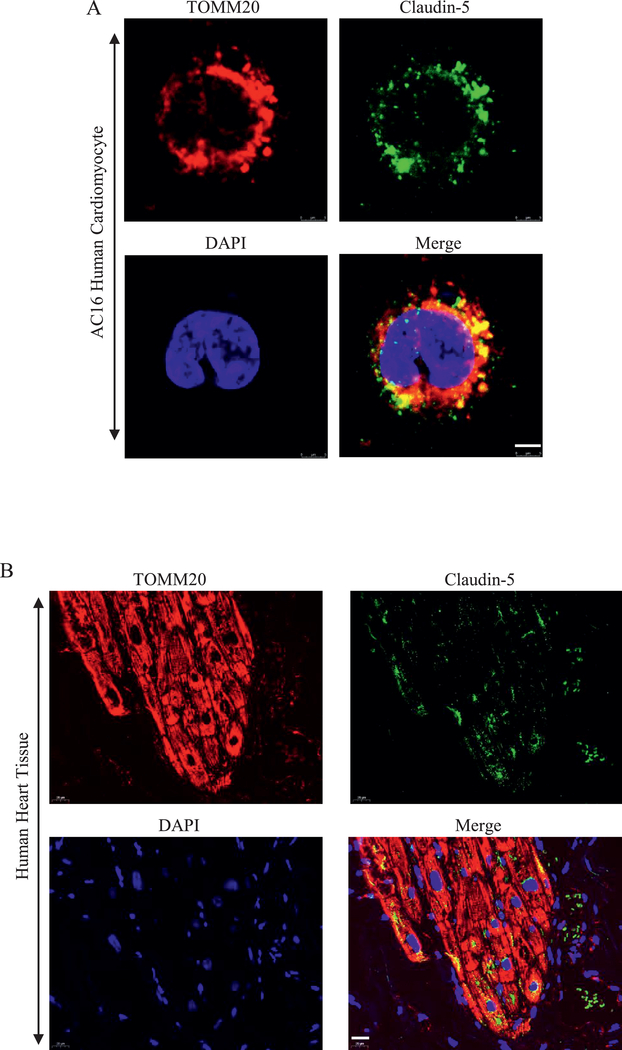
The previous study showed that claudin-5 was expressed in the lateral membrane of human cardiomyocyte10. However, whether claudin-5 is expressed on mitochondria of cardiomyocyte remains unclear. We cultured AC16 human cardiomyocyte cell line in vitro and investigated the subcellular localization of claudin-5 and TOMM20, a mitochondrial marker. As shown in Fig. 1A, there was a strong expression of claudin-5 with TOMM20. We further confirmed the expression of claudin-5 on mitochondria in human heart tissue (Fig. 1B).
Fig. 1. The expression of claudin-5 in human cardiomyocyte.
(A) Subcellular localization of claudin-5 in AC16 human cardiomyocyte. Anti-claudin-5 antibody (Alexa Fluor 488, green) was used to detect endogenous claudin-5; Anti-TOMM20 antibody, a mitochondrial marker, was used to stain mitochondria (red). Scale bar, 5 μm. (B) Subcellular localization of claudin-5 in human heart tissue. Scale bar, 20 μm.
Claudin-5 in the heart is downregulated after myocardial I/R injury in vivo
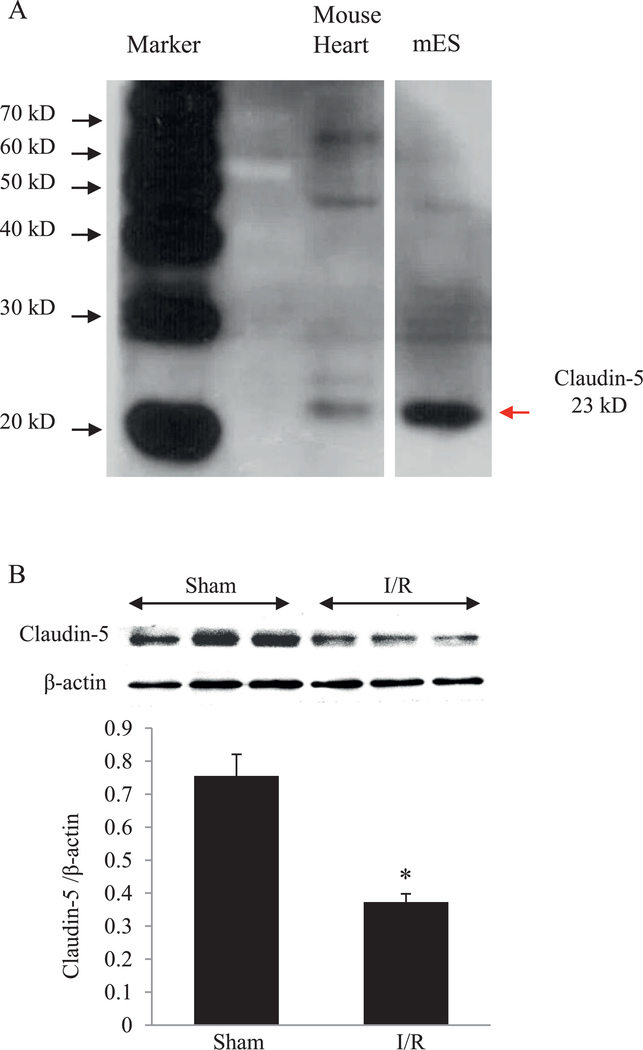
Cell lysates from mouse embryonic stem cells (mES) was used as a positive control for claudin-5 expression since claudin-5 is highly expressed during embryogenesis 7, 24. As shown in Fig. 2A and Figure S1 in Supplementary Materials, the expression of claudin-5 was detected in mouse heart tissue. After myocardial ischemia/reperfusion (I/R), the protein level of claudin-5 in the left ventricle was significantly decreased compared with the sham group (Fig. 2B).
Fig. 2. The protein level of claudin-5 in the heart tissue.
(A) Western blot of claudin-5 in mouse heart tissue. mES (mouse embryonic stem cells) was used as positive control. (B) Western blot and quantitative analysis of the protein level of claudin-5 in the mouse heart after myocardial ischemia/reperfusion (I/R). * P < 0.05 vs. Sham; n = 5.
Claudin-5 is downregulated in cardiomyocyte after H/R in vitro
We then isolated neonatal rat cardiomyocyte (NRCM) and detected the expression of claudin-5 in cellular level. Claudin-5 mRNA and protein were found in NRCM (Fig. 3A, Fig. 3B and Figure S1). We then produced hypoxia/reoxygenation (H/R) on NRCM, as shown in Fig. 3C, claudin-5 was decreased after H/R.
Fig. 3. The mRNA and protein level of claudin-5 in cardiomyocytes.
(A) mRNA level of claudin-5 in neonatal rat cardiomyocytes (NRCM). M indicates DNA marker. (B) Western blot of claudin-5 in NRCM. A549 and H293 cell lines are used as positive control. (C) Western blot and quantitative analysis of the protein level of claudin-5 in NRCM after hypoxia/reoxygeneration (H/R). * P < 0.05 vs. Normoxia (N); n = 5.
Downregulation of claudin-5 and mitochondrial fragmentation within cardiomyocyte after H/R in vitro
We further investigated the subcellular localization of claudin-5 in cardiomyocytes. Costaining NRCM with claudin-5 antibody and Mito-tracker, a mitochondrial label, revealed that much of claudin-5 was present intracellularly, and not just abundantly expressed at the lateral membrane as previously reported 9, and that it co-localized with mitochondria (Fig. 4A and Figure S2). We then isolated the mitochondrial protein, and confirmed the expression of claudin-5 in mitochondria by western blotting. COX IV was used as a mitochondrial marker 20, 23. Under stress triggered by H/R, known to cause mitochondrial dysfunction, there was a significant decrease in the mitochondrial claudin-5 level (Fig. 4B).
Fig. 4. Downregulation of claudin-5 and mitochondrial fragmentation in cardiomyocyte induced by hypoxia/reoxygenation (H/R).
(A) Subcellular localization of claudin-5 in neonatal rat cardiomyocytes (NRCM). A549 cell lines without anti-claudin-5 antibody incubation were used as negative control; A549 cell lines incubated with anti-claudin-5 antibody was used as positive control. Anti-claudin-5 antibody (green) was used to detect endogenous claudin-5 in cells; Mito-tracker was used to stain mitochondria (red) in cells. The images are merged to co-localize claudin-5 and mitochondria (orange). Scale bar, 50 μm. (B) Western blot and quantitative analysis of claudin-5 in mitochondria in NRCM. * P<0.05 vs. Normoxia (N); n=5. M indicates protein marker. (C) Downregulation of claudin-5 and mitochondrial fragmentation in NRCM after H/R. Anti-claudin-5 antibody (green) was used to detect endogenous claudin-5 in cells; Mito-tracker was used to stain mitochondria (red) in cells. The images are merged to co-localize claudin-5 and mitochondria (orange). Scale bar, 50 μm.
In order to further study the relationship between claudin-5 and mitochondria in cardiomyocytes, confocal microscopy of cardiomyocytes at normoxia and H/R was performed. Data revealed a marked downregulation of endogenous claudin-5 and fragmentation of mitochondria in NRCM after H/R that away closely associated with mitochondria degradation post H/R (Fig. 4C).
Claudin-5 upregulates Mfn2 and downregulates Drp-1
We next investigated the role of claudin-5 on mitochondrial dynamics by silencing the expression of endogenous claudin-5 by siRNA or overexpressing the protein by claudin-5-expressing recombinant adenovirus infection. Mitochondrial dynamics involve continuous mitochondrial fusion and fission, opposing processes that impact mitochondrial morphology, distribution and function, and regulated by mitofusins and dynamin-related protein-1 (Drp-1) 14. We measured the protein level of mitofusin 2 (Mfn2) and Drp-1 in NRCM under the condition of claudin-5 knockdown and overexpression. Transfecting NRCM with claudin-5 siRNA for 24 h drastically reduced the native claudin-5 expression in cardiomyocytes, as expected (Fig. 5A). Downregulating claudin-5 led to a significant reduction in the expression of Mfn2, one of the key molecular determinants of mitochondrial fusion (Fig. 5B). On the other hand, claudin-5 siRNA had an opposite effect on Drp-1, a promoter of mitochondrial fission. As seen in Fig. 5C, downregulating claudin-5 augmented the expressed protein level of Drp-1. By contrast, overexpressing claudin-5 in NRCM via adenovirus infection led to a significant increase in the Mfn2 expression and inhibition of Drp-1 expression, a reverse of what was observed with claudin-5 silencing (Fig. 5D, 5E, and 5F).
Fig. 5. The role of claudin-5 on the expression of Mfn2 and Drp-1 in cardiomyocytes.
(A). Effective knockdown of claudin-5 expression by siRNA. (B) The effect of claudin-5 knockdown on the protein level of Mfn2. (C) The effect of claudin-5 knockdown on the protein level of Drp-1. (D) Effective overexpression of claudin-5 by adenovirus (Adv). (E) The effect of claudin-5 overexpression on the protein level of Mfn2. (F) The effect of claudin-5 overexpression on the protein level of Drp-1. *P < 0.05 vs. Control (C), n=5. In control group, equal amount of PBS was added into the cell culture medium; Cont siRNA indicates nontargeted control siRNA. Cont Adv indicates LacZ adenovirus. Claudin-5 Adv indicates claudin-5 adenovirus.
Claudin-5 overexpression in cardiomyocytes prevents H/R-induced mitochondrial swelling and fission
Mitochondrial fusion is essential to maintaining normal mitochondrial functions 25, whereas mitochondrial fission participates in apoptosis via Drp-1 26. Given the effect of claudin-5 on Drp-1 expression, we investigated whether claudin-5 would modulate mitochondrial dynamics during apoptosis triggered by H/R. Following H/R stress, the expression of Mfn2 was dramatically decreased, while the Drp-1 expression more than doubled. Silencing claudin-5 further decreased the level of Mfn2 and, by contrast, increased the level of Drp-1. Furthermore, overexpression of claudin-5 by adenoviral infection expressing claudin-5 reversed these effects despite H/R (Fig. 6A). The changes in Mfn2 and Drp-1 by H/R and claudin-5 level accompanied alteration in the mitochondrial morphology in NRCM, observed by transmission electron microscopy. H/R induced a transformation of the mitochondrial phenotype consistent with the apoptotic process-swelling, rounding and increased fissions (Fig. 6B right upper panel, 6C and 6D). The morphological changes progressed further with claudin-5 silencing, which induced notable increase in fission (Fig. 6B left lower panel, 6C and 6D). On the other hand, claudin-5 overexpression prevented the H/R-induced swelling and fission, and nearly normalized the mitochondrial morphology (Fig. 6B right lower panel, 6C and 6D).
Fig. 6. The role of claudin-5 on mitochondrial dynamics in cardiomyocytes.
(A) The western blot and quantitative analysis of the protein level of Mfn2 and Drp-1 in neonatal rat cardiomyocytes (NRCM). *P < 0.05 vs. normoxia (N); †P < 0.05 vs. hypoxia/reoxygenation (H/R); ‡ P < 0.05 vs. H/R+Claudin-5 siRNA; n=5 in each group. (B) Representative pictures of mitochondrial morphology observed under transmission electron microscope. Red arrows indicate mitochondrial fission. Amplification, 11000 folds; Scale bar, 200 nm. (C) Quantitative analysis of the mean area of mitochondria. *P < 0.05 vs. N; †P < 0.05 vs. H/R, n=10; (D) Quantitative analysis of the mitochondrial fission. *P < 0.05 vs. N; †P < 0.05 vs. H/R.
Claudin-5 inhibits H/R-induced apoptosis
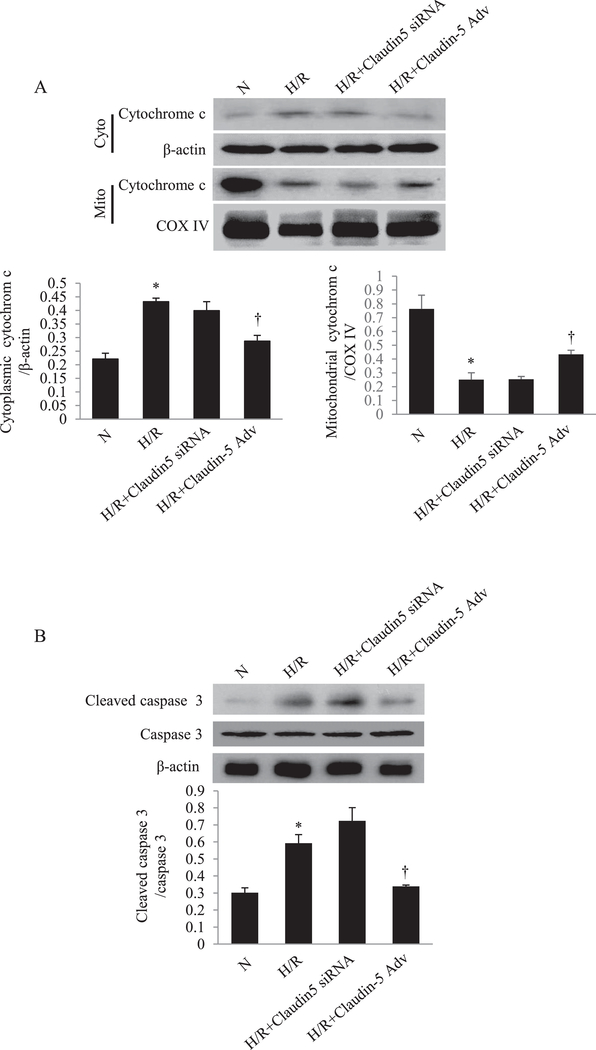
Given the effects of claudin-5 on the mitochondrial dynamics, namely promoting Mfn2, downregulating Drp-1 and fission triggered by H/R, we hypothesized that these actions of claudin-5 would confer overall protection of cardiomyocytes from undergoing H/R-triggered apopotosis. We measured the apoptotic markers, cytochrome c release and caspase 3 cleavage, in NRCM after H/R. As shown in Fig. 7A and B, the release of cytochrome c from mitochondria into cytoplasm was significantly increased after H/R, as was the cleaved caspase 3. Claudin-5 overexpression by adenovirus pretreatment markedly reduced the apoptotic process, as indicated by decreased cytochrome c release and caspase 3 cleavage.
Fig. 7. The role of claudin-5 on cardiomyocyte apoptosis induced by hypoxia/reoxygenation (H/R) in vitro.
(A) The western blot and quantitative analysis of the cytochrome c expression in cytoplasm and in mitochondria in neonatal rat cardiomyocytes (NRCM) (top, lower left and lower right). *P < 0.05 vs. normoxia (N); † P < 0.05 vs. H/R; n = 5 in each group. (B) The western blot and quantitative analysis of the caspase 3 cleavage in NRCM. *P<0.05 vs. N; † P < 0.05 vs. H/R; n = 5 in each group.
Overexpression of claudin-5 limits I/R-induced infarct size and improves heart function
Based on the anti-apoptotic role of claudin-5 in cardiomyocytes in H/R condition, we next tested the therapeutic role of claudin-5 in vivo in mouse heart with and/or without I/R injury. As shown in Fig. 8A, the injection of the purified claudin-5 adenovirus for 24 h significantly increased the protein level of claudin-5 in the left ventricle. The expression of cleaved caspase-3 was increased after I/R while attenuated by claudin-5 overexpression (Fig. 8B). TTC staining confirmed this anti-apoptotic effect of claudin-5 overexpression. The infarct size was significantly decreased in claudin-5 overexpressed heart (Fig. 8C). We also observed the improved FS after I/R injury in the claudin-5 overexpressed heart compared with the control group (Fig. 8D).
Fig. 8. The role of claudin-5 on infarct size and heart function after myocardial ischemia/reperfusion (I/R) in vivo.
(A) The western blot and quantitative analysis of claudin-5 after adenovirus (Adv) infection in left ventricle. *P < 0.05 vs. Control. (B) The western blot and quantitative analysis of claudin-5, cleaved caspase 3 and caspase 3 in the heart tissue in wild-type and claudin-5 adenovirus (Claudin-5 Adv) mice with sham operation and I/R. *P < 0.05 vs. Sham; † P < 0.05 vs. I/R in wild-type group; n = 5 in each group. (C) Representative 2,3,5-Triphenyl-2H-tetrazolium chloride (TTC) staining of the heart sections in wild-type and claudin-5 Adv mice with sham operation and I/R. *P < 0.05 vs. Sham; n = 5 in each group. (D) Representative echocardiographic imaging and quantitative analysis of fractional shortening (FS) in wild-type and claudin-5 Adv mice with sham operation and I/R. *P < 0.05 vs. Sham; † P < 0.05 vs. Wild-type I/R; n = 5 in each group.
Discussion
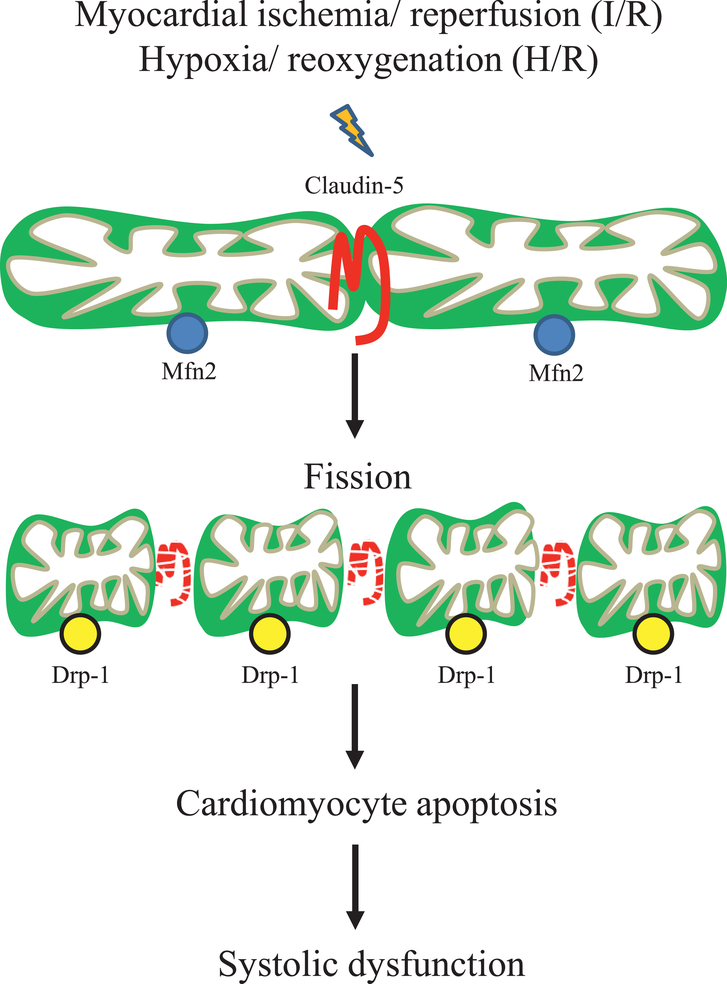
The role of claudin-5 located at the plasma membrane of endothelial cells in the regulation of paracellular permeability has been well studied for many decades, whereas the functional role of claudin-5 on intracellular organelle mitochondria has never been explored. Here we show for the first time that claudin-5 is expressed on the mitochondria of cardiomyocytes and participates in the regulation of mitochondrial dynamics. Our data demonstrate that claudin-5 [1] upregulates Mfn2, an important determinant of mitochondrial fusion, [2] downregulates Drp-1, a key molecule in promoting mitochondrial fission and apoptosis, [3] attenuates H/R-associated mitochondrial swelling and fission, [4] inhibits cardiomyocyte apoptosis following H/R in vitro, and [5] protects the heart from I/R injury in vivo. These findings are summarized in Fig. 9. Although the mechanism underlying claudin-5 regulates the expression of Mnf2 and Drp-1 is not investigated in this study, we found the intracellular function of claudin-5 on mitochondria besides its well-recognized role on plasma membrane.
Fig. 9. Diagram of claudin-5-mediated signaling.
Myocardial ischemia/ reperfusion (I/R) or hypoxia/ reoxygenation (H/R) decreases the expression of mitochondrial claudin-5, which in turn down-regulates the expression of Mfn2 (responsible for mitochondrial outer membrane fusion) and up-regulates the expression of Drp-1 (responsible for mitochondrial fission). The increased mitochondrial fission leads mitochondrial swelling and mitochondrial dependent apoptosis in cardiomyocyte, which finally impairs the systolic function of the heart.
Claudin-5 is a member of the claudin superfamily proteins making up integral components of tight junctions in paracellular transport and paracellular permeability 27. Downregulation of claudin-5 was found in cardiomyocytes and myocardial endothelium of patients with heart failure, although its role in the heart have not been fully defined 8, 10. In the murine heart, the tight junction protein is localized to the lateral membranes of cardiomyocytes 9. Our results suggest that mitochondrial membranes may be an additional intracellular target for intracellular claudin-5. However, there are data showing that the claudin-5 localization at cardiomyocyte lateral membranes and on the subsarcolemmal mitochondria under the surface crest of the cardiomyocyte 10, 28. These differences may be attributable to the species difference, difference of antibody for claudin-5 and experimental condition. The other possibility is that the integrity of mitochondrial and sarcolemmal membrane may cause such a difference of localization 29. In such a case, we believe that claudin-5 is located in the mitochondrial membrane, which would be target for the myocardial cellular injury as well as that in cardiomyocyte lateral membranes. Integrity of the mitochondrial structure was closely associated with claudin-5 expression. The translocation of claudin-5 to mitochondria may be facilitated by protein kinase activity important for the regulation and localization of claudin proteins, as shown in other cell types 30–32. Whether this post-translational modification holds for claudin-5 translocation into mitochondria in cardiomyocytes is not clear, and further investigation is needed.
Work presented here demonstrated that H/R-induced claudin-5 degradation in cardiomyocytes was accompanied by mitochondrial fragmentation, and overexpression of claudin-5 by adenovirus significantly prevented mitochondrial fission and cardiomyocyte apoptosis, suggesting a new role of claudin-5 in maintaining mitochondrial dynamics of fusion and fission. Mfn1 and Mfn2 mediate fusion of the outer mitochondrial membranes, while the fusion of inner membranes is mediated by optic atrophy (Opa) 1. Mitochondrial fusion in adult cardiac myocytes is necessary to maintain normal mitochondrial morphology, organelle function and cardiac homeostasis 33. Combined Mfn1/Mfn2 ablation was lethal after e9.5 in embryogenensis. Conditional Mfn1/Mfn2 ablation in adult hearts induced mitochondrial fragmentation, cardiomyocyte and mitochondrial respiratory dysfunction, and rapidly progressive and lethal dilated cardiomyopathy 33. Increasing the level of Mfn2 in cardiomyocyte attenuated the DOX-induced increase in mitochondrial fission and prevented cardiomyocyte apoptosis 34. Our data support that claudin-5 promotes upregulation of Mfn2 and thereby Mfn-mediated mitochondrial fusion processes and cytoprotection under H/R stress.
Mitochondrial fission, on the other hand, is closely related to apoptosis 26,35. Fission creates smaller mitochondria more capable of generating reactive oxygen species 36. Inhibiting the fission process was cardioprotective in the context of myocardial I/R 37–39. Mitochondrial fission is mainly controlled by Drp-1. Inhibition of mitochondrial fission via Mdivi-1 (25 μM), a Drp-1 GTPase inhibitor, reduced I/R-induced mitochondrial fission and diastolic dysfunction of the right ventricle in rats 40. Similarly, inhibition of Drp-1 reduced Drp-1 translocation to the mitochondria and mitochondrial fission, and protected against myocardial ischemia-reperfusion injury in diabetic mice 41. Our results show that at normoxic baseline, Mfn2 is present abundantly while Drp-1 expression is at a low level. However, when cardiomyocytes are under hypoxic stress triggering mitochondria-centered apoposis, the protein level of Drp-1 is upregulated and that of Mfn2 reduced, reversing the Mfn2:Drp-1 ratio, which can be observed in heart failure 42. The ratio can represent the perturbed balance between mitochondrial fusion and fission during H/R. Overexpressing claudin-5 attenuated this effect, blunting the H/R-induced downregulation of Mfn2, elevating the Mfn2:Drp-1 ratio, and greatly reducing mitochondrial fission rate. In addition, mitochondria-driven apoptosis during H/R was also reduced when claudin-5 was overexpressed. The mechanism by which mitochondrial claudin-5 regulates the the Mfn2:Drp-1 ratio remains unclear at this time. As we did not see evidence of claudin-5 in the cardiomyocyte nucleus, we suspect that the mechanism behind the altered expression of Mfn2 and Drp-1 by claudin-5 may be via a non-genomic pathway. Since the individual post-translational modifications that are known to affect mitochondrial dynamics include SUMOylation, ubiquitination, phosphorylation, S-nitrosylation, acetylation, O-linked N-acetylglucosamine glycosylation, ADP-ribosylation, and proteolytic cleavage 43, the post-translational modifications of claudin-5 on mitochondrial dynamics is hard to define at this paper and further investigation is needed.
We also found overexpression of claudin-5 in mouse left ventricle by claudin-5 adenovirus significantly upregulated the protein level of claudin-5 and decreased the cleaved caspase 3 after I/R. This anti-apoptotic effect was consistent with the echocardiographic observation and TTC staining, in which, the systolic function of the heart was improved and the infarct size of left ventricle was limited. Our result shows the anti-apoptotic role of claudin-5 on myocardium against myocardial I/R injury and hints the therapeutic target for ischemic heart disease.
Conclusions
We present a novel finding that claudin-5 is present in mitochondria of cardiomyocytes and that it modulates the key molecular components of mitochondrial dynamics and cell fate during H/R and/or I/R stress. These findings may be considered for development of promising cardioprotective therapeutics based on claudin-5 to maintain healthy mitochondrial function and reduce hypoxia-induced cardiac apoptosis.
Supplementary Material
Acknowledgments
We thank Farah Akhtar for excellent technical assistance for transmission electron microscope. We thank Adrian Manuel Almodovar Irizarry for providing helpful contributions in the writing of this manuscript.
Funding Sources
This work was supported by a grant from the National Natural Science Foundation of China (grant no.82060045, to Dr. Luo), two Funded Projects from Zunyi Medical University (grant no. [2018]5772-025, to Dr. Luo and [2018]5772-026 for Dr. Chou), a grant from Guizhou province of China (grant no. [2021]224, to Dr. Luo), two grants from the National Heart, Lung, Blood Institute at the National Institutes of Health (R01 HL111180 to Dr. Kim and R01 HL124266 to Dr. Abdel-Latif) and a grant from the University of Kentucky COBRE Early Career Program (P20 GM103527 to Dr. Abdel-Latif).
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. American journal of physiology. Renal physiology. 2008;295:F867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taddei A, Giampietro C, Conti A, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nature cell biology. 2008;10:923–934. [DOI] [PubMed] [Google Scholar]
- 3.Huang P, Zhou CM, Qin H, et al. Cerebralcare Granule(R) attenuates blood-brain barrier disruption after middle cerebral artery occlusion in rats. Experimental neurology. 2012;237:453–463. [DOI] [PubMed] [Google Scholar]
- 4.Kashiwamura Y, Sano Y, Abe M, et al. Hydrocortisone enhances the function of the blood-nerve barrier through the up-regulation of claudin-5. Neurochemical research. 2011;36:849–855. [DOI] [PubMed] [Google Scholar]
- 5.Zhu H, Wang Z, Xing Y, et al. Baicalin reduces the permeability of the blood-brain barrier during hypoxia in vitro by increasing the expression of tight junction proteins in brain microvascular endothelial cells. Journal of ethnopharmacology. 2012;141:714–720. [DOI] [PubMed] [Google Scholar]
- 6.Burek M, Arias-Loza PA, Roewer N, Forster CY. Claudin-5 as a novel estrogen target in vascular endothelium. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:298–304. [DOI] [PubMed] [Google Scholar]
- 7.Yamagishi M, Ito Y, Ariizumi T, et al. Claudin5 genes encoding tight junction proteins are required for Xenopus heart formation. Development, growth & differentiation. 2010;52:665–675. [DOI] [PubMed] [Google Scholar]
- 8.Mays TA, Binkley PF, Lesinski A, et al. Claudin-5 levels are reduced in human end-stage cardiomyopathy. Journal of molecular and cellular cardiology. 2008;45:81–87. [DOI] [PubMed] [Google Scholar]
- 9.Sanford JL, Edwards JD, Mays TA, Gong B, Merriam AP, Rafael-Fortney JA. Claudin-5 localizes to the lateral membranes of cardiomyocytes and is altered in utrophin/dystrophin-deficient cardiomyopathic mice. Journal of molecular and cellular cardiology. 2005;38:323–332. [DOI] [PubMed] [Google Scholar]
- 10.Swager SA, Delfin DA, Rastogi N, et al. Claudin-5 levels are reduced from multiple cell types in human failing hearts and are associated with mislocalization of ephrin-B1. Cardiovascular pathology: the official journal of the Society for Cardiovascular Pathology. 2015;24:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delfin DA, Xu Y, Schill KE, et al. Sustaining cardiac claudin-5 levels prevents functional hallmarks of cardiomyopathy in a muscular dystrophy mouse model. Molecular therapy: the journal of the American Society of Gene Therapy. 2012;20:1378–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calo L, Dong Y, Kumar R, Przyklenk K, Sanderson TH. Mitochondrial dynamics: an emerging paradigm in ischemia-reperfusion injury. Current pharmaceutical design. 2013;19:6848–6857. [DOI] [PubMed] [Google Scholar]
- 13.Marin-Garcia J, Akhmedov AT, Moe GW. Mitochondria in heart failure: the emerging role of mitochondrial dynamics. Heart failure reviews. 2013,18:439–456. [DOI] [PubMed] [Google Scholar]
- 14.Nan J, Zhu W, Rahman MS, et al. Molecular regulation of mitochondrial dynamics in cardiac disease. Biochimica et biophysica acta. 2017;1864:1260–1273. [DOI] [PubMed] [Google Scholar]
- 15.Dorn G II. Mitochondrial fission/fusion and cardiomyopathy. Current opinion in genetics & development. 2016;38:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorn GW 2nd. Mitochondrial dynamism and heart disease: changing shape and shaping change. EMBO molecular medicine. 2015; 7:865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorn GW 2nd. Mitochondrial dynamics in heart disease. Biochimica et biophysica acta. 2013;1833:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amasheh S, Schmidt T, Mahn M, et al. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell and tissue research. 2005;321:89–96. [DOI] [PubMed] [Google Scholar]
- 19.Luo T, Chen B, Zhao Z, et al. Histamine H2 receptor activation exacerbates myocardial ischemia/reperfusion injury by disturbing mitochondrial and endothelial function. Basic research in cardiology. 2013;108:342. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Yanamandala M, Lee TC, Kim JK. Mitochondrial p38beta and manganese superoxide dismutase interaction mediated by estrogen in cardiomyocytes. PloS one. 2014;9:e85272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Pedram A, Kim JK. Oestrogen prevents cardiomyocyte apoptosis by suppressing p38alphamediated activation of p53 and by down-regulating p53 inhibition on p38beta. Cardiovascular research. 2011;89:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo T, Kim JK, Chen B, Abdel-Latif A, Kitakaze M, Yan L. Attenuation of ER stress prevents postinfarction-induced cardiac rupture and remodeling by modulating both cardiac apoptosis and fibrosis. Chemico-biologicalinteractions. 2015;225:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo T, Liu H, Kim JK. Estrogen protects the female heart from ischemia/reperfusion injury through manganese superoxide dismutase phosphorylation by mitochondrial p38beta at threonine 79 and serine 106. PloS one. 2016;11:e0167761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins MM, Baumholtz Al, Ryan AK. Claudin-5 expression in the vasculature of the developing chick embryo. Gene expression patterns: GEP. 2012;12:123–129. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. The Journal of biological chemistry. 2005;280:26185–26192. [DOI] [PubMed] [Google Scholar]
- 26.Frank S, Gaume B, Bergmann-Leitner ES, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Developmental cell. 2001;1:515–525. [DOI] [PubMed] [Google Scholar]
- 27.Heiskala M, Peterson PA, Yang Y. The roles of claudin superfamily proteins in paracellular transport. Traffic. 2001;2:93–98. [DOI] [PubMed] [Google Scholar]
- 28.Guilbeau-Frugier C, Cauquil M, Karsenty C, et al. Structural evidence for a new elaborate 3D-organization of the cardiomyocyte lateral membrane in adult mammalian cardiac tissues. Cardiovascular research. 2019;115:1078–1091. [DOI] [PubMed] [Google Scholar]
- 29.Perez AC, Cabral de Oliveira AC, Estevez E, Molina AJ, Prieto JG, Alvarez Al. Mitochondrial, sarcoplasmic membrane integrity and protein degradation in heart and skeletal muscle in exercised rats. Comparative biochemistry and physiology. Toxicology & pharmacology: CBP. 2003;134:199–206. [DOI] [PubMed] [Google Scholar]
- 30.D'Souza T, Agarwal R, Morin PJ. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. The Journal of biological chemistry. 2005;280:26233–26240. [DOI] [PubMed] [Google Scholar]
- 31.French AD, Fiori JL, Camilli TC, et al. PKC and PKA phosphorylation affect the subcellular localization of claudin-1 in melanoma cells. International journal of medical sciences. 2009;6:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lippoldt A, Liebner S, Andbjer B, et al. Organization of choroid plexus epithelial and endothelial cell tight junctions and regulation of claudin-1, −2 and −5 expression by protein kinase C. Neuroreport. 2000;11:1427–1431. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Liu Y, Dorn GW 2nd. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circulation research. 2011;109:1327–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang H, Tao A, Song J, Liu Q, Wang H, Rui T. Doxorubicin-induced cardiomyocyte apoptosis: Role of mitofusin 2. The international journal of biochemistry & cell biology. 2017;88:55–59. [DOI] [PubMed] [Google Scholar]
- 35.Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nature reviews. Molecular cell biology. 2005;6:657–663. [DOI] [PubMed] [Google Scholar]
- 36.Archer SL. Mitochondrial dynamics-mitochondrial fission and fusion in human diseases. The New England journal of medicine. 2013;369:2236–2251. [DOI] [PubMed] [Google Scholar]
- 37.Disatnik MH, Ferreira JC, Campos JC, et al. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. Journal of the American Heart Association. 2013;2:e000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. [DOI] [PubMed] [Google Scholar]
- 39.Sharp WW, Fang fH, Han M, et al. Dynamin-related protein 1 (Drpl)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drpl inhibition to reduce mitochondrial fission. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian L, Neuber-Hess M, Mewburn J, et al. Ischemia-induced Drpl and Fisl-mediated mitochondrial fission and right ventricular dysfunction in pulmonary hypertension. Journal of molecular medicine. 2017;95:381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding M, Dong Q, Liu Z, et al. Inhibition of dynamin-related protein 1 protects against myocardial ischemia-reperfusion injury in diabetic mice. Cardiovascular diabetology. 2017;16:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Givvimani S, Pushpakumar S, Veeranki S, Tyagi SC. Dysregulation of Mfn2 and Drp-1 proteins in heart failure. Canadian journal of physiology and pharmacology. 2014;92:583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klimova N, Long A, Kristian T. Significance of mitochondrial protein post-translational modifications in pathophysiology of brain injury. Translational stroke research. 2018;9:223–237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.