Abstract
Chronic stress is a major risk factor in the pathophysiology of many neuropsychiatric disorders. Further, chronic stress conditions can promote neuroinflammation and inflammatory responses in both humans and animal models. Type I interferons (IFN-I) are critical mediators of the inflammatory response in the periphery and responsible for the altered mood and behavior. However, the underlying mechanisms are not well understood. In the present study, we investigated the role of IFN-I signaling in chronic stress-induced changes in neuroinflammation and behavior. Using the chronic restraint stress model, we found that chronic stress induces a significant increase in serum IFNβ levels in mice, and systemic blockade of IFN-I signaling attenuated chronic stress-induced infiltration of macrophages into prefrontal cortex and behavioral abnormalities. Furthermore, complement component 3 (C3) mediates systemic IFNβ-induced changes in neuroinflammation and behavior. Also, we found significant increases in the mRNA expression levels of IFN-I stimulated genes in the prefrontal cortex of depressed suicide subjects and significant correlation with C3 and inflammatory markers. Together, these findings from animal and human postmortem brain studies identify a crucial role of C3 in IFN-I-mediated changes in neuroinflammation and behavior under chronic stress conditions.
Introduction
Chronic stress is a major risk factor for developing diseases in which persistent mood and cognitive deficits are observed (1, 2). Understanding how stressful experiences can induce behavioral abnormalities such as depressive symptoms, social deficits, and cognitive decline is important for improved treatments for such disorders. Once believed to be immune-privileged, the brain is now shown to interact with the immune system via multiple mechanisms that facilitate the action of inflammatory signals from the periphery. The signaling of cytokines released by immune cells plays a critical role in such altreations in mood and behavior induced by neuroinflammation (3–5). Further, chronic stress conditions can also promote the inflammation and increased inflammatory responses in both humans and animal models (6); however, the mechanisms that link chronic stress to inflammation and behavioral abnormalities are not well understood.
Accumulating evidence demonstrate a causal relationship between immune activation and behavioral changes. Peripheral administration of antigens has been shown to induce changes in behavior and cognition in humans (7–10). In rodents, cytokines, or exposure to chronic stress conditions have resulted in increased neuroinflammation and changes in behavior. In particular, chronic unpredictable stress (11, 12), chronic adolescent stress (13), and repeated social defeat (14), have been shown to promote neuroinflammation and behavior changes such as behavioral despair, anhedonia, anxiety-like behavior, reduced social behavior and cognitive deficits in animals. Also, inhibition of neuroimmune activation prevents stress-induced behavioral changes (15–17), further supporting the interaction between immune pathways and behavioral changes.
Type I interferons (IFN-I) are critical elements in the cytokine family that play essential roles in activating both the innate and adaptive immune systems (18). The most studied members of the IFN-I family are IFNα and IFNβ; both are known to be critical mediators of the peripheral inflammatory response and also regulate the function of the immune system by inducing the recruitment of immune cell to sites of infection and promote their effector functions (19, 20). Further, they play important roles in CNS plasticity (21, 22) and emotion (23, 24). IFNα treatment is frequently associated with the development of symptoms of depression, fatigue, anxiety, and cognitive disturbances in multiple sclerosis (MS) (25) and hepatitis C patients (26, 27). Similarly, IFNβ treatment has been shown to trigger sickness behavior and depressive symptoms in MS patients (28, 29). Also, IFNβ administration in healthy volunteers resulted in reduced appetitive motivation in psychopathology ratings (30). Further, increased IFN-I signaling has been reported in the blood of depressed subjects (31). In rodents, peripheral IFN-I administration has been shown to induce depressive-like behavior, and inhibits neurogenesis (32) but increases the expression of IFN-I -stimulated genes (ISGs) in the hippocampus (33).
Chronic stress has been shown to enhance microglia activation in the hippocampus and prefrontal cortex (PFC); regions implicated in mood, social and cognitive functions (15, 17, 34–37). Further, microglia become reactive in response to increased IFN-I activity (38) and promotes engulfment of synaptic material resulting in synapse loss (39). It is known that the complement pathway plays a key role in the phagocytic activity of hyperactivated microglia leading to synapse elimination (40, 41). The complement system is a part of the innate immune mechanism and plays a key role in synaptic function and inflammatory processes (41). Further, chronic stress has been shown to increase complement component 3 (C3) levels (12) and synapse loss in mouse PFC (42, 43). However, it is not known whether peripheral IFN-I activates microglia and complement system to mediate chronic stress-induced effects on neuroinflammation and behavior.
In the present study, we tested the hypothesis that IFN-I signaling mediates chronic stress-induced neuroinflammation and behavior changes. Chronic restraint stress (RS) is a well-validated stress model known to induce many behavior changes, including social behavior and cognitive deficits in rodents (44–58). We found that RS induced significant increases in serum IFNβ levels in mice and systemic blockade of IFN-I signaling attenuated RS-induced infiltration of macrophages into PFC and behavioral abnormalities. Furthermore, complement component 3 (C3) mediates IFNβ-induced changes in neuroinflammation and behavior. Also, we found significant increases in the mRNA levels of ISGs in the PFC of depressed suicide subjects, and their levels correlated with C3 and inflammatory markers. Together, these findings from animal and human postmortem brain studies identify a crucial role of C3 in IFN-I-mediated changes in neuroinflammation and behavior under chronic stress conditions.
Materials and Methods
Animals
Adult (8 weeks old) male C57BL/6J mice, C3−/− (129S4-C3tm1Crr/J) and their age-matched wild type (WT) controls were purchased from The Jackson Laboratory, and ICR mice were purchased from Envigo RMS, Inc. Animals were housed in the animal facility at Augusta University. Mice were housed in groups of 5 mice in standard polypropylene cages in a 12-h light-dark cycle in compliance with the US National Institute of Health guidelines and approved by Augusta University animal welfare guidelines. Mice were assigned to experimental groups based on their genotype. The selection of animals for treatment was performed randomly and in a blinded manner.
Interferon alpha/beta receptor 1 (IFNAR-1) antibody and IFNβ treatment
Mice were intraperitoneally (i.p., 250μg per mouse) injected with anti-mouse IFNAR-1 antibody (IFNAR) or IgG control antibody (Leinco Technologies, MO, USA) every fourth day during the 21-day chronic stress paradigm. For recombinant IFNβ treatment, WT and C3−/− mice were injected (i.p.) with IFNβ (25000IU; PBL Assay Science, NJ, USA) or vehicle (PBS supplemented with 0.1% FBS) one time for 8h followed by behavior tests.
Clodrosome treatment
A Clodrosome Macrophage Depletion Kit, containing control liposomes (Encapsome) and clodronate liposomes (Clodrosome) (Encapsula NanoSciences, Brentwood, TN), was used to deplete endogenous monocytes/macrophages. Encapsome or clodronate liposomes were administered (i.p.; 200 μl; 5 mg/ml) three times at seven days apart during the RS paradigm for 21 days. Following RS, myeloid cell (CD11b+ F4/80+) depletion was confirmed by flow cytometry.
Restraint stress (RS) procedure
Mice were restrained in well-ventilated 50ml Falcon tubes for 2 h/day for 21 consecutive days. Control mice were housed in their usual cages under normal conditions. Mice were tested the day following the last restraint session.
Behavioral Tests
Behavior tests were performed in the room with ambient lighting [approx. 25–30 lux (lumen/m2 )] and with constant background sound unless noted. The temperature and pressure of the behavioral rooms are monitored and kept constant. Animals were transferred to the behavioral rooms in their home cages at least 1 hour before testing and allowed to habituate to the behavioral rooms.
All behavioral experiments were scored blind to the treatment.
Three-Chamber Test
The three-chamber test measures sociability and social deficits. The dimension of each chamber is 19 cm × 45 cm × 22 cm. The dividing walls of the chambers are made from clear Plexiglas® and have openings on each wall for free access to the other two chambers. Two identical wire containers that can house a single mouse were placed vertically in the apparatus’s side chambers. The test mouse was placed in a box with three chambers and allowed to move freely for 5 minutes to get habituated to the apparatus. After the habituation period of 5 min, the stranger mouse was introduced and placed in one of the wire containers (diameter: 9 cm) while the test mouse was still allowed to move outside of the container for another 5 min freely. The holes in wire containers allow air exchange but are small enough to prevent direct physical contact between stranger mouse and test mouse. Time spent by test mouse in chambers (stranger mouse chamber, empty chamber, and center chamber) was recorded. The stranger mouse chamber is a chamber with a stranger mouse inside the wire container. An empty cage chamber is a chamber containing an empty wire container. Stranger mouse was a mouse of similar age, same-sex, and similar weight as the test mouse.
Reciprocal Social Interaction Test
This test was performed to measure the social approach behavior of the mouse. The test mouse was placed in a neutral box and allowed to habituate for 5 min. The box was made from clear Plexiglas® with the dimensions 57 cm × 45 cm × 22 cm. After habituation, a stranger mouse was introduced and placed in the box. The test mouse was allowed to move and interact with the stranger mouse freely. The interaction between the mouse is defined as close physical contact, nose-to-nose sniffing, anogenital sniffing, and grooming. Time spent interacting (initiated by the test mouse) was video recorded. The stranger was of similar age, same-sex, and similar weight as the test mouse.
Y-maze test
Y-maze (Maze Engineers) was used to perform a continuous spontaneous alternation test. There were three arms in Y-maze at 120° and was made of beige plastic. Each arm was 32 cm long, 6 cm wide, and the wall was 20 cm high. Visual distal cues were placed around the Y-maze. The test mouse was placed in the center of the Y-maze and allowed to explore for 7 min. Mouse movement was monitored, recorded, and analyzed by web camera (C920, Logitech, Newark, CA) and the Any-Maze software (Stoelting, Wood Dale, IL). A mouse was considered to be entered in a particular arm if the whole body entered the arm, except for the tail, and similarly, to have exited if the whole body exited the arm, except for the tail. When a mouse consecutively entered three different arms, it was counted as an alternating triad. The maximum number of triads is the total number of arm entries minus 2; the score of alternation was calculated as “the number of alternating triads/(the total number of arm entries − 2).”
Flow cytometry
Mice were anesthetized with isoflurane and were sacrificed by cervical dislocation. Brain tissues (PFC and hippocampus) were collected according to a mouse brain atlas (12). Tissues were homogenized in cold PBS and sieved through a 100-μM cell strainer (BD Biosciences, San Diego, CA), followed by centrifugation (1500 rpm, 10 min) to prepare single-cell suspensions. Cells were incubated with Abs against cell surface markers, CD11b (#101228; BioLegend, San Diego, CA), CD45 (#103138; BioLegend) and Iba-1 (#019–19741, Wako Chemicals USA, Inc. Richmond, VA). Following a PBS wash, cells were fixed and permeabilized using a Fixation/Permeabilization Concentrate (Affymetrix eBioscience). All samples were run through a 4- Laser LSR II flow cytometer and analyzed using FlowJo software (BD Biosciences, San Jose, CA). Isotype matched controls were analyzed to set the appropriate gates for each sample. Samples were analyzed in duplicate for each marker. The number of double-positive events detected with the isotype controls was subtracted from the number of double-positive cells stained with the corresponding Abs (not isotype control) to reduce false-positive events. Cells that expressed a specific marker were reported as a percentage of the number of gated events. Mean channel fluorescence intensity (MFI) derived from a fluorescence graph was used to study the level of cell surface expression.
Quantitative reverse transcriptase PCR (qRT-PCR)
For qRT-PCR analyses, the mouse PFC and hippocampus tissues were collected immediately following decapitation under anesthesia. RNA from the mouse as well as human PFC samples were purified using a commercially available kit (SV RNA Isolation, Promega, Madison, WI, USA). qRT-PCR was performed on a MasterCycler (Eppendorf, Westburg, NY, USA) using a iTaq™ Universal SYBR® Green Supermix (Bio-Rad, CA, USA). Gene-specific primers were synthesized by Integrated DNA Technologies. Ct values of genes of interest were normalized to that of housekeeping genes (beta2-microglobulin (B2m) or 18S Ribosomal RNA (18S)). A list of primers used is given in Table S1.
IFN protein levels by enzyme-linked immunosorbent assay (ELISA)
Mouse serum samples were collected immediately following decapitation under anesthesia. IFNα (#MLA00) and IFNβ (#MLB00C) protein levels in mouse serum samples were measured by ELISA according to the manufacturer’s instructions (R&D Systems). The values are expressed as pg/ml.
Cytokine array
Simultaneous quantification of cytokines in mouse sera was performed using LEGENDplex Mouse Inflammation Panel (13-plex) with V-bottom Plate (BioLegend Cat# 740446) according to manufacturer’s instructions. In brief, samples were thawed completely, mixed, and centrifuged to remove particulates prior to use. To achieve measurement accuracy, serum samples were diluted 2-fold with Assay Buffer, and standards were mixed with Matrix solution (Biolegend) to account for additional components in the serum samples. Standards and samples were plated with capture beads for tumor necrosis factor-alpha (TNFα), IFN-γ, interleukin-1alpha (IL-1α), IL-1β, IL-6, IL-10, IL-17A, IL-12p70, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-23, IFNβ, monocyte chemoattractant protein-1 (MCP-1), IL-27 and incubated for two h at room temperature on a plate shaker (800 rpm). After washing the plate with Wash Buffer, Detection Antibodies were added to each well. The plate was incubated on a shaker for 1h at room temperature. Finally, without washing, streptavidin R-phycoerythrin (SA-PE) was added and incubated for 30 min. Samples were acquired on CytoFLEX flow cytometer (Beckman Coulter Life Sciences). Standard curves and protein concentration were calculated using R package DrLumi (59) installed on R 3.5.2 (https://www.r-project.org/). The limit of detection was calculated as the average of background samples plus 2.5 × SD. Assay and data calculations were performed at the Immune Monitoring Shared Resource (Augusta University).
Human postmortem samples
The postmortem PFC tissues for the analysis were obtained from 30 Caucasian men, including 15 suicide completers with a lifetime diagnosis of major depressive disorder and 15 unaffected controls (Table 1). The samples were obtained from the Quebec Suicide Brain Bank (QSBB; Douglas Institute; www.douglas.qc.ca/suicide). The study was granted by the Douglas Hospital Institutional Review Board and, written informed consent was obtained from each participating family (Declaration of Helsinki, 1964). The demographic characteristics of subjects are provided in Table S2.
Table 1.
Comparison of depressed/suicide and controls
| Measure | Depressed-Suicide | Control | F (1, 28) | p |
|---|---|---|---|---|
| M (SD) | ||||
| Age | 42.60 (15.54) | 42.80 (14.97) | 0.001 | 0.972 |
| PMI | 21.80 (17.55) | 20.43 (13.35) | 0.060 | 0.816 |
| pH | 6.59 (0.46) | 6.63 (0.21) | 0.100 | 0.754 |
| RNA Integrity | 5.93 (2.56) | 5.65 (1.98) | 0.118 | 0.734 |
Note. PMI = Postmortem Interval.
Statistical Analysis
No statistical methods were used to predetermine sample sizes in mouse studies, but our sample sizes are similar to those reported in previous publications (12, 60). For mouse studies, data were analyzed using two-tailed Student’s t-tests (for two-group comparisons) or Analysis of Variance (ANOVA; for multiple-group comparisons). p < 0.05 was considered significant. Bonferroni’s posthoc test was performed within the comparison. For the clinical studies, Multivariate Analysis of Variance (MANOVA) was used to examine the differences between suicide-depressed and the control subjects to compare the expression of IFN-induced protein-44 (IFI44), MX dynamin-like GTPase 1 (Mx1), C3, Inducible nitric oxide synthase (iNOS), C-X3-C Motif Chemokine Receptor 1 (Cx3CR1), and TNFα genes. A statistically significant multivariate effect was followed-up with examining the univariate effects to determine which of the genes mentioned above showed significant differences. The following covariates were included and evaluated in the MANOVA model to control their effects on gene expression: age, post mortem interval (PMI), pH, RNA integrity, lifetime substance use, substance use at death, and psychiatric medication use in the last three months. Partial eta-square (η2p) was used to index effect size differences between depressed-suicide and control subjects on IFI44, Mx1, C3, iNOS, Cx3CRI, and TNFα genes. Gene to gene interaction was observed using simple zero-order correlations. Holms-Bonferroni correction was applied for correlation p-values to control the elevated risk of type-I errors due to multiple pairwise correlations analysis. MANOVA and zero-order correlations were completed using SPSS Statistics 20 software (IBM).
Results
IFNAR antibody treatment attenuates chronic stress-induced deficits in social behavior and spatial working memory
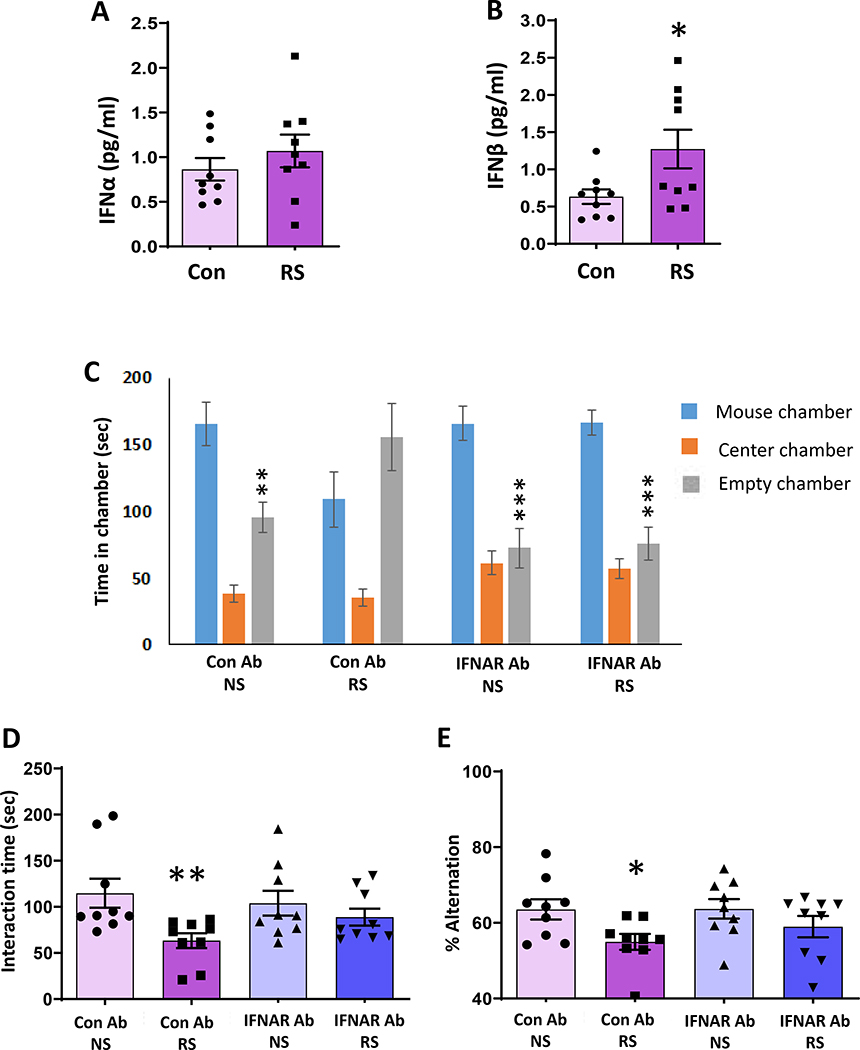
To examine the role of IFN-I in mediating chronic stress-induced inflammation and behavior deficits, we first examined the protein levels of two most studied members of the IFN-I family, IFNα and IFNβ, in mice exposed to RS. ELISA results showed a significant increase in serum IFNβ protein levels, but no change in serum IFNα protein levels in mice following RS compared to no stressed (NS) mice (Fig. 1A, B). To determine the role of IFN-I on chronic stress-induced changes in social behavior, we performed three-chamber and reciprocal interaction tests in mice treated with IFNAR antibody for the systemic blockade of IFN-I signaling or control IgG antibody. IgG antibody-injected NS mice spent more time in the chamber housing stranger mouse than the empty cage chamber (Fig. 1C). In contrast, IgG antibody-injected RS mice had no preference for either chamber. However, RS mice injected with the IFNAR antibody spent more time in the chamber housing stranger mouse than the empty cage chamber (Fig. 1C). Furthermore, in the reciprocal social interaction test, IgG antibody-injected RS mice showed decreased interaction with a stranger mouse compared with IgG antibody-injected NS mice (Fig. 1D). However, IFNAR antibody treatment significantly attenuated RS-induced decrease in interaction time in mice (Fig. 1D). Next, the Y-maze test was performed to evaluate the spatial working memory of the mice and the test is based on the natural tendency of animals to explore a novel environment. In the Y maze test, mice generally visit a new arm of the maze instead of going to the previously visited arm. Spontaneous alternation performance is considered as an index of spatial working memory (61, 62). The number of total arm entries was similar in all treatment groups of mice, showing intact general locomotor activity (data not shown). However, the IgG antibody-injected RS mice showed a significantly reduced percentage of alternation compared with IgG antibody-injected NS mice. In contrast, IFNAR antibody-treated RS mice exhibited a percentage of alternation similar to those of IgG antibody-injected NS mice (Fig. 1E). Together, these results suggest the role of IFN-I signaling in chronic stress-mediated social behavior and spatial working memory deficits in the mice.
Figure 1. IFNAR antibody treatment attenuates chronic stress-induced deficits in social behavior and spatial working memory.
(A-B) Chronic stress-induced changes in serum (A) IFNα and (B) IFNβ protein levels; *p =0.0348 vs NS; Student’s t test: n=9. (C-E) IFNAR antibody treatment attenuated chronic stress-induced deficits in social behavior and spatial working memory. C, Time in chamber in the three-chamber social interaction test. Two-way ANOVA, chamber (F(2,102)=53.75, p<0.0001); chamber X treatment interaction (F(6,102)=6.364, p<0.0001). **p<0.01 and ***p<0.001 (mouse chamber vs empty chamber); n=9–10 per group. D, Reciprocal social interaction test; Two-way ANOVA, stress (F(1,32)=7.617, p=0.0095); **p<0.01 vs Con Ab NS; n=9 per group. E, Y-maze test. Two-way ANOVA, stress (F(1,35)=6.898, p=0.0127); *p<0.05 vs Con Ab NS; n=9–10 per group. NS: no stress; RS: restraint stress.
IFNAR antibody treatment attenuates chronic stress-induced infiltration of macrophages and increases in IFN-I-stimulated gene in the PFC
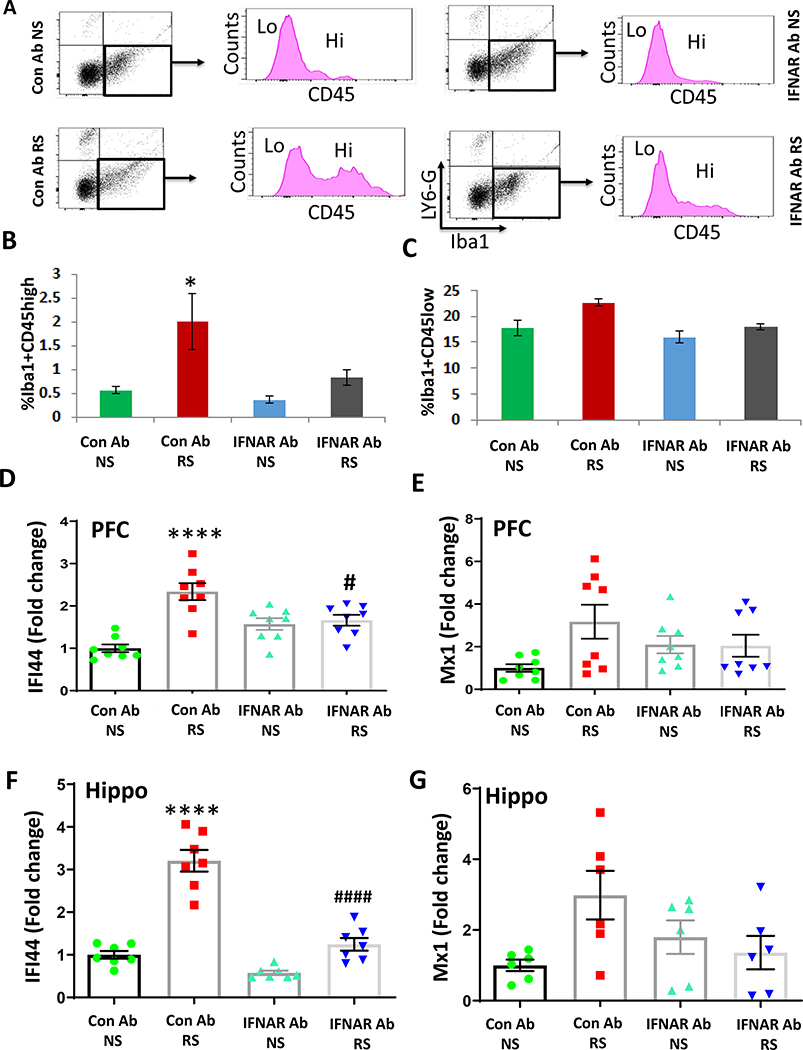
Next, we sought to determine if the peripheral increase of IFN-I signaling following chronic stress induced the recruitment of immune cells into the brain. We used flow cytometry analysis to identify the cell types in PFC and hippocampus following chronic stress. Differences in the levels of CD45 expression were used to determine if Lymphocyte antigen 6 complex locus G6D (Ly6-G) and ionized calcium-binding adapter molecule 1 (Iba1)–positive cells are infiltrating macrophages from the peripheral circulation, which show high levels of CD45, or part of a resident microglial population that express CD45 at low levels. We found that chronic stress increased the proportion of Iba1+CD45hi cells, but not Iba1+CD45low cells in PFC, while IFNAR antibody treatment significantly attenuated chronic stress-mediated infiltration of peripheral macrophages (Fig. 2A–C). However, the increases in Iba1+CD45hi cells and Iba1+CD45low cells in the hippocampus following RS treatment did not reach statistical significance (Fig. S1A, B). It is known that the binding of IFNα or IFNβ to IFNα/β receptors triggers the signaling pathway mediated by Janus kinases (JAKs; JAK1 and JAK2), signal transducer and activator of transcriptions (STATs; STAT1 and STAT2), and IRF9 (63). The above signaling pathways result in the modulation of a number of gene expressions. These IFN-stimulated genes (ISGs) play important roles in inhibiting viral infections and inflammatory stimuli. Mx1 and IFI44 are representative ISGs induced by IFN-I. To determine whether the increased levels of IFNβ in the periphery following chronic stress upregulated IFN-I signaling in the brain, we analyzed the mRNA expression of IFI44 and Mx1 in the PFC and hippocampus. Quantitative PCR (qPCR) analysis revealed the upregulation of IFI44, but not Mx1 mRNA levels in the PFC (Fig. 2D, E) and hippocampus (Fig. 2F, G) of the RS mice. However, the increases of IFI44 were attenuated in the PFC and hippocampus of IFNAR antibody-treated mice exposed to RS compared with IgG antibody-treated mice subjected to RS (Fig. 2D, F). To determine whether the infiltration of peripheral macrophages into the brain contributes to RS-induced increase in IFI44 expression, we treated mice with liposome-encapsulated clodronate (Clodrosome) to deplete myeloid cells during the RS paradigm. The dose and duration of Clodrosome used in our experiment have been shown to significantly reduce the number of circulating CD11b+ F4/80+ myeloid cells compared with Encapsome (control)-treated mice (12). Clodrosome administration significantly attenuated RS-induced increase in IFI44 mRNA levels in both PFC (Fig. S2A) and hippocampus (Fig. S2B). These results suggest that peripheral macrophages play a key role in RS-induced activation of IFN-I signaling in the brain.
Figure 2. IFNAR antibody treatment attenuates chronic stress-induced infiltration of macrophages and increases in IFN-I-stimulated gene in the PFC.
(A-C) Flow cytometry analysis of infiltrating monocytes and resident microglia in the PFC of mice exposed to chronic stress in presence or absence of IFNAR antibody. A, Flow cytometry gating strategy showing Iba1+ cells were analyzed using CD45 to differentiate infiltrating macrophages (CD45hi) from resident microglia (CD45low). B, % of Iba1+ cells expressing CD45high in PFC of no stress (NS) and restraint stress (RS) mice treated with control (Con) Ab or IFNAR Ab. Two-way ANOVA, stress (F(1,8)=2.708, p=0.0142); *p<0.05 vs Con Ab NS; n=3 per group. C, % of Iba1+ cells expressing CD45low in PFC of mice exposed to RS as compared to NS. Two-way ANOVA, stress (F (1, 8) = 7.848, p=0.0231); treatment ( F (1, 8) = 6.283, p=0.0366); n=3 per group. (D-G) IFN-I signaling in the brain of chronic stressed mice treated with Con Ab or IFNAR Ab. D, mRNA expression of IFI44 in PFC. Two-way ANOVA, stress (F (1, 28) = 24.27 p<0.0001); stress X treatment interaction (F (1, 28) = 18.47, p=0.0002); ****p<0.0001 vs Con Ab NS,; #p<0.05 vs Con Ab RS; n=8 per group. E, mRNA expression of Mx1 in PFC. Two-way ANOVA, n=8–9 per group. F, mRNA expression of IFI44 in hippocampus. Two-way ANOVA, stress (F (1, 24) = 85.35 p<0.0001); treatment (F (1, 24) = 58.65, p<0.0001); stress X treatment interaction (F (1, 24) = 24.46, p<0.0001); ****p<0.0001 vs Con Ab NS,; ####p<0.0001 vs Con Ab RS; n=7 per group. G, mRNA expression of Mx1 in hippocampus. Two-way ANOVA, stress (F (1, 20) = 6.196, p=0.0217); n=6 per group.
IFNAR antibody treatment attenuates chronic stress-induced changes in serum inflammatory markers, and M1 and M2 markers in the PFC and hippocampus
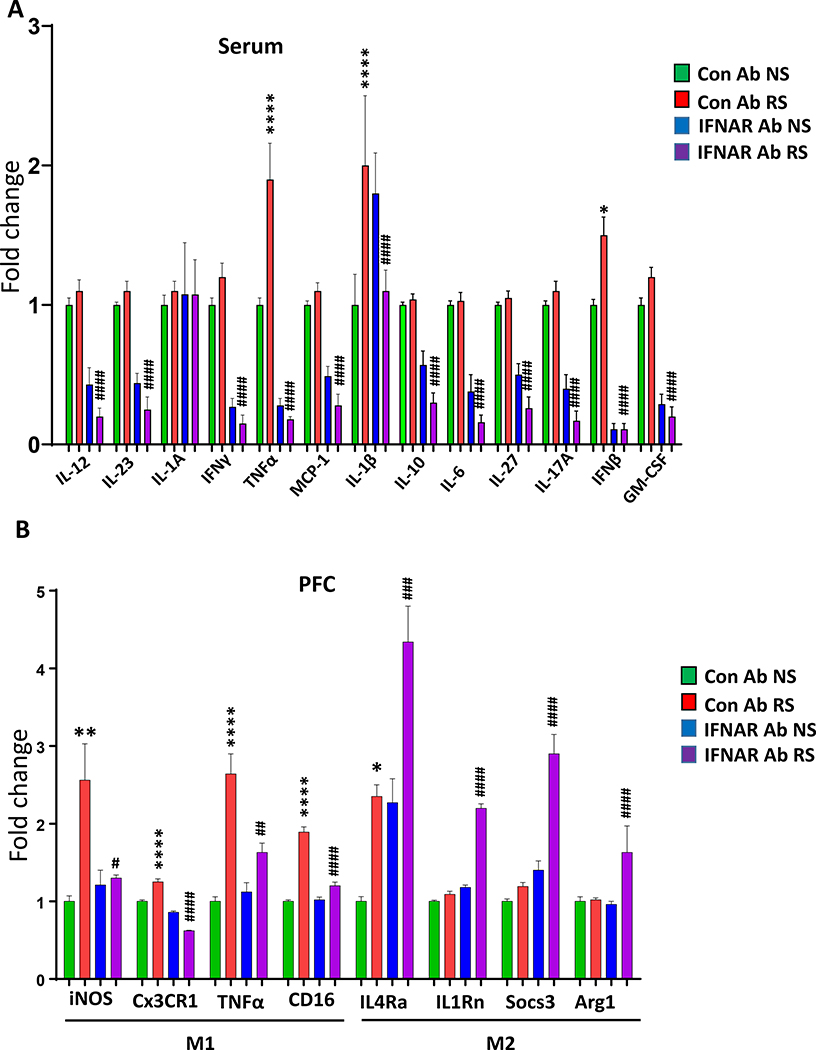
There is growing evidence that abnormal inflammatory responses such as increased serum levels of certain pro-inflammatory cytokines, particularly TNFα and IL-1β, increase susceptibility to neuropsychiatric disorders like depression (64–69). Moreover, chronic stress conditions increase serum levels of pro-inflammatory markers in animal models (70). We examined the role of IFN-I in chronic stress-induced changes in serum inflammatory markers. Cytokine array data showed significant upregulation of the TNFα and IL-1β in the serum of mice exposed to RS compared to the NS group (Fig. 3A). Also, IFNAR antibody treatment significantly attenuated the RS-induced increases in TNFα and IL-1β levels (Fig. 3A). As a result of the disturbance of tissue homeostasis, macrophages switch between pro- (M1) and anti-inflammatory (M2) phenotypes (71–73). We analyzed the mRNA levels of M1 markers (iNOS, Cx3CR1, TNFα, and CD16) and M2 markers (interleukin-4 receptor α (IL4Rα), interleukin-1 receptor antagonist (IL1Rn), Suppressor of cytokine signaling 3 (Socs3), and Arginase 1 (Arg1)) in the PFC and hippocampus of mice following RS. qPCR results showed significant increases in the expression levels of all M1 markers (Fig. 3B). In contrast, no change was found in the M2 markers (IL1Rn, Socs3, and Arg1) in PFC of mice following RS, except IL4Rα, which was significantly increased as a result of RS (Fig. 3B). Interestingly, IFNAR antibody treatment significantly attenuated the increases in M1 markers, whereas it increased the M2 markers in the PFC of RS mice (Fig. 3B). A similar change in the expression of the above markers was seen in the hippocampus except that Cx3CR1 and IL4Rα were unchanged, whereas Arg1 was increased following RS (Fig. S3). These results suggest that chronic stress induces M1 pro-inflammatory phenotype and blocking IFNAR systemically attenuates the increase in the levels of M1 markers.
Figure 3. IFNAR antibody treatment attenuates chronic stress-induced changes in serum inflammatory markers, and in M1 and M2 markers in the PFC.
(A) Array of 13 cytokines in the serum samples from no stress (NS) and restraint stress (RS) mice treated with control (Con) Ab or IFNAR Ab. IL-12. Two-way ANOVA, treatment (F (1, 19) = 81.67 p<0.001); ####p<0.0001 vs Con Ab RS; n =5–7 per group. IL-23. Two-way ANOVA, treatment (F (1, 20) = 91.68p<0.0001); ####p<0.05 vs Con Ab RS; n=5–7 per group. IL-1A. Two-way ANOVA, NSp>0.05 vs Con Ab RS; n=5–7 per group. IFNγ. Two-way ANOVA, treatment (F (1, 20) = 155.2 p<0.001); ####p<0.0001 vs Con Ab RS; n=5–7 per group. TNFα. Two-way ANOVA, stress (F (1, 20) = 9.92, p<0.01); treatment (F (1, 20) = 95.54 p<0.001); stress X treatment interaction (F (1, 20) = 14.62, p<0.01); ****p<0.0001 vs Con Ab NS; ####p<0.0001 vs Con Ab RS; n=5–7 per group. MCP1. Two-way ANOVA, treatment (F (1, 20) = 98.27, p<0.001); ####p<0.0001 vs Con Ab RS; n=5–7 per group. IL1β. Two-way ANOVA, stress X treatment interaction (F (1, 20) = 6.88, p<0.05); ****p<0.0001 vs Con Ab NS, ####p<0.0001 vs Con Ab RS; n=5–7 per group. IL-10. Two-way ANOVA, treatment (F (1, 20) = 79.09, p<0.0001);###p<0.001 vs Con Ab RS; n=5–7 per group. IL-6. Two-way ANOVA, treatment (F (1, 20) = 102.0, p<0.0001); ####p<0.0001 vs Con Ab RS; n=5–7 per group. IL-27. Two-way ANOVA, treatment (F (1, 20) = 85.32, p<0.0001); ####p<0.0001 vs Con Ab RS; n=5–7 per group. IL-17A. Two-way ANOVA, treatment (F (1, 20) = 108.3, p<0.001); ####p<0.0001 vs Con Ab RS; n=5–7 per group. IFNβ. Two-way ANOVA, stress (F (1, 17) = 5.309, p<0.05); treatment (F (1, 17) = 146.7 p<0.001); stress X treatment interaction (F (1, 17) = 5.309, p<0.05); *p<0.05 vs Con Ab NS, ####p<0.0001 vs Con Ab RS; n=5–7 per group. GM-CSF. Two-way ANOVA, treatment (F (1, 20) = 153.5, p<0.001); ####p<0.0001 vs Con Ab RS; n=5–7 per group. (B) mRNA expressions of M1/M2 phenotype in the PFC of no stress (NS) and restraint stress (RS) mice treated with control (Con) Ab or IFNAR Ab.. mRNA expressions of M1 markers; iNOS. Two-way ANOVA, stress (F (1, 20) = 10.32, p=0.0044); stress x treatment interaction (F (1, 20) = 7.985, p=0.0104); **p<0.01 vs Con Ab NS, #p<0.05 vs Con Ab RS; n=6 per group. Cx3CR1. Two-way ANOVA, treatment (F (1, 20) = 231.9, p<0.0001); stress x treatment interaction (F (1, 20) = 60.84, p<0.0001); ****p<0.0001 vs Con Ab NS, ####p<0.0001 vs Con Ab RS; n=6 per group. TNFα. Two-way ANOVA, Stress (F (1, 20) = 46.33, p<0.0001), treatment (F (1, 20) = 7.940, p=0.0106); stress x treatment interaction (F (1, 20) = 12.80, p=0.0019); ****p<0.0001 vs Con Ab NS, ##p<0.01 vs Con Ab RS; n=6 per group. CD16. Two-way ANOVA, Stress (F (1, 20) = 129.7, p<0.0001), treatment (F (1, 20) = 50.84, p<0.0001); stress x treatment interaction (F (1, 20) = 57.09, p<0.0001); ****p<0.0001 vs Con Ab NS, ####p<0.0001 vs Con Ab RS; n=6 per group. IL4Rα. Two-way ANOVA, Stress (F (1, 20) = 41.45, p<0.0001), treatment (F (1, 20) = 37.66, p<0.0001); *p<0.05 vs Con Ab NS, ###p<0.001 vs Con Ab RS; n=6 per group. IL1Rn. Two-way ANOVA, Stress (F (1, 20) = 277.3, p<0.0001), treatment (F (1, 20) = 380.9, p<0.0001); stress x treatment interaction (F (1, 20) = 194.3, p<0.0001); ####p<0.0001 vs Con Ab RS; n=6 per group. Socs3. Two-way ANOVA, Stress (F (1, 20) = 48.16, p<0.0001), treatment (F (1, 20) = 87.03, p<0.0001); stress x treatment interaction (F (1, 20) = 28.23, p<0.0001); ####p<0.0001 vs Con Ab RS; n=6 per group. Arg1. Two-way ANOVA, Stress (F (1, 20) = 70.76, p<0.0001), treatment (F (1, 20) = 48.29, p<0.0001); stress x treatment interaction (F (1, 20) = 62.80, p<0.0001); ####p<0.0001 vs Con Ab RS; n=6 per group.
Like microglia, astrocytes also play important role in maintainance of brain homeostasis. It is known that activated microglia induce the transformation of naive astrocytes into reactive astrocytes (74). A1 astrocytes gain neurotoxic function whereas A2 astrocytes show neuroprotective properties (74, 75). Here, we examined the expression levels of a group of astrocyte reactivity markers, such as “pan,” “A1,” and “A2” markers. In pan-markers, we found significant increases in serine (or cysteine) peptidase inhibitor, clade A, member 3N (Serpina3n) and vimentin (Vim), but decrease in lipocalin-2 (Lcn2) mRNA levels in the PFC following RS (Fig. S4A). IFNAR antibody treatment significantly attenuated the increase in serpina3n mRNA levels. In A1 markers, IFNAR antibody treatment significantly attenuated the RS-induced increases in C3, histocompatibility 2, T region locus 23 (H2-T23) and Serpin family G member 1 (Serping 1) mRNA levels (Fig. S4A). We found a significant increase in the A2 marker, CD109 with no change in Epithelial Membrane Protein 1 (EMP1) and S100 calcium-binding protein A (S100a) mRNA levels following RS (Fig. S4A). In the hippocampus, Vim mRNA levels were increased following RS (Fig. S4B). In A1 markers, the RS-induced increases in C3 and Serping 1 mRNA levels were significantly attenuated by IFNAR treatment (Fig. S4B). No significant change in A2 markers were found in the hippocampus following RS (Fig. S4B).
Complement component 3 mediates IFNβ–induced neuroinflammation and deficits in social behavior.
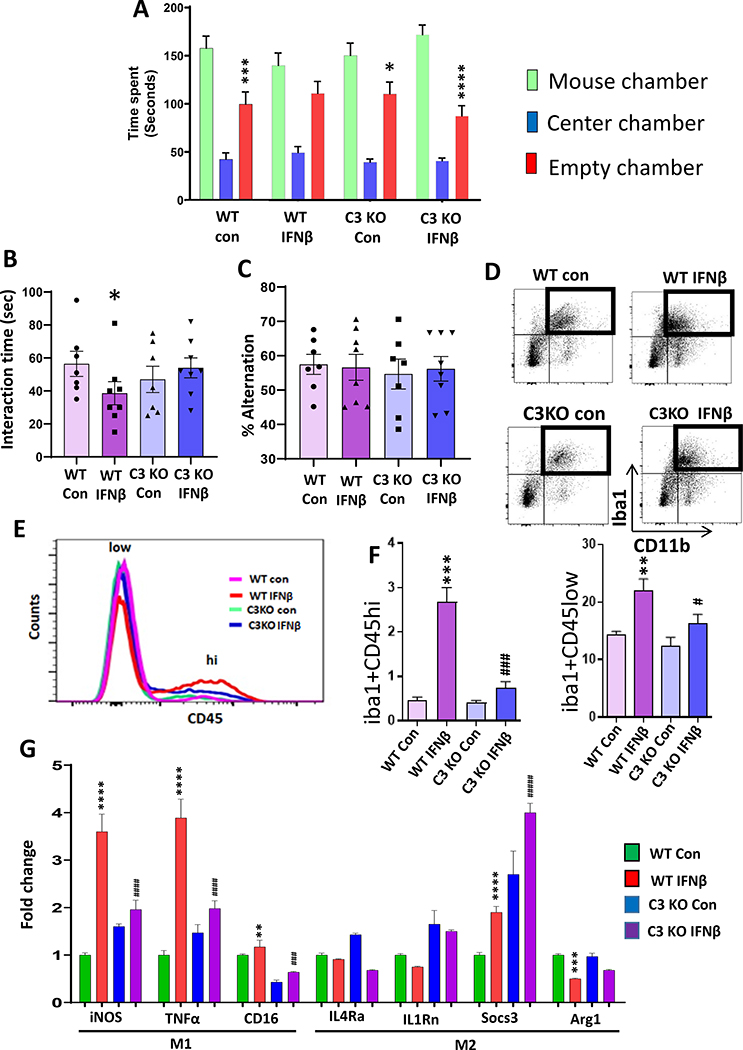
The classical complement pathway is involved in microglia-mediated synaptic loss (76) and the inflammatory response (77). However, the role of the classical pathway in peripheral IFN-I-mediated neuroinflammation and behavior is not known. Since we found a significant increase in C3 mRNA levels in the PFC and hippocampus following RS, we examined the expression levels of another classical complement marker, C1q in the PFC and hippocampus of mice following RS. We found no significant change in the mRNA levels of C1q in the PFC (Fig. S5A) and hippocampus (Fig. S5B) of RS-exposed mice. Next, we examined the role of C3 in IFNβ-mediated behavior deficits. Similar to the data observed following RS exposure, IFNβ administration induced significant upregulation of IFI44, but not Mx1 mRNA levels in the PFC (Fig. S5C–D) and hippocampus (Fig. S5E–F). C3-deficient mice (C3 KO) and age-matched wild type (WT) were injected with recombinant mouse IFNβ or vehicle, and social behavior and spatial working memory were examined. WT mice injected with IFNβ showed no preference for either chamber, whereas C3 KO injected with IFNβ showed a preference for the chamber housing stranger mouse than the empty cage chamber (Fig. 4A). Similarly, WT mice injected with IFNβ showed decreased interaction with a stranger mouse compared to C3 KO injected with IFNβ (Fig. 4B). In the Y maze test, no significant difference in the percentage of alternation between the treatment groups (Fig. 4C). Overall, these results suggest that C3 deletion attenuates IFNβ-induced deficits in social behavior.
Figure 4. Complement component 3 mediates IFNβ–induced neuroinflammation and deficits in social behavior.
(A-C) C3 deletion attenuated IFNβ-induced deficits in social behavior. WT and C3 KO mice were treated with vehicle (PBS; Con) or IFNβ. A, Time in chamber in the three-chamber social interaction test. Two-way ANOVA, chamber (F (2, 78) = 119.2, p<0.0001); *p<0.05, ***p<0.001, ****p<0.0001 and (mouse chamber vs empty chamber); n=7–8 per group). B, Reciprocal social interaction test. Two-way ANOVA, genotype X treatment interaction (F (1, 24) = 8.221, p=0.0085); *p<0.05 vs WT Con; n=7 per group). C, Y-maze test for spatial working memory. Two-way ANOVA; n=7–8 per group. (D-F) Flow cytometry analysis of infiltrating monocytes and resident microglia in the PFC of WT and C3 KO mice treated with Con or IFNβ. D-E, Flow cytometry gating strategy showing Iba1+ cells were analyzed using CD45 to differentiate infiltrating monocytes (CD45hi) from resident microglia (CD45low). F, % of Iba1+ cells expressing CD45high. Two-way ANOVA, genotype (F (1, 8) = 28.57, p=0.0007), treatment (F (1, 8) = 45.84, p=0.0001), genotype X treatment interaction (F (1, 8) = 24.89, p=0.0011); ***p<0.001 vs WT con, ###p<0.001 vs WT IFNβ; n=3 per group). % of Iba1+ cells expressing CD45low. Two-way ANOVA, genotype (F (1, 8) = 19.59, p=0.0022), treatment (F (1, 8) = 45.37, p=0.0001); **p<0.01 vs WT con, #p<0.05 vs WT IFNβ; n=3 per group). (G) mRNA expressions of M1/M2 phenotype in the PFC of WT and C3 KO mice treated with Con or IFNβ. mRNA expressions of M1 markers; iNOS. Two-way ANOVA, genotype (F (1, 20) = 36.35, p<0.0001); treatment (F (1, 20) = 294.5, p<0.0001), genotype X treatment interaction (F (1, 20) = 168.6, p<0.0001); ****p<0.0001 vs WT Con, ####p<0.0001 vs WT IFNβ; n=6 per group. TNFα. Two-way ANOVA, genotype (F (1, 20) = 56.80, p<0.0001); treatment (F (1, 20) = 316.6, p<0.0001), genotype X treatment interaction (F (1, 20) = 155.1, p<0.0001); ****p<0.0001 vs WT Con, ####p<0.0001 vs WT IFNβ; n=6 per group. CD16. Two-way ANOVA, genotype (F (1, 20) = 341.6, p<0.0001); treatment (F (1, 20) = 41.19, p<0.0001); **p<0.0001 vs WT Con, ###p<0.0001 vs WT IFNβ; n=6 per group. mRNA expression of M2 markers; IL4Rα. Two-way ANOVA, treatment (F (1, 20) = 5.027, p=0.0364), n=6 per group. IL1Rn. Two-way ANOVA, genotype (F (1, 20) = 119.8, p<0.0001); treatment (F (1, 20) = 6.709, p=0.0175); n=6 per group. Socs3. Two-way ANOVA, genotype (F (1, 20) = 236.6, p<0.0001); treatment (F (1, 20) = 74.92, p<0.0001), ****p<0.0001 vs WT Con, ####p<0.0001 vs WT IFNβ; n=6 per group. Arg1. Two-way ANOVA, treatment (F (1, 20) = 42.80, p=0.1909), genotype X treatment interaction (F (1, 20) = 4.569, p=0.0451); ***p<0.001 vs WT Con; n=6 per group.
Next, we examined the effect of C3 deletion on IFNβ-mediated infiltration of macrophages into the PFC and hippocampus. Results from flow cytometry analysis showed an increased proportion of Iba1+CD45hi and Iba1+CD45low cells in the PFC (Fig. 4D–F) and hippocampus (Fig. S5G–H) of IFNβ-treated WT mice. In contrast, the IFNβ-induced increases in Iba1+CD45hi, and Iba1+CD45low cells were significantly attenuated in C3 KO mice. Furthermore, we examined the macrophage phenotype by analyzing M1/M2 phenotype markers in the PFC and hippocampus. We found significant increases in mRNA expression levels of iNOS, TNFα, and CD16 in the PFC (Fig. 4G) and of iNOS and TNFα in the hippocampus (Fig. S5I) of WT mice following IFNβ treatment. IFNβ treatment resulted in a significant increase in Socs3, but a decrease in Arg1 mRNA levels in PFC (Fig. 4G), and decreases in IL1Rn and Arg1 mRNA levels in the hippocampus (Fig. S5I) of WT mice. However, C3 deletion significantly attenuated IFNβ-mediated changes in M1 and M2 markers in PFC (Fig. 4G) and hippocampus (Fig. S5I). These results suggest that C3 plays a critical role in IFNβ-induced neuroinflammation and deficits in social behavior.
Increased expression of IFN-I-stimulated genes, IFI44 and Mx1 in the PFC of depressed subjects
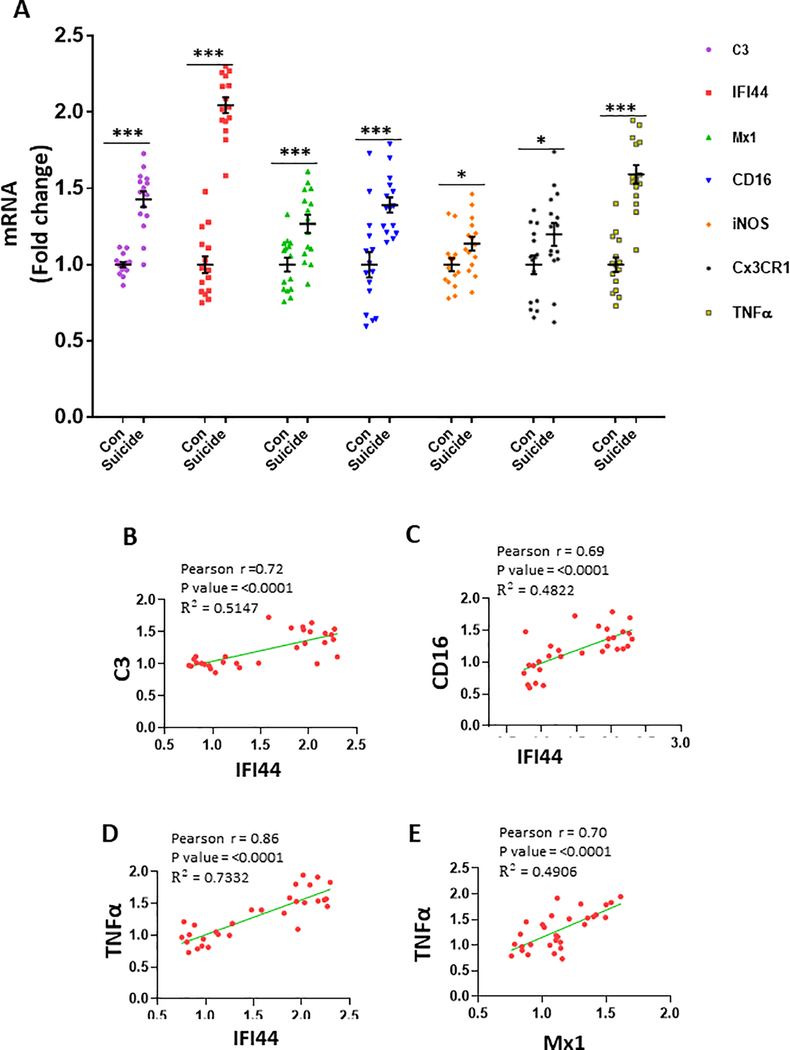
We examined the mRNA levels of IFN-I-stimulated genes, IFI44, and Mx1 by qPCR analysis. Since we found significant increases in the levels of M1 markers and C3 in PFC of mice following RS, the expression of those genes were also examined in the human PFC samples. None of the evaluated covariates had a statistically significant association with gene expression. Therefore, none of the covariates were included in the MANOVA model. In the overall multivariate model, depressed-suicide status was a significant predictor of gene expression [Wilk’s λ = 0.08, F(6, 23) = 44.19, p < 0.001, η2p = 0.920, Observed Power = 1.00]. An examination of the univariate between-subject effects showed that depressed-suicide status was associated with an increased expression of C3, IFI44, Mx1, CD16, iNOS, Cx3CRI and TNFα (Fig. 5a). The mean values for the depressed-suicide group were higher in every case, suggesting that increased expression of each of these genes may be implicated in depression and/or suicidal behavior. Next, we examined if the mRNA levels of IFI44 and Mx1 are correlated with those of M1 markers and C3 in our sample cohort. Following Holm-Bonferroni correction of significance levels, IFI44 showed significant positive correlations with C3 (Fig. 5b), CD16 (Fig. 5c), and TNFα (Fig. 5d), whereas Mx1 was positively correlated with TNFα (Fig. 5e). Another interesting observation is that the expression level of TNFα shows strong significant positive correlations with the expression levels of all other genes examined (Table S3).
Figure 5. Increased expression of IFI44 and Mx1 in the PFC of depressed subjects.
(A) Increase in IFN-I stimulated genes (IFI44 and Mx1), complement component (C3) and M1 phenotyppe markers in the PFC of depressed suicide subjects. mRNA levels of these genes in the PFC of depressed suicide (n=15) and control (n=15) subjects were measured by qRT-PCR. mRNA levels were normalized to housekeeping genes 18S and B2m. C3 (***p <0.001), IFI44 (***p <0.001), Mx1 (***p <0.001), CD16 (***p <0.001), iNOS (*p <0.05), Cx3CR1 (*p <0.005) and TNFα (***p =0.001) as compared to the controls, multivariate analysis of variance (MANOVA). (B-E) Representative graphs showing correlation between the mRNA levels. B, Correlation between IFI44 and C3 (Pearson r =0.72; p<0.0001; R2 = 0.5147). C, Correlation between IFI44 and CD16 (Pearson r =0.69; p<0.0001; R2 = 0.4822). D, Correlation between IFI44 and TNFα (Pearson r =0.86; p<0.0001; R2 = 0.7332). E, Correlation between Mx1 and TNFα (Pearson r =0.70; p<0.0001; R2 = 0.4906).
Discussion
Our results demonstrated that chronic restraint stress induces serum IFNβ levels and deficits in social behavior and spatial working memory. Conversely, inhibition of IFN-I signaling using the IFNAR antibody blocks chronic stress-induced infiltration of macrophages into the brain and behavioral abnormalities. Further, IFNβ-induced changes in behavior and neuroinflammation were observed to be C3-dependent. Also, depressed suicide subjects have increased levels of IFN-I-stimulated genes in their PFC and correlated with C3 and inflammatory markers compared to the controls.
In the present study, we have used the RS paradigm to investigate the effects of chronic stress on behavior and inflammatory markers. In the above paradigm, mice were exposed to continuous and predictable stress that mimic chronic stress conditions, such as daily repetition of stressful jobs and familial stress in humans (78). Several earlier studies support the validity of this model, in particular on the effects of chronic stress on anxiety and depression-like behaviors (44–47), functional connectivity of the brain (48, 49), hippocampal volume (50, 57), social behavior (51) and cognitive functions (53–56, 58) similar to those in human subjects with neuropsychiatric disorders such as depression (78). RS is known to induce persistent low-grade inflammation, as evidenced by increases in peripheral levels of pro-inflammatory markers such as TNFα (79, 80). Further, RS induces microglial activation, as shown by altered microglial morphology and expression of immune molecules in PFC (81). In addition, stress-induced microglial activation was associated with deficits in social behavior (82) and spatial memory (83). Further, the cotreatment of minocycline, an inhibitor of microglial activation, could reverse stress-induced cognitive impairment (83).
We found a significant increase in the number of infiltrating macrophages in the PFC; a brain region known to play critical roles in mood and cognition following RS. Also, an increase in IFN-I signaling was observed in PFC and hippocampus following RS. Mice treated with the IFNAR antibody showed prevention of macrophages’ recruitment into the PFC, indicating that an increased number of infiltrating macrophages is attributed mainly to the elevated peripheral levels of IFN-I following RS. These findings suggest that activation of IFN-I signaling plays a key role in linking the central and peripheral immune systems. It is important to note that IFNAR antibody treatment could also attenuate RS-induced increases in serum pro-inflammatory cytokines such as TNFα, IL1β, and IFNβ. Indeed, IFNβ has been shown to enhance TNFα production in murine macrophages (84) and LPS-activated monocytes (85). It is also known that IFNβ participates in enhancing the signals of other cytokines such as IL-6 (86). Although our findings show the critical role of peripheral IFNβ in mediating RS-induced effects on behavior, the cellular origin of IFNβ is not known. It is possible that IFNβ is produced in the brain, leaking to the periphery, or is produced by peripheral immune cells and has its effect in the brain regions mediating behavioral changes. A compromised blood-brain barrier (BBB) can allow macrophage infiltration and trafficking of IFNβ from the peripheral system to the brain. RS is known to damage the BBB in the PFC and hippocampus of adult rats (87). Further, it has been shown that the BBB endothelial-derived chemokine ligand CXCL10 (C-X-C motif chemokine 10) mediates IFNβ-induced behavioral changes in mice through impairment of synaptic plasticity (88). Our data showing inhibition in RS-induced increase in the expression of IFI44 in Clodrosome-treated mice suggest that IFNβ present in peripheral macrophages may play a critical role in RS-induced deficits in social behavior and spatial working memory. Additional studies using mice lacking IFNβ in peripheral macrophages vs. resident microglia are warranted.
We found an increase in M1 markers in PFC and hippocampus following RS, whereas IFNAR antibody-treated mice show a greater M2 phenotype compared to the control group following RS. In addition, the IFNAR antibody significantly attenuated the increased levels of M1 markers in mice exposed to RS. The macrophage polarization is tightly controlled by the balance between the M1-mediated inflammation and M2-mediated anti-inflammation/neuroprotection. Unexpectedly, we found a significant increase in the mRNA levels of IL4Rα, an M2 marker in PFC following RS. IL4Rα is a shared receptor subunit for IL-4 and IL-13 and represents a central switch for M1 to M2 macrophage polarization (89). In agreement with our results, many previous studies have reported pro-inflammatory-like functions of IL4Rα. Wound macrophages from mice deficient in the IL4Rα did not differ from wild-type mice in M2 markers (90). Also, macrophages from mice lacking IL4Rα responded to the TLR4 agonist LPS similarly to those from WT mice on the expression of inflammatory cytokines, including TNFα (91). These results imply that the analysis of both IL4Rα-dependent and IL4Rα-independent markers is important for identifying the M2 phenotype under stress conditions. Further, our data on M1/M2 markers do not differentiate the cell types (infiltrating macrophages vs. resident microglia) expressing these proteins. It is known that microglia and monocyte-derived macrophage expression profiles and characteristics differ dramatically even when these two cell types are exposed to the same stimuli (92, 93). Further, recent studies show that resident microglia and infiltrating immune cells regulate astrocyte reactivity (74). Specifically, microglia induce A1 reactive astrocytes through the release of cytokines such as IL1α, TNFα, and C1q (74). The increases in A1 makers including C3 following RS suggest that reactive astrocytes were induced by chronic stress conditions.
IFN-I and complement are both key components of the innate immune system. Abnormalities in the complement pathway have been found in many neurological and neuropsychiatric disorders (94–97). The complement pathway is inappropriately reactivated in these disease conditions leading to synaptic deficits, disrupted neuronal connectivity, and cognitive impairment (98, 99). C3 is considered the hub of all three complement activation pathways, the classical pathway, the lectin pathway, and the alternative pathway. Our earlier study has found significant increases in C3 mRNA levels in PFC of mice exposed to chronic unpredictable stress and depressed suicide subjects (12). In the present study, we found that the deletion of C3 decreased systemic IFNβ-induced deficits in social behavior and the infiltration of macrophages into PFC and hippocampus. Further, IFNβ-induced increases in M1 markers were attenuated in C3 knockout mice. The above findings on the role of C3 in mediating IFNβ-induced changes in CNS inflammation are further supported by our human postmortem data showing a positive association between C3 and IFI44 in PFC. Our results provide a possible mechanistic link between IFN-I signaling and the complement pathway in chronic stress conditions.
Although we found deficits in both social behavior and spatial working memory in RS-exposed mice, no change in spatial working memory was found in mice systemically treated with IFNβ. The reason behind these differing effects on spatial working memory is not clear. However, we suspect that the efficacy of IFN-I signaling needed to result in spatial working memory deficits may differ between the models. RS-exposed mice may have an overall higher magnitude of inflammation than IFNβ-injected mice. Additional studies with long-term IFNβ treatment are warranted to test the above possibility.
It is important to note that IFN-I exerts pathogenic or protective roles in the CNS depending on their concentration (100). In addition to their strong antiviral and immunomodulatory functions, IFN-I also regulates homeostatic processes in the periphery (101) and within the CNS (102). IFNβ functions as a growth factor for neural progenitor stem cells and enhances their differentiation into oligodendroglia (103). Baseline IFNβ expression is critical for neuronal homeostasis, whereas spontaneous pathologies similar to human neurodegeneration such as Parkinson’s disease and dementia with Lewy bodies were observed in IFNβ–/– mice (104). In MS patients, IFNβ therapy has been shown to prevent and shorten relapses and reduces cognitive loss (105, 106). However, IFNβ treatment has been shown to exacerbate depression in MS patients with a history of depression (107–110). A recent rodent study has demonstrated that systemically blocking maternal IFN-I signaling decreased poly(I:C)-induced behavioral abnormalities in the offspring (111).
A number of transcriptional activators and repressors have been implicated in the regulation of IFNα and IFNβ. Specifically, the IFNβ promoter contains four positive regulatory domains (PRDI-IV), which are occupied by transcription factor complexes: IRF-3 and IRF-7 bind PRDI and III; the p50/RelA NF-κB complex binds PRDII, and the ATF-2/c-Jun AP-1 complex binds PRDIV (112). The coordinated binding of these complexes is important for the activation of IFNβ promoter by viral infection or inflammatory stimuli. In contrast to IFNβ, the activation of IFNα promoter requires binding of IRF7 but not AP-1 and NF-κB (113). Since NF-κB has been well implicated in mediating chronic stress-induced behavioral impairments (114), it is possible that the selective increase in IFNβ observed in our study following chronic stress conditions could be a result of stress-induced NF-κB activation. However, additional studies are warranted to examine the role of NF-κB in mediating chronic stress-induced IFN-I induction. In addition to the PFC and hippocampus, chronic stress conditions as well as peripheral immune challenge have been shown to alter neuronal activity and morphology in the basolateral amygdala (BLA) in rodents (115–118). Further, chronic social defeat stress has been shown to induce microglia activation and altertations in immune signaling molecules in amygdala (119, 120). Moereover, restraint stress and repeated stimulation of corticotrophin releasing factor (CRF) receptors within the BLA have been shown to induce anxiety-like behavior and social interaction deficits in rats (121). The potential role of IFN-1 signaling in amygdala in mediating chronic stress-induced alterations in behavior and neuroinflammation will be investigated in future studies.
In summary, our findings provide a novel mechanism linking IFN-I signaling and complement pathway in chronic stress-induced neuroinflammation and changes in behavior. Additional studies are warranted to investigate the cell type in the brain that plays a central role in IFN-I-mediated macrophage recruitment during inflammation and contribute to complement-dependent behavioral alterations under chronic stress conditions. Collectively, these results support the rationale that targeting the peripheral IFN-I pathway represents a promising therapeutic option, especially for patients with an elevated immune profile, as seen in many depressed subjects.
Supplementary Material
Acknowledgments:
The authors acknowledge the funding support from US National Institute of Health/ National Institute of Mental Health (NIMH) grants (MH120876 and MH121959), and the Merit Review Award (BX004758) from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development to AP. The contents do not represent the views of the Department of Veterans Affairs or the United States Government. The research funding support from Augusta University is acknowledged. The authors would like to acknowledge Quebec Suicide Brain Bank for human postmortem tissue samples.
Footnotes
Conflict of Interest:
None.
Supplementary information
Supplementary information is available at MP’s website.
References:
- 1.Marshall PS, Watson D, Steinberg P, Cornblatt B, Peterson PK, Callies A, et al. An assessment of cognitive function and mood in chronic fatigue syndrome. Biol Psychiatry. 1996;39(3):199–206. [DOI] [PubMed] [Google Scholar]
- 2.Beck AT. Depression: Clinical, experimental, and theoretical aspects.1967. [Google Scholar]
- 3.Enns MW, Bernstein CN, Kroeker K, Graff L, Walker JR, Lix LM, et al. The association of fatigue, pain, depression and anxiety with work and activity impairment in immune mediated inflammatory diseases. PLoS One. 2018;13(6):e0198975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feinstein A, Brochet B, Sumowski J. The cognitive effects of anxiety and depression in immune-mediated inflammatory diseases. Neurology. 2019;92:211–2. [DOI] [PubMed] [Google Scholar]
- 5.Whitehouse CE, Fisk JD, Bernstein CN, Berrigan LI, Bolton JM, Graff LA, et al. Comorbid anxiety, depression, and cognition in MS and other immune-mediated disorders. Neurology. 2019;92:e406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140(3):774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schedlowski M, Engler H, Grigoleit JS. Endotoxin-induced experimental systemic inflammation in humans: a model to disentangle immune-to-brain communication. Brain Behav Immun 2014;35:1–8. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun 2010;24(4):558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63(11):1022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corona AW, Fenn AM, Godbout JP. Cognitive and behavioral consequences of impaired immunoregulation in aging. J Neuroimmune Pharmacol 2012;7(1):7–23. [DOI] [PubMed] [Google Scholar]
- 11.Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, et al. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci 2006;26(14):3813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crider A, Feng T, Pandya CD, Davis T, Nair A, Ahmed AO, et al. Complement component 3a receptor deficiency attenuates chronic stress-induced monocyte infiltration and depressive-like behavior. Brain Behav Immun 2018;70:246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pyter LM, Kelly SD, Harrell CS, Neigh GN. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain Behav Immun 2013;30:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci 2011;31(17):6277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19(6):699–709. [DOI] [PubMed] [Google Scholar]
- 16.McKim DB, Patterson JM, Wohleb ES, Jarrett BL, Reader BF, Godbout JP, et al. Sympathetic Release of Splenic Monocytes Promotes Recurring Anxiety Following Repeated Social Defeat. Biol Psychiatry. 2016;79(10):803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci 2014;34(7):2583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiber G, Piehler J. The molecular basis for functional plasticity in type I interferon signaling. Trends Immunol 2015;36(3):139–49. [DOI] [PubMed] [Google Scholar]
- 19.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol 2006;7(2):131–7. [DOI] [PubMed] [Google Scholar]
- 20.de Weerd NA, Samarajiwa SA, Hertzog PJ. Type I interferon receptors: biochemistry and biological functions. J Biol Chem. 2007;282(28):20053–7. [DOI] [PubMed] [Google Scholar]
- 21.Blank T, Prinz M. Type I interferon pathway in CNS homeostasis and neurological disorders. Glia. 2017;65(9):1397–406. [DOI] [PubMed] [Google Scholar]
- 22.Mendoza-Fernandez V, Andrew RD, Barajas-Lopez C. Interferon-alpha inhibits long-term potentiation and unmasks a long-term depression in the rat hippocampus. Brain Res 2000;885(1):14–24. [DOI] [PubMed] [Google Scholar]
- 23.Schrott LM, Crnic LS. Increased anxiety behaviors in autoimmune mice. Behav Neurosci 1996;110(3):492–502. [DOI] [PubMed] [Google Scholar]
- 24.Prinz M, Knobeloch KP. Type I interferons as ambiguous modulators of chronic inflammation in the central nervous system. Front Immunol 2012;3:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vattakatuchery JJ, Rickards H, Cavanna AE. Pathogenic mechanisms of depression in multiple sclerosis. J Neuropsychiatry Clin Neurosci 2011;23(3):261–76. [DOI] [PubMed] [Google Scholar]
- 26.Dowell NG, Cooper EA, Tibble J, Voon V, Critchley HD, Cercignani M, et al. Acute Changes in Striatal Microstructure Predict the Development of Interferon-Alpha Induced Fatigue. Biol Psychiatry. 2016;79(4):320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNutt MD, Liu S, Manatunga A, Royster EB, Raison CL, Woolwine BJ, et al. Neurobehavioral effects of interferon-alpha in patients with hepatitis-C: symptom dimensions and responsiveness to paroxetine. Neuropsychopharmacology. 2012;37(6):1444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alba Pale L, Leon Caballero J, Samso Buxareu B, Salgado Serrano P, Perez Sola V. Systematic review of depression in patients with multiple sclerosis and its relationship to interferonbeta treatment. Mult Scler Relat Disord 2017;17:138–43. [DOI] [PubMed] [Google Scholar]
- 29.Hoyo-Becerra C, Schlaak JF, Hermann DM. Insights from interferon-alpha-related depression for the pathogenesis of depression associated with inflammation. Brain Behav Immun 2014;42:222–31. [DOI] [PubMed] [Google Scholar]
- 30.Coch C, Viviani R, Breitfeld J, Munzer K, Dassler-Plencker J, Holdenrieder S, et al. Interferon-beta-induced changes in neuroimaging phenotypes of appetitive motivation and reactivity to emotional salience. Neuroimage Clin 2019;24:102020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mostafavi S, Battle A, Zhu X, Potash JB, Weissman MM, Shi J, et al. Type I interferon signaling genes in recurrent major depression: increased expression detected by whole-blood RNA sequencing. Mol Psychiatry. 2014;19(12):1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng LS, Hitoshi S, Kaneko N, Takao K, Miyakawa T, Tanaka Y, et al. Mechanisms for interferon-alpha-induced depression and neural stem cell dysfunction. Stem Cell Reports. 2014;3(1):73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Campbell IL, Zhang H. Systemic interferon-alpha regulates interferon-stimulated genes in the central nervous system. Mol Psychiatry. 2008;13(3):293–301. [DOI] [PubMed] [Google Scholar]
- 34.Bian Y, Pan Z, Hou Z, Huang C, Li W, Zhao B. Learning, memory, and glial cell changes following recovery from chronic unpredictable stress. Brain Res Bull. 2012;88(5):471–6. [DOI] [PubMed] [Google Scholar]
- 35.Kopp BL, Wick D, Herman JP. Differential effects of homotypic vs. heterotypic chronic stress regimens on microglial activation in the prefrontal cortex. Physiol Behav 2013;122:246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.London A, Cohen M, Schwartz M. Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Front Cell Neurosci 2013;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 2012;12(9):623–35. [DOI] [PubMed] [Google Scholar]
- 38.Goldmann T, Zeller N, Raasch J, Kierdorf K, Frenzel K, Ketscher L, et al. USP18 lack in microglia causes destructive interferonopathy of the mouse brain. EMBO J 2015;34(12):1612–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bialas AR, Presumey J, Das A, van der Poel CE, Lapchak PH, Mesin L, et al. Microglia-dependent synapse loss in type I interferon-mediated lupus. Nature. 2017;546(7659):539–43. [DOI] [PubMed] [Google Scholar]
- 40.Lynch MA. The multifaceted profile of activated microglia. Mol Neurobiol 2009;40(2):139–56. [DOI] [PubMed] [Google Scholar]
- 41.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–78. [DOI] [PubMed] [Google Scholar]
- 42.Krugers HJ, Lucassen PJ, Karst H, Joels M. Chronic stress effects on hippocampal structure and synaptic function: relevance for depression and normalization by anti-glucocorticoid treatment. Front Synaptic Neurosci 2010;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Csabai D, Wiborg O, Czeh B. Reduced Synapse and Axon Numbers in the Prefrontal Cortex of Rats Subjected to a Chronic Stress Model for Depression. Front Cell Neurosci 2018;12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suvrathan A, Tomar A, Chattarji S. Effects of chronic and acute stress on rat behaviour in the forced-swim test. Stress. 2010;13(6):533–40. [DOI] [PubMed] [Google Scholar]
- 45.Ulloa JL, Castaneda P, Berrios C, Diaz-Veliz G, Mora S, Bravo JA, et al. Comparison of the antidepressant sertraline on differential depression-like behaviors elicited by restraint stress and repeated corticosterone administration. Pharmacol Biochem Behav 2010;97(2):213–21. [DOI] [PubMed] [Google Scholar]
- 46.Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(1):112–9. [DOI] [PubMed] [Google Scholar]
- 47.Bogdanova OV, Kanekar S, D’Anci KE, Renshaw PF. Factors influencing behavior in the forced swim test. Physiol Behav 2013;118:227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henckens MJ, van der Marel K, van der Toorn A, Pillai AG, Fernandez G, Dijkhuizen RM, et al. Stress-induced alterations in large-scale functional networks of the rodent brain. Neuroimage. 2015;105:312–22. [DOI] [PubMed] [Google Scholar]
- 49.Seewoo BJ, Hennessy LA, Feindel KW, Etherington SJ, Croarkin PE, Rodger J. Validation of Chronic Restraint Stress Model in Young Adult Rats for the Study of Depression Using Longitudinal Multimodal MR Imaging. eNeuro. 2020;7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alemu JL, Elberling F, Azam B, Pakkenberg B, Olesen MV. Electroconvulsive treatment prevents chronic restraint stress-induced atrophy of the hippocampal formation-A stereological study. Brain Behav 2019;9(2):e01195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park MJ, Seo BA, Lee B, Shin HS, Kang MG. Stress-induced changes in social dominance are scaled by AMPA-type glutamate receptor phosphorylation in the medial prefrontal cortex. Sci Rep 2018;8(1):15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Zhuang X, Gou L, Ling X, Tian X, Liu L, et al. Protective effects of nizofenone administration on the cognitive impairments induced by chronic restraint stress in mice. Pharmacol Biochem Behav 2013;103(3):474–80. [DOI] [PubMed] [Google Scholar]
- 53.Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav 2003;43(1):48–59. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Kan H, Yin Y, Wu W, Hu W, Wang M, et al. Protective effects of ginsenoside Rg1 on chronic restraint stress induced learning and memory impairments in male mice. Pharmacol Biochem Behav 2014;120:73–81. [DOI] [PubMed] [Google Scholar]
- 55.Huang P, Li C, Fu T, Zhao D, Yi Z, Lu Q, et al. Flupirtine attenuates chronic restraint stress-induced cognitive deficits and hippocampal apoptosis in male mice. Behav Brain Res 2015;288:1–10. [DOI] [PubMed] [Google Scholar]
- 56.Woo H, Hong CJ, Jung S, Choe S, Yu SW. Chronic restraint stress induces hippocampal memory deficits by impairing insulin signaling. Mol Brain. 2018;11(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee T, Jarome T, Li SJ, Kim JJ, Helmstetter FJ. Chronic stress selectively reduces hippocampal volume in rats: a longitudinal magnetic resonance imaging study. Neuroreport 2009;20(17):1554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Y, Mao Y, Zhou D, Hu X, Wang J, Ma Y. Environmental enrichment and chronic restraint stress in ICR mice: effects on prepulse inhibition of startle and Y-maze spatial recognition memory. Behav Brain Res 2010;212(1):49–55. [DOI] [PubMed] [Google Scholar]
- 59.Sanz H, Aponte JJ, Harezlak J, Dong Y, Ayestaran A, Nhabomba A, et al. drLumi: An open-source package to manage data, calibrate, and conduct quality control of multiplex bead-based immunoassays data analysis. PLoS One. 2017;12(11):e0187901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pandya CD, Hoda N, Crider A, Peter D, Kutiyanawalla A, Kumar S, et al. Transglutaminase 2 overexpression induces depressive-like behavior and impaired TrkB signaling in mice. Mol Psychiatry. 2017;22(5):745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci 1996;110(6):1321–34. [DOI] [PubMed] [Google Scholar]
- 62.Wright RL, Conrad CD. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8(2):151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soper A, Kimura I, Nagaoka S, Konno Y, Yamamoto K, Koyanagi Y, et al. Type I Interferon Responses by HIV-1 Infection: Association with Disease Progression and Control. Front Immunol 2017;8:1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun 2007;21(2):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–57. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord 2012;139(3):230–9. [DOI] [PubMed] [Google Scholar]
- 68.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163(9):1630–3. [DOI] [PubMed] [Google Scholar]
- 69.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun 2007;21(7):901–12. [DOI] [PubMed] [Google Scholar]
- 70.Manikowska K, Mikolajczyk M, Mikolajczak PL, Bobkiewicz-Kozlowska T. The influence of mianserin on TNF-alpha, IL-6 and IL-10 serum levels in rats under chronic mild stress. Pharmacol Rep 2014;66(1):22–7. [DOI] [PubMed] [Google Scholar]
- 71.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 2009;27:451–83. [DOI] [PubMed] [Google Scholar]
- 72.Fernandes A, Miller-Fleming L, Pais TF. Microglia and inflammation: conspiracy, controversy or control? Cell Mol Life Sci 2014;71(20):3969–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003;3(1):23–35. [DOI] [PubMed] [Google Scholar]
- 74.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, et al. Genomic analysis of reactive astrogliosis. J Neurosci 2012;32(18):6391–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Presumey J, Bialas AR, Carroll MC. Complement System in Neural Synapse Elimination in Development and Disease. Adv Immunol 2017;135:53–79. [DOI] [PubMed] [Google Scholar]
- 77.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol 2013;190(8):3831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Q, Timberlake MA 2nd, Prall K, Dwivedi Y. The recent progress in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller ES, Apple CG, Kannan KB, Funk ZM, Plazas JM, Efron PA, et al. Chronic stress induces persistent low-grade inflammation. Am J Surg 2019;218(4):677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu Y, Klomparens EA, Guo S, Geng X. Neuroinflammation caused by mental stress: the effect of chronic restraint stress and acute repeated social defeat stress in mice. Neurol Res 2019;41(8):762–9. [DOI] [PubMed] [Google Scholar]
- 81.Walker FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 2013;14(11):1262–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lehmann MLWT, Poffenberger CN and Herkenham M. The Behavioral Sequelae of Social Defeat Require Microglia and Are Driven by Oxidative Stress in Mice. J Neurosci 2019;39(28):5594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hinwood M, Morandini J, Day TA, Walker FR. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex. 2012;22(6):1442–54. [DOI] [PubMed] [Google Scholar]
- 84.O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104(5):1604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Molnarfi N, Gruaz L, Dayer JM, Burger D. Opposite effects of IFN beta on cytokine homeostasis in LPS- and T cell contact-activated human monocytes. J Neuroimmunol 2004;146(1–2):76–83. [DOI] [PubMed] [Google Scholar]
- 86.Taniguchi T, Takaoka A. A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol 2001;2(5):378–86. [DOI] [PubMed] [Google Scholar]
- 87.Santha P, Veszelka S, Hoyk Z, Meszaros M, Walter FR, Toth AE, et al. Restraint Stress-Induced Morphological Changes at the Blood-Brain Barrier in Adult Rats. Front Mol Neurosci 2015;8:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blank T, Detje CN, Spiess A, Hagemeyer N, Brendecke SM, Wolfart J, et al. Brain Endothelial- and Epithelial-Specific Interferon Receptor Chain 1 Drives Virus-Induced Sickness Behavior and Cognitive Impairment. Immunity. 2016;44(4):901–12. [DOI] [PubMed] [Google Scholar]
- 89.Barner MMM, Brombacher Fand Kopf M. Differences between IL-4R alpha-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr Biol 1998;8(11):669–72. [DOI] [PubMed] [Google Scholar]
- 90.Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol 2010;87(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, et al. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation. 2013;36(4):921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med 2014;211(8):1533–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci 2014;17(1):131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu T, Dejanovic B, Gandham VD, Gogineni A, Edmonds R, Schauer S, et al. Complement C3 Is Activated in Human AD Brain and Is Required for Neurodegeneration in Mouse Models of Amyloidosis and Tauopathy. Cell Rep 2019;28(8):2111–23 e6. [DOI] [PubMed] [Google Scholar]
- 95.Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352(6286):712–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tenner AJ, Stevens B, Woodruff TM. New tricks for an ancient system: Physiological and pathological roles of complement in the CNS. Mol Immunol 2018;102:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morgan BP. Complement in the pathogenesis of Alzheimer’s disease. Semin Immunopathol 2018;40(1):113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hajishengallis G, Reis ES, Mastellos DC, Ricklin D, Lambris JD. Novel mechanisms and functions of complement. Nat Immunol 2017;18(12):1288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Whalley K. Neurodegenerative disease: Complement mediates pathological pruning. Nat Rev Neurosci 2016;17(6):336. [DOI] [PubMed] [Google Scholar]
- 100.Owens T, Khorooshi R, Wlodarczyk A, Asgari N. Interferons in the central nervous system: a few instruments play many tunes. Glia. 2014;62(3):339–55. [DOI] [PubMed] [Google Scholar]
- 101.Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36(2):166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deczkowska A, Baruch K, Schwartz M. Type I/II Interferon Balance in the Regulation of Brain Physiology and Pathology. Trends Immunol 2016;37(3):181–92. [DOI] [PubMed] [Google Scholar]
- 103.Arscott WT, Soltys J, Knight J, Mao-Draayer Y. Interferon beta-1b directly modulates human neural stem/progenitor cell fate. Brain Res 2011;1413:1–8. [DOI] [PubMed] [Google Scholar]
- 104.Ejlerskov P, Hultberg JG, Wang J, Carlsson R, Ambjorn M, Kuss M, et al. Lack of Neuronal IFN-beta-IFNAR Causes Lewy Body- and Parkinson’s Disease-like Dementia. Cell. 2015;163(2):324–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lacy M, Hauser M, Pliskin N, Assuras S, Valentine MO, Reder A. The effects of long-term interferon-beta-1b treatment on cognitive functioning in multiple sclerosis: a 16-year longitudinal study. Mult Scler 2013;19(13):1765–72. [DOI] [PubMed] [Google Scholar]
- 106.Reder AT, Feng X. How type I interferons work in multiple sclerosis and other diseases: some unexpected mechanisms. J Interferon Cytokine Res 2014;34(8):589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kremenchutzky M, Morrow S, Rush C. The safety and efficacy of IFN-beta products for the treatment of multiple sclerosis. Expert Opin Drug Saf 2007;6(3):279–88. [DOI] [PubMed] [Google Scholar]
- 108.Lugaresi A, Rottoli MR, Patti F. Fostering adherence to injectable disease-modifying therapies in multiple sclerosis. Expert Rev Neurother 2014;14(9):1029–42. [DOI] [PubMed] [Google Scholar]
- 109.Reder AT, Oger JF, Kappos L, O’Connor P, Rametta M. Short-term and long-term safety and tolerability of interferon beta-1b in multiple sclerosis. Mult Scler Relat Disord 2014;3(3):294–302. [DOI] [PubMed] [Google Scholar]
- 110.Ziemssen T. Multiple sclerosis beyond EDSS: depression and fatigue. J Neurol Sci 2009;277 Suppl 1:S37–41. [DOI] [PubMed] [Google Scholar]
- 111.Ben-Yehuda H, Matcovitch-Natan O, Kertser A, Spinrad A, Prinz M, Amit I, et al. Maternal Type-I interferon signaling adversely affects the microglia and the behavior of the offspring accompanied by increased sensitivity to stress. Mol Psychiatry. 2020;25(5):1050–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goodbourn S. The regulation of beta-interferon gene expression. Semin Cancer Biol 1990;1(1):89–95. [PubMed] [Google Scholar]
- 113.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol 2008;26:535–84. [DOI] [PubMed] [Google Scholar]
- 114.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107(6):2669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;67(12):1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang W, Rosenkranz JA. Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience. 2012;226:459–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Munshi S, Rosenkranz JA. Effects of Peripheral Immune Challenge on In Vivo Firing of Basolateral Amygdala Neurons in Adult Male Rats. Neuroscience. 2018;390:174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Munshi S, Loh MK, Ferrara N, DeJoseph MR, Ritger A, Padival M, et al. Repeated stress induces a pro-inflammatory state, increases amygdala neuronal and microglial activation, and causes anxiety in adult male rats. Brain Behav Immun 2020;84:180–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37(9):1491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Azzinnari D, Sigrist H, Staehli S, Palme R, Hildebrandt T, Leparc G, et al. Mouse social stress induces increased fear conditioning, helplessness and fatigue to physical challenge together with markers of altered immune and dopamine function. Neuropharmacology. 2014;85:328–41. [DOI] [PubMed] [Google Scholar]
- 121.Ray B, Gaskins DL, Sajdyk TJ, Spence JP, Fitz SD, Shekhar A, et al. Restraint stress and repeated corticotrophin-releasing factor receptor activation in the amygdala both increase amyloid-beta precursor protein and amyloid-beta peptide but have divergent effects on brain-derived neurotrophic factor and pre-synaptic proteins in the prefrontal cortex of rats. Neuroscience. 2011;184:139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.