Abstract
Biological diversity is the basis for, and an indicator of biosphere integrity. Together with climate change, its loss is one of the two most important planetary boundaries. A halt in biodiversity loss is one of the UN Sustainable Development Goals. Current changes in biodiversity in the vast landmass of Siberia are at an initial stage of inventory, even though the Siberian environment is experiencing rapid climate change, weather extremes and transformation of land use and management. Biodiversity changes affect traditional land use by Indigenous People and multiple ecosystem services with implications for local and national economies. Here we review and analyse a large number of scientific publications, which are little known outside Russia, and we provide insights into Siberian biodiversity issues for the wider international research community. Case studies are presented on biodiversity changes for insect pests, fish, amphibians and reptiles, birds, mammals and steppe vegetation, and we discuss their causes and consequences.
Supplementary information
The online version of this article (10.1007/s13280-021-01570-6) contains supplementary material, which is available to authorized users.
Keywords: Biodiversity change, Climate change, Ecosystem services, Land cover change, Siberia
Introduction
The Planetary Boundaries concept developed by the Stockholm Resilience Centre identifies nine global priorities relating to human-induced changes to the environment (Rockström et al. 2009). The science shows that these nine processes and systems regulate the stability and resilience of the Earth System—the interactions of land, ocean, atmosphere and life that together provide conditions upon which our societies depend. Four of the nine planetary boundaries have now been crossed because of human activity: climate change, loss of biosphere integrity, land-system change and altered biogeochemical cycles (phosphorus and nitrogen). Two of these, climate change and biosphere integrity, are “core boundaries”.
Halting biodiversity loss is also a UN Sustainable Development Goal (United_Nations 2019), one that is strongly linked to all other SDGs. Keeping ecosystems resilient and safeguarding our planet’s biodiversity is fundamental to poverty eradication and human health and wellbeing. These biodiversity-dependent ecosystem services include the provision of potable water, food and fibres, soil fertility, maintenance of the genetic databank of biodiversity, climate regulation, and recreational and aesthetic values among others. Biodiversity and cultural diversity are intricately linked.
Siberia is a unique region representing some 13 million km2 in the northeast of Eurasia (Shumilova 1962) which is the largest of the six continents on Earth. Siberia is bounded from the west by the Ural Mountains, from the east by the Pacific Ocean, from the north by the Arctic Ocean and from the south by the borders of Kazakhstan, Mongolia and China. In this vast territory, with a length of more than 5000 km from the west to the east, climatic and ecosystem gradients are expressed more sharply and dramatically in comparison with other regions of the planet, where they are mitigated by the warming effect of the oceans. The degree of continentality (defined as the difference between the monthly average temperature of the hottest (July) and coldest (January) months of the year) of Siberia reaches 92.2 (Bezrukov and Korytny 2009) and one of the coldest places of the Northern Hemisphere is located here. The degree of continentality of climate results in four meridional sectors: Western Siberia, Middle Siberia, Eastern Siberia and the Far East (Shumilova 1962).
Siberia’s land mass is continuous and vast also from north to south, exhibiting a gradient of natural zones connected by major rivers such as the Ob which stretches for 2500 km. The natural zones range from polar desert and Arctic tundra in the north to the steppes and even semi-deserts in the south with tundra, forest-tundra, taiga (northern, middle, southern), sub-taiga, forest-steppe, steppe and semi-desert in-between (Shumilova 1962). In addition, large mountain ranges, especially in the Middle and East Siberian sectors, as well as the presence of the world’s largest wetland in Western Siberia, contribute to the great range of Siberian environments and habitat diversity. Land use ranges from open, range-land use by nomads in the north and south, to agriculture, forestry, resource extraction and urbanization which are present to varying degrees in the separate provinces of Siberia. Superimposed on these natural gradients are dynamic changes in climate and land use that affect the boundaries and interactions among geographical regions and their biodiversity.
Thousands of biodiversity studies have been carried out in Siberia (and new species are being discovered) but they are devoted to the study of individual taxonomic groups and their dynamics (Camacho et al. 2020). Consequently, an initial inventory of the biodiversity of this vast territory exists (Zyryanova et al. 2007). Also, there is a number of studies focussed on individual geographical regions. However, there are apparently no studies on various drivers of biodiversity changes that link them together and build an understanding of the possible impact of these processes on land-use practices throughout Siberia. This paper addresses this important gap.
Our main aim is to present a selection of case studies representing the most significant examples of biodiversity changes in Siberia, and particularly, but not exclusively, in Western Siberia. We present a discussion of their environmental, land-use and social transformation causes, and wide-ranging consequences. The case studies are based on a review and analysis of a vast literature (little known outside Russia) and our own data sets. Another aim is to increase global awareness of important processes in Siberia and Russian studies as Siberia is grossly under-represented in western literature (Callaghan et al., unpubl.). To fulfil these aims, we bring together the joint expertise of 25 researchers with detailed knowledge of their fields and data gained over decades of field work in Siberia. The importance of such local knowledge is acknowledged widely today, e.g. by the Arctic Council (https://www.arctic-council.org/en/explore/topics/arctic-peoples/). We support the various case studies with additional, relevant references in online Supplementary Material (SS1). This paper also contributes to a range of studies dedicated to Siberian Environmental Change (Callaghan et al. 2021). We start by presenting the major drivers of biodiversity change in Siberia.
Major drivers of biodiversity change
Climate change
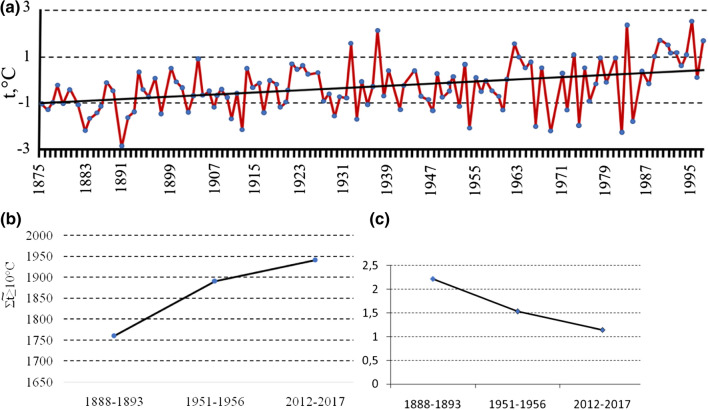
The Arctic is warming twice as fast as the Earth as a whole (Overland et al. 2016) and during the last 10 years, the Russian Arctic has been warming even four times faster (Yurganov 2020). In Siberia, the linear average annual temperature increase was about 0.76 °C in each decade (ROSHYDROMET 2019), and total warming over 50 years (from 1968 to 2017), recorded at Dikson, shows an increase in average annual temperature of almost 4 °C (Callaghan et al. 2020). Siberian meteorological archives for 140 years (www.pogodaiklimat.ru —accessed March 13, 2020) clearly demonstrate an increase in mean annual and growing season temperatures (Fig. 1a, b), as well as a decrease in growing season precipitation (Fig. 1c), particularly in the southern steppes and forest-steppes. Locally, however, in the Tuva region, evidence suggests soil moisture is increasing. In the forest zone (taiga) and in the northern regions (tundra), humidity is increasing, and by 2050 the amount of precipitation is predicted to rise by 10–15% compared to the modern period (Gruza 2004).
Fig. 1.
Climate changes in Tomsk, Western Siberia, over 120 years. a Changes in the average annual temperature, b increases in biologically “active” temperatures, Change 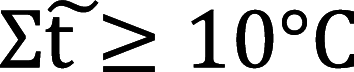 , c decrease in the Hydrothermal coefficient (HTC).
, c decrease in the Hydrothermal coefficient (HTC). 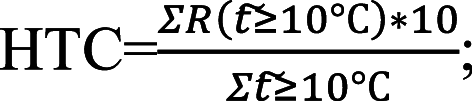
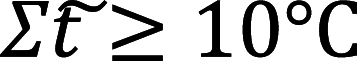 —is the sum of daily average temperatures above or equal to 10 °C.
—is the sum of daily average temperatures above or equal to 10 °C.  —is the amount of precipitation at an average daily temperature above or equal to 10 °C
—is the amount of precipitation at an average daily temperature above or equal to 10 °C
The most serious consequences of the observed and predicted climate change for the Russian Federation are increase in dangerous hydrometeorological phenomena as a result of increasing instability of the climate system (Golitsyn and Vasiliev 2019): heat waves or cold weather, extreme rainfall and its consequences (floods, landslides, erosion of roads, dam failures, soil failures, pollution of drinking water), and drought and its consequences (for example, crop loss and increased fire hazard). The consequences of long-term trends in temperature increase lead to an increase in permafrost thaw (Anisimov and Zimov 2021) with a decrease in the stability of infrastructures (IPCC 2019b), and the spread of vector-borne diseases (Everett 2020) and agricultural and forest pests (Popova and Popov 2019).
This powerful climate change in Siberia is the most significant driver of dramatic changes in natural landscapes, vulnerable ecosystems and biodiversity (Kirpotin et al. 2009). An increasing list of documented climate-driven changes in ecosystems and populations is being reported, showing potentially significant impacts on ecological processes and ecosystem services that support basic life support systems (EPA 2017; Malhi et al. 2020) (online Supplementary Material SS1). These changes affect land-use practices that also respond to changes in society, resource extraction and infrastructure development (Oliver and Morecroft 2014). Perhaps the most important changes from a Siberian biodiversity perspective are a longer and warmer growing season, warmer and shorter winters, changes in snow duration and depth, aridization, extreme weather events in summer and winter and increasing active layer depths.
The impacts of climate change on the diversity of natural ecosystems will significantly change the distribution of bioclimatic regions in Siberia by the end of the century. In a drier climate, the forest region will shrink by half. The border between forests and steppes in Central Siberia will shift northward by 10° latitude and the area of steppes in the south of Siberia will increase by 30%, while the area of desert steppes will double (ROSHYDROMET 2014). Predictions suggest a 60–65% increase in the area of dark-conifer “chern” taiga (dominated by fir (Abies sibirica), Siberian pine (Pinus sibirica) and aspen (Populus tremula) of the southern Siberian mountains (Kokorin 2011). Natural afforestation in inter-mountain depressions (steppe ecosystems) in the Tuva region has been observed already (Kirpotin et al., unpubl, see below). Transformations of bioclimatic regions also include a reduction in mountain-tundra vegetation and biodiversity (Sergienko and Konstantinov 2016).
Forest fires, that have natural cycles, have increased in frequency and area due to human factors and temperature increases (Kharuk et al. 2021). Their consequences include rejuvenation of vegetation, but their effects on biodiversity of terrestrial vertebrates are not well understood. Fires, especially large ones, lead to a change in species and quantitative composition of animal populations. Some species, such as bears, are displaced to near settlements (Shaduyko 2019) while species of open spaces move to former forested areas.
In the Arctic, climate warming is leading to “greening” and “shrubification” of the tundra, an increase in phytomass reserves and a decrease in lichen mass (Lavrinenko and Lavrinenko 2013; Myers-Smith et al. 2020). Consequently, there is degradation of reindeer pastures where reindeer lichens grow only slowly. Degradation of pastures is especially pronounced along the main paths of the seasonal migrations of Taimyr reindeer (Rangifer tarandus sibiricus), and particularly where animals gather before guiding hedges, and overgraze and trample lichens. In contrast to greening, some areas of tundra have remained stable over several decades (Matveyeva and Zanokha 2013).
Land-use change
Drastic changes occurred in the land-use system in Siberia after the collapse of the Soviet Union. Agricultural practices changed dramatically, hundreds of small villages disappeared (Krivov et al. 2020) and huge areas of croplands and pastures were abandoned and began to revert to forest and scrublands (Gutman and Radeloff 2017; Prishchepov et al. 2012). In addition, tens of millions of hectares of Siberian arable land have been withdrawn from use due to the negative impacts of natural and anthropogenic processes (e.g. water and wind erosion). Although the removal of vast areas from agriculture has negative social and economic consequences (Edelgeriev 2019), reversion to forest has clear potential to mitigate climate change through increased soil formation and sequestration of atmospheric CO2, while providing more natural habitats to sustain biodiversity. However, such increases in forest areas combined with climate warming and deterioration of the effective Soviet forestry system are leading to an increase of forest fire frequency and intensity, which has doubled during the last decade (Kharuk et al. 2021). Drainage of wetlands and land reclamation, and construction of clearings and roads, also add to habitat loss and biodiversity change.
Resource extraction and development
Direct human impacts on biodiversity arise from infrastructure development and unintentional release of pollutants. The most important factor adversely affecting the ichthyofauna of Western Siberia is the construction of hydroelectric dams in the upper reaches of the Ob and Irtysh rivers. The resulting drastic decrease of water level during spring floods leads to a reduction of spawning areas and abundance of most spring-spawning fish species such as sterlet (Acipenser ruthenus), Northern pike (Esox Lucius), ide (Leuciscus idus), roach (Rutilus rutilus) and European perch (Perca fluviatilis) (Popkov 1995). In addition, the dam of the Novosibirsk hydroelectric station blocked the migration routes of semi-migratory fish species (Siberian sturgeon (Acipenser baerii) and nelma (Stenodus nelma), decreasing their spawning areas by 40% (Petkevich 1952) and natural reproduction in the basin.
Development effects on biodiversity include incidents such as oil spills (Hese and Schmullius 2008). Other impacts are less clear, for example, the growth of populations of reindeer predators is facilitated by industrial waste thrown in the tundra (Kolpashchikov et al. 2019). Reindeer winter pastures are degraded by pollution from heavy metals (e.g. cadmium, copper) resulting from enterprises of the Norilsk industrial zone and they are also damaged by heavy and tracked vehicles used for geological work. The cadmium content in the reindeer liver in the winter pastures of East Taimyr exceeded 1.2 of the maximum permissible concentrations (Kochkarev and Mikhailov 2016). A great influence on fish is exerted by the pollution of natural water, which, in particular, caused the disappearance of tugun (Coregonus tugun) from the Tom river (Popov and Trifonova 2007). Establishment of the raccoon dog (Nyctereutes procyonoides) is probably associated with intensive development of oil and gas fields (construction of pipelines, shift camps, access roads and power lines), and particularly with the growth of refuse dumping grounds. The common lizard is colonizing villages and disturbed lands (power lines, landfills, road embankments, etc.) in high latitudes and has reached greater population numbers than that in its natural environments. Frogs use technological ponds and quarries for spawning, where in the absence of predators, the success of their breeding is often higher than in natural water bodies.
Hunting, poaching, introductions, exterminations and conservation
Regulated and unregulated hunting are the greatest direct anthropogenic factors affecting the size of the wild Taimyr reindeer population. Expert estimates of poaching suggest 60 to 70 thousand wild reindeer are shot annually, which is more than 2 times higher than the hunting quota. Added to this are increased quotas for Indigenous people totaling 80 thousand reindeer per year. This is several times higher than the scientifically based hunting quota (Kolpashchikov et al. 2019). The catch from IUU (illegal, unreported, unregulated) fishing of economically valuable fish species is still not estimated in Western Siberia, although the negative impact of it is clear (Krokhalevsky et al. 2018). Hunting outside of Russia affects migrant bird populations. Over the past 15–25 years, the number of yellow-breasted banting (Ocyris aureola) in the southern taiga of the Tomsk region has decreased from 250–300 individuals per km2 to complete extirpation (Gureev et al. 2019), possibly because of capture by “spider web” nets on flight paths and during wintering in China and Southeast Asia (in addition to negative effect of climate change on their natural habitats). The badger (Meles leucurus) population also disappeared as a result of extermination and, together with sable (Martes zibellina), the pine marten (M. martes), the wild boar (Sus scrofa) and roe deer (Capreolus pygargus) are restoring their former distributional areas (Ivanova et al. 2016).
Both currently inappropriate conservation regulations and deteriorating control of some animal populations can lead to unwanted impacts on target animal populations. Lack of control of wolf populations is decreasing the wild Taimyr reindeer population leading to the deaths of 40–50 thousand reindeer annually (Kolpashchikov et al. 2019) while regulations to conserve beaver populations combined with lack of economic incentives to hunt them have led to population growth (Krivov et al. 2020) and adverse environmental impacts (Popkov et al. 2018). Many fish populations are changing due to accidental or intentional introductions into the water bodies of Western Siberia.
Deforestation
Russia contains the largest area of natural forests in the world, covering 49% of Russia’s landmass and 815 million hectares, i.e. 23% of the planet’s total forest area (FAO 2020). The greatest forest cover is in the Irkutsk region of Siberia, covering 82.6% of the territory (69.4 million hectares) (Kravchuk 2020).
Much of Russia’s forest area is experiencing rapid anthropogenic deforestation in addition to damage from insect pests, forest fires and wind-throw. The development of industry has cleared tens of millions of hectares of forests through felling, flooding and industrial emissions (Bryukhanov 2009). From 2001 to 2019, Russia lost 64 million hectares of relative tree cover, equivalent to an 8.4% decrease since 2000 and 17% of the global total. In 2018 alone, Russia lost 5.6 million hectares of tree cover (Yorke 2020).
The Irkutsk and Krasnoyarsk regions experience the largest volume of logging (70% of that in Siberia). In 2008, 19.5 million m3 were harvested in the Irkutsk region, and 9.5 million m3 in the Krasnoyarsk territory (Bryukhanov 2009). However, there is widespread illegal logging. According to the Federal Forestry Agency, the most acute problem of illegal procurement is in Siberia. In 2019, in the Irkutsk Region, Krasnoyarsk and Trans-Baikal Territories, the total volume of illegal logging exceeded 650 thousand m3 (Shmatkov 2020).
Illegal deforestation has reached crisis proportions on the valuable temperate hardwoods in the Russian Far East. In the period 2004–2011, the volume of Mongolian oak (Quercus mongolica) (the most valuable hardwood species) logged for export to China exceeded authorized logging volumes by 2–4 times (WWF Russia 2013).
Although forest thinning or narrow linear felling can increase biodiversity in specific areas due to the ecotone effect, in general, logging, especially illegal logging, has a negative impact on biodiversity. This impact affects the forest tree species and the general biodiversity of the forest ecosystems. The value to society of non-timber forest resources is significant. The harvest of wild berries, nuts and mushrooms in Russia is measured in millions of tons and in certain categories of forests, the economy of these resources exceeds the value of wood. However, most of these resources are outside commercial reach (MNRE 2014).
Far Eastern forest biodiversity is especially vulnerable to deforestation. These forests are characterized by the highest biodiversity, including endemic forest-forming species; they are in close proximity to the border with China, the main buyer of illegal timber; and they are home to many rare species of animals such as the Amur tiger (Panthera tigris altaica) and the Far Eastern Leopard (Panthera pardus orientalis) (Vandergert and Newell 2003; WWF Russia 2013). Other regions of Siberia, especially the Irkutsk and Krasnoyarsk territories, also risk serious biodiversity loss due to large-scale logging (Bryukhanov 2009).
Changes in biodiversity in siberia
Insect pests
The responses of insects to ongoing climate change, particularly warming, are extremely diverse due to many reasons. These responses can be divided into four main categories: changes in geographic range, population dynamics/life cycle, trophic interactions and community structure (Musolin 2007). Here, we review the literature and evaluate the climate change-induced responses of the most dangerous agricultural and forestry pests in Siberia (Fig. 2) which have great environmental and socio-economic importance, and contribute to the species, population and ecosystem biodiversity of the region.
Fig. 2.
Insect pests and the damage they cause. a Adult and b larvae of Colorado beetle (photo by © V. Alekseev, used with permission), c larvae of Siberian moth photo by © V.V. Remorov, used with permission). d Mass mortality of Pinus sibirica trees in its outbreak focus. e Adult of four-eyed fir bark beetle and f damaged Abies sibirica forests. g Adult of small spruce bark beetle and h dead Pinus sibirica stand (photos by I.A. Kerchev)
Colorado beetle
(Leptinotarsa decemlineata Say) is a global pest of potatoes and other Solanaceae plants. The pest damages potatoes both on big agricultural farms and on the personal, subsidiary farms of the local Siberian people, for which this crop is one of the traditional foods. A wide-scale spreading of the pest is facilitated by climate changes such as a decrease in soil freezing that facilitates over-wintering of beetles (Babenko 2009). In the early 1980s, as a result of active resettlement of people, the pest reached latitude 56° in the west of Siberia, and now it has spread throughout Western Siberia. Today its northern border is latitude 58°. The early onset of spring observed in recent decades and hot summer temperatures are favourable conditions for two generations of Colorado beetle to develop and further migration is expected.
Siberian moth
(Dendrolimus sibiricus Tschetv, hereafter SM) is the most important defoliator of Siberian “dark coniferous” taiga stands composed of Siberian pine, fir and spruce (Picea obovata Ledeb.). This insect feeds also on needles of larch (Larix spp.), although larches are more resistant to defoliation. The SM outbreak onset is linked with warmer and dryer pre-outbreak years with sum of average daily temperatures exceeding +10 °C equal to 1200 °C (Rozhkov 1965), and decreased ambient humidity. Periodic SM outbreaks (e.g. 2.5 million ha in 1953–1957 (Kolomiets 1957)) were one of the major factors driving taiga forest successions. During the recent panzonal outbreak (2014–2017) in Central Siberia, the stands were damaged over 1.3 million ha, and the weakened trees were then subsequently attacked by bark-beetles. Similar large-scale outbreaks seem no longer feasible in the southern taiga because the pest’s “food base” was exhausted by former outbreaks and human-induced forest fragmentation.
SM outbreaks were historically observed within the latitudinal range of 52–59°N (Kolomiets and Mayer 1963). The northern and alpine SM outbreak ranges are approximated by the sum of active temperatures. Meanwhile, during a recent outbreak, SM surpassed its northern historical boundary by about 50 km (to 60°26′ N latitude; Kharuk et al. 2017). At the current warming rate, SM outbreak boundaries are predicted to shift 150–300 km northward by 2030. The combined impact of pests, deforestation and fires led to widespread degradation of indigenous forests in the southern taiga, where the natural zonal formation of dark coniferous taiga forests is now fragmented. The alpine boundary of SM outbreak range in the Altai-Sayan Mountain region has shifted uphill by about 370 m since the 1950s (Kharuk et al. 2020), leading to degradation of forests that stabilize soils on slopes. The SM range expansion and consequent stand mortality may facilitate wildfire rate in the future as fire frequency and burned area steadily increase (up to ten times) in insect-affected forest stands (Kharuk and Antamoshkina 2017).
Four-eyed fir bark beetle
(Polygraphus proximus Blandf.) (abbreviation FFBB) is an invasive species of Far Eastern origin that was introduced into southern Siberia and the European part of Russia, where it became the most aggressive pest of fir forests and caused their unprecedented degradation (Krivets and Baranchikov 2015). The onset of the FFBB invasion in Siberia is dated to the last quarter of the 20th century (Baranchikov et al. 2014), but it was identified there as recently as only 2008. The area of FFBB covers many and various types of administrative regions in Siberia (the Kemerovo, Tomsk and Novosibirsk Oblasts, the Altai and Krasnoyarsk Krais, and the Khakassia and Altai Republic) (Krivets et al. 2015). Recently, this invasive species was also found in the Baikal region (Bystrov and Antonov 2019). The northern boundary of the FFBB range in Siberia is latitude 59°.
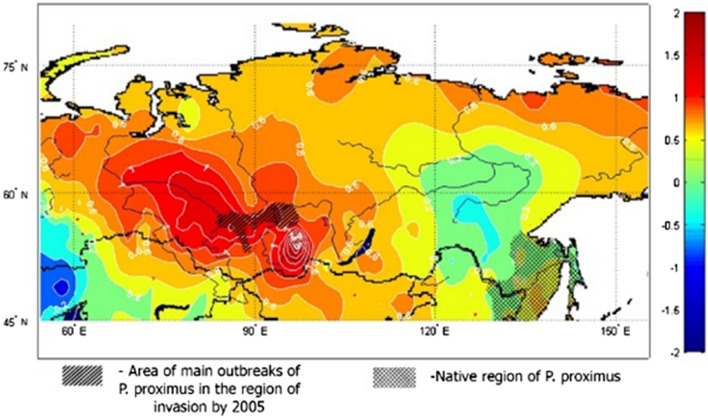
FFBB outbreaks in various regions of Siberia began almost synchronously with the period of intensive warming in 1975–2005 (Kerchev et al. 2017). The temperature trends (0.55 °C per decade) in May show that southern Siberia, where the main fir stands damaged by FFBB are found, was almost the epicentre of warming in Russian Asia (Fig. 3). In this period, precipitation decreased by 5 mm per decade (Ippolitov et al. 2008). Intensive warming, especially at the beginning of the growing season, resulted in two generations of the pests, which led to a dramatic increase of its total population (Kerchev et al. 2017). The temperature rise along with periodic acute droughts made fir even more vulnerable to the SM and bark-beetle attacks. Furthermore, trees weakened by SM defoliation were subsequently killed by FFBB (Kharuk et al. 2017). The resulting fir mortality has been observed over 5% of its habitat within the southern taiga and is increasing. Warming in synergy with pest attacks is also leading to upward displacement of fir from its low and middle elevation range in southern Siberia (Kharuk et al. 2020).
Fig. 3.
The boundaries of the native and invasive changes of Polygraphus proximus shaded on the fields of temperature trends in May for the period 1975–2005 (°C/10 years) in the Asian territory of Russia (according to Ippolitov et al. 2008)
Warming, in synergy with pest attacks, is leading to large economic losses and negative environmental consequences in southern Siberia far beyond the loss of fir forests. Effects on ecosystems and biodiversity include changes in the structure of tree layers and lower tiers of vegetation, soil structure, accumulation of soil organic matter (in litter and humus horizons), the composition and abundance of native stem insects, and the composition and structure of their natural enemy’s communities (Kerchev 2014; Krivets and Kerchev 2016).
Small spruce bark beetle
(Ips amitinus Eichh.) is new alien forest pest in Western Siberia. This originally mountainous Central European species is spread throughout almost all of Europe in the 20th century (Cognato 2015). In recent decades, I. amitinus has been actively expanding in Sweden, Norway and Finland, associated primarily with climatic changes (Økland et al. 2019). In the European part of Russia, I. amitinus is found in the north-western regions, where it reached the north of the Kola Peninsula and Arkhangelsk Oblast (Økland et al. 2019). In 2019, the bark beetle was identified in Western Siberia for the first time (Kerchev et al. 2019), i.e. in the Tomsk and Kemerovo regions, at a distance of more than 3000 km from known locations. In Western Siberia, the alien bark beetle is now abundant on a new host plant—Siberian pine—causing massive death rates of trees in unique pristine forests and areas protected for traditional harvesting of pine nuts. The pest colonizes the upper trunk and branches of standing and windfall trees. Intensive outbreak foci of I. amitinus have been observed in all Siberian pine forests in the north of the Kemerovo region, particularly near the Trans-Siberian Railway that, apparently, was an invasive corridor.
The spread of I. amitinus has been presumably facilitated by dry and hot summers in the southeast of Western Siberia during the last decade and more frequent heavy winter snowfalls, which weaken trees and increase the food supply for the beetles (Kerchev et al. 2019). The total outbreak area in the Kemerovo region in 2019 was more than 1000 ha. From here, the beetles spread to the nearby Tomsk region, where the forest was already weakened by attacks of SM in 2016 and 2017. In 2018–2019, the bark beetle killed thousands of Siberian pine trees over an area of 300 ha in the Luchanovsky forest near Tomsk (Fig. 2d). Currently, the southern regions of the West Siberian taiga are affected by the invader, but the largest ranges of Siberian pine forests are located to the north in the middle taiga, where the invasion can further spread in future decades of climate change.
Fishes
Native fish fauna of Western Siberia
There are about 40 freshwater fish species in Western Siberia (Pavlov and Mochek 2006; Popov 2009). The systematic status of some species is currently being reviewed (Dyldin et al. 2017; Levin et al. 2017), which is likely to lead to a change in the total number of species in the near future, independent of climate change! We review the significant changes that occurred in fish diversity during the 20th century in Western Siberia.
The abundance of the majority of economically valuable species fell i.e. Siberian sturgeon (Acipenser baerii), sterlet (Acipenser ruthenus), muksun (Coregonus muksun), broad whitefish (Coregonus nasus), peled (Coregonus peled), nelma and taimen (Hucho taimen) (Pavlov and Mochek 2006). Also, the range of some species has significantly decreased, for example, the tugun (Coregonus tugun) has disappeared from the Tom and Chulym rivers in the Ob river basin (Popov and Trifonova 2007). The decrease of economically valuable species is also typical for the Yenisei river basin (Vyshegorodtsev 2000; Perepelin et al. 2020), and the rivers of Yakutia (Karpova et al. 2015).
Introduction of non-native species
Throughout the 20th century, not only economically valuable fish species but also non-commercial alien species have been introduced into Western Siberia. In total, 23 non-native freshwater fish species occur in the Ob river basin. At present, 19 of these species occur in natural waters of the region. A few attempts to introduce chum salmon (Oncorhynchus keta), Baikal whitefish (Coregonus migratorius), black buffalo (Ictiobus niger) and black carp (Mylopharyngodon piceus) were unsuccessful. Of the 23 species, two (pink salmon—Oncorhynchus gorbuscha and rainbow trout—Oncorhynchus mykiss) have been caught, but there is no information about their natural reproduction. Eight species (bighead carp—Aristichthys nobilis, silver carp—Hypophthalmichthys molitrix, grass carp—Ctenopharyngodon idella, bigmouth buffalo—Ictiobus cyprinellus, channel catfish—Ictalurus punctatus, brown trout—Salmo trutta, vendace—Coregonus albula and European smelt—Osmerus eperlanus) probably formed local self-reproducing populations, but did not begin self-redistribution. And finally, 9 species (pikeperch—Sander lucioperca, common bream Abramis brama, common carp Cyprinus carpio, sunbleak Leucaspius delineatus, bleak—Alburnus alburnus, topmouth gudgeon—Pseudorasbora parva, Chinese sleeper—Perccottus glenii, Nikolski’s loach—Misgurnus nikolskyi and southern ninespine stickleback—Pungitius platygaster) have been fully naturalized (Interesova 2016). Currently, alien species make up a significant part of fish communities and catches (Fig. 4) in diverse water bodies of the region (Yadrenkina 2012; Interesova and Bogomolova 2013). Sometimes their share in the structure of fish communities reaches 100% (Interesova et al. 2020a). In the east of Siberia there are fewer naturalized alien species of fish: 4 in the Yenisei (Zuev et al. 2016); 2 in the Lena; and none in the rivers Anabar, Olenek, Indigirka and Kolyma (Karpova et al. 2015).
Fig. 4.
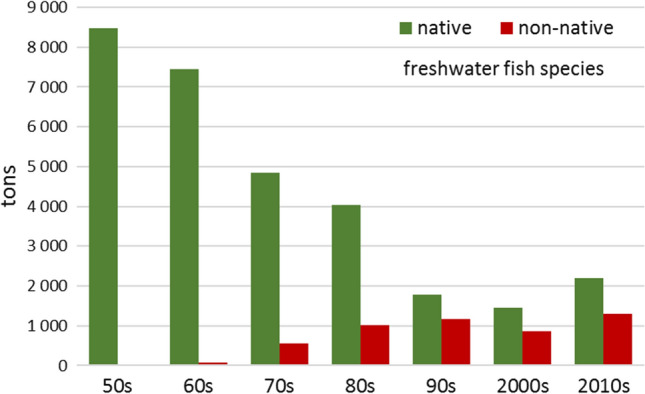
Catches of freshwater fish in the River Ob in the south of Western Siberia (Altai, Novosibirsk, Tomsk and Omsk regions). The graph is derived from various data sources (Popov 2007; Rostovtsev and Interesova 2015; Krokhalevsky et al. 2018)
Current and potential climate change impacts
In scientific publications, little attention is paid to fish fauna in Siberia in the context of climate change. However, in the north of Western Siberia, there is evidence that warming water led to an increase in the growth rates of cyprinids (possibly related to both an increase in the productivity of water bodies and extension of feeding season), and a decrease in reproduction of coregonids due to a reduction in their feeding season resulting from their high demands on the water content of dissolved oxygen (Matkovsky 2019). The warmer climate projected in Siberia (Groisman et al. 2013) will lead to more significant changes of living conditions for fish populations.
Warming can affect the timing of fish spawning. For spring-spawning species, warming will lead to an earlier appearance of juveniles and, consequently, to an extension of the growing season. For autumn-spawning species, warming will lead to a shortening of the feeding season and the total duration of incubation of eggs with unclear consequences. The most likely effect of warming could be the range expansion to the north of non-native fish species, most of which are relatively thermophilic species (Interesova 2016). Perhaps, this is already happening. In the last years, there has been a self-redistribution of some of the non-native species (sunbleak, bleak and Chinese sleeper) to the north (Reshetnikov et al. 2017). For example, there was a sharp increase in the share of non-native common bream in total fish catches in the Lower Ob at the beginning of the 20th century (Matkovsky 2019). This is not surprising as the minimal temperature for spawning of common bream is 10 °C (Popov 2007) and the water temperature in the Lower Ob now, at least in June, stably reaches this value.
The risk of increased invasiveness of non-native fishes in the River Ob basin is high (Interesova et al. 2020b). As invasive fish species affect their new ecosystems through predation, competition, hybridization, environmental changes and transmission of diseases, they impact significantly the structure and major functioning of natural ecosystems impacting biodiversity and the national economy (Williams et al. 2010).
Amphibians and reptiles
General relationships with climate
Increase in spring–summer temperatures in temperate regions and the Subarctic is causing generally predictable changes in populations of poikilothermic animals. Summer warming extends the active season and, therefore, shifts the timing of individual phenological events such as breeding periods. It also prolongs growth periods, increases the accumulation of reserves for winter, and accelerates embryonic and larval development. Prediction of the consequences of changes in winter conditions is much more complicated, since over-wintering is the most vulnerable period in the life of amphibians and reptiles in cold regions, and it has been insufficiently studied for many species.
Experimental data on the physiological capabilities of amphibians and reptiles in northern Asia related to negative temperatures on land and hypoxic conditions in water, together with data on distribution, allow us to make initial predictions of changes in distributional ranges of nine species: seven species are widespread in the forests of Western Siberia, and two species are widespread in the forest-steppes and steppes.
Amphibians and reptiles in Western Siberia use three types of adaptive strategies for low winter temperatures. They either (a) endure the formation of ice in the body (freeze) and spend the winter on land, (b) tolerate negative temperatures close to zero Celsius in the state of super cooling or (c) they avoid the effect of the cold by wintering in the water or deep in the ground. These strategies largely determine the distribution of species in the region and their possible responses to climate change.
Freeze-resistant species
The distribution of only three species of amphibians and reptiles over-wintering on land in Western Siberia is not limited by winter temperatures: Siberian salamander (Salamandrella keyserlingii), moor frog (Rana arvalis) and common lizard (Zootoca vivipara). These animals are supercooled at temperatures slightly below zero (− 0.1 to − 3 °C), i.e. their body fluids remain unfrozen; but at lower temperatures they freeze, and survive winter in the upper soil horizons at significant negative temperatures.
The Siberian salamander has the greatest cold resistance among terrestrial poikilothermic vertebrates: its tolerance reaches − 50 to − 55 °C (Berman et al. 2016b). It is also highly tolerant of summer conditions. In tundra on the northern coast of the Sea of Okhotsk, population characteristics of this species are comparable to those in other parts of its distribution range with the highest possible abundance (Bulakhova and Berman 2014; Berman and Bulakhova 2016). The moor frog, spreading from the Atlantic to Yakutia and from the steppes to the tundra in Western Siberia, can withstand temperatures down to − 16 °C (Berman et al. 2020b). It survives winters in the soil even under a thin snow cover: evidently, the absence of this species in the far north is not driven by winter factors. The common lizard is the most cold-resistant species of reptiles studied to date. It survives winters in shallow shelters (burrows, cracks and cavities) with temperatures down to −10 °C (Berman et al. 2016a) and, like the moor frog, is common in Western Siberia from the steppes to the tundra. We hypothesize that an increase in winter temperature would not directly affect the distribution of these three species in Western Siberia.
Supercooled species
Three of the nine species do not survive freezing, but tolerate temperatures slightly below zero in a supercooled state for a long time. These are two species of toads (Bufotes sitibundus and Bufo bufo) and the common adder (Vipera berus). They stay deep in the soil to avoid low winter temperatures. Both species of toads tolerate supercooling at − 1 °C for at least one month (Bulakhova et al. 2017), while common adder survives up to four months in the range of − 0.1 to − 1.9 °C (Berman et al. 2020a). Due to this adaptive strategy, Bufotes sitibundus populates Western Siberia and even territories with little snow and as cold as the steppes and forest-steppes of Northern Kazakhstan; the common toad and common adder occur in the northern taiga almost as far as 65° N. However, the low temperatures tolerated are not preferred temperatures, which are higher. Therefore, a slight increase in soil temperatures at the depth of wintering in Western Siberia will weaken the limiting factor leading to the expansion of the species’ ranges.
Species that avoid low winter temperatures
Three species of anurans in the region are not cold resistant: common frog (Rana temporaria), Siberian wood frog (R. amurensis) and Pallas’ spadefoot toad (Pelobates vespertinus). Both species of frogs are only able to tolerate temperatures slightly below zero for several days and consequently, in Western Siberia, they survive winter only in water: R. temporaria in rivers and R. amurensis in ponds. Many water bodies of the Ob-Irtysh basin are characterized by an annual winter drop in oxygen content (winterkill) so the oxyphilic common frog is distributed in Western Siberia only in the upper reaches of rivers flowing from the Ural Mountains. On plains with swamps, rivers have oxygen-poor (hypoxic) waters and after freeze-up become unfit for the over-wintering of the common frog. As the species range is limited to river sections with oxygen contents in winter higher than 2 mg/L (Berman and Bulakhova 2019), if warming leads to the thawing of river ice to create holes that provide aeration of water, the common frog could possibly spread from the mountain foothills into the West Siberian Plain. The Siberian wood frog is the only amphibian able to exist for months in a state of “sluggish wakefulness” at oxygen concentrations below 0.2 mg/L (less than 1.5% of saturation at 3 °C) during wintering in small lakes, including thermokarst lakes (Berman and Bulakhova 2019). In winter, the Siberian wood frog does not breathe, using the anaerobic pathway to generate energy (Berman et al. 2019). Obviously, its distribution in the region is not limited by low winter temperatures, however, an increase in the number of waterbodies that do not freeze to the bottom can lead to an expansion of the species range. The Pallas’ spadefoot toad is unable to tolerate temperatures below 0 °C (Berman et al. 2019) and hibernates in non-freezing soils at depths of 40–70 cm in the European part of Russia and over 1.5 m in the Asian part. Its distribution in Western Siberia is limited to the farthest southwest, to the east of which the ground freezing reaches even greater depths (Bulakhova et al. 2020). If warming continues, an eastward shift of the spadefoot toad’s range may occur.
Implications of the adaptations
The biodiversity of amphibians and reptiles in Western Siberia, especially in the north, is extremely low, and one would expect similar or even the same adaptations (behavioural, physiological, biochemical, etc.) to survive the winter environment. However, it is not so, each listed species has specific adaptations to negative temperatures and low oxygen contents in water. This individuality of adaptations requires a re-thinking of some principles of faunogenesis in temperate and northern regions and requires an autecological approach to predict possible changes of biodiversity. In Western Siberia we can expect the following:
A broadening of the distributional ranges of low- or non-cold-resistant species due to an increase in soil temperatures e.g. the three species of toads and common adder.
No range expansion of species that are not limited by low winter temperatures.
Range expansion not related to climate change, but following anthropogenic changes in the environment (the moor frog, the common adder and the common lizard). At the beginning of this century, the adder had already advanced to the north by almost 1° latitude in the Yamal-Nenets Autonomous Area (Emtsev et al. 2012). As well as the common lizard in the north, it colonizes settlements and disturbed areas (power lines, landfills, embankments of road, pipelines, etc.) where it reaches greater population numbers compared to natural habitats (as a result of the formation of patches that warm up well in summer, have food available and do not freeze deeply in winter). Frogs use technogenic ponds and quarries for spawning, where in the absence of predators, the success of their breeding is often higher than in natural water bodies (Fig. 5).
Fig. 5.

Overgrown anthropogenic and technogenic reservoirs (drainage ditches, quarries, etc.) are convenient spawning places for frogs such as Rana arvalis (photo by N.A. Bulakhova)
Birds
The impacts of climate change on the biodiversity of terrestrial vertebrates in Siberia are the most obvious for birds, and here we review the literature on these biodiversity changes. After the last cold snap in the late 18th–early 19th centuries (the Little Ice Age), the avifauna of Siberia was subsequently formed during a warm and arid climate (the so-called “Warm-dry Era”) (Gashev and Kurkhinen 2015). Significant changes in the Siberian regional fauna, the dynamics of bird ranges and their numbers have been observed since the middle of the last century, and especially during the last 15–20 years (Table 1; Supplementary Material SII; Fig. 6a and b). Comparing the biodiversity indices of bird communities in the same regions between the 1960–1990ies and 2010–2011, the greatest differences occurred in the most severe conditions of tundra and northern taiga, where climate warming was most evident. In contrast, the changes were minimal in the ecotonic bird communities of the forest-steppe and forest-tundra (Gashev 2012).
Table 1.
Synthesis of biodiversity dynamics throughout Siberia, their probable causes and expert judgements of likely societal consequences
| Biodiversity component | Region | Dynamics | Driver(s) | Societal consequences | |
|---|---|---|---|---|---|
| Group | Species | ||||
| Insects | Central Asian and Mongolian species | Western Siberia | Northwards spread | Climate change: warmer and dryer climate. | Irritation of reindeer and people. |
| Insect pests | Colorado Beetle | Western Siberia | Northwards spread | Climate change: decrease in soil freezing and earlier and longer active season. |
Damage to potato crops Reduced economy of agricultural and subsidiary farms. |
| Siberian moth |
Central Siberian Southern Taiga Subzone Altai-Sayan Mts. |
Northwards spread Upwards spread |
Climate change: warmer and dryer pre-outbreak years, decreased ambient humidity. |
Damage to dark coniferous stands and increased fire risk Reduced forest economy and biodiversity impacts. |
|
| Four-eyed fir bark beetle | Siberian plain and mountain taiga | Invasion from the Far East to Central and West Siberia, reinvasion to Baikal Region. | Early season warming (insect) and drought (trees). |
Damage to dark coniferous stands and increased fire risk Reduced forest economy and biodiversity impacts. |
|
| Small spruce bark beetle | Southern regions of the West Siberian taiga | Eastwards spread from North European Russia along the Trans-Siberian Railway. | Climate change: dry and hot summers and frequent heavy snowfalls weakening trees. |
Damage to dark coniferous stands and increased fire risk Reduced forest economy and biodiversity impacts. |
|
| Fish | Native fish | Western Siberia | Abundance and range decreases. | Climate change and anthropogenic | Reductions in commercially important fish species. |
| Non-native fish | Western Siberia | Of 23 species, 9 have become naturalized. Some are moving northwards. | Introductions; warmer waters; the dam of the Novosibirsk hydroelectric station blocking migration of Siberian sturgeon and nelma. | Loss of biodiversity of native fish, economic loss by non-commercial fish introductions. | |
| Amphibians and reptiles | Freeze-resistant species (Siberian salamander, moor frog and common lizard) | Forest-steppe, forest and tundra zones of Western Siberia |
Predicted lack of response of all to winter warming: increase of the moor frog and lizard on anthropogenic disturbed territories. |
Wide climatic tolerances to summer and winter weather: Increase in technogenic ponds useful in breeding of amphibians and that lack predators. |
Not assessed |
| Supercooled species (green toad, common toad and common adder) | Forest-steppe and forest zones of Western Siberia | Predicted expansion to response to winter warming. | Broadening of the ranges following increase in soil temperatures. Increase of common adder on anthropogenic disturbed territories. | Not assessed | |
| Freeze-intolerant species (common frog) | Forest zone of Western Siberia | Predicted expansion from foothills to plain. | Climate change: greater oxygenation of river water by thinner and discontinuous ice cover. | Not assessed | |
| Freeze-intolerant species (Siberian wood frog) | Forest zone of Western Siberia | Could respond to changing abundance of water bodies that do not freeze to the bottom (including thermokarst) lakes, but not directly to warmer winter temperatures. | Climate change: changes in abundance of water bodies that do not freeze to the bottom thermokarst lakes due to permafrost thaw. | Not assessed | |
| Freeze-intolerant species (Pallas’ spadefoot toad) | Steppe and forest-steppe zones of Western Siberia | Could move eastwards | Climate change: decreasing winter soil freeze depths responding to warming climate. | Not assessed | |
| Birds | For species names see Supplementary Material SII | Eastern Siberia (Transbaikalia and the basin of Lake Baikal) |
Range extension and restoration of previous distribution. |
Climate change: series of large droughts in Central and Southeast Asia. | Not assessed |
| General | Range extension and shift in geographical optima. | Aridization of steppe and forest-steppe landscapes, warming of the taiga zone. | Not assessed | ||
| Yakutia | Range extension northwards. | Climate change: warming and economic development impacts on habitat. | Not assessed | ||
| Yamal | Range extension northwards. | Abundance of carrion following extreme winter weather. | Not assessed | ||
| Climate change: warming and economic development impacts on habitat. | |||||
| West Siberian Plain | Range expansion (a) to the north and east and (b) from east to west. | a) Climate change: aridization and decrease in water reserves in the southern regions of Western Siberia and Kazakhstan. | Not assessed | ||
| West Siberian Plain—middle and northern taiga |
Decrease in many forest and meadow-bog species of birds, e.g. yellow-breasted bunting Ocyris aureola Increase in species inhabiting forest edges and open spaces. |
Intensive development of oil and gas fields. | Reductions in commercially important bird species. | ||
| Southeast of Western Siberia (Tomsk Ob River region) | 20–30% decrease in past 30 to 40 years. |
Climate change: (a) and (b) drying out of open habitats; Overgrowing and increased drainage of agricultural land. |
Not assessed | ||
| Decrease in past 10–15 years. | |||||
| Local absence | c) Drying out of floodplain habitats and mass capture on flight paths and during wintering in China and Southeast Asia. | ||||
| d) Range extension to the north and east of the forest and taiga species, new nesting found, regular flights and nesting observations of new species. | d) Climate change: warming, northward displacement of the taiga zone and changes in land-use patterns. | Not assessed | |||
| Mountains of the Altai-Sayan region, Southern Siberia | a) Range expansion to the east and west | Natural dispersal of species |
Not assessed Not assessed |
||
| b) Population decline in high mountain habitats |
b) and c) Warming and high humidity, leading to the loss of habitat: melting of glaciers, spread of mountain taiga to great heights, reduction of the area of mountain tundra; Altitudinal relocation of habitat zones. |
||||
| c) Upwards altitudinal expansion | |||||
| Mammals | Roe deer, red deer, American mink, Asian badger | Western Siberia | Northwards advance |
Infrastructure development of the northern regions and for the badger, increased active layer thickness new infrastructure corridors. |
New options for commercial hunting. Impact on native species not found. |
| Badger, sable, roe deer, pine martin, wild boar | Western Siberia | Restoration of range prior to extermination | Reduced killing and new infrastructure corridors. | New options for commercial hunting. Impact on native species not found. | |
| Wild boar | Western Siberia | Expansion from west to the north and northeast | Climate change and new infrastructure corridors. | New options for commercial hunting. Impact on native species not found. | |
| Northern pika | Yakutia | Range expansion | Climate change | Not assessed | |
| Forest voles | Yakutia | Disruption to population dynamics and peaks. | Climate change: warm winters. | Not assessed | |
| Grey-sided vole | Yakutia | Increase in populations | Climate change: warm winters. | Not assessed | |
| Steppe species such as Red-cheeked ground squirrel, small gopher | Tyumen region | Northwards expansion | Climate change | Not assessed | |
| Wolverines, arctic foxes, sables | Western Siberia | In winter, southward extension to the sub-taiga and northern forest-steppe | Presumably: Increasing continental regional climate | New options for hunting. Impact on native species not found. | |
| Beaver | Siberia | Increase to former distributions and colonization of “unsuitable” areas. | Re-introductions and reduced hunting | Great damage to the ecosystems of the Ob River basin. | |
| Raccoon dog | Western Siberia | Range expansion from the west to the east and north including “unsuitable” areas. |
Climate change: milder winters; intensive development of oil and gas fields; New infrastructure corridors. |
They can begin to compete with native carnivorous species. Uncertain consequences, but it is expected to be negative. | |
| Taimyr Reindeer | Taimyr Peninsula | Range expansion followed by collapse to half previous population numbers. |
Climate change: increased over-heating, increased populations of blood-sucking insects, increased migration paths; Anthropogenic activities: poaching, taking antlers from live animals, lack of control of wolf predator population, increase in legal hunting quotas, local pollution of pastures. |
Taimyr reindeer are the largest wild reindeer population in Russia and the basis for the traditional nature management of Taimyr indigenous peoples. Reduced numbers will lead to radical breakdown in the management and ecological conditions of the region and culture of its indigenous people. | |
| Vegetation | Tundra and treeline | Northern Siberia |
Stability of tundra vegetation; “greening” of tundra vegetation; Browning of tundra vegetation; Northwards and upwards movement of the treeline. |
Climatic tolerances of species; Numerous aspects of climate change; Often following extreme weather events; Numerous aspects of climate change; |
Impacts on biodiversity, economic reindeer herding and hunting and indigenous cultures. |
| Taiga | Siberia |
Forest damage; Afforestation |
Logging, fire, oil and gas infrastructure development, insect pest outbreaks; Abandonment of agricultural lands. |
Impacts on biodiversity and economy. | |
| Steppes | Altai-Sayan Ecoregion | Afforestation—“greening of the steppes” |
Possible increased moisture in depressions from thawing permafrost. Possible reduce of land-use intensity and fewer grazing animals; Possible increase of fire frequency led to thawing of permafrost. |
The transformations can significantly affect the traditional land use by the nomads of Tuva via dramatic decreases of grazing areas and they are, therefore, of great importance for the sustainable development of the region. | |
Fig. 6.

The avifauna is changing in the Tomsk region. a The last recorded nest of the yellow-breasted bunting Ocyris aureola in the Tomsk region was found in 2006 (photos by Sergey Gureev). b The first recorded nest of the grey heron Ardea cinerea in the Tomsk region was found in 2020 (photos by Alexander Berezin)
In Eastern Siberia (Transbaikalia and the basin of Lake Baikal), there is widespread northwards movement of southern bird species from the deserts and steppes of Central Asia to the Arctic tundra covering the subarctic mountains and islands of the Arctic Ocean. There is also restoration of the northern part of the ranges of those species that once lived there, but subsequently disappeared. These distributional changes are a result of a shift in atmospheric circulation patterns and a series of large droughts in Central and Southeast Asia (Lebedeva et al. 2019). Changes in the numbers of common species that have large populations, and of near-water and waterfowl are more significant and reflected in the expansion of their northern ranges and a shift of their optima to northern latitudes (Popov 2011; Melnikov et al. 2018). In winter, the dynamics of the bird fauna (an increase in the number of new species and abundance of some numerous species) is caused not by their resettlement from other regions, but by a noticeable improvement of the living conditions at the nesting sites because of climate warming. Common and numerous settled species are expanding their ranges far to the north.
In Yakutia, there is an obvious expansion of many bird species to the north of the taiga and to the tundra. This is especially noticeable in a number of gulls, e.g. little and black-headed gull (Larus minutus and L. ridibundus) (Supplementary Material SII) (Vartapetov et al. 2019). Some bird species can relocate very quickly. The scavengers crow (Corvus sp.) and magpie (Pica pica) successfully moved to Yamal tundra after the mass death of reindeer due to an extreme weather event in 2012 (Sokolov et al. 2016).
Similar processes of bird resettlement towards the north and the east were observed in Western Siberia due to aridization and decrease in water reserves in the southern regions of Western Siberia and Kazakhstan (unpublished data, Table 1; Supplementary Material SII). This led to the further advance of a number of Central Asian and Mediterranean species, steppe and forest-steppe species, and birds of the wetland fauna from the steppe and forest-steppe regions. However, some species (Table 1, Supplementary Material SII) continue to move from east to west (Gashev and Kurkhinen 2015). In the middle and northern taiga of Western Siberia, intensive development of the oil and gas industry in recent decades has transformed natural habitats, and hunting pressure has increased. These processes have led to a decreased number of species of the forest and wetland avifauna. At the same time, a number of species inhabiting forest edges and open spaces, including forest-tundra up to northern Yamal, have increased (Vartapetov 1998; Vartapetov 2014). The most noticeable decline in the number of birds, such as the starling (Sturnus vulgaris), in the southeast of Western Siberia has resulted from climate change-induced aridization, drainage of agricultural land and abandonment of open fields (Milovidov and Nekhoroshev 2007). Some species completely disappeared (e.g. yellow-breasted bunting—Ocyris aureola) (Gureev et al. 2019).
In the mountainous Altai-Sayan region and in other regions of southern Siberia, numerous species of the European avifauna have expanded their range to the east over the last fifty years, whereas range expansion to the west is observed in far fewer species and has occurred at a much slower rate (Zabelin 2018). Global warming has led to a reduction in areas of glaciers and snowfields, increased humidity and higher temperatures in the highlands with a corresponding spread of forest and shrub vegetation to greater altitudes. Consequently, the mountain-tundra bird habitats of have decreased in area and the number of mountain species has declined dramatically. In contrast, the species of foothill and low mountain habitats have moved to higher altitudes, creating serious competition with populations of local species. In addition, the birds of boreal forests have penetrated into the steppes along river valleys (Baranov and Voronina 2016), (Table 1, Supplementary Material SII).
Mammals
Range extensions and population increases
A review of the literature shows many changes in the biodiversity and ranges of mammals in Siberia. The northward advances of the roe deer (Capreolus pygargus), red deer (Cervus elaphus) and American mink (Neovison vison) are determined primarily by infrastructure development in the northern regions. In the past, the sable (Martes zibellina), the pine marten (M. martes), the wild boar (Sus scrofa) and roe deer (Capreolus pygargus) disappeared as a result of extermination in Western Siberia. However, these species have been restoring their populations in their former ranges (Ivanova et al. 2016). In the last decade, the expansion of the range of wild boar (Sus scrofa) has been observed in Western Siberia from the west to the north and northeast directions, up to the Khanty-Mansiysk and Tomsk administrative regions. This is due to a warming climate and increase of its population in the neighbouring regions (Markov et al. 2019). The construction of roads, pipelines and other infrastructure elements creates unique channels for the expansion of such animals.
Both the sable and the pine marten lived in the southeast of Western Siberia during the second half of the Holocene up to the present. The sable range occupied the entire forest and forest-steppe regions, while the pine marten was found between the south of the forest and forest-steppes up to the Yenisei River. By the end of the 18th century, pine marten remained only near the Ural Mountains, and the range of sable was fragmented in the boreal region and in the mountain forests of Altai. The population of sable began to recover one hundred years ago and now it is almost completely restored. The range of the pine marten advanced northeast up to the Yenisei river, and reached the northern border of the forest-steppes at the beginning of the 20th century. In recent decades, the pine marten has started to expand towards the east and south, where it has settled along tape burs of the south of Western Siberia extending to the foothills of Altai (Tyutenkov and Budz 2014; Devyashin et al. 2016). Tape burs are unique “ribbon” forests in the middle of the Altai steppes.
In Yakutia, global warming is causing the range expansion of the northern pika (Ochotona hyperborea), decreases in the populations of wintering small rodents, disturbances in the cycles of forest voles (Clethrionomys rutilus, Cl. Rufocanus) and an increase in the number of grey voles (Microtus) (Safronov 2016). Warming and aridization, and particularly warm and dry extreme summer weather, are forcing the relocation of steppe species to the north in the Tyumen region (e.g. red-cheeked, small gopher.). Also, in the winter season, some animals (wolverines (Gulo gulo), arctic foxes (Vulpes lagopus), sables, etc.) penetrated further to the south (to the sub-taiga and northern forest-steppes). The reasons for northern species moving south are speculative, but could be explained by an increase in the frequency and level of extreme and adverse weather events at high latitudes (Gashev 2017).
The indigenous West Siberian and Tuvan subspecies of the beaver have completely disappeared due to extermination. However, the beaver population in Siberia has been restored from the middle of the 20th century due to the introduction of individuals of the East European (Castor fiber orientoeuropaeus) and Belarusian (C. f. belorussicus) subspecies to the Omsk, Tomsk, Tyumen, and Irkutsk regions and the Krasnoyarsk Territory. In the last decades of the 20th century in Western Siberia and from the end of the 20th century in Eastern Siberia, the range of beaver has expanded and its population has increased due to conservation policy and poor hunting economy (Malyshev and Prelovsky 2009; Krivov et al. 2020; Trenkov and Saveljev 2020). Today, a growing and uncontrolled breeding beaver population is doing great damage to Siberian ecosystems. In the Ob River basin, beaver ponds increase the stream emission of methane by about 15 times by building their dams (Cazzolla Gatti et al. 2018) and they reduce fish movements and reproduction (Popkov et al. 2018). In other areas, they destroy tracks between villages and block drains (Fedorov 2017) and their foraging affects vegetation succession and invertebrate fauna (Fedorov and Yakimova 2012).
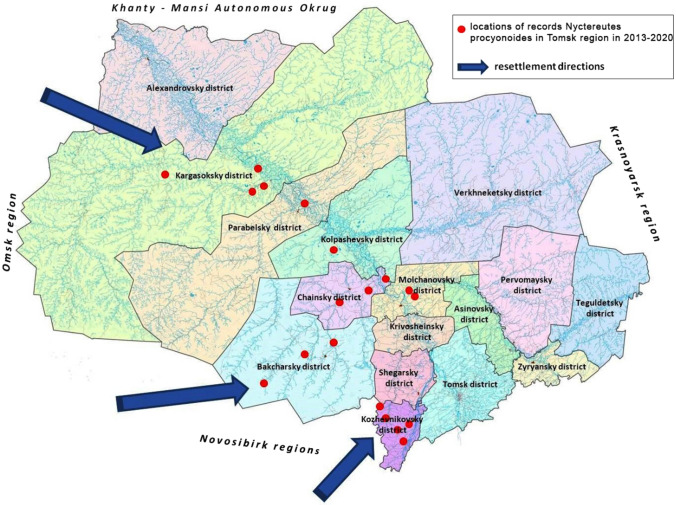
The raccoon dog (Nyctereutes procyonoides) is settling in Western Siberia. Its natural distribution area is Southeast Asia, the Amur river basin and Primorye. In the 1930–1950s, the introductions of raccoon dog into the European part of the USSR were successful, but failed in Siberia and the Altai region (Pavlov et al. 1974). However, after successful acclimatization in the European part of the country, it independently settled eastwards to the Urals. Since the 1970s, the species has been actively moving further eastward, being recorded in the west of Western Siberia in the 1980s (Bogdanov 1998), in the forest-steppe (near Novosibirsk) in 1998, (Kiryukhin et al. 2012) and in the middle taiga (Khanty-Mansiysk administrative district) at the end of the 1990s (Starikov 2003). In the Tomsk region, the first raccoon dog was recorded in January 2013, and since then the animals have been recorded annually in all climatic regions (from the sub-taiga to the middle taiga) (Fig. 7). Despite apparently unfavourable conditions (e.g. extensive swamps, low temperatures), this rapid spread has been probably facilitated by the intensively developing oil and gas industry and climate warming (Baranov 2007).
Fig. 7.
Mammals are spreading in Western Siberia. Paths of resettlement and more than 20 records of the raccoon dog (Nyctereutes procyonoides) in the Tomsk Region between 2013 and 2020 (Nekhoroshev 2017)
Population decline
In the Arctic region of Taimyr, the spatial heterogeneity, exceptional diversity and high productivity of natural pastures have created favourable conditions for Russia’s largest commercial animal population—the Taimyr population of the wild reindeer—which is the basis for the traditional life-styles of Taimyr’s Indigenous People. The estimated carrying capacity in Taimyr is 800–850 thousand head (Kolpashchikov et al. 2019) but in 2000, the reindeer population reached 1 million head, which naturally led to the degradation of pastures. In 2017, the population decreased to 400 thousand animals in the same area. If this trend persists, estimates suggest a catastrophic decline of Taimyr reindeer to 50,000 individuals by 2030) (Fig. 8). These individuals will live in disparate small groups, and hunting will not be economically profitable (Kolpashchikov et al. 2019). The first priority to preserve and maintain this large Taimyr wild reindeer population is for the local and wide international research communities to find the major drivers that led to its dramatic and rapid decline. The current understanding of the drivers of population decline focuses on climate change and anthropogenic pressure. As temperatures increases, feeding activity of reindeer decreases. When they overheat, they stop feeding, body fat reserves decrease and animal mortality increases in the autumn/winter period. Reindeer start migrating to mitigate high temperatures and recent estimates suggest a mean annual temperature increase of 2 °C leads to a shift the summer range of reindeer to the north by 100–150 km or upward by 200–300 m to the Byrranga Mountains where food reserves are very scarce. Elongation of migration paths and change of calving locations limit population growth. The farther from the places of summer pastures the calves are born, the greater is the likelihood of their death in the first year of life. The earlier onset of warmer summers and earlier appearance of blood-sucking diptera stimulates an earlier start and longer duration of migration. Fleeing from the insects, herds of wild reindeer often swim across rivers and lakes, which leads to overcooling and the death of weaker calves. Many surviving calves catch chronic pneumonia, which reduces their reproductive abilities two years later. In 2014–2017, tens of thousands of reindeer aggregated on the unproductive pastures of the polar deserts and Arctic tundra to escape the heat and insect pests (Kolpashchikov et al. 2019). A marked increased migration speed of females in the last month of pregnancy increased calf loss. Calf mortality rate increased by almost 1.5 times (from 10% between 1977 and 1993 to 14% in-between 2000 and 2009) (Mikhailov and Kolpashchikov 2012). The young generation contributed an average of 23.5% to the total population of reindeer between 1969 and 1993, but steadily decreased to 15.5% in 2017 (Kolpashchikov et al. 2019).
Fig. 8.
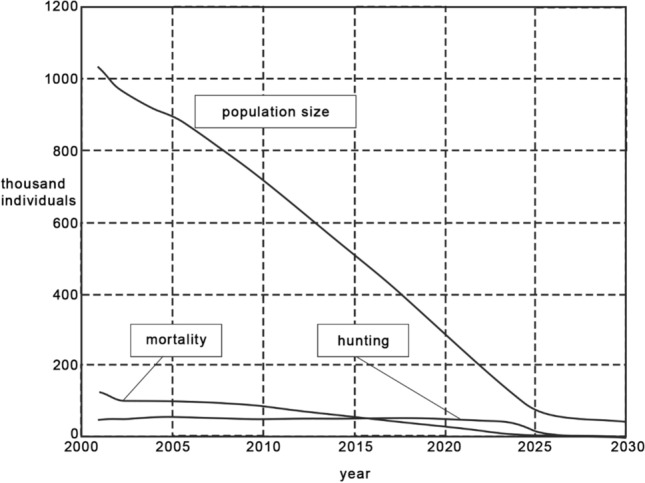
Population dynamics of Taimyr wild reindeer, natural mortality and hunting. Kolpashchikov (unpubl.) observed data to the present, and modelled data from the present to 2030
Hunting is the main direct anthropogenic factor affecting the number of Taimyr reindeer. Expert estimates of poaching suggest 60 to 70 thousand wild reindeer are shot annually, which is more than 2 times higher than the hunting quota (Kolpashchikov et al. 2019). Only the largest individuals are selected for poaching but these are the most valuable for reproduction and the gene pool is weakened. Enormous damage to reindeer reproduction results from cutting (including illegally) antlers from live deer in the spring because hornless animals are not able to mate. In contrast to increased hunting pressure on reindeer, decreased control over the wolf population which was successfully organized between 1971 and 1993, has led to deaths of 40–50 thousand reindeer annually (Kolpashchikov et al. 2019). Constant pollution of pastures by heavy metals (e.g. cadmium, copper) around the Norilsk industrial zone since 2015 has likely decreased population stability: the cadmium content in reindeer liver in the winter pastures of East Taimyr exceeded 1.2 of the maximum permissible concentrations (Kochkarev and Mikhailov 2016).
Hunting and poaching will inevitably lead to a catastrophic decrease in the number of Taimyr wild reindeer already within one generation of Taimyr Indigenous People. The decree of the Government of the Krasnoyarsk Territory No. 103 dated 09/25/2008 gives legal rights for Indigenous People to increase their hunting allowance to 8 deer per person, which amounts to 80 thousand deer per year. This is several times higher than the scientifically based hunting quota. If current trends continue, in 10–15 years hunting should no longer be possible by the Indigenous People. This will threaten their economy and national identity and will lead to a radical breakdown in the ecology of the region.
Vegetation
Tundra
Profound climate-induced changes of landscapes and ecosystems have been observed in the Arctic (e.g. Myers-Smith et al. 2020), especially in its Siberian sector (Volkov et al. 2020). Observed spatial expansion of pristine young forests over un-forested areas (so-called, afforestation), as well as displacement (or shift) of the forest and shrub border towards the far north—the concept of “Greening of the Arctic” have been a focus of many publications (some presented in Supplementary material SI). However, the studies on stable vegetation that represents over 50% of the northern landmasses are under-represented, and particularly in Siberia (Callaghan et al., unpubl.) despite research by Matveyeva and Zanokha (2013) and Shevtsova et al. (2020).
Taiga
In the taiga region of Western Siberia, land cover changes are predominately driven by logging and fire (Kharuk et al. 2021) along with the development of industrial infrastructure, and abandonment of agricultural lands. In the steppes, vegetation changes are similar to those in northern and Arctic regions i.e. the process of afforestation, or “Greening of the steppes”. This has not been previously described in scientific publications, but has been observed in the Altai-Sayan Mountain region by local people and is documented here by our recent studies and field observations (unpublished data).
Greening of the Steppes: changes in vegetation and their possible drivers in the inter-mountain steppe basins of south Siberia
The Altai-Sayan Ecoregion (ASE) is located in the south of Siberia near the geographical centre of Asia (at Kyzyl, Tuva). Its location inside the huge continent of Eurasia and remoteness from the oceans determines the main features of climate there, which is arid-continental (dry and severe). The steppe ecosystems contribute some 17% to the total land area in the region. The multi-cereal steppe is the main type of “true steppe” in Tuva. It occupies flat areas on mountain slopes, piedmont edging, terraces of rivers and lakes, and the bases of inter-mountain depressions. The vegetation mainly consists of (a) the (short) cereals: Stipa krylovii Roshev, Agropyron cristatum L. Beauv, Cleistogenes squarrosa Trin. Keng, Koeleria cristata L. Pers, Poa attenuata Trin; b) plants resistant to grazing: Artemisia frigida Willd, Potentilla acaulis L, Carex duriuscula C.A. Mey, Leymus chinensis Trin. Tzvel; and c) in many communities of the true steppes, a shrub layer of Caragana bungei Ledeb, C. spinosa DC, and C. pygmaea L. DC. Dry steppes are the most common type of steppes in Tuva, occupying the flat bases of depressions with the constant presence of Stipa krylovii Roshev, S. orientalis Trin, Agropyron cristatum L. Beauv, Cleistogenes squarrosa Trin, Festuca valesiaca Gaud, and Artemisia frigida Willd. The shrubs Caragana pygmaea L. DC. and C. bungei Ledeb. are usually abundant with a coverage up to 60–70% (Sobolevskaya 1950; Namzalov 1994).
The centre of Asian anticyclone in winter is located directly in the south of the Tuva region. The topographical features of the region (undulating ground or mountain ridges and inter-mountain steppe depressions) affect atmospheric circulation and define a wide variety of local climates, especially in the southern regions (Kokorin 2011).
Increasing aridization and even desertification are expected in the steppe and dry steppe areas according to the most recent IPCC predictions (IPCC 2019a) and other climate assessments focussing on arid regions (Zalibekov 2011; Kulikov et al. 2014; Berdugo et al. 2020). Furthermore, existing aridization and desertification (Gashev and Kurkhinen 2015), www.pogodaiklimat.ru (accessed March 13, 2020) constitute a threat to the ASE. In particular, the Republic of Tuva is projected to experience a 20–65% increase in steppe area, including dry steppes, and semi-deserts will increase in area several times compared to the present (Kokorin 2011).
In contrast to these observations and results from model simulations, a few studies suggest that climate change affects local atmospheric circulation (Yao et al. 2017) leading to “atmospheric blocking” (Mokhov et al. 2013) and increased meridional circulation that can eventually lead to humidization, at least in some regions of Tuva. However, the ongoing rise of average annual air temperature estimated at the rate of 0.4 °C per decade in the Tuva region balanced against the rise in annual precipitation (only 1.27 mm per year) (Unpublished data) suggests that the losses of moisture via evaporation will probably increase. In contrast, permafrost thaw on mountain slopes could lead to accumulation of moisture in the inter-mountain depressions and in lower accumulative positions, a process that provides one of the main sources of moisture in the cryoarid conditions for larch forest growth (Sugimoto et al. 2002). As soil carbonates are important indicators of the hydrothermal regime and are able to reflect changes in the bioclimatic situation in a very short period (Khokhlova 2008), new observations of a shift of the upper boundary of carbonates to a greater depth indicate new sources of additional moisture in lower steppe depressions. This local humidization process is so contrary to generally accepted models and forecasts that it makes the phenomenon unique, and it could be one reason (in addition to changing land use) for the ongoing colonization of dry steppe areas by trees (Fig. 9) i.e. “the greening of the steppes”. However, it is not yet known how common and how widespread this effect is.
Fig. 9.
Natural expansion of the forest: young trees are moving into the steppe against the backdrop of a mature forest, deluvial foothill plain of East Tannu-Ola ridge, Northern macroslope, Tuva Republic, Russia. a Ground view of colonizing young larches in the pristine true steppe, N 51°03′25.4″, E 94°33′44.9″, 22/08/2020 (photo by Sergey Kirpotin). b Ground view of young larches colonizing the previously once ploughed meadow steppe, N 51°03′38.9″, E 94°33′23.9″, 27/07/2020 (photo by Sergey Kirpotin)
In addition to the “greening of the steppes” that we hypothesize to be climate induced via local increases in soil moisture, some steppe ecosystems are greening through afforestation as the result of land-use change (i.e. reduction in grazing pressure) and increasing wild fires that accelerate permafrost thaw. All of these drivers act either individually or synergistically.
The ongoing transformations in soil and vegetation related to changes in climate, land use and fire events have great potential to affect the traditional land use by the nomads of Tuva via dramatic decreases of grazing areas and they are, therefore, of great importance for the sustainable development of the region.
Conclusions
This synthesis of expert assessments of biodiversity case studies in Siberia shows that climate change currently dominates or is expected to dominate biodiversity dynamics in the future (Table 1). Many climate change impacts are direct, for example, acting through temperature increases on winter survival of some amphibians and acting through over-heating of wild reindeer. Impacts of warming can operate gradually over time and become increasingly evident in changes of population ranges or they can be expressed as increases in frequency of extreme weather events driving rapid, increased mortality, for example, when reindeer die after a rain-on-snow event or vole cycles crash due to changing snow conditions. Climate change also indirectly impacts biodiversity i.e. when drying leads to increased forest fires and insect pest outbreaks. However, these direct and indirect effects of climate change are not the only drivers of biodiversity change in Siberia (Table 1). Abandonment of agricultural lands, oil and gas infrastructure development, hunting and poaching, all affect biodiversity and in different ways. In addition, some human activities allow species to expand their population ranges: the alien bark beetle spreads rapidly along the Trans-Siberian Railway and the moor frogs escape predators in technogenic ponds. Superimposed on such drivers of biodiversity dynamics are legal (national and regional) regulations that are intended to strike a balance between the exploitation and conservation of biodiversity. Unambiguously, these legal regulations need to be based on solid scientific findings and expert assessments and they need to be constantly reviewed, revised and renewed during a period of rapid environmental change. Such science-based management of Siberian ecosystems needs to be made more effective by improving enforcement. Although policing a vast and sparsely populated region such as Siberia is a huge challenge, unless action is taken against poaching and particularly illegal logging, biodiversity will be decreased and iconic species lost. The goal of protecting biodiversity is imperative in its own right, but it has in addition important cultural and economic values. Gaining a scientific understanding of biodiversity issues and formulating management options are huge and complex tasks, particularly in the vast and rapidly changing territory of Siberia. Implementing these tasks requires a multi-disciplinary approach with international cooperative research projects that involve national/regional stakeholders and policy makers, and Indigenous Peoples’ perspectives and knowledge.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
SK, AP, APyak, ZK and AK are grateful for support of the Russian Science Foundation, Grant 20-67-46,018. The work of AP was carried out according to the State assignment of ISSA SB RAS. The research was partly supported by the State assignment of the Ministry of Science and Higher Education of the Russian Federation (Project No. 0721-2020-0019) and RFBR Grants No. 20-04-00587, 19-04-00312A, 18-45-240003, 18-05-00432. Finally, we thank the EU project INTERACT II GA 730938. We also thank Guest Editor Olga Shaduyko for constructive comments and perspectives.
Biographies
Sergey N. Kirpotin
is a Professor, and a Leading Researcher at Tuvan State University and Director of the “Bio-Clim-Land” Center of Excellence at Tomsk State University. His research interests include landscape ecology, Arctic studies, geocryology, remote sensing, plant ecology and biogeochemistry.
Terry V. Callaghan
CMG is D.Sc, and a Professor of Arctic Ecology at Sheffield University, UK, and Professor of Botany at Tomsk State University, Russia. He is a Founder of INTERACT—the international network of terrestrial research stations for research and monitoring in the Arctic and its scientific adviser. He is a Co-founder and Scientific Director of the Siberian Environmental Change Network. His main interests include Arctic Ecology, Climate change, Traditional Indigenous Knowledge, Adaptation and Science Diplomacy.
Anna M. Peregon
is a Ph.D. in Biology (Soil Science), and is a Leading Researcher at Tuvan State University and Senior Researcher in the Institute of Soil Science and Agrochemistry, Siberian Branch of the Russian Academy of Sciences (ISSA SB RAS). Her research interests include climate change in the Northern Eurasia, remote sensing applications for land cover/land-use change analysis, biogeochemistry and carbon cycle in terrestrial ecosystems: regional, national and global assessments.
Andrei S. Babenko
is a Professor, and Head of Department of Agricultural Biology, Institute of Biology, Ecology, Soil Science, Agriculture and Forestry at Tomsk State University. His research interests include biodiversity of insects, soil zoology and environmental management.
Daniil I. Berman
is a Professor, D.Sc. in Biology, and a Chief Researcher of Laboratory of Biocenology, Institute of Biological Problems of the North of the Far Eastern Branch of the RAS. His research interests include the ecology of northern organisms and paleoecology of the Pleistocene.
Nina A. Bulakhova
is Head of Laboratory of Biocenology, Institute of Biological Problem of the North of the Far Eastern Branch of the RAS and Senior Researcher, Laboratory of Biodiversity and Ecology, Tomsk State University. Her research interests include population ecology and adaptive strategy of poikilothermic animals in the North.
Arysia A. Byzaakay
is an early Career Researcher at Tuvan State University, and a PhD student of Institute of Biology, Ecology, Soil Science, Agriculture and Forestry at Tomsk State University. Her research interests include water ecology, microplastics, biogeochemistry and meteorology.
Tatiana M. Chernykh
is a Ph.D. Student of Institute of Biology, Ecology, Soil Science, Agriculture and Forestry at Tomsk State University. Her research interests include landscape ecology, biodiversity, ecotoxicology, radioecology, geoecology and aerosol pollution.
Vladislav Chursin
is a Ph.D. Student in the Department of Meteorology and climatology, Geology and Geography Faculty, at Tomsk State University and Junior Researcher at Siberian Center of State Research Center for Space Hydrometeorology « Planeta ». His research interests include remote sensing, machine learning and climate change.
Elena A. Interesova
Ph.D., is a Leading Researcher at the Novosibirsk Branch of Russian Federal Research Institute of Fisheries and Oceanography; Associate Professor, Department of Ichthyology and Hydrobiology, Institute of Biology, Ecology, Soil Science, Agriculture and Forestry at Tomsk State University. Her research interests include biodiversity of aquatic ecosystems, freshwater ecology, non-native organisms and fisheries science.
Sergey P. Gureev
Ph.D., is a Senior Researcher, Biodiversity and Ecology Laboratory of Research Institute of Biology and Biophysics at Tomsk State University. His research interests include ornithology, landscape ecology, landscape zoogeography, bird ecology and the conservation of rare species.
Ivan A. Kerchev
Ph.D., is a Senior Researcher of Institute of Monitoring of Climatic and Ecological Systems of Siberian Branch of Russian Academy of Sciences; Associate Professor, Department of Forestry and landscape design, Institute of Biology, Ecology, Soil Science, Agriculture and Forestry at Tomsk State University. His research interests include biological invasions, ecology of alien species and forestry pest insects.
Viacheslav I. Kharuk
is a Professor, Head of Forest Monitoring Laboratory at Sukachev Institute of Forests, Russian Academy of Sciences, Siberian Branch; Professor at GIS Chair at Siberian Federal University, Krasnoyarsk, Russia. Scientific interests: forest ecology, climate change, wildfires, remote sensing and GIS.
Aldynai O. Khovalyg
Ph.D., is a Senior Lecturer at the Department of Geography and Tourism at Tuvan State University. Her research interests include geoecology and biogeochemistry.
Leonid A. Kolpashchikov
Ph.D., is a Senior Researcher, and Head of Scientific Department, Joint Directorate of Taimyr Nature Reserves. His research interests include studies of reindeer population and nature conservation.
Svetlana A. Krivets
Ph.D., is a Leading Researcher of Institute of Monitoring of Climatic and Ecological Systems of Siberian Branch of Russian Academy of Sciences. Her research interests include biodiversity of forest ecosystems, biological invasions and ecology of forestry pest insects.
Zoya N. Kvasnikova
Ph.D., is a Senior Researcher at Tuvan State University and Associate Professor in the Department of Geography, Faculty of Geology and Geography at Tomsk State University. Her research interests include geography, landscape science, landscape geochemistry and landscape geophysics.
Irina V. Kuzhevskaia
Ph.D., is an Associate Professor in the Department of Meteorology and Climatology, Faculty of Geology and Geography, at Tomsk State University. Her research interests include remote sensing, regional climate and statistical methods in climatology.
Oleg E. Merzlyakov
Ph.D., is an Associate Professor in the Department of Soil Science and Soil Ecology, Institute of Biology, Ecology, Soil Science, Agriculture and Forestry at Tomsk State University. His research interests include soil science, environmental design, information and statistical methods in soil science, environmental management and audit, soil mapping and soil physics.
Oleg G. Nekhoroshev
is a Researcher of Biodiversity and Ecology laboratory of Research Institute of Biology and Biophysics at Tomsk State University. His research interests include ornithology, biodiversity of birds and nature conservation.
Viktor K. Popkov
Ph.D., is a Senior Researcher of Biodiversity and Ecology laboratory of Research Institute of Biology and Biophysics at Tomsk State University. His research interests include hydrobiology, biodiversity and biology of fish and freshwater ecology.
Andrei I. Pyak
is a Professor, and a Senior Researcher at Tuvan State University and Professor at Botany Department, Institute of Biology, Ecology, Soil Science, Agriculture and Forestry at the Tomsk State University. His research interests include botany, biodiversity of plants, biogeography and nature conservation.
Tatyana O. Valevich
is a Ph.D. Student in the Department of Soil Science and Soil Ecology, Institute of Biology, Ecology, Soil Science, Agriculture and Forestry at Tomsk State University. Her research interests include soil science.
Igor V. Volkov
is an Associate Professor at the Department of General Biology and Methods of Teaching Biology at Tomsk State Pedagogical University. His research interests include plant ecology, adaptations to extreme environments, geobotany, landscape ecology, nature wise use and conservation.
Irina I. Volkova
is an Associate Professor at the Department of Botany and Coordinator of Wetland Centre at Tomsk State University. Her research interests include mire science, geobotany, bryology, landscape ecology, nature wise use and conservation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sergey N. Kirpotin, Email: kirp@mail.tsu.ru
Terry V. Callaghan, Email: terry_callaghan@btinternet.com
Anna M. Peregon, Email: anya.peregon@gmail.com
Andrei S. Babenko, Email: babenko.56@mail.ru
Daniil I. Berman, Email: dber@yandex.ru
Nina A. Bulakhova, Email: sigma44@mail.ru
Arysia A. Byzaakay, Email: belekmaa_byzaakay@mail.com
Tatiana M. Chernykh, Email: tatiana.chernykh94@gmail.com
Vladislav Chursin, Email: skriptym@mail.ru.
Elena A. Interesova, Email: elena.interesova@gmail.com
Sergey P. Gureev, Email: vitagur@mail.ru
Ivan A. Kerchev, Email: ikea86@mail.ru
Viacheslav I. Kharuk, Email: v7sib@mail.ru
Aldynai O. Khovalyg, Email: aldyn@mail.ru
Leonid A. Kolpashchikov, Email: ntnt69@yandex.ru
Svetlana A. Krivets, Email: krivec_sa@mail.ru
Zoya N. Kvasnikova, Email: zojkwas@rambler.ru
Irina V. Kuzhevskaia, Email: ivk@ggf.tsu.ru
Oleg E. Merzlyakov, Email: molege@mail.ru
Oleg G. Nekhoroshev, Email: oleg@green.tsu.ru
Viktor K. Popkov, Email: hydra@mail.tsu.ru
Andrei I. Pyak, Email: a_pyak@rambler.ru
Tatyana O. Valevich, Email: tvalevitch@gmail.com
Igor V. Volkov, Email: volkovhome2016@gmail.com
Irina I. Volkova, Email: volkovhome@yandex.ru
References
- Anisimov O, Zimov S. Thawing permafrost and methane emission in Siberia: Synthesis of observations, reanalysis, and predictive modelling. Ambio. 2021 doi: 10.1007/s13280-020-01392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko A. The agricultural pests of West Siberia in a changing climate. International Journal of Environmental Studies. 2009;66:495–501. doi: 10.1080/00207230902836356. [DOI] [Google Scholar]
- Baranchikov YN, Demidko DA, Laptev AB, Pet’ko VM. Dynamics of Siberian fir dieback in the outbreak area of the four-eyed fir bark beetle. Lesnoi Vestnik. 2014;18:132–138. [Google Scholar]
- Baranov, A.A, and K.K. Voronina. 2016. Reasons and main trends in the dynamics of bird ranges on the territory of Central Siberia. In: Geography and ecology at the service of science and innovative education: Materials XI International Scientific and Practical Conference Krasnoyarsk, 2016. pp. 93–97 (in Russian, English summary).
- Baranov PV. The dynamics of the raccoon dog (Nyctereutes procyonoides) range in Transbaikal region. Russian Journal of Zoology. 2007;86:894–896. [Google Scholar]
- Berdugo M, Delgado-Baquerizo M, Soliveres S, Hernández-Clemente R, Zhao Y, Gaitán JJ, Gross N, Saiz H, et al. Global ecosystem thresholds driven by aridity. Science. 2020;367:787–790. doi: 10.1126/science.aay5958. [DOI] [PubMed] [Google Scholar]
- Berman DI, Bulakhova NA. Ecological characteristics of the Siberian salamander (Salamandrella keyserlingii, Caudata, Hynobiidae) from the coastal part of the Sea of Okhotsk. Biology Bulletin. 2016;43:643–653. doi: 10.1134/s1062359016070037. [DOI] [Google Scholar]
- Berman DI, Bulakhova NA. How winterkill suffocations stop the common frog spreading from Europe to Asia. Priroda. 2019;7:12–26. doi: 10.7868/s0032874x19070020. [DOI] [Google Scholar]
- Berman DI, Bulakhova NA, Alfimov AV, Meshcheryakova EN. How the most northern lizard, Zootoca vivipara, overwinters in Siberia. Polar Biology. 2016;39:2411–2425. doi: 10.1007/s00300-016-1916-z. [DOI] [Google Scholar]
- Berman DI, Bulakhova NA, Korosov AV, Ganyushina ND. Cold hardiness and overwintering of the common viper Vipera berus (Reptilia, Viperidae) on the island of Kizhi, Karelia. Russian Journal of Zoology. 2020;99:1014–1022. [Google Scholar]
- Berman DI, Bulakhova NA, Meshcheryakova EN. The Siberian wood frog survives for months underwater without oxygen. Scientific Reports. 2019;9:13594. doi: 10.1038/s41598-018-31974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman DI, Bulakhova NA, Meshcheryakova EN, Shekhovtsov SV. Overwintering and cold tolerance in the moor frog (Rana arvalis, Anura) across its range. Canadian Journal of Zoology. 2020;98:697–706. doi: 10.1139/cjz-2019-0179. [DOI] [Google Scholar]
- Berman DI, Meshcheryakova EN, Bulakhova NA. Extreme negative temperatures and body mass loss in the Siberian salamander (Salamandrella keyserlingii, amphibia, hynobiidae) Doklady Biological Sciences. 2016;468:137–141. doi: 10.1134/s001249661603011x. [DOI] [PubMed] [Google Scholar]
- Bezrukov LA, Korytny LM. The role of Siberia in the economic development of Russia. Geography and Natural Resources. 2009;30:229–235. doi: 10.1016/j.gnr.2009.09.005. [DOI] [Google Scholar]
- Bogdanov II. Mammals of the Omsk region: textbook. Omsk: Publishing house OmGPU; 1998. [Google Scholar]
- Bryukhanov A. Ecological Assessment of the state of forests in Siberia: Alarming results. Sustainable Forest Management. 2009;2:21–31. [Google Scholar]
- Bulakhova N, Berman D. Male reproductive cycle of the Siberian salamander Salamandrella keyserlingii (Caudata: Hynobiidae) in coastal tundra of the Sea of Okhotsk. Polar Biology. 2014;37:123–133. doi: 10.1007/s00300-013-1418-1. [DOI] [Google Scholar]
- Bulakhova NA, Alfimov AV, Berman DI. The eastern boundary of the geographic range of the Pallas’ spadefoot Pelobates vespertinus (Anura, Amphibia) is limited by overwintering temperatures. Herpetozoa. 2020;33:171–175. doi: 10.3897/herpetozoa.33.e58050. [DOI] [Google Scholar]
- Bulakhova, N.A, E.N. Meshcheryakova, and D.I. Berman. 2017. Cryoresistance of three toad species from North Asia. In: Abstract book of the 19th Ordinary General Meeting of SEHI, Salzburg, Austria. p. 186.
- Bystrov SO, Antonov IA. First Record of the Four-Eyed Fir Bark Beetle Polygraphus proximus Blandford, 1894 (Coleoptera, Curculionidae: Scolytinae) from Irkutsk Province, Russia. Entomological Review. 2019;99:54–55. doi: 10.1134/s001387381901007x. [DOI] [Google Scholar]
- Callaghan TV, Kulikova O, Rakhmanova L, Topp-Jørgensen E, Labba N, Kuhmanen L-A, Kirpotin S, Shaduyko O, et al. Improving dialogue among researchers, local and indigenous peoples and decision-makers to address issues of climate change in the North. Ambio. 2020;49:1161–1178. doi: 10.1007/s13280-019-01277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan, T.V., O. Shaduyko, and S. Kirpotin. 2021. Siberian Environmental Change. Ambio. Special Issue 50. [DOI] [PMC free article] [PubMed]
- Camacho AI, Mas-Peinado P, Iepure S, Perina G, Dorda BA, Casado A, Rey I. Novel sexual dimorphism in a new genus of Bathynellidae from Russia, with a revision of phylogenetic relationships. Zoologica Scripta. 2020;49:47–63. doi: 10.1111/zsc.12387. [DOI] [Google Scholar]
- Cazzolla Gatti R, Callaghan T, Rozhkova-Timina I, Dudko A, Lim A, Vorobyev S, Kirpotin S, Pokrovsky O. The role of Eurasian beaver (Castor fiber) in the storage, emission and deposition of carbon in lakes and rivers of the River Ob flood plain, western Siberia. Science of the Total Environment. 2018;644:1371–1379. doi: 10.1016/j.scitotenv.2018.07.042. [DOI] [PubMed] [Google Scholar]
- Cognato AI. Biology, systematics, and evolution of Ips. In: Hofstetter RW, editor. Vega FE. Biology and Ecology of Native and Invasive Species. Oxford: Elsevier Academic Press; 2015. pp. 351–370. [Google Scholar]
- Devyashin MM, Kosintsev PA, Tyutenkov OY, Ovodov ND, Vasiliev SK. The formation of modern ranges of martens (genus Martes Pinel 1792) in the southeast of Western Siberia. Russian Journal of Zoology. 2016;95:728–738. doi: 10.7868/s0044513416060088. [DOI] [Google Scholar]
- Dyldin YV, Hanel L, Romanov VI, Plesnik J. Review of the genus Thymallus (Pisces: Salmoniformes, Salmonidae, Thymallinae) with taxonomic notes. Bulletin Lampetra. 2017;8:103–126. [Google Scholar]
- Edelgeriev, R.S.-K. 2019. National Report. Global Climate and Soil Cover of Russia: Desertification and Land Degradation, Institutional, Infrastructure, Technological Adaptation Measures (Agriculture and Forestry). vol 2. Moscow, MBA Publishing House LLC (in Russian).
- Emtsev AA, Bernikov KA, Akopyan EK. About the expansion of the areas borders of some animals species in the northern part of Western Siberia. Mir nauki kil’tury obrazovaniya. 2012;6:471–477. [Google Scholar]
- EPA. 2017. Climate Impacts on Ecosystems 2017. Available at https://www.19january2017snapshot.epa.gov/climate-impacts/climate-impacts-ecosystems.html.
- Everett, L. 2020. Proceedings of a Workshop. In: Understanding and Responding to Global Health Security Risks from Microbial Threats in the Arctic. National Academies of Sciences, Engineering, and Medicine, 2020. Washington, DC: The National Academies Press. [PubMed]
- FAO. 2020. Global Forest Resources Assessment 2020 (FRA 2020), UN Food and Agricultural Organization. Available at http://www.fao.org/forest-resources-assessment/2020/.
- Fedorov FV. On the question of the negative role of the beaver in human economic activity Scientific notes of Petrozavodsk State University. 2017;8:88–94. [Google Scholar]
- Fedorov FV, Yakimova AE. Changes in ecosystems of the middle taiga due to the impact of beaver activities, Karelia, Russia. Baltic Forestry. 2012;18:278–287. [Google Scholar]
- Gashev SN. The bird population of the West Siberian Plain under global climate change. Bulletin of the Tyumen State University Medical and Biological Sciences. 2012;6:6–15. [Google Scholar]
- Gashev SN. Mammal phenological response to climate change: Analysis of Russian-language sources. Bulletin of the Tyumen State University: Ecology and Nature Management. 2017;3:125–146. [Google Scholar]
- Gashev SN, Kurkhinen YP. Dynamic processes in the vertebrate fauna of Western Siberia and their drivers. Bulletin of the Tyumen State University Ecology and Nature Management. 2015;1:80–89. [Google Scholar]
- Golitsyn GS, Vasiliev AA. Climate change and its impact on the frequency of extreme hydrometeorological events. Meteorology and Hydrology. 2019;11:9–12. [Google Scholar]
- Groisman PY, Blyakharchuk TA, Chernokulsky AV. Climate Changes in Siberia. In: Groisman PY, Gutman G, editors. Regional Environmental Changes in Siberia and Their Global Consequences. Springer Environmental Science and Engineering. Dordrecht: Springer; 2013. pp. 57–109. [Google Scholar]
- Gruza GV. Climate change detection: State, climate variability and extremeness. Meteorology and Hydrology. 2004;4:50–67. [Google Scholar]
- Gureev, S.P., O.G. Nekhoroshev, and P.J. Mitchell. 2019. Spatial Heterogeneity of Bird Communities in the Natural Landscapes of the Southern Taiga of the Ob – Yenisei Interfluve and the Chulym River Valley (Tomsk Region). Paper presented at the Bio-Clim-Land 2019. IOP Conference Series. Earth and Environmental Science. 400: 012014. 10.1088/1755-1315/400/1/012014.
- Gutman G, Radeloff V, editors. Land-Cover and Land-Use Changes in Eastern Europe after the Collapse of the Soviet Union in 1991. Cham: Springer; 2017. [Google Scholar]
- Hese S, Schmullius C. Object oriented oil spill contamination mapping in West Siberia with Quickbird data. In: Blaschke T, Lang S, Hay GJ, editors. Object-Based Image Analysis. Berlin: Springer; 2008. [Google Scholar]
- Interesova EA. Alien fish species in the Ob River basin. Russian Journal of Biological Invasions. 2016;7:156–167. doi: 10.1134/s2075111716020089. [DOI] [Google Scholar]
- Interesova EA, Bogomolova IN. Fish in the tributaries of the Novosibirsk reservoir. Contemporary Problems of Ecology. 2013;6:597–602. doi: 10.1134/s1995425513060048. [DOI] [Google Scholar]
- Interesova EA, Rostovtsev AA, Suslyaev VV, Blokhin AN, Bogomolova IN, Lyalina MI. Fish communities in water bodies of the Southern Taiga of Western Siberia (within Tomsk Oblast) Russian Journal of Ecology. 2020;51:157–165. doi: 10.1134/s1067413620020034. [DOI] [Google Scholar]
- Interesova EA, Villizzi L, Copp GH. Risk screening of the potential invasiveness of non-native freshwater fishes in the River Ob basin (West Siberian Plain, Russia) Regional Environmental Change. 2020;20:64. doi: 10.1007/s10113-020-01644-3. [DOI] [Google Scholar]
- IPCC. 2019a. Climate Change and Land. An IPCC Special Report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. Summary for Policymakers. Approved Draft. vol Intergovernmental Panel on Climate Change (IPCC). Available at https://www.ipcc.ch/srccl/.
- IPCC. 2019b. IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. In press. Retrieved 6 December 2019 from https://www.ipcc.ch/srocc/.
- Ippolitov II, Kabanov MV, Loginov SV, Kharyutkina EV. Structure and dynamic of meteorological fields on the Asian region of Russia in the period of the global warming for 1975–2005. Journal of the Siberian Federal University: Biology. 2008;1:323–344. doi: 10.17516/1997-1389-0255. [DOI] [Google Scholar]
- Ivanova NV, Tyutenkov OY, Fadeev KD, Devyashin MM. Features of the distribution and ecology of wild boar (Sus scrofa L, 1758) in the southeastern part of Western Siberia (Tomsk Priobye) Principles of Ecology. 2016;5:54. [Google Scholar]
- Karpova LN, Kirillov AF, Sivtseva LV, Zhirkov FN, Apsolikhova OD, Venediktov EY, Venediktov SY, Karpov SO, et al. Results of monitoring of aquatic biological resources in water bodies of the Republic of Sakha (Yakutia) Bulletin of Fishery Science. 2015;2:3–17. [Google Scholar]
- Kerchev IA. Ecology of four-eyed fir bark beetle Polygraphus proximus Blandford (Coleoptera; Curculionidae, Scolytinae) in the west Siberian region of invasion. Russian Journal of Biological Invasions. 2014;5:176–185. doi: 10.1134/s2075111714030072. [DOI] [Google Scholar]
- Kerchev, I.A., S.A. Krivets, S.V. Loginov, and E.V. Kharutkina. 2017. Climate extremes and formation of outbreaks foci of four-eyed fir bark beetle in Western Siberia. In: Biological diversity and conservation problems in the fauna, 118–122. Yerevan (Armenia) (in Russian).
- Kerchev IA, Mandelshtam MY, Krivets SA, Ilinsky YY. Small Spruce Bark Beetle Ips amitinus (Eichhoff, 1872) (Coleoptera, Curculionidae: Scolytinae): A New Alien Species in West Siberia. Entomological Review. 2019;99:639–644. doi: 10.1134/s0013873819050075. [DOI] [Google Scholar]
- Kharuk V, Im S, Soldatov V. Siberian silkmoth outbreaks surpassed geoclimatic barrier in Siberian Mountains. Journal of Mountain Sciences. 2020;17:1891–1900. doi: 10.1007/s11629-020-5989-3. [DOI] [Google Scholar]
- Kharuk VI, Antamoshkina OA. Impact of silkmoth outbreak on taiga wildfires Contemporary Problems of. Ecology. 2017;10:556–562. doi: 10.1134/s1995425517050055. [DOI] [Google Scholar]
- Kharuk VI, Im ST, Ranson KJ, Yagunov MN. Climate-induced northerly expantion of Siberian silk moth range. Forests. 2017;8:301. doi: 10.3390/f8080301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharuk VI, Ponomarev EI, Ivanova GI, Dvinskaya ML, Coogan SCP, Flannigan MD. Wildfires in the Siberian taiga. Ambio. 2021 doi: 10.1007/s13280-020-01490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlova, O.S. 2008. Pedogenic carbonates as a carrier of memory of soil-forming factors (Case study in steppe areas of the Russian plain). In: Soil memory: Soil as a Memory of Biosphere-Geosphere-Anthroposphere interactions. ed. V.O. Targulian and S. V. Goryachkin SV, 406–437. Moscow: LKI Publ. (in Russian).
- Kirpotin S, Berezin A, Bazanov V, Polishchuk Y, Vorobiov S, Mironycheva-Tokareva N, Kosykh N, Volkova I, et al. Western Siberia wetlands as indicator and regulator of climate change on the global scale. International Journal of Environmental Studies. 2009;66:409–421. doi: 10.1080/00207230902753056. [DOI] [Google Scholar]
- Kiryukhin, S.T., V.G. Telepnev, V.S. Kryuchkov, and E.V. Kuznetsov. 2012. Resettlement of a raccoon dog across the territory of the Novosibirsk Region. In: Modern problems of nature management, hunting and animal husbandry. vol. 1, 400 (in Russian).
- Kochkarev PV, Mikhailov VV. Complex analysis of the heavy metals content in bodies and tissues of the wild reindeer (Rangifer tarandus L. 1758) Bulletin of the Krasnoyarsk GAU. 2016;8:21–26. [Google Scholar]
- Kokorin, A.O. 2011. Assessment Report: Climate change and its impacts on ecosystems, populations and economies Russian part of Altae-Sayan ecoregion. Moscow. Available at https://wwf.ru/en/resources/publications/booklets/assessment-report-climate-change-and-its-impact-on-ecosystems-population-and-economy-of-the-russian.
- Kolomiets NG. Siberian moth is a pest of the plain taiga. Proceedings on forestry in Siberia. 1957;3:61–76. [Google Scholar]
- Kolomiets NG, Mayer EI. The Most Important Forest Pests in Tomskaya Oblast and Pest Control. Tomsk: Kn. izd-vo; 1963. [Google Scholar]
- Kolpashchikov LA, Bondar MG, Mikhailov VV. The modern history of the Taimyr population of wild reindeer: Dynamics, management, threats and conservation methods. Proceedings of the Karelian Scientific Center of the Russian Academy of Sciences. 2019;11:5–20. [Google Scholar]
- Kravchuk, M. 2020. Forests and Biodiversity: Too valuable to lose. Materials of the portal “Scientific Russia”. Available at https://www.scientificrussiaru/articles/mezhdunarodnyj-den-lesov.
- Krivets, S.A., and Y.N. Baranchikov. 2015. Four-eyed fir bark beetle in Siberian forests: Distribution, biology, ecology, detection and survey of damaged stands. Tomsk – Krasnoyarsk (in Russian).
- Krivets SA, Bisirova EM, Kerchev IA, Pats EN, Chernova NA. Transformation of taiga ecosystems in the Western Siberian invasion focus of four-eyed fir bark beetle Polygraphus proximus Blandford (Coleoptera: Curculionidae, Scolytinae) Russian Journal of Biological Invasions. 2015;6:94–108. doi: 10.1134/s2075111715020058. [DOI] [Google Scholar]
- Krivets SA, Kerchev IA. Insects inhabiting the galleries of the four-eyed fir bark beetle Polygraphus proximus Blandf. (Coleoptera, Curculionidae: Scolytinae) in Siberia. Entomological Review. 2016;96:545–558. doi: 10.1134/s0013873816050043. [DOI] [Google Scholar]
- Krivov, M.A., Y.V. Lunyeva, E.V. Zhuravleva. 2020. State report on the state and protection of the environment of Tomsk Region in 2019. Tomsk (in Russian).
- Krokhalevsky BP, Babkina IB, Wieser AM, Dorogin MA, Zhirkov FN, Zaytsev VF, Interesova EA, Karpova LN, et al. Status of sturgeon stocks in Siberian water bodies. Problems of Fisheries. 2018;19:269–284. doi: 10.36038/0234-2774-2018-19-3-269-284. [DOI] [Google Scholar]
- Kulikov AI, Ubugunov LL, Mangataev AT. Global climate change and its impact on ecosystems. Arid Ecosystems. 2014;4:135–141. doi: 10.1134/s2079096114030032. [DOI] [Google Scholar]
- Lavrinenko IA, Lavrinenko OV. The impact of climate change on the plant cover of the Barents Sea islands. Proceedings of Karelian Scientific Center RAS. 2013;6:4–16. [Google Scholar]
- Lebedeva MG, Lupo AR, Chendev YG, Krymskaya OV, Solovyev AB. Changes in the atmospheric circulation conditions and regional climatic characteristics in two remote regions since the mid-20th century. Atmosphere. 2019;10:11. doi: 10.3390/atmos10010011. [DOI] [Google Scholar]
- Levin BA, Simonov EP, Ermakov OA, Levina MA, Interesova EA, Kovalchuk OM, Malinina YA, Mamilov NS, et al. Phylogeny and phylogeography of the roaches, genus Rutilus (Cyprinidae), at the Eastern part of its range as inferred from mtDNA analysis. Hydrobiologia. 2017;788:33–46. doi: 10.1007/s10750-016-2984-3. [DOI] [Google Scholar]
- Malhi Y, Franklin J, Seddon N, Solan M, Turner MG, Field CB, Knowlton N. Climate change and ecosystems: Threats, opportunities and solutions. Philosophical Transactions of the Royal Society B: Biological Sciences. 2020;375:20190104. doi: 10.1098/rstb.2019.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyshev YS, Prelovsky VA. Alien species of mammals in reserves and national parks of Eastern Siberia. Baikal Journal of Zoology. 2009;2:88–97. [Google Scholar]
- Markov N, Pankova N, Filippov I. Wild boar (Sus scrofa L.) in the north of Western Siberia: History of expansion and modern distribution. Mammal Research. 2019;64:99–107. doi: 10.1007/s13364-018-0378-9. [DOI] [Google Scholar]
- Matkovsky, A.K. 2019. The ongoing changes in the ichthyocenoses of the Ob-Irtysh fisheries region under the influence of anthropogenic factors and global climate warming. In: Problems of ensuring environmental safety and sustainable development of the Arctic territories, 488–492 (in Russian).
- Matveyeva, N.V., and L.L. Zanokha. 2013. Plant cover stability under significant landscape transformation in Western Taymyr tundras. In: Trudy Vserossiiskoi nauchnoi konferentsii “Biodiversity of ecosystems of the Far North: inventarization, monitoring, protection”, Syktyvkar, Komi Republik (3–7 July, 2013), 100–109 (in Russian).
- Melnikov, Y.I., T.N. Gagina-Skalon, V.V. Popov, T.Z. Dorzhiev, A.A. Ananin, O.A. Goroshko, E.E. Malkov, and Y.A. Durnev. 2018. The fauna of birds of Eastern Siberia and the features of its dynamics (late XIX - early XXI centuries). Paper presented at the Modern problems of ornithology in Siberia and Central Asia: Materials of the VI International Ornithological Conference (in Russian).
- Mikhailov VV, Kolpashchikov LA. Three stages in the recorded history of the Taimyr wild reindeer population. Russian Journal of Zoology. 2012;91:486–492. [Google Scholar]
- Milovidov SP, Nekhoroshev OG. The dynamics of the bird population of Tomsk. Vestnik of Tomsk State University (Series Biology) 2007;300:182–185. [Google Scholar]
- MNRE. 2014. Strategy and Action Plan for the Conservation of Biological Diversity of the Russian Federation. Available at https://www.cbd.int/doc/world/ru/ru-nbsap-v2-ru.pdf.
- Mokhov II, Akperov M, Prokofyeva MA, Timazhev AV, Lupo AR, Le Treut H. Blockings in the Northern hemisphere and Euro-Atlantic region: Estimates of changes from reanalysis data and model simulations. Doklady Earth Sciences. 2013 doi: 10.1134/s1028334x13040144. [DOI] [Google Scholar]
- Musolin DL. Insects in a warmer world: Ecological, physiological and life-history responses of true bugs (Heteroptera) to climate change. Global Change Biology. 2007;13:1565–1585. doi: 10.1111/j.1365-2486.2007.01395.x. [DOI] [Google Scholar]
- Myers-Smith IH, Kerby JT, Phoenix GK, Bjerke JW, Epstein HE, Assmann JJ, John C, Andreu-Hayles L, et al. Complexity revealed in the greening of the Arctic. Nature Climate Change. 2020;10:106–117. doi: 10.1038/s41558-019-0688-1. [DOI] [Google Scholar]
- Namzalov, B.B. 1994. Steppes of southern Siberia. BNZ SB RAS. Novosibirsk: Ulan-Ude (in Russian).
- Nekhoroshev O. The raccoon dog (Nyctereutes procyonoides Gray), 1834 in Tomsk Region. Vestnik IrGSHA. 2017;82:120–125. [Google Scholar]
- Økland B, Flø D, Schroeder M, Zach P, Cocos D, Martikainen P, Siitonen J, Mandelshtam M, et al. Range expansion of the small spruce bark beetle Ips amitinus: A newcomer in northern Europe. Agricultural and Forest Entomology. 2019;21:286–298. doi: 10.1111/afe.12331. [DOI] [Google Scholar]
- Oliver TH, Morecroft MD. Interactions between climate change and land use change on biodiversity: Attribution problems, risks, and opportunities. WIREs Climate Change. 2014;5:317–335. doi: 10.1002/wcc.271. [DOI] [Google Scholar]
- Overland JE, Dethloff K, Francis JA, Hall RJ, Hanna E, Kim S-J, Screen JA, Shepherd TG, Vihma T. Nonlinear response of mid-latitude weather to the changing Arctic Nature. Climate Change. 2016;6:992–999. doi: 10.1038/nclimate3121. [DOI] [Google Scholar]
- Pavlov DS, Mochek AD. Ecology of fish of the Ob-Irtysh basin. Moscow: KMK; 2006. [Google Scholar]
- Pavlov MP, Korsakova IB, Lavrov NP. Acclimatization of hunting and fishing animals and birds in the USSR. Kirov: Volga-Vyatka Prince Publishing House; 1974. [Google Scholar]
- Perepelin, Y.V., G.I. Bogdanova, V.A. Zadelenov, V.V. Zvantsev. 2020. Characteristics of fishing for aquatic biological resources in the Krasnoyarsk Territory at the beginning of the 21st century. In: Resources of game and fish: Use and reproduction. Materials of the 1st All-Russian Scientific and Practical Conference, Krasnoyarsk. 114–122 (in Russian).
- Petkevich AN. Biology and reproduction of sturgeon in the Middle and Upper Ob in connection with hydro construction. Trudy Tomsk University. 1952;119:39–64. [Google Scholar]
- Popkov, V.K. 1995 The dynamics of the stocks of the main commercial fish in the floodplain-river system of the Middle Ob and its determining factors. In: Nature complex of the Tomsk region. T. 2. Biological and water resources, 169–177. TSU Publishing House, Tomsk (in Russian).
- Popkov, V.K., V.V. Drozdov, and O.G. Nekhoroshev. 2018. Influence of the environment-transforming activity of beavers on the formation of fish resources in the floodplain of the Middle Ob. In: Ecology and environmental management. Collection of scientific papers, vol 2. Izd-vo Literaturnoe byuro, Tomsk (in Russian).
- Popov PA. Fish of Siberia: Distribution, ecology, catch. Novosibirsk: Novosibirsk University Press; 2007. [Google Scholar]
- Popov P. Species composition and pattern of fish distribution in Siberia. Journal of Ichthyology. 2009;49:483–495. doi: 10.1134/s0032945209070017. [DOI] [Google Scholar]
- Popov PA, Trifonova OV. The content and features of the accumulation of metals in fish from the river of Tom’. Siberian Journal of Ecology. 2007;6:961–967. [Google Scholar]
- Popov, V.V. 2011. Influence of climate change on the biodiversity of terrestrial vertebrates by the example of the Baikal region. Successes in Modern Biology 131: 466–468 (in Russian, English summary).
- Popova EN, Popov IO. Climatic causes of the current expansion of the Italian locust range in Russia and neighboring countries. Reports of the Russian Academy of Sciences. 2019;488:658–660. doi: 10.31857/s0869-56524886658-660. [DOI] [Google Scholar]
- Prishchepov AV, Radeloff VC, Baumann M, Kuemmerle T, Müller D. Effects of institutional changes on land use: agricultural land abandonment during the transition from state-command to market-driven economies in post-Soviet Eastern Europe. Environmental Research Letters. 2012;7:024021. doi: 10.1088/1748-9326/7/2/024021. [DOI] [Google Scholar]
- Reshetnikov AN, Golubtsov AS, Zhuravlev VB, Lomakin SL, Rezvyi AS. Range expansion of rotan Perccottus glenii, sunbleak Leucaspius delineatus, and bleak Alburnus alburnus in the Ob River basin. Contemporary Problems of Ecology. 2017;10:612–620. doi: 10.1134/s1995425517060105. [DOI] [Google Scholar]
- Rockström, J., W. Steffen, K. Noone, A. Persson, I. F.S. Chapin, E. Lambin, T.M. Lenton, M. Scheffer, et al. 2009. Planetary boundaries: Exploring the safe operating space for humanity. Ecology and Society 14: 32. http://www.ecologyandsociety.org/vol14/iss2/art32/.
- ROSHYDROMET. 2014. The second assessment report of Roshydromet on climate change and its consequences on the territory of the Russian Federation. Moscow, Roshydromet (in Russian). Available at http://www.downloads.igce.ru/publications/OD_2_2014/v2014/htm/1.htm Moscow (in Russian).
- ROSHYDROMET. 2019. Report on the features of climate on the territory of the Russian Federation 2018. Moscow, ROSHYDROMET. Available at http://www.climatechange.igce.ru/ (in Russian).
- Rostovtsev AA, Interesova EA. Fish resources of Tomsk region. Rybnoe khoziaystvo (Fisheries) 2015;5:48–49. [Google Scholar]
- Rozhkov AS. Siberian Moth Outbreaks and Pest Control. Moscow: Nauka; 1965. [Google Scholar]
- Safronov VM. Climate change and mammals of Yakutia. Russian Journal of Zoology. 2016;95:1459–1474. doi: 10.7868/s004451341612014x. [DOI] [Google Scholar]
- Sergienko VG, Konstantinov AV. Forecast of influence of climate change on ecosystems and natural diversity species of Russian flora and fauna biotic complexes. Proceedings of the Saint Petersburg Forestry Research Institute. 2016;2:29–44. doi: 10.21178/2079-6080.2016.2.27. [DOI] [Google Scholar]
- Shaduyko, O. 2019. INTERACT Internal Report. www.eu-interact.org.
- Shevtsova I, Heim B, Kruse S, Schröder J, Troeva EI, Pestryakova LA, Zakharov ES, Herzschuh U. Strong shrub expansion in tundra-taiga, tree infilling in taiga and stable tundra in central Chukotka (north-eastern Siberia) between 2000 and 2017. Environmental Research Letters. 2020;15:085006. doi: 10.1088/1748-9326/ab9059. [DOI] [Google Scholar]
- Shmatkov N. On illegal logging in Russia: How serious the problem is and can FSC reduce risks? Sustainable Forest Management. 2020;5:20–21. [Google Scholar]
- Shumilova LV. Botanical geography of Siberia. Tomsk: Tomsk State University Press; 1962. [Google Scholar]
- Sobolevskaya KA. Vegetation of Tuva. Novosibirsk: ZSF AN SSSR; 1950. [Google Scholar]
- Sokolov AA, Sokolova NA, Ims RA, Brucker L, Ehrich D. Emergent rainy winter warm spells may promote boreal predator expansion into the Arctic. Arctic. 2016;69:121–129. doi: 10.14430/arctic4559. [DOI] [Google Scholar]
- Starikov VP. Mammals of the Khanty-Mansiysk Autonomous Okrug (distribution, ecology, practical value): textbook. Surgut: State Unitary Enterprise Khanty-Mansi Autonomous Okrug Surgut Printing House; 2003. [Google Scholar]
- Sugimoto A, Yanagisawa N, Naito D, Fujita N, Maximov TC. Importance of permafrost as a source of water for plants in east Siberian taiga. Ecological Research. 2002;17:493–503. doi: 10.1046/j.1440-1703.2002.00506.x. [DOI] [Google Scholar]
- Trenkov I, Saveljev A. The formation and current state of the beaver population in the Kuznetskiy Alatau Mountains. South Siberia Agricultural science Euro-North-East. 2020;21:605–613. [Google Scholar]
- Tyutenkov, O.Y., and A.V. Budz. 2014. Dynamics of geographical range and occurrence of pine marten (Martes martes L.) in the southeast of the forest zone of Western Siberia. Achievements in the Life Sciences 8: 150–152. https://www.scipedia.com/public/Tyutenkov_Budz_2015a.
- United_Nations. 2019. Sustainable development goals report 2019, Jensen, L. edn, United Nations, New York. Available at https://unstats.un.org/sdgs/report/2019/.
- Vandergert P, Newell JP. Illegal logging in the Russian Far East and Siberia. International Forestry Review. 2003;5:303–306. doi: 10.1505/ifor.5.3.303.19150. [DOI] [Google Scholar]
- Vartapetov LG. Birds of the northern taiga of the West Siberian Plain. Novosibirsk: Nauka; 1998. [Google Scholar]
- Vartapetov LG. The classification of bird communities from the middle taiga of West Siberia. Bulletin of the Irkutsk State University Ecologia. 2014;8:31–39. [Google Scholar]
- Vartapetov, L.G., Germogenov, N.I, and Shemyakin, E.V. 2019. Results and prospects of studying the fauna and bird population of Yakutia. In: Safe North - the clean Arctic: Materials of the II All-Russian Scientific and Practical Conference. Electronic edition, Surgut State University, 139–146 (in Russian).
- Volkov IV, Volkova II, Kirpotin SN. Chapter 6. Vegetation of the Subarctic and arctic of Siberia: Some types and approaches to the study. In: Pokrovsky OS, Kirpotin SN, Malov AI, editors. The Arctic: Current Issues and Challenges. New York: Nova Science Publishers; 2020. pp. 147–184. [Google Scholar]
- Vyshegorodtsev AA. Fish of the Yenisei: A Handbook. Science. Novosibirsk: Siberian Publishing Company RAS; 2000. [Google Scholar]
- Williams, F., R. Eschen, A. Harris, D. Djeddour, C. Pratt, R. Shaw, S. Varia, J. Godwin, et al. 2010. The Economic Cost of Invasive Non-Native Species on Great Britain. November 2010, CAB/001/09.
- WWF-Russia. 2013. Illegal logging in the Russian Far East: Global demand and taiga destruction, ed. A.G. Smirnov, B.J. Milakovsky, E.A. Lepeshkin, and D.V. Sychikov, 39. Moscow. https://www.worldwildlife.org/publications/illegal-logging-in-the-russian-far-east-global-demand-and-taiga-destruction.
- Yadrenkina EN. Distribution of alien fish species in Lakes within the temperate climatic zone of Western Siberia. Russian Journal of Biological Invasions. 2012;3:145–157. doi: 10.1134/s2075111712020117. [DOI] [Google Scholar]
- Yao Y, Luo D, Dai A, Simmonds I. Increased quasi stationarity and persistence of winter ural blocking and Eurasian extreme cold events in response to Arctic warming. Part I: insights from observational analyses. Journal of Climate. 2017;30:3549–3568. doi: 10.1175/jcli-d-16-0261.1. [DOI] [Google Scholar]
- Yorke, O. 2020. Deforestation in Russia: Depleting the Lungs of the World. Retrieved 20 November, 2020, from https://www.earthorg/deforestation-in-russia/.
- Yurganov, L. 2020. Methane flow from the Arctic seas: A view from space. Science and Life 5, The Circle 2.2019 (in Russian).
- Zabelin VI. On changes in the composition of the avifauna of the Altai-Sayan region due to climate warming. Russian Ornithological Journal. 2018;27:3803–3806. [Google Scholar]
- Zalibekov ZG. The arid regions of the world and their dynamics in conditions of modern climatic warming. Arid Ecosystems. 2011;1:1. doi: 10.1134/s2079096111010094. [DOI] [Google Scholar]
- Zuev IV, Vyshegorodtsev AA, Chuprov SM, Zlotnik DV. Modern composition and distribution of alien fish species in the water bodies of Krasnoyarsk territory. Russian Journal of Biological Invasions. 2016;4:324–332. doi: 10.1134/s2075111716040123. [DOI] [Google Scholar]
- Zyryanova OA, Milyutin L, Muratova EN, Ryzhkova VA, Larionova AY, Sedel'nikova TS, Korets MA, Mikhailova IA. Boreal forests of Siberia: Genetic, species and ecosystem diversity. Siberian Journal of Ecology. 2007;14:149–158. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.