Abstract
Calcium carbonate micro- and nanoparticles are considered as chemically inert materials. Therefore, they are widely considered in the field of biosensing, drug delivery, and as filler material in plastic, paper, paint, sealant, and adhesive industries. The unusual properties of calcium carbonate-based nanomaterials, such as biocompatibility, high surface-to-volume ratio, robust nature, easy synthesis, and surface functionalization, and ability to exist in a variety of morphologies and polymorphs, make them an ideal candidate for both industrial and biomedical applications. Significant research efforts have been devoted for developing novel synthesis methods of calcium carbonate particles in micrometer and nanometer dimensions. This review highlights different approaches of the synthesis of calcium carbonate micro- and nanoparticles, such as precipitation, slow carbonation, emulsion, polymer-mediated method, including in-situ polymerization, mechano-chemical, microwave-assisted method, and biological methods. The applications of these versatile calcium carbonate micro- and nanoparticles in the biomedical field (such as in drug delivery, therapeutics, tissue engineering, antimicrobial activity, biosensing applications), in industries, and environmental sector has also been comprehensively covered.
Keywords: Calcite, Vaterite, Drug delivery, Filler material, Precipitation
Introduction
Since few decades, nanomaterials have gained significant attention due to their wide applications in various fields, such as electronics, textiles, and various sectors of food and agriculture, environment, healthcare, etc. (Mauter and Elimelech 2008; Avouris 2010; Servin and White 2016; Boles et al. 2016; Yao et al. 2018; He et al. 2019). These applications are based on few intrinsic and unique properties of nanomaterials, including high surface-area-to-volume ratio, low-cost synthesis, easy surface modification, and high catalytic efficiency. Such properties make nanomaterials a suitable candidate for exhibiting controlled chemical reactions, drug delivery at the desired site, efficient energy and mass transfer, other industrial and biomedical applications (Wang et al. 2007, 2009; Helland et al. 2008; Byrappa et al. 2008; Du et al. 2013; Das et al. 2013; Stark et al. 2015). Nanomaterials could be broadly classified based on their composition, including metallic, metal oxides, carbon-based, and semiconductor-based particles. Among these, metal oxide-based nanomaterials have made tremendous progress due to their plethora of applications in industrial processes, such as catalysis, plasmonics, and fuel production (Rodríguez and Fernández-García 2007; Li et al. 2012; Hanus and Harris 2013; Dave and Chopda 2014; Dizaj et al. 2014; Corr 2016). Among oxides, calcium carbonate (CaCO3) nanoparticles (CCNPs) have attracted the attention of researchers due to their distinct physical and chemical properties (Combes et al. 2006; Murakami et al. 2007; Fakhrullin et al. 2009; Kim et al. 2014; Shaikh and Supit 2014). Calcium is the 5th most abundant element and 3rd most commonly found metal in the earth’s crust. The most common calcium-based compound is CaCO3 which accounts for around 4% of the entire earth’s crust. It is an essential raw material for both industries, fundamental and applied research. This allows CaCO3 to make its place in industrial applications, including paper, construction, pharmaceuticals, and biomedicines (Won et al. 2010; Lin and Chan 2012; Kawashima et al. 2013; Barhoum et al. 2014; Munyemana et al. 2018; Kiranda et al. 2018).
CaCO3 exists in three types of polymorphs: vaterite, aragonite, and calcite with varying shapes, such as needle (Chakrabarty and Mahapatra 1999; Lee et al. 2001), spherical, and rhombohedral, respectively (Barhoum et al. 2014; Hu et al. 2019b). Aragonite is the metastable form of CaCO3 with an orthorhombic crystal system, which is an important constituent of pearl secreted by mollusks and other related invertebrates (Boyjoo et al. 2014). It is a thermodynamically unstable isoform at standard pressure and temperature, however, can be converted into the calcite form over 107–108 years (Sulpis et al. 2018). Yu and co-workers have reported that at elevated temperatures, aragonite can be converted to vaterite. Secondly, vaterite is also a metastable phase of CaCO3 with a hexagonal crystal system (Demichelis et al. 2013). It is also known as mu-calcium carbonate (µ-CaCO3) (Ropp 2013) and found rarely in some fresh and seawater crustaceans (Luquet 2012). Vaterite shows better solubility in water than the corresponding three phases of calcium. Therefore, when vaterite is exposed to water at low and high (60 °C) temperature, it gets converted to stable calcite and aragonite, respectively (Ropp 2013). Calcite (also known as “Ubiquitous Mineral”) is the most common polymorph of CaCO3, which is found throughout the earth’s crust in sedimentary, metamorphic, and igneous rocks.
Unique chemical and physical properties of CaCO3 micro- and nanoparticles have attracted tremendous attention for research work (Won et al. 2010; Lin and Chan 2012; Kawashima et al. 2013; Barhoum et al. 2014). Owing to the low-cost synthesis, biocompatible, and biodegradable nature, CaCO3 micro- and nanoparticles have gained much attention in biomedical applications, including delivery of drugs, genes, and enzymes (Cai et al. 2006; Zhao et al. 2010; Qiu et al. 2012). The CaCO3 micro- and nanoparticles are also capable of acting as delivery vehicles for various hydrophobic and hydrophilic drugs. Because of the slow biodegradation of matrices, these CaCO3 micro- and nanoparticles can also be used as sustained drug release systems as they can aid in retaining the drug into the system for a longer time. The pH-sensitive CCNPs are preferred as a drug delivery agent (Shi et al. 2015), especially in case of cancers where the acidic tumor environment could trigger controlled drug release (Qiu et al. 2012). CaCO3 microparticles (CCMPs) are used in the manufacturing of plastics (Erdoğan and Eken 2017), whereas CCNPs are employed for developing silicones, urethanes, polypropylene (Sahebian et al. 2009), plastisols, polyvinyl chloride (PVC), etc. (Schlickmann et al. 2019). It also acts as a basic material for synthesizing adhesives (Elkassas and Arafa 2017), cement and sealants (Zamani et al. 2020), etc. In personal healthcare products, CCNPs are used in talcum powder, concealer, foundation, and eyeshadow, whereas the CCMPs work as additives in the body/face scrub, bath salts, and face wash. Figure 1 represents the applications of CaCO3 micro- and nanoparticles in various sectors, such as various industries, environmental and biological applications (Table 1).
Fig. 1.
Flowchart representing applications of CaCO3 micro- and nanoparticles in various sectors
Table 1.
Different properties of CaCO3 polymorph types. Images reprinted from open access article (Costa et al. 2017)
| Properties | Calcite | Vaterite | Aragonite | References |
|---|---|---|---|---|
| Stability | Most stable | Metastable | Thermodynamically unstable | Boyjoo et al. (2014), Demichelis et al. (2013) |
| Cleavage/shape | Rhombohedral | Spherical Shape | Rod-like/ needle like Shape | Chakrabarty and Mahapatra (1999), Lee et al. (2001), Barhoum et al. (2014), Hu et al. (2019b) |
| Chemical composition | Carbonate | Carbonate | Carbonate | Boyjoo et al. (2014) |
| Crystal lattice | Hexagonal | Hexagonal | Orthorhombic | Boyjoo et al. (2014), Demichelis et al. (2013) |
| Uses | Used in the paper industry, water treatment, construction material, agricultural soil treatment, pigment, pharmaceutical, acid neutralizer, sorbents, etc | Present in the molluscs shells, regenerative medicines, drug delivery, personal care products, etc | Used in cement and steel production, ornamental carvings, as a main component of pearl, coral, eggshells, helps removal of pollutants, like zinc, cobalt, and lead from contaminated wastewaters | Boyjoo et al. (2014), Mydin et al. (2018) |
| Density | 2.71 g/cm3 | 2.54 g/cm3 | 2.93 g/cm3 | Duraisamy (2016) |
| Crystals |
 〹 〹 |
 〹 〹 |

|
(taken from wikipedia) |
| SEM-Micrograph |

|

|

|
Costa et al. (2017) |
Synthesis methods of calcium carbonate micro- and nanoparticles
Depending on the need for applications, several techniques to synthesize CaCO3 micro- and nanoparticles are reported. Therefore, this section has been developed to provide a comprehensive analysis and discussion of the different methods of synthesis. Precipitation, emulsion, polymerization, and mechanical and biological methods are some of the common methods for synthesis of CaCO3 micro- and nanoparticles. Figures 2 and 3 depict how the CaCO3 micro- and nanoparticles can be synthesized using various methods, like mechanical, emulsion, precipitation, carbonation, and polymer-mediated technique.
Fig. 2.
Flowchart representing strategies for the synthesis of CMNPs
Fig. 3.
Methods for synthesis of CaCO3 micro- and nanoparticles: a the precipitation, b the CO2 bubbling (carbonation), c the emulsion (W/O), and d the mechanical (ball milling) method
Precipitation methods
The precipitation method is considered to be one of the most widely used methods to synthesize CaCO3 micro- and nanoparticles which includes slow carbonation and spontaneous precipitation reaction. The carbonation method involves the production of CO3−2 ions upon dissolution of CO2 in water or slow hydrolysis of compounds, like ammonium carbonate or dimethyl carbonate under alkaline conditions (Fig. 3a) (Guillemet et al. 2006). The precipitation method is one of the most studied approaches to synthesize naked CCMPs. This method does not involve any additives and allows simple mixing of the supersaturated solutions of sodium carbonate (Na2CO3) and calcium chloride (CaCl2) (Fig. 3a). In a typical synthesis, rapid mixing of equimolar (0.33 M) concentration of the reactants is performed for 30 s, which results in the formation of highly porous and spherical calcite microparticles of 4–6 µm (Fujii et al. 2010). The size of the CCMPs could be increased to 15–20 µm when the reaction time increases (Volodkin et al. 2004). Babou-Kammoe et al. reported the synthesis of CCNPs (~ 54 nm) by using the controlled precipitation method. The saturated solutions of Na2CO3 (0.1 M) and calcium nitrate (Ca(NO3)2) (0.1 M) were mixed at varying speed (300–30,000 rpm) and temperature (25–100 °C). The study revealed that the agitation speed was inversely proportional to the size of the CCNPs, whereas the temperature of the reaction system was directly proportional to the size of the CCNPs (Babou‐Kammoe et al. 2012).
Depending on the application, the precipitation method is reported to involve the addition of different molecules, including synthetic polymers, surfactants, and biomolecules, to synthesize CaCO3 micro- and nanoparticles of different sizes and shapes (Bing et al. 2007; Wang et al. 2010; Donatan et al. 2016). A few of the additive-based synthesis methods of novel CaCO3 micro- and nanoparticles are discussed below.
Lower concentration of biomolecular additives, such as soluble starch (Salek et al. 2016), dextran (Donatan et al. 2016), cellulose (Mohamadzadeh-Saghavaz et al. 2014), collagen (Tovani et al. 2019), and some proteins (BSA, bovine serum albumin), favor the formation of biomolecule incorporated CaCO3 micro- and nanoparticles (Fujiwara et al. 2008). The addition of these biomolecules to CaCO3 micro- and nanoparticles can synthesize a hybrid nanoparticle with better stability and bioavailability. Balabushevich et al. have demonstrated co-encapsulation of doxorubicin (DOX) and mucin [a high molecular weight (M.W.) glycoprotein] into the porous vaterite nanoparticles. The synthesis of the nanocomposite involved the mixing of (0.167 mg/mL) DOX and 0.167–10 mg/mL mucin, to 1 mL of 1 M CaCl2 and 1 mL of 1 M Na2CO3. The so-produced porous vaterite could encapsulate mucin as well as DOX and exhibited sustained release for long period of time. Subsequently, the metastable vaterite nanoparticles were recrystallized to thermodynamically stable polymorph calcite that led to the loss of porosity and thus release of the contents. The size of bare and hybrid complex was 47 ± 7 nm and 109 ± 25 nm, respectively (Balabushevich et al. 2019). Wei et al. have demonstrated the formation of carbonated hydroxyapatite microspheres from CaCO3 spheres within a matrix formed by collagen. In this synthesis method, CaCl2 was dissolved in acidic collagen and then added to sodium bicarbonate (NaHCO3) and concentrated aqueous phosphate solution. The resulted collagen–hydroxyapatite microsphere hydrogels were used as a scaffold for drug delivery and tissue engineering (Wei et al. 2015). Tovani et al. reported the synthesis of type-I collagen adsorbed on tube-like CCNPs of 100 nm, 200 nm, and 400 nm size. This complex was used for bone–tissue mimetic matrix formation to promote cell proliferation and as a filler to provide mechanical strength to organic scaffolds (Tovani et al. 2016). Wei et al. established a one-pot synthesis of ~ 500 nm hollow spheres of vaterite. In this method, 2 mM concentration of calcium and carbonate precursor ions were reacted in the presence of soluble starch. Upon altering the concentration of the precursor ions to 5 mM and 12.5 mM, the polymorphs transformed into cubic calcite, and spherical vaterite nanoparticles, respectively (Wei et al. 2008). Subsequently, Wang et al. synthesized carboxymethyl chitosan-based CaCO3 (CMC/CaCO3) hybrid nano- and microspheres. Hybrid microspheres of 5 µm were prepared by addition of aqueous CMC to equimolar (0.5 M) Na2CO3 and CaCl2 followed by 12 h incubation. Upon lowering the concentrations of the reactants (0.02 M) and the reaction time (5 h), nanospheres of 400 nm were obtained. These synthesized micro- and nanoparticles exhibited high encapsulation efficiency along with sustained drugs release (hydrophilic drugs) (Wang et al. 2010).
The addition of an appropriate concentration of synthetic polymers led to the development of spherical vaterite particles of various shapes, including pyramid, hollow spherical, and peanut like structure (Cölfen and Qi 2001; Yue et al. 2004; Bastakoti et al. 2011). The spheres formed were usually reported to have a rough surface with high porosity because of nanocrystalline aggregates embedded in the polymer scaffold. The shape, size, and surface morphology of the so-developed CaCO3 micro- and nanoparticles depend on the polymer used in the reaction. Polyvinyl alcohol (PVA) with high M.W. (89,000–98,000 g/mol) was demonstrated to stabilize the synthesized polylactic acid-based CCMPs of size ~ 2.5 µm (Kudryavtseva et al. 2017). The change in the PVA concentration did not affect the shape, size, and morphology of CCMPs. However, with an increase in the PVA concentration, the polydispersity of the particles was found to be lowered (Kudryavtseva et al. 2017). Saveleva et al., developed polycaprolactone-based CaCO3 (PCL-CaCO3) scaffolds, where porous vaterite particles were coated on to PCL matrix (Saveleva et al. 2018). Here, the thermodynamically unstable vaterite gets converted to stable crystalline cubic calcite crystals. This complex with particles embedded in the PCL matrix has shown potential application as a scaffold for bone reconstruction and drug delivery (Saveleva et al. 2018). In a report by Donatan et al. a new strategy is developed to form stable vaterite particles (Donatan et al. 2016). They demonstrated the layer-by-layer coating of polyelectrolytes, poly alkylamine hydrochloride (PAH, M.W. 70 kDa), and poly(sodium 4-styrene sulfonate) (PSS, M.W.70 kDa) on to ethylene glycol (EG)-coated vaterite particles. The high viscosity of EG prevents the diffusion of ions and the solubility of the crystal. EG along with PAH and PSS prevents the recrystallization of metastable vaterite to calcite. This method involved the various concentrations of EG (25%, 50%, and 80%) along with the varying ratio of CaCl2/Na2CO3 from saturated equimolar (1 M:1 M) to non-equimolar (0.1 M:1 M and 1 M:0.1 M) concentrations. High and lower concentration of CO3−2 were responsible for the formation of anisotropic and spherical nanoparticles, respectively. The synthesis resulted in the production of anisotropic vaterite isoforms of CCNPs of distinct shapes of ellipsoidal, rhomboidal, star-like, and isotropic spherical particles (Donatan et al. 2016).
Apart from the above-mentioned methods, other precipitation methods include the use of surfactants, like sodium dodecyl sulfate (SDS), diaminodiphenyl sulfone (DDS), sodium dodecylbenzene sulfonate (SDBS), and cetrimonium bromide (CTAB) (Kang et al. 2005; Barhoum et al. 2014). The synthesis of CaCO3 micro- and nanoparticles is also mediated by several other components, such as amino acids, copolymers, and chelating agents.
Carbonation is another most common approach for the large-scale synthesis of CCNPs. This technique includes the bubbling of CO2 into slaked lime (Ca (OH)2) to produce CaCO3 particles (Fig. 3b). The crystals synthesized by this method are rhombohedral and cubic calcite. The synthesis of the calcite phase is not affected by high pressure, minor fluctuations in temperature, or the addition of polymers, and surfactants. Rautaray et al. have demonstrated the bubbling of CO2 into the mixture of equimolar concentration (10–3 M) of CaCl2 and phosphotungstic acid, which resulted in the formation of monodisperse calcite particles just after 1 h. A clear transition of the particles from a single rhombohedral structure of size ~ 500 nm at 12 h to a fascinating star-shaped assembly of calcite particles sized over a few microns was observed at 48 h (Rautaray et al. 2003). Wang et al. have also explored the in-situ carbonation method for the synthesis of CCNPs in the presence of organic substrate. Hydrophobic CCNPs was yielded due to addition of C18H37OPO3Ca which exhibited spindle shape, with a diameter of < 100 nm (aspect ratio of 1:3.5) (Wang et al. 2006).
Thus, the precipitation route for synthesizing CaCO3 micro- and nanoparticles is found to be a facile, cost-effective, and one-pot approach to produce particles of controlled size, shape, and morphology. Also, it offers a variety of applications in the fields of biomedicine, such as drug delivery, biosensing, and tissue engineering (Kiranda et al. 2018). Table 2 summarizes different applications of CCMPs and CCNPs synthesized by precipitation method.
Table 2.
Applications of CaCO3 micro- and nanoparticles synthesized by precipitation method
| Sr no | Size (nm) | Shape/Nature | Reactants | Experimental conditions | Applications | References |
|---|---|---|---|---|---|---|
| 1 | 2000 | No | CaCl2, Na2CO3 = 615 µL (1 M), BSA 1 mL | The solution was stirred for 30 s and then washed thrice with water and ethanol. Precipitates were dried in an oven overnight | Targeted delivery of bone growth factors | Kudryavtseva et al. (2017) |
| 2 | 500–1000 | Spherical, Semi-crystalline | Polycaprolactone solution = 9.4% weight (wt) CaCl2 and Na2CO3 = 1 M | All experiments were performed using ultrasonication with 35 kHz frequency and 0.64 W/cm2 radiation intensity | Tissue engineering and tissue regeneration showed antioxidant properties and also helped in bone reconstruction with improved functionality of targeting drug delivery | Saveleva et al. (2018) |
| 3 | 64 | Spherical at 25 ºC Rods at a higher temperature, Crystalline | Na2CO3 and Ca (NO3)2 = 0.1 M, NaOH and NaNO3 = 0.2 M | The temperature and stirring rate were varied from 25 to 85 °C and 3425 to 15,900 rpm respectively | Used to replace conventional films, like fumed silica and carbon black, for improving the mechanical and physical properties of the developed product | Nassar et al. (2015) |
| 4 | 47 ± 7 | Spherical, Crystalline |
Tris buffer = 3 mL (0.05 M) Mucin = 0.167 to 10 mg mL−1 DOX = 0.167 mg mL−1, CaCl2 and Na2CO3 = 1 mL (1 M) |
The suspension was stirred for 5 min at 100 rpm. Precipitates were separated using centrifugation for 2 min at 1000 rpm and were then air-dried | Controlled discharge of small hydrophilic drugs | Balabushevich et al. (2019) |
| 5 | 10 | Spherical, Amorphous |
Glycine, NaCl and NaOH = 1 mol/L, Ca (OH)2 = 1% Hyaluronic acid = 1 g/L |
High-pressure liquid chromatography was used for 10 mL/min. The solution was stirred at 1000 rpm for 5 min at the same time in the autoclave. The final pressure of the autoclave was kept at the range of 220–240 bars. The solution was centrifugated at 4000 rpm for 15 min and washed with DI water and air-dried for 48 h | Used as an intranasal carrier of insulin and hydrophilic compounds | Achour et al. (2017) |
| 6 | 2000 | Spherical, Crystalline |
Collagen solution = 20 mL (4 mg mL−1) Na2CO3 = 21 mL (0.22 M) |
The experiment involves slow continous stirring at 4 °C. The solution was degassed using a centrifuge and later incubated at 35 °C for 48–120 h | Used in bone tissue designing and supports scaffolds for surgical repair and medical reconstruction | Wei et al. (2015) |
| 7 | 100 | Spherical, Crystalline |
PAA = 0.1 wt% CaCl2 = 0.05 mol L−1 |
PAA/CaCl2 hybrid membranes were kept in a sealed desiccator containing (Na4)2CO3 at RT for 12 h. The templates were removed using CHCl3 using centrifugation for 5 min at 11,000 rpm. The precipitates were reserved |
Used as a drug delivery system Used as molecular detection agents for imaging, and the proliferation of osteoblasts |
Tovani et al. (2016) |
| 8 | 400 | Tubular, Crystalline |
PAA = 0.1 wt% CaCl2 = 0.05 mol L−1 Titanium disk = 13 mm × 1.5 mm span 20 = 4 × 10–5 mol L−1, Acetic acid = 4.5 mL (5 mmol L−1) n-Propanol = 500 µl |
PAA/CaCl2 hybrid membranes were kept in a sealed desiccator containing (Na4)2CO3 at RT for 12 h. The templates were removed using CHCl3 using centrifugation for 5 min at 11,000 rpm. Titanium disk was immersed in CaCO3 containing aqueous solutions for 15 min to obtain PAA coated CCNPs | The scaffold could be used as a substitute material for bone | Tovani et al. (2019) |
| 9 | 70–100 | Spherical/Tubular, Amorphous |
CaCO3 solution = 500 mL (8 wt%) sodium silicate = 180 mL, SiO2 = 2 wt% |
The temperature was kept at 80 °C with vigorous stirring for 2 h. Rinsed with DI water and dried at 100 °C | Not Specified | Chen et al. (2004) |
| 10 | 30–50 | Spherical, Amorphous |
AgNO3 = 45 mg 1% trisodium citrate = 5 mL, Ca(NO3) and Na2CO3 = 50 mL (0.03 M), (PSS) Poly(sodium 4-styrene sulfonate) = 4.8 g/L |
The prepared mixture of particles was kept in an ultrasonic bath and heated at 75 °C for 60 min. The resultant was sonicated at 25 °C for 5 min. The colloid suspension was rinsed with DI water and centrifuged for 5 min at 4000 rpm |
Anti-microbial activity and effective in preventing the propagation of microorganisms | Długosz et al. (2012) |
| 11 | 1.68 |
Rhombohedral Crystalline |
CaCO3 = 11.7 g CO2 = 2 dm3 |
Dissolution time (2.5, 5.0, 7.5, and 10.0 min), calcination temperature (800, 850, 900, 950, and 1000 °C), and precipitation time (2.5, 5.0, 7.5, 10.0, 12.5, and 15.0 min) | Used in paper and plastic industry for coating and as a filler material | Erdoğan and Eken (2017) |
| 12 | 5000–10,000 | Sphere and Needle-shaped, Crystalline | CaCl2 and K2CO3 = 2 M | The procedure was done at 30 °C and refluxed at 100 °C. Precipitates obtained were filtered by Whatman 40 filter paper and dried for 24 h at room temperature | Used in a variety of personal care products | Sarkar and Mahapatra (2010) |
| 13 | 20–30 | Tubular, Crystalline |
PAA = 1800 g mol−1 (0.1 wt%) CaCl2 = 0.05 mol L−1 |
PAA/CaCl2 hybrid membranes were kept in a sealed desiccator containing (Na4)2CO3 at room temperature for 12 h. The templates were removed using CHCl3 using centrifugation for 5 min at 11,000 rpm | Used in varied areas, such as microelectronics, catalysis, optical, electronic, and attractive gadgets, high-performance ceramics, sensors, and biomaterials | Tovani et al. (2016) |
| 14 | 50–90 | Ellipsoidal, Crystalline |
NH4CO3 = 0.5 M SDS = 0.7 g and 1.25 g, CaCl2 = 0.25 M |
An ultrasonication process was used | Acts as nanofillers, as a binder in the polymer and paint industry | Shimpi et al. (2015) |
| 15 | ~ 400 | Spherical, Crystalline |
CaCl2 and Na2CO3 solution = 10 mL (1:1 M; 0.1:1 M; 1:0.1 M) EG = 25%, 50%, 80% |
The mixture was stirred for 30, 60, and 90 min at 600 rpm. The resulting suspension was rinsed thrice with DI water and ethanol and later dried for 60 min at 70 °C | Acts as a good nano-drug delivery platform in clinical applications | Xing et al. (2020) |
| 16 | 40 | Spherical, Crystalline |
CaCl2 and Na2CO3 solution = 10 mL (1:1 M; 0.1:1 M; 1:0.1 M) EG = 25%, 50%, 80% PAH (poly(allylamine hydrochloride) and PSS = 2 mg/mL, NaCl = 0.5 M |
The mixture was stirred for 30, 60, and 90 min at 600 rpm. The resulting suspension was rinsed thrice with DI water and ethanol and then dried for 60 min at 70 °C. PAH, PSS, and NaCl having a concentration of 2 mg/mL each was mixed along with CaCO3 templates using the layer-by-layer deposition method. After centrifugation, the precipitates were collected by centrifugation at 3000 rpm for 3 min and washed with distilled water | As a carrier for the delivery of drugs due to its highly porous arrangement and biocompatibility | Donatan et al. (2016) |
| 17 | 500 | Spherical/ Rods/ Ellipsoidal, Crystalline | CaCl2 = 40 mL (0.2 M) CTAB = 0.01 M, 1,3-dimorpholin-propylene = 10–3 mol, Na2CO3 = 40 mL (0.21 M) | The experiment was done at room temperature. The obtained precipitates were washed by DI water and ethanol. They were then dried for 24 h at RT | Used for coating in the cosmetic industry | Gao et al. (2011) |
Emulsion methods
Emulsion method is a type of controlled precipitation reaction frequently used to synthesize micro- and nanosized particles (Desgouilles et al. 2003; UskokoviĆ and Drofenik 2005; Anton et al. 2008). The microemulsion method was first reported by T.P Hoar and J.H. Shulman in 1943, which involves a mixture comprising of oil, water, surfactants (Tween 20 or Tween 80) and, co-surfactants (hexadecane or cetyl alcohol). The interaction of the oil phase and aqueous phase shows a repulsive effect; however, the presence of surfactant molecules imparts an attractive effect (Fig. 3c). The whole system is stabilized by the surfactant, which produces different types of microstructures similar to micelles with strong electrostatic interaction. The nano–microemulsions can be of three distinct types, O/W (also known as a reverse emulsion, oil in water, where water is in persistent phase), W/O (water in oil where oil is in persistent phase), and O/W/O (also known as a double emulsion, where oil is dispersed in water, which is again dispersed in oil) (Hirai et al. 1997; Wu et al. 2008; Shum et al. 2008, 2009). O/W/O is the most complicated method compared to the former two approaches (Sun and Deng 2004).
CaCO3 micro- and nanoparticles are mostly synthesized by reverse emulsion (O/W), i.e., oil in water emulsion approach, where emulsion is stabilized by the presence of surfactants and co-surfactants. This complex often referred to as micro/nanoreactors, functions as a template for the synthesis of nano- and microsized particles (Sun and Deng 2004; Du et al. 2013; Han et al. 2019). In this method, the reactants (Ca+2 and CO3−2 ions) were enclosed in micellar structures formed by surfactant and were separated by hydrophobic phase (Pai and Pillai 2008). When the microemulsion was mixed, the micelles collide and resulted in the mixing of reactants. This controlled mixing of the reactants leads to the synthesis of CaCO3 micro- and nanoparticles with controlled shapes, sizes, and morphology (Shen et al. 2007). This approach could also be used for the microgram–gram-scale synthesis of the product. The shape, size, morphology, and phase of the synthesized CaCO3 micro- and nanoparticles depend on various parameters, including reaction temperature, pH, oil–water ratio, water:surfactant concentration ratio, reactant ions (Ca+2: CO3−2) molar ratio (¥), and the type of additive used. In the case of the microemulsion, the surfactant facilitates the formation of a micelle that acts as a template for controlling the size and shape of the nanoparticles. This approach aids the solubility of the lipophilic drug as well as water-soluble drugs encapsulated in their aqueous phase. The microemulsion method is also resistant to harsh conditions, like high temperature, extreme pH, salinity, and pressure. The emulsion system is also reported to retain the morphology even with the change in oil volume fraction (Chen and Tai 2010). This method can be applied in food, paper, pharmaceutical industries, and also for the preparation of various types of nanoparticle composites (Ganguli et al. 2010; Jha et al. 2011; Lu et al. 2013, 2016; Pournajaf et al. 2014; Tojo et al. 2014; Liu et al. 2015a; Pineda-Reyes and Olvera 2018).
Kang et al. demonstrated the crystallization of CaCO3 by the reaction of Na2CO3 and CaCl2 in an aqueous medium. The so-formed micelles were stabilized by anionic SDS and sodium bis(2-ethylhexyl) sulfosuccinate (AOT), which controls shape, size, and crystal structure of CaCO3 (Kang et al. 2005). It was observed that upon increasing the surfactant concentration in the reverse micelle, unstable vaterite formation was favored. When the ratio of water:surfactant (S) was kept lower (S < 30), it resulted in a stable reverse micelle of spherical shape containing crystal mixture of vaterite and calcite crystals, whereas upon reducing the volume of surfactant (S > 30), a two-phase separation was observed. This resulted in the formation of crystals of vaterite and calcite of irregular shape (Kang et al. 2005). Tai and Chen reported that, when surfactants, like AOT, and additive, like sodium triphosphate is used, the water:surfactant concentration ratio had greater influence than water:oil ratio on size and shape of CaCO3 micro- and nanoparticles. Among the different water:oil ratios, spherical-shaped particles of submicron dimension (100–500 nm) were produced at 12:3 water:surfactant ratio. Irregular shapes or submicron- and micron-sized disks were formed with water:surfactant concentration ratio of 14.0 and 16.0 (Tai and Chen 2008). The alteration in ¥ leads to various effects on morphology, size, and shape of nanoparticles. The addition of additives also helps in influencing the polymorph of the crystals (Kang et al. 2005). Chen and Tai reported the formation of doughnut-shaped particles having the ¥ value as 1 and 4. The size of the particles obtained was about 1–3 microns; however, with a ¥ of 0.5, hexagonal plates (1 micron diameter) were formed at 25 ºC but without any additives (Chen and Tai 2010). Thachepan et al. demonstrated the formation of aragonite phase at ambient temperature using AOT as a surfactant and additives, like diethylenetriamine (DETA) and ethylenediamine (EDA) at ¥ = 1 where the formation of calcite crystals was favored (Thachepan et al. 2006).
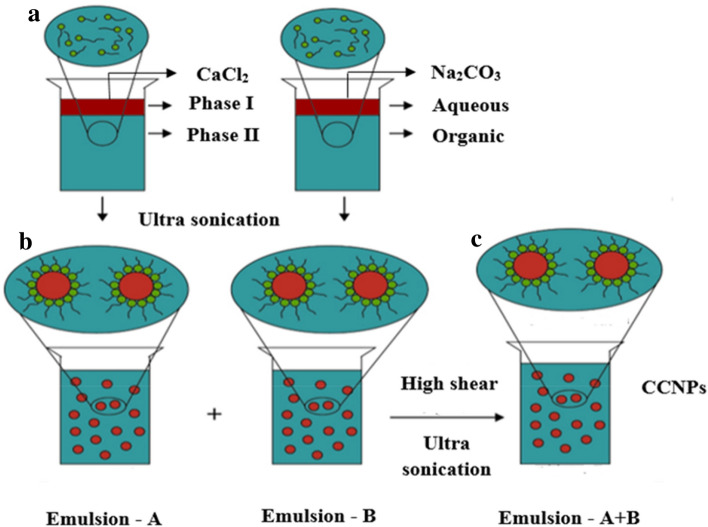
Using the W/O/W approach Hirai et al. demonstrated the biomimetic synthesis of CCNPs in the pseudo-vascular double emulsion (Hirai et al. 1997). Micron-sized particles were derived by aqueous precipitation of W/O/W double emulsion method. Upon increasing the pH of internal aqueous phase, the polymorphic form of CaCO3 particles changed to micron-sized crystalline rhombohedral calcite from aggregated vaterite (Hirai et al. 1997). Subsequently, Badnore and Pandit used a novel sono-chemical method for CCNPs synthesis by reverse miniemulsion technique using ultrasonication. This method was found to be more effective than the conventional reverse miniemulsion method because it gives 100% yield and significantly reduces the time of reaction and also saves ~ 80% energy than the conventional method. Figure 4 indicates CCNPs synthesis method through the miniemulsion technique using the ultrasonication method. The method produced uniform particles of size 24 ± 5 nm. The highest yield (100%) was obtained at 40% amplitude with spherical vaterite particles of 20 nm size (Badnore and Pandit 2015). Chen and Nan demonstrated the change in the morphology and polymorph of CaCO3 crystals by using a mixture of surfactants in the emulsion. Here, SDS and CTAB were used as surfactants to mediate growth and nucleation of CaCO3 crystals. At a fixed SDS concentration (0.1 mM) and with increase in CTAB concentration, the particles phase changed to pure aragonite from vaterite. Various morphologies, such as cluster-shaped aragonite and flower-shaped calcite, were obtained by varying the concentration of SDS and CTAB in the emulsion mixture (Chen and Nan 2011). Time-resolved experiments were also performed, which concluded that eventually the hexagonal subunits were clustered by the surface-induced aggregation to form flower-like CaCO3 crystals (Chen and Nan 2011).
Fig. 4.
Schematic representation of synthesis of CCNPs. Two miniemulsions preparation consisting of Ca+2 and CO3−2 ions (a) was performed by mixing two miniemulsions (b) and then applying high shear to synthesize CCNPs (c).
“Reprinted with permission from 2015 Elsevier” Synthesis of Nanosized CaCO3 Using Reverse Miniemulsion Technique: Comparison between Sonochemical and Conventional Method
In summary, the emulsion method is a facile one-pot synthesis procedure that facilitates the formation of CaCO3 micro- and nanoparticles of various morphologies by varying the reactants and additives. These synthesized CaCO3 micro- and nanoparticles could be used for a wide range of applications. Despite several advantages, the emulsion method also involves certain limitations, for example, the need for high amount of co-surfactant and surfactant to stabilize the droplets, and it also limits the phase separation and suffers from a lack of homogeneity. Table 3 comprises synthesis of CCMPs and CCNPs using emulsion method.
Table 3.
CaCO3 micro- and nanoparticles synthesized by emulsion method.
| No. | Size (nm) | Microemulsion Composition | Surfactants | Results | References |
|---|---|---|---|---|---|
| 1 | 60 | Bis(2ethylhexyl) phosphoric acid, sodium hydroxide (NaOH), NaNO3, Na2CO3, span 83, Ca(NO3)2 | Span 83 | CCNPs were synthesized in a pseudo-vesicular system by double emulsion method in which size, structure, and mineralizing phase within the vesicle can be controlled using the aqueous phase composition. It was found that with increase in pH of the aqueous phase the vaterite particles aggregate changed to calcite of micro-sized single-crystalline rhombs | Hirai et al. (1997) |
| 2 | 10–60 /80–100 | Lubrizol (polybutene–succinimide pentamine), cyclohexane, CaCl2, Na2CO3 | Lubrizol (polybutene–succinimide Pentamine) | Amorphous CaCO3 was synthesized by miniemulsion by using surfactants. Higher surfactant concentration which increases the reactive functional groups could help in better CaCO3 binding efficiency and adsorption ability | Pai and Pillai (2008) |
| 3 | 1000–5000 | n-Dodecane oil, 1% w/v stearic acid, 0.01 M NaOH, CaCl2/MgCl2 solution | Stearic acid, DHBC |
A core–shell particle with an outer wall of CaCO3 was synthesized using the microemulsion method. The method is useful in templating porosity into the mineral shells Such a system is used in extraction processes, such as overdosed drug uptake from the blood |
Patel et al. (2004) |
| 4 | 5–20 | CaCl2, Na2CO3, span 80, tween 80, toluene, methanol (CH3OH) |
Tween 80 Span-80 |
The CCNPs were synthesized using growth and collapse of microbubbles under the ultrasonic field influence in the solution The method shortens the reaction time and saves more than 80% of energy, and controls the morphology and size of the nanoparticles |
Badnore and Pandit (2015) |
| 5 | 5–25 | P-octyl polyethylene glycol phenyl ether (OP), and n-amyl alcohol, cyclohexane, CaCl2, Na2CO3, NaOH, acetone, ethyl alcohol, HCl, DL-Aspartic acid (Asp) | P-octyl polyethylene glycol phenyl ether (OP), and n-amyl alcohol, Cyclohexane, DL-Asp | CaCO3 nanocrystals with various polymorphs and multi-morphologies were controlled in the DL-Asp/OP bicontinuous microemulsion by maintaining the pH. Nucleation and growth of CaCO3 nanocrystal in OP bicontinuous microemulsion were done. DL-Asp was an important factor inducing the vaterite stable phase | Shen et al. (2007) |
| 6 | Not reported | Span -60, Tween-80, non-ionic surfactant, benzene, CaCl2, potassium carbonate, polypropylene | Span -60, Tween-80, Non-ionic surfactant | The shape and crystal structure were affected by the mixing method in the emulsion phase where the vaterite was used as a nucleating agent for polypropylene and the composite’s tensile strength with vaterite was greater than other crystal forms | Lyu et al. (1999) |
| 7 | 40/36/21 | CaCl2, Na2CO3, span-80, tween-80, toluene (99%), CH3OH, Stearic acid, | Tween-80, Span-80 |
Different sizes of surface-modified CCNPs were synthesized using a reverse microemulsion method With the increase in the concentration of surfactants, the water–surfactant molar ratio reduces, resulting in the developed nanoparticles size reduction |
Ghadami Jadval Ghadam and Idrees (2013) |
| 8 | < 100 | APS, SDS and n-pentanol, triethoxyvinyl silane (TEVS), PMMA | SDS and n-pentanol | Nano-CaCO3/PMMA core–shell particles with nano-CaCO3 acting as a core and PMMA working as a shell were synthesized successfully with the help of an atomized microemulsion method. This method of synthesis leads to wider industrial usage of core–shell nanoparticles because of their thermal stability and better dispersion | Chatterjee and Mishra (2013) |
| 9 | 1–3 | AM, MBAM, PVA, CaO, FC4430 and potassium persulfate (K2S2O8, KPS) | FC4430 (Fluro surfactant), PVA | Porous CaCO3/PAM composites were formed using CO2 in the water emulsion templating method. It involves the production of inorganic/polymer composites hydrogels, which are porous, by combining the emulsion templating with a reaction | Bing et al. (2007) |
| 10 | 562 | CaCl2 and Na2CO3 solutions, SDS, DTAB (dodecyl trimethylammonium bromide), n-Hexane | n-Hexane, DTAB, SDS | Vaterite nanocrystals were obtained in the stable water–oil emulsion with a high amount of non-ionic surfactants. Any decrease in surfactant content results in the precipitation of vaterite and aragonite crystals of a bigger size | Szcześ (2009) |
| 11 | 100 | CaCl2.2H2O, Na2CO3, SDS; CH3–(CH2)11–O–SO3–Na + ; iso-octane, CH3OH and 10X phosphate-buffered saline (PBS) | SDS | Micron-sized vaterite CaCO3 capsules were synthesized by the double emulsion method. The sulfate group of surfactants SDS which is negatively charged plays an important role to stabilize the vaterite polymorph which is metastable. The calcite capsules were developed from vaterite capsules after calcination at 500 °C. After being immersed in a PBS solution for 5 days, these vaterite capsules begin to collapse, showing their biodegradability which can have promising applications in controlled release of drug | Wu et al. (2008) |
Mechano-chemical methods
Mechano-chemical approach of synthesis is also known as mechano-chemistry. It is a process that involves mechanical breaking and chemical reaction for the production of the CaCO3 micro- and nanoparticles. Mechanical methods that are widely practiced for particle synthesis include milling, membrane filtration, microwave-assisted synthesis, etc. (Khulbe and Matsuura 2018; Hu et al. 2019b). Ball milling is one of the common methods that involves grinding of big particles using a reactor with high pressure and energy (Fig. 3d). The grinding, blending, and mixing of materials is carried out for several applications, such as in paints and synthesis of ceramics, etc. (Alshora et al. 2016). Industries working on ceramics, pebbles, and stainless steel use high-power milling for the synthesis of nanosized particle powder, which is also known as mechanical alloying or attrition (Piras et al. 2019). Membrane filtration is a pressure-driven separation technique that involves the separation of the particles based on the pressure applied, involving the use of a membrane for the separation of particles (Tsuzuki et al. 2000). Here, the basis of separation is not only the pore size of the filter used but also the physical size, charge, or affinity of the particles (Rojac and Kosec 2010). Using the mechano-chemical process, several nanoparticles of cobalt oxide, zinc oxide, silicon metal sulfide, CaCO3, barium titanate, etc., are reported to be synthesized (Shi et al. 2000; Ao et al. 2006; Serra-Gómez et al. 2012; Xu et al. 2014). The equipment setup for the mechano-chemical method is cost-effective and enables the agglomeration-free production of nanoparticles with narrow particles size distribution. Apart from these above-mentioned advantages, this method also offers nano-powders with improved sinterability that helps in the preparation of thick films by screen printing technology (Rojac and Kosec 2010). All these advantages are thought to be due to high rate of mass transfer, i.e., the net movement of mass across the membrane. This method can also have some drawbacks, such as the rusting of milling balls which may hinder the synthesis process of nanoparticles and cause contamination (Wang et al. 2007).
In another approach, Qi and Zhu developed a quick microwave-assisted method to synthesize vaterite form of CCNPs. They used a water/EG system with CTAB and SDS to obtain various morphologies. The reagents were mixed at room temperature followed by microwave-assisted nucleation at 100 ºC (Qi and Zhu 2006). They studied all the possible reaction conditions, such as microwave heating time, surfactant type and its concentration, reaction time, and water-EG mixed solvent. From these parameters, it was observed that these parameters play a crucial role in controlling morphology of the synthesized vaterite nanoparticles. Results revealed the formation of self-assembled dagger-shaped vaterite particles in the presence of SDS as a surfactant. However, spherical microspheres were observed when CTAB was used (Qi and Zhu 2006). Similarly, Skubiszewska-Zięba et al. synthesized calcite particles by mechano-chemical technique and microwave-assisted hydrothermal process (Skubiszewska-Zięba et al. 2017). Their study revealed that the synthesized particles were smaller in size (~ 917 Å) at reaction time 30 min, and of slightly bigger size (1126 Å) after 60 min of reaction. The particles synthesized using hydrothermal method in a high-pressure microwave reactor resulted in the large-sized crystallites (2084 Å) at 30 min of reaction, whereas the size of particles was noted to be 2500 Å after 60 min of reaction (Skubiszewska-Zięba et al. 2017). Tsuzuki et al. used a ball milling technique for the synthesis of calcite nanoparticles, where a solid displacement reaction takes place between Na2CO3 and CaCl2 in a miller which resulted in an ultrafine powder of CaCO3 and NaCl. The milled ultrafine powder was then given a heat treatment at 350 ºC to form a stable calcite form in the NaCl matrix. Then, the calcite particles were isolated from the matrix. The obtained results showed that the isolated particles had a narrow size distribution. Also, the volume fraction of CaCO3 in the milled product affected the mean particle size; 10% and 20% volume fraction of CaCO3 in the product resulted in particles of size ~ 77 nm and ~ 142 nm, respectively (Tsuzuki et al. 2000). Similarly, other mechano-chemical techniques, such as planetary ball milling (Hu et al. 2019b) and roller ball-mill methods (Kiranda et al. 2018), have also been utilized for the formation of different polymorphs of calcium with varying morphologies.
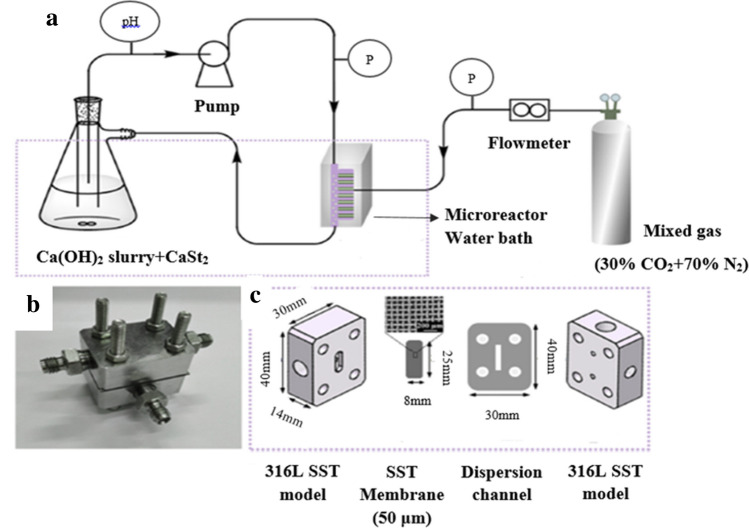
Apart from the microwave heating and milling techniques, another mechanical approach for the synthesis of CCNPs is the membrane filtration method. This technique involves the use of membrane as a mode of filtration; thus, the pore size of the membrane is a crucial component to determine the particle size. Wang et al. have used a membrane dispersion minireactor for synthesizing CCNPs using a simple CO2–Ca (OH)2 precipitation reaction. Upon tuning the experimental conditions, like mass transfer rates of CO2, the flow rate of disperse phase and continuous phase, and the volume fraction of calcium hydroxide [Ca (OH)2], they obtained 34.3–110 nm size-ranged CCNPs with a narrow size distribution. It was also found that the mass transfer density of CO2 was inversely related to the particle size of CaCO3 formed (Wang et al. 2007). Recently, Han et al. used a similar approach for the synthesis of CCNPs. They used an in-situ approach to prepare and modify the surface of CCNPs using membrane dispersion microreactor. Figure 5a illustrates the synthesis of CCNPs in a microreactor using Ca(OH)2 slurry, calcium stearate (CaSt2), and mixed gas (30% CO2 and 70% N2). The digital image of the microreactor used shown in Fig. 5b, c represents the parts of the membrane dispersion microreactor in detail, which includes a 316L stainless steel (SST) model having the dimensions 14 mm × 40 mm × 30 mm, a SST Membrane (50 μm) having dimensions of 25 mm × 8 mm and a dispersion channel of 40 mm × 30 mm. The method involves the carbonation of Ca(OH)2 in the microreactor in the presence of a surfactant and CaSt2 for the synthesis of hydrophobic CCNPs of ~ 34 nm with surface area of ~ 30 m2/gram. The particles also exhibited excellent hydrophobicity with a contact angle of 107.8º and the hydrophobic powder had aggregate size of ~ 250 nm. However, the aggregates of hydrophilic powder showed a size limit of 2000–4000 nm (Han et al. 2019). From the membrane-assisted methods for synthesizing the CCNPs, it was also presumed that the relation between temperature and particle size/diameter have a direct proportional relation.
Fig. 5.
a Schematic diagram of synthesizing CCNPs using in-situ modification process. b The microreactor’s digital image. c Membrane dispersion microreactor’s detailed structures.
Reprinted with permission from reference (Han et al. 2019) Copyright © Elsevier
In summary, this technique can be used to produce agglomeration-free nanoparticles with narrow size distribution (Tsuzuki et al. 2000). The microfiltration membrane method can be used for the synthesis of contamination-free CCNPs (Wang et al. 2007). The use of the milling technique showed the key role of these CCNPs in cytotoxicity assay and anticancer effects. The as-synthesized particles can be used for various environmental and biological applications, such as to monitor the quality of drinking water, bacterial contamination in pharmaceuticals, cosmetics, food, electronics, beverage industries, dairy processing, as pigments, adsorbent to remove heavy metals from water, and in biogas purification, without any further treatment or surface modification (Liu et al. 2015b; Kiranda et al. 2018). Table 4 contains different CCMPs and CCNPs synthesized using mechano-chemical method.
Table 4.
CaCO3 micro- and nanoparticles synthesized by mechano-chemical method
| No. | Size (nm) | Nature | Method of synthesis | Results/Observations | References |
|---|---|---|---|---|---|
| 1 | 77–142 | Amorphous and Crystalline | Mechano-chemical | Vaterite and calcite (ACC) form of CCNPs were synthesized at low and high milling speed in the presence and absence of sodium hexametaphosphate, respectively | Hu et al. (2019a) |
| 2 | 11.56–180.06 | Crystalline | Ball-milling | The energy-dispersive spectroscopy (EDX) data confirmed that the mechano-chemical method helped to obtain CCNPs using Achatina fulica shell. TEM data suggested that CCNPs were in semi-spherical shape. The synthesized CCNPs had potential to modify the mechanical strength and stiffness of the polymer | Gbadeyan et al. (2020) |
| 3 | 3200–6800 | Crystalline | Ball-milling | The synthesized CCNPs’ size was depended on milling speed, number of zirconia balls used, and the collision duration. Using this method, industrial grade CCNPs can be synthesized | Sulimai et al. (2018) |
| 4 | 19–51 | Not specified | Roller ball-milling | The CCNPs was environment friendly as synthesized using biogenic raw material, i.e., cockle–shell (obtained naturally from the molluscs from sea water). The CCNPs can act as good biomaterial for biomedical applications due to stability and ability to act as a nontoxic nanocarrier | (Kiranda et al. 2018) |
| 5 | ~ 300 | Crystalline | Microwave-assisted technique and planetary milling | The synthesized CCNPs was in calcite form. This NPs can be used as polymer filler, pigment, an encapsulation template of bioactive compounds or as an adsorbent to eliminate heavy metals from water | Skubiszewska-Zięba et al. (2017) |
| 6 | 34 | Not specified | Membrane dispersion mini reactor | It was observed that the morphology and size of the CCNPs was dependent on various factors, such as the effect of flow rates of dispersed and continuous phases, amount of CaSt2 surfactant, and the temperature. It was also found that these hydrophobic CCNPs can be produced industrially and their surfaces could be modified | Han et al. (2019) |
Polymer-mediated synthesis
Polymerization is a process that involves connecting monomers resulting in large macromolecules of different shapes and sizes (Peppas 1992). Due to the ability of the polymers to act as a surface protecting agents, they can help prevent nanoparticles from getting aggregated. The polymers also form scaffolds which further facilitate nanoparticles synthesis (Lauster et al. 2017). Polymer-mediated synthesis of nanoparticles can be divided into two types: In-situ polymerization and polymer-mediated growth.
In-situ polymerization
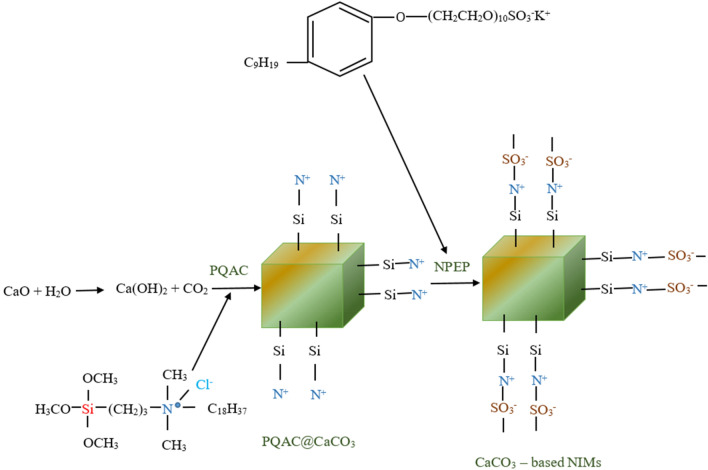
The term in-situ is a Latin word meaning “in position.” It is carried out within the polymerization mixture and utilized for development of “polymer nanocomposites.” In this technique, the initial step of adding nanofillers to a monomer solution is followed by a series of polymerization steps, which results in the synthesis of hybrid “polymer nanocomposites” after the solvent present in the mixture has evaporated (Persistence market research 2021). Bhanvase et al. used ultrasonic radiations coupled with an in-situ polymerization technique for the synthesis of PMMA (poly (methyl methacrylate))/CaCO3 nanocomposite. Here, firstly, hydrophobic nanosized CaCO3 were formed by the carbonation of Ca (OH)2 in the presence of myristic acid. These hydrophobic nano-inorganic particles were dispersed into the PMMA matrix using cavitation generated due to ultrasonic irradiation. Thermogravimetric analysis showed that PMMA alone decomposed completely in the temperature from 290 to 485 ºC, whereas the modified PMMA/CaCO3 nanocomposite failed to decompose below 625 ºC. Also, 5% weight loss was observed due to decomposition of myristic acid proving that the in-situ synthesized PMMA/CaCO3 nanocomposite showed enhanced thermal stability in comparison to PMMA alone (Bhanvase et al. 2011). Recently, Wang et al. reported an in-situ method for the formation of a polysiloxane quaternary ammonium salt/CaCO3 (PQAC/CaCO3) nanocomposite (Wang et al. 2018). Here, the PQAC forms a corona around the CCNPs. The nanocomposite was further modified by an ionic exchange reaction with a long chain polymeric anion forming a CaCO3-based nanoscale ionic material (CaCO3-NIMs). As illustrated in Fig. 6, the CaCO3-NIMs was formed by a reaction between CaO and water in the presence of PQAC and NPEP. The shape of the PQAC/CaCO3 NPs was rhombohedral with 60–70 nm size. As a form of modification of CaCO3-NIMs, an outer layer was formed with an extra thickness of around 4–6 nm, which is considered to be the canopy of the anionic polymer. The synthesized CaCO3-NIMs exhibited temperature-dependent high electrical conductivity because of the conductive effect of ions (Wang et al. 2018). Guillemet et al. have synthesized amorphous CaCO3 (ACC) nanoparticles by in-situ carbonation where CaCl2 was reacted with slow releasing CO2 gas under alkaline hydrolysis of dimethyl carbonate in water leading to the formation of CaCO3 and HCl (Guillemet et al. 2006). These particles were stabilized in the presence of trace amounts of double hydrophilic block copolymers (DHBC), composed of poly (acrylic acid) (PAA) and poly (ethylene oxide) (PEO). The addition of trace amount (3–10 ppm) of DHBC resulted in the synthesis of spherical ACC nanoparticles of 60–100 nm (Guillemet et al. 2006).
Fig. 6.
Schematic diagram showing in-situ formation of PQAC modified with CCNPs that was utilized for the synthesis of CCNPs-based NIMs using NPEP.
Reprinted with permission from reference (Wang et al. 2018) Copyright © The Royal Society
Polymer-assisted growth
Polymer-mediated growth is another approach to synthesize CCNPs. In this method, one of the constituent ions binds to the polymer matrix while another ion coming from external solution subsequently binds to the first ion to form the desired compound (Mydin et al. 2018; Persistence market research 2021). Sonawane et al. showed that cross-linking of the gel-forming polymer matrix affected the ions diffusivity into the polymer matrix and particle size (Sonawane et al. 2007). In their method, methylene bis-acrylamide (MBAM) worked as a cross-linker and ammonium persulfate (APS) (1%) as the initiator to form the matrix. Results revealed that the size of the particles were dependent on the extent of cross-linking with the lowest size of the particle as 7 nm that could be obtained. The diffusivity of the ions depends on the dispersion growth medium’s nature. With an increase in the cross-linking, the diffusivity reduces, which leads to the formation of smaller-sized nanoparticles (Sonawane et al. 2007). Additionally, Ramasamy et al. showed a polymer-mediated synthesis of CaCO3 utilizing a biomimetic method involving limestone (Ramasamy et al. 2018). Their synthesis protocol was extended to a variety of polymers, including PMMA, polyvinylpyrrolidone (PVP), and poly (ethylene glycol) (PEG). The resultant composites were rhombohedral in crystal structure with calcite morphology of ~ 20–30 nm diameter. The synthesized CCNPs showed higher thermal stability and large-scale synthesis to meet the industrial demand without using hazardous chemicals (Ramasamy et al. 2018).
In conclusion, the CCNPs produced by the polymer-mediated method offer easy synthesis and monodispersed particles. Unlike mechano-chemical methods, polymeric methods do not require sophisticated instruments. The particles synthesized by this method find their application for the fabrication of organic/inorganic nanohybrid materials (Guillemet et al. 2006), as semiconductors (Ramasamy et al. 2018), in the bio-electro analysis of trace compounds (Cai et al. 2006), as molecular targets for tumor, and as a system for nano-drug delivery (Maleki Dizaj et al. 2015; Dong et al. 2018). Table 5 summarizes the different CCMPs and CCNPs synthesized by polymer mediated method.
Table 5.
CaCO3 micro- and nanoparticles synthesized by polymer mediated method
| No. | Size (nm) | Nature | Method of synthesis | Results/Observations | References |
|---|---|---|---|---|---|
| 1 | 20 | Crystalline | Polymer-mediated | CCNPs coated with PEG or PMMA or PVP nanocomposites were synthesized using natural limestone rock. The developed nanocomposites were spherical in shape, monodispersed, and porous-like structure. The value of band gap was 3.15–3.36 eV and the resultant product also showed good luminescence intensity in blue region | Ramasamy et al. (2018) |
| 2 | ~ 47 | Crystalline | Polymerization | The co-synthesis of DOX and mucin significantly improved the DOX loading efficiency by ten times and offered a great control over drug release from CCNPs. Retention of DOX inside CCNPs was administered by strong electrostatic attraction between CaCO3 and mucin matrix which significantly narrowed of nanocrystal pores. Hence, this system works as a great drug delivery agent to release small hydrophilic drugs | Balabushevich et al. (2019) |
| 3 | ~ 90 | Amorphous | Ultrasound sonication | CCNPs were synthesized using ultrasound-assisted precipitation technique. Surface modification of CCNPs was done using triethoxy vinyl silane (TEVS). CCNPs-TEVS showed good dispersibility in the polymer matrix as compared to the bare CCNPs. Hence, the surface modified CCNPs can be used as nanofillers and as a binder in the polymer and paint industry | Shimpi et al. (2015) |
| 4 | 60–70 | Crystalline | In-situ technique | The CCNPs was surface modified using polysiloxane quaternary ammonium salt (PQAC) corona (PQAC-CCNPs). The developed NIMs had a rhombohedral shape. Its shell was made up of poly(ethylene glycol)-tailed sulfonate anion (NPEP) canopy (thickness of 4– 6 nm). The inner core of CCNPs had calcite crystalline structure and NPEP canopy was amorphous. NPEP canopy showed a characteristic crystallization–melting behavior in the presence of ion bonding with PQAC-CCNPs. The CCNP-based NIMs exhibited a high electrical conductivity with temperature dependency due to the ionic conductive effect | Wang et al. (2018) |
| 5 | Not specified | Crystalline | Electrostatic interactions | The developed AuNP-CaCO3 hybrid material was utilized for HRP assembly and biosensor fabrication. The amperometric response exhibited by biosensor was in the linear range of 4.0 × 10–5–8.0 × 10–3 mol/L and detection limit was 1.0 × 10–6 mol/L | Cai et al. (2006) |
| 6 | ~ 300 | Not specified | Layer-by-layer assembly | The mesoporous CCNPs (MCNs) was loaded with DOX (MCNs-DOX) using layer-by-layer assembly method. The MCNs-DOX was found stable at a pH 7.4. The HeLa cells showed low cytotoxicity, high cell uptake efficiency, and strong molecular targeting property for MCNs-DOX | Xing et al. (2020) |
| 7 | ~ 34 | Not specified | In-situ polymerization | The synthesized CCNPs were modified in situ using calcium hydroxide slurry and CO2/nitrogen mixed gas. The developed nanoparticles had a surface area of 30 m2/g with a contact angle of 107.8°. CCNps showed good hydrophobicity and dispersity | Han et al. (2019) |
Biological synthesis methods
The biological method of synthesis is a green approach of CaCO3 micro- and nanoparticles synthesis without the use of any harmful chemicals and harsh physical conditions. In the biosynthesis route, CCNPs are synthesized by the use of naturally occurring plant extracts and biomolecules, like proteins, peptides, enzymes, and microbes, including bacteria and fungi (Singh et al. 2018). Various nanoparticles are reported to be synthesized using biosynthesis approach, such as silver, gold, copper, and metal oxides of zinc, iron, tungsten, etc. (Deng et al. 2016; Saif et al. 2016; Singh et al. 2018).
Eggshell-mediated synthesis
In this method, eggshells are used as raw material for CaCO3 micro- and nanoparticle synthesis. Eggshell is composed of both, organic as well as inorganic, components that can be utilized in different applications. The outer most layers of eggshell contain 95% of CaCO3 and 5% of magnesium carbonate, calcium phosphate, and some insoluble/soluble proteins. Hence, it proves to be a good biological source for CaCO3 synthesis. Other reasons behind using eggshell include its low cost, easy accessibility, biocompatibility with human body, and high porosity (Render et al. 2016). In a report by Khan et al., the CCNPs were synthesized using agar and calcined eggs as precursors (Khan et al. 2019). The synthesis was performed by a solvothermal process where the precursor mixture was mixed and heated at 700 ºC for 5 h. The synthesized CCNPs was found to act as good catalyst in degradation reaction of Congo red dye and enhanced the calorific value of diesel (Khan et al. 2019). Render et al. demonstrated the synthesis of CCNPs by eggshell which was coupled with the top-down-ball-milling method along with the use of polymer (Render et al. 2016). The milled product was passed through a filter and then sonicated to achieve size of the particle ranging from ~ 10 to 60 nm (Render et al. 2016). Unlike other chemically synthesized CCNPs, eggshell-derived particles were porous in nature, thus offered effective drug loading capacity. Additionally, the developed CCNPs were found to be biocompatible, thus making them an ideal material for biomedical applications. Rinu et al. demonstrated the synthesis of CCNPs from hen eggshell using a crucible and a muffle furnace at 900 ºC. It was also reported that from these CCNPs, calcium hydrogen phosphate nanoparticles can also be further prepared and it was found that both these CCNPs demonstrated antibacterial properties against gram-positive bacteria (Rinu et al. 2020).
Microbial synthesis
In this synthesis method, microorganisms, including yeast, fungi, and bacteria, act as a new resource (nano-factories) for synthesizing nanoparticles. Microorganisms have several advantages, such as eco-friendly nature, cost-effectiveness, and also avoiding the use of harsh and toxic chemicals (Singh et al. 2016). Wu and Zeng reported the use of microorganisms to precipitate the CCNPs (Wu and Zeng 2017). In this method, Sporosarcina pasteurii was immobilized on a substrate using calcium alginate gel followed by exposure of CaCO3 precursor, which led to the cross-linking with sodium alginate. From calcium alginate gel, CO3−2 was generated by ureolysis, which can directly react with Ca+2 and can be cross-linked in the sodium alginate matrix to develop CaCO3 precipitate in situ. The size of synthesized particles was found to be ~ 3.5 to 6.5 µm. The alginate molecules were found to act as an organic stabilizer to regulate the polymorphism and morphology of CaCO3 which resulted from the collapse of calcium alginate gel. Upon changing the concentration of sodium alginate (1–3%) the morphology of the CCMPs also changed from hexagonal to capsule-like vaterite particles (Wu and Zeng 2017). Caicedo-Pineda et al. demonstrated the application of garden Bacillus cereus to synthesize CCNPs. Before CaCO3 biomineralization, bacteria were sub-cultured in M3 media consisting of 1.0% tryptone and 0.5% calcium acetate followed by washing of extracts with ethanol. It was hypothesized that the CaCO3 was produced during the metabolic activity of the bacteria under the influence of calcium acetate. Also, it was found that with the increase and decrease in tryptone concentration, particles with high vaterite phase and calcite phase were synthesized, respectively (Caicedo-Pineda et al. 2018). Several other bacterial species, such as Gluconacetobacter xylinus (Mohammadkazemi et al. 2016) and Shewanella piezotolerans WP3 (a deep-sea bacterium) (Li et al. 2019), were also employed for the green synthesis of CCNPs in situ.
Biomolecules-mediated synthesis
In this method, the nanomaterials are synthesized using biomolecules, like antibodies, DNA, and enzymes. These nanoparticles are employed for various biomedical applications (Willner and Willner 2010). Apart from the eggshells and microbes, Ru et al. have recently reported formation of CCNPs in erythrocytes (Ru et al. 2019). The study reported a concept of “cyborg cells,” living cells that have built-in nano-scaffolds, which integrate biological function retaining nanoparticles functionality. In this report, they have constructed “cyborg erythrocytes” and demonstrated an in-situ reaction of the exogenous Ca+2 and CO3−2 to form CaCO3 nanodots. The results presented in Fig. 7 shows the morphological analysis of nanodots imaged under various microscopes. The transmission electron microscope (TEM) image illustrates the size distribution histogram for the synthesized CaCO3 nanodots, while the high-resolution transmission electron microscope (HRTEM) shows the area for each CaCO3 nanodot and the image from field emission scanning electron microscope (FESEM) shows functionalized erythrocytes. Nanodots synthesized in situ were analyzed under a TEM and it showed narrow particle size distribution with a mean particle size of 3.89 ± 0.03 nm. These cyborg erythrocytes were used in the efficient removal of extracellular lead ions (Ru et al. 2019). Yang et al. (2020), reported the synthesis of CCNPs using phenylalanine and observed that with an increase in the concentration of phenylalanine, calcite and vaterite particles were formed at ≤ 6 g/L and > 8 g/L concentration of phenylalanine, respectively (Yang et al. 2020).
Fig. 7.
Characterization of “cyborg erythrocytes” and CaCO3 NDs. a TEM image showing intracellular CaCO3 NDs; inset: histogram of CaCO3 NDs for the corresponding size distribution. b HRTEM image showing several individual CaCO3 NDs (inset: selected area electron diffraction (SAED) pattern of CaCO3 NDs, bottom: magnified image of the area denoted by the red dashed boxes). c FESEM image of functionalized erythrocytes.
Reprinted from open access article (Ru et al. 2019) Copyright © Springer Nature
In summary, the CCNPs synthesized by biological methods are mostly biocompatible and environment friendly. Therefore, they can be utilized for various applications (Khan et al. 2019), such as catalyst /photocatalyst (Wu and Zeng 2017), antibacterial, antitumor, (Render et al. 2016; Rinu et al. 2020), and in drug delivery (Render et al. 2016). However, this method suffers from the control of shape and size of CCNPs. Table 6 comprises of different CCMPs and CCNPs synthesized using biological methods.
Table 6.
Applications of CaCO3 micro- and nanoparticles synthesized by biological method
| No. | Size (nm) | Nature | Microorganism/Biomaterial | Applications | References |
|---|---|---|---|---|---|
| 1 | Not-Specified | Not-Specified | Gluconacetobacter xylinus | In the biomedical field to obtain scaffold for bone regeneration | Mohammadkazemi et al. (2016) |
| 2 | Not-Specified | Crystalline | Kappa (type III; from Eucheuma cottonii) and Iota (type V; from E. cottonii) carrageenans | As the synthesized particles are biocompatible and have many biocompatible applications | Nogueira et al. (2016) |
| 3 | ~ 80 | Amorphous | PL-MIT (phospholipid-Mitoxantrone) | The synthesized particles could be used for drug release and to enhance the drug penetration for the antitumor effect in cancer therapy | Wang et al. (2019) |
| 4 | Not-Specified | Crystalline | Eggshell | The synthesized particles showed antibacterial effects specifically on the gram-positive bacteria | Rinu et al. (2020) |
| 5 | ~ 10–60 | Crystalline | Eggshell | The particles can be used in tablets for use in enteric drug delivery and other biomedical purposes | Render et al. (2016) |
| 6 | ~ 10 | Crystalline | Sporosarcina pasteurii | The synthesized particles could be used as fuel cells and as photocatalyst | Wu and Zeng (2017) |
| 7 | ~ 1000 | Crystalline | Bacillus subtilis, Bacillus amyloliquefaciens, Bacillus cereus | As the shells did not collapse after washing, the precipitates could help manufacture osseous structures to carry out microencapsulation for the medicinal purpose because of their uniform and hollow distribution | Caicedo-Pineda et al. (2018) |
| 8 | 80–120 | Crystalline | Chicken eggshell and agar | Can be used as fuel additives and as a catalyst for degrading Congo Red dye in aqueous media | Khan et al. (2019) |
| 9 | 20–50 | Crystalline | From aqueous extract and leach solution of Myrtus communis plant | Used for color removal from an aqueous solution containing basic dye compounds | Uzunoğlu and Özer (2018) |
| 10 | 19–51 | Not specified | Cockleshell | Acts as a starting material for biomedical applications | Kiranda et al. (2018) |
| 11 | 14 µm | Crystalline | DNA | Can be used as catalyst and as chromatography column packaging material | Cheng et al. (2010) |
| 12 |
20 × 5 µm Film |
Crystalline | BSA | Can be used in the synthesis of advanced materials | Xue et al. (2009) |
| 13 | 1–2 µm | Crystalline | Egg White | Can be used for synthesizing inorganic/organic hybrid materials | Zheng et al. (2012) |
Applications of CaCO3 micro- and nanoparticles
CaCO3 micro- and nanoparticles are employed in a plethora of industries, including plastics, paint, paper, and pharmaceuticals. CaCO3 micro- and nanoparticles act as a filler material for the improvement and reinforcement of the physical properties of the products. In biomedicines, CaCO3 micro- and nanoparticles are used for drug/gene delivery as well as bone engineering. CaCO3 micro- and nanoparticles are also used for the treatment of wastewater and environmental pollution detection sensors. They also contribute to the food industry as a unique supplement material.
Biomedical applications
CaCO3 micro- and nanoparticles are found to possess excellent biocompatibility and negligible toxicity to the mammalian cells/tissues (Boyjoo et al. 2014). The CCNPs can be used for tasks, like drug delivery, for tissue injury repair, and for biosensing.
Drug delivery
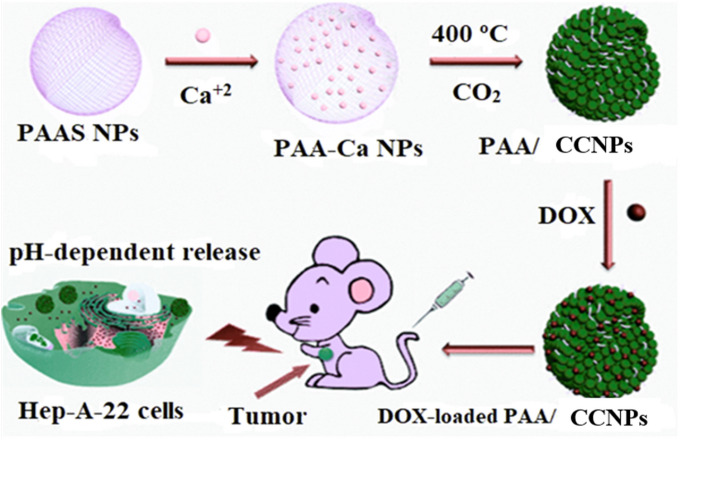
Nanoparticles have an increased scope in the area of drug delivery because of their small size, target specificity, pH-responsive behavior, biocompatibility, easy biodegradability, site-specific actions, non-toxic in both micrometer and nanometer range, etc. (Boyjoo et al. 2014). When nanoparticles are conjugated with the drugs, they increase the efficiency of the drugs and help improve the stability and bioavailability. Nanoparticle-conjugated drug formulation offers enhanced time of blood circulation to provide better efficacy against the targeted disease (Roberti et al. 2019). CCNPs have also shown a promising role in cancer therapy because they facilitate drug delivery and increase the therapeutic efficacy due to being safe, easily available, slow biodegradability, and ability to reduce the drawbacks of the remedial agents. Targeting ability leads to increase in their efficacy and stability due to better penetration and high payload delivery (Maleki Dizaj et al. 2015). The bioactive agents, such as anticancer drugs, enzymes, and other biomacromolecules, are reported to be effectively loaded inside the hollow and porous CCNPs to enable a sustained release in the biological system (Maleki Dizaj et al. 2015). Shi et al. have demonstrated the efficiency of mesoporous PAA/CCNPs encapsulating doxorubicin (DOX) to treat liver cancer. Due to the mesoporosity, this complex exhibited high loading of DOX and pH-dependent sustained release in both in vivo and in vitro experimental models. The results showed that this complex had an excellent antitumor effect against the hepatic cancer model without significant toxicity (Shi et al. 2015). Figure 8 shows the synthesis of the PAA/CCNPs from poly-acrylic acid sodium salt (PAAS) and their effect on treating cancer tumors. Wang et al. synthesized CaCO3-CMC hybrid nano- and microspheres by precipitation. Due to the nanoporous structure and negatively charged surface, loading of positively charged DOX was successfully realized on the surface of the hybrid. The hybrid spheres also showed sustained release for a longer duration, which makes them a go-to method to deliver water-soluble drugs. The encapsulation efficiency in both the micro- and nanoparticle was above 60%, indicating easy loading of the drug in the porous structure. It was also reported that high surface-to-volume ratio of nanospheres was responsible for the fast release of drug in comparison with hybrid microspheres (Wang et al. 2010). Recently, Li et al. synthesized DOX-loaded CaCO3 NPs using emulsion method to study the pH-responsive drug release. The synthesized particles were of ~ 100 nm size with ~ 49% drug loading capacity (Li et al. 2020). The results revealed that at pH 5.0, CaCO3 NPs released Ca+2 ions causing blood coagulation by catalyzing the formation of thrombin from prothrombin. This conversion was found to be dependent on the concentration of Ca2+ ions. Such pH-sensitive blood coagulation by increase in acidity leads to the blocking of tumor vessels.
Fig. 8.
Schematic illustration for synthesizing Polyacrylic acid (PAA)/CCNPs from polyacrylic acid sodium salt (PAAS) and their applications for treating cancer as pH-dependent drug delivery vehicles in vivo and in vitro.
Reprinted with permission from reference (Shi et al. 2015) Copyright © Royal Society of Chemistry
However, at pH 7.0, thrombus was absent. This formulation was biocompatible with mouse fibroblast cells (NIH/3T3 cell line) and toxic to breast cancer cells (MCF-7 and MDA-MD-231 cell lines). Apart from cancer, CCNPs are also reported to deliver various molecules to realize biological applications. For example, CaCO3-based mineralized nanoparticles were coated with S-nitrosothiol [a nitric acid donor (NO)] (SNT-CCNPs) and monitored its effect on regulation of cell signaling pathways of osteogenic differentiation (mouse stem cells) (An et al. 2020). SNT-CCNPs were found biocompatible and increased the expression of osteogenic targeted genes, such as alkaline phosphatase, osteocalcin, and osterix in stem cells.
Tissue engineering
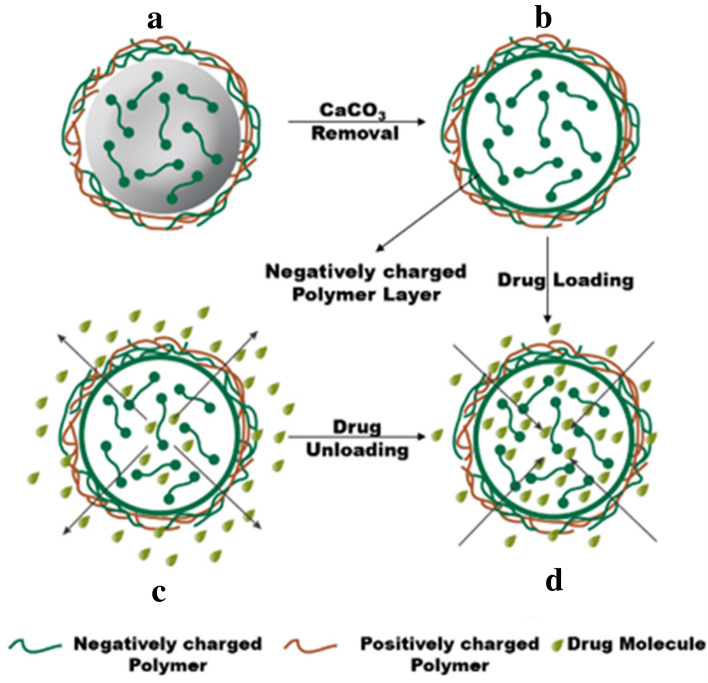
Tissue engineering is a technique of growing organs and tissues in the laboratory. Nanoparticles due to their properties, like small size, antimicrobial properties (silver nanoparticles), fluorescence properties (quantum dots), electrical, and thermal conductivity (carbon-based nanoparticles), make them an ideal choice for tissue engineering (Hasan et al. 2018). It has been reported that hydroxyapatite microspheres were synthesized in a collagen matrix to act as hybrid gel and it was found to support scaffold during the surgical reconstruction of bones and other tissue engineering applications. Since the suspension of the collagen-based mixture is injectable, the resultant composite gel can act as multifunctional gel and be constructed by the facile introduction of external additives, such as cells, bioactive factors, and drug molecules into the system before the activation of gelation (Wei et al. 2015). Figure 9 shows the loading and unloading of the drug on a polymer matrix. A recent report by Ru et al. revealed that CaCO3 nanodots (3.9 nm) could be synthesized inside the erythrocytes and exhibited degradation of Pb+2 under both in vivo and in vitro experimental models. The extracellular lead ions were found to diffuse through membrane transport by forming PbCO3 and the functionalized erythrocytes removed ~ 80% of the lead ions. However, in an in vivo model, accumulation of a negligible amount of Pb+2 was observed in the liver and kidneys (Ru et al. 2019). Recently, Sokolova et al. developed a plasmid DNA [containing Functional Enhanced Green Fluorescent Protein (EGFP)]-loaded calcium phosphate nanoparticles decorated on a porous scaffold containing poly(lactide-co-glycolide) (PLGA) and nano-hydroxyapatite for bone tissue engineering (Sokolova et al. 2020). Results revealed that nanoparticles were taken up by the bone cells and induced the expression of EGFP, thus stimulated the growth of bone tissues by modulating VEGF pathway. In another attempt, calcium peroxide nanoparticles (CPO NPs) were used as an oxygen generator to engineer bone tissues (Mohseni-Vadeghani et al. 2021). The scaffold was prepared using poly (l-lactic acid) (PLLA) and loaded with CPO NPs. The surface of scaffold was coated with catalase enzyme to accelerate the H2O2 degradation and prevent cell damage.
Fig. 9.
Steps involved for synthesizing, loading, and unloading of drugs into the microcapsules: a synthesized CaCO3 core loaded with a negatively charged polymer along with a mixed polymer shell microparticle, b CaCO3 dissolution, c drug loading through electrostatic attraction, and (d) drug unloading through diffusion or environmental pH.
Reprinted with permission from reference (Boyjoo et al. 2014) Copyright © Royal Society of Chemistry
Biosensing
Nanoparticles have been very useful in biosensing due to their unique optical and electronic properties. For example, the quantum dots, metal nanoparticles and nanoclusters, etc., have shown to be effective for biosensing of important biomolecule (Pirzada and Altintas 2019). CCNPs are also used to develop sensitive biosensors displaying rapid response with almost no interference. They are also used as a catalyst as they can withstand harsh reaction conditions without alteration in the activity (Jeevanandam et al. 2018). These nanoparticles can also act as ideal imaging agents used for cancer therapy and show several applications in the field of biosensing and protein encapsulation in pharmaceuticals. These CCNPs can act as biomarkers for detecting biomolecules in food. For example, detection of glucose (range of nM–µM) from food for a diabetic patient (Yashchenok et al. 2012). Other potential applications of CaCO3 micro- and nanoparticles include the development of scaffold matrix immobilized with biomacromolecules, including enzymes, such as lipases and xanthine oxidases. The multiwalled carbon nanotube-CCNP composites can be used as dopamine biosensors to detect dopamine levels (15 nM) for the neuronal disorder treatment (Bujduveanu et al. 2013). The nano-CaCO3-polyphenol oxidase (PPO) (PPO/nano-CaCO3 film) act as good biosensors for the detection of phenols (0.44 nM) due to the phenolic toxicity present in the environment. Thus PPO/nano-CaCO3 film is used for detecting phenols due to their properties, like good biocompatibility, high hydrophilicity, and surface activity (Shan et al. 2007a). These enzymes are essential for the food industry due to their application in the manufacturing of monoacylglycerol and testing the freshness of seafood (Groboillot et al. 1994; Suppes et al. 2001). In another attempt, imiquimod (antagonist to toll, like receptor 7, IMQ) and indocyanine green (dye used in angiography, IDG)-loaded and Mn+2-doped amorphous CCNPs (IMQ/IDG-ACC(Mn) NPs) were synthesized to enhance the photoimmunotherapy in breast cancer model (Liu et al. 2021). The IMQ/IDG-ACC(Mn) NPs showed specificity toward acidic environment of cancer cells and co-delivered IMQ and IDG into the tumor tissues. Also, IMQ/IDG-ACC(Mn) NPs in combination with phototherapy (using near-infrared laser) triggered tumor-associated antigens followed by increase in CD+8 and CD+4 immune responses. Further, the formulation was traceable using magnetic resonance imaging (due to Mn+2) and fluorescence microscopy (due to IDG). Sabbarwal et al. synthesized CaCO3 nanoclusters (CNPs) using bovine serum albumin (FCNPs) and M. oleifera leaf extract (with ascorbic acid) and used them for cell imaging (Sabbarwal et al. 2020). The FCNPs exhibited blue, red, green, and yellow fluorescence emission when excited with 366, 595, 472, and 516 nm wavelengths, respectively. The average fluorescence life time was found to be 1.05 (blue), 6.23 (green), and 30.60 (red/yellow) ns. FCNPs showed biocompatibility as well as different fluorescence wavelengths when incubated with MG-63 cells for 7 days.
In dentistry
Teeth are important part of the oral cavity along with other parts, such as enamel, pulp, cementum, dentin, and periodontal ligament. Various compounds are used in dentistry to protect teeth, such as bone filling products, teeth bleaching products, ceramics, and amalgam. Hydroxyapatite (Ca5(PO4)3(OH)) is a primary mineral component of enamel and dentin. Therefore, calcium-based nanomaterials are expected to be helpful to control the tooth mineral loss and reduction of biofilm formation (due to frequent sugar intake) (Balhaddad et al. 2019). Calcium phosphate NPs inhibit demineralization of tooth due to the release of constituent ions (Fig. 10a). Incorporating different metal NPs with calcium (alloy NPs) may reduce the biofilm formation (Fig. 10b) (Balhaddad et al. 2019). Nayak et al. reported inorganic dental nanocomposites, calcium fluoride (CaF2), releasing fluoride ions to exhibit antibacterial as well as inhibition of biofilm formation from Streptococcus mutans (Nayak et al. 2019). Mechanistically, the nanoparticles adversely affected the metabolism of bacteria and decreased the adhesion of microbes on the tooth surface.
Fig. 10.
Schematics showing application of CCNPs in dentistry. a Control of tooth mineral loss via CCNPs and b reduction and modulation of biofilm formation via antibacterial CCNP-conjugated metallic nanoparticles
Environmental applications
Owing to high porosity, surface area, and facile synthesis procedures, CCMPs and CCNPs are considered as the best option for the fabrication of simple, cost-effective, and reliable point-of-care biosensors. They are also exploited for various environmental applications, such as wastewater treatment and other bioremediation applications. Shan et al. synthesized a hybrid enzyme particle (polyphenol oxidase/CaCO3 nanoparticle) electrode for the detection of phenols at sub-nanomolar ranges with a limit of detection at 0.44 nM. The electrode shows rapid response (< 12 s) and is effective over a large range of concentrations (6 × 10–9 to 2 × 10–5 M) (Shan et al. 2007a). The same group synthesized glucose oxidase/CaCO3 nanoparticles for the detection of glucose (Shan et al. 2007b). Haložan et al. used CaCO3 modified with a dye tagged polymer as a pH indicator. The system was robust enough to give results in the presence of salt in the mixture (Haložan et al. 2009). The CCMPs and CCNPs also showed applications for the elimination of heavy metal ions, like nickel, chromium, lead from the water via membrane adsorption, and precipitation method (Sdiri and Higashi 2013; Khulbe and Matsuura 2018; Mallakpour et al. 2018; Jacob et al. 2018). Radioactive contaminants, like Eu3+ could also be removed from the system with a removal efficiency of 83%. In another study by Alsohaimi et al., silver nanoparticle-loaded calcium oxide nanoparticles (Ag–CaO NPs) were synthesized as antibacterial agent and to treat industrial waste water (Alsohaimi et al. 2020). Indigo carmine dye (hazardous dye used in textile industry) was shown to be degraded (99.45%) in the presence of solar energy and Ag–CaO NPs. Also, Ag–CaO NPs showed antibacterial activity against Bacillus cereus, Bacillus subtilis, Enterococcus faecalis, E. coli, Klebsiella sp., Pseudomonas fluorescence, Enterobacter sp., and Micrococcus luteus. Walsh et al. demonstrated the use of CaCO3/magnetite hybrid crystals synthesized by microwave-assisted technique for effective photocatalytic splitting of water to generate oxygen. The reuse and separation of the particles ensures its recyclability (Walsh et al. 2011). CaCO3-based nanoscale ionic materials with liquid-like properties are known to exhibit high electrical conductivity and are explored for various industrial applications (Wang et al. 2018). Recently, Banihashemi et al. fabricated CCNPs for the removal of fluoride from aqueous solutions (Banihashemi et al. 2021). The synthesized CCNPs was enabled to remove 460 mg fluoride ions from 1 L solutions at 298 K and neutral pH.
Conclusion
This review article summarizes various approaches for synthesis and prospective applications of CaCO3 micro- and nanoparticles. CaCO3 micro- and nanoparticles offer high surface-area-to-volume-ratio, high porosity, negligible cytotoxicity, and easy surface modification. Therefore, several attempts have been made to realize various applications of CaCO3 micro- and nanoparticles in the fields of pharmacy and medicine, food processing, plastic and paper industry, and environmental sciences (Boyjoo et al. 2014). Among various synthesis methods discussed in this review paper, it can be concluded that precipitation method could result in to precisely controlled particle size with variety of polymorphs and morphologies. The carbonation method, a subtype of precipitation method, on the other hand, does not provide ability to easily control the size of the particle, morphology, and the phase of product. The polymerization method is usually preferred for the synthesis of particles with precise isotropic and anisotropic shapes with narrow size distribution in micro- and nanometer range. The emulsion method and mechano-chemical methods are comparatively complex processes and require precise control of reagents and the use of sophisticated instruments for the synthesis. The biological method is a completely green approach for the synthesis of the particles, as it does not use any harmful chemicals as precursors, rather it involves the use of naturally existing biological materials, like proteins, bacteria, and eggshell, as starting materials. The porous spherical and hollow CCNPs are widely used as a drug delivery vehicle and as a reservoir for drug storage. Once embedded in the biocompatible polymer matrix, they could be used for bone regeneration and grafting, as catalysts, filler material, and biosensors for environmental applications. To recapitulate, the process of synthesizing CaCO3 micro- and nanoparticles is a continuous area of research to deliver facile synthesis methods for controlled manufacturing of particles of varied shape, size, morphologies, and polymorphs at a larger-scale and explore different horizons in the application of this material.
Acknowledgements
S. Singh acknowledges the financial support from Ahmedabad University as seed grant (AU/SG/SAS/DBLS/17-18/03) and the funding for undergraduate research projects. The funding from BRNS (58/14/14/2019-BRNS/37020) and Winner Trade (URBSASE20A2/2019-20/12_SS_04.20) is also duly acknowledged. S. Bhagat thanks Indian Council of Medical Research for providing senior research fellowship.
Declarations
Conflict of interest
All the authors confirm that there is no conflict of interest to declare.
References
- Achour A, Arman A, Islam M, Zavarian AA, Basim Al-Zubaidi A, Szade J. Synthesis and characterization of porous CaCO3 micro/nano-particles. Eur Phys Jl plus. 2017;132(6):267. doi: 10.1140/epjp/i2017-11531-8. [DOI] [Google Scholar]
- Alshora DH, Ibrahim MA, Alanazi FK. Chapter 6—nanotechnology from particle size reduction to enhancing aqueous solubility. In: Grumezescu AM, editor. Surface chemistry of nanobiomaterials. William Andrew Publishing; 2016. pp. 163–191. [Google Scholar]
- Alsohaimi IH, Nassar AM, Seaf Elnasr TA, Ba C. A novel composite silver nanoparticles loaded calcium oxide stemming from egg shell recycling: A POTENT photocatalytic and antibacterial activities. J Clean Prod. 2020;248:119274. doi: 10.1016/j.jclepro.2019.119274. [DOI] [Google Scholar]
- An SY, Lee HJ, Lee SC, Heo JS. Supplement of nitric oxide through calcium carbonate-based nanoparticles contributes osteogenic differentiation of mouse embryonic stem cells. Tissue Cell. 2020;66:101390. doi: 10.1016/j.tice.2020.101390. [DOI] [PubMed] [Google Scholar]
- Anton N, Benoit J-P, Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates—a review. J Control Release. 2008;128(3):185–199. doi: 10.1016/j.jconrel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Ao W, Li J, Yang H, Zeng X, Ma X. Mechanochemical synthesis of zinc oxide nanocrystalline. Powder Technol. 2006;168(3):148–151. doi: 10.1016/j.powtec.2006.07.014. [DOI] [Google Scholar]
- Avouris P. Graphene: electronic and photonic properties and devices. Nano Lett. 2010;10(11):4285–4294. doi: 10.1021/nl102824h. [DOI] [PubMed] [Google Scholar]
- Babou-Kammoe R, Hamoudi S, Larachi F, Belkacemi K. Synthesis of CaCO3 nanoparticles by controlled precipitation of saturated carbonate and calcium nitrate aqueous solutions. Can J Chem Eng. 2012;90(1):26–33. doi: 10.1002/cjce.20673. [DOI] [Google Scholar]
- Badnore AU, Pandit AB. Synthesis of nanosized calcium carbonate using reverse miniemulsion technique: comparison between sonochemical and conventional method. Chem Eng Process. 2015;98:13–21. doi: 10.1016/j.cep.2015.10.003. [DOI] [Google Scholar]
- Balabushevich NG, Kovalenko EA, Le-Deygen IM, Filatova LY, Volodkin D, Vikulina AS. Hybrid CaCO3-mucin crystals: effective approach for loading and controlled release of cationic drugs. Mater Des. 2019;182:108020. doi: 10.1016/j.matdes.2019.108020. [DOI] [Google Scholar]
- Balhaddad AA, Kansara AA, Hidan D, Weir MD, Xu HHK, Melo MAS. Toward dental caries: Exploring nanoparticle-based platforms and calcium phosphate compounds for dental restorative materials. Bioactive Mater. 2019;4:43–55. doi: 10.1016/j.bioactmat.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihashemi A, zareJavanbakhtMohammadifard KVK. Calcium carbonate nanoparticles fabricated by a facile method based on the colloidal gas aphrons for removal of fluoride ions from aqueous solutions. Mater Chem Phys. 2021;258:123934. doi: 10.1016/j.matchemphys.2020.123934. [DOI] [Google Scholar]
- Barhoum A, Rahier H, Abou-Zaied RE, Rehan M, Dufour T, Hill G, Dufresne A. Effect of cationic and anionic surfactants on the application of calcium carbonate nanoparticles in paper coating. ACS Appl Mater Interfaces. 2014;6(4):2734–2744. doi: 10.1021/am405278j. [DOI] [PubMed] [Google Scholar]
- Bastakoti BP, Guragain S, Yokoyama Y, Yusa S-i, Nakashima K. Synthesis of hollow CaCO3 nanospheres templated by micelles of poly(styrene-b-acrylic acid-b-ethylene glycol) in aqueous solutions. Langmuir. 2011;27(1):379–384. doi: 10.1021/la103660x. [DOI] [PubMed] [Google Scholar]
- Bhanvase BA, Pinjari DV, Gogate PR, Sonawane SH, Pandit AB. Process intensification of encapsulation of functionalized CaCO3 nanoparticles using ultrasound assisted emulsion polymerization. Chem Eng Process. 2011;50(11):1160–1168. doi: 10.1016/j.cep.2011.09.002. [DOI] [Google Scholar]
- Bing Z, Lee JY, Choi SW, Kim JH. Preparation of porous CaCO3/PAM composites by CO2 in water emulsion templating method. Eur Polymer J. 2007;43(11):4814–4820. doi: 10.1016/j.eurpolymj.2007.08.014. [DOI] [Google Scholar]
- Boles MA, Ling D, Hyeon T, Talapin DV. The surface science of nanocrystals. Nat Mater. 2016;15(2):141–153. doi: 10.1038/nmat4526. [DOI] [PubMed] [Google Scholar]
- Boyjoo Y, Pareek VK, Liu J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J Mater Chem A. 2014;2(35):14270–14288. doi: 10.1039/C4TA02070G. [DOI] [Google Scholar]
- Bujduveanu M-R, Yao W, Le Goff A, Gorgy K, Shan D, Diao G-W, Ungureanu E-M, Cosnier S. Multiwalled carbon nanotube-CaCO3 nanoparticle composites for the construction of a tyrosinase-based amperometric dopamine biosensor. Electroanalysis. 2013;25(3):613–619. doi: 10.1002/elan.201200245. [DOI] [Google Scholar]
- Byrappa K, Ohara S, Adschiri T. Nanoparticles synthesis using supercritical fluid technology—towards biomedical applications. Adv Drug Deliv Rev. 2008;60(3):299–327. doi: 10.1016/j.addr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Cai W-Y, Xu Q, Zhao X-N, Zhu J-J, Chen H-Y. Porous gold-nanoparticle−CaCO3 hybrid material: preparation, characterization, and application for horseradish peroxidase assembly and direct electrochemistry. Chem Mater. 2006;18(2):279–284. doi: 10.1021/cm051442i. [DOI] [Google Scholar]
- Caicedo-Pineda GA, Prada-Fonseca MC, Casas-Botero AE, Martínez-Tejada HV. Effect of the tryptone concentration on the calcium carbonate biomineralization mediated by Bacillus cereus. DYNA. 2018;85:69–75. doi: 10.15446/dyna.v85n205.60637. [DOI] [Google Scholar]
- Chakrabarty D, Mahapatra S. Aragonite crystals with unconventional morphologies. J Mater Chem. 1999;9(11):2953–2957. doi: 10.1039/a905407c. [DOI] [Google Scholar]
- Chatterjee A, Mishra S. Novel synthesis with an atomized microemulsion technique and characterization of nano-calcium carbonate (CaCO3)/poly(methyl methacrylate) core–shell nanoparticles. Particuology. 2013;11(6):760–767. doi: 10.1016/j.partic.2012.11.005. [DOI] [Google Scholar]
- Chen C-K, Tai CY. Competing effects of operating variables in the synthesis of CaCO3 particles using the reverse microemulsion technique. Chem Eng Sci. 2010;65(16):4761–4770. doi: 10.1016/j.ces.2010.05.019. [DOI] [Google Scholar]
- Chen Z, Nan Z. Controlling the polymorph and morphology of CaCO3 crystals using surfactant mixtures. J Colloid Interface Sci. 2011;358(2):416–422. doi: 10.1016/j.jcis.2011.02.062. [DOI] [PubMed] [Google Scholar]
- Chen J-F, Wang J-X, Liu R-J, Shao L, Wen L-X. Synthesis of porous silica structures with hollow interiors by templating nanosized calcium carbonate. Inorg Chem Commun. 2004;7(3):447–449. doi: 10.1016/j.inoche.2004.01.003. [DOI] [Google Scholar]
- Cheng B, Cai W, Yu J. DNA-mediated morphosynthesis of calcium carbonate particles. J Colloid Interface Sci. 2010;352(1):43–49. doi: 10.1016/j.jcis.2010.08.050. [DOI] [PubMed] [Google Scholar]
- Cölfen H, Qi L. A systematic examination of the morphogenesis of calcium carbonate in the presence of a double-hydrophilic block copolymer. Chem Eur J. 2001;7(1):106–116. doi: 10.1002/1521-3765(20010105)7:1<106::AID-CHEM106>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Combes C, Miao B, Bareille R, Rey C. Preparation, physical–chemical characterisation and cytocompatibility of calcium carbonate cements. Biomaterials. 2006;27(9):1945–1954. doi: 10.1016/j.biomaterials.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Corr SA (2016) Metal oxide nanoparticles. In: O’Brien P, John Thomas P (eds) Nanoscience, vol 3. The Royal Society of Chemistry, pp 31–56. 10.1039/9781782623717-00031
- Costa LMM, Olyveira G, Salomão R. Precipitated calcium carbonate nano-microparticles: applications in drug delivery. Adv Tissue Eng Regen Med Open Access. 2017;3(2):00059. [Google Scholar]
- Das S, Mitra S, Khurana SMP, Debnath N. Nanomaterials for biomedical applications. Front Life Sci. 2013;7(3–4):90–98. doi: 10.1080/21553769.2013.869510. [DOI] [Google Scholar]
- Dave PN, Chopda LV. Application of iron oxide nanomaterials for the removal of heavy metals. J Nanotechnol. 2014 doi: 10.1155/2014/398569. [DOI] [Google Scholar]
- Demichelis R, Raiteri P, Gale JD, Dovesi R. The multiple structures of vaterite. Cryst Growth Des. 2013;13(6):2247–2251. doi: 10.1021/cg4002972. [DOI] [Google Scholar]
- Deng J, You Y, Sahajwalla V, Joshi RK. Transforming waste into carbon-based nanomaterials. Carbon. 2016;96:105–115. doi: 10.1016/j.carbon.2015.09.033. [DOI] [Google Scholar]
- Desgouilles S, Vauthier C, Bazile D, Vacus J, Grossiord J-L, Veillard M, Couvreur P. The design of nanoparticles obtained by solvent evaporation: a comprehensive study. Langmuir. 2003;19(22):9504–9510. doi: 10.1021/la034999q. [DOI] [Google Scholar]
- Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C. 2014;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- Długosz M, Bulwan M, Kania G, Nowakowska M, Zapotoczny S. Hybrid calcium carbonate/polymer microparticles containing silver nanoparticles as antibacterial agents. J Nanopart Res. 2012;14(12):1313. doi: 10.1007/s11051-012-1313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatan S, Yashchenok A, Khan N, Parakhonskiy B, Cocquyt M, Pinchasik B-E, Khalenkow D, Möhwald H, Konrad M, Skirtach A. Loading capacity versus enzyme activity in anisotropic and spherical calcium carbonate microparticles. ACS Appl Mater Interfaces. 2016;8(22):14284–14292. doi: 10.1021/acsami.6b03492. [DOI] [PubMed] [Google Scholar]
- Dong Z, Feng L, Hao Y, Chen M, Gao M, Chao Y, Zhao H, Zhu W, Liu J, Liang C, Zhang Q, Liu Z. Synthesis of hollow biomineralized CaCO3–polydopamine nanoparticles for multimodal imaging-guided cancer photodynamic therapy with reduced skin photosensitivity. J Am Chem Soc. 2018;140(6):2165–2178. doi: 10.1021/jacs.7b11036. [DOI] [PubMed] [Google Scholar]
- Du L, Wang Y, Luo G. In situ preparation of hydrophobic CaCO3 nanoparticles in a gas–liquid microdispersion process. Particuology. 2013;11(4):421–427. doi: 10.1016/j.partic.2012.07.009. [DOI] [Google Scholar]
- Duraisamy Y (2016) Strength and stiffness improvement of bio-cemented sydney sand. Doctoral Dessertation, University of Sydney
- Elkassas D, Arafa A. The innovative applications of therapeutic nanostructures in dentistry. Nanomed Nanotechnol Biol Med. 2017;13(4):1543–1562. doi: 10.1016/j.nano.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Erdoğan N, Eken H. Precipitated Calcium carbonate production, synthesis and properties. Fizykochemiczne Problemy Mineralurgii Physicochem Probl Mineral Process. 2017;53:57–68. doi: 10.5277/ppmp170105. [DOI] [Google Scholar]
- Fakhrullin RF, Bikmullin AG, Nurgaliev DK. Magnetically responsive calcium carbonate microcrystals. ACS Appl Mater Interfaces. 2009;1(9):1847–1851. doi: 10.1021/am9003864. [DOI] [PubMed] [Google Scholar]
- Fujii A, Maruyama T, Ohmukai Y, Kamio E, Sotani T, Matsuyama H. Cross-linked DNA capsules templated on porous calcium carbonate microparticles. Colloids Surf A. 2010;356(1):126–133. doi: 10.1016/j.colsurfa.2010.01.008. [DOI] [Google Scholar]
- Fujiwara M, Shiokawa K, Morigaki K, Zhu Y, Nakahara Y. Calcium carbonate microcapsules encapsulating biomacromolecules. Chem Eng J. 2008;137(1):14–22. doi: 10.1016/j.cej.2007.09.010. [DOI] [Google Scholar]
- Ganguli AK, Ganguly A, Vaidya S. Microemulsion-based synthesis of nanocrystalline materials. Chem Soc Rev. 2010;39(2):474–485. doi: 10.1039/B814613F. [DOI] [PubMed] [Google Scholar]
- Gao G, Huang P, Wang K, He R, Cui D. Gram-scale synthesis and shape evolution of micro-CaCO3. Powder Technol. 2011;205(1):270–275. doi: 10.1016/j.powtec.2010.09.032. [DOI] [Google Scholar]
- Gbadeyan OJ, Adali S, Bright G, Sithole B, Onwubu S. Optimization of milling procedures for synthesizing nano-CaCO3 from Achatina fulica shell through mechanochemical techniques. J Nanomater. 2020;2020:4370172. doi: 10.1155/2020/4370172. [DOI] [Google Scholar]
- Ghadami Jadval Ghadam A, Idrees M. Characterization of CaCO3 nanoparticles synthesized by reverse microemulsion technique in different concentrations of surfactants. Ira J Chem Chem Eng (IJCCE) 2013;32(3):27–35. doi: 10.30492/ijcce.2013.5739. [DOI] [Google Scholar]
- Groboillot A, Boadi DK, Poncelet D, Neufeld RJ. Immobilization of cells for application in the food industry. Crit Rev Biotechnol. 1994;14(2):75–107. doi: 10.3109/07388559409086963. [DOI] [PubMed] [Google Scholar]
- Guillemet B, Faatz M, Gröhn F, Wegner G, Gnanou Y. Nanosized amorphous calcium carbonate stabilized by poly(ethylene oxide)-b-poly(acrylic acid) block copolymers. Langmuir. 2006;22(4):1875–1879. doi: 10.1021/la052419e. [DOI] [PubMed] [Google Scholar]
- Haložan D, Riebentanz U, Brumen M, Donath E. Polyelectrolyte microcapsules and coated CaCO3 particles as fluorescence activated sensors in flowmetry. Colloids Surf A. 2009;342(1):115–121. doi: 10.1016/j.colsurfa.2009.04.024. [DOI] [Google Scholar]
- Han C, Hu Y, Wang K, Luo G. Preparation and in-situ surface modification of CaCO3 nanoparticles with calcium stearate in a microreaction system. Powder Technol. 2019;356:414–422. doi: 10.1016/j.powtec.2019.08.054. [DOI] [Google Scholar]
- Hanus MJ, Harris AT. Nanotechnology innovations for the construction industry. Prog Mater Sci. 2013;58(7):1056–1102. doi: 10.1016/j.pmatsci.2013.04.001. [DOI] [Google Scholar]
- Hasan A, Morshed M, Memic A, Hassan S, Webster TJ, Marei HE-S. Nanoparticles in tissue engineering: applications, challenges and prospects. Int J Nanomed. 2018;13:5637–5655. doi: 10.2147/IJN.S153758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Deng H, Hwang H-m. The current application of nanotechnology in food and agriculture. J Food Drug Anal. 2019;27(1):1–21. doi: 10.1016/j.jfda.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helland A, Scheringer M, Siegrist M, Kastenholz HG, Wiek A, Scholz RW. Risk assessment of engineered nanomaterials: a survey of industrial approaches. Environ Sci Technol. 2008;42(2):640–646. doi: 10.1021/es062807i. [DOI] [PubMed] [Google Scholar]
- Hirai T, Hariguchi S, Komasawa I, Davey RJ. Biomimetic synthesis of calcium carbonate particles in a pseudovesicular double emulsion. Langmuir. 1997;13(25):6650–6653. doi: 10.1021/la9705266. [DOI] [Google Scholar]
- Hu C, Chen C, Wu Y, Li J, Hu Y, Wei L, Jiang J. The mechanochemical route of vaterite synthesis using sodium hexametaphosphate as an inorganic additive. J Am Ceram Soc. 2019;102(12):7116–7124. doi: 10.1111/jace.16624. [DOI] [Google Scholar]
- Hu C, Chen C, Wu Y, Li J, Hu Y, Wei L, Jiang J. The mechanochemical route of vaterite synthesis using sodium hexametaphosphate as an inorganic additive. J Am Ceram Soc. 2019 doi: 10.1111/jace.16624. [DOI] [Google Scholar]
- Jacob JJ, Varalakshmi R, Gargi S, Jayasri MA, Suthindhiran K. Removal of Cr (III) and Ni (II) from tannery effluent using calcium carbonate coated bacterial magnetosomes. Npj Clean Water. 2018;1(1):1. doi: 10.1038/s41545-018-0001-2. [DOI] [Google Scholar]
- Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol. 2018;9:1050–1074. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha SK, Dey S, Karki S. Microemulsions-potential carrier for improved drug delivery. Asian J Biomed Pharm Sci. 2011;1(1):1–12. [Google Scholar]
- Kang SH, Hirasawa I, Kim W-S, Choi CK. Morphological control of calcium carbonate crystallized in reverse micelle system with anionic surfactants SDS and AOT. J Colloid Interface Sci. 2005;288(2):496–502. doi: 10.1016/j.jcis.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Kawashima S, Hou P, Corr DJ, Shah SP. Modification of cement-based materials with nanoparticles. Cement Concr Compos. 2013;36:8–15. doi: 10.1016/j.cemconcomp.2012.06.012. [DOI] [Google Scholar]
- Khan SR, Jamil S, Rashid H, Ali S, Khan SA, Janjua MRSA. Agar and egg shell derived calcium carbonate and calcium hydroxide nanoparticles: synthesis, characterization and applications. Chem Phys Lett. 2019;732:136662. doi: 10.1016/j.cplett.2019.136662. [DOI] [Google Scholar]
- Khulbe KC, Matsuura T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl Water Sci. 2018;8(1):19. doi: 10.1007/s13201-018-0661-6. [DOI] [Google Scholar]
- Kim Y-Y, Schenk AS, Ihli J, Kulak AN, Hetherington NBJ, Tang CC, Schmahl WW, Griesshaber E, Hyett G, Meldrum FC. A critical analysis of calcium carbonate mesocrystals. Nat Commun. 2014;5(1):4341. doi: 10.1038/ncomms5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiranda HK, Mahmud R, Abubakar D, Zakaria ZA. Fabrication, characterization and cytotoxicity of spherical-shaped conjugated gold-cockle shell derived calcium carbonate nanoparticles for biomedical applications. Nanoscale Res Lett. 2018;13(1):1. doi: 10.1186/s11671-017-2411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryavtseva VL, Zhao L, Tverdokhlebov SI, Sukhorukov GB. Fabrication of PLA/CaCO3 hybrid micro-particles as carriers for water-soluble bioactive molecules. Colloids Surf B. 2017;157:481–489. doi: 10.1016/j.colsurfb.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Lauster D, Glanz M, Bardua M, Ludwig K, Hellmund M, Hoffmann U, Hamann A, Böttcher C, Haag R, Hackenberger CPR, Herrmann A. Multivalent peptide-nanoparticle conjugates for influenza-virus inhibition. Angew Chem Int Ed Engl. 2017;56(21):5931–5936. doi: 10.1002/anie.201702005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Han SW, Choi HJ, Kim K. Nanoparticle-directed crystallization of calcium carbonate. Adv Mater. 2001;13(21):1617–1620. doi: 10.1002/1521-4095(200111)13:21<1617::AID-ADMA1617>3.0.CO;2-W. [DOI] [Google Scholar]
- Li Y, Yang X-Y, Feng Y, Yuan Z-Y, Su B-L. One-dimensional metal oxide nanotubes, nanowires, nanoribbons, and nanorods: synthesis, characterizations, properties and applications. Crit Rev Solid State Mater Sci. 2012;37(1):1–74. doi: 10.1080/10408436.2011.606512. [DOI] [Google Scholar]
- Li H, Yao Q-Z, Wang F-P, Huang Y-R, Fu S-Q, Zhou G-T. Insights into the formation mechanism of vaterite mediated by a deep-sea bacterium Shewanella piezotolerans WP3. Geochim Cosmochim Acta. 2019;256:35–48. doi: 10.1016/j.gca.2018.06.011. [DOI] [Google Scholar]
- Li H, Zhang X, Lin X, Zhuang S, Wu Y, Liu Z, Rong J, Zhao J. CaCO3 nanoparticles pH-sensitively induce blood coagulation as a potential strategy for starving tumor therapy. J Mater Chem B. 2020;8(6):1223–1234. doi: 10.1039/C9TB02684C. [DOI] [PubMed] [Google Scholar]
- Lin Y, Chan CM. 3—Calcium carbonate nanocomposites. In: Gao F, editor. Advances in polymer nanocomposites. Woodhead Publishing; 2012. pp. 55–90. [Google Scholar]
- Liu F, He X, Zhang J, Chen H, Zhang H, Wang Z. Controllable synthesis of polydopamine nanoparticles in microemulsions with pH-activatable properties for cancer detection and treatment. J Mater Chem B. 2015;3(33):6731–6739. doi: 10.1039/C5TB01159K. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhou J, Zhang Y, Liu X, Chen Y, Yong X, Wang S, Zheng T, Yuan H. Continuous process of biogas purification and co-production of nano calcium carbonate in multistage membrane reactors. Chem Eng J. 2015;271:223–231. doi: 10.1016/j.cej.2015.02.086. [DOI] [Google Scholar]
- Liu Y, Yu B, Dai X, Zhao N, Xu F-J. Biomineralized calcium carbonate nanohybrids for mild photothermal heating-enhanced gene therapy. Biomaterials. 2021;274:120885. doi: 10.1016/j.biomaterials.2021.120885. [DOI] [PubMed] [Google Scholar]
- Lu H, Ju H, Yang Q, Li Z, Ren H, Xin X, Xu G. Synthesis of Ag@SiO2 hybrid nanoparticles templated by a Triton X-100)/1-hexanol/cyclohexane/H2O water-in-oil microemulsion. CrystEngComm. 2013;15(33):6511–6517. doi: 10.1039/C3CE40432C. [DOI] [Google Scholar]
- Lu CY, Puig T, Obradors X, Ricart S, Ros J. Ultra-fast microwave-assisted reverse microemulsion synthesis of Fe3O4@SiO2 core–shell nanoparticles as a highly recyclable silver nanoparticle catalytic platform in the reduction of 4-nitroaniline. RSC Adv. 2016;6(91):88762–88769. doi: 10.1039/C6RA19435D. [DOI] [Google Scholar]
- Luquet G. Biomineralizations: insights and prospects from crustaceans. Zookeys. 2012;176:103–121. doi: 10.3897/zookeys.176.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu SG, Park S, Sur GS. The synthesis of vaterite and physical properties of PP/CaCO 3 composites. Korean J Chem Eng. 1999;16(4):538–542. doi: 10.1007/BF02698281. [DOI] [Google Scholar]
- Maleki Dizaj S, Barzegar-Jalali M, Zarrintan MH, Adibkia K, Lotfipour F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin Drug Deliv. 2015;12(10):1649–1660. doi: 10.1517/17425247.2015.1049530. [DOI] [PubMed] [Google Scholar]
- Mallakpour S, Abdolmaleki A, Tabesh F. Ultrasonic-assisted manufacturing of new hydrogel nanocomposite biosorbent containing calcium carbonate nanoparticles and tragacanth gum for removal of heavy metal. Ultrason Sonochem. 2018;41:572–581. doi: 10.1016/j.ultsonch.2017.10.022. [DOI] [PubMed] [Google Scholar]
- Mauter MS, Elimelech M. Environmental applications of carbon-based nanomaterials. Environ Sci Technol. 2008;42(16):5843–5859. doi: 10.1021/es8006904. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh-Saghavaz K, Resalati H, Ghasemian A. Cellulose-precipitated calcium carbonate composites and their effect on paper properties. Chem Pap. 2014;68(6):774–781. doi: 10.2478/s11696-013-0513-7. [DOI] [Google Scholar]
- Mohammadkazemi F, Faria M, Cordeiro N. In situ biosynthesis of bacterial nanocellulose-CaCO3 hybrid bionanocomposite: One-step process. Mater Sci Eng C. 2016;65:393–399. doi: 10.1016/j.msec.2016.04.069. [DOI] [PubMed] [Google Scholar]
- Mohseni-Vadeghani E, Karimi-Soflou R, Khorshidi S, Karkhaneh A. Fabrication of oxygen and calcium releasing microcarriers with different internal structures for bone tissue engineering: Solid filled versus hollow microparticles. Colloids Surf B. 2021;197:111376. doi: 10.1016/j.colsurfb.2020.111376. [DOI] [PubMed] [Google Scholar]
- Munyemana JC, He H, Fu C, Wei W, Tian J, Xiao J. A trypsin–calcium carbonate hybrid nanosphere based enzyme reactor with good stability and reusability. New J Chem. 2018;42(22):18388–18394. doi: 10.1039/C8NJ04282A. [DOI] [Google Scholar]
- Murakami F, Rodrigues P, Campos C, Silva M. Physicochemical study of CaCO3 from egg shells. Ciencia E Tecnol De Aliment Ciencia Tecnol Aliment. 2007 doi: 10.1590/S0101-20612007000300035. [DOI] [Google Scholar]
- Mydin SMNRB, Nadhirah I, Ishak N, Shaida N, Moshawih S, Siddiquee S. Potential of calcium carbonate nanoparticles for therapeutic applications. Malays J Med Health Sci. 2018;14:2636–9346. [Google Scholar]
- Nassar MM, Farrag TE, Mahmoud MS, Abdelmonem S, Khalil KA, Barakat NAM. Influence of the operating conditions on the morphology of CaCO3 nanoparticles prepared by modified co-precipitation with pulse mode feeding. Adv Powder Technol. 2015;26(3):914–919. doi: 10.1016/j.apt.2015.03.006. [DOI] [Google Scholar]
- Nayak AK, Mazumder S, Ara TJ, Ansari MT, Hasnain MS. 2—Calcium fluoride-based dental nanocomposites. In: Asiri AM, Inamuddin I, Mohammad A, editors. Applications of nanocomposite materials in dentistry. Woodhead Publishing; 2019. pp. 27–45. [Google Scholar]
- Nogueira LFB, Maniglia BC, Pereira LS, Tapia-Blácido DR, Ramos AP. Formation of carrageenan-CaCO3 bioactive membranes. Mater Sci Eng C. 2016;58:1–6. doi: 10.1016/j.msec.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Pai RK, Pillai S. Nanoparticles of amorphous calcium carbonate by miniemulsion: synthesis and mechanism. CrystEngComm. 2008;10(7):865–872. doi: 10.1039/B717057B. [DOI] [Google Scholar]
- Patel VM, Sheth P, Kurz A, Ossenbeck M, Shah DO, Gower LB (2004) Synthesis of calcium carbonate-coated emulsion droplets for drug detoxification. In: Somasundaran P, Markovic B (eds) Concentrated dispersions. ACS symposium series, vol 878. American Chemical Society, pp 15–25. 10.1021/bk-2004-0878.ch002
- Peppas NA (1992) Principles of polymerization: G. Odian, 3rd edition, Wiley, New York, 1991,768 pages, $59.95. JControlled Release 22 (3): 294. 10.1016/0168-3659(92)90108-4
- Persistence market research Nano Calcium Carbonate Market (2021) Persistence market research https://www.persistencemarketresearch.com/market-research/nano-calcium-carbonate-market.asp. Accessed 5 Mar 2021
- Pineda-Reyes AM, Olvera MDIL. Synthesis of ZnO nanoparticles from water-in-oil (w/o) microemulsions. Mater Chem Phys. 2018;203:141–147. doi: 10.1016/j.matchemphys.2017.09.054. [DOI] [Google Scholar]
- Piras C, Fernandez Prieto S, Borggraeve W. Ball milling : A green technology for the preparation and functionalisation of nanocellulose derivatives. Nanoscale Adv. 2019 doi: 10.1039/C8NA00238J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirzada M, Altintas Z. Nanomaterials for healthcare biosensing applications. Sensors. 2019;19(23):5311. doi: 10.3390/s19235311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournajaf R, Hassanzadeh-Tabrizi SA, Jafari M. Reverse microemulsion synthesis of CeO2 nanopowder using polyoxyethylene(23)lauryl ether as a surfactant. Ceram Int. 2014;40(6):8687–8692. doi: 10.1016/j.ceramint.2014.01.086. [DOI] [Google Scholar]
- Qi R-J, Zhu Y-J. Microwave-assisted synthesis of calcium carbonate (Vaterite) of various morphologies in water−ethylene glycol mixed solvents. J Phys Chem B. 2006;110(16):8302–8306. doi: 10.1021/jp060939s. [DOI] [PubMed] [Google Scholar]
- Qiu N, Yin H, Ji B, Klauke N, Glidle A, Zhang Y, Song H, Cai L, Ma L, Wang G, Chen L, Wang W. Calcium carbonate microspheres as carriers for the anticancer drug camptothecin. Mater Sci Eng C. 2012;32(8):2634–2640. doi: 10.1016/j.msec.2012.08.026. [DOI] [Google Scholar]
- Ramasamy V, Anand P, Suresh G. Synthesis and characterization of polymer-mediated CaCO3 nanoparticles using limestone: a novel approach. Adv Powder Technol. 2018;29(3):818–834. doi: 10.1016/j.apt.2017.12.023. [DOI] [Google Scholar]
- Rautaray D, Sainkar SR, Sastry M. Ca2+−Keggin anion colloidal particles as templates for the growth of star-shaped calcite crystal assemblies. Langmuir. 2003;19(24):10095–10099. doi: 10.1021/la034128g. [DOI] [Google Scholar]
- Render D, Samuel T, King H, Vig M, Jeelani S, Babu RJ, Rangari V. Biomaterial-derived calcium carbonate nanoparticles for enteric drug delivery. J Nanomater. 2016;2016:3170248. doi: 10.1155/2016/3170248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinu S, Queen AJ, Udhaya PA. Synthesis, structural characterization and antibacterial applications of calcium nanoparticles. J Adv Sci Res. 2020;11(1):83–87. [Google Scholar]
- Roberti A, Valdes AF, Torrecillas R, Fraga MF, Fernandez AF. Epigenetics in cancer therapy and nanomedicine. Clin Epigenet. 2019;11(1):81. doi: 10.1186/s13148-019-0675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez JA, Fernández-García M. Synthesis, properties, and applications of oxide nanomaterials. Wiley; 2007. [Google Scholar]
- Rojac T, Kosec M. 6—mechanochemical synthesis of complex ceramic oxides. In: Sopicka-Lizer M, editor. High-energy ball milling. Woodhead Publishing; 2010. pp. 113–148. [Google Scholar]
- Ropp RC. Chapter 5—group 14 (C, Si, Ge, Sn, and Pb) alkaline earth compounds. In: Ropp RC, editor. Encyclopedia of the alkaline earth compounds. Amsterdam: Elsevier; 2013. pp. 351–480. [Google Scholar]
- Ru X, Guo Y, Bai Z, Xie X, Ma X, Zhu L, Wang K, Wang F, Yang L, Lu J. Synthesis of calcium carbonate nanoparticles in erythrocytes enables efficient removal of extracellular lead ions. Commun Chem. 2019;2(1):105. doi: 10.1038/s42004-019-0199-z. [DOI] [Google Scholar]
- Sabbarwal S, Dubey AK, Pandey M, Kumar M. Synthesis of biocompatible, BSA capped fluorescent CaCO3 pre-nucleation nanoclusters for cell imaging applications. J Mater Chem B. 2020;8(26):5729–5744. doi: 10.1039/D0TB00881H. [DOI] [PubMed] [Google Scholar]
- Sahebian S, Zebarjad SM, Khaki JV, Sajjadi SA. The effect of nano-sized calcium carbonate on thermodynamic parameters of HDPE. J Mater Process Technol. 2009;209(3):1310–1317. doi: 10.1016/j.jmatprotec.2008.03.066. [DOI] [Google Scholar]
- Saif S, Tahir A, Chen Y. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials. 2016 doi: 10.3390/nano6110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek SS, Bozkurt OD, van Turnhout AG, Kleerebezem R, van Loosdrecht MCM. Kinetics of CaCO3 precipitation in an anaerobic digestion process integrated with silicate minerals. Ecol Eng. 2016;86:105–112. doi: 10.1016/j.ecoleng.2015.10.025. [DOI] [Google Scholar]
- Sarkar A, Mahapatra S. Synthesis of all crystalline phases of anhydrous calcium carbonate. Cryst Growth Des. 2010;10(5):2129–2135. doi: 10.1021/cg9012813. [DOI] [Google Scholar]
- Saveleva MS, Ivanov AN, Kurtukova MO, Atkin VS, Ivanova AG, Lyubun GP, Martyukova AV, Cherevko EI, Sargsyan AK, Fedonnikov AS, Norkin IA, Skirtach AG, Gorin DA, Parakhonskiy BV. Hybrid PCL/CaCO3 scaffolds with capabilities of carrying biologically active molecules: synthesis, loading and in vivo applications. Mater Sci Eng C. 2018;85:57–67. doi: 10.1016/j.msec.2017.12.019. [DOI] [PubMed] [Google Scholar]
- Schlickmann KP, Howarth JLL, Silva DAK, Pezzin APT. Effect of the incorporation of micro and nanoparticles of calcium carbonate in poly (Vinyl Chloride) matrix for industrial application. Mater Res. 2019;22:1–12. doi: 10.1590/1980-5373-mr-2018-0870. [DOI] [Google Scholar]
- Sdiri A, Higashi T. Simultaneous removal of heavy metals from aqueous solution by natural limestones. Appl Water Sci. 2013;3(1):29–39. doi: 10.1007/s13201-012-0054-1. [DOI] [Google Scholar]
- Serra-Gómez R, González-Gaitano G, Gonzalez-Benito J. Composites based on EVA and barium titanate submicrometric particles: preparation by high-energy ball milling and characterization. Polym Compos. 2012 doi: 10.1002/pc.22291. [DOI] [Google Scholar]
- Servin AD, White JC. Nanotechnology in agriculture: next steps for understanding engineered nanoparticle exposure and risk. NanoImpact. 2016;1:9–12. doi: 10.1016/j.impact.2015.12.002. [DOI] [Google Scholar]
- Shaikh FUA, Supit SWM. Mechanical and durability properties of high volume fly ash (HVFA) concrete containing calcium carbonate (CaCO3) nanoparticles. Constr Build Mater. 2014;70:309–321. doi: 10.1016/j.conbuildmat.2014.07.099. [DOI] [Google Scholar]
- Shan D, Zhu M, Han E, Xue H, Cosnier S. Calcium carbonate nanoparticles: A host matrix for the construction of highly sensitive amperometric phenol biosensor. Biosens Bioelectron. 2007;23(5):648–654. doi: 10.1016/j.bios.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Shan D, Zhu M, Xue H, Cosnier S. Development of amperometric biosensor for glucose based on a novel attractive enzyme immobilization matrix: calcium carbonate nanoparticles. Biosens Bioelectron. 2007;22(8):1612–1617. doi: 10.1016/j.bios.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Shen Y, Xie A, Chen Z, Xu W, Yao H, Li S, Huang L, Wu Z, Kong X. Controlled synthesis of calcium carbonate nanocrystals with multi-morphologies in different bicontinuous microemulsions. Mater Sci Eng, A. 2007;443(1):95–100. doi: 10.1016/j.msea.2006.08.105. [DOI] [Google Scholar]
- Shi Y, Ding J, Yin H. CoFe2O4 nanoparticles prepared by the mechanochemical method. J Alloy Compd. 2000;308(1):290–295. doi: 10.1016/S0925-8388(00)00921-X. [DOI] [Google Scholar]
- Shi H, Li L, Zhang L, Wang T, Wang C, Zhu D, Su Z. Designed preparation of polyacrylic acid/calcium carbonate nanoparticles with high doxorubicin payload for liver cancer chemotherapy. CrystEngComm. 2015;17(26):4768–4773. doi: 10.1039/C5CE00708A. [DOI] [Google Scholar]
- Shimpi N, Mali A, Hansora D, Mishra S. Synthesis and surface modification of calcium carbonate nanoparticles using ultrasound cavitation technique. Nanosci Nanoeng. 2015;3(1):8–12. doi: 10.13189/nn.2015.030102. [DOI] [Google Scholar]
- Shum HC, Lee D, Yoon I, Kodger T, Weitz DA. Double emulsion templated monodisperse phospholipid vesicles. Langmuir. 2008;24(15):7651–7653. doi: 10.1021/la801833a. [DOI] [PubMed] [Google Scholar]
- Shum HC, Bandyopadhyay A, Bose S, Weitz DA. Double emulsion droplets as microreactors for synthesis of mesoporous hydroxyapatite. Chem Mater. 2009;21(22):5548–5555. doi: 10.1021/cm9028935. [DOI] [Google Scholar]
- Singh P, Kim Y-J, Zhang D, Yang D-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34(7):588–599. doi: 10.1016/j.tibtech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Singh J, Dutta T, Kim K-H, Rawat M, Samddar P, Kumar P. ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol. 2018;16(1):84. doi: 10.1186/s12951-018-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubiszewska-Zięba J, Charmas B, Waniak-Nowicka H. Hydrothermal and mechanochemical synthesis of crystalline CaCO3. Adsorpt Sci Technol. 2017;35:026361741770429. doi: 10.1177/0263617417704298. [DOI] [Google Scholar]
- Sokolova V, Kostka K, Shalumon KT, Prymak O, Chen J-P, Epple M. Synthesis and characterization of PLGA/HAP scaffolds with DNA-functionalised calcium phosphate nanoparticles for bone tissue engineering. J Mater Sci Mater Med. 2020;31(11):102. doi: 10.1007/s10856-020-06442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane PS, Biradar SS, Radhakrishnan S, Kulkarni BD. Role of ionic diffusion in polymer gel mediated growth (PMG) technique for the synthesis of nanoparticulate fillers. Mater Chem Phys. 2007;105(2):348–353. doi: 10.1016/j.matchemphys.2007.04.071. [DOI] [Google Scholar]
- Stark WJ, Stoessel PR, Wohlleben W, Hafner A. Industrial applications of nanoparticles. Chem Soc Rev. 2015;44(16):5793–5805. doi: 10.1039/C4CS00362D. [DOI] [PubMed] [Google Scholar]
- Sulimai NH, Rani RA, Khusaimi Z, Abdullah S, Salifairus MJ, Alrokayan S, Khan H, Rusop M. Effect of ball-milling to the surface morphology of CaCO3. AIP Conf Proc. 2018;1963(1):020057. doi: 10.1063/1.5036903. [DOI] [Google Scholar]
- Sulpis O, Boudreau BP, Mucci A, Jenkins C, Trossman DS, Arbic BK, Key RM. Current CaCO3 dissolution at the seafloor caused by anthropogenic CO2. Proc Natl Acad Sci. 2018;115(46):11700. doi: 10.1073/pnas.1804250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Deng Y. Synthesis of micrometer to nanometer CaCO3 particles via mass restriction method in an emulsion liquid membrane process. J Colloid Interface Sci. 2004;278(2):376–382. doi: 10.1016/j.jcis.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Suppes GJ, Bockwinkel K, Lucas S, Botts JB, Mason MH, Heppert JA. Calcium carbonate catalyzed alcoholysis of fats and oils. J Am Oil Chem Soc. 2001;78(2):139–146. doi: 10.1007/s11746-001-0234-y. [DOI] [Google Scholar]
- Szcześ A. Influence of the surfactant nature on the calcium carbonate synthesis in water-in-oil emulsion. J Cryst Growth. 2009;311(4):1129–1135. doi: 10.1016/j.jcrysgro.2008.12.044. [DOI] [Google Scholar]
- Tai CY, Chen C-k. Particle morphology, habit, and size control of CaCO3 using reverse microemulsion technique. Chem Eng Sci. 2008;63(14):3632–3642. doi: 10.1016/j.ces.2008.04.022. [DOI] [Google Scholar]
- Thachepan S, Li M, Davis SA, Mann S. Additive-mediated crystallization of complex calcium carbonate superstructures in reverse microemulsions. Chem Mater. 2006;18(15):3557–3561. doi: 10.1021/cm060847f. [DOI] [Google Scholar]
- Tojo C, de Dios M, Buceta D, López-Quintela MA. Cage-like effect in Au–Pt nanoparticle synthesis in microemulsions: a simulation study. Phys Chem Chem Phys. 2014;16(36):19720–19731. doi: 10.1039/C4CP02936D. [DOI] [PubMed] [Google Scholar]
- Tovani CB, Zancanela DC, Faria AN, Ciancaglini P, Ramos AP. Bio-inspired synthesis of hybrid tube-like structures based on CaCO3 and type I-collagen. RSC Adv. 2016;6(93):90509–90515. doi: 10.1039/C6RA18984A. [DOI] [Google Scholar]
- Tovani CB, Faria AN, Ciancaglini P, Ramos AP. Collagen-supported CaCO3 cylindrical particles enhance Ti bioactivity. Surf Coat Technol. 2019;358:858–864. doi: 10.1016/j.surfcoat.2018.11.071. [DOI] [Google Scholar]
- Tsuzuki T, Pethick K, McCormick PG. Synthesis of CaCO3 nanoparticles by mechanochemical processing. J Nanopart Res. 2000;2(4):375–380. doi: 10.1023/A:1010051506232. [DOI] [Google Scholar]
- UskokoviĆ VUK, Drofenik M. Synthesis of materials within reverse micelles. Surf Rev Lett. 2005;12(02):239–277. doi: 10.1142/S0218625X05007001. [DOI] [Google Scholar]
- Uzunoğlu D, Özer A. Biosynthesis and characterization of CaCO3 nanoparticles from the leach solution and the aqueous extract of Myrtus communis plant. Intl Adv Res Eng J. 2018;2(3):245–253. [Google Scholar]
- Volodkin DV, Petrov AI, Prevot M, Sukhorukov GB. Matrix polyelectrolyte microcapsules: new system for macromolecule encapsulation. Langmuir. 2004;20(8):3398–3406. doi: 10.1021/la036177z. [DOI] [PubMed] [Google Scholar]
- Walsh D, Kim Y-Y, Miyamoto A, Meldrum FC. Synthesis of macroporous calcium carbonate/magnetite nanocomposites and their application in photocatalytic water splitting. Small. 2011;7(15):2168–2172. doi: 10.1002/smll.201100268. [DOI] [PubMed] [Google Scholar]
- Wang C, Xiao P, Zhao J, Zhao X, Liu Y, Wang Z. Biomimetic synthesis of hydrophobic calcium carbonate nanoparticles via a carbonation route. Powder Technol. 2006;170(1):31–35. doi: 10.1016/j.powtec.2006.08.016. [DOI] [Google Scholar]
- Wang K, Wang YJ, Chen GG, Luo GS, Wang JD. Enhancement of mixing and mass transfer performance with a microstructure minireactor for controllable preparation of CaCO3 nanoparticles. Ind Eng Chem Res. 2007;46(19):6092–6098. doi: 10.1021/ie061502+. [DOI] [Google Scholar]
- Wang X, Liu L-H, Ramström O, Yan M. Engineering nanomaterial surfaces for biomedical applications. Exp Biol Med. 2009;234(10):1128–1139. doi: 10.3181/0904-MR-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen J-S, Zong J-Y, Zhao D, Li F, Zhuo R-X, Cheng S-X. Calcium carbonate/carboxymethyl chitosan hybrid microspheres and nanospheres for drug delivery. J Phys Chem C. 2010;114(44):18940–18945. doi: 10.1021/jp105906p. [DOI] [Google Scholar]
- Wang X, Shi L, Zhang J, Cheng J, Wang X. In situ formation of surface-functionalized ionic calcium carbonate nanoparticles with liquid-like behaviours and their electrical properties. R Soc Open Sci. 2018;5(1):170732. doi: 10.1098/rsos.170732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Han M, Liu X, Chen S, Hu F, Sun J, Yuan H. Mitoxantrone-preloaded water-responsive phospholipid-amorphous calcium carbonate hybrid nanoparticles for targeted and effective cancer therapy. Int J Nanomedicine. 2019;14:1503–1517. doi: 10.2147/IJN.S193976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Ma G-H, Hu G, Yu D, McLeish T, Su Z-G, Shen Z-Y. Preparation of hierarchical hollow CaCO3 particles and the application as anticancer drug carrier. J Am Chem Soc. 2008;130(47):15808–15810. doi: 10.1021/ja8039585. [DOI] [PubMed] [Google Scholar]
- Wei Q, Lu J, Wang Q, Fan H, Zhang X. Novel synthesis strategy for composite hydrogel of collagen/hydroxyapatite-microsphere originating from conversion of CaCO3templates. Nanotechnology. 2015;26(11):115605. doi: 10.1088/0957-4484/26/11/115605. [DOI] [PubMed] [Google Scholar]
- Willner I, Willner B. Biomolecule-based nanomaterials and nanostructures. Nano Lett. 2010;10(10):3805–3815. doi: 10.1021/nl102083j. [DOI] [PubMed] [Google Scholar]
- Won Y-H, Jang HS, Chung D-W, Stanciu LA. Multifunctional calcium carbonate microparticles: synthesis and biological applications. J Mater Chem. 2010;20(36):7728–7733. doi: 10.1039/C0JM01231A. [DOI] [Google Scholar]
- Wu J, Zeng RJ. Biomimetic regulation of microbially induced calcium carbonate precipitation involving Immobilization of Sporasarcina pasteurii by sodium alginate. Cryst Growth Des. 2017;17(4):1854–1862. doi: 10.1021/acs.cgd.6b01813. [DOI] [Google Scholar]
- Wu GX, Ding J, Xue JM. Synthesis of calcium carbonate capsules in water-in-oil-in-water double emulsions. J Mater Res. 2008;23(1):140–149. doi: 10.1557/JMR.2008.0017. [DOI] [Google Scholar]
- Xing J, Cai Y, Wang Y, Zheng H, Liu Y. Synthesis of polymer assembled mesoporous CaCO3 nanoparticles for molecular targeting and pH-responsive controlled drug release. Adv Polym Technol. 2020 doi: 10.1155/2020/8749238. [DOI] [Google Scholar]
- Xu C, Zhang Z, Zhang J, Lei L, Zhang D, Fu Z. A new route to fabricate barium titanate with high permittivity. Cerams Int. 2014;40(7, Part B):10927–10931. doi: 10.1016/j.ceramint.2014.03.090. [DOI] [Google Scholar]
- Xue Z-H, Hu B-B, Jia X-L, Wang H-W, Du Z-L. Effect of the interaction between bovine serum albumin Langmuir monolayer and calcite on the crystallization of CaCO3 nanoparticles. Mater Chem Phys. 2009;114(1):47–52. doi: 10.1016/j.matchemphys.2008.07.002. [DOI] [Google Scholar]
- Yang T, Fu J, Ma L, Du H, Yue X, Zhao B, Wang C. Biomimetic synthesis of calcium carbonate under phenylalanine: control of polymorph and morphology. Mater Sci Eng C. 2020;114:111019. doi: 10.1016/j.msec.2020.111019. [DOI] [PubMed] [Google Scholar]
- Yao S, Swetha P, Zhu Y. Nanomaterial-Enabled Wearable Sensors for Healthcare. Adv Healthc Mater. 2018;7(1):1700889. doi: 10.1002/adhm.201700889. [DOI] [PubMed] [Google Scholar]
- Yashchenok AM, Borisova D, Parakhonskiy BV, Masic A, Pinchasik B, Möhwald H, Skirtach AG. Nanoplasmonic smooth silica versus porous calcium carbonate bead biosensors for detection of biomarkers. Ann Phys. 2012;524(11):723–732. doi: 10.1002/andp.201200158. [DOI] [Google Scholar]
- Yue L, Jin D, Shui M, Xu Z. Study on the synthesis of spherulitic calcium carbonate composite in amphiphilic PS-b-PAA solution and its crystalline structure. Solid State Sci. 2004;6(9):1007–1012. doi: 10.1016/j.solidstatesciences.2004.04.011. [DOI] [Google Scholar]
- Zamani M, Nikafshar S, Mousa A, Behnia A. Bacteria encapsulation using synthesized polyurea for self-healing of cement paste. Constr Build Mater. 2020;249:118556. doi: 10.1016/j.conbuildmat.2020.118556. [DOI] [Google Scholar]
- Zhao Y, Lu Y, Hu Y, Li J-P, Dong L, Lin L-N, Yu S-H. Synthesis of superparamagnetic CaCO3 mesocrystals for multistage delivery in cancer therapy. Small. 2010;6(21):2436–2442. doi: 10.1002/smll.201000903. [DOI] [PubMed] [Google Scholar]
- Zheng L, Hu Y, Ma Y, Zhou Y, Nie F, Liu X, Pei C. Egg-white-mediated crystallization of calcium carbonate. J Cryst Growth. 2012;361:217–224. doi: 10.1016/j.jcrysgro.2012.09.003. [DOI] [Google Scholar]