Abstract
Excessive alcohol and iron intake can reportedly cause liver damage. In the present study, we investigated the effect of Lactobacillus casei on liver injury in rats co‐exposed to alcohol and iron and evaluated its possible mechanism. Sixty male Wistar rats were randomly divided into three groups for 12 weeks: the Control group (administered normal saline by gavage and provided a normal diet); alcohol +iron group (Model group, treated with alcohol [3.5–5.3 g/kg/day] by gavage and dietary iron [1,500 mg/kg]); Model group supplemented with L. casei (8 × 108 CFU kg−1 day−1) (L. casei group). Using hematoxylin and eosin (HE) staining and transmission electron microscopy, we observed that L. casei supplementation could alleviate disorders associated with lipid metabolism, inflammation, and intestinal mucosal barrier injury. Moreover, levels of serum alanine aminotransferase, gamma‐glutamyl transferase, triglyceride (TG), and hepatic TG were significantly increased in the model group; however, these levels were significantly decreased following the 12‐week L. casei supplementation. In addition, we observed notable improvements in intestinal mucosal barrier function and alterations in T lymphocyte subsets and natural killer cells in L. casei‐treated rats when compared with the model group. Furthermore, L. casei intervention alleviated serum levels of tumor necrosis factor‐α and interleukin‐1β, accompanied by decreased serum endotoxin levels and downregulated expression of toll‐like receptor 4 and its related molecules MyD88, nuclear factor kappa‐B p65, and TNF‐α. Accordingly, supplementation with L. casei could effectively improve liver injury induced by the synergistic interaction between alcohol and iron. The underlying mechanism for this improvement may be related to immune regulation and inhibition of enterogenic endotoxin‐mediated inflammation.
Keywords: alcohol and iron, immunity, inflammatory, Lactobacillus casei, liver injury
Lactobacillus casei could effectively improve liver injury induced by co‐exposure to alcohol and iron. The mechanism may be related to the immune regulation and enterogenic endotoxin‐mediated inflammation inhibition.

1. INTRODUCTION
The liver is an essential organ for alcohol metabolism and iron storage; therefore, it is the primary target organ for alcohol injury and iron overload (Lainé et al., 2017; Lakhal‐Littleton et al., 2016). The intake of both excessive alcohol and iron (iron or iron‐rich foods) can lead to liver damage via oxidative stress and lipid peroxidation (Osna et al., 2017; Pietrangelo, 2016); excessive alcohol intake can also promote iron absorption, resulting in excessive iron deposition in the liver (Corradini & Pietrangelo, 2012; Grochowski et al., 2019). In addition, studies have revealed that co‐exposure to alcohol and iron results in synergistic oxidation and cumulative effects that aggravate hepatocyte injury (Fletcher & Powell, 2003; Sumida et al., 2001).
Reportedly, the immune status of T lymphocytes is closely related to the occurrence and development of alcoholic liver injury (Matos et al., 2013). Long‐term drinking can inhibit innate immune cells and reduce the number and activity of lymphocyte subsets (Støy et al., 2015). Alcohol consumption also impacts the functions of hepatic natural killer (NK) cells (Cui et al., 2017). In addition, disturbances in iron homeostasis have been associated with altered immune functions and liver injury. Iron overload can affect lymphocyte proliferation/maturation, induce the apoptosis of T lymphocytes, and selectively affect peripheral T lymphocytes, with hepatocellular ballooning injury (Buracco et al., 2017; Handa et al., 2016). Based on these previous reports, it can be postulated that the immune state caused by excessive alcohol and iron intake mediates the development of liver injury.
Endotoxins are a major component of Gram‐negative bacterial cell walls (Clementi et al., 2017). Notably, excessive alcohol intake is known to result in enhanced intestinal mucosal permeability, which, in turn, causes the leakage of large amounts of gut‐derived endotoxins from the gut lumen into the systemic circulation (Meroni & Longo, 2019). These endotoxins enter the liver through the portal vein and bind to toll‐like receptor 4 (TLR4) on the Kupffer cell surface. Subsequently, a cascade reaction triggers downstream signaling molecules, including MyD88 and nuclear factor kappa‐B (NF‐κB), resulting in the activation of Kupffer cells to secrete and release excessive proinflammatory cytokines such as tumor necrosis factor (TNF)‐α and interleukin (IL)‐1β (Li et al., 2012; Zannetti et al., 2016), which ultimately causes inflammatory injury to hepatocytes. Additionally, excessive iron uptake by macrophages can enhance NF‐κB activity, further promoting inflammatory factor production and intensifying liver injury (Liu Meng‐na & Zhang, 2019).
Probiotics are living microorganisms that are beneficial to the health of the host. Appropriate probiotic supplementation improves the repair of intestinal mucosal damage (Deng et al., 2017) and affords anti‐inflammatory (Li et al., 2016), antioxidant (Wang et al., 2017), and immune regulation (La Fata et al., 2018). As a well‐known probiotic, Lactobacillus casei, in addition to the above characteristics, has attracted extensive attention, especially for its protective effect against alcoholic liver injury. Several studies have achieved good therapeutic effects following L. casei supplementation in patients with alcoholic cirrhosis (Koga et al., 2013; Macnaughtan et al., 2020; Stadlbauer et al., 2008), during which iron deposition is a known marker (Zhang & Krinsky, 2004). Currently, the mechanism underlying the protective effect of L. casei on alcoholic liver injury remains poorly understood. Accordingly, we aimed to explore the effects of L. casei on liver injury in rats induced by the synergistic interaction between alcohol and iron. Furthermore, we investigated the mechanism underlying immune regulation and inhibition of enterogenic endotoxin‐mediated inflammation.
2. MATERIALS AND METHODS
2.1. Animals and ethics statement
Adult male Wistar rats (180–220 g, aged 2 months) were provided by the Animal Experiment Centre (Qingdao, China). The experimental protocol was approved by the Animal Ethics Committee of Qingdao University.
2.2. Experimental design
After one week of adaptive feeding, 60 male Wistar rats were randomly divided into 3 groups (20 animals/group): the control group, normal saline by oral gavage and a standard diet; the Model group (alcohol +iron group), fed a standard diet containing high dietary iron (1,500 mg/kg) and 56% v/v alcohol by oral gavage (3.5g kg−1 day−1 2 weeks +5.3g kg−1 day−1 10 weeks); the L. casei group (L. casei treatment group), fed a standard diet containing high dietary iron (1,500 mg/kg) and oral alcohol and L. casei (the dose of alcohol was the same as in the model group; L. casei, 8 × 108 CFU/kg−1 day−1). The experiment was performed over a 12‐week period.
Lactobacillus casei was provided by Yangle Duo China Investment Co., Ltd. (L. casei content ≥1 × 108 CFU/ml). The alcohol (56% [v/v] ethanol) used was Red Star Erguotou (Beijing Red Star Co., Ltd., Beijing, China) (Ma et al., 2018).
After 12 weeks, the animals were sacrificed, and the blood (serum or plasma), liver, and small intestinal tissues were harvested and stored at −80°C. Fresh liver tissues were quickly excised, fixed in 10% formaldehyde, and embedded in paraffin.
2.3. Determination of serum iron (SI), liver iron concentration (LIC), inflammatory factors, endotoxin, liver function, and lipid metabolism
The levels of SI and LIC were measured using inductively coupled plasma mass spectrometry (ICP‐MS) by strictly following the manufacturer's instructions. In addition, the levels of interleukin‐1β (IL‐1β), tumor necrosis factor‐α (TNF‐α), and endotoxin were measured by a competitive enzyme immunoassay using an enzyme‐linked immunosorbent assay (ELISA) kit (Cloud‐Clone Corp, Katy, USA), in accordance with the manufacturer's instructions.
Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma‐glutamyltransferase (GGT), total cholesterol (TC), and triglyceride (TG), as well as hepatic TG levels, were measured using commercially available kits on a HITACHI 7020 chemistry analyzer.
2.4. Histopathological analysis
The formalin‐fixed liver tissues were subjected to hematoxylin–eosin (HE) staining, and morphological changes were examined under a light microscope (Olympus BX60, Tokyo, Japan). Ultrastructures of the small intestinal and liver tissues were observed using a JEM‐1200EX transmission electron microscope (TEM; JEOL, Tokyo, Japan).
2.5. Detection of T lymphocyte subsets and NK cells
The number of CD4+, CD8+ T lymphocyte subsets, and NK cells in the peripheral blood of rats was determined flow cytometrically. Monoclonal antibodies labeled with fluorochrome, TCRαβ‐FITC, CD3‐FITC, CD4‐PE, and CD8‐PE were procured from BD Pharmingen. For each test, 100 μl of fresh heparinized rat whole blood was incubated with indicated antibodies for 15 min, then lysed with FACS™ lysing solution, washed with phosphate‐buffered saline, fixed, and eventually detected by BD FACSAria with DB FACSDiva software.
2.6. Small intestine tracer permeability assay
In brief, the ends small intestinal middle segment tissues (2 cm) were ligated, and 2 mg/ml EZ‐link Sulfo‐NHS‐Biotin was slowly injected; this was followed by incubation for 5 min at room temperature. The tissues were fixed in 4% paraformaldehyde. After 24 hr, the sections were washed three times with phosphate‐buffered saline, embedded in paraffin, and cut into 5‐μm thick sections. The sections were incubated with streptavidin DyLightTM 488 conjugated (1:500 dilution) for 30 min in the dark. The distribution of EZ‐link Sulfo‐NHS‐biotin was observed using a fluorescence microscope.
2.7. Expressions of TLR4 and MyD88 signaling pathway‐related proteins by Western blot analysis
Proteins were extracted from liver tissues using the Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, Jiangsu, China), in accordance with the manufacturer's instructions. Total protein content was determined using the BCA Protein Assay Kit (Beyotime). Equal amounts of protein were subjected to sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) on 5% to 10% polyacrylamide gels and then transferred onto PVDF membranes (Millipore, Bedford, MA). The membranes were blocked with 10% nonfat milk in TBST (Tris‐buffered saline, 0.1% Tween 20) and incubated with anti‐NF‐κB p65 and anti‐IκB (Cell Signalling Technology, Danvers, MA.), anti‐histone H3, and anti‐β‐actin (1:1,000 dilution; Proteintech, Rosemont, IL) antibodies at 4°C overnight. Subsequently, membranes were washed three times with TBST for 10 min, followed by incubation with the corresponding secondary antibodies (1:3,000 dilution; Zhongshan Goldenbridge, Beijing, China) for 2 hr at 37°C. Finally, protein bands were visualized using an enhanced chemiluminescence detection kit (Beyotime). Histone H3 and β‐actin served as internal controls for nucleoprotein and cytoplasmic proteins, respectively.
2.8. Statistical analysis
All data values are expressed as mean ±standard deviation using SPSS (version 18; IBM Corp., Chicago, IL, USA). One‐way analysis of variance (ANOVA) was used to compare multiple groups, followed by Duncan's multiple range test. p ˂ 0.05 was deemed statistically significant.
3. RESULTS
3.1. Effects of L. casei on body weight
The recorded body weights before and after the intervention are shown in Figure 1. No significant difference in body weight was observed between the groups before intervention (p > .05). However, the weight of the model group reached (348.17 ± 29.18) g after the 12‐week intervention, which was significantly decreased when compared with the control group (401.17 ± 18.93) g (p < .05). Lactobacillus casei supplementation significantly improved the weight loss observed in rats cotreated with alcohol and iron (p <.05) (Figure 1).
FIGURE 1.
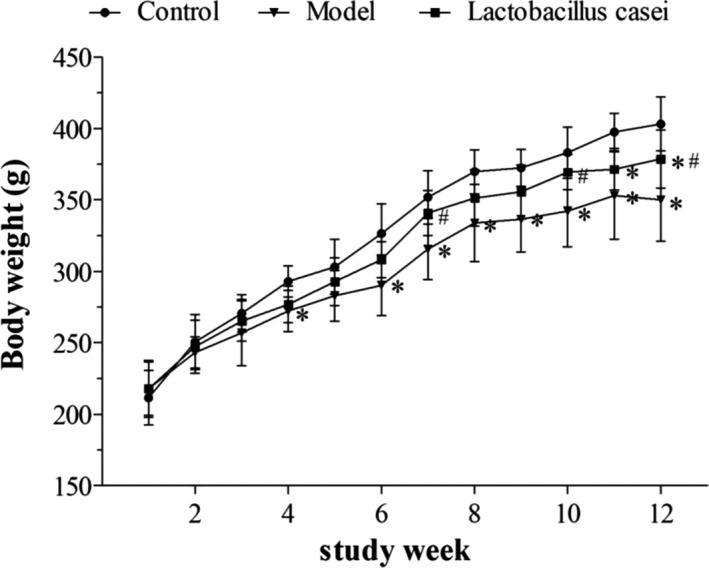
Effects of L. casei on body weight. Control: the control group; Model: the model group; Lactobacillus casei: the L. casei group. * p < .05 versus the control group, # p < .05 versus the model group
3.2. Effects of L. casei on SI and LIC
As shown in Figure 2, SI and LIC were significantly increased (58.17% and 24.46%, respectively) in the model group when compared with those in the control group (p < .05); these levels were significantly decreased following L. casei supplementation by 20.60% and 24.65%, respectively (p < .05).
FIGURE 2.
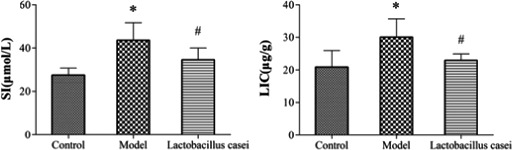
Effects of L. casei on serum iron and liver iron concentration. SI: serum iron; LIC: liver iron concentration; Control: the control group; Model: the model group; Lactobacillus casei: the L. casei group. * p < .05 versus the control group, # p < .05 versus the model group
3.3. Effects of L. casei on pathological changes in the liver
We examined liver sections from each group using HE staining and light microscopy. The control group presented a typical lobular structure, with an ordered hepatic cord, normal hepatocyte structure, and the absence of steatosis or inflammatory infiltration (Figure 3a). Liver damage was observed in the model group, with hepatic cord derangement, liver cell swelling, inflammatory infiltration, microvesicular steatosis, specific Mallory bodies, and fat vacuoles (Figure 3b). Compared with the model group, L. casei supplementation significantly ameliorated these histopathological changes and alleviated steatosis in the liver. In the L. casei group, the hepatic lobular structure was typical, the hepatic cord was orderly, hepatocyte swelling was alleviated, and only a few fat vacuoles were observed (Figure 3c).
FIGURE 3.
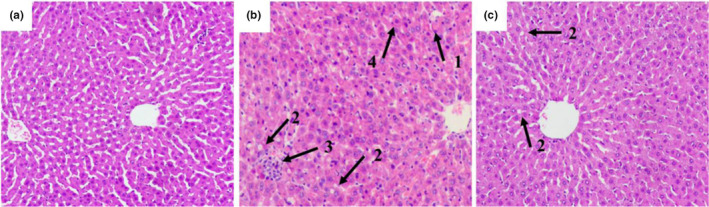
Effects of L. casei on pathological changes in the liver (Hematoxylin–eosin [HE] Staining, ×200). 1: Mallory body; 2: fat vacuoles; 3: inflammatory cell infiltration; 4: vitreous degeneration of hepatocytes. (a) The control group; (b) the model group; (c) the L. casei group
The control group displayed a normal mitochondrial structure, with clearly visible cristae, regularly arranged endoplasmic reticulum, and typically shaped bile canaliculi (Figure 4a). In the model group, large amounts of lipid droplets were observed. The bile canaliculi and microvilli were swollen, and the mitochondrial ridge was slightly blurred. In addition, the endoplasmic reticulum was arranged in a disorderly fashion (Figure 4b). Supplementation with L. casei alleviated the swelling in the microvilli and bile canaliculi. Structurally, mitochondria and endoplasmic reticulum tended to be normal, with only a few lipid droplets observed (Figure 4c). This indicated that L. casei supplementation could alleviate the abnormal liver tissue ultrastructure induced by cotreatment with alcohol and iron in rats.
FIGURE 4.
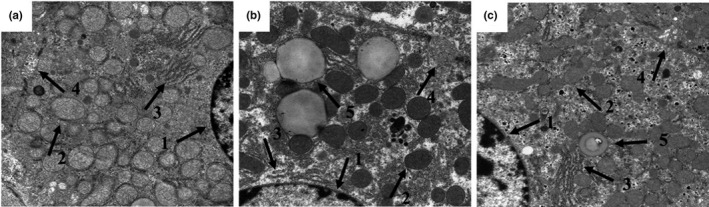
Effects of L. casei on ultrastructure changes in the liver (transmission electron microscopy, ×15,000). 1: nucleus; 2: mitochondria; 3: endoplasmic reticulum; 4: bile canaliculi; 5: lipid droplets. (a) The control group; (b) the model group; (c) the L. casei group
3.4. Effects of L. casei on liver function and lipid metabolism
As shown in Figure 5, the serum levels of ALT, GGT, TG, and hepatic TG were increased by 116.7%, 50.42%, 78.97%, and 86.08%, respectively, in the model group when compared with those in the control group (p < .05); these levels were significantly decreased by 33.33%, 38.59%, 32.38%, and 28.31%, respectively, (p < .05) after Lactobacillus casei supplementation.
FIGURE 5.
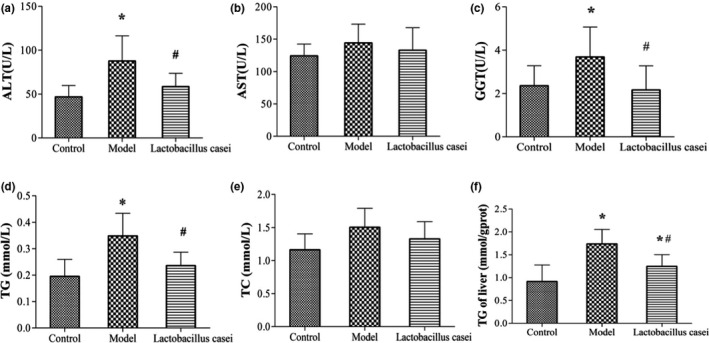
Effects of L. casei on liver function and lipid metabolism. ALT: alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyltransferase; TC, total cholesterol; TG, triglyceride. Control: the control group; Model: the model group; Lactobacillus casei: the L. casei group. * p < .05 versus the control group, # p < .05 versus the model group
3.5. Effects of L. casei on T lymphocyte subsets and NK cells
We observed that the CD4+ lymphocyte percentage and the CD4+/CD8+ ratio in the model group were significantly decreased when compared with the control group, while the CD8+ lymphocyte percentage and NK cell lymphocyte percentage were significantly increased (p < .05). Lactobacillus casei supplementation significantly decreased the percentage of CD8+ lymphocytes and NK cells (p < .05); simultaneously, the CD4+/CD8+ ratio was significantly increased (p < .05). Accordingly, L. casei supplementation could effectively improve the immune response in rats cotreated with alcohol and iron (Figure 6, Table 1).
FIGURE 6.
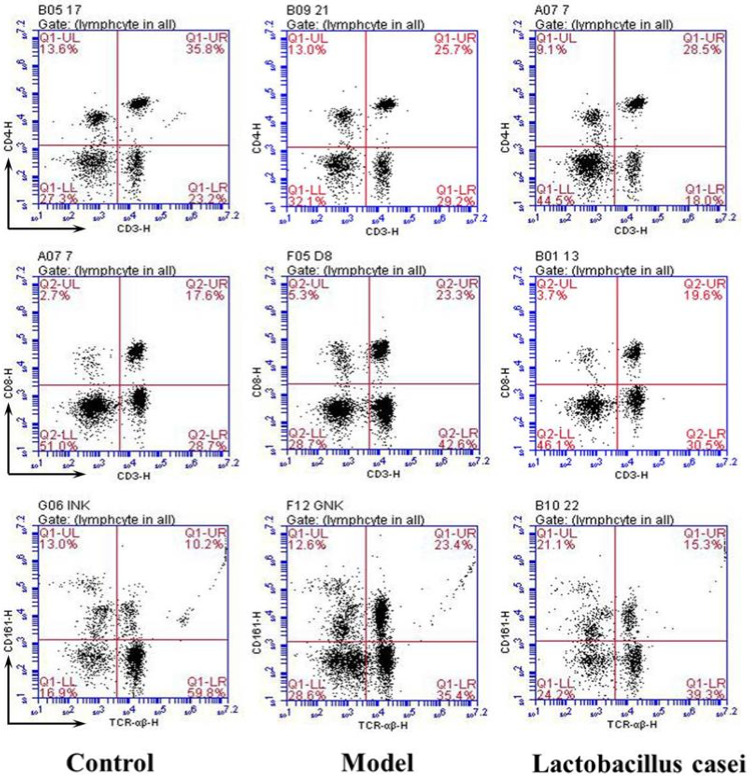
Effects of L. casei on T lymphocyte subsets and NK cells. Control: the control group; Model: the model group; Lactobacillus casei: the L. casei group
TABLE 1.
Proportion of T lymphocyte subsets and NK cells in peripheral blood of rats
| Group | CD3+CD4+ lymphocytes (%) | CD3+CD8+ lymphocytes (%) | CD4+/CD8+ ratio | TCRαβ+ CD161+ NK lymphocytes (%) |
|---|---|---|---|---|
| Control | 35.90 ± 2.15 | 18.03 ± 1.11 | 1.99 ± 0.04 | 11.00 ± 0.98 |
| Model | 25.90 ± 1.71* | 23.90 ± 1.97* | 1.08 ± 0.02* | 23.90 ± 2.29* |
| Lactobacillus casei | 27.73 ± 1.78* | 20.27 ± 2.08 # | 1.41 ± 0.17*# | 15.63 ± 1.72*# |
Control: the control group; Model: the model group; Lactobacillus casei: L. casei group.
p < .05 versus the control group.
p < .05 versus the model group.
3.6. Effects of L. casei on intestinal permeability
3.6.1. Effects of L. casei on the small intestinal tracer permeability
In the control group, the fluorescent tracer was continuous and mostly confined to the intestinal lumen of the small intestine (Figure 7a). In the model group, the fluorescent tracer lacked continuity, demonstrating varying degrees of infiltration (Figure 7b). We observed that intestinal permeability was effectively improved by L. casei supplementation (Figure 7c).
FIGURE 7.
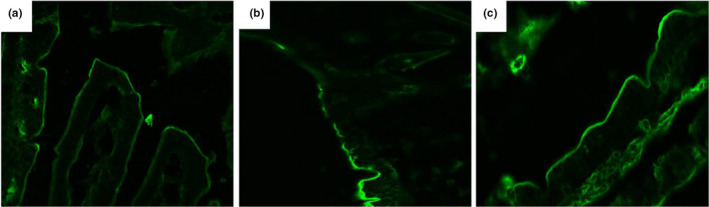
Effects of L. casei on the small intestinal tracer permeability (×400). (a) The control group; (b) the model group; (c) the L. casei group
3.6.2. Effects of L. casei on tight junctions of intestinal epithelia
The control group revealed a normal ultrastructure of the intercellular junction of epithelial cells (Figure 8a). Compared with the control group, the tight junctions and adherens junctions in the model group showed a widened gap, with a reduced electron density (Figure 8b). Lactobacillus casei supplementation improved the intercellular junctional structures in the small intestine tissue, narrowing the gap closer to that observed in the control group (Figure 8c).
FIGURE 8.
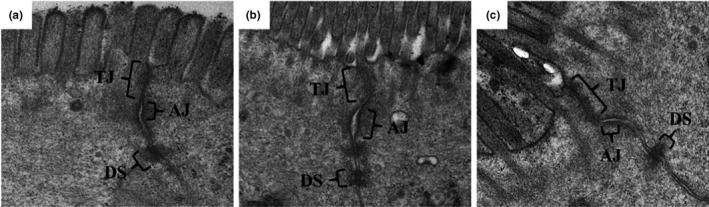
Effects of L. casei on tight junctions of intestinal epithelia (transmission electron microscope ×50,000). (a) The control group; (b) the model group; (c) the L. casei group. TJ, tight junctions; AJ, adherens junctions; DS, desmosomes
3.6.3. Effects of L. casei on endotoxin
As shown in Figure 9, the serum level of endotoxin in the model group was significantly increased by 72.41% when compared with that in the control group (p < .05); however, this level was significantly decreased by 22% (p < .05) following L. casei supplementation.
FIGURE 9.
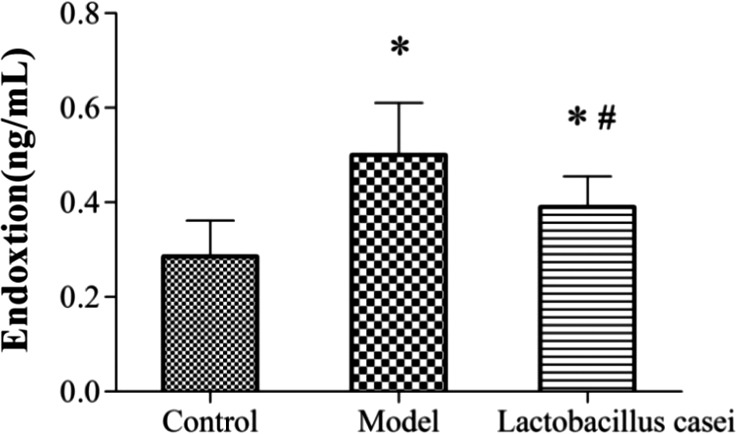
Effects of L. casei on endotoxin. Control: the control group; Model: the model group; Lactobacillus casei: the L. casei group. * p < .05 versus the control group, # p < .05 versus the model group
3.7. Effects of L. casei on TLR4 signaling pathway and inflammation
As shown in Figure 10, the expression of TLR4, MyD88, TNF‐α, and NF‐κB p65 proteins was upregulated following cotreatment with alcohol and iron when compared with the control group (p < .05). Notably, the expression of TLR4, MyD88, TNF‐α, and NF‐κB p65 was downregulated following L. casei supplementation by 39.84%, 34.25%, 38.10%, and 51.16%, respectively (p < .05).
FIGURE 10.
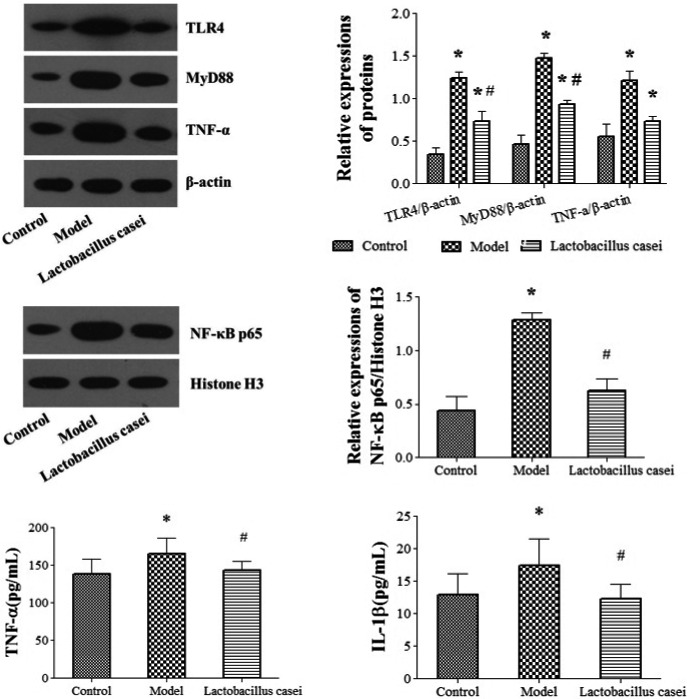
Effects of L. casei on TLR4 signaling pathway and inflammation. TNF‐α, tumor necrosis factor‐α; IL‐1β, interleukin‐1β. Control: the control group; Model: the model group; Lactobacillus casei: the L. casei group. * p < .05 versus the control group, # p < .05 versus the model group
The serum levels of TNF‐α and IL‐1β in the model group were significantly increased by 25.28% and 27.36% when compared with those in the control group (p < .05); these levels were significantly decreased by 14.36% and 30.17%, respectively (p < .05), following L. casei supplementation.
4. DISCUSSION
Epidemiological studies have indicated that excessive intake of alcohol and iron can damage liver functions. Alcohol is a leading cause of liver diseases worldwide (Leggio & Lee, 2017). The number of proliferating hepatocytes was significantly increased in mice fed an excess‐iron diet, with the iron overload reportedly inducing mitochondrial injury (Furutani et al., 2006). Even under low alcohol intake, a certain amount of iron overload can cause significant liver damage (Gao et al., 2017). In the present study, a rat model of liver injury was established by co‐administering alcohol (by gavage) and a high‐iron feed, and the impact of L. casei supplementation was evaluated. Our study demonstrated that L. casei supplementation affords superior protection against liver injury in rats induced by the synergistic interaction between alcohol and iron.
Herein, we observed that L. casei significantly improved the weight of rats treated with alcohol plus iron, indicating that nutritional intake and absorption in rats were affected. This finding is consistent with our previous report (Ma et al., 2018). One possible explanation is damage to liver function. In addition, serological and pathological examinations indicated that the liver damage caused by combined exposure to alcohol and iron, including liver function decline, lipid metabolism disorders, and inflammatory cell infiltration, were significantly alleviated by L. casei supplementation, with a significant reduction in SI and MIC. These results indicate that L. casei could effectively improve liver injury in rats induced by the synergistic interaction between alcohol and iron.
Previous studies have revealed that liver injury is closely associated with immune system disorders. Matos et al. have reported that T lymphocytes are closely related to the occurrence and development of alcoholic liver injury, with all patients with alcoholic liver disease presenting decreased lymphocyte counts (Matos et al., 2013). Annie et al. have observed that NK cells play a crucial role in regulating chronic inflammatory diseases by modulating the balance between liver inflammation and cell repair (Annie et al., 2017). Reportedly, NK cell depletion can improve hepatic injury and survival (Kawabata et al., 2008; Qu et al., 2015). In addition, studies have shown that iron overload is closely related to the immune system. Chen et al. have revealed that iron overload induces T lymphocyte apoptosis and decreases the percentage of CD3+ T cells while increasing the percentage of regulatory T (Treg) cells and the ratio of CD4/CD8 (Chen et al., 2017). Previous reports have also demonstrated that L. casei plays a role in regulating immunity. Vaisberg et al. have documented that L. casei can modulate systemic and airway immune responses postmarathon (Vaisberg & Paixão, 2019). Aktas et al. have reported that L. casei can alter the gut microbiota composition and modulate the host immune response (Aktas et al., 2016). Song et al. have indicated that mice demonstrate humoral immunity and cellular immunity after L. casei supplementation (Song et al., 2018). We have previously reported that L. casei can regulate the proportion of T lymphocyte subsets and NK cells, thus improving the immune function of rats with alcoholic liver injury or breast cancer (Zhengyan et al., 2017; Yiyun et al., 2019). The findings of our present study indicate that L. casei supplementation significantly decreased the percentage of CD8+ lymphocytes and NK cells, while the CD4+/CD8+ ratio was significantly increased. This suggests that the protective effect of L. casei on alcohol plus iron‐induced liver injury may be related to the proportion of CD4+ and CD8+ T lymphocyte subsets and NK cells regulated by L. casei.
Previous studies have revealed that enterogenic endotoxin‐mediated inflammation is involved in the progression of alcoholic liver injury. Xiao et al. have demonstrated that treatment with rice bran phenolic extract represses the alcohol‐induced trigger of the hepatic endotoxin‐TLR4‐NF‐κB pathway, followed by mitigated liver inflammation (Xiao & Zhang, 2020). Perea et al. have suggested that treatment with pentraxin‐3 attenuates lipopolysaccharide (LPS)‐induced liver injury and inflammation (Perea et al., 2017). Moreover, iron overload‐induced inflammation causes further damage to the liver tissue (Preziosi et al., 2017). Our previous studies have revealed that L. casei improves intestinal injury induced by acrylamide in rats and D‐galactose in aging mice (JiaH Fang et al., 2016; Tianjiao et al., 2018). In the present study, TEM and tracer experiments showed that L. casei supplementation could effectively repair the intestinal mucosal barrier damage induced by co‐exposure to alcohol and iron in rats. We observed that the intestinal leakage was significantly alleviated, further confirmed by the serum endotoxin test. Xiao et al. have reported that the phenolic extract of lychee pulp phenolic improves the intestinal barrier function and decreases serum endotoxin levels (Xiao et al., 2017). Subsequently, Western blotting analysis of TLR4, MyD88, NF‐κB p65, and TNF‐α in liver tissue revealed that L. casei significantly downregulated the expression of these proteins. Serological tests further confirmed that L. casei supplementation inhibited the release of TNF‐α and IL‐1β. Li et al. have reported that ethanol exposure could induce microglia‐mediated neuroinflammation through TLR4 activation and elevated TNF‐α and IL‐1β in rats (Li et al., 2019). The TLR4 MyD88 signaling pathway plays a vital role in the inflammatory response (Ikebe et al., 2009; Takeda et al., 2003). Based on the above evidence, we speculate that the inhibition of inflammation by suppressing the enterogenic endotoxin‐mediated TLR4 signaling pathway could be one of the possible protective mechanisms of L. casei on liver injury in rats induced by the synergistic interaction between alcohol and iron.
5. CONCLUSIONS
In summary, our findings demonstrate that L. casei supplementation could effectively improve liver injury induced by the synergistic interaction between alcohol and iron. The underlying mechanism may involve improved immunity and inhibition of enterogenic endotoxin‐mediated inflammation. These findings provide a scientific basis for developing novel treatment strategies for alcoholic liver disease with coexisting iron overload.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This work was funded and supported by Shandong Provincial Natural Science Foundation (No. ZR2020MH215) and Major Scientific & Engineering Projects of Innovation in Shandong Province (2019JZZY010818).
Li, X. , Han, J. , Liu, Y. , & Liang, H. (2021). Lactobacillus casei relieves liver injury by regulating immunity and suppression of the enterogenic endotoxin‐induced inflammatory response in rats cotreated with alcohol and iron. Food Science & Nutrition, 9, 5391–5401. 10.1002/fsn3.2486
Xuelong Li and Jianmin Han contributed equally to this work.
REFERENCES
- Aktas, B. , De Wolfe, T. J. , Safdar, N. , Darien, B. J. , & Steele, J. L. (2016). The impact of Lactobacillus casei on the composition of the cecal microbiota and innate immune system is strain specific. PLoS One, 11(5), e0156374. 10.1371/journal.pone.0156374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annie, T. T. , Surette, F. A. , Ewald, S. E. , & Hahn, Y. S. (2017). Immunoregulatory role of NK cells in tissue inflammation and regeneration. Frontiers in Immunology, 8, 301‐. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buracco, S. , Peracino, B. , Andreini, C. , Bracco, E. , & Bozzaro, S. (2017). Differential effects of iron, zinc, and copper on Dictyostelium discoideum cell growth and resistance to Legionella pneumophila. Frontiers in Cellular and Infection Microbiology, 7, 536. 10.3389/fcimb.2017.00536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Lu, W.‐Y. , Zhao, M.‐F. , Cao, X.‐L. , Jiang, Y.‐Y. , Jin, X. , Xu, P. , Yuan, T.‐T. , Zhang, Y.‐C. , Chai, X. , Meng, J.‐X. , Li, Q. , Xiao, X. , Mu, J. , Li, D.‐G. , & Qi, A.‐P. (2017). Reactive oxygen species mediated T lymphocyte abnormalities in an iron‐overloaded mouse model and iron‐overloaded patients with myelodysplastic syndromes. Annals of Hematology, 96(7), 1085–1095. 10.1007/s00277-017-2985-y [DOI] [PubMed] [Google Scholar]
- Clementi, A. , Virzì, G. M. , Brocca, A. , & Ronco, C. (2017). The role of endotoxin in the setting of cardiorenal syndrome type 5. Cardiorenal Medicine, 7(4), 276–283. 10.1159/000475846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini, E. , & Pietrangelo, A. (2012). Iron and steatohepatitis. Journal of Gastroenterology and Hepatology, 27(Suppl 2), 42–46. 10.1111/j.1440-1746.2011.07014.x [DOI] [PubMed] [Google Scholar]
- Cui, K. , Yan, G. , Zheng, X. , Bai, L. , Wei, H. , Sun, R. , & Tian, Z. (2017). Suppression of natural killer cell activity by regulatory NKT10 cells aggravates alcoholic hepatosteatosis. Frontiers in Immunology, 8, 1414. 10.3389/fimmu.2017.01414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, B. , Wu, J. , Li, X. , Men, X. , & Xu, Z. (2017). Probiotics and probiotic metabolic product improved intestinal function and ameliorated LPS‐induced injury in rats. Current Microbiology, 74(11), 1306–1315. 10.1007/s00284-017-1318-7 [DOI] [PubMed] [Google Scholar]
- Fletcher, L. M. , & Powell, L. W. (2003). Hemochromatosis and alcoholic liver disease. Alcohol, 30(2), 131–136. 10.1016/S0741-8329(03)00128-9 [DOI] [PubMed] [Google Scholar]
- Furutani, T. , Hino, K. , Okuda, M. , Gondo, T. , Nishina, S. , Kitase, A. , Korenaga, M. , Xiao, S. Y. , Weinman, S. A. , Lemon, S. M. , Sakaida, I. , & Okita, K. (2006). Hepatic iron overload induces hepatocellular carcinoma in transgenic mice expressing the hepatitis C virus polyprotein. Gastroenterology, 130(7), 2087–2098. 10.1053/j.gastro.2006.02.060 [DOI] [PubMed] [Google Scholar]
- Gao, W. , Zhao, J. , Gao, Z. , & Li, H. (2017). Synergistic interaction of light alcohol administration in the presence of mild iron overload in a mouse model of liver injury: Involvement of triosephosphate isomerase nitration and inactivation. PLoS One, 12(1), e0170350. 10.1371/journal.pone.0170350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grochowski, C. , Blicharska, E. , Baj, J. , Mierzwińska, A. , Brzozowska, K. , Forma, A. , & Maciejewski, R. (2019). Serum iron, magnesium, copper, and manganese levels in alcoholism: A systematic review. Molecules, 24(7), 1361. 10.3390/molecules24071361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa, P. , Morgan‐Stevenson, V. , Maliken, B. D. , Nelson, J. E. , Washington, S. , Westerman, M. , Yeh, M. M. , & Kowdley, K. V. (2016). Iron overload results in hepatic oxidative stress, immune cell activation, and hepatocellular ballooning injury, leading to nonalcoholic steatohepatitis in genetically obese mice. American Journal of Physiology. Gastrointestinal and Liver Physiology, 310(2), G117–G127. 10.1152/ajpgi.00246.2015 [DOI] [PubMed] [Google Scholar]
- Ikebe, M. , Kitaura, Y. , Nakamura, M. , Tanaka, H. , Yamasaki, A. , Nagai, S. , Wada, J. , Yanai, K. , Koga, K. , Sato, N. , Kubo, M. , Tanaka, M. , Onishi, H. , & Katano, M. (2009). Lipopolysaccharide (LPS) increases the invasive ability of pancreatic cancer cells through the TLR4/MyD88 signaling pathway. Journal of Surgical Oncology, 100(8), 725–731. 10.1002/jso.21392 [DOI] [PubMed] [Google Scholar]
- JiaH Fang, Y. L. , Xue, M. L. , Yong, F. U. , & Hui, L. (2016). Effects of Lactobacillus casei on antioxidant system and intest inalmucosal barrier in D‐galactose induced aging mice. Acta Nutrimenta Sinica, 38(6), 580–585. [Google Scholar]
- Kawabata, T. , Kinoshita, M. , Inatsu, A. , Habu, Y. , Nakashima, H. , Shinomiya, N. , & Seki, S. (2008). Functional alterations of liver innate immunity of mice with aging in response to CpG‐oligodeoxynucleotide. Hepatology, 48(5), 1586–1597. 10.1002/hep.22489 [DOI] [PubMed] [Google Scholar]
- Koga, H. , Tamiya, Y. , Mitsuyama, K. , Ishibashi, M. , Matsumoto, S. , Imaoka, A. , Hara, T. , Nakano, M. , Ooeda, K. , Umezaki, Y. , & Sata, M. (2013). Probiotics promote rapid‐turnover protein production by restoring gut flora in patients with alcoholic liver cirrhosis. Hepatology International, 7(2), 767–774. 10.1007/s12072-012-9408-x [DOI] [PubMed] [Google Scholar]
- La Fata, G. , Weber, P. , & Mohajeri, M. H. (2018). Probiotics and the gut immune system: Indirect regulation. Probiotics Antimicrob Proteins, 10(1), 11–21. 10.1007/s12602-017-9322-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainé, F. , Ruivard, M. , Loustaud‐Ratti, V. , Bonnet, F. , Calès, P. , Bardou‐Jacquet, E. , Sacher‐Huvelin, S. , Causse, X. , Beusnel, C. , Renault, A. , Bellissant, E. , & Deugnier, Y. (2017). Metabolic and hepatic effects of bloodletting in dysmetabolic iron overload syndrome: A randomized controlled study in 274 patients. Hepatology, 65(2), 465–474. 10.1002/hep.28856 [DOI] [PubMed] [Google Scholar]
- Lakhal‐Littleton, S. , Wolna, M. , Chung, Y. J. , Christian, H. C. , Heather, L. C. , Brescia, M. , Ball, V. , Diaz, R. , Santos, A. , Biggs, D. , Clarke, K. , Davies, B. , & Robbins, P. A. (2016). An essential cell‐autonomous role for hepcidin in cardiac iron homeostasis. eLife, 5, e19804. 10.7554/eLife.19804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio, L. , & Lee, M. R. (2017). Treatment of alcohol use disorder in patients with alcoholic liver disease. American Journal of Medicine, 130(2), 124–134. 10.1016/j.amjmed.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. , Duan, K. , Wang, C. , McClain, C. , & Feng, W. (2016). Probiotics and alcoholic liver disease: Treatment and potential mechanisms. Gastroenterology Research and Practice, 2016, 5491465. 10.1155/2016/5491465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , Yu, L. , She, T. , Gan, Y. , Liu, F. , Hu, Z. , Chen, Y. , Li, S. , & Xia, H. (2012). Astragaloside IV attenuates Toll‐like receptor 4 expression via NF‐κB pathway under high glucose condition in mesenchymal stem cells. European Journal of Pharmacology, 696(1–3), 203–209. 10.1016/j.ejphar.2012.09.033 [DOI] [PubMed] [Google Scholar]
- Li, Q. , Liu, D. , Pan, F. (2019). Ethanol exposure induces microglia activation and neuroinflammation through TLR4 activation and SENP6 modulation in the adolescent rat hippocampus. Neural Plasticity, 2019, 1648736. 10.1155/2019/1648736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Meng‐na, X. T. M. , & Zhang, K. (2019). Iron‐overload and its relation with the development of endometriosis. International Journal of Obstetrics and Gynecology, 46(2), 224–228. [Google Scholar]
- Ma, Y. , Li, R. , Liu, Y. , Liu, M. , & Liang, H. (2018). Protective effect of Aplysin supplementation on intestinal permeability and microbiota in rats treated with ethanol and iron. Nutrients, 10(6), 681. 10.3390/nu10060681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnaughtan, J. , Figorilli, F. , García‐López, E. , Lu, H. , Jones, H. , Sawhney, R. , Suzuki, K. , Fairclough, S. , Marsden, J. , Moratalla, A. , Cox, I. J. , Thomas, L. , Davies, N. , Williams, R. , Mookerjee, R. , Wright, G. , & Jalan, R. (2020). A double‐blind, randomized placebo‐controlled trial of probiotic Lactobacillus casei Shirota in stable cirrhotic. Patients, 12(6), 1651. 10.3390/nu12061651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos, L. C. , Batista, P. , Monteiro, N. , Ribeiro, J. , Cipriano, M. A. , Henriques, P. , Girao, F. , & Carvalho, A. (2013). Lymphocyte subsets in alcoholic liver disease. World Journal of Hepatology, 5(2), 46–55. 10.4254/wjh.v5.i2.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni, M. , Longo, M. , & Dongiovanni, P. (2019). Alcohol or gut microbiota: Who is the guilty? International Journal of Molecular Sciences, 20(18), 4568. 10.3390/ijms20184568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osna, N. A. , Donohue, T. M. Jr , & Kharbanda, K. K. (2017). Alcoholic liver disease: Pathogenesis and current management. Alcohol Research, 38(2), 147–161. [PMC free article] [PubMed] [Google Scholar]
- Perea, L. , Coll, M. , Sanjurjo, L. , Blaya, D. , Taghdouini, A. E. , Rodrigo‐Torres, D. , Altamirano, J. , Graupera, I. , Aguilar‐Bravo, B. , Llopis, M. , Vallverdú, J. , Caballeria, J. , van Grunsven, L. A. , Sarrias, M.‐R. , Ginès, P. , & Sancho‐Bru, P. (2017). Pentraxin‐3 modulates lipopolysaccharide‐induced inflammatory response and attenuates liver injury. Hepatology, 66(3), 953–968. 10.1002/hep.29215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrangelo, A. (2016). Iron and the liver. Liver International: Official Journal of the International Association for the Study of the Liver, 36(Suppl 1), 116–123. 10.1111/liv.13020 [DOI] [PubMed] [Google Scholar]
- Preziosi, M. E. , Singh, S. , Valore, E. V. , Jung, G. , Popovic, B. , Poddar, M. , Nagarajan, S. , Ganz, T. , & Monga, S. P. (2017). Mice lacking liver‐specific β‐catenin develop steatohepatitis and fibrosis after iron overload. Journal of Hepatology, 67(2), 360–369. 10.1016/j.jhep.2017.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, M. , Cui, J. , Zhu, J. , Ma, Y. , Yuan, X. U. , Shi, J. , Guo, D. , & Li, C. (2015). Bone marrow‐derived mesenchymal stem cells suppress NK cell recruitment and activation in PolyI:C‐induced liver injury. Biochemical and Biophysical Research Communications, 466(2), 173–179. 10.1016/j.bbrc.2015.08.125 [DOI] [PubMed] [Google Scholar]
- Song, B. , Cui, H. , Ju, L. , Song, L. , Tang, L. , & Li, Y. (2018). Expression of the alpha toxin of Clostridium perfringens in Lactobacillus casei genome and evaluation of its immune effects in mice. Microbial Pathogenesis, 118, 1–8. 10.1016/j.micpath.2018.03.010 [DOI] [PubMed] [Google Scholar]
- Stadlbauer, V. , Mookerjee, R. P. , Hodges, S. , Wright, G. A. , Davies, N. A. , & Jalan, R. (2008). Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. Journal of Hepatology, 48(6), 945–951. 10.1016/j.jhep.2008.02.015 [DOI] [PubMed] [Google Scholar]
- Støy, S. , Dige, A. , Sandahl, T. D. , Laursen, T. L. , Buus, C. , Hokland, M. , & Vilstrup, H. (2015). Cytotoxic T lymphocytes and natural killer cells display impaired cytotoxic functions and reduced activation in patients with alcoholic hepatitis. American Journal of Physiology. Gastrointestinal and Liver Physiology, 308(4), G269–G276. 10.1152/ajpgi.00200.2014 [DOI] [PubMed] [Google Scholar]
- Sumida, Y. , Nakashima, T. , Yoh, T. , Kakisaka, Y. , Nakajima, Y. , Ishikawa, H. , Mitsuyoshi, H. , Okanoue, T. , Nakamura, H. , & Yodoi, J. (2001). Serum thioredoxin elucidates the significance of serum ferritin as a marker of oxidative stress in chronic liver diseases. Liver, 21(5), 295–299. 10.1034/j.1600-0676.2001.210501.x [DOI] [PubMed] [Google Scholar]
- Takeda, K. , Kaisho, T. , & Akira, S. (2003). Toll‐like receptors. Annual Review of Immunology, 21(1), 335–376. 10.1146/annurev.immunol.21.120601.141126 [DOI] [PubMed] [Google Scholar]
- Tianjiao, L. , Yongjie, Z. , Zilong, W. , Ying, L. , & Hui, L. (2018). Protective effect of Lactobacillus casei on acrylamide‐induced intestinal injury in rats. Food Science, 39, 121–126. [Google Scholar]
- Vaisberg, M. , Paixão, V. , Almeida, E. , Santos, J. , Foster, R. , Rossi, M. , Pithon‐Curi, T. , Gorjão, R. , Momesso, C. , Andrade, M. , Araujo, J. , Garcia, M. , Cohen, M. , Perez, E. , Santos‐Dias, A. , Vieira, R. , & Bachi, A. (2019). Daily intake of fermented milk containing Lactobacillus casei Shirota (Lcs) modulates systemic and upper airways immune/inflammatory responses in marathon runners. Nutrients, 11(7), 1678. 10.3390/nu11071678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Wu, Y. , Wang, Y. , Xu, H. , Mei, X. , Yu, D. , Wang, Y. , & Li, W. (2017). Antioxidant properties of probiotic bacteria. Nutrients, 9(5), 521. 10.3390/nu9050521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, J. , Zhang, R. , Wu, Y. , Wu, C. , Jia, X. , Dong, L. , Liu, L. , Chen, Y. , Bai, Y. , & Zhang, M. (2020). Rice bran phenolic extract protects against alcoholic liver injury in mice by alleviating intestinal microbiota dysbiosis, barrier dysfunction, and liver inflammation mediated by the endotoxin–TLR4–NF‐κB pathway. Journal of Agricultural and Food Chemistry, 68(5), 1237–1247. 10.1021/acs.jafc.9b04961 [DOI] [PubMed] [Google Scholar]
- Xiao, J. , Zhang, R. , Zhou, Q. , Liu, L. , Huang, F. , Deng, Y. , & Zhang, M. (2017). Lychee (Litchi chinensis Sonn.) pulp phenolic extract provides protection against alcoholic liver injury in mice by alleviating intestinal microbiota dysbiosis, intestinal barrier dysfunction, and liver inflammation. Journal of Agricultural and Food Chemistry, 65(44), 9675–9684. 10.1021/acs.jafc.7b03791 [DOI] [PubMed] [Google Scholar]
- Yiyun, Z. , Jing, L. , Ying, L. , Zilong, W. , Man, L. , & Hui, L. (2019). Nattokinase alleviates alcoholic liver injury and modulates immune function in rats. Food Science, 40(7), 156–162. [Google Scholar]
- Zannetti, C. , Roblot, G. , Charrier, E. , Ainouze, M. , Tout, I. , Briat, F. , Isorce, N. , Faure‐Dupuy, S. , Michelet, M. , Marotel, M. , Kati, S. , Schulz, T. F. , Rivoire, M. , Traverse‐Glehen, A. , Luangsay, S. , Alatiff, O. , Henry, T. , Walzer, T. , Durantel, D. , & Hasan, U. (2016). Characterization of the inflammasome in human Kupffer cells in response to synthetic agonists and pathogens. The Journal of Immunology, 197(1), 356–367. 10.4049/jimmunol.1502301 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , & Krinsky, G. A. (2004). Iron‐containing nodules of cirrhosis. Nmr in Biomedicine, 17(7), 459–464. 10.1002/nbm.926 [DOI] [PubMed] [Google Scholar]
- Zhengyan, L. , Ying, L. , Meilan, X. , Jia, L. , & Hui, L. (2017). Inhibitory effect and immunological mechanism of Lactobacillus casei on induction of breast cancer by 7,12‐dimethylbenz(a)anthracene in rats. Carcinogenesis, Teratogenesis & Mutagenesis, 29(1), 7–12. [Google Scholar]


