Abstract
Gastric bypass surgery leads to significant and sustained weight loss and a reduction in associated health risks in individuals with severe obesity. While reduced energy intake (EI) is the primary driver of weight loss following surgery, the underlying mechanisms accounting for this energy deficit are not well understood. The evidence base has been constrained by a lack of fit-for-purpose methodology in assessing food intake coupled with follow-up studies that are relatively short-term. This paper describes the underlying rationale and protocol for an observational, fully residential study using covert, objective methodology to evaluate changes in 24-hr food intake in patients (n = 31) at 1-month pre-surgery and 3-, 12- and 24-months post-surgery, compared to weight-stable controls (n = 32). The main study endpoints included change in EI, macronutrient intake, food preferences, and eating behaviours (speed, frequency, and duration of eating). Other physiological changes that may influence EI and weight regulation including changes in body composition, circulating appetite hormones, resting metabolic rate, total energy expenditure and gastrointestinal symptoms were also evaluated. Understanding which mechanisms contribute to a reduction in EI and weight loss post-surgery could potentially help to identify those individuals who are most likely to benefit from gastric bypass surgery as well as those that may need more targeted intervention to optimise their weight loss post-surgery. Furthermore, clarification of these mechanisms may also inform targeted approaches for non-surgical treatments of obesity.
Keywords: Gastric bypass, Study protocol, Objective validation, Food intake
1. Introduction
Gastric bypass surgery is a safe, effective treatment for individuals with severe obesity [1] and leads to improvements in associated conditions including type 2 diabetes mellitus (T2DM) [2] and cardiovascular disease [3]. The most frequently performed procedure is the Roux-en-Y gastric bypass (RYGB), and more recently the One-Anastomosis gastric bypass (OAGB) which are equally effective for both weight loss [4] cardiovascular and quality-of-life outcomes [5,6].
The exact mechanisms underlying the profound weight loss and subsequent weight maintenance remain elusive and involve a complex interaction between physiological, psychological, and behavioural factors. Although a decrease in energy intake (EI) is the main driver of weight loss [[7], [8], [9], [10]] this cannot be fully explained by purely restrictive and malabsorptive mechanisms [11,12].Other proposed mechanisms include changes in hunger and satiety [13,14] caused by changes in circulating gut hormones [15,16], changes in eating patterns such as reduced meal sizes without compensatory increases in meal frequency or duration [17,18], or shifts in dietary energy density (ED) [19] resulting from changes in food selection and/or changes in food preferences [20,21]. Change in macronutrient intakes and the associated impact on EI is a particularly contentious issue. Evidence from animal studies suggest that there is a post-surgical decrease in fat and sugar intakes [[22], [23], [24], [25]]. However, the evidence from human studies regarding changes in relative macronutrient intake in the short-term is equivocal [26], with studies variously reporting a decrease [20,22,27] or no change [18,28] in the intake of high fat/high sugar foods.
The elucidation of the underlying mechanisms of post-operative weight loss has been severely hampered by inconsistencies in bariatric research methodology and further compounded by differences in the analysis, interpretation, and presentation of results [[29], [30], [31]]. In particular, there has been overwhelming reliance on and acceptance of the purported validity of subjectively reported food intake and food preference data in bariatric research without proper acknowledgement that biased food intake data are a fundamental obstacle in understanding the dynamics of food selection and intake [32]. To date, only one research group has objectively observed food intake behaviour in a bariatric surgery population [[33], [34], [35]]. The observed reduction in EI was not macronutrient specific and was accounted for by consumption of smaller portion sizes of the same foods that were consumed pre-surgery. However, these potentially significant and independently validated findings are confined to one eating event which limits their extrapolation. Further verification of these findings is required across multiple eating events.
The integrity of the existing evidence base is further constrained by the frequency and duration of study follow-up. Most of the current evidence regarding shifts in post-operative EI is based on short-term (up-to 12 months post-surgery) and/or single time point studies. However, this is the stage when patients are losing significant weight and it is inconceivable that these studies will capture the dynamics of food intake behaviour and subsequent impact on the longer-term weight trajectory.
Full clarification of the mechanisms underpinning the dynamics of food selection and intake post-surgery can only be resolved by the application of fit-for-purpose methodology and reporting criteria. Consequently, the overall aim of this research was to evaluate the transition in food intake in patients pre- and post-gastric bypass surgery during a dynamic phase of weight change through covert and objective tracking of food intake and eating behaviours assessed under fully residential conditions. In addition, associations with Resting Metabolic Rate (RMR), free-living total energy expenditure (TEE), appetite hormones, and body composition were evaluated. It was hypothesised that the interplay between the various dimensions of dietary intake, eating behaviour, energy expenditure, and gut hormone responses are key in driving both weight loss and longer-term weight regulation.
2. Methods
This observational study was conducted on patients scheduled to undergo gastric bypass surgery and time-matched, weight-stable controls under fully residential conditions at 4 time points (1-month pre-surgery and 3-, 12-, and 24-months post-surgery) to evaluate changes in energy and macronutrient intake, eating behaviours (eating speed (g/min, kJ/min), timing of eating) and food preferences over 2 years following surgery. Concurrent changes in circulating appetite hormones, body composition and RMR were also assessed and, in addition, free-living TEE and patterns of physical activity (PA) were evaluated in a subset of patients.
2.1. Participants
Patients scheduled to undergo gastric bypass surgery (n = 34) and weight-stable controls (n = 32) were recruited. Patients were referred for either RYGB or OAGB at several hospitals/health trusts across the United Kingdom (UK) and Republic of Ireland (ROI). Patients recruited in England were referred for surgery (provided by the National Health Service) by their General Practitioner whereas those recruited in Northern Ireland were self-referred and having their treatment privately. The referral criteria for both groups followed the UK guidelines, namely: a body mass index (BMI) ≥40 kg/m2, or a BMI ≥35 kg/m2 and an obesity-related condition (such as T2DM or high blood pressure) that might improve with weight loss and where previous weight loss methods have been unsuccessful.
Patients from ROI were recruited from a group who were clinically selected to undergo gastric bypass surgery as part of a pilot programme that offered the surgery primarily for the management of T2DM in individuals who were unable to manage the condition with lifestyle changes and medication.
Control participants were weight-stable (>6 months) individuals time-matched to the patient group and with no planned weight changes.
For all participants, the exclusion criteria were: <18years of age, pregnancy/lactation, food allergies/dietary restrictions and/or gastrointestinal conditions or medications that may affect food intake.
2.1.1. Recruitment strategy
Dietitians (UK) or Ulster University researchers (ROI) recruited patients at hospital clinics prior to surgery. During these initial screenings, a detailed explanation of the study protocol was provided and, following an expression of interest, full screening determined eligibility. The baseline (pre-surgery) study time point for the patient group was scheduled before the commencement of the requisite, energy-restricted diet prior to surgery (approximately 1-month pre-surgery).
The control group was recruited by Ulster Univeristy researchers through word-of-mouth, social media, and via emails and posters. The study time points for the control volunteers were matched with the patient group. Recruitment of all study participants took place from October 2016 to March 2018.
All participants provided fully informed written consent (REC 16/WS/0056, IRAS 200567). To divert attention from the main purpose of the study, participants were informed that the primary purpose of the study was to measure changes in RMR following gastric bypass surgery. At the conclusion of the study participants will be debriefed on study outcomes.
2.1.2. Sample size
As this study protocol was both novel and intensive, there was no existing literature to inform a power calculation and so sample size was estimated using a randomised controlled trial (RCT) by le Roux et al. [22]. This RCT, which assigned participants to undergo either RYGB or Vertical Banded Gastrectomy (VBG) and assessed dietary intake by self-report measures, detected significant differences in EI in 16 (VBG (n = 7), RYGB (n = 9)) participants at 6 years post-surgery. The sample size was calculated using the standard deviation (SD) associated with the change in dietary fat (% energy) intake from pre-to post-surgery and a 95% confidence interval as follows:
| n=(confidence level*standard deviation/margin of error)2 |
| n=(1.96*1.9/1) 2 |
| n = 14 |
Applying a 14% attrition rate as reported by Kenler et al. [20] in which changes in self-reported dietary intake were reported at 2 years post-surgery it was estimated that a minimum of 16 patients should be recruited in the present study. However, given the intensity of the proposed protocol, possible participant attrition was accounted for by recruiting 32 patients scheduled to undergo gastric bypass surgery and 32 weight-stable control participants.
2.2. Study protocol
All participants were studied at 4 time points: at baseline (1-month pre-surgery) and 3 post-surgery time-points (3-, 12- and 24-months post-surgery). Fig. 1 provides an overview of participant recruitment and progress.
Fig. 1.
Overview of participant recruitment, progression and retention
NHS National Health Service ROI Republic of Ireland. a Body weight, gastrointestinal symptoms and medication data collected via telephone.
At each of the 4 study time points, participants were required to undertake a 36hr fully residential period, starting late afternoon on day 1 and ending at lunchtime on day 3, in the Human Intervention Studies Unit (HISU) within the Nutrition Innovation Centre for Food and Health (NICHE), Coleraine Campus, Ulster University. This unit consists of 9 en-suite bedrooms, communal living and dining areas for participants and a closed-access kitchen (access for researchers only). All communal areas were monitored by closed circuit television cameras (CCTV) for verifying food intake and eating behaviours (timing/duration of eating, size/frequency of eating occasions, food interest, food selection, eating speed (g/min, kJ/min)). Participants were fully informed of and consented to the presence of CCTV monitoring within the unit.
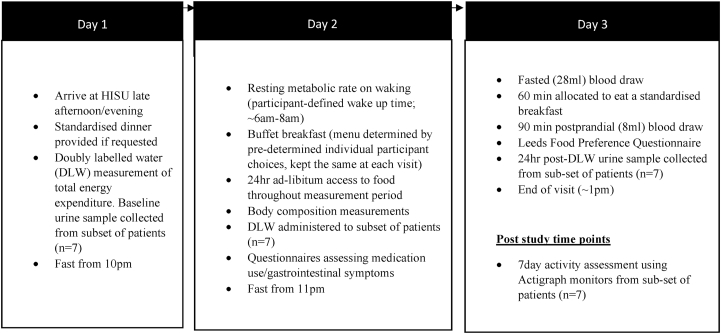
All participants followed the same general protocol (Fig. 2). Participants arrived at the HISU on the late afternoon/early evening of day 1 and a pre-set dinner (Spaghetti Bolognese) was provided if requested, followed by fasting from 10pm. All measurements, including the covert monitoring of 24hr food intake, began on the morning of day 2 (∼7am) until bedtime (11pm). Participants remained in the unit for the duration of each study visit but had access to a range of sedentary activities including reading and crafts, with televisions in communal areas and bedrooms.
Fig. 2.
Residential study protocol with scheduled measurements.
2.3. Food provision
2.3.1. Food choice questionnaire
In order to ensure that the foods/beverages served were compatible with the usual food intake of each participant a food choice questionnaire was administered prior to the baseline visit. Participants rated their liking for 96 food options on a Likert scale (1–9; 1 = dislike extremely, 9 = like extremely) with food items listed in no particular order. These foods were chosen to be representative of 6 macronutrient (expressed as %energy) mix groups (high fat/low fat, high complex carbohydrate/low complex carbohydrate, high simple sugar/low simple sugar, high protein/low protein) (Table 1) (adapted from Geiselman et al. [36]. Stated food choices were then used to establish the foods served to each participant. Individual participant menus consisted of 9 food options from each of the 6 macronutrient mix groups for which participants had scored the highest hedonic response.
Table 1.
Macronutrient paradigm for the foods served to study participants.
| High Simple Sugar (%energy) | High Complex Carbohydrate (%energy) | High Protein (%energy) | |
|---|---|---|---|
| High Fat (%energy) | n = 9 | n = 9 | n = 9 |
| Fat >40% energy | Fat >40% energy | Fat >40% energy | |
| Sugar >30% energy | CCHO >30% energy | Protein >13% energy | |
| e.g. chocolate muffin, caramel chocolate bar, ice cream | e.g. croissant, steak pies, apple pies | e.g. salted peanuts, smoked bacon, mature cheddar cheese | |
| Low Fat (%energy) | n = 9 | n = 9 | n = 9 |
| Fat <20% energy | Fat <20% energy | Fat <20% energy | |
| Sugar >30% energy | CCHO >30% | Protein >13% energy | |
| e.g. banana, grapes, sugar-free meringues | e.g. sesame bagel, white bread, sugar-free jelly | e.g. turkey bacon, crumbed ham, fat-free cottage cheese |
Macronutrient mix groups adapted from Geiselman et al., [36].
CCHO Complex carbohydrate.
2.3.2. Participant menus
The same personalised menu of 54 foods was provided at each study time point. As far as possible the foods presented (n = 54), including pack size and branding were consistent for each study visit. If foods were discontinued or modified over the course of the study, these were replaced with suitable alternatives that had similar macronutrient content and pack sizing. Sugar-sweetened and sugar-free beverages, tea, coffee, milk, and water were freely available to participants throughout the day, with condiments including salt, pepper, salad cream, tomato sauce, mayonnaise, butter, low-fat spread and mixed jams available. Foods and snacks were prepared and stored according to the manufacturer's instructions. An example of a participant menu is provided in Table 2.
Table 2.
Example of a participant menu of foods and beverages (n = 84a) served at each study time point.
| HFHSS foods | HFHCCHO foods | HFHP foods | LFHSS foods | LFHCCHO foods | LFHP foods | Additional foods | |
|---|---|---|---|---|---|---|---|
| Buffet | Granola Fruit and Nut | Croissant | Sausages (fried | Banana | Porridge | Turkey rashers (fried | Orange juice |
| Fridge/Cupboard | Blueberry Muffins | Steak & gravy pie | Quiche Lorraine | Apple | Baked Potato | Ham | Apple juice |
| Chocolate cake bars | Pain au chocolat | Mature cheddar | Grapes | Minestrone soup | Tuna in brine | White bread | |
| KitKat | Crisps | Peanut butter | Pears | Lentil soup | Quorn turkey slices | Brown bread | |
| Snickers | Cheese crackers | Salami | Salad tomatoes | Baked beans | Fat-free cottage cheese | Coca cola | |
| Nutella | Shortbread | Boiled eggs | Marshmallows | White bread | Turkey slices | Diet coca cola | |
| Peanuts and Raisins | Apple pie | Salted peanuts | Fruit pastel sweets | Brown bread | 0%fat protein yoghurt | Fanta | |
| Fanta Zero | |||||||
| Sugar-free cordial | |||||||
| Tea | |||||||
| Coffee | |||||||
| Milk | |||||||
| Sugar | |||||||
| Sweetener | |||||||
| White bread | |||||||
| Brown bread | |||||||
| Honey | |||||||
| Jam | |||||||
| Butter | |||||||
| Margarine | |||||||
| Salad Cream | |||||||
| Ketchup | |||||||
| Salt | |||||||
| Pepper | |||||||
| Menu Options | Pork medallions in a cider jus | Vegetable loaf with tomato sauce | Poached chicken coconut curry | Sweet and sour chicken | Smoked haddock pie | Braised beef with vegetables and red wine sauce | Rice |
| Sticky toffee pudding | Mini eclairs | Almonds | Raspberry sorbet | Low-fat rice pudding | Vanilla soya dessert | Pasta | |
| Boiled Potatoes | |||||||
| Salad | |||||||
| Mixed vegetables | |||||||
| Cream |
HFHSS; High fat, High Simple Sugar HFHCCO; High Fat High Complex Carbohydrate HFHP; High Fat, High Protein LFHSS; Low Fat, High Simple Sugar LFHCCHO; Low Fat, High Complex Carbohydrate LFHP; Low Fat, High Protein.
n = 84 foods comprised of 54 foods based on the macronutrient mix groups [36] identified using the participant food choice questionnaire administered prior to time point 1 and 30 additional food items (e.g. tea, coffee, sauces) that were available to every participant.
A qualified chef prepared all composite evening dishes using modified (to meet macronutrient mix requirements) standardised recipes of popular savoury dishes (e.g. sweet and sour dishes, smoked haddock pie). These modified dishes underwent sensory testing prior to the start of the study to determine acceptability in relation to flavour, texture and colour. The side dishes, which accompanied the evening meal included pasta, potatoes (boiled), rice (white, boiled), salad and mixed vegetables with participants able to select any combination of these. Participants were also able to select sweet/dessert items representative of the 6 macronutrient mix groups from this menu. Desserts were recognisable, branded desserts (e.g., sugar-free jelly, apple pie) that participants were able to select in any combination. Double cream (served whipped or as pouring cream depending on participant specification) was available as an optional accompaniment for all desserts.
Foods were presented in different formats; hot and cold traditional ‘breakfast’ foods (n = 6) were presented as a buffet, while lunch/snack foods (n = 36) were available ad-libitum from each participant's assigned refrigerators and cupboard for storing non-perishable foods. Evening meals (n = 12 dishes) were selected from individually tailored menus featuring hot savoury dishes (n = 6) and desserts (n = 6), with no restriction on the number of choices that could be made.
Participants were advised to consume only the foods provided to them and not to share food items. Researchers were not present while participants were eating. Meal and snack times were not researcher prescribed in advance, rather participants could select to eat at time(s) of their choosing.
2.4. Outcome measures
2.4.1. Dietary intake
The ad-libitum food intake of each participant was directly and covertly measured by weighing all foods before serving together with leftovers over a 24hr period (from participant wake up (∼6am–8am) to 11pm) on day 2 of each study visit. Throughout this period, participants had ad-libitum access to foods and beverages stored in individually assigned fridges and store cupboards for non-perishable foods. Food intake was verified using the CCTV footage which also provided information on associated eating behaviours: frequency (n), duration (min), size (g), energy content (kJ) and distribution of eating occasions, eating rate (kJ/min, g/min), foods selected). All CCTV data were double-entered and checked, with a third member of the research team reconciling any discrepancies in the data entry.
Dietary intake data were calculated using a database developed specifically for this study. Estimated energy and nutrient content of foods were obtained from manufacturer websites [37], and other food composition databases [38].The main outcome measures were total EI (kJ/d) and relative (%energy) macronutrient intake. Other outcome measures were macronutrient mix group contribution to overall EI (%EI), ratio of sugar:sugar free beverages, % contribution of energy-containing fluids to overall EI.
2.4.2. . Dietary energy density
Dietary energy density (ED) (kJ/g), the amount of energy in food relative to weight, is primarily influenced by the fat and water content of foods [39]. Currently, there is no standardised definition of ED and methodological differences in its calculation can lead to inconsistencies in the interpretation of data, particularly if beverages are included in the calculation [40,41].
This study evaluated how the application of different definitions impacted the measurement of dietary ED and study outcomes. Previous work [42] identified 8 methods for deriving ED, from which the following 4 definitions were identified as being most applicable to this study:
-
⁃
Food only; solid/liquid items consumed as food.
-
⁃
Food and milk; solid/liquid items consumed as food plus dairy beverages.
-
⁃
Food and energy-containing beverages; solid/liquid items consumed as food plus beverages containing >21kJ/100 ml.
-
⁃
Food and all beverages: solid/liquid items consumed as food plus all beverages except water.
2.4.3. definition of an eating occasion
By design, this protocol did not impose researcher- or participant-defined ‘meals’ and ‘snacks’, but instead applied the term ‘eating occasion’. An eating occasion has been defined as ‘an event which provides at least 210 kJ with a separation in time from a preceding or following eating event of at least 15 min’ [43]. However, this arbitrary definition has not been subjected to independent evaluation.
The CCTV data (patient (n = 31) and control (n = 30) group data merged) from the baseline study time point were used to determine both pause duration between eating occasions and energy content of eating occasions. A pause was operationally defined as ‘a pause in eating where a start and finish time can be clearly recorded, with termination defined as a break in eating for more than 5 s’. The minimum of 5 s was selected as this was the shortest pause time that could be clearly verified from the CCTV data. In total, 1577 pauses of ≥5 s were recorded for analyses.
All pauses were recorded on a scatter plot to examine any patterns in the data and plotted by frequency when grouped into minutes (>15min). There were a high concentration of pauses within the first 100 s (63.1%), with the majority (83%) occurring within 300 s (5 min). A plateau occurred at approximately 5 min, suggesting that the previously defined 15-min pause may not be the most applicable for these data as eating occasions could merge. It was established that an interval of ≥5 min was more appropriate to apply to this data set.
To evaluate the efficacy of the definition that an eating event should provide >210 kJ, baseline CCTV data were evaluated in combination with directly measured available EI data for 61 participants (patients n = 31, controls n = 30. Nearly a fifth (18.7%) of all eating occasions (n = 538) were <210 kJ and mainly consisted of sugar-free beverages and/or black tea or coffee. Given the small proportion and energy content of eating occasions that fell below the 210 kJ cut-off, it was concluded that only EOs ≥210 kJ should be analysed.
In summary, the proposed definition for an eating occasion employed in this study was ‘an eating event that provided at least 210 kJ with a separation in time from a preceding or subsequent eating event of at least 5 min’
2.4.4. . Eating patterns
The distribution of EI across the measurement period was divided into four eating epochs: wake-up-11am, 11.01am-3pm, 3.01pm–7pm and 7.01pm-11pm. These eating epochs loosely represent the periods that would encompass traditional mealtimes in the UK and ROI (i.e., breakfast, lunch, dinner, supper) and were used to determine the distribution of eating occasions, EI, relative macronutrient intake and ED across the day.
CCTV data were also used to evaluate the frequency, duration, and size of eating occasions as well as eating rate (g/min), food interest (% of total number of visits to food storage areas when food was removed and consumed) and food selection (first food and its associated macronutrient mix composition from buffet table in eating epoch 1). Frequency/duration of eating occasions and eating rate were included in the analysis only if the start and finish time could be clearly observed.
2.4.5. . Food preferences
Prior to leaving the HISU on day 3, 2 h after breakfast and after all other dietary measurements had been completed, participants self-administered the Leeds Food Preference Questionnaire (LFPQ) [44]. This instrument has been validated in different populations [45,46] and has previously been used to measure food preferences in individuals with obesity [47,48].
The LFPQ is a computer-based measure of both explicit and implicit components of food preference and is a validated measure of food ‘liking’ (hedonic pleasure) and food ‘wanting’ (desire to consume). The LFPQ presented participants with a range of pre-validated pictures of common food items that are either high fat (>50% energy content) or low fat (<20% energy content) but similar in familiarity, palatability and sweet/savoury taste [49]. If participants expressed dislike for any of the foods presented these were substituted with a different food with similar macronutrient content and taste (sweet/savoury). Any food picture substitutions were made at baseline only, then kept consistent at subsequent visits.
Explicit measures of food reward were determined by presenting participants with an image of a food item that is either high-/low-fat and sweet/savoury and requiring them to rate on a visual analogue scale either; ‘How much would you like some of this food now?’ or ‘How much do you want some of this food now?’. Average responses to each category (n = 4) were calculated, with a higher score representing higher explicit preference for that food category. Examples of the food pictures included chocolate (high fat/sweet), cheese (high fat/savoury), fruit salad (low fat sweet) and bread roll (low fat/savoury).
The LFPQ measured implicit wanting for food by presenting participants with a forced-choice paradigm which required them to choose between a high-fat vs. low-fat food and a sweet vs. savoury food. Participants were asked to respond quickly to the question ‘Which food do you most want to eat now?’. Responses and reaction times were subsequently used to calculate implicit wanting score, where selection and speed positively contribute to the score. Data were analysed using a frequency-weighted algorithm which has been developed to assess which foods have been avoided or selected, with non-selection negatively contributing to the implicit wanting score [49].
2.4.6. . Body composition
Anthropometric measurements were made on day 2 of each study visit. Body weight was measured using the GE Lunar Dual X-Ray Absorptiometry (DXA) scanner (GE Healthcare). Participants were weighed while wearing light clothing and with no shoes or jewellery, and measurements were made to the nearest 0.1 kg.
Height to the nearest 0.1 cm was measured only at baseline under standardised conditions using a standing stadiometer. Participants were asked to stand on a base plate with their back against the stadiometer background with an upright spine and their feet flat and together.
BMI was calculated as (weight(kg)/height(m)2) and applying the BMI categories defined by using WHO cut offs (World Health Organisation, 2000).
Total weight loss (%TWL) was calculated using the following equation:
| %TWL = ((Weight at baseline(kg) – weight at time point(kg))/baseline)* | 100 |
DXA scans were used to determine lean mass (LM) (kg, %) and total fat mass (FM) (kg, %) at each study time point, with the software additionally able to measure visceral fat (g, %) in participants who had a BMI <40 kg/m2. Bone mineral density was assessed at baseline and 24-months post-surgery.Where body width exceeded the scanning area a half-body scan was used as a valid substitute for a whole-body scan [50,51] to estimate body composition. Scans take between 7 and 15 min, depending on participant size.
Body composition was measured across multiple regions (arms, legs, trunk, android, gynoid). A qualified practitioner performed scans with outputs subsequently assessed by the same radiographer at each time point.
2.4.7. . Energy expenditure
2.4.7.1. . Free living total energy expenditure
Free living TEE was measured under free-living conditions over 14 consecutive days by the doubly labelled water (DLW) method [[52], [53], [54], [55]] in a subgroup of patients (n = 7). TEE is estimated by enriching the participant's body water with two stable isotopes: deuterium (2H2) and oxygen-18 (18O) and determining the difference in elimination rate between both isotopes. The method is based on the principle that 2H2 is eliminated as water, corresponding to water output, and 18O exits the body as both water and expired CO2 with the difference between the elimination rates providing a measure of CO2 production from which TEE is calculated from classical indirect calorimetric equations.
The DLW dose for each participant was based on total body weight (0.07 g/kg of 99% 2H2 and 1.74 g/kg of 10% 18O, as advised by Iso-Analytical Limited, United Kingdom). The dose was administered orally (under supervision) followed by a 100 ml regular water rinse to ensure all the labelled water was consumed. Five time-measured urine samples (5 mls x 5) were collected from each participant: pre-dose, 4hr-, 24hr-,7days-, and 14days post-dose. Samples were subsequently analysed at the Scottish Universities Environmental Research Centre (SUERC) (University of Glasgow, UK).
2.4.7.2. . Resting metabolic rate
On waking on day 2, RMR was measured using indirect calorimetry in a well-ventilated room and with the participant in a supine position (ECAL, Metabolic Health Solutions). Participants were woken (∼7am), asked to empty their bladder and rest a further 30 min before the measurement was made. Distractions such as use of mobile phones were not permitted. The first 2 min of the measurement period were automatically discarded by the ECAL software, with any other anomalous recordings (e.g. coughing, removal of mouthpiece) also discarded as ‘false’ readings. Data were recorded for a minimum of 5min, and was terminated after readings had been stable for 45 s.
2.4.7.3. . Physical activity
Physical activity related energy expenditure (PAEE) was assessed both subjectively and objectively in the same subgroup of patients (n = 7) whose TEE was measured by DLW.
Subjective assessment of PAEE was made using the Recent Physical Activity Questionnaire (RPAQ) [56] which is based on the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Physical Activity Questionnaire (EPAQ2) [57] and has shown good validity for an assessing individual's PAEE [58]. The RPAQ assessed activities according to 4 domains of physical activity (home activity, work activity, commuting, leisure time) over the previous 4 weeks, with closed questions pertaining to the type, frequency, and duration of both physical and sedentary activities. This questionnaire was self-administered, and responses were coded and used to calculate the Metabolic Equivalent of Task (METs). One MET is equivalent to an individual's RMR, and METs are calculated based on activity level (sedentary (≤1.5x RMR); light (1.5–2.99x RMR); moderate (3-5.99x RMR); vigorous (≥6.0x RMR) [[58], [59], [60]], and applying the following standard formula:
| PAEE = (METs x 3.5 x body weight(kg)/200) x duration of activity (minutes) |
Objective assessment of PAEE was completed using an Actigraph (ActiGraph™ GT3X+, ActiGraph, Florida, USA). Actigraphs are small, lightweight activity monitors that measure triaxial activity (acceleration x magnitude x time). The monitor was worn for 7 consecutive days following each study time-point, and participants were included in the analysis if ≥ 10hrs wear for ≥3 days was recorded. Measured activity was categorised into 4 intensities (sedentary, moderate, vigorous, very vigorous) [60]. Reported outcomes included daily step count, PAEE and duration per intensity category (hr/day).
2.4.8. Biochemistry
The following biochemical measurements were made to determine nutrient status (glucose, vitamin D, B6 and B12), gut hormones (glicentin and GLP-1) and bile acids. At all study time points fasted (28 ml) and postprandial (8 ml) blood samples (plasma EDTA and serum) were drawn on the morning of day 3 of each study visit. Postprandial blood samples were drawn 90 min after a standardised breakfast, during which participants were instructed to eat until they were comfortably full. Processing of plasma EDTA tubes was immediate (<5 min) in refrigerated centrifuges (15 min, 2600 rpm, 4 °C), with fasted blood glucose measurements (<15 min from draw) (Hemocue Hb 201+) and full blood counts completed on sample arrival at the laboratory (SYSMEX KX-21 N). Serum tubes were coagulated at room temperature for 30 min prior to processing. All bloods samples were stored at −80 °C until analysis.
2.4.9. . Gastrointestinal symptoms
Self-reported gastrointestinal symptoms were assessed using a modified post-meal digestion questionnaire (PDMQ) which was developed by merging the Sigstad Clinical Diagnostic Index (SCDI) [61] and the more recent Dumping Symptom Rating Scale (DSRS) [19,62]. The SCDI rates the intensity of 16 symptoms suggestive of Dumping Syndrome (DS). Two of the 16 symptoms (eructation/vomiting) are scored negatively to distinguish DS from other syndromes such as afferent loop syndrome or small stomach syndrome, but as vomiting is a common symptom following gastric bypass [63,64], data were analysed with and without these negative scores. The DSRS evaluated severity and frequency of symptoms, and the assessment of symptom frequency was incorporated into the PMDQ (<once a week; once a week; several times per week; once a day; several times per day) along with additional questions on food avoidance. To determine if the gastrointestinal symptoms were experienced during early or late DS, an additional question on the timing of symptoms (within 1hr of eating; 1–3hr after eating; both) was also incorporated into the PMDQ.
2.4.10. . Medication
At each study time point medical information pertaining to any pre-existing medical conditions and details of any medications and dietary supplements taken were recorded. Information was obtained on dose, indication, frequency and start/stop dates of medications and supplements.
2.4.11. . Qualitative data
Patient experiences following gastric bypass surgery were assessed using a qualitative semi-structured interview at the final study time point. Based on the Socioecological Model (SEM) framework [[65], [66], [67], [68]], a series of open-ended interview questions was developed to explore the impact of gastric bypass surgery on post-operative health-related behaviours. The SEM categorises determinants of health behaviour into 3 groups: intra-personal, inter-personal and environmental. Questions were designed to elicit patient experiences following gastric bypass surgery, their current eating behaviours relative to pre-operative behaviour, the impact of surgery on personal relationships, personal and professional support received, their clinical experience and factors which were perceived to contribute to an individual's weight trajectory following surgery. Following audio-recorded interviews data were professionally transcribed verbatim and coded thematically [69] using NVivo 12 (QSR International Pty Ltd, Doncaster, Victoria, Australia). Research team members independently read and coded one randomly selected transcript to ensure the validity of the application of codes to the data.
2.5. Statistical analysis
Statistical analyses were performed using SPSS (Statistical Package for the Social Sciences software, IBM). Available-case analysis was used. Where participants missed a study time point, missing value regression imputation was used where possible to predict results rather than exclude them from analysis. Imputed values were included only when the adjusted R square value was greater than 0.5. Continuous variables were presented as mean±SEM and categorical variables were presented as n (%) unless otherwise stated. Data were tested for normal distribution and log10 transformed where necessary. Two-way mixed Analysis of Variance (ANOVA) was used to determine changes in overall group mean data (patients vs. weight-stable controls) following gastric bypass surgery and changes in mean data based on surgery type (RYGB vs OAGB). Subsequently, Bonferonni post-hoc tests (controlling for multiple comparisons) were conducted to explore all valid multiple pairwise comparisons within the dataset. Linear regression analyses were used to assess relationships between different outcome variables. Further in-depth analysis was conducted to determine individual differences in response to the surgery. Significance was considered at the p=<0.05 level.
3. . Discussion
There are multiple mediators involved in weight loss following bariatric surgery. While a decrease in EI is the main driver of weight loss, the literature presents a complex and inconsistent picture of the consequences of bariatric surgery on macronutrient intake, food selection and food reward/aversion processes, all of which have been implicated to a greater or lesser extent in the diminution in EI.
From a methodological standpoint there are two plausible explanations for this confusion which were addressed in the proposed protocol. Firstly, there has been a paucity of follow-up studies with sufficiently robust methodology which acknowledge that food intake behaviour is likely to transition over time as body weight decreases, then stabilizes and perhaps even rebounds following surgery. By studying patients and controls at 4 time points from 1-month pre-surgery to 24-months post-surgery it is more likely that potentially misleading conclusions about the dynamics of food intake behaviour following bariatric surgery based on single time point studies can be avoided.
Secondly, most of the studies have placed overwhelming and unquestioning reliance on the purported validity of self-reported food intake data. Unfortunately, all techniques for dietary assessment in current use are fraught with inherent and extrinsic methodological problems making accurate measurements of food intake data under free-living conditions one of the most intractable problems facing nutrition research [70,71]. Independent validation of EI data using DLW estimates of TEE [70,72] has conclusively demonstrated that people with obesity consistently underreport their EI by self-reported measures. By implication, under-reporting of EI also implies an under-reporting of dietary factors which may be food and/or macronutrient specific. However, the question of whether there is distortion in macronutrient reporting in patients after bariatric surgery has not been fully answered and is difficult to prove given that macronutrients are highly interrelated when expressed as %energy.
Consequently, an imperative in bariatric research must be to obtain objective and unbiased measures of appetite and eating behaviour. While the semi-naturalistic laboratory conditions of HISU can reduce participant self-awareness compared to standard laboratories [73] they cannot replicate the free-living situation. However, it can legitimately be argued that this is not their intention. Rather, this fully residential study permitted the isolation and systematic testing of specific variables associated with appetite and eating behaviour free from the constraints of external influences which are an inevitable part of a free-living scenario.
Another novel feature of this study protocol has been the opportunity to test the validity in a bariatric population of several definitions of ED [41,42] and validate a definition of an EO which has been widely applied in the literature [43,74,75]. Other strengths of this protocol included the control over the period of fasting prior to the measurement period at each time point and the steps taken to facilitate ‘normal’, free-living behaviour including tailoring of menus to individual food preferences, the absence of researcher-imposed mealtimes, and researcher absence when food was being eaten. Finally, measurement of different outcomes including RMR, body composition and food preferences were standardised, with the LFPQ administered in the satiated state and after the covert measurements had been completed.
There were some limitations of this protocol which need to be acknowledged. While the aim was to encourage and mimic free-living behaviours, several factors including dietary advice delivered as standard post-operative care, social desirability bias and perceived negative and positive connotations associated with certain foods may have influenced food consumption decisions. Furthermore, the availability of a large variety of foods could have inadvertently heightened food interest and impacted eating behaviour. In addition, while recruitment was initially focused on RYGB patients, an increase in OAGB procedures in the U.K. led to the inclusion of patients having one or other of these procedures. However, there is evidence that the OAGB procedure may lead to greater malabsorption and adverse nutritional events compared to the RYGB procedure [76], which could potentially impact EI and food selection. In the patient cohort, differences in pre-operative care may also have an impact on the outcomes. For example, patients from the UK may be enrolled in weight management services for up-to 24-months prior to surgery, while patients in the ROI are clinically selected and not enrolled in pre-operative weight management programmes.
4. Conclusions
A robust methodology to assess the various components of eating and other associated behaviours is imperative for understanding the causal mechanisms underlying changes in food intake after bariatric surgery. While the proposed study design represented a compromise between the demands of external and internal validity it may help to fill a critical void in understanding the dynamics of food selection and intake behaviour following bariatric surgery which, hitherto, has suffered from overreliance on and uncritical acceptance of the purported integrity of self-reported food intake data. However, while objective laboratory observations of food intake are essential for providing crucial experimental data to complement free-living studies it is essential that laboratory and field research in this area should advance together to fully understand the clinical significance of changes in food selection and intake following gastric bypass surgery.
Understanding the underlying mechanisms and post-operative eating behaviours that contribute to individual variability in the reduction of EI and body weight following surgery could help identify those who are most likely to benefit from gastric bypass surgery and provide more individually targeted approaches to optimise sustained weight regulation. It may also have the potential to inform the development of more targeted approaches for the majority of people with obesity who will manage the condition by non-surgical treatments and allow patients to make more informed decisions regarding their treatment approaches.
Authors’ contributions
The study was initiated by ACS, CWleR and MBEL. The study design was conceived and developed by ACS, CWleR, MBEL and RKP. Collection of data and data analysis were carried out by MBEL, RKP, TR, FN, AB and MM. All authors contributed to the writing of and approved the final manuscript.
Funding
This research was supported by a US-Ireland Research and Development Partnership grant (R01DK106112) through the Health and Social Care R&D Division of Northern Ireland (STL/5062/14) and the UK Medical Research Council (MC_PC_16,017), Health Research Board of the Republic of Ireland (USIRL-2006), and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01DK106112).
The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Ethical approval
This study was approved by the West of Scotland Research Ethics Service (WoSRES) (REC 16/WS/0056, IRAS 200567) (Clinical trial number: NCT03113305)
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
Research reported in this publication was supported in part by the US-Ireland Research and Development Partnership program through the Health and Social Care R&D Division of Northern Ireland (STL/5062/14) and the Medical Research Council (MC_PC_16,017), Health Research Board of the Republic of Ireland (USIRL-2006-), and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01DK106112). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Contributor Information
Tamsyn Redpath, Email: Redpath-T@ulster.ac.uk.
Fathimath Naseer, Email: Naseer-F@ulster.ac.uk.
Ruth Karen Price, Email: rk.price@ulster.ac.uk.
Adele Boyd, Email: a.boyd@ulster.ac.uk.
Melanie Martin, Email: melaniemartin1610@yahoo.com.
Carel Wynand le Roux, Email: carel.leroux@ucd.ie.
Alan C. Spector, Email: spector@psy.fsu.edu.
Margaret Barbara Elizabeth Livingstone, Email: mbe.livingstone@ulster.ac.uk.
References
- 1.Colquitt J.L., Pickett K., Loveman E., Frampton G.K. Surgery for weight loss in adults. Cochrane Database Syst. Rev. 2014;8:1645–1858. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings D.E., Arterburn D.E., Westbrook E.O., Kuzma J.N., Stewart S.D., Chan C.P., Bock S.N., Landers J.T., Kratz M., Foster-Schubert K.E., Flum D.R. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia. 2016;59(5):945–953. doi: 10.1007/s00125-016-3903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrostowska M., Szyndler A., Hoffman M. Impact of obesity on cardiovascular health. Best Pract. Res. Clin. Endocrinol. Metabol. 2013;27(2):147–156. doi: 10.1016/j.beem.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Solouki A., Kermansaravi M., Davarpanah Jazi A.H., Kabir A., Farsani T.M., Pazouki A. One-anastomosis gastric bypass as an alternative procedure of choice in morbidly obese patients. J. Res. Med. Sci. 2018;23:84. doi: 10.4103/jrms.JRMS_386_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee W., Yu P., Wang W., Chen T., Wei P., Huang M. Laparoscopic Roux-en-Y versus Mini-Gastric Bypass for the treatment of morbid obesity: a prospective randomized controlled clinical trial. Ann. Surg. 2005;242(1):20–28. doi: 10.1097/01.sla.0000167762.46568.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magouliotis D., Tasiopoulou V.S., Tzovaras G. One anastomosis gastric bypass versus roux-en-Y gastric bypass for morbid obesity: an updated meta-analysis. Obes. Surg. 2019;29:2721–2730. doi: 10.1007/s11695-019-04005-0. [DOI] [PubMed] [Google Scholar]
- 7.Warde-Kamar J., Rogers M., Flancbaum L., Laferrere B. Calorie intake and meal patterns up to 4 years after Roux-en-Y gastric bypass surgery. Obes. Surg. 2004;14(8):1070–1079. doi: 10.1381/0960892041975668. [DOI] [PubMed] [Google Scholar]
- 8.Kruseman M., Leimgruber A., Zumbach F., Golay A. Dietary, weight, and psychological changes among patients with obesity, 8 Years after gastric bypass. J. Am. Diet Assoc. 2010;110(4):527–534. doi: 10.1016/j.jada.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Moize V., Andreu A., Flores L., Torres F., Ibarzabal A., Delgado S., Lacy A., Rodriguez L., Vidal J. Long-term dietary intake and nutritional deficiencies following sleeve gastrectomy or roux-en-y gastric bypass in a Mediterranean population. J. Acad. Nutr. Diet. 2013;113(3):400–410. doi: 10.1016/j.jand.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Janmohammadi P., Sajadi F., Alizadeh S., Daneshzad E. Comparison of energy and food intake between gastric bypass and sleeve gastrectomy: a meta-analysis and systematic review. Obes. Surg. 2019;29(3):1040–1048. doi: 10.1007/s11695-018-03663-w. [DOI] [PubMed] [Google Scholar]
- 11.Abdeen G., le Roux C.W. Mechanism underlying the weight loss and complications of roux-en-Y gastric bypass. Review. Obes. Surg. 2016;26(2):410–421. doi: 10.1007/s11695-015-1945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahawar K.K., Sharples A.J. Contribution of malabsorption to weight loss after roux-en-Y gastric bypass: a systematic review. Obes. Surg. 2017;27(8):2194–2206. doi: 10.1007/s11695-017-2762-y. [DOI] [PubMed] [Google Scholar]
- 13.Morinigo R., Moize V., Musri M., Lacy A.M., Navarro S., Marin J.L., Delgado S., Casamitjana R., Vidal J. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J. Clin. Endocrinol. Metab. 2006;91(5):1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 14.Le Roux C.W., Welbourn R., Werling M., Osborne A., Kokkinos A., Laurenius A., Lonroth H., Fandriks L., Ghatei M.A., Bloom S.R., Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 2007;246(5):780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 15.Falken Y., Hellstrom P.M., Holst J.J., Naslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J. Clin. Endocrinol. Metab. 2011;96(7):2227–2235. doi: 10.1210/jc.2010-2876. [DOI] [PubMed] [Google Scholar]
- 16.Holst J.J., Madsbad S., Bojsen-Moller K.N., Svane M.S., Jorgensen N.B., Dirksen C., Martinussen C. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg. Obes. Relat. Dis. 2018;14(5):708–714. doi: 10.1016/j.soard.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng H., Shin A.C., Lenard N.R., Townsend R.L., Patterson L.M., Sigalet D.L., Berthoud H. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297(5):R1273–R1282. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurenius A., Larsson I., Bueter M., Melanson K.J., Bosaeus I., Forslund H.B., Lonroth H., Fandriks L., Olbers T. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int. J. Obes. 2012;36(3):348–355. doi: 10.1038/ijo.2011.217. [DOI] [PubMed] [Google Scholar]
- 19.Laurenius A., Larsson I., Melanson K.J., Lindroos A.K., Lonroth H., Bosaeus I., Olbers T. Decreased energy density and changes in food selection following Roux-en-Y gastric bypass. Eur. J. Clin. Nutr. 2013;67(2):168–173. doi: 10.1038/ejcn.2012.208. [DOI] [PubMed] [Google Scholar]
- 20.Kenler H.A., Brolin R.E., Cody R.P. Changes in eating behavior after horizontal gastroplasty and Roux-en-Y gastric bypass. Am. J. Clin. Nutr. 1990;52(1):87–92. doi: 10.1093/ajcn/52.1.87. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen M.S., Schmidt J.B., le Roux C.W., Sjödin A. Effects of roux-en-Y gastric bypass and sleeve gastrectomy on food preferences and potential mechanisms involved. Curr Obes Rep. 2019;8:292–300. doi: 10.1007/s13679-019-00354-0. [DOI] [PubMed] [Google Scholar]
- 22.le Roux C.W., Bueter M., Theis N., Werling M., Ashrafian H., Lowenstein C., Athanasiou T., Bloom S.R., Spector A.C., Olbers T., Lutz T.A. Gastric bypass reduces fat intake and preference. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301(4):R1057–R1066. doi: 10.1152/ajpregu.00139.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathes C.M., Bohnenkamp R.A., le Roux C.W., Spector A.C. Reduced sweet and fatty fluid intake after Roux-en-Y gastric bypass in rats is dependent on experience without change in stimulus motivational potency. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309(8):R864–R874. doi: 10.1152/ajpregu.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathes C.M., Letourneau C., Blonde G.D., Roux C.W.L., Spector A.C. Roux-en-y gastric bypass in rats progressively decreases the proportion of fat calories selected from a palatable cafeteria diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;310(10):R952–R959. doi: 10.1152/ajpregu.00444.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyde K.M., Blonde G.D., Bueter M., le Roux C.W., Spector A.C. Gastric bypass in female rates lowers concentrated sugar solution intake and preference without affecting brief-access licking after long-term sugar exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020;318(5):R870–R885. doi: 10.1152/ajpregu.00240.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathes C.M., Spector A.C. Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: a direct-measures approach. Physiol. Behav. 2012;107(4):476–483. doi: 10.1016/j.physbeh.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Olbers T., Björkman S., Lindroos A.K., Maleckas A., Lönn L., Sjöstrom L., Lönorth H. Body composition, dietary intake and energy expenditure after laparoscopic roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann. Surg. 2006;244(5):715–722. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brolin R.E., Robertson L.B., Kenler H.A., Cody R.P. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann. Surg. 1994;220(6):782–790. doi: 10.1097/00000658-199412000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coulman K.D., Abdelrahman T., Owen-Smith A., Andrews R.C., Welbourn R., Blazeby J.M. Patient-reported outcomes in bariatric surgery: a systematic review of standards of reporting. Obes. Rev. 2013;14(9):707–720. doi: 10.1111/obr.12041. [DOI] [PubMed] [Google Scholar]
- 30.Hopkins J.C., Howes N., Chalmers K., Savovic J., Whale K., Coulman K.D., Welbourn R., Whistance R.N., Andrews R.C., Byrne J.P., Mahon D., Blazeby J.M. By-Band Trial Management Group. Outcome reporting in bariatric surgery: an in-depth analysis to inform the development of a core outcome set, the BARIACT Study. Obes. Rev. 2015;16(1):88–106. doi: 10.1111/obr.12240. [DOI] [PubMed] [Google Scholar]
- 31.Mocanu V., Nasralla A., Dang J., Jacobson M., Switzer N., Madsen K., Birch D.W., Karmali S. Ongoing inconsistencies in weight loss reporting following bariatric surgery: a systematic review. Obes. Surg. 2019;29(4):1375–1387. doi: 10.1007/s11695-018-03702-6. [DOI] [PubMed] [Google Scholar]
- 32.Redpath T.L., Livingstone M.B.E., Dunne A.A., Boyd A., le Roux C.W., Spector A.C., Price R.K. Methodological issues in assessing change in dietary intake and appetite following gastric bypass surgery: a systematic review. Obes. Rev. 2021 doi: 10.1111/obr.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen M.S., Christensen B.J., Ritz C., Rasmussen S., Hansen T.T., Bredie W.L.P., Le Roux C.W., Sjodin A., Schmidt J.B. Roux-En-Y gastric bypass and sleeve gastrectomy does not affect food preferences when assessed by an ad libitum buffet meal. Obes. Surg. 2017;27(10):1–7. doi: 10.1007/s11695-017-2678-6. [DOI] [PubMed] [Google Scholar]
- 34.Christensen B.J., Schmidt J.B., Nielsen M.S., Taekker L., Holm L., Lunn S., Bredie W.L.P., Ritz C., Holst J.J., Hansen T., Hilbert A., le Roux C.W., Hulme O.J., Siebner H., Morville T., Naver L., Floyd A.K., Sjodin A. Patient Profiling for success after weight loss surgery (GO Bypass study): an interdisciplinary study protocol. Contemp Clin Trials Commun. 2017;10:121–130. doi: 10.1016/j.conctc.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen M.S., Rasmussen S., Just Christensen B., Ritz C., le Roux C.W., Berg Schmidt J., Sjödin A. Bariatric surgery does not affect food preferences, but individual changes in food preferences may predict weight loss. Obesity. 2018;26(12):1879–1887. doi: 10.1002/oby.22272. [DOI] [PubMed] [Google Scholar]
- 36.Geiselman P.J., Anderson A.M., Dowdy M.L., West D.B., Redmann S.M., Smith S.R. Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiol. Behav. 1998;63(5):919–928. doi: 10.1016/s0031-9384(97)00542-8. [DOI] [PubMed] [Google Scholar]
- 37.Nutritics . 2019. Nutritics Research Edition v5.095, Dublin. [Google Scholar]
- 38.Finglas P., Roe M.A., Pinchen H.M., Berry R., Church S.M., Dodhia S.K., Farran-Wilson M., Swan G. McCance and Widdowson's the Composition of Foods. Seventh Summary Edition. Royal Society of Chemistry; Cambridge: 2015. [Google Scholar]
- 39.Rolls B.J. The relationship between dietary energy density and energy intake. Physiol. Behav. 2009;97(5):609–615. doi: 10.1016/j.physbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox D., Mela D. Determination of energy density of freely selected diets: methodological issues and implications. Int. J. Obes. 2000;24:49–54. doi: 10.1038/sj.ijo.0801084. [DOI] [PubMed] [Google Scholar]
- 41.McCaffery T.A., Rennie K.L., Kerr M.A., Wallace J.M., Hannon-Fletcher M.P., Coward M.A., Jebb S.A., Livingstone M.B.E. Energy density of the diet and change in body fatness from childhood to adolescence; is there a relation? Am. J. Clin. Nutr. 2008;87(5):1230–1237. doi: 10.1093/ajcn/87.5.1230. [DOI] [PubMed] [Google Scholar]
- 42.Ledikwe J.H., Blanck H.M., Khan L., Serdula M.K., Seymour J.D., Tohill B.C., Rolls B.J. Dietary energy density determined by eight calculation methods in a nationally representative United States population. J. Nutr. 2005;135(2):273–278. doi: 10.1093/jn/135.2.273. [DOI] [PubMed] [Google Scholar]
- 43.Gibney M., Wolever T. Periodicity of eating and human health: present perspective and future directions. Br. J. Nutr. 1997;77(S1):S3–S5. doi: 10.1079/bjn19970099. [DOI] [PubMed] [Google Scholar]
- 44.Finlayson G., King N., Blundell J. The role of implicit wanting in relation to explicit liking and wanting for food: implications for appetite control. Appetite. 2008;50(1):120–127. doi: 10.1016/j.appet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Alkahtni S.A., Dalton M., Abuzaid O., Obeid O., Finlayson G. Validation of the Leeds food preference questionnaire in arabs. Asia Pac. J. Clin. Nutr. 2016;25(2):257–264. doi: 10.6133/apjcn.2016.25.2.07. [DOI] [PubMed] [Google Scholar]
- 46.Oustric P., Thivel D., Dalton M., Beaulieu K., Gibbons C., Hopkins M., Blundell J., Finlayson G. Measuring food preference and reward: application and cross-cultural adaptation of the Leeds Food Preference Questionnaire in human experimental research. Food Qual. Prefer. 2020;80:103841. [Google Scholar]
- 47.Dalton M., Finlayson G. Psychobiological examination of liking and wanting for fat and sweet taste in train binge eating females. Physiol. Behav. 2014;136:128–134. doi: 10.1016/j.physbeh.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 48.Blundell J., Finlayson G., Axelsen M., Flint A., Gibbons C., Kvist T., Hjerpsted J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242–1251. doi: 10.1111/dom.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalton M., Finlayson G. Psychobiological examination of liking and wanting for fat and sweet taste in train binge eating females. Physiol. Behav. 2014;136:128–134. doi: 10.1016/j.physbeh.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 50.Tataranni P., Ravussin E. Use of dual-energy x-ray absorptiometry in obese individuals. Am. J. Clin. Nutr. 1995;62(4):730–734. doi: 10.1093/ajcn/62.4.730. [DOI] [PubMed] [Google Scholar]
- 51.Rothney M., Brychta R.J., Schaefer E.V., Chen K.Y., Skanilis M.C. Body composition measured by dual-energy x-ray absorptiometry half-body scans in obese adults. Obesity. 2009;17(6):1281–1286. doi: 10.1038/oby.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lifson N., McClintock R. Theory of use of the turnover rates of body water for measuring energy and material balance. J. Theor. Biol. 1966;12(1):46–74. doi: 10.1016/0022-5193(66)90185-8. [DOI] [PubMed] [Google Scholar]
- 53.Schoeller D.A., Van Santen E. Measurement of energy expenditure in humans by doubly labeled water. J. Appl. Physiol. 1982;53:955–959. doi: 10.1152/jappl.1982.53.4.955. [DOI] [PubMed] [Google Scholar]
- 54.Bhutanni S., Racine N., Shriver T., Schoeller D.A. Special considerations for measuring energy expenditure with doubly labeled water under atypical conditions. J. Obes. Weight Loss Ther. 2015;5(S5) doi: 10.4172/2165-7904.S5-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westerterp K.R. Doubly labelled water assessment of energy expenditure: principle, practice, and promise. Eur. J. Appl. Physiol. 2017;177(7):1277–1285. doi: 10.1007/s00421-017-3641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goulbic R., May A.M., Borch K.B., Overvad K., Charles M., Diaz M.J.T., Amiano P., Palli D., Valanou E., Vigl M., Franks P.W., Wareham N., Ekelund U., Brage S. Validity of electronically administered recent physical activity questionnaire (RPAQ) in ten European countries. PloS One. 2014;9(3) doi: 10.1371/journal.pone.0092829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wareham N.J., Jakes R.W., Renni K.L., Mitchel J., Henning S., Day N.E. Validity and repeatability of the EPIC-norfolk physical activity questionnaire. Int. J. Epidemiol. 2002;31(1):168–174. doi: 10.1093/ije/31.1.168. [DOI] [PubMed] [Google Scholar]
- 58.Besson H., Brage S., Jakes R.W., Ekelund U., Wareham N.J. Estimating physical activity energy expenditure, sedentary time, and physical activity intensity by self-report in adults. Am. J. Clin. Nutr. 2010;91(1):106–114. doi: 10.3945/ajcn.2009.28432. [DOI] [PubMed] [Google Scholar]
- 59.Ainsworth B.E., Haskell W.L., Leon A.S., Jacobs D.R., Montoye H.J., Sallis J.F., Paffenbarger R.S. Compendium of physical activities: classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 60.Ainsworth B.E., Bassett, Strath S.J., Swartz A.M., O'Brien W.L., Thompson R.W., Jones D.A., Macera C.A., Kimsey C.D. Comparison of three methods for measuring the time spent in physical activity. Med. Sci. Sports Exerc. 2000;32(S9):S457–S464. doi: 10.1097/00005768-200009001-00004. [DOI] [PubMed] [Google Scholar]
- 61.Sigstad H. A clinical diagnostic index in the diagnosis of the dumping syndrome. J. Intern. Med. 1970;188(1–6):479–486. [PubMed] [Google Scholar]
- 62.van Beek A.P., Emous M., Laville M., Tack J. Dumping syndrome after esophageal, gastric or bariatric surgery: pathophysiology, diagnosis, and management. Obes. Rev. 2017;18(1):68–85. doi: 10.1111/obr.12467. [DOI] [PubMed] [Google Scholar]
- 63.Powers P.S., Perez A., Boyd F., Rosemurgy A. Eating Pathology before and after bariatric surgery: a prospective study. Int. J. Eat. Disord. 1999;25(3):293–300. doi: 10.1002/(sici)1098-108x(199904)25:3<293::aid-eat7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 64.Mitchell J.E., Lancaster K.L., Burgard M.A., Howell L.M., Krahn D.D., Crosby R.D., Wonderlich S.A., Gosnell B.A. Obes. Surg. 2001;11(4):464–468. doi: 10.1381/096089201321209341. [DOI] [PubMed] [Google Scholar]
- 65.Bronfenbrenner U. Toward an experimental ecology of human development. Am. Psychol. 1977;32(7):513–531. [Google Scholar]
- 66.Bronfenbrenner U. Ecology of the family as a context for human development: research perspectives. Dev. Psychol. 1986;22(6):723–742. [Google Scholar]
- 67.Stokols D. Translating social ecological theory into guidelines for community health promotion. Am. J. Health Promot. 1996;10(4):282–298. doi: 10.4278/0890-1171-10.4.282. [DOI] [PubMed] [Google Scholar]
- 68.Kilanowski J.F. Breadth of the socio-ecological model. J. Agromed. 2017;22:295–297. doi: 10.1080/1059924X.2017.1358971. [DOI] [PubMed] [Google Scholar]
- 69.Braun V., Clarke C. What can “thematic analysis” offer health and wellbeing researchers? Int. J. Qual. Stud. Health Well-Being. 2014;9:26152. doi: 10.3402/qhw.v9.26152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Livingstone M.B., Black A.E. Markers of the validity of reported energy intake. J. Nutr. 2003;133(S3):895S–920S. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
- 71.Subar A.F., Freedman L.S., Tooze J.A., Kirkpatrick S.I., Boushey C., Neuhouser M.L., Thompson F.E., Potischman N., Guenther P.M., Tarasuk V., Reedy J., Krebs-Smith S.M. Addressing current criticism regarding the value of self-report dietary data. J. Nutr. 2015;145(12):2639–2645. doi: 10.3945/jn.115.219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pietilainen K.H., Korkeila M., Bogl L.H., Westerterp K.R., Jarvinen Y., Kaprio J., Rissanen A. Inaccuracies in food and physical activity diaries of obese subjects: complementary evidence from double labelled water and co-twin assessments. Int. J. Obes. 2010;34:437–445. doi: 10.1038/ijo.2009.251. [DOI] [PubMed] [Google Scholar]
- 73.Gough T., Haynes A., Clarke K., Hansell A., Kaimkhani M., Price B., Roberts A., Hardman C.A., Robinson E. Out of the lab and into the wild: the influence of portion size on food intake in laboratory vs. real-world settings. Appetite. 2021;162:105160. doi: 10.1016/j.appet.2021.105160. [DOI] [PubMed] [Google Scholar]
- 74.Drummond S., Crombie N., Cursiter M., Kirk T. Evidence that eating frequency is inversely related to body weight status in male, but not female, non-obese adults reporting valid dietary intakes. Int. J. Obes. 1998;22:102–112. doi: 10.1038/sj.ijo.0800552. [DOI] [PubMed] [Google Scholar]
- 75.Murakami K., Livingstone M. Eating frequency in relation to body mass index and waist circumference in British adults. Int. J. Obes. 2014;38:1200–1206. doi: 10.1038/ijo.2014.1. [DOI] [PubMed] [Google Scholar]
- 76.Robert M., Espalieu R., Pelascini E., Caiazzo R., Sterkers A., Khamphommala L., Poghosyan T., Chevallier J., Malherbe V., Chouillard E., Reche F., Torcivia A., Maucirt-Boulch D., Bin-Dorel S., Langlois-Jacques C., Delauney D.M. Pattou F., Disse E. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): a multicentre, randomised, open-label, non-inferiority trial. Lancet. 2019;393(10178):1299–1309. doi: 10.1016/S0140-6736(19)30475-1. [DOI] [PubMed] [Google Scholar]