Abstract
Objectives
COVID-19 is a viral transmissible disease and there is limited evidence on vertical transmission and prevalence of SARS-CoV-2 during pregnancy, birth, and the postnatal period. This descriptive cross-sectional study aimed to evaluate the possible perinatal transmission of SARS-CoV-2 in mothers and neonates in a Mexican population.
Methods
A total of 133 nasopharyngeal swab samples from mothers, 131 swab samples from neonates, and 140 colostrum samples were obtained, and the presence of SARS-CoV-2 was determined by qPCR.
Results
One in eight asymptomatic 38–39 weeks’ pregnant women were positive for the presence of SARS-CoV-2 in nasopharyngeal swabs taken just before delivery; and one in 12 nasopharyngeal swabs collected from neonates immediately after delivery without breast feeding were also positive. It was also determined that one in 47 colostrum/milk samples were positive for the test. In addition, there was no association between positive results and any collected metadata of mothers or newborns.
Conclusions
Asymptomatic women carried the SARS-CoV-2 virus during delivery, with perinatal transmission of SARS-CoV-2 to newborns. Since neonates were sampled immediately after birth, the detection of positive cases might be due to infection by the virus in utero.
Keywords: Perinatal transmission, COVID-19, Human milk, SARS-CoV-2, RT-qPCR, RT-ddPCR
1. Introduction
COVID-19, a novel coronavirus, was reported in China in December 2019 and spread around the world in a short period of time, reaching the Americas by the beginning of 2020. The severe outbreak was declared a “public-health emergency of international concern” by the World Health Organization (WHO) by 30 January 2020 (Wu et al., 2020). This situation led to healthcare centers facing a critical infection inflicted by this novel coronavirus, which was identified as the causative pathogen and named SARS-CoV-2 (Karimi-Zarchi et al., 2020).
The SARS-CoV-2 virus is transmitted by small liquid droplets produced by an infected person during coughing, sneezing, speaking, or breathing (CDC, 2021; Patel et al., 2020). A person affected by COVID-19 commonly has fever, cough, and dyspnea, and occasionally watery diarrhea, as symptoms. It is estimated that 20–30% of the infected patients require mechanical ventilation, with a 10% probability of dying (Paules et al., 2020). According to Mexican health authorities, the Mexican population has a higher risk of fatal outcomes in older people or persons with medical comorbidities like hypertension, diabetes, and being overweight (Secretaria de Salud, 2020).
The first reported COVID-19 case in Mexico was in January 2020 (Suárez et al., 2020) and, according to the Mexican Government, there were 1,011,153 confirmed cases and 99,026 deaths by November 2020. Similar situations have caused the implementation of extraordinary public health measures in several countries to reduce further viral spreading (Wang et al., 2020). Nevertheless, despite the health measures taken by authorities, COVID-19 cases have continued an upward trend (WHO, 2021). There have now been more than 2.5 million confirmed cases and more than 2.4 thousand deaths in Mexico (CONACYT, 2021). According to official reports, there is a higher risk of fatal outcome in older people or persons with medical comorbidities like hypertension, diabetes, and being overweight in the Mexican population (Secretaria de Salud, 2020).
At a population level, one vulnerable group of special interest is pregnant women. Some studies have shown that pregnant women are at greater risk of COVID-19 morbidity and mortality, which increase adverse pregnancy outcomes; however, although the results suggest that vertical transmission is infrequent, there is insufficient information about the factors that influence it (Qiao, 2020, Plüddemann et al., 2021).
Despite reasonable doubts about the safety of breastfeeding in COVID-19 pandemic times, the beneficial impact of human milk (HM) has been widely investigated and demonstrated, especially on cases of extremely low birth weight or newborns in neonatal intensive care units (Mercado et al., 2019). In addition, HM represents the ideal nutrition source, and exclusive breastfeeding is recommended by the WHO at least during the first 6 months after birth (WHO, 2003). HM has been related to positive effects in the successive phases of life, as it provides protection against the development of obesity, diabetes, and cardiovascular disease, improving neurological development in newborns (Bardanzellu et al., 2019). HM also promotes immune system development, by provision of passive immunity via immunoglobulins and other bioactive factors produced in response to viral infections. In women previously affected by COVID-19, it has been reported that HM contains IgA immunoglobulins, reactive to the receptor binding domain (RBD) of SARS-CoV-2 spike protein, conferring protection to the neonate against the virus (Pace et al., 2021). A recent review of 13 studies testing HM for the presence of SARS-CoV-2 (Lackey et al., 2020) reported the presence of virus in one milk sample, and SARS-CoV-2-specific IgG in the milk of another study. In China, HM was analyzed at delivery time in mothers with a SARS-CoV-2-positive diagnosis, finding that all milk samples were negative for the virus (Yang and Liu, 2020). In fact, in 2020, the WHO determined that no evidence of SARS-CoV-2 transmission was detected in mother's milk with suspected or confirmed COVID-19; therefore, it is highly recommended to continue breastfeeding because of the abundant health benefits HM provides to newborn and which are maintained throughout life (Centeno-Tablante et al., 2021).
Additional reports have described newborns with positive nasopharyngeal swabs after birth, which could be the consequence of horizontal transmission by contact with the infected mother or with positive asymptomatic healthcare personnel at the time of delivery (Auriti et al., 2020). Another study found no evidence of virus transmission through milk, and it was shown that transmission might have occurred via respiratory droplets from the mother during breastfeeding (Salvatori et al., 2020).
However, there is limited evidence on vertical transmission, prevalence, and clinical features of COVID-19 during pregnancy, birth, and the postnatal period in the Mexican population; hence, there is a need for evidence of SARS-CoV-2 vertical transmission from mothers to infants. However, continuing with breastfeeding is highly recommended, as there is evidence that the HM of infected mothers carries antibodies against SARS-CoV-2 (Mardani and Pourkaveh, 2020).
There is no conclusive evidence of intrauterine vertical transmission of COVID-19 from infected pregnant mothers to their fetuses (Deniz et al., 2020). Infected mothers are at high risk of severe respiratory complications (Karimi et al., 2020). Nine neonates born to infected mothers were found to be negative for SARS-CoV2 infection in China, suggesting that there is not intrauterine infection (Cao et al., 2020). In another case report, detailed studies conducted to test viral vertical transmission found no vertical transmission caused by intrauterine infection in asymptomatic COVID‐19 pregnant women (Lu et al., 2020). However, in the first reported case of a SARS-CoV-2-positive nasopharyngeal swab from a neonate delivered vaginally, both stool and rectal swabs from the mother were positive for the virus, suggesting neonate infection due to fecal contamination during vaginal delivery (Carosso et al., 2020). In the same sense, there is a report of SARS-CoV-2 RNA detection in one umbilical cord blood and in two at-term placentas (Fenizia et al., 2020).
SARS CoV-2 infection has been associated with negative perinatal outcomes like fetal distress, premature labor, respiratory distress, thrombocytopenia, abnormal liver function tests, and even death (Zhu et al., 2020; Hosier et al., 2020; Zheng et al., 2020). In a study in COVID-19 mothers, where the placenta tested positive for SARS CoV-2 and all nasopharyngeal newborn swabs tested negative for the virus, fetal vascular malperfusion was detected in only one of the cases, but the infection was associated with thrombosis in fetal circulation (Baergen and Heller, 2020). Attention has been drawn to the maternal immune response against the COVID-19 virus, which may also negatively affect fetal development due to the symptoms derived from the infection, like high fever, which may damage the fetal neurons, leading to negative neurodegenerative outcomes (Deniz and Tezer, 2020; Forestieri et al., 2020).
There are high numbers of COVID-19 infections in the Mexican population and there is therefore constant concern about whether childbirth is safe, due to the potential risk of vertical transmission or not; therefore, it is a priority to quickly establish preventive measures by healthcare systems. The purpose of this study was to explore the possible vertical transmission of the SARS-CoV-2 virus from an asymptomatic mother with presence of SARS-CoV-2 RNA to her newborn at the time of delivery.
2. Methods
2.1. Study type
This was a transversal, descriptive, cross-sectional study performed in the “Dr. Gustavo Baz Prada” General Hospital located in the municipality of Ciudad Netzahualcoyotl (19°25′19′'N 99°00′53′'W) (Figure 1 a). The study was approved by the hospital's Bioethics Committee in Research, with registry number 208C0101110500T-3157_2020-08. All participants consented to the collection of data and signed informed consent in accordance with the Declaration of Helsinki.
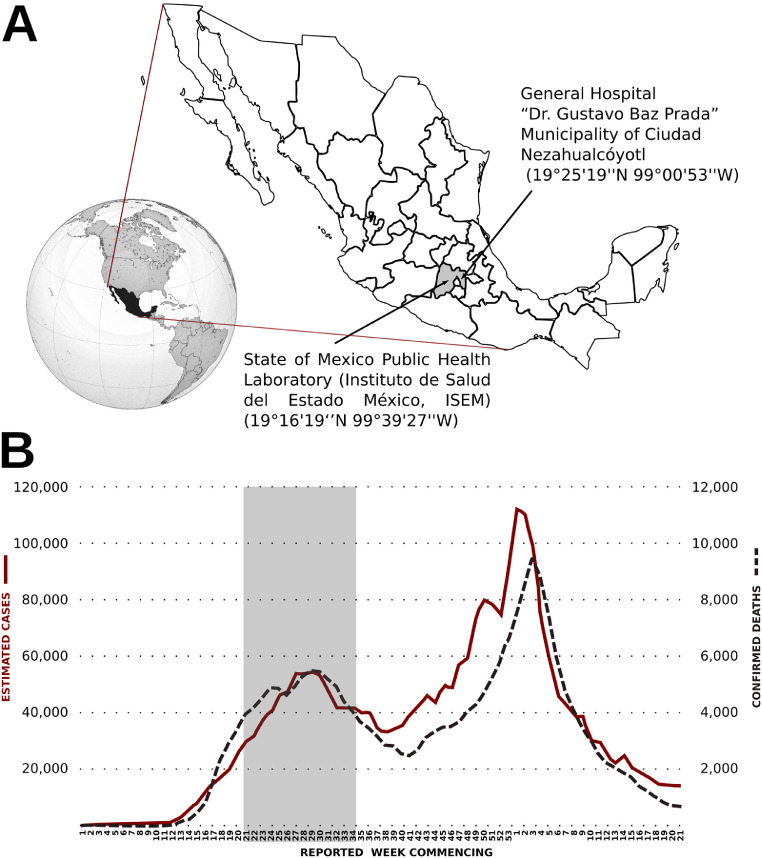
Figure 1.
Location of the study in place and time during the COVID-19 pandemic. A: The figure shows the location of the Mexican republic in the American continent, the sampling site General Hospital “Dr. Gustavo Baz Prada”, Municipality of Ciudad Netzahualcoyotl (19°25′19′'N 99°00′53′'W), and the analysis site State of Mexico Public Health Laboratory (Instituto de Salud del Estado México, ISEM) (19°16′19‘’N 99°39′27′'W). The hospital and laboratory are at 2235 m (7333 ft), and 2681 m (8796 ft) above average sea level, respectively. The source of the globe is https://es.wikipedia.org/wiki/M%C3%A9xico#/media/Archivo:MEX_orthographic.svg, and the source for the map is https://commons.wikimedia.org/wiki/File:Mexico_Map.svg B: The graph shows the sampling period during the COVID-19 pandemic in the Mexican Republic. Samples were taken from July 17th to October 13th, 2020 (vertical shaded area), equivalent to the reported week commencing 21 to 34 (x-axis). The estimated cases are plotted using solid cherry color line (left y-axis), and the confirmed deaths are shown with the dashed black line (right y-axis). Data were retrieved from https://www.gob.mx/salud/documentos/coronavirus-covid-19-comunicado-tecnico-diario-238449 on June 10th, 2021.
2.2. Sample
For this study, 150 samples were collected from 17 July to 13 October 2020, during the first epidemiological wave (Figure 1b). Inclusion criteria were apparently healthy, pregnant Mexican women in the third trimester of pregnancy, with no COVID-19 clinical symptoms, no hormonal treatment, and no antibiotic treatment. The exclusion criterion was pregnant women with COVID-19 clinical symptoms. Entries with incomplete or inadequate data were excluded. Sampling was made according to the Standard Guidelines for Epidemiological Surveillance and SARS-CoV-2 Disease Laboratory manual from the Mexican Government Health Ministry (Secretaría de Salud, Gobierno de México, 2020). The SARS-CoV-2 matrix was obtained from pharyngeal and nasopharyngeal swabs following Laboratory testing of 2019 novel coronavirus (2019-nCoV) in suspected human cases: interim guidance, 17 January 2020 (LVIR-P-05 procedure) https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117. Mother and infant nasopharyngeal exudate swab samples, in the gynecological area of the hospital, were taken using sterile polyester swabs, and each swab was placed in viral culture medium. For mothers, samples were taken immediately after vaginal delivery or cesarean section; for neonates, before cleaning the upper airways. Human colostrum/milk was collected in the recovery area or gynecological room by specialized medical staff within the 48-hour postpartum recovery period. The nipple area was cleaned using a sterile swab and water, and approximately 500 μL of colostrum was extracted in a sterile tube. Of all samples obtained, after applying the exclusion and inclusion criteria, a total of 133 nasopharyngeal swab samples from mothers, 131 swab samples from neonates, and 140 colostrum samples were taken. Instructions from the Laboratory testing of 2019 novel coronavirus (2019-nCoV) in suspected human cases: interim guidance, 17 January 2020 (REMU-MA-01 manual guidelines) were followed for shipment delivery to the Molecular Biology laboratory, Laboratorio Estatal de Salud Pública del Estado de México (19°16′19′'N 99°39′27′'W) (ISEM Secretaría e Instituto de Salud del Estado de México), where they were analyzed (Figure 1a).
2.3. RNA extraction
RNA extraction was made from 140 μL of viral sample in transport medium (VTM), using the MagNA Pure LC RNA Isolation Kit - High Performance (Cat 03542394001, Roche). A total of 260 μL of the lysis buffer was added to the sample and the mixture was placed in a cartridge along with other kit reagents in the MagNA Pure 96 Instrument equipment (Roche, Germany). The HP RNA program Blood_external_lysis' DNA and Viral NA Small Volume purification protocols were followed.
2.4. qRT-PCR methodology
SARS-CoV-2 virus detection in samples was made by real-time RT-PCR, using the One-Step Real-Time RT-PCR kit (Cat. MAD-003941M, Thermo Fisher Scientific, USA), in which the primers and probes are designed for detecting the SARS-CoV-2 N gene and the E gene, as described by the CDC. The RT-PCR reaction mixture was in a final volume of 20 μL, with 12 μL of SARS CoV-2 MMix and 8 μL of total extracted RNA. The RT-PCR cycling protocol was: 25°C for 5 min, 50°C for 20 min for reverse transcription, 95°C for 5 min enzyme deactivation, and 45 cycles of (95°C for 30 s denaturation, 60°C for 1 min alignment and extension) in the CFX96 Touch System (BIO-RAD). A negative control, reaction control, and positive control of non-infectious synthetic DNA contained in the kit (PC SARS CoV2) were used (Corman et al., 2020).
2.5. Digital droplet PCR (ddPCR) methodology
The viral load of the SARS-CoV-2 virus was measured in the samples by RT-ddPCR, using the One-Step RT-ddPCR kit (Cat. 1864021 BIO-RAD, USA). The 20 μL reaction mixture was set up with: 5 μL of 4X of Supermix; 2 μL of 25 U/μL reverse transcriptase enzyme; 1 μL of 300 mM DTT; 0.5 μL of 100 nM primers and probes targeting the N gene of SARS-CoV-2 described by the CDC; 3 μL of total RNA; and 8.5 μL of H2O. Once mixed, the reactions were transferred to the GCR96 cartridge (Cat. 12006858) and sealed using PX1 PCR plate sealer (Cat. 1814000). The RT-ddPCR cycling protocol was: 50°C for 20 min for reverse transcription, followed of enzyme deactivation at 95°C for 5 min, and 45 cycles of (95°C for 30 s denaturation, 60°C for 1 min for alignment and extension) in the QX ONE Droplet Digital PCR (ddPCR) System (BIO-RAD) (Corman et al., 2020).
2.6. Data analysis
Data corresponding to samples were filtered and compiled. To assess whether there were significant differences between the positive and negative groups for SARS-CoV-2 RNA content, the Mann-Whitney U test was used, and p<0.05 was considered statistically significant. Epidemiological and clinical data were represented as percentages and reported as mean±standard deviation (SD). Statistical analysis and graphics were evaluated in RStudio v.1.3.1093 program.
3. Results
3.1. Sample collection and processing
The samples were collected in a public hospital (Figure 1a) from asymptomatic mothers during a 14-week period encompassing the first of the two peaks of the COVID-19 pandemic in Mexico (Figure 1b). The nasopharyngeal swabs and human colostrum/milk samples were collected from 150 mother-neonate pairs; however, there was a reduction in the number of samples in each category due to low sample quality or incomplete data. As a result, the study was finally made with 133 nasopharyngeal swab samples from mothers, 131 swab samples from neonates, and 140 colostrum/milk samples.
3.2. Asymptomatic women carried the SARS-CoV-2 virus during delivery
Although the participating women were clinically asymptomatic for COVID-19 on admission to the delivery room, the qPCR results for SARS-CoV-2 RNA virus detection revealed that 16 of the 133 women or approximately 12% of them were positive for the test (Table 1 ). According to Mann-Whitney U test, no association was observed between gestational age, the number of parities or the anthropometry, which included assessment of being overweight and obesity, with the positive results for SARS-CoV-2. Similar results were observed for the blood tests, including total white cells and platelet counts and fasting glucose. Additional data evaluating risk factors, educational level, marital status, and main activity of the mothers did not appear to be associated with the detection of coronaviral RNA (Table 1).
Table 1.
Data for the 133 mothers with SARS-CoV-2 diagnosis in the study.
| Variable | Positive | Negative | p-value* | |
| Number of subjects | 16 | 117 | ‒ | |
| Age (years) | 23.00 (±4.93) | 24.11 (±6.37) γ | 0.621 | |
| Gestational age (weeks) | 38.76 (±1.89) | 39.07 (±1.54) γ | 0.469 | |
| Anthropometry | Height (m) | 1.56 (±0.06) | 1.56 (±0.06) δ | 0.796 |
| Weight (kg) | 70.31 (±14.20) | 73.03 (±12.29) δ | 0.309 | |
| BMI | 29.00 (±5.47) | 29.96 (±4.48) δ | 0.338 | |
| Normal (BMI 18.5–24.9) | 4 (25.00%) | 18 (16.22%) δ | ‒ | |
| Overweight (BMI 25.0–29.9) | 6 (37.50%) | 38 (34.23%) δ | ‒ | |
| Obesity (BMI >30.0) | 6 (46.15%) | 55 (47.00%) δ | ‒ | |
| Grade I (BMI 30.0–34.9) | 4 (25.00%) | 38 (34.23%) δ | ‒ | |
| Grade II (BMI 35.0–39.9) | 1 (6.25%) | 16 (14.41%) δ | ‒ | |
| Grade III (BMI >40.0) | 1 (6.25%) | 1 (0.91%) δ | ‒ | |
| Blood test | Reference range | |||
| Leukocytes (x109/L) | 4.5–10 | 9.51 (±2.65) β | 10.39 (±7.52) ε | 0.747 |
| Neutrophils (x109/L) | 1.8–8.0 | 6.79 (±2.54) β | 7.17 (±2.88) ζ | 0.961 |
| Neutrophils (%) | 43.0–65.0 | 74 (±8.18) β | 72.53 (±10.19) ζ | 0.840 |
| Lymphocytes (x109/L) | 1.1–3.2 | 1.69 (±0.80) β | 1.76 (±0.62) ζ | 0.596 |
| Lymphocytes (%) | 21–48 | 18.35 (±8.31) β | 19.15 (±7.59) ζ | 0.846 |
| Platelet count (x109/L) | 150–450 | 227.33 (±47.64) β | 227.70 (±66.63) ε | 0.866 |
| Fasting glucose (mg/dL) | 74–106 | 85.27 (±9.51) β | 87.56 (±32.42) ε | 0.283 |
| Creatinine (mg/dL) | 0.5–0.9 | 0.68 (±0.13) β | 0.68 (±0.13) η | 0.752 |
| Risk factors | Alcoholism | 1 (6.25%) | 4 (3.54%) κ | – |
| Smoking | 2 (12.50%) | 5 (4.42%) κ | – | |
| Drug addiction | 0 (0.00%) | 0 (0.00%) κ | – | |
| Parity | Total parities | 29 | 271 | |
| Average | 1.81 (±0.98) | 2.42 (±1.34) | 0.083 | |
| Uniparous | 8 (50%) | 35 (31.25%) | – | |
| Multiparous | 8 (50%) | 77 (68.75%) | – | |
| Total previous parities μ | 12 | 127 | ||
| Average | 0.75 (±1.00) | 1.13 (±1.16) | 0.191 | |
| Total vaginal (previous) | 8 (66.66%) | 89 (70.08%) | – | |
| Total cesarean (previous) | 4 (33.33%) | 38 (29.92%) | – | |
| Abortions | 1 | 32 | – | |
| Socioeconomic data | ||||
| Educational level | Primary school (6 years) | 2 (12.5%) | 21 (18.75%) γ | – |
| Secondary school (3 years) | 7 (43.75%) | 63 (56.25%) γ | – | |
| High school (3 years) | 7 (43.75%) | 21 (18.75%) γ | – | |
| University (4–5 years) | 0 (0.00%) | 7 (6.25%) γ | – | |
| Marital status | Free union | 13 (81.25%) | 74 (66.07%) γ | – |
| Married | 1 (6.25%) | 14 (12.50%) γ | – | |
| Single | 2 (12.50%) | 23 (20.54%) γ | – | |
| Widow | 0 (0.00%) | 1 (0.89%) γ | ||
| Main activity | Housewife | 15 (93.75%) | 87 (77.68%) γ | – |
| General employee | 1 (6.25%) | 18 (16.07%) γ | – | |
| Student | 0 (0.00%) | 7 (6.25%) γ | – | |
*Mann-Whitney U test, significant p-value <0.05.
Standard deviation is shown as ± values between parentheses
–, no value was calculated for categorical data
= 15 subjects
= 112 subjects
= 111 subjects
= 106 subjects
= 103 subjects
=107 subjects
= 113 subjects
= data for the current pregnancy were unavailable, and only the previous pregnancies reported
3.3. There was perinatal vertical transmission of the SARS-CoV-2 virus to newborns and colostrum/milk samples
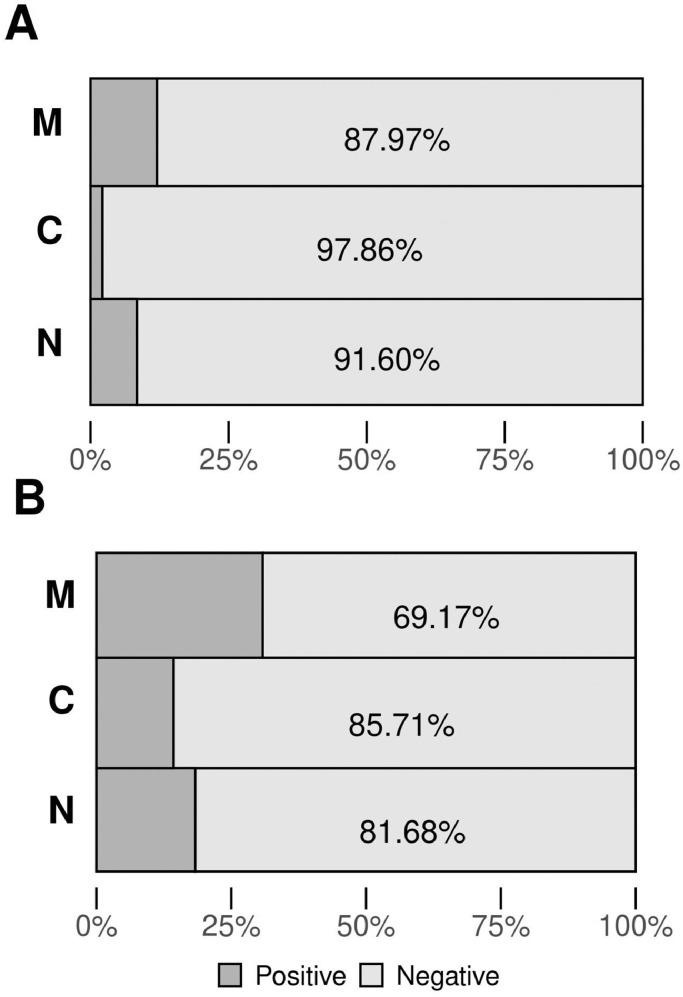
For the case of the neonates, nasopharyngeal swabs were immediately taken after birth and before cleaning the upper airways. Neonates were not breastfed and did not have contact with their mothers prior to sampling. For these samples, 11 of 131 (∼8.40%) were positive for the presence of SARS-CoV-2 RNA, according to the qPCR test (Table 2 , Figure 1, Table S1). On the other hand, there was no association between newborn sex, qualification status, somatometry, or complications during delivery and the positive results for SARS-CoV-2 (Table 2). Exploration of the presence of SARS-Cov-2 in 140 human colostrum/milk samples collected from the asymptomatic mothers within the 48 hours immediately after delivery showed three positive samples, giving a percentage of 2.14% (Figure 2 A, Table S1).
Table 2.
Data for 131 neonates with a SARS-CoV-2 diagnosis in the study.
| Variable | Positive | Negative | p-value* | |
| Number of subjects | 11 | 120 | – | |
| Sex | Male | 6 (54.55%) | 55 (47.83%) β | – |
| Female | 5 (45.45%) | 60 (52.17%) β | – | |
| Qualification status | ||||
| Apgar (1 to 10) | 6/8 | 0 (0.00%) | 1 (0.95%) σ | – |
| 6/9 | 0 (0.00%) | 1 (0.95%) σ | – | |
| 7/8 | 1 (9.09%) | 5 (4.50%) σ | – | |
| 7/9 | 0 (0.00%) | 15 (13.25%) σ | – | |
| 8/8 | 0 (0.00%) | 1 (0.95%) σ | – | |
| 8/9 | 9 (81.82%) | 80 (69.72%) σ | – | |
| 9/9 | 1 (9.09%) | 11 (9.68%) σ | – | |
| Silverman Andersen κ | 0 | 7 (63.64%) | 80 (69.72%) σ | – |
| 1 | 0 (0.0%) | 2 (1.8%) σ | – | |
| 0/1 | 3 (27.27%) | 30 (26.58%) σ | – | |
| 0/3 | 1 (9.09%) | 0 (0.00%) σ | – | |
| 3 | 0 (0.0%) | 1 (0.95%) σ | – | |
| 9 | 0 (0.0%) | 1 (0.95%) σ | – | |
| Capurro λ (weeks) | 39.55 (±0.82) | 38.96 (±1.38) μ | 0.259 | |
| Somatometry | Reference range | |||
| Macrosomia (g) | >4000 | 0 (0.0%) | 2 (1.8%) | – |
| Proper weight (g) | 2500 <4000 | 11 (100%) | 103 (87.83%) | – |
| Low weight (g) | ≤2500 | 0 (0.0%) | 10 (7.83%) | – |
| IUGR | 0 (0.0%) | 1 (0.95%) | – | |
| Weight (kg) | 3.19 (±0.33) | 2.98 (±0.41) π | 0.086 | |
| Size (cm) | 50.82 (±2.27) | 49.59 (±2.03) π | 0.129 | |
| Cephalic perimeter (cm) | 34.23 (±1.29) | 33.65 (±1.45) ε | 0.178 | |
| Abdominal perimeter (cm) | 31.64 (±1.96) | 30.17 (±2.57) ε | 0.055 | |
| Perinatal asphyxiation | 0 (0.0%) | 2 (1.80%) | – | |
| Breathing difficulty | 0 (0.0%) | 1 (0.95%) | – | |
| Infection | 0 (0.0%) | 1 (0.95%) | – | |
| Sepsis | 0 (0.0%) | 0 (0.0%) | – | |
| Death | 0 (0.0%) | 0 (0.0%) | – | |
IUGR, intrauterine growth restriction
*Mann-Whitney U test, significant p-value <0.05
Standard deviation is shown as ± values between parentheses
–, no value was calculated for categorical data
= estimated gestational age in weeks (Engle WA, 2004)
= respiratory severity score (Hedstrom et al., 2018)
= of 115 subjects
= of 115 subjects
= of 114 subjects
= of 114 subjects
= of 113 subjects
Figure 2.
Graphic representation of RNA SARS-CoV-2 detection. A: Quantitative PCR detection, positive results for M are 12.03%; C, 2.14%, and N, 8.40%. B: Digital PCR detection, positive results for M are 30.83%; C, 14.29%, and N, 18.32%.
Horizontal bars represent the relative percentage for M, mother nasopharyngeal swab (n=133); C, colostrum/human milk samples (n=140), and N, neonate nasopharyngeal swab (n=131). For N, results contain data for three additional samples with incomplete data for qualification status, somatometry, and complications during delivery. Numbers in the x-axis show the percentage scale. Dark grey color indicates positive results, light grey color indicates negative results.
3.4. The use of ddPCR revealed a higher percentage of SARS-CoV-2 RNA presence
The qPCR test showed the presence of SARS-CoV-2 RNA in 16 of the 133 samples of the mothers’ nasopharyngeal swabs (12.03%) (Figure 2A, Table S1). Further exploration of the presence of viral RNA by digital droplet PCR (ddPCR) confirmed the positive qPCR results and revealed the existence of 25 more samples, giving a total of 41 (30.83%) (Figure 2B, Table S2). For the case of SARS-CoV-2 RNA presence in the neonate nasopharyngeal swabs determined by qPCR, the percentage was 8.40% (11 samples of 131) (Figure 2A, Table S1). Further retesting of these samples using ddPCR analysis confirmed the 11 positive qPCR results, and revealed the existence of 13 more positive samples, giving a total of 24 positive among the 131 collected neonate nasopharyngeal swabs; this indicated a vertical transmission of approximately 18.32% (Figure 2B, Table S2). A similar situation occurred for positivity in the 140 human colostrum/milk samples collected from asymptomatic mothers within the 48 hours immediately after delivery and before feeding the newborns, where it increased from 2.14% by qPCR (3 of 140) (Figure 2A, Table S1) to 14.29% (20 of 140) by ddPCR (Figure 2B, Table S2).
4. Discussion
The persistence of the global COVID-19 pandemic is demanding continuous study of the nature of this disease from all scientific areas of expertise. One important aspect of this virus is the mode and frequency of transmission, mostly in vulnerable members of the population like pregnant women. The mother-neonate pair represents an excellent opportunity to assess the frequency of the SARS-CoV-2 virus vertical transmission, since the binomial link usually involves intimate contact and the baby feeding with HM.
The current results from the 133 mothers in this study suggest that one in eight asymptomatic pregnant Mexican women (12.03%), aged 23–24 years, with 38–39 weeks’ gestational age, were positive for the presence of the SARS-CoV-2 genome in the nasopharyngeal swabs taken just before delivery. The frequency shown in this population seems to be greater compared with other countries sampled at the same period in the first COVID-19 wave. The results obtained in a UK cohort estimated the incidence of positivity for SARS-CoV-2 in asymptomatic pregnant women to be 1.2/1000 maternities (∼0.12%) from March to September 2020 (Vousden et al., 2021). One study during April 2020, by the Mass General Brigham Health system in Boston, found that nine of 618 (1.5%) asymptomatic mothers tested positive (Goldfarb et al., 2020). However, the current results are comparable with another study from 4–15 April 2020, in the Mount Sinai Health system in New York City, where 24 of 155 (15.5%) COVID-19 asymptomatic mothers tested positive for SARS-CoV-2 RNA (Bianco et al., 2020).
The same test performed in the nasopharyngeal swabs collected from the 131 neonates immediately after delivery showed that approximately one in ∼12 (∼8.40%) were positive for the presence of the SARS-CoV-2 genome. Likewise, in a study in an Indian population, 12 of 155 neonates (7.74%) tested positive for SARS CoV-2 infection (Kalamdani et al., 2020). It is important to note in the current study that none of the neonates had been breastfed by their mothers before the samples were taken. This supports the idea that the possibility of vertical transfer of the virus through breast feeding or a mother's handling is not possible. However, as mentioned above, a frequency of 11 of 131 (∼8.40%) positive nasopharyngeal swabs was observed.
The source of SARS-Cov-2 RNA in neonates might be transmission from the placenta. There is some published evidence regarding this: a report that the SARS-CoV-2 virus spread in the body of an Italian pregnant woman through the bloodstream, reaching the placenta and finally infecting the fetus (Fenizia et al., 2020); the birth of Chinese neonates of mothers with COVID-19 pneumonia (Zhu et al., 2020); evidence of vertical transition of SARS-CoV-2 to one neonate in an Iranian mother (Zamaniyan et al., 2020); and the report of SARS-CoV-2 RNA presence in the placenta of two Italian mothers and neonates at birth (Patanè et al., 2020). In addition, the current study provides evidence of SARS-CoV-2 virus vertical transmission from mothers to neonates, which might help to answer valid questioning about the absence of evidence (Auriti et al., 2020).
Contrary to the results in other reports, there was no association between the neonate's data collected at birth by the pediatrician (somatometry, sex and newborn qualification status) and a positive result by qPCR, and any collected metadata of the mothers, as reported in other populations like British healthy pregnant women, where positivity of SARS-CoV-2 was associated to preterm neonates and cesarean section delivery (Vousden et al., 2021), or Turkish healthy pregnant women where positivity was associated with cesarean section, prematurity and low birth weight (Oncel et al., 2021).
All of the 140 studied mothers expressed a sample of colostrum or milk to be studied. It was observed that most samples were negative for the presence of the SARS-CoV-2 genome by qPCR test (one positive of 47 samples or 2.14%). These findings are comparable with other work: for example, in a recent evaluation of 37 peer-reviewed studies where the presence of SARS-CoV-2 RNA in human milk was assessed, nine of 68 samples in all the reported studies had detectable levels of SARS-CoV-2 RNA (Centeno-Tablante et al., 2021). In addition, another mini-review summarizing 19 case-report studies in different populations tested the presence of SARS-CoV-2 in HM. These studies were sub-grouped into four categories, according to the infected member in the binomials, finding that in SARS-CoV-2–positive lactating mother with a negative neonate, two (5.2%) of 38 samples were positive for SARS-CoV-2 (Vassilopoulou et al., 2021). The low frequency of virus in HM samples in the current work agrees with these findings, supporting the idea of low risk of vertical transmission and highlighting the protective role of passive antibodies provided by HM to the neonate (Bardanzellu et al., 2021). For these reasons, breastfeeding is encouraged following appropriate sanitary procedures, like using a facemask, during the pandemic.
The results obtained in this study strongly suggest that asymptomatic Mexican women carried the SARS-CoV-2 virus during delivery, with perinatal transmission of SARS-CoV-2 virus to the newborns. However, since the presence of SARS-CoV-2 RNA in colostrum/milk was comparatively small and sampling was immediately after birth, the detection of positive neonates might have been due to infection by the virus in utero.
This study re-assayed most of the nasopharyngeal swabs and colostrum/milk samples by droplet digital PCR. It was found that this more sensitive technique increased the percentage of positive samples by approximately 2.6-fold for nasopharyngeal swabs of mothers, 6.7-fold for colostrum/milk, and 2.2-fold for nasopharyngeal neonate swabs (Figures 2A, 2B, Tables S1, S2).
In terms of the scope of this work, there were limitations like the sample size and the need to expand the studies, sampling not only nasopharyngeal swabs in mothers-neonates as well as colostrum/human milk, but also collecting other important samples like amniotic fluid, umbilical cord blood, and peripheral blood from the binomials, which are informative in evaluating the presence of the SARS-CoV-2 genome during delivery. In addition, the conclusions may not be generalized to other populations.
Funding
This work was financed by Secretaría de Relaciones Exteriores México (SRE), No. SRE/027/2021; and Agencia Mexicana de Cooperación Internacional para el Desarrollo (AMEXCID), No. AMEXCID 2020-5.
Ethical approval
The study was approved by the Bioethics Committee in Research of the General Hospital “Dr. Gustavo Baz Prada” with registry 208C0101110500T-3157_2020-08. All participants signed informed consent in accordance with the Declaration of Helsinki.
Conflict of interest
The authors declare no conflicts of interest.
Contributions
Conceptualization: FB-G, PZ-S, JG-M; Data curation: TB-G, KC-C, FB-G, PZ-S, JG-M; Formal analysis: AR-R, TB-G, KC-C, JMV-I, NGZ-T, FB-G, PZ-S, JG-M; Funding acquisition: FB-G, PZ-S, JG-M; Investigation: AR-R, FB-G, PZ-S; Methodology: KC-C, JMV-I, NGZ-T; Project administration: FB-G, PZ-S, JG-M; Resources: AR-R, JC-L, SM-P, MEL-M, FB-G, PZ-S; Software: TB-G, KC-C, JMV-I, NGZ-T; Supervision: FB-G, PZ-S, JG-M; Validation: FB-G, PZ-S, JG-M; Visualization: TB-G, KC-C, JMV-I, NGZ-T; Writing – original draft: TB-G, KC-C, JMV-I, NGZ-T, JG-M; Writing – review & editing: AR-R, TB-G, KC-C, JMV-I, NGZ-T, JC-L, SM-P, MEL-M, FB-G, PZ-S, JG-M.
Acknowledgments
We are grateful to all mothers who participated in the study, to Carolina Miranda-Brito for technical support in data curation, and Viridiana Rosas Ocegueda for administrative assistance. PZ-S (43142), FB-G (225525), and JG-M (19815) are Fellows from the Sistema Nacional de Investigadores, Mexico. PZ-S is also a EDI-IPN and COFAA-IPN fellow.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.10.006.
Appendix. Supplementary materials
References
- Auriti C, De Rose DU, Tzialla C, Caforio L, Ciccia M, Manzoni P, et al. Vertical Transmission of SARS-CoV-2 (COVID-19): Are Hypotheses More than Evidences? Am J Perinatol. 2020;37(S 02):S31–S38. doi: 10.1055/s-0040-1714346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baergen RN, Heller DS. Placental Pathology in Covid-19 Positive Mothers: Preliminary Findings. Pediatr Dev Pathol. 2020;23(3):177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardanzellu F, Fanos V, Reali A. Human Breast Milk-acquired Cytomegalovirus Infection: Certainties, Doubts and Perspectives. Curr Pediatr Rev. 2019;15(1):30–41. doi: 10.2174/1573396315666181126105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardanzellu F, Puddu M, Fanos V. Breast Milk and COVID-19: From Conventional Data to "Omics" Technologies to Investigate Changes Occurring in SARS-CoV-2 Positive Mothers. International journal of environmental research and public health. 2021;18(11):5668. doi: 10.3390/ijerph18115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A, Buckley AB, Overbey J, Smilen S, Wagner B, Dinglas C, et al. Testing of Patients and Support Persons for Coronavirus Disease 2019 (COVID-19) Infection Before Scheduled Deliveries. Obstet Gynecol. 2020;136(2):283–287. doi: 10.1097/AOG.0000000000003985. [DOI] [PubMed] [Google Scholar]
- Cao Q, Chen YC, Chen CL, Chiu CH. SARS-CoV-2 infection in children: Transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119(3):670–673. doi: 10.1016/j.jfma.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carosso A, Cosma S, Borella F, Marozio L, Ghisetti Coscia A, et al. Pre-labor anorectal swab for SARS-CoV-2 in COVID-19 pregnant patients: is it time to think about it? Eur J Obstet Gynecol Reprod Biol. 2020;249:98–99. doi: 10.1016/j.ejogrb.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno-Tablante E, Medina-Rivera M, Finkelstein JL, Rayco-Solon P, Garcia-Casal MN, Rogers L, et al. Transmission of SARS-CoV-2 through breast milk and breastfeeding: a living systematic review. Ann NY Acad Sci. 2021;1484(1):32–54. doi: 10.1111/nyas.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC - Center for Disease Control and Prevention [Internet]. How COVID-19 Spreads c 2021 [cited 2021 Jun 2]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- CONACYT – Consejo Nacional de Ciencia y Tecnología [Inernet]. COVID-19 Tablero México - CONACYT - CentroGeo - GeoInt - DataLab c 2021. [cited 2021 Jun 2]. Available from: https://datos.covid-19.conacyt.mx/Spanish.
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR [published correction appears in Euro Surveill. 2020 Apr;25(14):] [published correction appears in Euro Surveill. 2020 Jul;25(30):] [published correction appears in Euro Surveill. 2021 Feb;26(5):] Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniz M, Tezer H. Vertical transmission of SARS CoV-2: a systematic review. J Matern Fetal Neonatal Med. 2020:1–8. doi: 10.1080/14767058.2020.1793322. [published online ahead of print, 2020 Jul 21] [DOI] [PubMed] [Google Scholar]
- Fenizia C, Biasin M, Cetin I, Vergani P, Mileto D, Spinillo A, et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun. 2020;11(1):5128. doi: 10.1038/s41467-020-18933-4. Published 2020 Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestieri S, Marcialis MA, Migliore L, Panisi C, Fanos V. Relationship between pregnancy and coronavirus: what we know [published online ahead of print, 2020 Jun 4] J Matern Fetal Neonatal Med. 2020:1–12. doi: 10.1080/14767058.2020.1771692. [DOI] [PubMed] [Google Scholar]
- Goldfarb IT, Diouf K, Barth WH, et al. Universal SARS-CoV-2 testing on admission to the labor and delivery unit: Low prevalence among asymptomatic obstetric patients. Infect Control Hosp Epidemiol. 2020;41(9):1095–1096. doi: 10.1017/ice.2020.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom AB, Gove NE, Mayock DE, Batra M. Performance of the Silverman Andersen Respiratory Severity Score in predicting PCO2 and respiratory support in newborns: a prospective cohort study. J Perinatol. 2018;38(5):505–511. doi: 10.1038/s41372-018-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosier H, Farhadian SF, Morotti RA, Deshmuhk U, Lu-Cullingan A, Campbell KH, et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130(9):4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamdani P, Kalathingal T, Manerkar S, Mondkar J. Clinical Profile of SARS-CoV-2 Infected Neonates From a Tertiary Government Hospital in Mumbai. India. Indian Pediatr. 2020;57(12):1143–1146. doi: 10.1007/s13312-020-2070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Zarchi M, Neamatzadeh H, Dastgheib SA, Abbasi H, Mirjalili SR, Behforouz A, et al. Vertical Transmission of Coronavirus Disease 19 (COVID-19) from Infected Pregnant Mothers to Neonates: A Review. Fetal Pediatr Pathol. 2020;39(3):246–250. doi: 10.1080/15513815.2020.1747120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey KA, Pace RM, Williams JE, Bode L, Donovan SM, Järvinen KM, et al. SARS-CoV-2 and human milk: What is the evidence? Matern Child Nutr. 2020;16(4):e13032. doi: 10.1111/mcn.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Sang L, Du S, Li T, Chang Y, Yang XA. Asymptomatic COVID-19 infection in late pregnancy indicated no vertical transmission. J Med Virol. 2020;92(9):1660–1664. doi: 10.1002/jmv.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardani M, Pourkaveh B. A controversial debate: vertical transmission of COVID-19 in pregnancy. Arch Clin Infect Dis. 2020;15(1) doi: 10.5812/archcid.102286. Feb 29. [DOI] [Google Scholar]
- Mercado K, Vittner D, Drabant B, McGrath J. Neonatal Intensive Care Unit-Specific Lactation Support and Mother's Own Breast Milk Availability for Very Low Birth-Weight Infants. Adv Neonatal Care. 2019;19(6):474–481. doi: 10.1097/ANC.0000000000000684. [DOI] [PubMed] [Google Scholar]
- Oncel MY, Akın IM, Kanburoglu MK, Tayman C, Coskun S, Narter F, et al. A multicenter study on epidemiological and clinical characteristics of 125 newborns born to women infected with COVID-19 by Turkish Neonatal Society. European journal of pediatrics. 2021;180(3):733–742. doi: 10.1007/s00431-020-03767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace RM, Williams JE, Järvinen KM, Belfort M, Pace CDW, Lackey KA, et al. Characterization of SARS-CoV-2 RNA, Antibodies, and Neutralizing Capacity in Milk Produced by Women with COVID-19. mBio. 2021;12(1):e03192-20. Published 2021 Feb 9. doi: https://doi.org/10.1128/mBio.03192-20 [DOI] [PMC free article] [PubMed]
- Patanè L, Morotti D, Giunta MR, Sigismomdi C, Piccoli MG, Frigerio L, et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020;2(3) doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KP, Vunnam SR, Patel PA, Krill KL, Korbitz PM, Callagher JP, et al. Transmission of SARS-CoV-2: an update of current literature. Eur J Clin Microbiol Infect Dis. 2020;39(11):2005–2011. doi: 10.1007/s10096-020-03961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paules CI, Marston HD, Fauci AS. Coronavirus Infections-More Than Just the Common Cold. JAMA. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- Plüddemann A, Spencer EA, Heneghan C, Brassey J., Onakpoya IJ, Rosca EC, et al. SARS-CoV-2 and the role of vertical transmission from infected pregnant women to their fetuses: systematic review. medRxiv. 2021. doi: https://doi.org/10.1101/2021.06.30.21259750
- Qiao J. What are the risks of COVID-19 infection in pregnant women? Lancet. 2020;395(10226):760–762. doi: 10.1016/S0140-6736(20)30365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatori G, De Rose DU, Concato C, Alario D, Olivini N, Dotta A, Campana A. Managing COVID-19-Positive Maternal-Infant Dyads: An Italian Experience. Breastfeed Med. 2020;15(5):347–348. doi: 10.1089/bfm.2020.0095. [DOI] [PubMed] [Google Scholar]
- Secretaría de Salud, Gobierno de México [Internet] Lineamiento Estadarizado Para la Vigilancia Epidemiológica y por Laboratorio de Covid-19 [Standarized Lineament for Epidemiological Surveillance and by Covid-19 Laboratory] 2020 [Cited 2021 June 2] Available from: http://cvoed.imss.gob.mx/wp-content/uploads/2020/01/LinVigEpiLab_COVID19.pdf.pdf.pdf Spanish.
- Suárez V, Suarez Quezada M, Oros Ruiz S, Ronquillo De Jesús E. Epidemiología de COVID-19 en México: del 27 de febrero al 30 de abril de 2020 [Epidemiology of COVID-19 in Mexico: from the 27th of February to the 30th of April 2020] Rev Clin Esp. 2020;220(8):463–471. doi: 10.1016/j.rce.2020.05.007. Spanish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilopoulou E, Feketea G, Koumbi L, Mesiari C, Berghea EC, Konstantinou GN. Breastfeeding and COVID-19: From Nutrition to Immunity. Frontiers in immunology. 2021;12 doi: 10.3389/fimmu.2021.661806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden N, Bunch K, Morris E, Simpson N, Gale C, Patrick O'Brien, et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: A national cohort study using the UK Obstetric Surveillance System (UKOSS) PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0251123. Published 2021 May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern [published correction appears in Lancet. 2020 Jan 29;:] Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO - World Health Organization [Internet]. Global Strategy for Infant and Young Child Feeding 2003 [cited 2021 Jul 16]. Available from: https://apps.who.int/iris/bitstream/handle/10665/42590/9241562218.pdf;sequence=1
- WHO - World Health Organization [Internet] WHO Coronavirus (COVID-19) c2021 [cited 2021 July 16] Dashboard. Available from: https://covid19.who.int/
- Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: What we know. Int J Infect Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Liu Y. Vertical Transmission of Severe Acute Respiratory Syndrome Coronavirus 2: A Systematic Review. Am J Perinatol. 2020;37(10):1055–1060. doi: 10.1055/s-0040-1712161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamaniyan M, Ebadi A, Aghajanpoor S, Rahmani Z, Haghshenas M, Azizi S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat Diagn. 2020;40(13):1759–1761. doi: 10.1002/pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QL, Duan T, Jin LP. Single-cell RNA expression profiling of ACE2 and AXL in the human maternal–Fetal interface. Reproductive and Developmental Medicine. 2020;4(1):7. doi: 10.4103/2096-2924.278679. Jan 1. [DOI] [Google Scholar]
- Zhu H, Wang L, Fang C, Peng S, Zhang L, Chang G, Xia S, Zhou W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Translational pediatrics. 2020;9(1):51. doi: 10.21037/tp.2020.02.06. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.