Abstract
Arbovirus transmission by Aedes mosquitoes has long been a significant problem in Africa. In West Africa, Aedes vector management faces significant challenges; lack of recent Aedes distributional data and potential distributional modeling hinder effective vector control and pose serious public health issues. In this study, larval and adult mosquitoes were collected from four study sites in Enugu State, Nigeria every other month between November 2017 and September 2018. A total number of 2997 Aedes mosquitoes were collected and identified, and 59 positive field occurrence points for both Aedes adult and larvae were recorded. A total of 18 positive occurrence points were used for modeling. Ecological Niche Models (ENMs) were used to estimate the current geographic distribution of Aedes species (spp.) in Enugu State, south-east Nigeria, and mosquito presence was used as a proxy for predicting risk of disease transmission. Maximum Entropy distribution modeling or “MaxEnt” was used for predicting the potential suitable habitats, using a portion of the occurrence records. A total of 23 environmental variables (19 bioclimatic and four topographic) were used to model the potential geographical distribution area under current climatic conditions. The most suitable habitat for Aedes spp. was predicted in the northern, central, and southeastern parts of Enugu State with some extensions in Anambra, Delta, and Edo States in the west, and Ebonyi State in the east. Seasonal temperature, precipitation of the wettest month, mean monthly temperature range, elevation, and precipitation of the driest months were the highest estimated main variable contributions associated with the distribution of Aedes spp. We found that Aedes spp. prefer to be situated in environmental conditions where precipitation of wettest month ranged from 265 to 330 mm, precipitation of driest quarter ranged from 25 to 75 mm while precipitation of wettest quarter ranged from 650 to 950 mm. Aedes mosquitoes, such as Ae. aegypti and Ae. albopictus, pose a significant threat to human health, hence, the results of this study will help decision makers to monitor the distribution of these species and establish a management plan for future national mosquito surveillance and control programs in Nigeria.
Keywords: Arboviruses, Habitat suitability, Prediction model, West Africa, Mosquito, MaxEnt, Ecological niche modeling
1. Introduction
Arboviral infections are a public health concern that is receiving increased attention globally (Wilder-Smith et al., 2017) and particularly in Africa due to the wide distribution of Aedes (Ae.) spp. Mosquitoes and the escalating burden and risk of Aedes-borne acute febrile diseases (Weetman et al., 2018). Two major mosquito vectors, Ae. aegypti (Linnaeus) and Ae. albopictus (Skuse), are responsible for the transmission of viruses such as dengue, Zika, yellow fever (YF) and chikungunya in West Africa, and have shown tremendous global expansion (Kraemer et al., 2015). These viruses have also been isolated from Ae. vittatus (Bigot), which is thought to play an important role in the maintenance and transmission of these diseases based on experimental evidence of transmission (Sudeep and Shil, 2017). In East Africa, epidemics of YF may be vectored by Ae. bromeliae (Theobald), the only anthropophilic member of the Ae. simpsoni (Theobald) species complex which contains 10 subspecies (Huang, 1986). Additionally, the distribution and frequencies of Zika virus (ZIKV) infected mosquitoes suggest that Ae. africanus and Ae. lutocephalus are involved in sylvatic ZIKV transmission cycles, however, ZIKV detection in field-collected mosquitoes does not prove its role as vector (Kraemer et al., 2015).
The emergence or re-emergence of arboviruses involved in human epidemics is associated with the coincidence of several factors including: the presence of sufficient numbers of susceptible hosts, the presence of the vectors, and the ecological conditions allowing for the establishment of a transmission cycle (Sall et al., 2010; Pfeffer and Dobler, 2010; Weaver and Reisen, 2010; Vasilakis et al., 2011). Lack of recent Aedes distributional data and potential distributional modeling hinders effective vector control and poses a serious public health issue Nigeria. Therefore, applying techniques such as Ecological Niche Modeling (ENM) are important in bridging knowledge gaps in the distribution of organisms and hence the risk of disease transmission (Peterson et al., 2005; Escobar et al., 2016).
The ecological niche can be characterized as the set of natural conditions (abiotic variables) in which a species is able to preserve reasonable population sizes without migration (Grinnell, 1917, Grinnell, 1924; Fath, 2018). ENM provides a prediction of the suitable habitats for mosquito species in an explicit spatial manner derived from extracting and comparing environmental factors that are similar to the areas where the species occurs, thus filling the gaps resulting from surveillance data shortages (Jiménez-Valverde et al., 2011). It is not only used to analyze species distributions, but also to predict the presence or absence of species or their habitats in unrecorded areas (Guisan and Hofer, 2003; Araújo et al., 2005; Wintle et al., 2005; Elith et al., 2006; Elith and Leathwick, 2009).
An accurate and thorough understanding of the roles that geographical distribution of vectors and ecological effects play on arboviral transmission dynamics may allow the prediction, and potential prevention, of epidemics and reduce the disease impact on humans. The goal of this study is to model the potential geographical distribution of Aedes spp. in Enugu, south-east Nigeria and develop an ecological niche model that will address information gaps and provide accurate data for public health decision makers for future national surveillance and control programs. Vector surveillance data that couples climatological and mapping data offers robust tools for predict population dynamics of vectors (Foley et al., 2011).
2. Material and methods
2.1. The study area
Enugu State is one of five states within the south-east geopolitical zone of Nigeria and lies inside the tropical rainforest zone. Enugu State is at approximately 223 m (732 ft) above sea level; the soil is well depleted amid its rainy seasons. The mean temperature in Enugu State within the hottest month of February is around 87.16 °F (30.64 °C), and the lowest temperatures typically occur within the month of November, at 60.54 °F (15.86 °C). Precipitation is highest in July (35.7 cu cm [2.18 cu in]), and lowest in February (0.16 cu cm [0.0098 cu in]).
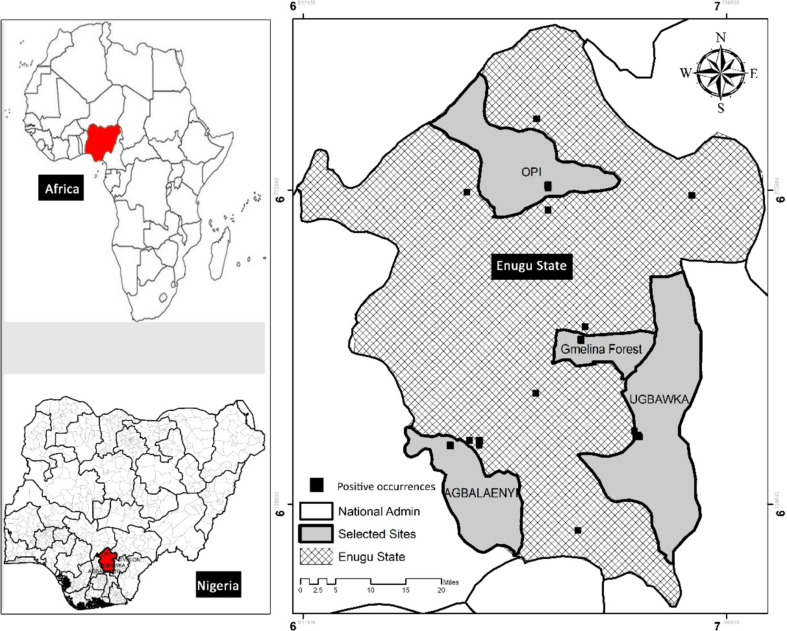
Study sites: Four sites (Ugbawka, Agbalenyi, Gmelina Forest and Opi) were selected for collecting adult Aedes spp. according to the three senatorial zones of the state (Fig. 1).
Fig. 1.
Enugu State map presenting the selected adult mosquito surveillance sites and the positive occurrences of Aedes spp.
Enugu East Senatorial Zone: Ugbawka, in Nkanu West local government area (LGA), is located on Latitude 6.2761 and Longitude 7.5936. It is approximately 25 km (16 miles) away from Enugu metropolis. Gmelina Forest, in Enugu North LGA, is located on Latitude 6.4660 and Longitude 7.4872.
Enugu West Senatorial Zone: Agbalenyi, in Oji River LGA, is located on the coordinates within plus/minus 10Km from Latitude 6.2677 and Longitude 7.2828.
Enugu North Senatorial Zone: Opi, in Nsukka LGA, has coordinates of Latitude 6.7777 and Longitude 7.4210.
The four communities selected (Ugbawka, Agbalenyi, Gmelina Forest and Opi) were largely agrarian, growing varieties of both food and cash crops, and determined to be favourable mosquito breeding habitats. Five houses in each community were randomly selected for trap placement. This was done to adequately cover the community, ensuring that the mosquito population was fairly represented.
2.2. Mosquito sampling and identification
Adult mosquitoes were collected during the period between November 2017 and September 2018 for seven consecutive days every other month. The same set of houses were used for the two periods of sampling (0600–1800 and 1800–0600) in each site. The Biogents® Sentinel Traps (BGS) baited with a lure were used from 0600 to 1800, after which they were removed and replaced with the Centers for Disease Control and Prevention (CDC) Ultra Violet (UV) traps baited with lure and CO2 which lasted from 1800 to 0600. In each period of sampling, one trap was set around one house. The trapped mosquitoes were retrieved and transported to National Arbovirus and Vectors Research Centre (NAVRC), Enugu Laboratory for identification and processing. All collected Aedes adult mosquitoes were identified to species level using the morphological keys of Rueda (2004). Aedes mosquito larvae were also collected from potential breeding sites in Enugu State, on-site identification of Aedes larvae was made and the corresponding geographical coordinate was recorded as positive or negative for Aedes larvae.
2.3. Ecological Niche Modeling (ENM)
2.3.1. Environmental variables
To determine the possible distribution of Aedes spp.in Enugu State using the prediction model, a total of 59 positive occurrences for Aedes spp. were recorded from the field study (including Aedes adult and larvae). The autocorrelation problems were addressed by eliminating redundant presences on the scale of the bioclimatic variables used in each 1 × 1 km grid (de Luis et al., 2018). In addition, records for spatial autocorrelation were screened in ArcGIS 10.4.1 using average nearest neighbor analyses to remove spatially correlated data points (Bosso et al., 2016; Smeraldo et al., 2018). After this selection, a total of 18 Aedes positive occurrence points were used to create a prediction model. We considered twenty-three environmental variables (19 bioclimatic and 4 topographic) as potential predictors of the target species habitat distribution (Khafagi et al., 2011, Khafagi et al., 2013). These variables were chosen based on their biological relevance to the target species distributions and other habitat modeling studies (Kumar et al., 2006; Guisan et al., 2007; Pearson et al., 2007). Nineteen bioclimatic variables, biologically more meaningful to define eco-physiological tolerances of a species (Graham and Hijmans, 2006; Murienne et al., 2009), with a 30 arc-second spatial resolution (about. 1 km2) were downloaded from the WorldClim database (http://www.worldclim.org/) (Hijmans et al., 2005). Elevation data 1 km2-resolution was obtained from the Shuttle Radar Topography Mission (SRTM). The elevation data was used to generate slope, aspect, and hillshade (all in degrees) using the Spatial Analyst tool/surface in using ArcGIS 10.4.1 software. Then we utilized the standard geographic coordinates in decimal degrees (to five decimal places) in WGS 84. Then we spatialized and checked the geographic coordinates on Google Earth. After downloading the climatic files (covering the period 1950–2000), the Nigeria layer was extracted by using a boundary mask. After that, extracted files were converted to ASCII format via using ArcGIS 10.4.1 software to be use later with Maxent software.
All combinations of the 23 environmental variables have been tested for multi-collinearity through the calculation of R-squared in linear regression analysis in SPSS ver. 25. In this study, because topographic, and bioclimatic variables were strongly correlated (R2 ≥ 0.7), only those variables that showed little correlation with other predictors were retained; following Kalle et al. (2013), and Omar and Elgamal (2021). A total of 13 environmental variables were selected in this study (R2 < 0.7); elevation, slope, aspect, hillshade, mean diurnal range (max. temp – min. temp) (bio2), temperature seasonality (SD × 100) (bio 4), max temperature of warmest month (bio5), min temperature of coldest month (bio 6), mean temperature of wettest quarter (bio 8), precipitation of wettest month (bio13), Precipitation seasonality (Coefficient of variation) (bio 15), precipitation of driest quarter (bio17), and precipitation of coldest quarter (bio19) (Table 1).
Table 1.
Environmental variables used for modeling the potential distribution of Aedes spp. in the present study.
| No | Variable | Code/Unit | Source |
|---|---|---|---|
| 1 | Elevation | Elev (m) | WorldClim |
| 2 | Slope | SL (%) | Derived from DEM |
| 3 | Aspect | AS (degrees) | Derived from DEM |
| 4 | Hill Shad | Degrees | Derived from DEM |
| 5 | Mean diurnal range (max. temp – min. temp) | Bio2 (°C) | WorldClim |
| 6 | Temperature seasonality (SD × 100) | Bio4 (°C) | WorldClim |
| 7 | Max temperature of warmest month | Bio5 (°C) | WorldClim |
| 8 | Min temperature of coldest month | Bio6 (°C) | WorldClim |
| 9 | Mean temperature of wettest quarter | Bio8 (°C) | WorldClim |
| 10 | Precipitation of wettest month | Bio13 (mm) | WorldClim |
| 11 | Precipitation seasonality (Coefficient of variation) | Bio15 | WorldClim |
| 12 | Precipitation of driest quarter | Bio17 (mm) | WorldClim |
| 13 | Precipitation of coldest quarter | Bio19 (mm) | WorldClim |
2.3.2. Modeling procedure
The modeling technique maximum entropy distribution or Maxent were used in this study; which has been found to be the most effective among several different modeling methods (Elith et al., 2006; Ortega-Huerta and Peterson, 2008; Khafagi et al., 2013), and may continue to be effective even with small sample sizes (Hernandez et al., 2006; Papes and Gaubert, 2007; Pearson et al., 2007; Wisz et al., 2008; Khafagi et al., 2011). For the study area, it only requires species presence data (not absence) and environmental variable (continuous or categorical) layers. We used the freely available Maxent software, version 3.3.3, which generates an estimate of the probability of the presence of the species that varies from 0 “unsuitable” to 0.99 “best habitat suitability”. ASCII files of the 13 selected environmental variables and a CSV file of species presence coordinates in decimal degrees were used to create the module. Maxent's performance was assessed using a threshold independent Receiver-Operating Characteristic (ROC) analysis and Area Under Receiver-Operating Characteristic Curve (AUC) values (0.5 = random to 1 = perfect discrimination). The algorithm either runs 1000 iterations of these processes or continues until convergence is reached (threshold 0.00001).
For the model, the relative importance of each environmental predictor was evaluated using the percentage contribution of the Jackknife test, which is the best index for small sample sizes according to Pearson et al. (2007). The default logistic output format was chosen, i.e. related to the probability of suitable conditions, ranging from 0 to 1. A total of 75% of the location point data were used for training, and the remaining 25% to test the predictive ability of the model, in addition 10 replicates were considered. Average and Standard deviation values for training and test AUC for the 10 models were extracted from the Maxent text result output. The ASCII output map for the average model for the target species was loaded in ArcGIS 10.4.1 where the prediction models of habitat suitability were divided based on Choudhury et al. (2016) into 4 classes; ≤0.10 very low to unsuitable, 0.11–0.30 Low Probability, 0.31–0.70 Moderate Probability, and ≥0.71 High Probability for the presence of the species.
3. Results
3.1. Mosquito sampling and identification
Between November 2017 and September 2018, a total number of 2997 Aedes adult mosquitoes were collected and identified from the four sites in Enugu State, south-east Nigeria. Also, potential mosquito breeding sites were checked for the presence or absence of Aedes larvae. Out of 92 sites checked, 59 coordinates were positive for Aedes adults and larvae. Aedes larvae were found mainly in used tyres, plastic containers and earthen pots for cooking.
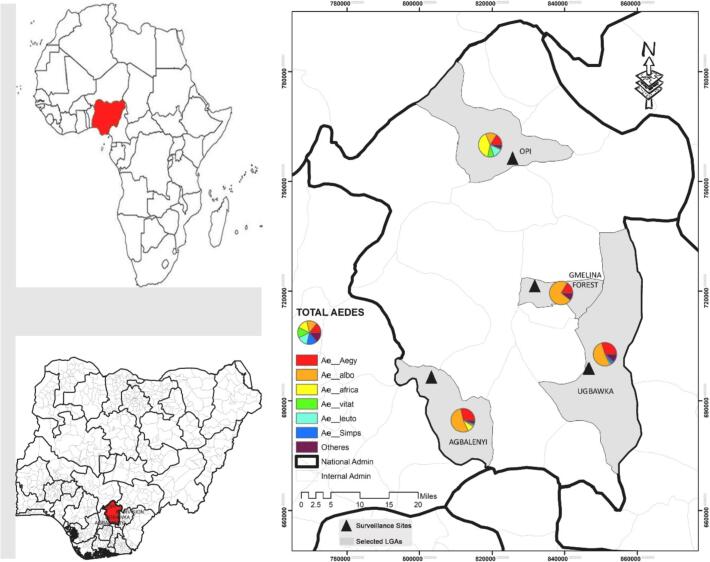
Eight Aedes spp. were recorded; Ae. albopictus (51.6%), Ae. aegypti (22.3%), Ae. africanus (15.8%), Ae. luteocephalus (7.7%), Ae. simpsoni (1.6%), Ae. vittatus (0.5%), Ae. circumluteolus (0.5%) and Ae. longipalpis (0.03%) (Table 2, Fig. 2). Three of the eight Aedes species recorded (Ae. aegypti, Ae. albopictus and Ae. simpsoni) were found in all surveyed sites in both dry and rainy seasons, while Ae. africanus was collected from Agbalenyi and Opi only (Table 2).
Table 2.
Aedes spp. distributed within the four communities in Enugu State, Nigeria.
| Species/Study area | Gmelina | Ugbawka | Agbalenyi | Opi | Total |
|---|---|---|---|---|---|
| Ae. albopictus | 759 | 216 | 425 | 147 | 1547 |
| Ae. aegypti | 205 | 122 | 189 | 152 | 668 |
| Ae. africanus | 0 | 2 | 71 | 400 | 473 |
| Ae. simpsoni | 7 | 18 | 12 | 10 | 47 |
| Ae. luteocephalus | 0 | 13 | 13 | 205 | 231 |
| Ae. vittatus | 0 | 1 | 0 | 15 | 16 |
| Ae. circumluteolus | 0 | 10 | 2 | 2 | 14 |
| Ae. longipalpis | 0 | 0 | 0 | 1 | 1 |
Fig. 2.
Spatial distribution of adult Aedes spp. collected from 4 sites in Enugu State during November 2017 to September 2018.
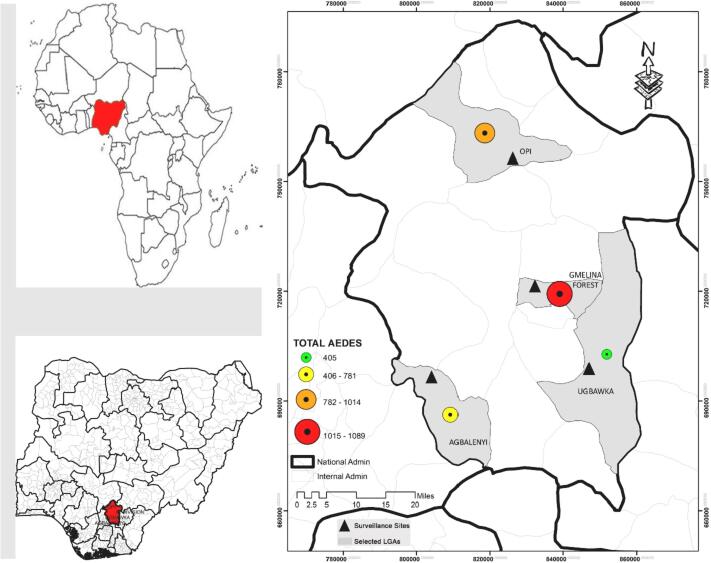
Fig. 3 shows the total number of Aedes mosquitoes collected across the four sites. Gmelina forest recorded the highest number which represented 32.4% (n = 971) of the overall Aedes collected followed by Opi 31.1% (n = 932), and then Agbalenyi 23.8% (n = 712) and lastly Ugbakwa 12.7% (n = 382).
Fig. 3.
Comparison of the total number of Aedes mosquitoes collected from the 4 study areas.
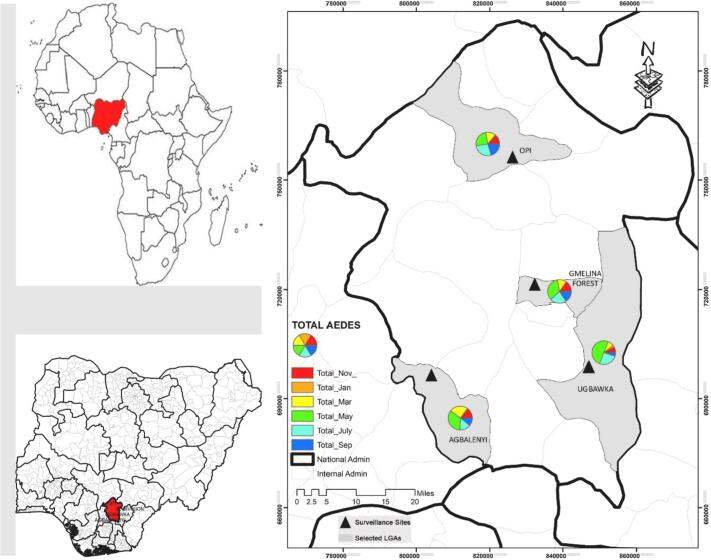
In the area investigated by the current study, Aedes species were present year-round. However, seasonal variation markedly affected the number of collected Aedes mosquitoes with the highest number collected in May 33.1% (n = 992) and July 26.6% (n = 798) (rainy season starts April through July 2018). However, during September, November, January and March, Aedes mosquitoes were collected in low numbers 17.2% (n = 514), 6.3% (n = 190), 0.9% (n = 27) and 15.9% (n = 476), respectively (Fig. 4).
Fig. 4.
Comparison of the number of Aedes spp. collected in different months.
3.2. Potential habitat distribution
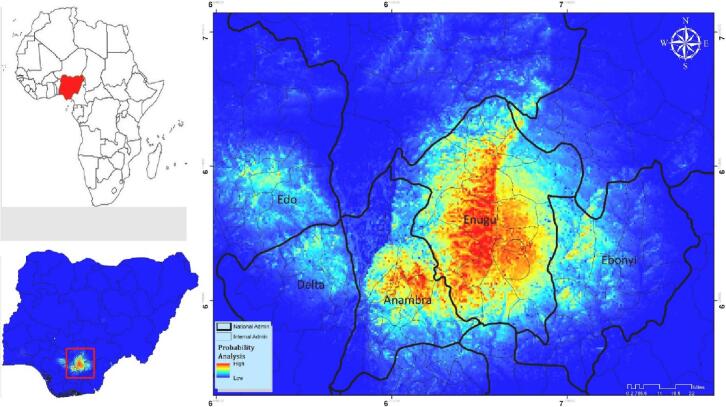
The MaxEnt model predicted potential suitable habitat with high success rates, where training AUC 0.98 ± 0.001 and test AUC 0.98 ± 0.007 at Lowest Presence Threshold (LPT). The most suitable habitat for Aedes spp. was predicted in the northern, central, southwestern parts of the Enugu State with some extensions in Anambra, Delta, and Edo States from the west and Ebonyi State from the east (Fig. 5), and its distribution is quite fragmented.
Fig. 5.
Potential geographical distribution of Aedes spp. in Enugu state using MaxEnt. Warmer colors show areas with better habitat suitability.
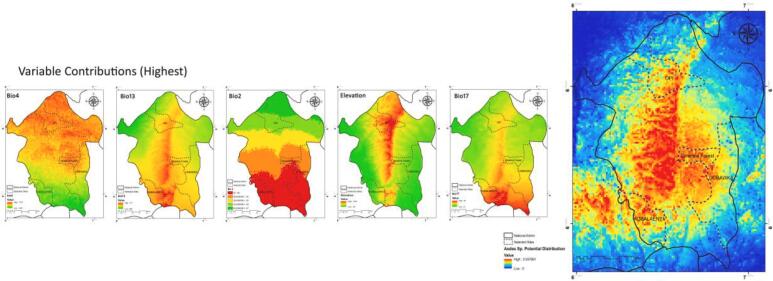
The MaxEnt model's internal jackknife test of variables' contribution showed that temperature seasonality (STD * 100) (Bio4) (24.5%), precipitation of wettest month (Bio13) (20.1%), mean monthly temperature range (Bio2) (18.7%), Elevation (16.7%), and precipitation of driest quarter (Bio17) (8.8%) are the highest mean contributions that determine the distribution of Aedes spp. in Enugu state (Table 3).
Table 3.
Contributions, importance gain of the environmental variables used in MaxEnt modeling of Aedes spp. in Enugu state.
| Variables | Contribution | Training gain (Importance) |
|---|---|---|
| bio2 | 18.799 | 0.543 |
| bio4 | 24.530 | 0.591 |
| bio5 | 0.120 | 0.119 |
| bio6 | 3.529 | 0.004 |
| bio8 | 0.834 | 0.128 |
| bio13 | 20.172 | 1.139 |
| bio15 | 1.163 | 0.587 |
| bio17 | 8.887 | 0.962 |
| bio19 | 1.202 | 0.948 |
| Elevation | 16.718 | 0.226 |
| aspect | 0.811 | 0.041 |
| hill_shad | 2.433 | 0.047 |
| slope | 0.779 | 0.208 |
Variables with the highest importance gain (>0.85) were: Precipitation of the wettest month (Bio13), precipitation of the driest quarter (Bio17), and precipitation of coldest quarter (Bio19) (Table 3). Table 3 provides an indicative estimate of the relative contributions and training acquisition of environmental variables in the MaxEnt model. To determine and quantify that estimate to the contribution of the corresponding variable, the increment of uniform gain is added to each iteration of the training algorithm.
Fig. 6 shows the main highest estimated environmental variables (contributions) that affect the distribution of Aedes spp. in Enugu State and area around it. Spatial distribution analysis was done to determine the geographical variability in the mentioned variables among Enugu State.
Fig. 6.
The Highest environmental variables that estimate to control the geographical distribution of Aedes spp. in Enugu State. Variable contributions (Bio4, 13, 2, Elevation, and Bio17).
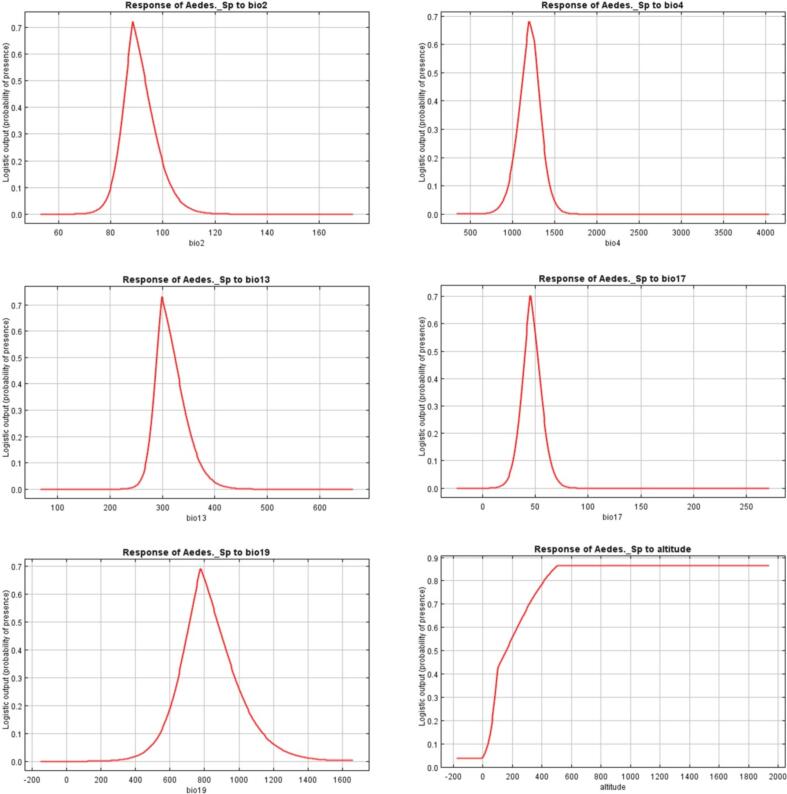
The response curves of eight variables to Aedes spp. habitat suitability are shown in (Fig. 7). From our model we find that precipitation is one of the most important environmental variables controlling the distribution of Aedes spp. in Enugu State. We found that precipitation of wettest month (Bio13) ranged from 265 to 330 mm, precipitation of driest quarter (Bio17) ranged from 25 to 75 mm while precipitation of wettest quarter (Bio16) ranged from 650 to 950 mm. Annual precipitation (Bio12) ranged from 1250 to 2300 mm, and elevation ranged from 100 to 1800 m.
Fig. 7.
Response curves of eight environmental predictors used in MaxEnt model for Aedes spp.
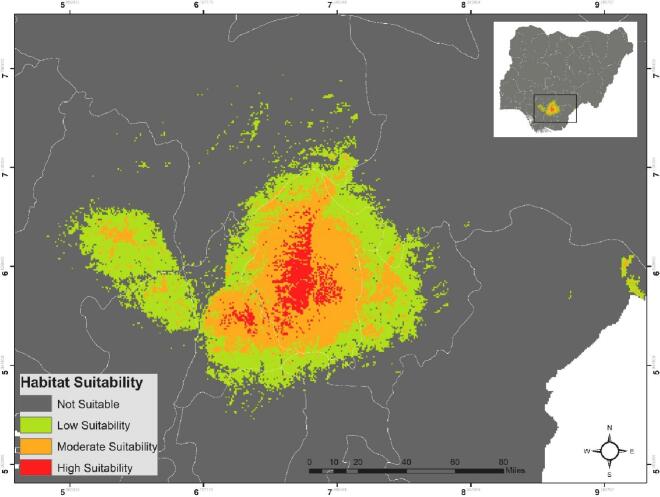
The prediction model of habitat suitability was divided into 4 classes; ≥0.71 High Suitability, 0.70–0.31 Moderate Suitability, and 0.30–0.11 Low Suitability, and ≤0.10 very low to unsuitable for the presence of the species (Fig. 8). It was recorded that Aedes spp. potential distribution cover an area of 16,420 km2. This area divided as; 1011 km2 high probability (≥0.71), 6119 km2 moderate probability (0.70–0.31), and 9289 km2 low probability (0.30–0.11); and 890,495 km2 recorded as unsuitable.
Fig. 8.
Map for potential current habitat suitability of Aedes spp. according to occurrence records in Enugu State, Nigeria. Habitat suitability classes include: not suitable (0–0.1), low suitability (0.11–0.30), moderate suitability (0.31–0.70) and high suitability (0.71–1.0).
4. Discussion
In West Africa, lack of recent Aedes potential distributional modeling hinders effective vector control interventions and poses a serious public health issue. This kind of information is incredibly important to public health. To support these efforts, this study was conducted in Enugu State, Nigeria. During the current study, Aedes mosquitoes (adults and larvae) were collected in close proximity to human habitation, and they were found to breed in transient water collections, including tree holes, rock pools, vehicle tires, and water storage containers.
Three of the eight Aedes species recorded (Ae. aegypti, Ae. albopictus and Ae. simpsoni) were found in all surveyed sites in both dry and rainy seasons, while Ae. africanus was collected from Agbalenyi and Opi only. All recorded Aedes spp. with the exception of the Ae. circumluteolus and Ae. longipalpis are known to transmit viruses of public health concerns.
Aedes. albopictus was the most abundant Aedes spp. collected throughout the study. This agrees with the findings of Chukwuekezie et al., 2018 who reported that Ae. albopictus was the dominant species from their collections. This result was predicted since it had been described as one of the most invasive species based on their global spread (Bonizzoni et al., 2013).
A. aegypti was the second most abundant Aedes species in all collections. During the study, it was demonstrated that Ae. aegypti and Ae. albopictus shared many of the same biological and behavioral characteristics; both were found to compete with each other for surrounding resources, which supports the findings of Richards et al. (2012). This study, consistent with the findings of Fatima et al. (2016), demonstrated that the distribution of Ae. aegypti is highly correlated with the presence and proximity of urban infrastructure and proximity to humans.
The discovery of Ae. bromeliae, which is part of Ae. simpsoni complex, as a vector for the largest outbreak YF virus registered in Ethiopia in 1961–1962 (Sérié et al., 1964), and in laboratory studies, is a major vector for the newly emergent East or Central African viral genotype (Ellis et al., 2012).
The low capture numbers of Aedes africanus in this study was anticipated as this species is expected to be more prominent in tropical rain forest zones as Delta and Abia states than in Enugu state.
Aedes vittatus has been found only in Opi, and mainly in rainy season. This agrees with the findings of Diallo et al. (2011) who recorded Ae. vittatus in rural areas in Africa and Diallo et al. (2012) who reported its presence mainly from June to October in village land covers. Little importance has been given to mosquitoes as vectors, despite the retention of bio-retroviruses, specifically dengue, chikungunya, YF and Zika infections (Sudeep and Shil, 2017).
The MaxEnt-created model for Aedes spp. highlighted the northern, central, southwestern parts of the Enugu state as the most suitable habitats for Aedes spp. Also noteworthy was the eastern zone (Agbalenyi) that was not predicted to be suitable. This is in agreement with the low numbers of Aedes spp. collected in this area during the current study compared to the rest of the state.
As predicted, Aedes mosquito occurrence showed a positive association with variables reflecting precipitation. This finding was also supported by Richman et al. (2018) who reported similar findings while mapping Aedes mosquito vectors of chikungunya virus in south-east Senegal. Relative humidity is always high if rainfall is high. The current study supports previous studies reporting a positive correlation of Aedes larval population with the amount of rainfall and relative humidity (Goncalves Neto and Rebelo, 2004; Ratho et al., 2005; Zeicler et al., 2008). Mouchet et al. (1996) reported that rainfall can promote transmission by creating breeding sites therefore, adults Aedes can lay eggs and increase their population size. Also, survival and growth of mosquito is facilitated when the relative humidity is high (Nakhapakorn and Tripathi, 2005).
Elevation showed a great effect on the distribution of Aedes spp. as the response curve showed that the probability of finding Aedes spp. increases with increased elevation (Fig. 7). Many studies have acknowledged the effect of elevation on the distribution of Aedes species. Brady et al. (2014) concluded that elevation is a potential intermediary for Ae. aegypti extent as it could be more promptly comprehended and operationalized than time-subordinate meteorological elements. Elevation is a possible alternative to interpretation of Ae. aegypti range because it can be understood, interpreted and measured more easily than time-dependent meteorological factors. Elevation is an alternative clear environmental factor to elucidating the Ae. aegypti range because it is associated with a variety of other essential dynamic environmental factors critical to mosquito development such as temperature. Coblentz and Riitters (2004), and Stein et al. (2014) concluded that the major environmental predictor of species wealth is topographical heterogeneity, including elevation, aspect, and slope. As the topography and complexity are increased, the environmental heterogeneity is expected to increase. The high complexity of topography probably leads to a high diversity of habitats and therefore to a great local niche (Scherrer and Körner, 2011).
Although the prediction model showed high success rates, there are some limitations, where the situation was evaluated based on presence data only in addition to relying exclusively on climatic and topographic data only, while Hamlet et al. (2021) mentioned that there are other factors that may be responsible for the YF virus spread, including the presence of agricultural areas and other variables.
Currently, the World Health Organization utilizes elevation as a factor in advising travelers on the risks of acquiring YF virus, pardoning the prescribed immunization for explorers whose agendas are constrained to territories above 2300 m in some African and South American areas (Shlim et al., 2010; Jentes et al., 2011).
5. Conclusions
In this study we demonstrated predictive models for habitat distribution patterns for Aedes spp. using a limited number of records and natural factors using Maxent. This study provides the first predictive habitat distribution map for these species in Enugu State. Pearson (2007) and Murienne et al. (2009) previously concluded that Maxent map can predict base niche (different from the actual occupied niche) of species by using appropriate climate and topographic variables of Aedes spp. Our distribution map of potential habitats for arboviral vectors benefits health protection planning and land-use management around Enugu State's current populations. It also supports discovering new potential disease clusters, prioritizing vector survey sites, habitat restoration efforts, and effectively utilize vector control resources. Our results support Enugu State's public health efforts by providing data that will aid in developing mitigation strategies to contain and prevent vector borne disease outbreaks before they spread. Further research is needed to determine whether the existing study area adequately covers all suitable habitats for these species. Area-wide, species-focused studies will provide granularity to data and help reveal additional geographical variation among species based on topography, ecology, climate, and social aspects.
Declaration of Competing Interest
All authors declare that there is no conflict of interest.
Acknowledgments
The authors are grateful to Country Director of the U.S. Army Medical Research Directorate Africa/Nigeria (USAMRD-A/N), Walter Reed Army Institute of Research and the team for their support and assistance in study execution. The authors are military service members or employees of the U.S. Government. This work was prepared as part of their official duties. Title 17, U.S·C. §105 provides that copyright protection under this title is not available for any work of the U.S. Government. Title 17, U.S·C. §101 defines a U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person's official duties. This work was supported by Armed Forces Health Surveillance Division, Global Emerging Infections Surveillance (GEIS) Branch, ProMIS ID P0021_17_N3 for FY17. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
References
- Araújo M.B., Pearson R.G., Thuiller W., Erhard M. Validation of species-climate impact models under climate change. Glob. Chang. Biol. 2005;11:1504–1513. [Google Scholar]
- Bonizzoni M., Gasperi G., Chen X.G., James A.A. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 2013;29(9):460–468. doi: 10.1016/j.pt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosso L., Di Febbraro M., Cristinzio G., Zoina A., Russo D. Shedding light on the effects of climate change on the potential distribution of Xylella fastidiosa in the Mediterranean basin. Biol. Invasions. 2016;18:1759–1768. [Google Scholar]
- Brady O.J., Golding N., Pigott D.M., Kraemer M.U.G., Messina J.P., Reiner R.C. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit. Vectors. 2014;7:338. doi: 10.1186/1756-3305-7-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury M.R., Deb P., Singha H., Chakdar B., Medhi M. Predicting the probable distribution and threat of invasive Mimosa diplotricha Suavalle and Mikania micrantha Kunth in a protected tropical grassland. Ecol. Eng. 2016;97:23–31. doi: 10.1016/j.ecoleng.2016.07.018. [DOI] [Google Scholar]
- Chukwuekezie O.C., Nwankwo A.C., Nwosu E.O. Diversity and distribution of Aedes mosquitoes in Nigeria. New York Sci. J. 2018;11(2):50–57. doi: 10.7537/marsnys110218.07. [DOI] [Google Scholar]
- Coblentz D.D., Riitters K.H. Topographic controls on the regional-scale biodiversity of the South-Western USA. J. Biogeogr. 2004;31(7):1125–1138. [Google Scholar]
- Diallo D., Sall A.A., Diagne C.T., Faye O., Faye O., Hanley K.A., Buenemann M., Weaver S.C., Diallo M. Zika virus emergence in mosquitoes in southeastern Senegal. PLoS One. 2011;9(10) doi: 10.1371/journal.pone.0109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo D., Diagne C., Hanley K.A., Sall A.A., Buenemann M., Ba Y., Dia I., Weaver S.C., Diallo M. Larval ecology of mosquitoes in sylvatic arbovirus foci in southeastern Senegal. Parasit. Vectors. 2012;5:286. doi: 10.1186/1756-3305-5-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elith J., Leathwick J.R. Species distribution models: ecological explanation and prediction across space and time. Evol. Syst. 2009;40:677–697. [Google Scholar]
- Elith J., Graham C.H., Anderson R.P., Dudik M., Ferrier S., Guisan A., Hijmans R.J., Huettman F., Leathwick J.R., Lehmann A., Li J., Lohmann L.G., Loiselle B.A., Manion G., Moritz C., Nakamura M., Nakazawa Y., Overton J.M., Peterson A.T., Phillips S.J., Richardson K., Scachetti-Pereira R., Schapire R.E., Soberón J., Williams S.E., Wisz M.S., Zimmermann N.E. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- Ellis B.R., Sang R.C., Horne K.M., Higgs S., Wesson D.M. Yellow fever virus susceptibility of two mosquito vectors from Kenya, East Africa. Trans. R. Soc. Trop. Med. Hyg. 2012;106:387–389. doi: 10.1016/j.trstmh.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Escobar L.E., Romero-Alvarez D., Leon R., Lepe-Lopez M.A., Craft M.E., Borbor-Cordova M.J., Svenning J.C. Declining prevalence of disease vectors under climate change. Sci. Rep. 2016;6:39150. doi: 10.1038/srep39150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath B.D. Elsevier; 2018. Encyclopedia of Ecology. [Google Scholar]
- Fatima S.H., Atif S., Rasheed S.B., Zaidi F., Hussain E. Species distribution modelling of Aedes aegypti in two dengue-endemic regions of Pakistan. Tropical Med. Int. Health. 2016;21:427–436. doi: 10.1111/tmi.12664. [DOI] [PubMed] [Google Scholar]
- Foley D., Maloney F.A., Harrison F.J., Wilkerson R.C., Rueda L.M. Online spatial database of US Army public health command region-west mosquito surveillance records: 1947–2009. Army Med. Depart. J. July–September. 2011;2011:29–36. http://www.cs.amedd.army.mil/dasqaDocuments.aspx?type=l [PubMed] [Google Scholar]
- Goncalves Neto V.S., Rebelo J.M. Epidemiological characteristics of dengue in the Municipality of Sao Luis, Maranhao, Brazil, 1997–2002. Cad Saude Publica. 2004;20:1424–1431. doi: 10.1590/s0102-311x2004000500039. [DOI] [PubMed] [Google Scholar]
- Graham C.H., Hijmans R.J. A comparison of methods for mapping species ranges and species richness. Glob. Ecol. Biogeogr. 2006;15(6):578–587. [Google Scholar]
- Grinnell J. Field tests of theories concerning distributional control. Am. Nat. 1917;51:115–128. [Google Scholar]
- Grinnell J. Geography and evolution. Ecology. 1924;5:225–229. [Google Scholar]
- Guisan A., Hofer U. Predicting reptile distributions at the mesoscale: relation to climate and topography. J. Biogeogr. 2003;30:1233–1243. [Google Scholar]
- Guisan A., Graham C.H., Elith J., Huettmann F., The NCEAS Species Distribution Modelling Group Sensitivity of predictive species distribution models to change in grain size. Divers. Distrib. 2007;13:332–340. [Google Scholar]
- Hamlet A., Ramos D.G., Gaythorpe K.A., Romano A.P.M., Garske T., Ferguson N.M. Seasonality of agricultural exposure as an important predictor of seasonal yellow fever spillover in Brazil. Nat. Commun. 2021;12(1):1–11. doi: 10.1038/s41467-021-23926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez P.A., Graham C.H., Master L.L., Albert D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography. 2006;29:773–785. [Google Scholar]
- Hijmans R.J., Cameron S.E., Parra J.L., Jones P.G., Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. [Google Scholar]
- Huang Y.M. Aedes (Stegomyia) bromeliae (Diptera: Culicidae), the yellow fever virus vector in East Africa. J. Med. Entomol. 1986;23:196–200. doi: 10.1093/jmedent/23.2.196. [DOI] [PubMed] [Google Scholar]
- Jentes E.S., Poumerol G., Gershman M.D., Hill D.R., Lemarchand J., Lewis R.F., Staples J.E., Tomori O., Wilder-Smith A., T.P. Monath; informal WHO working group on geographic risk for yellow fever. 2011. The revised global yellow fever risk map and recommendations for vaccination: consensus of the informal WHO working group on geographic risk for yellow fever. Lancet Infect. Dis. 2011;11:622–632. doi: 10.1016/S1473-3099(11)70147-5. [DOI] [PubMed] [Google Scholar]
- Jiménez-Valverde A., Peterson A.T., Soberón J., Overton J.M., Aragón P., Lobo J.M. Use of niche models in invasive species risk assessments. Biol. Invasions. 2011;13:2785–2797. [Google Scholar]
- Kalle R., Ramesh T., Qureshi Q., Sankar K. Predicting the distribution pattern of small carnivores in response to environmental factors in the Western Ghats. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0079295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khafagi O., Hatab E.E., Omar K. Predicting the potential geographical distribution of Nepeta septemcrenata in Saint Katherine Protectorate, South Sinai, Egypt using Maxent. Academia Arena. 2011;3(7):45–50. [Google Scholar]
- Khafagi O., Hatab E.E., Omar K. Ecological niche modeling as a tool for conservation planning: suitable habitat for Hypericum sinaicum in South Sinai, Egypt. Univers J Environ Res Technol. 2013;2(6):515–524. [Google Scholar]
- Kraemer M.U.G., Sinka M.E., Duda K.A., Mylne A.Q., Shearer F.M., Barker C.M., Moore C.G., Carvalho R.G., Coelho G.E., Van Bortel W., Hendrickx G., Schaffner F., Elyazar I.R., Teng H.J., Brady O.J., Messina J.P., Pigott D.M., Scott T.W., Smith D.L., Wint G.R., Golding N., Hay S.I. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife. 2015;4 doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stohlgren T.J., Chong G.W. Spatial heterogeneity influences native and nonnative plant species richness. Ecol. 2006;87:3186–3199. doi: 10.1890/0012-9658(2006)87[3186:shinan]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- de Luis M., Bartolomé C., Cardo Ó.G., Álvarez-Jiménez J. Gypsophila bermejoi G. López: a possible case of speciation repressed by bioclimatic factors. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchet J., Faye O., Juivez J., Manguin S. Drought and malaria retreat in the Sahel, West Africa. Lancet. 1996;348:1735–1736. doi: 10.1016/s0140-6736(05)65860-6. [DOI] [PubMed] [Google Scholar]
- Murienne J., Guilbert E., Grandcolas P. Species’ diversity in the new Caledonian endemic genera Cephalidiosus and Nobarnus Insecta: Heteroptera: Tingidae, an approach using phylogeny and species’ distribution modelling. Bot. J. Linn. Soc. 2009;97:177–184. [Google Scholar]
- Nakhapakorn K., Tripathi N.K. An information value based analysis of physical and climatic factors affecting dengue fever and dengue haemorrhagic fever incidence. Int. J. Health Geogr. 2005;4(13):1–11. doi: 10.1186/1476-072X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar K., Elgamal I. Can we save critically endangered relict endemic plant species? A case study of Primula boveana Decne ex Duby in Egypt. J. Nat. Conserv. 2021;61:126005. [Google Scholar]
- Ortega-Huerta M.A., Peterson A.T. Modeling ecological niches and predicting geographic distributions: a test of six presence-only methods. Revista Mexicana De Biodiversidad. 2008;79:205–216. [Google Scholar]
- Papes M., Gaubert P. Modelling ecological niches from low numbers of occurrences: assessment of the conservation status of poorly known viverrids (Mammalia, Carnivora) across two continents. Divers. Distrib. 2007;13:890–902. [Google Scholar]
- Pearson R.G. Synthesis; American Museum of Natural History: 2007. Species’ Distribution Modeling for Conservation Educators and Practitioners.http://ncep.amnh.org Available at. [Google Scholar]
- Pearson R.G., Raxworthy C.J., Nakamura M., Peterson A.T. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J. Biogeogr. 2007;34:102–117. [Google Scholar]
- Peterson A.T., Martínez-Campos C., Nakazawa Y., Martínez-Meyer E. Time-specific ecological niche modeling predicts spatial dynamics of vector insects and human dengue cases. Trans. R. Soc. Trop. Med. Hyg. 2005;99(9):647–655. doi: 10.1016/j.trstmh.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Pfeffer M., Dobler G. Emergence of zoonotic arboviruses by animal trade and migration. Parasit. Vectors. 2010;3(1):35. doi: 10.1186/1756-3305-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratho R.K., Mishra B., Kaur J., Kakkar N., Sharma K. An outbreak of dengue fever in peri urban slums of Chandigarh, India with social reference to entomological and climatic factors. Indian J. Med. Sci. 2005;59(12):519–527. [PubMed] [Google Scholar]
- Richards S.L., Anderson S.L., Alto B.W. Vector competence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) for dengue virus in Florida Keys. J. Med. Entomol. 2012;48:942–946. doi: 10.1603/me11293. [DOI] [PubMed] [Google Scholar]
- Richman R., Diallo D., Diallo M., Sall A.A., Faye O., Diagne C.T., Dia I., Weaver S.C., Hanley K.A., Buenemann M. Ecological niche modeling of Aedes mosquito vectors of chikungunya virus in southeastern Senegal. Parasit. Vectors. 2018;1(1):255. doi: 10.1186/s13071-018-2832-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda Leopoldo. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with Dengue Virus Transmission. Zootaxa. 2004;589 doi: 10.11646/zootaxa.589.1.1. [DOI] [Google Scholar]
- Sall A.A., Faye O., Diallo M., Firth C., Kitchen A., Holmes E.C. Yellow fever virus exhibits slower evolutionary dynamics than dengue virus. J. Virol. 2010;84(2):765–772. doi: 10.1128/JVI.01738-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer D., Körner C. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J. Biogeogr. 2011;38(2):406–416. doi: 10.1111/j.1365-2699.2010.02407.x. [DOI] [Google Scholar]
- Sérié C., Andral L., Lindrec A., Neri P. Epidémie de fièvre jaune en Ethiopie (1960–1962): observations préliminaires. Bull. World Health Organ. 1964;30(3):299. [PMC free article] [PubMed] [Google Scholar]
- Shlim D.R., Freedman D.O., Keystone J.S., Tan K.R., Kozarsky P.E., Borwein S.T. In: CDC Health Information for International Travel. Brunette Gary W., editor. vol. 2009. Oxford University Press; New York: 2010. CDC health information for international travel 2010; pp. 242–269. [Google Scholar]
- Smeraldo S., Di Febbraro M., Bosso L., Flaquer C., Guixé D., Lisón F., Meschede A., Juste J., Prüger J., Puig-Montserrat X., Russo D. Ignoring seasonal changes in the ecological niche of non-migratory species may lead to biases in potential distribution models: lessons from bats. Biodivers. Conserv. 2018;27:2425–2441. [Google Scholar]
- Stein A., Gerstner K., Kreft H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014;17(7):866–880. doi: 10.1111/ele.12277. [DOI] [PubMed] [Google Scholar]
- Sudeep A.B., Shil P. Aedes vittatus (Bigot) mosquito: an emerging threat to public health. J. Vector Borne Dis. 2017;54(4):295–300. doi: 10.4103/0972-9062.225833. [DOI] [PubMed] [Google Scholar]
- Vasilakis N., Cardosa J., Hanley K.A., Holmes E.C., Weaver S.C. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat. Rev. Microbiol. 2011;9(7):532–541. doi: 10.1038/nrmicro2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S.C., Reisen W.K. Present future arboviral threats. Antivir. Res. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman D., Kamgang B., Badolo A., Moyes C.L., Shearer F.M., Coulibaly M., Pinto J., Lambrechts L., McCall P.J. Aedes mosquitoes and Aedes-borne arboviruses in Africa: current and future threats. Int. J. Environ. Res. Public Health. 2018;15(2):220. doi: 10.3390/ijerph15020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A., Gubler D.J., Weaver S.C., Monath T.P., Heymann D.L., Scott T.W. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect. Dis. 2017;17:e101–e106. doi: 10.1016/S1473-3099(16)30518-7. [DOI] [PubMed] [Google Scholar]
- Wintle B.A., Elith J., Potts J.M. Fauna habitat modelling and mapping: a review and case study in the Lower Hunter Central Coast region of NSW. Aust. Ecol. 2005;30:719–738. [Google Scholar]
- Wisz M.S., Hijmans R.J., Li J., Peterson A.T., Graham C.H., Guisan A., NCEAS Predicting Species Distributions Working Group Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008;14:763–773. [Google Scholar]
- Zeicler J.D., Acosta P.O.A., Barreto P.P., Cordeiro J.D.S. Dengue virus in Aedes aegypti larvae and infestation dynamics in Roraima, Brazil. Rev. Saude Publica. 2008;42(6):9–14. doi: 10.1590/s0034-89102008005000055. [DOI] [PubMed] [Google Scholar]