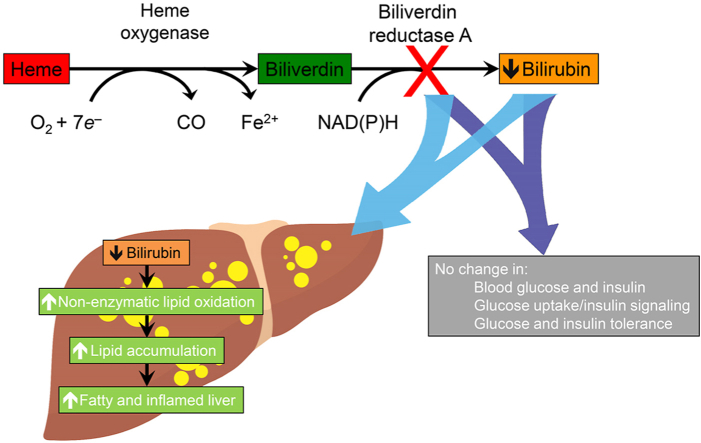
Abstract
Background & aims
Plasma concentrations of bilirubin, a product of heme catabolism formed by biliverdin reductase A (BVRA), inversely associate with the risk of metabolic diseases including hepatic steatosis and diabetes mellitus in humans. Bilirubin has antioxidant and anti-inflammatory activities and may also regulate insulin signaling and peroxisome proliferator-activated receptor alpha (PPARα) activity. However, a causal link between bilirubin and metabolic diseases remains to be established. Here, we used the global Bvra gene knockout (Bvra–/–) mouse as a model of deficiency in bilirubin to assess its role in metabolic diseases.
Approach & results
We fed mice fat-rich diets to induce hepatic steatosis and insulin resistance. Bile pigments were measured by LC-MS/MS, and hepatic lipids by LC-MS/MS (non-targeted lipidomics), HPLC-UV and Oil-Red-O staining. Oxidative stress was evaluated measuring F2-isoprostanes by GC-MS. Glucose metabolism and insulin sensitivity were verified by glucose and insulin tolerance tests, ex vivo and in vivo glucose uptake, and Western blotting for insulin signaling. Compared with wild type littermates, Bvra–/– mice contained negligible bilirubin in plasma and liver, and they had comparable glucose metabolism and insulin sensitivity. However, Bvra–/– mice exhibited an inflamed and fatty liver phenotype, accompanied by hepatic accumulation of oxidized triacylglycerols and F2-isoprostanes, in association with depletion of α-tocopherol. α-Tocopherol supplementation reversed the hepatic phenotype and observed biochemical changes in Bvra–/– mice.
Conclusions
Our data suggests that BVRA deficiency renders mice susceptible to oxidative stress-induced hepatic steatosis in the absence of insulin resistance.
Keywords: Bilirubin, Insulin signaling, Lipid oxidation, F2-isoprostanes, Vitamin E
Graphical abstract
Highlights
-
•
Low plasma levels of bilirubin associate with increased metabolic disease risk.
-
•
A direct link between bilirubin and metabolic disease remains to be established.
-
•
Global BVRA deficiency causes global bilirubin deficiency and a fatty, inflamed liver.
-
•
This hepatic phenotype is linked to decreased vitamin E and increased lipid oxidation.
-
•
Vitamin E supplements restore normal liver phenotype in BVRA deficiency.
List of abbreviations:
- BOXes
bilirubin/biliverdin oxidation
- BVRA
biliverdin reductase A
- Bvra–/– mouse
global Bvra gene knockout mouse
- CE
cholesterylesters
- DB
double bonds
- GLUT4
glucose transporter type 4
- GTT
glucose tolerance tests
- HF
high fat diet
- HFHS
high fat high sucrose diet
- ITT
insulin tolerance tests
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- PPARα
peroxisome proliferator-activated receptor alpha
- TAG
triacylglycerides
- α-TOH
α-tocopherol
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) has an estimated worldwide prevalence of 25% [1] and is associated with features of metabolic syndrome that confer increased risk of cardiovascular and metabolic diseases [2,3]. NAFLD is characterized by hepatic lipid accumulation and its progression leads to hepatic steatosis (fatty liver) to non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and hepatocellular carcinoma [4,5]. The mechanisms responsible for NAFLD remain unclear, although a commonly accepted hypothesis is that the disease progresses by multiple interrelated mechanisms, including insulin resistance, inflammation and oxidative stress [5]. It is conceivable that modifying these factors may attenuate the development and progression of NAFLD [6].
Moderate to high concentrations of plasma bilirubin, a product of biliverdin reductase A (BVRA)-dependent conversion of biliverdin generated by heme catabolism, are associated with low incidence of NAFLD [[7], [8], [9], [10]], insulin resistance and type 2 diabetes [[11], [12], [13], [14], [15]], suggesting that bilirubin may protect against hepatic steatosis and metabolic diseases. This could be achieved by the pigment's inhibition of lipid oxidation alone [16,17] or via interaction with α-tocopherol (α-TOH, the most active form of vitamin E) [18]. Consistent with such antioxidant activity, we recently reported mice with very low concentrations of endogenous bilirubin as a result of genetic Bvra-deficiency (Bvra–/–) to have elevated circulating concentrations of oxidized lipids [19]. Bilirubin also has anti-inflammatory properties ameliorating acute and chronic hepatic inflammation [[20], [21], [22]]. Finally, bilirubin may modulate hepatic metabolism of glucose and lipids via the glycogen synthase kinase/peroxisome proliferator-activated receptor-α and insulin receptor/PI3K/Akt signaling pathways, as suggested by experiments with liver-specific BVRA-deficient mice, in which plasma bilirubin concentrations were not shown to be regulated [[23], [24], [25], [26], [27], [28]].
Despite these multiple lines of evidence for bilirubin and/or BVRA protecting against hepatic steatosis, the underlying mechanism remains unclear. We hypothesized that bilirubin/BVRA protect against NAFLD by decreasing oxidative stress, inflammation, and insulin resistance. To test this hypothesis, we fed global Bvra–/– mice high fat (HF) or high fat high sucrose diet (HFHS) to induce hepatic steatosis and insulin resistance. We observed Bvra–/– and littermate Bvra+/+ mice to have comparable fasted plasma glucose and insulin, glucose and insulin tolerance, glucose uptake, as well as insulin signaling. However, Bvra–/– mice developed a fatty and inflamed liver associated with vitamin E depletion and increased concentrations of oxidized lipids. Administration of vitamin E reversed this phenotype and biochemical changes, indicating that bilirubin/BVRA protect mice from high fat diet-induced hepatic steatosis via antioxidant protection rather than prevention of insulin resistance.
2. Materials and methods
For extended Material and Methods, see online Supplementary materials.
2.1. Animals
iliverdin reductase a gene-deficient (Bvra–/–) mice have been described previously [19]. Bvra+/+ and Bvra–/– littermate male mice at 7 ± 1 weeks of age were obtained from Bvra+/– x Bvra+/– breeding and used for all experiments. All mice were housed in a temperature-controlled room (Physical Containment Level 2 certification) on a 12 h light/dark cycle and were allowed access to water and food ad libitum. All procedures were carried out according to the Australian National Health & Medical Research Council Guidelines for Animal Research and were approved by the Animal Care and Ethics Committee of the Garvan Institute of Medical Research/St Vincent's Hospital. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals.”
To fully characterize the metabolic phenotype of Bvra–/– mice required performing multiple assays. This was achieved by using minimal group sizes for each parameter as predicted by power calculations, and by prioritizing assays that required freshly collected tissue. Group sizes for different assays varied from 5 to 18. This information is contained in Figure Legends.
2.2. Diets
Animals were maintained on a standard chow containing 13% calories from fat, 22% calories from protein, and 65% calories from carbohydrate, 3.1 kcal/g (#27, Gordons Specialty Feeds, Australia) until they were allocated to a study involving a high fat (HF) or high fat high sucrose (HFHS) diet. Where indicated, mice were fed for up to 14 weeks a HF diet formulated based on D12492 (Research Diet, Inc) containing 5.2 kcal/g with 60, 20 and 20% calories from fat, protein, and carbohydrate, respectively (SF13-092, Specialty Feeds, Australia). Alternatively, mice were fed for up to 6 weeks an in-house HFHS diet based on D12451 (Research Diets, Inc) containing 4.7 kcal/g with 47, 21 and 32% calories from fat, protein, and carbohydrate, respectively. The RRR-α-tocopheryl succinate was kindly donated by Pharma Nord, Denmark. The RRR-α-tocopheryl succinate was incorporated as powder in the HF diet obtained a concentration of RRR-α-tocopherol 0.2%, w/w in the diet.
2.3. Statistical analyses
Statistical analysis was performed using GraphPrism 8 software. Results are expressed as mean ± SEM. Normality of the data distribution was assessed (D'Agostino-Pearson test) and a Student's t-test, 1- or 2-way ANOVA, Mann-Whitney, or Kruskal-Wallis test used as appropriate. A P-value of <0.05 was considered as statistically significant.
3. Results
3.1. Global BVRA deficiency does not impact glucose tolerance and insulin sensitivity in mice fed standard chow
Littermate Bvra–/– and Bvra+/+ mice fed standard chow exhibited comparable body weight (Fig. 1A), consistent with our previous observation [19]. Bvra–/– mice had very low concentrations of hepatic bilirubin compared with Bvra+/+ littermates (0.3 ± 0.1 versus 2.9 ± 0.3 pmol/mg protein) (Fig. 1B), as determined by LC-MS/MS. Instead, biliverdin was elevated in livers of Bvra–/– compared with Bvra+/+ mice (2.6 ± 0.1 versus 0.9 ± 0.1 pmol/mg protein) (Fig. 1C). Elevated concentrations of biliverdin were also observed in other tissues (e.g., arteries) of Bvra–/– mice (unpublished data). However, Bvra+/– mice had similar concentrations of biliverdin and bilirubin as in Bvra+/+ littermates [19].
Fig. 1.
Glucose tolerance, insulin sensitivity and insulin signaling are normal in Bvra–/– mice fed standard chow. (A) Body weights, (B, C) hepatic concentrations of biliverdin and bilirubin, and (D) non-fasted blood glucose in Bvra+/+ and Bvra–/– mice 7 ± 1 weeks of age. Bile pigments were measured by LC-MS/MS, with results normalized to protein content. (E) Blood glucose concentration following intraperitoneal glucose tolerance test (GTT, 1 g/kg glucose) in fasted (8 h) Bvra+/+ (n = 6) and Bvra–/– (n = 5) mice, with (F) incremental area under the curve (iAUC). (G) Plasma insulin concentrations following GTT (1 g/kg glucose) in fasted (8 h) Bvra+/+ (n = 6) and Bvra–/– mice (n = 5). (H) Blood glucose concentrations following insulin tolerance test (ITT, 0.75 U/kg insulin) in fasted (8 h) Bvra+/+ (n = 6) and Bvra–/– (n = 7) mice, with (I) area above the curve (AAC). (J) Protein standardized [3H] 2-deoxy-d-glucose (2-DOG) uptake into adipose tissue explants derived from the epididymal fat pads of Bvra+/+ (n = 5) and Bvra–/– (n = 5) mice after treatment with different concentrations of insulin. (K) Representative Western blot of glucose transporter type 4 (GLUT4) in adipose tissue of Bvra+/+ and Bvra–/– mice. GLUT4 expression in adipose tissue of littermate Bvra–/– and Bvra+/+ mice was quantified by densitometry and normalized to loading control (α-tubulin) and the expression in the Bvra+/+ group. (L) Representative Western blots of BVRA, Akt and AS160 phosphorylation in adipose tissue of Bvra+/+ and Bvra–/– mice after treatment with different concentrations of insulin. Phosphorylation of Ser473 and Thr308 of Akt, and Thr642 of AS160 as assessed by densitometry, and normalized to total protein and expressed relative to Bvra+/+ mice treated with 10 nM insulin; n = 5 per genotype. Open and filled circles correspond to Bvra–/– and Bvra+/+ mice, respectively. Numerical results show individual data as well as mean ± SEM, with data analyzed for statistical difference by the Mann-Whitney test. *P < 0.05.
There was no significant difference in non-fasted blood glucose concentrations in Bvra+/+ and Bvra–/– littermates 7 ± 1 weeks old (Fig. 1D). Similarly, blood glucose and plasma insulin concentrations were similar in Bvra–/– and Bvra+/+ littermates during glucose (GTT) and insulin tolerance tests (ITT) (Fig. 1E–I), indicating BVRA deficiency had no effect on glucose or insulin tolerance. Insulin resistance in adipose tissue appears early in the pathogenesis of metabolic disease [29] and can influence whole body insulin sensitivity [30,31]. Therefore, we assessed glucose uptake and insulin sensitivity in adipose tissue using [3H] 2-deoxy-d-glucose (2-DOG) and different doses of insulin. We observed no significant difference in glucose uptake between the two genotypes (Fig. 1J). Consistent with this, we observed no significant difference in the expression of glucose transporter type 4 (GLUT4), the major insulin-regulated controller of glucose uptake in adipose, between Bvra+/+ and Bvra–/– mice (Fig. 1K). Moreover, BVRA deficiency did not affect insulin signaling in adipose explants, as indicated by the comparable extent of phosphorylation of Akt and AS160, which are key regulators of insulin-stimulated glucose uptake (Fig. 1L).
3.2. BVRA deficiency does not alter high fat diet-induced glucose intolerance and insulin sensitivity
Insulin resistance, e.g., impaired tolerance to glucose and insulin, has been observed in mice on short- and long-term high fat (HF) diets [32,33]. Therefore, we next investigated whether BVRA deficiency exacerbated glucose intolerance and insulin resistance in the setting of HF diet. Compared with our previous results from mice fed standard chow [19], HF diet had no significant effect on plasma concentrations of bile pigments in Bvra+/+ and Bvra–/– mice (Fig. 2A&B). Similarly, body weights, fasting blood glucose concentrations, and glucose and insulin tolerance remained comparable between the two genotypes (Fig. 2C–I), as did in vivo insulin sensitivity in epididymal fat pads assessed by 2-DOG uptake (Fig. 2J) and phosphorylation of Akt and AS160, as well as GLUT4 expression (Fig. 2K). There was also no difference in hepatic phosphorylation of Akt and GSK3β between Bvra+/+ and Bvra–/– mice (Fig. 2L). Finally, we examined a potential effect of BVRA deficiency on insulin sensitivity by providing Bvra+/+ and Bvra–/– mice high fat high sucrose (HFHS) diet which, due to the addition of sucrose, induces a more pronounced glucose intolerance [29]. Again, Bvra+/+ and Bvra–/– mice had comparable body weights, fasting blood glucose, and blood glucose and plasma insulin following injection of glucose (Supplemental Figs. 2A–E). Together, these findings suggest that global BVRA deficiency does not affect glucose metabolism and insulin sensitivity in mice, whether animals are fed standard chow, or are challenged with a HF or HFHS diet.
Fig. 2.
Bvra deficiency does not aggravate glucose intolerance, insulin resistance and insulin signaling in mice fed high fat (HF) diet. (A, B) Plasma biliverdin and bilirubin in Bvra+/+ and Bvra–/– mice fed HF diet for 12 weeks. (C) Weekly body weights of Bvra+/+ and Bvra–/– mice fed a HF diet over 12 weeks (n = 12 per genotype). (D) Blood glucose concentrations in fasted (8 h) Bvra+/+ (n = 11) and Bvra–/– mice (n = 11) fed HF diet for 5, 28, 56 and 84 days. (E) Blood glucose concentrations following GTT (1 g/kg glucose) in fasted (8 h) Bvra+/+ (n = 11) and Bvra–/– mice (n = 11) fed HF diet for 12 weeks, with (F) iAUC for GTT performed after HF diet for 5, 28, 56 and 84 days. (G) Plasma insulin concentrations following GTT (1 g/kg glucose) in fasted (8 h) Bvra+/+ (n = 11) and Bvra–/– mice (n = 11) fed HF diet for 84 days. (H) Blood glucose concentrations following ITT (0.75 U/kg insulin) in fasted (8 h) Bvra+/+ (n = 6) and Bvra–/– (n = 7) mice fed HF diet for 12 weeks, with (I) AAC for blood glucose concentrations following ITT performed after HF diet for 5, 28, 56 and 84 days. (J) Insulin-induced 2-DOG uptake into epididymal adipose tissue of Bvra+/+ and Bvra–/– mice fed HF diet for 14 weeks. Data was normalized to tissue weight. (K) Representative Western blots of BVRA, GLUT4, and phosphorylated Akt and AS160 in epididymal adipose tissue of Bvra+/+ and Bvra–/– mice fed HF diet for 14 weeks (two independent experiments). Densitometry analysis of expression of GLUT4, phosphorylation of Ser473 and Thr308 of Akt, and Thr642 of AS160 in adipose tissue of Bvra+/+ (n = 12) and Bvra–/– (n = 11) mice. Data was normalized to total protein content or loading control (14-3-3) respectively. (L) Western blots of BVRA, phosphorylated Akt and GSK3β in liver of Bvra+/+ and Bvra–/– mice fed HF diet for 14 weeks (two independent experiments). (L) Densitometry analysis of phosphorylated Ser473 and Thr308 of Akt, and Ser9 of GSK3β in liver of Bvra+/+ (n = 12, filled circles) and Bvra–/– (n = 11, open circles) mice. Numerical results show individual data as well as mean ± SEM, with data analyzed for statistical difference by the Mann-Whitney test. *P < 0.05.
3.3. Deficiency of Bvra enhances HF diet-induced hepatic lipid accumulation
Chronic feeding of fat-rich diet leads to obesity-related steatosis, a manifestation of the metabolic syndrome characterized by excessive hepatic lipid accumulation [27,34]. After 14 weeks HF diet or 6 weeks HFHS diet, livers in Bvra–/– mice showed evidence for lipid accumulation compared with control, as indicated by increased Oil Red O-positive staining and the presence of lipid droplets and vacuoles (Fig. 3A and Supplemental Fig. 2H). HPLC analysis confirmed this observation and revealed that compared with Bvra+/+ mice, livers of HF-fed Bvra–/– mice contained more triacylglycerides (TAG, 237 ± 34 versus 551 ± 134 nmol/mg protein) and cholesterol (25 ± 3 versus 38 ± 5 nmol/mg protein) (Fig. 3B and C) but not cholesterylesters (CE) (Fig. 3D). In mice fed HFHS diet, BVRA deficiency also increased hepatic TAG (93.8 versus 70.4 nmol/mg protein), while cholesterol and CE were not different (Supplemental Figs. 2I–K). There was no significant difference in plasma cholesterol, CE and TAG between Bvra+/+ and Bvra–/– mice fed HF diet (Supplemental Figs. 3F–H).
Fig. 3.
Bvra deficiency enhances HF diet-induced hepatic lipid accumulation in mice. (A) Representative H&E and Oil Red O-stained liver sections. Scale bar = 100 μm. Quantification of Oil Red O-stained area of liver sections of Bvra+/+ and Bvra–/– mice. (B–D) Concentrations of hepatic TAG, cholesterol and CE analyzed by HPLC-UV, with results normalized to protein. Semi-quantitative RT-PCR of hepatic Acaca, Fasn and Srebf1 mRNA transcripts (E–G), liver weight and liver-to-body-weight ratio (H, I), and plasma ALT (J) in male Bvra+/+ and Bvra–/– mice fed HF diet for 14 weeks. (K) Representative immunohistochemistry (IHC) of F4/80 and rat IgG isotype control in liver sections of Bvra+/+ and Bvra–/– mice fed HF diet, with corresponding quantitative data of F4/80 positive area. Scale bar = 100 μm. (L) Semi-quantitative RT-PCR of Il-1β, Il-6, Il-10, Mcp-1 and Tnf-α mRNA transcripts in liver of Bvra+/+ (n = 18, filled circles) and Bvra–/– mice (n = 17, open circles) fed HF diet for 14 weeks. Results shown are expressed as mean ± SEM, with data analyzed for statistical difference by the Mann-Whitney test. *P < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Development and progression of hepatic steatosis is linked to de novo synthesis of lipid [35]. In agreement with enhanced lipid accumulation in Bvra–/– mice fed HF diet, mRNA of enzymes involved in lipid synthesis, i.e., acetyl-CoA carboxylase (Acaca) and fatty acid synthase (Fasn), but not sterol regulatory element-binding transcription factor 1 (Srebf1), were increased compared with Bvra+/+ littermates (Fig. 3E–G). Liver weights, liver-to-bodyweight ratios, and plasma ALT activities (Fig. 3H–J) were comparable in Bvra–/– and Bvra+/+ mice. As excess fat can cause chronic inflammation, we determined F4/80 expression as a marker of Kupffer cells. Compared with Bvra+/+ mice fed HF diet, Bvra–/– mice had increased F4/80 (Fig. 3K) and mRNA of the pro-inflammatory cytokines MCP-1 and TNF-α, while mRNA of interleukins-1β, -6 and -10 remained similar (Fig. 3L).
The above results suggest that Bvra deficiency in combination with a HF diet enhances hepatic fat accumulation and inflammation. Fatty liver can progress to hepatic fibrosis. However, we observed only mild fibrosis, as assessed by Fast green Sirius red staining, in the liver Bvra+/+ or Bvra–/– mice fed HF diet, and no significant difference was observed between the genotypes (Supplementary Fig. 4).
3.4. Bvra deficiency moderately regulates PPARα activity
Stec and co-workers reported liver-specific BVRA deficiency caused hepatic steatosis via a decrease peroxisome proliferator-activated receptor-α (PPARα) activity and resulting attenuated hepatic lipid metabolism [27]. Hepatic concentrations of bilirubin and biliverdin in mice with liver specific BVRA deficiency were not reported, which may be important as biliverdin has been shown to activate PPARα more efficiently than bilirubin in a cellular reporter system [25]. We therefore first determined hepatic concentrations of bile pigments in Bvra–/– and Bvra+/+ mice fed a HF diet using LC-MS/MS. Global BVRA deficiency significantly decreased hepatic bilirubin, and this was associated with a commensurate increase in biliverdin, such that total bile pigment concentrations were comparable in the two genotypes (Fig. 4A–C). Consistent with this, we observed only a modest (∼30%) increase in PPARα phosphorylation, a marker of decreased PPARα expression [27], in Bvra–/– compared with Bvra+/+ mice as assessed by Western blotting, while PPARα protein expression was not different (Fig. 4D). This increase in PPARα phosphorylation was associated with a small albeit significant decrease in mRNA levels of some (carnitine palmitoyltransferase 1A, Cpt1a; cytochrome P450, family 2 (Cyp2j6) and 4, (Cyp4a12)), but not other PPARα-targeted genes (cluster of differentiation 36, Cd36; glucose 6-phosphatase, G6pase) (Fig. 4E). Together, these results indicate that global BVRA deficiency at most decreases hepatic PPARα activity modestly, and that other factors need to be considered to explain the observed steatosis in Bvra–/– mice.
Fig. 4.
Bvra and bilirubin deficiency modestly regulates hepatic PPARα activity in mice fed HF diet. (A, B, C) Hepatic concentration of biliverdin, bilirubin and total bile pigments in Bvra+/+ and Bvra–/– mice determined by LC-MS/MS with results normalized to protein. (D) Representative Western blots of PPARα phosphorylation in liver of Bvra+/+ and Bvra–/– mice fed HF diet for 14 weeks. Densitometry analysis of expression of hepatic PPARα and phosphorylation of Ser73. Data was normalized to total protein content or loading control (Actin). (E) Semi-quantitative RT-PCR of Cpt1a, Cyp2j6, Cyp4a12, Cd36, G6pase mRNA transcripts in liver of Bvra+/+ (n = 13, filled circles) and Bvra–/– mice (n = 11, open circles) fed HF diet for 14 weeks. Open and filled circles correspond to Bvra–/– and Bvra+/+ mice respectively. Results shown are expressed as mean ± SEM, with data analyzed for statistical difference by the Mann-Whitney test. *P < 0.05.
3.5. Bvra deficiency increases hepatic lipid oxidation associated with a decrease in α-tocopherol
We next considered non-enzymatic lipid oxidation as an alternative cause of hepatic lipid accumulation in Bvra–/– mice [36], as bilirubin protects lipids more effectively from non-enzymatic oxidation than biliverdin [16], and circulating lipids are more oxidized in Bvra–/– than Bvra+/+ mice [19]. Non-targeted lipidomic analysis of livers from Bvra+/+ and Bvra–/– mice fed HF diet identified 781 lipid species, with the top 50 regulated lipids presented as a heatmap (Fig. 5A). As can be seen, livers of Bvra–/– mice contained significantly more TAG (Fig. 5B), confirming the HPLC-UV data. Compared with livers from Bvra+/+ littermates, Bvra–/– mice had significantly elevated concentrations of oxidized TAG (oxTAG) (Fig. 5C), as well as increased ratios of oxTAG to total TAG (Fig. 5D). We also expressed oxTAG per TAG with more than three double bonds (DB > 3), reasoning that TAGDB>3 contain at least one fatty acid with an isolated double bond and associated pair of bisallylic hydrogens that render lipids susceptible to oxidation. The ratios of oxTAG to TAGBD>3 was significantly increased in livers of Bvra–/– than Bvra+/+ mice despite comparable TAGDB>3 (Fig. 5E and F). Similarly, the ratio of oxidized PC (oxPC) to PCBD > 2 was significantly increased in livers of Bvra–/– compared with Bvra+/+ mice despite comparable concentrations of PC and PCDB > 2 (Supplemental Fig. 3).
Fig. 5.
Bvra deficiency depletes hepatic α-tocopherol (α-TOH) and increases lipid oxidation in mice fed HF diet. (A) Untargeted lipidomic analysis of livers from Bvra+/+ and Bvra–/– mice. (B–F) Concentrations of hepatic total TAG, oxidized TAG (oxTAG), ratio of oxTAG-to-total TAG, ratio of TAG containing fatty acids with more than three double bonds (TAGDB>3) to total TAG, and oxTAG-to-TAGDB>3 ratio in Bvra+/+ and Bvra–/– mice. Lipids were determined by LC-MS/MS and their concentrations assessed by area comparison of individual lipid signals with that of the corresponding internal lipid standard. (G) Ratio of hepatic α-TOH-to-TAG and α-TOH-to-TAGDB>3 in Bvra+/+ and Bvra–/– mice. (H) Hepatic ascorbate in Bvra+/+ and Bvra–/– mice determined by HPLC. (I) Ratio of oxidation products of bile pigments (BOXes)-to-TAGDB>3 and BOXes -to-bile pigments in liver of Bvra+/+ and Bvra–/– mice. Results of hepatic TAG and oxTAG were normalized to tissue weight (mg), while ascorbate was normalized to protein. Results shown are mean ± SEM with data analyzed for statistical difference using the Mann-Whitney test. *P < 0.05.
To separately probe the implied presence of oxidative stress in livers of Bvra–/– mice, we determined liver concentrations of α-tocopherol (α-TOH), the most abundant lipid-soluble antioxidant in mammals that protects lipids with bisallylic hydrogens from non-enzymatic oxidation. Compared with Bvra+/+ littermates, Bvra–/– mice had decreased ratios of α-TOH to TAGDB>3 (Fig. 5G), indicating decreased antioxidant protection of lipids. Interestingly, hepatic concentrations of ascorbic acid, a water-soluble antioxidant able to maintain α-TOH in the antioxidant active form [37], were not different between Bvra+/+ and Bvra–/– mice (Fig. 5H). We also assessed the extent of bile pigment oxidation by determining their end products, or BOXes, by LC-MS/MS [38,39]. The ratio of hepatic BOXes to TAGDB>3 was significantly decreased in Bvra–/– compared with Bvra+/+ mice (Fig. 5I), indicating that BVRA deficiency decreased the combined antioxidant protection provided by biliverdin and bilirubin.
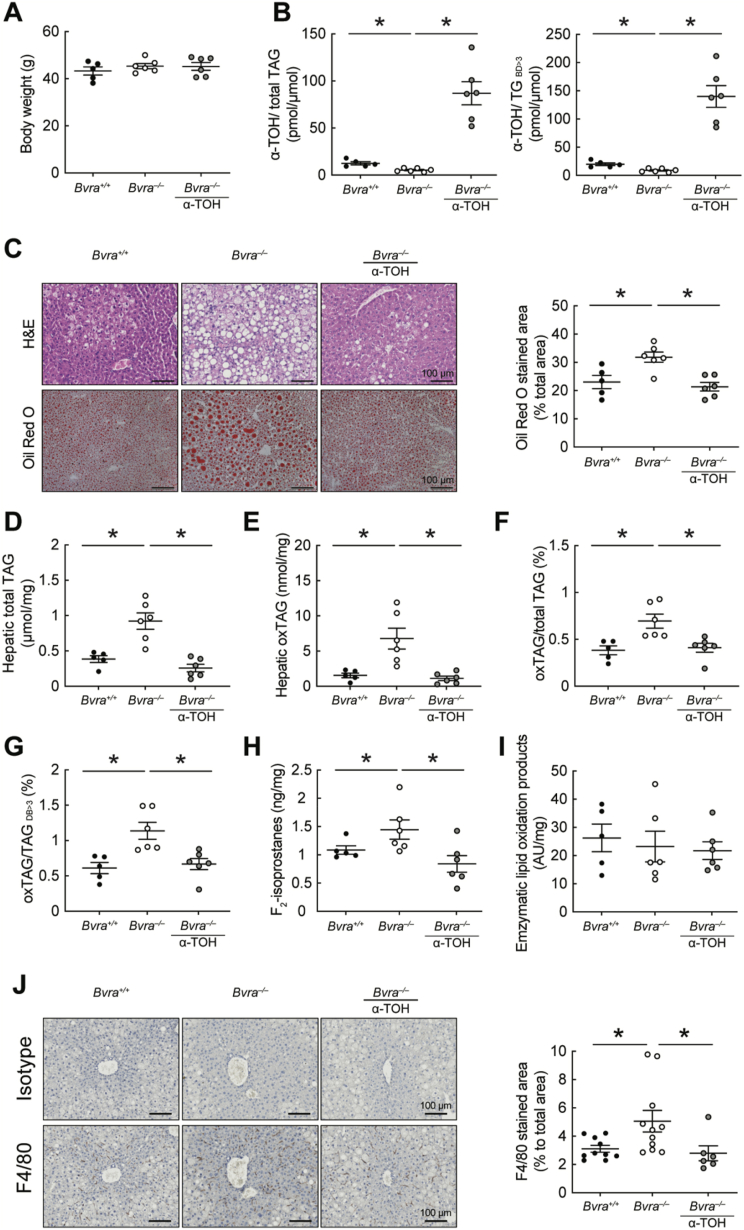
3.6. α-Tocopherol prevents hepatic lipid oxidation and lipid accumulation in Bvra–/– mice
The above results suggest that non-enzymatic lipid oxidation, resulting from decreased endogenous lipid-soluble antioxidants (bilirubin and α-TOH), may cause hepatic lipid accumulation in Bvra–/– mice. As bilirubin cannot be administered readily to animals, we therefore tested whether α-TOH supplements reverses hepatic lipid oxidation and steatosis in BVRA deficiency by feeding Bvra+/+ and Bvra–/– mice a HF diet ± α-TOH (0.2%, w/w, RRR-α-TOH, the most active form of vitamin E) for 14 weeks. Supplementation with α-TOH had no effect on body weights (Fig. 6A), although it increased hepatic α-TOH concentrations in Bvra–/– mice (Fig. 6B). This was associated with a decrease in oxTAG and the ratios of oxTAG-to-total TAG and oxTAG-to-TAGDB>3 (Fig. 6E–G). More importantly, α-TOH supplementation restored wild-type hepatic concentrations of TAG (Fig. 6D) and it prevented both the fatty liver and inflammatory phenotype in Bvra–/– mice, as assessed by Oil Red O staining (Fig. 6C) and F4/80 expression, respectively (Fig. 6J).
Fig. 6.
α-Tocopherol prevents hepatic lipid accumulation and lipid oxidation in Bvra–/– mice fed HF diet for 14 weeks. (A) Body weights of Bvra+/+ and Bvra–/– mice fed a HF diet ± α-TOH supplement (0.2% RRR-α-TOH, w/w). (B) Ratio of hepatic α-TOH-to-TAG and α-TOH-to-TAGDB>3 in Bvra+/+ and Bvra–/– mice fed HF diet ± α-TOH. α-TOH was measured by LC-MS/MS and results normalized to tissue weight (mg). (C) Representative H&E and Oil Red O-stained liver sections of Bvra+/+ and Bvra–/– mice fed HF ± α-TOH. Scale bar = 100 μm, with quantification also provided. (D–G) Concentrations of hepatic total TAG, oxTAG, oxTAG-to-total TAG, and oxTAG-to-TAGDB>3 in Bvra+/+ and Bvra–/– mice fed HF ± α-TOH. (H) Hepatic F2-IsoP in Bvra+/+ and Bvra–/– mice fed HF diet ± α-TOH. (I) Enzymatic lipid oxidation products in the liver of Bvra+/+ and Bvra–/– mice fed HF diet ± α-TOH. TAG and oxTAG were measured by untargeted lipidomic analysis using LC-MS/MS, F2-IsoP were measured by GC-MS and enzymatic lipid oxidation products by ion mobility Q-TOF LC/MS. (J) IHC of F4/80 and rat IgG isotype control in liver sections of Bvra+/+ and Bvra–/– mice fed HF diet ± α-TOH, with corresponding quantitative data of F4/80 positive area. Scale bar = 100 μm. Numerical results show individual data as well as mean ± SEM, with data analyzed for statistical difference using the Kruskal Wallis test. *P < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
As bilirubin and α-TOH inhibit non-enzymatic lipid oxidation and supplementation with α-TOH prevents such lipid oxidation and reverses the fatty liver phenotype, we next asked whether oxidative stress and associated non-enzymatic lipid oxidation causes the fatty liver phenotype in Bvra–/– mice fed HF diet. To do so, we used gas chromatography mass spectrometry to determine hepatic concentrations of F2-IsoP, a specific product of non-enzymatic lipid oxidation regarded as the gold standard biomarker of in vivo oxidative stress. Compared with the corresponding wild-type control, livers of Bvra–/– mice had increased concentration of F2-IsoP, and α-TOH supplementation restored wild type concentrations of F2-IsoP (Fig. 6H). In contrast, hepatic concentrations of enzymatic lipid oxidation products were comparable in Bvra+/+ and Bvra–/– littermate mice fed a HF diet, with or without α-TOH supplementation (Fig. 6I), as assessed by Ion Mobility Quadrupole Time-of-Flight mass spectrometry. Together, these results indicate that BVRA deficiency increases non-enzymatic lipid oxidation in the liver and that this, rather than enzymatic lipid oxidation, is a driver of both hepatic inflammation and formation of a fatty liver.
4. Discussion
This study shows that Bvra–/– mice lack bilirubin in the liver, and that they respond to high fat diets comparably to wildtype littermate animals with regards to glucose metabolism and insulin sensitivity. Despite this, Bvra–/– mice challenged with high fat diets develop a fatty and inflamed liver driven by increased non-enzymatic lipid oxidation and depletion of α-TOH, as restoration of hepatic α-TOH prevented such lipid oxidation, inflammation and steatosis. Together, the results indicate that protection from NAFLD observed in individuals with moderately to highly elevated bilirubin [[7], [8], [9], [10]] may be due to an antioxidant action by the bile pigment in the liver.
Evidence for the presence of steatosis in Bvra deficiency combined with a high fat diet (HF or HFHS) is based on histological (Red-O staining) and analytical evidence, the latter including both targeted (HPLC-UV) and non-targeted (LC-MS/MS) analyses. This revealed the presence of lipid droplets and elevated concentrations of TAG. In agreement with these finding, livers of Bvra–/– mice had increased expression of Acaca and Fasn that encode key enzymes in de novo lipogenesis. Moreover, catabolism of fatty acids appeared decreased, as mRNA of Cpt1a (an essential factor to trigger mitochondrial β-oxidation of long chain fatty acids) and Cyp2j6 and Cyp4a12 (catalysing the first step in ω-oxidation of fatty acids) [40] were decreased in Bvra–/– mice. In contrast, hepatic mRNA expression of Cd36 was not affected by BVRA deficiency, indicating that enhanced fatty acid esterification leading to TAG lipid droplets was likely taking place in the extracellular space [41]. Also, as plasma lipid concentrations were unaffected in Bvra–/– mice fed high fat diets, indicating that the observed steatosis was most likely caused by local changes in lipid metabolism. Accordingly, lipid peroxidation may exacerbate mitochondrial dysfunction, thereby promoting lipid accumulation because of decreased β-oxidation/lipid metabolism [6].
In parallel to the observed hepatic lipid accumulation, Bvra deficiency also increased hepatic inflammation, as indicated by the increase in F4/80 staining. As F4/80 is a macrophage marker, these results suggest that enhanced inflammation was likely due to the increase in Kupffer cells. If so, this could explain the concomitant increase in the expression of inflammatory cytokines TNFα and MCP-1 that potentially exacerbate hepatic inflammation via cross talk with hepatocytes.
We are not aware of previous studies reporting the presence of biliverdin in liver of wildtype mice or other mammals, whereas we observed ∼25% of hepatic bile pigments to be present as biliverdin in livers of Bvra+/+ mice, independent of the diet used. This is different to the situation in plasma, where biliverdin accounts for only ∼1% of bile pigments. In fact, biliverdin is commonly thought to be essentially undetectable in vivo [42] due to the kinetic efficiency of BVRA. Bile pigments were detected by an LC-MS/MS-based method, providing high analytical specificity. The presence of biliverdin in the liver of C57BL/6J mice fed standard chow or high fat diet indicates that available BVRA activity is insufficient to ‘deal with’ the amounts of biliverdin formed. Our data also suggests a potential limitation to the perceived role of BVRA in ‘releasing’ biliverdin from heme oxygenase to ‘accelerate’ the rate-limiting step in heme degradation by the oxygenase [43]. In any case, our findings warrant further investigation into the potential biological activities of biliverdin in the liver.
We provide direct evidence that Bvra deficiency increases oxidative stress and that this results in the oxidation of hepatic lipids. Evidence for the presence of oxidative stress comes from both, the observed decrease in hepatic concentrations of α-TOH and the increase in F2-IsoP, the latter regarded as the gold standard biomarker of in vivo oxidative stress [44]. Non-targeted lipidomics and ion mobility LC-MS analyses revealed that the inflammatory hepatic steatosis in the Bvra–/– mice was accompanied by an increase in enzymatically oxidized fatty acids in the form of oxTAG and oxPC. As expected, the concentration of these enzymatic lipid oxidation products was unaffected by α-TOH supplementation, and therefore not likely responsible for the observed steatosis in BVRA deficiency.
In contrast to enzymatic lipid oxidation, BVRA deficiency increased, and α-TOH supplementation decreased, the hepatic concentration of the non-enzymatic lipid oxidation products F2-IsoP. This suggests non-enzymatic lipid oxidation as a cause for the observed inflammatory steatosis in Bvra–/– mice. In support of this interpretation, in vivo administration of a free radical generator has been reported to cause non-enzymatic lipid oxidation and a fatty liver in mice [36]. Oxidative stress has also been implicated in the pathogenesis of NAFLD [6]. If oxidative stress and resulting non-enzymatic lipid oxidation is causally involved in the development of NAFLD, supplementation of lipid-soluble radical scavenging antioxidants should be effective in suppressing the disease. We addressed this question by supplementing Bvra–/– mice fed high fat diet with RRR-α-TOH. This not only inhibited non-enzymatic lipid oxidation but also reversed hepatic steatosis and inflammation. Our findings may have translational implications, because supplementation of humans with 800 IU/day α-TOH has been reported to inhibit the total NAFLD activity score in non-diabetic adults with nonalcoholic steatohepatitis [45]. A recent practice guidance for the diagnosis and management of NAFLD by the American Association for the Study of Liver Diseases [46] stated “Vitamin E (RRR α-tocopherol) administered at a daily dose of 800 IU/day improves liver histology in nondiabetic adults with biopsy-proven NASH and therefore may be considered for this patient population. Risks and benefits should be discussed with each patient before starting therapy. Until further data supporting its effectiveness become available, vitamin E is not recommended to treat NASH in diabetic patients, NAFLD without liver biopsy, NASH cirrhosis, or cryptogenic cirrhosis”.
The present study used BVRA-deficient mice as a model of bilirubin deficiency. A limitation of this model is that it does not allow to immediately assign any observed phenotype to either the absence of BVRA or bilirubin, or both. Previous studies suggested that in addition to catalysing the reduction of biliverdin to bilirubin, BVRA may also regulate glucose metabolism and insulin signaling [24,28,47,48]. Stec and co-workers reported hepatic steatosis and impaired hepatic insulin signaling in liver-specific BVRA knockout mice fed the same high fat diet for the same duration as in the present work [27]. They also reported BVRA and its enzymatic product bilirubin to attenuate hepatic steatosis via the activation of PPARα signaling pathway to promote β-oxidation of fatty acids [[25], [26], [27]]. We also observed global BVRA deficiency to indirectly decrease β-oxidation. However, any effect on the PPARα signaling pathway was modest, and Bvra–/– mice fed a high fat diet had comparable insulin sensitivity and signaling compared to littermate control animals, as assessed by multiple parameters, and using two different types of high fat diets. Others reported a deficiency in PPARα to not alter insulin sensitivity in mice maintained on high fat diet, as assessed by hyperinsulinemic-euglycemic clamp studies [49]. These results indicate potential differences between the two mouse models and suggest that the resulting phenotypic differences may be explained by differences in hepatic bilirubin concentrations and/or the fact that BVRA can activate or inhibit glucose uptake and insulin signaling [48]. Further studies are required to establish a role for BVRA in in vivo glucose metabolism and insulin sensitivity.
Hepatic steatosis is widely believed to result in insulin resistance [4,50]. However, there is also evidence indicating that hepatic lipid accumulation is insufficient to cause, and can occur independent of, insulin resistance [51,52], especially hepatic insulin resistance [53]. Intrahepatic accumulation of TAG may be more a marker than cause of insulin resistance, as exemplified by patients with familial hypobetalipoproteinemia in whom hepatic steatosis is dissociated from insulin resistance [54].
Bilirubin is a potent non-enzymatic antioxidant in its own right for both the lipid and aqueous phase [16], and the pigment can also afford effective inhibition of lipid oxidation through interaction with α-TOH [18]. Therefore, it is tempting to speculate that the observed increase in non-enzymatic lipid oxidation in the liver of Bvra–/– mice was due to their lack of bilirubin rather than the absence of BVRA. Consistent with this interpretation, we reported previously that lipid oxidation products are increased in blood plasma of Bvra–/– compared with Bvra+/+ mice fed standard chow [19]. Also, our observation that products of bilirubin/biliverdin oxidation (BOXes) are present in livers of Bvra+/+ mice fed high fat diet directly shows that hepatic bilirubin acts as an antioxidant in vivo. Interestingly, we detected biliverdin in the livers of both Bvra+/+ and Bvra–/– mice. As biliverdin also has antioxidant activity [16], and its liver concentrations were higher in Bvra–/– than wildtype mice, it is conceivable that biliverdin may compensate for bilirubin as a non-enzymatic antioxidant. Inconsistent with this idea, however, hepatic concentrations of α-TOH and BOXes were lower and non-enzymatic lipid oxidation products were higher in livers of Bvra–/– compared with Bvra+/+ mice. Also, in vitro biliverdin has lower antioxidant activity than bilirubin [16]. Surprisingly, we observed no change in hepatic ascorbate between Bvra–/– and Bvra+/+ mice. In contrast to bilirubin, ascorbate requires α-TOH to protect lipids from oxidation, so that the depletion of hepatic α-TOH in Bvra–/– mice may explain why tissue concentrations of ascorbate were unaffected and substantial non-enzymatic lipid oxidation, especially within large lipid droplets, took place despite the presence of normal concentrations of ascorbate. A prerequisite for this may be that lipid oxidation is initiated within the ‘lipid phase’, by a presently unknown cause.
The almost complete systemic absence of bilirubin makes Bvra–/– mice a useful tool to address the physiological roles of bilirubin, especially as provision of exogenous bilirubin is not a feasible approach due to the pigment's effective insolubility in physiological buffers. By comparison, there is currently no evidence that tissue-specific BVRA knock-out affects bilirubin concentrations systemically or locally, so that this approach is not suitable to directly assess the biological roles of bilirubin. A complicating factor of Bvra–/– mice is that they have elevated concentrations of biliverdin in plasma, bile, and other tissues, including liver and arteries (unpublished data), and the physiological roles of biliverdin in mammals are not well understood. Pharmacological induction of hyperbilirubinemia by atazanavir (that inhibits bilirubin conjugation), has been reported to protect against metabolic and cardiovascular diseases in HIV patients [55,56]. Therefore, pharmacological tools may be useful in unravelling the mechanisms underlying the protective effects of bilirubin, as well as in its translation as novel strategies for the treatment of cardiovascular and metabolic diseases. Although Bvra mRNA and BVRA protein are completely absent in Bvra–/– mice, very low concentrations of bilirubin were detected in the liver, plasma, and other tissues, consistent with our previous observation [19]. The residual bilirubin detected in Bvra–/– mice may be due to biliverdin reductase B (BVRB), which is present in mammals and can also reduce biliverdin to bilirubin.
In conclusion, our findings for the first time, support the hypothesis proposed nearly 35 years ago [16] that bilirubin has a biological function by acting as a physiological antioxidant. Our metabolic characterization of Bvra–/– mice indicates that the inflammatory steatosis is caused by the local depletion of the lipid-soluble antioxidants α-TOH and bilirubin, and its associated non-enzymatic lipid oxidation. As high concentrations of supplemented α-TOH may increase the risk of all-cause mortality [57,58], prostate cancer [59], and hemorrhagic stroke [60], elevating the endogenous antioxidant bilirubin deserves consideration as a potential alternative treatment in the management of NALFD in patients where conventional management fails.
Future investigations will utilize the Bvra deficient mouse line to establish a potential causative link between bilirubin and protection against cardiovascular disease, as increased plasma bilirubin is also inversely associated to low risk of cardiovascular disease.
Author contributions
WC and RS designed the studies and drafted the manuscript. WC characterized the metabolic phenotype in Bvra–/– mice. LD assisted GTT, ITT, histology, and molecular biology. DF, JC, and DJ assisted with the HF and HFHS studies, and initial glucose uptake and insulin signaling studies. CS and RS designed and assisted with the HPLC analysis of lipids. CS helped with the LC-MS/MS analysis of bile pigments. ST carried out lipidomic analysis. ST and TS carried GC-MS analysis of F2-isoprostanes. All authors read and approved the manuscript.
Declaration of competing interest
None declared.
Acknowledgments
We thank Darren Newington, Kristen Cooke, and Ciana Diskin for technical assistance, Drs Julian Griffin and Sonia Liggi (Imperial College, London) for generously providing their Ion Mobility mass spectrometry library of enzymatically oxidized fatty acids, and Dr Stefan Ryter for providing constructive critique on the manuscript. This work was supported by National Health & Medical Research Council Program Grant 1052616 and Senior Principal Research Fellowship 1111632 to RS. WC acknowledges support received in form of a University of New South Wales International Postgraduate Award. We thank New South Wales Government Office for Health and Medical Research for infrastructure support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.102152.
Financial support statement
This work was supported by National Health & Medical Research Council of Australia Program Grant 1052616 and Senior Principal Research Fellowship 1111632 to RS. WC acknowledges support received in form of a University of New South Wales International Postgraduate Award.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Perrone-Filardi P., Paolillo S., Costanzo P., Savarese G., Trimarco B., Bonow R.O. The role of metabolic syndrome in heart failure. Eur. Heart J. 2015;36:2630–2634. doi: 10.1093/eurheartj/ehv350. [DOI] [PubMed] [Google Scholar]
- 3.Bozkurt B., Aguilar D., Deswal A., Dunbar S.B., Francis G.S., Horwich T., Jessup M. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134:e535–e578. doi: 10.1161/CIR.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 4.Marchesini G., Bugianesi E., Forlani G., Cerrelli F., Lenzi M., Manini R., Natale S. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 5.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z., Tian R., She Z., Cai J., Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020;152:116–141. doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R., Rastogi A., Maras J.S., Sarin S.K. Unconjugated hyperbilirubinemia in patients with non-alcoholic fatty liver disease: a favorable endogenous response. Clin. Biochem. 2012;45:272–274. doi: 10.1016/j.clinbiochem.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Kwak M.S., Kim D., Chung G.E., Kang S.J., Park M.J., Kim Y.J., Yoon J.H. Serum bilirubin levels are inversely associated with nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2012;18:383–390. doi: 10.3350/cmh.2012.18.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Y.C., Chang P.F., Hu F.C., Chang M.H., Ni Y.H. Variants in the UGT1A1 gene and the risk of pediatric nonalcoholic fatty liver disease. Pediatrics. 2009;124:e1221–e1227. doi: 10.1542/peds.2008-3087. [DOI] [PubMed] [Google Scholar]
- 10.Puri K., Nobili V., Melville K., Corte C.D., Sartorelli M.R., Lopez R., Feldstein A.E. Serum bilirubin level is inversely associated with nonalcoholic steatohepatitis in children. J. Pediatr. Gastroenterol. Nutr. 2013;57:114–118. doi: 10.1097/MPG.0b013e318291fefe. [DOI] [PubMed] [Google Scholar]
- 11.Cheriyath P., Gorrepati V.S., Peters I., Nookala V., Murphy M.E., Srouji N., Fischman D. High total bilirubin as a protective factor for diabetes mellitus: an analysis of NHANES data from 1999 - 2006. J. Clin. Med. Res. 2010;2:201–206. doi: 10.4021/jocmr425w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin L.Y., Kuo H.K., Hwang J.J., Lai L.P., Chiang F.T., Tseng C.D., Lin J.L. Serum bilirubin is inversely associated with insulin resistance and metabolic syndrome among children and adolescents. Atherosclerosis. 2009;203:563–568. doi: 10.1016/j.atherosclerosis.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y., Li M., Xu M., Bi Y., Li X., Chen Y., Ning G. Low serum total bilirubin concentrations are associated with increased prevalence of metabolic syndrome in Chinese. J. Diabetes. 2011;3:217–224. doi: 10.1111/j.1753-0407.2011.00138.x. [DOI] [PubMed] [Google Scholar]
- 14.Kwon K.M., Kam J.H., Kim M.Y., Kim M.Y., Chung C.H., Kim J.K., Linton J.A. Inverse association between total bilirubin and metabolic syndrome in rural Korean women. J. Womens Health (Larchmt) 2011;20:963–969. doi: 10.1089/jwh.2010.2453. [DOI] [PubMed] [Google Scholar]
- 15.Choi S.H., Yun K.E., Choi H.J. Relationships between serum total bilirubin levels and metabolic syndrome in Korean adults. Nutr. Metabol. Cardiovasc. Dis. 2013;23:31–37. doi: 10.1016/j.numecd.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Stocker R., Yamamoto Y., McDonagh A.F., Glazer A.N., Ames B.N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 17.Stocker R., Glazer A.N., Ames B.N. Antioxidant activity of albumin bound bilirubin. Proc. Natl. Acad. Sci. U.S.A. 1987;84:5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuzil J., Stocker R. Free and albumin-bound bilirubin is an efficient co-antioxidant for a-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J. Biol. Chem. 1994;269:16712–16719. [PubMed] [Google Scholar]
- 19.Chen W., Maghzal G.J., Ayer A., Suarna C., Dunn L.L., Stocker R. Absence of the biliverdin reductase-a gene is associated with increased endogenous oxidative stress. Free Radic. Biol. Med. 2018;115:156–165. doi: 10.1016/j.freeradbiomed.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Wang W.W., Smith D.L., Zucker S.D. Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats. Hepatology. 2004;40:424–433. doi: 10.1002/hep.20334. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi S., Takamiya R., Yamaguchi T., Matsumoto K., Tojo S.J., Tamatani T., Kitajima M. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ. Res. 1999;85:663–671. doi: 10.1161/01.res.85.8.663. [DOI] [PubMed] [Google Scholar]
- 22.Dong H., Huang H., Yun X., Kim D.S., Yue Y., Wu H., Sutter A. Bilirubin increases insulin sensitivity in leptin-receptor deficient and diet-induced obese mice through suppression of ER stress and chronic inflammation. Endocrinology. 2014;155:818–828. doi: 10.1210/en.2013-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien L., Hosick P.A., John K., Stec D.E., Hinds T.D., Jr. Biliverdin reductase isozymes in metabolism. Trends Endocrinol. Metabol. 2015;26:212–220. doi: 10.1016/j.tem.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maines M.D. Potential application of biliverdin reductase and its fragments to modulate insulin/IGF-1/MAPK/PI3-K signaling pathways in therapeutic settings. Curr. Drug Targets. 2010;11:1586–1594. doi: 10.2174/1389450111009011586. [DOI] [PubMed] [Google Scholar]
- 25.Stec D.E., John K., Trabbic C.J., Luniwal A., Hankins M.W., Baum J., Hinds T.D., Jr. Bilirubin binding to PPARα inhibits lipid accumulation. PloS One. 2016;11 doi: 10.1371/journal.pone.0153427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinds T.D., Jr., Hosick P.A., Chen S., Tukey R.H., Hankins M.W., Nestor-Kalinoski A., Stec D.E. Mice with hyperbilirubinemia due to Gilbert's syndrome polymorphism are resistant to hepatic steatosis by decreased serine 73 phosphorylation of PPARα. Am. J. Physiol. Endocrinol. Metab. 2017;312:E244–E252. doi: 10.1152/ajpendo.00396.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinds T.D., Jr., Burns K.A., Hosick P.A., McBeth L., Nestor-Kalinoski A., Drummond H.A., AlAmodi A.A. Biliverdin reductase A attenuates hepatic steatosis by inhibition of glycogen synthase kinase (GSK)3β phosphorylation of serine 73 of peroxisome proliferator-activated receptor (PPAR)α. J. Biol. Chem. 2016;291:25179–25191. doi: 10.1074/jbc.M116.731703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapitulnik J., Maines M.D. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol. Sci. 2009;30:129–137. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Fazakerley D.J., Chaudhuri R., Yang P., Maghzal G.J., Thomas K.C., Krycer J.R., Humphrey S.J. Mitochondrial CoQ deficiency is a common driver of mitochondrial oxidants and insulin resistance. Elife. 2018;7 doi: 10.7554/eLife.32111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abel E.D., Peroni O., Kim J.K., Kim Y.B., Boss O., Hadro E., Minnemann T. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 31.Sugii S., Olson P., Sears D.D., Saberi M., Atkins A.R., Barish G.D., Hong S.H. PPARγ activation in adipocytes is sufficient for systemic insulin sensitization. Proc. Natl. Acad. Sci. U. S. A. 2009;106:22504–22509. doi: 10.1073/pnas.0912487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raher M.J., Thibault H.B., Buys E.S., Kuruppu D., Shimizu N., Brownell A.L., Blake S.L. A short duration of high-fat diet induces insulin resistance and predisposes to adverse left ventricular remodeling after pressure overload. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2495–H2502. doi: 10.1152/ajpheart.00139.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foley K.P., Zlitni S., Denou E., Duggan B.M., Chan R.W., Stearns J.C., Schertzer J.D. Long term but not short term exposure to obesity related microbiota promotes host insulin resistance. Nat. Commun. 2018;9:4681. doi: 10.1038/s41467-018-07146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau J.K., Zhang X., Yu J. Animal models of non-alcoholic fatty liver disease: current perspectives and recent advances. J. Pathol. 2017;241:36–44. doi: 10.1002/path.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Postic C., Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita M., Ishida N., Uchiyama K., Yamaguchi K., Itoh Y., Shichiri M., Yoshida Y. Fatty liver induced by free radicals and lipid peroxidation. Free Radic. Res. 2012;46:758–765. doi: 10.3109/10715762.2012.677840. [DOI] [PubMed] [Google Scholar]
- 37.Bowry V.W., Stocker R. Tocopherol-mediated peroxidation. The pro-oxidant effect of vitamin E on the radical-initiated oxidation of human low-density lipoprotein. J. Am. Chem. Soc. 1993;115:6029–6044. [Google Scholar]
- 38.Seidel R.A., Kahnes M., Bauer M., Pohnert G. Simultaneous determination of the bilirubin oxidation end products Z-BOX A and Z-BOX B in human serum using liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015;974:83–89. doi: 10.1016/j.jchromb.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 39.Kranc K.R., Pyne G.J., Tao L., Claridge T.D., Harris D.A., Cadoux-Hudson T.A., Turnbull J.J. Oxidative degradation of bilirubin produces vasoactive compounds. Eur. J. Biochem. 2000;267:7094–7101. doi: 10.1046/j.1432-1327.2000.01812.x. [DOI] [PubMed] [Google Scholar]
- 40.Wanders R.J., Komen J., Kemp S. Fatty acid omega-oxidation as a rescue pathway for fatty acid oxidation disorders in humans. FEBS J. 2011;278:182–194. doi: 10.1111/j.1742-4658.2010.07947.x. [DOI] [PubMed] [Google Scholar]
- 41.Xu S., Jay A., Brunaldi K., Huang N., Hamilton J.A. CD36 enhances fatty acid uptake by increasing the rate of intracellular esterification but not transport across the plasma membrane. Biochemistry. 2013;52:7254–7261. doi: 10.1021/bi400914c. [DOI] [PubMed] [Google Scholar]
- 42.McDonagh A.F. Green jaundice revisited. Am. J. Med. 2010;123:e23. doi: 10.1016/j.amjmed.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y., Ortiz de Montellano P.R. Reaction intermediates and single turnover rate constants for the oxidation of heme by human heme oxygenase-1. J. Biol. Chem. 2000;275:5297–5307. doi: 10.1074/jbc.275.8.5297. [DOI] [PubMed] [Google Scholar]
- 44.Morrow J.D. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler. Thromb. Vasc. Biol. 2005;25:279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 45.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M., Neuschwander-Tetri B.A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 47.Lerner-Marmarosh N., Miralem T., Gibbs P.E., Maines M.D. Human biliverdin reductase is an ERK activator; hBVR is an ERK nuclear transporter and is required for MAPK signaling. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6870–6875. doi: 10.1073/pnas.0800750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gibbs P.E., Lerner-Marmarosh N., Poulin A., Farah E., Maines M.D. Human biliverdin reductase-based peptides activate and inhibit glucose uptake through direct interaction with the kinase domain of insulin receptor. Faseb. J. 2014;28:2478–2491. doi: 10.1096/fj.13-247015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haluzik M., Gavrilova O., LeRoith D. Peroxisome proliferator-activated receptor-alpha deficiency does not alter insulin sensitivity in mice maintained on regular or high-fat diet: hyperinsulinemic-euglycemic clamp studies. Endocrinology. 2004;145:1662–1667. doi: 10.1210/en.2003-1015. [DOI] [PubMed] [Google Scholar]
- 50.Marchesini G., Brizi M., Bianchi G., Tomassetti S., Bugianesi E., Lenzi M., McCullough A.J. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 51.Monetti M., Levin M.C., Watt M.J., Sajan M.P., Marmor S., Hubbard B.K., Stevens R.D. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metabol. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Minehira K., Young S.G., Villanueva C.J., Yetukuri L., Oresic M., Hellerstein M.K., Farese R.V., Jr. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J. Lipid Res. 2008;49:2038–2044. doi: 10.1194/jlr.M800248-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asai A., Chou P.M., Bu H.F., Wang X., Rao M.S., Jiang A., DiDonato C.J. Dissociation of hepatic insulin resistance from susceptibility of nonalcoholic fatty liver disease induced by a high-fat and high-carbohydrate diet in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;306:G496–G504. doi: 10.1152/ajpgi.00291.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amaro A., Fabbrini E., Kars M., Yue P., Schechtman K., Schonfeld G., Klein S. Dissociation between intrahepatic triglyceride content and insulin resistance in familial hypobetalipoproteinemia. Gastroenterology. 2010;139:149–153. doi: 10.1053/j.gastro.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dekker D., Dorresteijn M.J., Pijnenburg M., Heemskerk S., Rasing-Hoogveld A., Burger D.M., Wagener F.A. The bilirubin-increasing drug atazanavir improves endothelial function in patients with type 2 diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2011;31:458–463. doi: 10.1161/ATVBAHA.110.211789. [DOI] [PubMed] [Google Scholar]
- 56.Li M., Chan W.W., Zucker S.D. Association between atazanavir-induced hyperbilirubinemia and cardiovascular disease in patients infected with HIV. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller E.R., 3rd, Pastor-Barriuso R., Dalal D., Riemersma R.A., Appel L.J., Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 58.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012 doi: 10.1002/14651858.CD007176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klein E.A., Thompson I.M., Jr., Tangen C.M., Crowley J.J., Lucia M.S., Goodman P.J., Minasian L.M. Vitamin E and the risk of prostate cancer: the selenium and vitamin E cancer prevention trial (SELECT) J. Am. Med. Assoc. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schürks M., Glynn R.J., Rist P.M., Tzourio C., Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. Br. Med. J. 2010;341 doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.