Abstract
SARS-CoV-2 is a positive-sense RNA virus and it is the causative agent of the global COVID-19 outbreak. COVID-19 is similar to the previous outbreaks for instance SARS in 2002-2003 and MERS in 2012. As the peptides have many advantages, peptide-based therapeutics might be one of the possible ways in the development of COVID-19 specific drugs. SARS-CoV-2 enters into a human via its S protein by attaching with human hACE2 present on the cell membrane in the lungs and intestines of humans. hACE2 cleaves S protein into the S1 subunit for viral attachment and the S2 subunit for fusion with the host cell membrane. The fusion mechanism forms a six-helical bundle (6-HB) structure which finally fuses the viral envelope with the host cell membrane. hACE2 based peptides such as SBP1 and Spikeplug have shown their potential as antiviral agents. S protein-hACE2 interaction and the SARS-CoV-2 fusion machinery play a crucial part in human viral infection. It is evident that if these interactions could be blocked successfully and efficiently, it could be the way to find the drug for COVID-19. Several peptide-based inhibitors are potent inhibitors of S protein-hACE2 interaction. Similarly, the antiviral activity of the antimicrobial peptide, lactoferrin makes it an important candidate for the COVID-19 drug development process. A candidate drug, RhACE2-APN01 based on recombinant hACE2 peptide has already entered phase II clinical trials. This review sheds light on different aspects of the feasibility of using peptide-based therapeutics as the promising therapeutic route for COVID-19.
Keywords: COVID-19, SARS-CoV-2, Pandemic, Peptides-based therapeutics, S protein-hACE2 interaction, In silico method
Abbreviations
- 6-HB
six-helical bundle
- ACE
angiotensin-converting enzyme
- AMPs
antimicrobial peptide database
- ARDS
acute respiratory distress syndrome
- AVPs
antiviral peptides
- BPPs
bradykinin potentiating peptides
- COVID-19
coronavirus disease 2019
- COVs
human coronaviruses
- CT
cytoplasmic tail
- CTD
carboxy(C)-terminal domain
- FDA
Food and Drug Administration
- FP
fusion peptide
- hACE2
human angiotensin-converting enzyme 2
- hAPN
human aminopeptidase
- hDPP4
human dipeptidyl pepsidase4
- HKU1
human coronavirus HKU1
- HR1
heptad repeat 1
- HR2
heptad repeat 2
- LF
lactoferrin
- MERS
Middle East respiratory syndrome
- NTD
amino(N)-terminal domain
- RBD
receptor-binding domain
- RNA
ribonucleic acid
- RTD-1
rhesus theta-defensin 1
- S (protein)
spike protein
- SARS
severe acute respiratory syndrome
- SARS-COV2
severe acute respiratory syndrome coronavirus 2
- SVM
support vector machine
- TM
tansmembrane region
- WHO
World Health Organization
Introduction
Origin of COVID-19 and symptoms
The novel Betacoronavirus named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the causative agent of coronavirus disease-19 (COVID-19) [1], [2]. The term COVID-19 was issued by World Health Organization (WHO) and the name SARS-CoV-2 was suggested by the Coronaviridae Study Group of the International Committee on Taxonomy of Viruses which published a detailed taxonomic classification on SARS-CoV-2 [3].
The majority of the COVID-19 patients reported mild symptoms like fever, nonproductive cough, and sore throat which are treated effectively. But the patients who develop fatal complications such as organ failure, acute cardiac and kidney injury, pulmonary edema, pneumonia, and acute respiratory distress syndrome (ARDS) are difficult to treat, and depending upon the severity of the case death may occur [4]. Patients with a weak immune system and prevailing comorbidities like cerebral and cardiovascular diseases, diabetes, obesity, cancer, and respiratory diseases, and old age are at maximum risk [5]. The virus has been confirmed to be of zoonotic origin [6].
Coronaviruses and their associated diseases
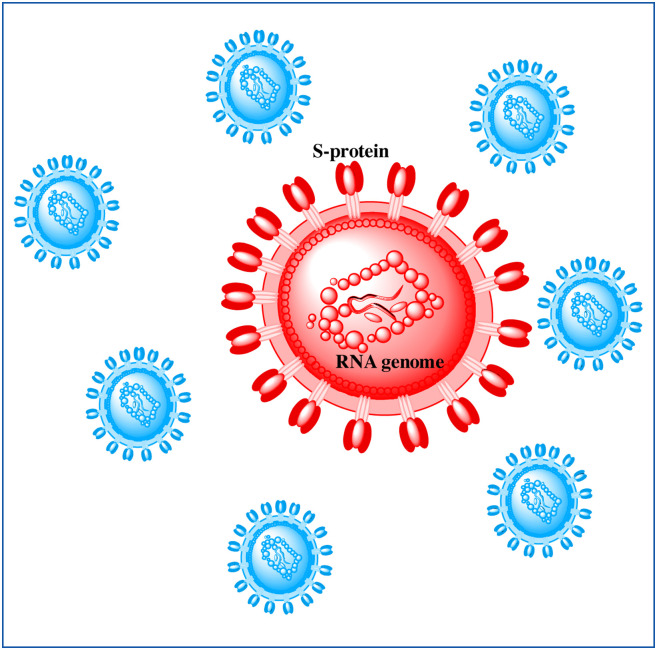
Coronaviruses are enveloped (spherical or pleomorphic) particles comprising a single-stranded positive-sense unsegmented ribonucleic acid (RNA) genome. A matrix of protein forms a capsid that is closely associated with the RNA. The envelope consists of club-shaped glycoproteins throughout its surface which are protruding outwards. These glycoproteins spikes resemble the shape of a crown, hence the name is corona (Fig. 1 ) [7]. Coronaviruses can be divided into four genera: Alphacoronaviruses, Betacoronaviruses, Gammacoronavirus, and Deltacoronavirus [8]. In general, coronaviruses are prominent throughout the animal kingdom including bats, camels, civet cats, pangolins, birds, rodents, and domestic animals as well. There are seven human coronaviruses known which fall under alpha (NL63, and 229E) and beta sub-groups (HKU1, OC43, Middle East respiratory syndrome coronavirus [MERS-CoV], SARS-CoV, and SARS-CoV-2) [9], [10]. The two coronavirus-related outbreaks like severe acute respiratory syndrome (SARS) and MERS were caused by SARS-CoV and MERS-CoV respectively. Both of these outbreaks were caused by zoonotic transmission possibly from bats to humans via intermediate hosts civet cats (MERS) and dromedary camels (SARS) [11], [12]. Currently, there are no specific treatments or antivirals available to battle any of the diseases. Antiviral drugs like ribavirin, interferon, lopinavir, ritonavir, interferons, and corticosteroids were used in the SARS and MERS patients, but their effectiveness is controversial. Traditional preventive measures, including travel restrictions and patient isolation, gradually halted the SARS epidemic in 2002-03 and MERS in 2012 [11]. The COVID-19 pandemic is far more rapid as compared to SARS and MERS. Since the emergence of COVID-19 in Dec-2019, the number of cases by mid-Sep-2020 has been recorded to be 29,729,993 out of which 939,188 corresponds to total deaths [13].
Figure 1.
The diagrammatic representation of SARS-CoV-2. RNA: ribonucleic acid;S protein: spike protein; SARS-CoV2: severe acute respiratory syndrome coronavirus 2.
Repurposed drugs for the pandemic
The pandemic needs to be checked as soon as it is possible. The COVID-19 pandemic has more or less stopped the world. To this date, there is no effective and specific therapy for COVID-19. The traditional drug discovery method is time taking. Scientists and researchers all over the world are working round the clock to find the cure for COVID-19. Antiviral drugs such as ribavirin, nafamostat, penciclovir, chloroquine, nitazoxanide, remdesivir, and favipiravir were repurposed against SARS-CoV-2 in vitro by Wang et al. (2020) [14]. At the earlier stage of the study, it was discovered that remdesivir and chloroquine as highly effective against SARS-CoV-2 infection. The researchers emphasized these pre-developed drugs as effective candidates for COVID-19, as these are Food and Drug Aministatration (FDA)-approved against Ebola virus infection (remdesivir), malaria, and auto-immune diseases (chloroquine) [14]. But in a recent randomized clinical trial with moderate COVID-19 patients, therapy with remdesivir did not show results with any clinical importance [15]. Presently, there are 24 ongoing trials with chloroquine and hydroxychloroquine [16]. No benefit of the use of hydroxychloroquine has been identified in COVID-19 and is rather unsafe [17].
Peptides as emerging therapeutics
Peptides are easy to develop both in terms of time, and technology; and the peptides are cost-effective. Peptides are small fragments of proteins consisting typically of 2-50 amino acid residues bound together by amide bonds. Peptide-based drug candidates or structurally modified peptides have several advantages such as easy availability, low production cost, better bioavailability, low immunogenic responses, high specificity, high biological activity, low intrinsic toxicity, convenient purification, and storage [18]. Peptides-based therapeutics can be developed to resolve some of their disadvantages such as instability in serum, low bioavailability, and hydrophobicity through different types of modifications in peptide backbone, amino acid side chains, and peptide structure (primary, secondary, tertiary, and quaternary) [19].
Peptides are suitably designed and formulated by adding additives to administer through the non-invasive routes like oral, pulmonary, buccal, vaginal, transdermal, and ocular drug delivery systems. Polymer carriers, penetration enhancers, enzyme inhibitors are used as additives in formulating the peptides. The suitably formulated peptide enhances the absorption and stability of peptide-based drugs. The peptides for the oral route of administration are designed to protect from proteolytic enzymes and an acidic environment. The use of permeability enhancers, design of peptide analogs, prodrug strategy, and mucoadhesive delivery is actively under the consideration to enable the peptide drug delivery by oral route. The lower molecular weight (< 75-100 D) of the peptide, entrapment of peptide in a liposome, and nanoscale formulation of the peptide have also shown significant improvement in bioavailability when the peptides were administered through the oral route [20].
Proteins are positively/negatively charged large molecules with hydrophilic properties in nature. Therefore, the peptides permeate the cell membrane poorly. The penetration of peptides across biological membranes like plasma membranes, and nuclear membrane limits their application as therapeutic agents. However, the problems associated with the membrane permeability of peptides can be overcome by the use of a viral/non-viral carrier delivery system. Cell-penetrating peptides are effective in penetrating the cells to deliver therapeutic agents. Cell-penetrating peptides are small molecules (∼30 amino acids), amphipathic and hydrophobic; and the basic mechanism may be endocytosis/direct penetration [21]. More than 60 US FDA-approved peptides are in the markets, more than 150 peptides are in clinical trials, 260 peptides are already tested in human clinical trials, and about 500 peptides are in preclinical phases [22], [23].
Peptides targetting the viral entry
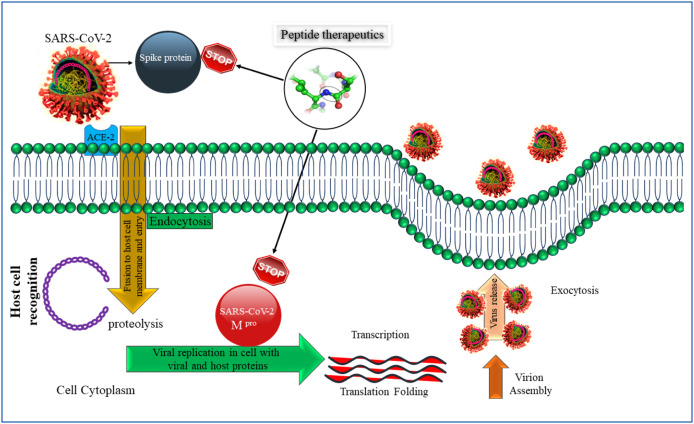
SARS-CoV-2 recognizes the human angiotensin-converting enzyme 2 (hACE2) domain present in the cell membrane. The attachment causes a downstream signaling process. Peptide-based therapeutics interacting and attaching with hACE2 would cease the virus-receptor interaction. Similarly, peptides might also attack SARS-CoV-2 Mpro protein inside the cell membrane thus stopping the virus replication (Fig. 2 ). At this moment, only a handful of published studies (Table 1 ) are available that have the perspectives of targeting the interaction of S protein of the viral particle with the cell surface receptor (hACE-2) of the host cell via peptide-based therapeutics. As the entry point of the virus is the most critical part of the onset of the infection, targeting this point of interaction might lead to the discovery and design of the specific drug for COVID-19. The objectives of this review are to throw light on the ongoing and pre-existing peptide-based antiviral therapeutics, their discovery methods, and their viability in this outbreak. One of the major objectives is to save human lives which could be done by the immediate development of a drug for COVID-19. The significance and potential of peptide-based therapeutics are elaborated in this review. Besides, it also reveals the importance of S protein–hACE2 interaction as a therapeutic target. The importance of AMPs, hACE2 fragments, and in silico method of peptide-based drug design are also elucidated in this review.
Figure 2.
Mechanistic pathway of peptide-based drug that interacts with Spike protein and SARS-CoV-2 Mpro protein. SARS-CoV2: severe acute respiratory syndrome coronavirus 2.
Table 1.
Peptide-based therapeutics for of SARS-CoV-2.
| Peptide-based therapeutics | Types | Targets | Stage of the development | References |
|---|---|---|---|---|
| SBP1 | hACE2 fragment | SARS-CoV-2-RBD | Preclinical | [33] |
| Spikeplug | hACE2 fragment | SARS-CoV-2-RBD | Preclinical | [34] |
| Inhibitors 1-4 | hACE2 fragment | SARS-CoV-2-RBD | Theoretical | [35] |
| Not available | In silico | SARS-CoV-2 RBD | Theoretical | [54] |
| EK1 | HR2 domain of HCoV-OC43 | S protein-HR1 | Preclinical | [37] |
| EK1C4 | HR2 domain of HCoV-OC43 | S protein-HR1 | Preclinical | [38] |
| 2019-nCoV-HR1P & HR2P | HR1 & HR2 of SARS-CoV-2 | S protein-HR1 | Preclinical | [37] |
| IBP01 | HR2 of SARS-CoV-2 | S protein-HR1 | Preclinical | [32] |
| P9R | AMP | Viral binding and endosomal acidification | Preclinical | [49] |
| AC20, AC23, DBP6, and cnCoVP-1- cnCoVP-7 | In silico | S protein- hACE2 interaction | Theoretical | [51] |
| HR2-anti-P | In silico | S protein- hACE2 interaction | Theoretical | [53] |
| RhACE2-APN01 | hACE2 | S protein- hACE2 interaction | Phase- II clinical trial (NCT04335136) | [59] |
Pathogenicity of SARS-CoV-2 in the human
The role of ACE2 in the viral entry
The binding of the viral particle to the host cell via a cell receptor is the initiation of the viral infection. Therefore, the host cell surface receptor is a critical attribute in the cell and tissue tropism of a viral particulate. Out of the seven human coronaviruses (CoVs), two (HKU1, and OC43) bind through sugar and the other five recognizes proteinaceous peptidases. MERS-CoV attaches itself to human dipeptidyl peptidase 4 (hDPP4) [24] while HCoV-229E complexes with human aminopeptidase N (hAPN) as its receptor [25]. SARS-CoV and HCoV-NL63 both use hACE2 as a receptor [6]. The receptor of the SARS-CoV-2 S protein is the human angiotensin-converting enzyme-2 (hACE2) similar to that of the spike (S) protein of SARS [26], [27]. hACE2 is a monocarboxypeptidase, and is a homolog of the angiotensin-converting enzyme (ACE). It is expressed abundantly in the lungs, intestines, kidneys, and heart. hACE2 takes part in the maturation of the peptide hormone, angiotensin, which controls vasoconstriction and maintains blood pressure [27], [28].
Structural segments of SARS-CoV-2 S-protein
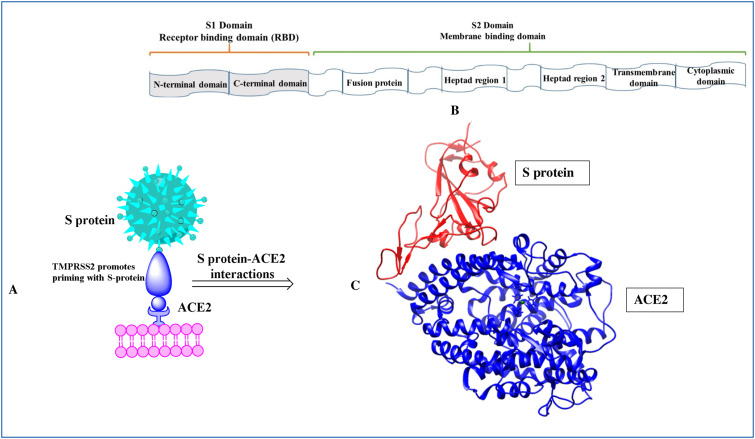
The core element of the host specificity of CoVs is the S glycoprotein embedded in the viral envelope [29]. S protein is a type of class-I fusion protein. S protein is a trimeric protein that possesses an N-terminal S1 subunit and a membrane-embedded C-terminal S2 region (Fig. 3 ). The S2 subunit exists in metastable pre-fusion conformation. Moreover, the S1 subunit of coronavirus is composed of two domains: an amino (N)-terminal domain (NTD) and a carboxy (C)-terminal domain (CTD). The CTD recognizes the receptors on host cells in SARS-CoV and MERS-CoV [30]. The S1 CTD domain is also known as the receptor-binding domain (RBD). A comparative analysis shows that the extent of atomic interaction between SARS-CoV-2 RBD and hACE2 is more than that of SARS-CoV RBD and hACE2 meaning a higher affinity between SARS-CoV-2 RBD and hACE2 [31]. The higher affinity might impart the meaning that SARS-CoV-2 has more infectivity and transmissibility than SARS-CoV.
Figure 3.
(A) The interaction of S-protein of SARS-CoV-2 with the ACE2 [transmembrane protease serine 2 (TMPRSS2) promotes priming of S-protein] (B) The structural segments of S-protein (C) The three-dimensional binding mode of S-protein with ACE2 (PDB ID: 7C8D) [29].
The viral entry into the host cell
The host protease, hACE2 cleaves the S protein into S1 and S2 subunits; the S1 subunit functions to recognize the host cell receptor hence easing the virus attachment to the host cell; while the S2 fuses with the host cell membrane. TMPRSS2, a serine protease has been identified to help in the priming of SARS-CoV-2 S protein. The priming of S protein is necessary for the entry of the virus into the cell. This involves the S protein cleavage at the S1/S2 and the S2′ sites. The S1/S2 cleavage site contains several basic arginine residues which render high cleavable characteristics [26]. Because of receptor binding, the S protein undergoes a considerable conformational change that destabilizes the pre-fusion trimer, leading to the detaching of the S1-subunit and activation of the S2-subunit's fusogenic activity. S2 subunit is composed of an N-terminal fusion peptide (FP), heptad repeat 1 (HR1), heptad repeat 2 (HR2), a transmembrane region (TM), and the cytoplasmic tail (CT). FP is exposed and incorporated into the target cell membrane during the fusion process, leading to S2 in a pre-hairpin intermediate that bridges the membranes of the virus and the cells. Then, a trimeric coiled-coil is self-assembled by three segments of HR1, and three segments of HR2 fold into the grooves on the surface of the inner core of HR1, this results in a formation of a six-helical bundle (6-HB) structure which drives cell and viral membrane together thus initiating the process of membrane fusion [32].
hACE2-based peptides as inhibitors
Zhang et al. (2020) [33] have reported an engineered peptide-based inhibitor as a first-in-class treatment for COVID-19 therapy. hACE2 which specifically binds the S protein was truncated into small fragments. Several hACE2 fragments demonstrate potent antiviral activity and attribute high-binding affinity to SARS-CoV-2. The authors reported a 23-mer peptide, SBP1 which they synthesized via automated fast-flow peptide synthesis in just 1.5 hours. SBP1 peptide showed a micromolar affinity with insect-derived SARS-CoV-2-RBD protein [33]. This discovery raises interest in using an engineered peptide as an antiviral agent for COVID-19 treatment.
In a preprint made available on bioRxiv, the authors engineered an 85-mer N-terminal truncate of the hACE2 mini-protein, Spikeplug with the motive of creating a stable and soluble S protein interactor. Spikeplug has an α-helical conformation and interacts at a nanomolar affinity with SARS-CoV-2-RBD protein. Spikeplug was designed based on the interaction between the S protein and hACE2 amino acid residues. According to the authors, the receptor mimic, Spikeplug would recognize the viral S protein and thus block the viral entry into the cells. Further, Spikeplug can efficiently cover the interaction with S protein and hence raise its potential to stop the interactions with the hACE2 receptor [34].
On analyzing the interacting amino acids from the crystal structure of hACE2 and SARS-CoV-2 RBD, Han et al. (2020) identified 15 critical amino acid residues in combination with α-helices- α1, and α2 and β-sheets- β-1and β-2 as critical binding components from hACE2. They designed peptide inhibitors extracted from hACE2 and showed that these are promising in blocking the SARS-CoV-2 virus through molecular dynamics simulations. Out of their designed inhibitors, inhibitors 2-4 were more stable than inhibitor 1. Because the former uses α1 and α2 which can support each other and they also retain their bent shapes thereby matching conformationally and covering the whole surface of RBD. The authors further explained that the binding affinity of the peptides could be improved and be used in the future to design more inhibitors [35].
Peptide inhibitors targeting S protein-hACE2 interaction
S protein-hACE2 interaction is the basis of the viral infection. The elucidation of the interaction between the RBD domain of S protein and hACE2 via electron microscope has been completed recently. It has revealed that polar residues from the peptidase recognize the RBD domain which can be a potential target for the development of a novel drug [27]. Alternatively, the SARS-CoV-2 fusion machinery can also be treated as an important target for coronavirus fusion inhibitor development.
EK1, a pan-coronavirus fusion inhibitor was developed to target the HR1 domains of HCoV S proteins. It was proved to be effective in MERS as well [36]. EK1 molecule, when tested on hACE2-expressing 293 T cells infected with SARS-CoV-2 pseudovirus, showed a half-maximal inhibitory concentration (IC50) of 2.38 μM [37]. On conjugation of a cholesterol molecule with EK1, EK1C4 lipopeptide was formed that displayed extremely potent inhibitory action against SARS-CoV-2 S protein mediated-membrane fusion. In vitro and in vivo experiments showed that EK1C4 was 240-times more potent than EK1 peptide. EK1C4 has also shown promising results with other coronaviruses, for example, HCoVOC43 and MERS-CoV [38]. Based on the previously designed inhibitors of SARS-CoV and MERS- CoV where S- heptad repeat 1 (HR1) of the fusion machinery was used as the target, HR1 and heptad repeat 2 (HR2) derived peptide inhibitors were designed for SARS-CoV-2 named as 2019-nCoV-HR1P and 2019-nCoV-HR2P. 2019-nCoV-HR2P showed significant fusion-inhibitory activity with an IC50 of 0.18 μM in vitro [37]. This observation points out the importance of targeting the HR1 region of the S protein for the development of a potent peptide inhibitor.
In a detailed study by Zhu et al. (2020) [32], it was confirmed that the S protein of SARS-CoV-2 has increased fusogenic properties as compared to the S protein of SARS-CoV. They developed an HR2 based lipopeptide to function as an inhibitor of the fusogenic machinery. Lipopeptides interact with viral envelopes increasing the concentration of the peptide inhibitor at the site where the fusion is supposed to take place. Thus, it increases the inhibition capacity of the peptide inhibitor. On the C-terminal end of a peptide IBP01, derived from HR2-a cholesterol moiety was added resulting in a peptide IBP02. DSP-based cell fusion assay was used to examine the inhibitory activity of IBP02 and it revealed that IBP02 inhibited the cell fusion with an IC50 value of 0.025 μM. Further, a single-cell infection assay was carried to evaluate the inhibitory potency of the lipopeptide with pseudovirus. The IC50 values were determined as 0.08 μM and 0.251 μM for SARS-CoV-2 and SARS-CoV respectively [32]. These results are promising and highly suggest that the peptide could be a potent inhibitor of the cell-fusion activity of the viral S protein.
Antimicrobial peptides as peptide-based therapeutics
Antimicrobial peptides (AMPs) can be considered promising candidates for COVID-19 therapy. Antimicrobial peptides are amphipathic molecules ranging from 5-50 amino acid sequences and show secondary α-helix, β-sheets, and other configurations [39]. The amphipathic structure is due to the presence of 40–50% hydrophobic residues. In general, AMPs are cationic molecules (+2 to +9). These properties are the basis of the mechanism of action against various pathogens. For example, cations cause electrostatic attraction with anionic phospholipids present on the microbial membrane and the hydrophobicity helps the AMPs to incorporate themselves with the viral membrane, causing membrane lysis. Also, the amphipathic nature of AMPs enables them to be soluble in both aqueous surroundings and lipid membranes [40].
The antimicrobial peptide database (APD) features 3241 AMPs including 190 peptides exhibiting antiviral activities [41]. In another database called AVPdb contains 2683 peptides, among which 624 are modified peptides evaluated for antiviral activity experimentally and 2059 are normal peptides tested on different cell lines with varying effectiveness. There are 76 peptides sourced from SARS-CoV [42]. These databases could be screened further to discover antiviral peptides that might be potent against COVID-19.
Lactoferrin (LF) is an iron-binding glycoprotein that is present in several mucosal secretions. LF, which is proved to be potent against several pathogens including viruses. It can also inhibit replication in DNA or RNA viruses including rotavirus, respiratory syncytial virus, herpes viruses, and HIV [43]. Lang et al. (2011) used LF against SARS-CoV and showed that LF prevented dose-dependent infection with the SARS pseudo-virus. They further suggested that LF exercised its inhibitory role at the viral attachment stage and could block the binding of spike protein to host cells [44]. LF prevents viral entry into the host cell by either blocking cell receptors or by direct binding to virus particles. It is not reported to function against the virus after infection. LF's substantial antiviral activity makes it a possible alternative to be used as a drug or a drug-conjugate with traditional antivirals [45].
Defensins are highly abundant and play a central role in innate immunity in humans [46]. Defensins have been found to show antiviral activities in SARS-CoV [47]. The theta-defensin, Rhesus theta-defensin 1 (RTD-1), a cyclic AMP with three disulfide bonds was first located in rhesus macaque leukocytes and is not expressed in humans. Wohlford-Lenane et al. (2009) used RTD-1 in mouse SARS-CoV models to evaluate its therapeutic properties and found that RTD-1 intranasal application has shown a 100% survival rate in the infected mouse whereas the untreated mouse death rate was 75%. The authors concluded that RDT-1 did not participate in the direct inactivation of the viral particle but rather revealed an immunomodulatory mechanism of action as indicated by the expression of the N gene antigen in lung tissue [48].
Zhao et al. (2020) developed an antiviral peptide with broad-spectrum activity, P9R from a pre-existing P9 peptide, and mouse β-defensin-4. P9R showed antiviral potency towards SARS-CoV-2, MERS-CoV, SARS-CoV, A(H1N1)pdm09, A(H7N9) virus, and rhinovirus. P9R's antiviral activity involved both viral binding, and endosomal acidification inhibition. The authors demonstrated that a rise in the positive charge of P9R raised the inhibition of endosomal acidification [49]. These types of broad-spectrum antiviral peptides are today's urgent need and are a must for fighting the emerging and re-emerging viral outbreaks.
In silico method of designing peptide-based therapeutics
Often peptides synthesized are not potent enough to advance the drug development process. Hence, it is advantageous to design peptides by in silico methods which could be time and cost-effective. AVPpred is a support vector machine (SVM)-based in silico method that could be used for the prediction of antiviral peptides (AVPs). The algorithm for AVPpred is based on positive and negative data sets of experimentally verified AVPs against several viral diseases. The authors have created four different models of predictions of AVPs based on parameters like composition, physicochemical, sequence, and alignment [50]. By the use of this webserver, researchers are anticipated to develop peptide-based therapeutics for SARS-CoV-2 on-chip and then to the laboratory for experimental verifications.
Barh et al. (2020) [51] utilized bioinformatics tools to design potential peptide therapeutics which could act on the S protein-hACE2 interaction. They utilized three strategies: first, analyzing the major amino acids residues that are interacting between S protein and hACE2; second, screening some databases of AMPs against SARS-CoV-2 S protein RBD domain; and lastly, designing a chimeric peptide from the two fragments of two different peptides obtained from the above first two steps. The authors designed several hybrid peptides and the resulting peptides were docked via HPEPDOCK server with the S protein RBD target residues [51]. HPEPDOCK is a blind peptide-peptide docking web server that works on a hierarchical algorithm [52]. Out of the cluster of about 500 peptides, only 10 peptides (AC20, AC23, DBP6, and cnCoVP-1- cnCoVP-7) showed the characteristics to block the S protein-hACE2 interaction [53].
Ling and co-workers (2020) [53] have designed an AVP that could target the S protein of SARS-CoV-2. They designed an HR2-based AVP based on biomolecular simulations of the fusion core and HR2 of SARS-CoV-2. They found that the binding energy of HR1 and HR2 of the virus is lower than that of HR1 and HR2-based AVP (HR2-anti-P) signifying that the HR2-anti-P binds competitively to HR1. Thus, the entry of the virus into the cell can be prohibited by blocking the formation of the fusion mechanism [53]. Based on the crystal structure of the SARS-CoV-2 RBD/hACE2 complex, hybrid peptides were created from two different peptide fragments of hACE2 that could block the binding of S protein with hACE2. The fragments were attached via a glycine residue. By the use of protein designing tools like EvoEF2 and EvoDesign, the amino acid sequences of the hybrid peptides were completely re-designed so that they could match the peptide scaffold and also increase the binding affinity to SARS-CoV-2 [54]. There is an urgent requirement of in vitro and in vivo studies to evaluate the above-mentioned peptides generated through in silico methods.
Baig et al. [55] identified the peptide inhibitors to restrict the entry of SARS-CoV-2 into the host cell using in silico methods like molecular docking and dynamics. SARS-CoV-2 enters into the cell based on the interaction between the S protein of the virus and the ACE2 of the cell. Therefore, the peptide has been designed by blocking the interaction of S protein-ACE2. A short sequence of twenty-three amino acids (PDB ID: (6M17-2019-nCoV RBD/ACE2-B0AT1 complex) from Glu23 to Leu45 was selected and the initial five amino acids were removed to design the eighteen amino acids peptide. The peptides were designed by the alanine scanning method by analyzing the importance of each of the contributing amino acids. The results of the designed peptides were analyzed based on the binding free energies and conformation. However, it is not validated with in-vitro/in-vivo studies [55].
The immunoinformatic approaches have been employed to design multiepitope-based vaccines for the treatment of COVID-19. The vaccine was designed by fusing B cells, cytotoxic T lymphocytes, and helper T lymphocytes and by linking β-defensin and pan-HLA DR binding epitopes through the EAAAK linker at the N-terminal. Further, a short TAT sequence comprising eleven amino acids was introduced at the C-terminal. The molecular docking was carried out to determine the interaction between the vaccine and immune receptors. However, the efficacy of the vaccine has not been evaluated experimentally [56].
Other peptide-based therapeutics
A recent literature review has suggested the use of snake-derived bradykinin-potentiating peptides (BPPs), particularly BPP-10c in developing the drug for COVID-19. BPP-10c increases bradykinin-related effects and decreases angiotensin II levels by inhibiting ACE. BPP-10c is also safe as no cytotoxic effects have been observed in its administration. BPP-10c exhibits organo-protective and antihypertensive effects. As COVID-19 is involved in organo-destructive effects, the administration of BPP-10c may help in reducing the severity of COVID-19 infection [57], [58].
A phase-II clinical trial (NCT04335136) with more than 200 participants is being conducted to study the therapeutic effect (RhACE2-APN01). The trial is being conducted at a multinational level consisting of Austria, Denmark, and Germany [59].
Interferon β is an acid-resistant glycoprotein. Recombinantly developed interferon β has been under consideration for the various therapeutic actions. The regulation of the immunological response of interferon β after the viral infection is well known. The safety and efficacy of interferon β-1a for the treatment of severe COVID-19 have been evaluated in a randomized clinical trial. The study employed forty-two patients who received interferon β-1a (44 μg/mL three times in a week for two consecutive weeks) along with hydroxychloroquine, lopinavir, and ritonavir or atazanavir-ritonavir. The control groups consisted of thirty-nine patients who received only hydroxychloroquine, lopinavir, and ritonavir, or atazanavir-ritonavir. The interferon treated group has decreased the 28 days mortality and increased the 14 days discharge rate among the COVID-19 patients [60], [61].
Conclusion
COVID-19 is growing unprecedently and has become a threat to humanity. The search for the cure of COVID-19 is indispensable; this has led the researchers to search the available domains for the development of COVID-19 specific drugs. Peptide-based therapeutics (structurally modified peptides) have many advantages in comparison to antibody-based treatment and small-molecule drugs. The advantages of peptides are easy availability, low production cost. The structurally modified peptides have shown improved bioavailability, low immunogenic responses, high biological activity, low intrinsic toxicity. Moreover, the time taken to develop a peptide drug is also less. The peptide-based therapeutics used in several preclinical studies reviewed in this paper imparts the importance of peptide and peptide-based therapeutics in the development of the drug for COVID-19. Further, as discussed herein S protein–hACE2 interaction is a very promising target for a potential drug investigation. AMPs might be a good candidate drug for the COVID-19 as the majority of them exist as natural entities and are even produced in the human body; hence they are non-toxic and safe. Time is a critical factor here as each passing day is costing thousands of lives. Computer-aided peptide drug discovery could play a crucial role in this aspect. Moreover, a peptide-based drug, RhACE2-APN01 based on recombinant hACE2 peptide has already entered phase II clinical trials. This review reveals many facets of the feasibility of using peptide-based therapeutics as the promising therapeutic route for COVID-19. More detailed preclinical studies and inputs from the scientific community are required for the development and screening of peptide-based therapeutics against COVID-19.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors gave consent to publication.
Funding
None.
Author's contributions
Conceived and designed the Review: JNS, GQG, KA, and MR. Draft the Manuscript: JNS, NKK, MS, MHA, SKS, and KD. All authors provided critical review in updating the final version of the review.
Data and material availability
Review data and materials available on request with first author and corresponding author.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
The first author (JNS) is supported by the Chinese Scholarship Council (2019SLJ018630). One of the authors (MHA) thankfully acknowledges the Taif University researcher supporting project Number TURSP/91, Taif University, Taif, Saudi Arabia.
References
- 1.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A., et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyrrell D.A.J., Myint S.H. In: Medical microbiology. 4th edition. Baron S., editor. The University of Texas Medical Branch at Galveston; Galveston (TX): 1996. Coronaviruses. [ISBN-10: 0-9631172-1-1] [Google Scholar]
- 8.International Committee on Taxonomy of Viruses. Virus taxonomy: 2019 Release 2020. https://talk.ictvonline.org/taxonomy/.[Accessed 30 September 2021].
- 9.Lu G., Wang Q., Gao G.F. Bat-to-human: spike features determining ‘host jump’ of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015;23:468–478. doi: 10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wevers B.A., van der Hoek L. Recently discovered human coronaviruses. Clin Lab Med. 2009;29(4):715–724. doi: 10.1016/j.cll.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 13.Worldometer . 2021. COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus/. [Accessed 30 September 2021] [Google Scholar]
- 14.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spinner C.D., Gottlieb R.L., Criner G.J., Arribas López J.R., Cattelan A.M., Soriano Viladomiu A., et al. Effect of remdesivir vsstandard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis J.S., Ferreira D., Denholm J.T., Tong S.Y. Clinical trials for the prevention and treatment of COVID-19: current state of play. Med J Aust. 2020;213:86–93. doi: 10.5694/mja2.50673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez M.A. Clinical trials of repurposed antivirals for SARS-CoV-2. Antimicrob Agents Chemother. 2020;64:e01101–e01120. doi: 10.1128/AAC.01101-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyagi A., Tuknait A., Anand P., Gupta S., Sharma M., Mathur D., et al. CancerPPD: a database of anticancer peptides and proteins. Nucleic Acids Res. 2015;43(Database issue):D837–D843. doi: 10.1093/nar/gku892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erak M., Bellmann-Sickert K., Els-Heindl S., Beck-Sickinger A.G. Peptide chemistry toolbox - Transforming natural peptides into peptide therapeutics. Bioorg Med Chem. 2018;26:2759–2765. doi: 10.1016/j.bmc.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Jitendra, Sharma P.K., Bansal S., Banik A. Noninvasive routes of proteins and peptides drug delivery. Indian J Pharm Sci. 2011;73:367–375. doi: 10.4103/0250-474X.95608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kardani K., Milani A., Shabani S.H., Bolhassani A. Cell penetrating peptides: the potent multi-cargo intracellular carriers. Expert Opin Drug Deliv. 2019;16:1227–1258. doi: 10.1080/17425247.2019.1676720. [DOI] [PubMed] [Google Scholar]
- 22.Fosgerau K., Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Lau J.L., Dunn M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg Med Chem. 2018;26:2700–2707. doi: 10.1016/j.bmc.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 24.Kim J., Yang Y.L., Jeong Y., Jang Y.S. Middle east respiratory syndrome-coronavirus infection into established hDPP4-transgenic mice accelerates lung damage via activation of the pro-inflammatory response and pulmonary fibrosis. J Microbiol Biotechnol. 2020;30:427–438. doi: 10.4014/jmb.1910.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z., Tomlinson A.C., Wong A.H., Zhou D., Desforges M., Talbot P.J., et al. The human coronavirus HCoV-229E S-protein structure and receptor binding. Elife. 2019;8:e51230. doi: 10.7554/eLife.51230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel V.B., Lezutekong J.N., Chen X., Oudit G.Y. Recombinant human ACE2 and the angiotensin 1-7 axis as potential new therapies for heart failure. Can J Cardiol. 2017;33:943–946. doi: 10.1016/j.cjca.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Wu L., Chen Q., Liu K., Wang J., Han P., Zhang Y., et al. Broad host range of SARS-CoV-2 and the molecular basis for SARS-CoV-2 binding to cat ACE2. Cell Discov. 2020;6:68. doi: 10.1038/s41421-020-00210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L., et al. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904.e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Y., Yu D., Yan H., Chong H., He Y. Design of potent membrane fusion inhibitors against SARS-CoV-2, an emerging coronavirus with high fusogenic activity. J Virol. 2020;94 doi: 10.1128/JVI.00635-20. [e00635-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang G., Pomplun S., Loftis A.R., Tan X., Loas A., Pentelute B.L. Investigation of ACE2 N-terminal fragments binding to SARS-CoV-2 spike RBD. bioRxiv. 2020 doi: 10.1101/2020.03.19.999318. https://www.biorxiv.org/content/10.1101/2020.03.19.999318v2.full. [Accessed 30 September 2021] [DOI] [Google Scholar]
- 34.Romano M., Ruggiero A., Squeglia F., Berisio R. An engineered stable mini-protein to plug SARS-Cov-2 Spikes. bioRxiv. 2020 doi: 10.1101/2020.04.29.067728. https://www.biorxiv.org/content/10.1101/2020.04.29.067728v1.full. [Accessed 30 September 2021] [DOI] [Google Scholar]
- 35.Han Y., Král P. Computational design of ACE2-based peptide inhibitors of SARS-CoV-2. ACS Nano. 2020;14:5143–5147. doi: 10.1021/acsnano.0c02857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia S., Yan L., Xu W., Agrawal A.S., Algaissi A., Tseng C.K., et al. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci Adv. 2019;5 doi: 10.1126/sciadv.aav4580. [eaav4580] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia S., Zhu Y., Liu M., Lan Q., Xu W., Wu Y., et al. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol Immunol. 2020;17:765–767. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fry D.E. Antimicrobial peptides. Surg Infect (Larchmt) 2018;19:804–811. doi: 10.1089/sur.2018.194. [DOI] [PubMed] [Google Scholar]
- 40.Cederlund A., Gudmundsson G.H., Agerberth B. Antimicrobial peptides important in innate immunity. FEBS J. 2011;278:3942–3951. doi: 10.1111/j.1742-4658.2011.08302.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang G., Li X., Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2015;44:D1087–D1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qureshi A., Thakur N., Tandon H., Kumar M. AVPdb: a database of experimentally validated antiviral peptides targeting medically important viruses. Nucleic Acids Res. 2014;42(Database issue):D1147–D1153. doi: 10.1093/nar/gkt1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Strate B.W.A., Beljaars L., Molema G., Harmsen M.C., Meijer D.K.F. Antiviral activities of lactoferrin. Antiviral Research. 2001;52:225–239. doi: 10.1016/s0166-3542(01)00195-4. [DOI] [PubMed] [Google Scholar]
- 44.Lang J., Yang N., Deng J., Liu K., Yang P., Zhang G., et al. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS One. 2011;6:e23710. doi: 10.1371/journal.pone.0023710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elnagdy S., AlKhazindar M. The potential of antimicrobial peptides as an antiviral therapy against COVID-19. ACS Pharmacol Transl Sci. 2020;3:780–782. doi: 10.1021/acsptsci.0c00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holly M.K., Diaz K., Smith J.G. Defensins in viral infection and pathogenesis. Annu Rev Virol. 2017;4:369–391. doi: 10.1146/annurev-virology-101416-041734. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed A., Siman-Tov G., Hall G., Bhalla N., Narayanan A. Human antimicrobial peptides as therapeutics for viral infections. Viruses. 2019;11:704. doi: 10.3390/v11080704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wohlford-Lenane C.L., Meyerholz D.K., Perlman S., Zhou H., Tran D., Selsted M.E., et al. Rhesus theta-defensin prevents death in a mouse model of severe acute respiratory syndrome coronavirus pulmonary disease. J Virol. 2009;83:11385–11390. doi: 10.1128/JVI.01363-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao H., To K.K.W., Sze K.H., Yung T.T., Bian M., Lam H., et al. A broad-spectrum virus- and host-targeting peptide against respiratory viruses including influenza virus and SARS-CoV-2. Nat Commun. 2020;11:4252. doi: 10.1038/s41467-020-17986-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thakur N., Qureshi A., Kumar M. AVPpred: collection and prediction of highly effective antiviral peptides. Nucleic Acids Res. 2012;40(Web Server issue):W199–W204. doi: 10.1093/nar/gks450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barh D., Tiwari S., Silva Andrade B., Giovanetti M., Almeida Costa E., Kumavath R., et al. Potential chimeric peptides to block the SARS-CoV-2 spike receptor-binding domain. F1000Res. 2020;9:576. doi: 10.12688/f1000research.24074.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou P., Jin B., Li H., Huang S.Y. HPEPDOCK: a web server for blind peptide-protein docking based on a hierarchical algorithm. Nucleic Acids Res. 2018;46:W443–W450. doi: 10.1093/nar/gky357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ling R., Dai Y., Huang B., Huang W., Yu J., Lu X., et al. In silico design of antiviral peptides targeting the spike protein of SARS-CoV-2. Peptides. 2020;130:170328. doi: 10.1016/j.peptides.2020.170328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang X., Pearce R., Zhang Y. De novo design of protein peptides to block association of the SARS-CoV-2 spike protein with human ACE2. Aging (Albany NY) 2020;12:11263–11276. doi: 10.18632/aging.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baig M.S., Alagumuthu M., Rajpoot S., Saqib U. Identification of a potential peptide inhibitor of SARS-CoV-2 targeting its entry into the host cells. Drugs R D. 2020;20:161–169. doi: 10.1007/s40268-020-00312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong R., Chu Z., Yu F., Zha Y. Contriving multi-epitope subunit of vaccine for COVID-19: Immunoinformatics approaches. Front Immunol. 2020;11:1784. doi: 10.3389/fimmu.2020.01784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gouda A.S., Mégarbane B. Snake venom-derived bradykinin-potentiating peptides: A promising therapy for COVID-19? Drug Dev Res. 2021;82:38–48. doi: 10.1002/ddr.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guerreiro J.R., Lameu C., Oliveira E.F., Klitzke C.F., Melo R.L., Linares E., et al. Argininosuccinate synthetase is a functional target for a snake venom anti-hypertensive peptide. J Biol Chem. 2009;284:20022–20033. doi: 10.1074/jbc.M109.021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo L., Bi W., Wang X., Xu W., Yan R., Zhang Y., et al. Engineered trimeric ACE2 binds viral spike protein and locks it in “Three-up” conformation to potently inhibit SARS-CoV-2 infection. Cell Res. 2021;31:98–100. doi: 10.1038/s41422-020-00438-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goloshchapova E.O., Runova O.B., Ustinnikova O.B. Recombinant interferons beta-1a and beta-1b: Protein structural features and problematic issues with identity confirmation. Pharm Chem J. 2018;52:749–752. [Google Scholar]
- 61.Davoudi-Monfared E., Rahmani H., Khalili H., Hajiabdolbaghi M., Salehi M., Abbasian L., et al. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob Agents Chemother. 2020;64(9):e01061–e1120. doi: 10.1128/AAC.01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]