Abstract
Background
Genital tract infection is associated with preterm birth (before 37 weeks' gestation). Screening for infections during pregnancy may therefore reduce the numbers of babies being born prematurely. However, screening for infections may have some adverse effects, such as increased antibiotic drug resistance and increased cost of treatment.
Objectives
To assess the effectiveness of antenatal lower genital tract infection screening and treatment programs for reducing preterm birth and subsequent morbidity.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 November 2014), the Cochrane Central Register of Controlled Trials (The Cochrane Library 2014, Issue 7) and reference lists of retrieved reports.
Selection criteria
We included all published and unpublished randomised controlled trials in any language that evaluated any described methods of antenatal lower genital tract infection screening compared with no screening.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked for accuracy.
Main results
One study (4155 women at less than 20 weeks' gestation) met the inclusion criteria. The intervention group (2058 women) received infection screening and treatment for bacterial vaginosis, trichomonas vaginalis and candidiasis; the control group (2097 women) also received screening, but the results of the screening program were not revealed and women received routine antenatal care. The rate of preterm birth before 37 weeks' gestation was significantly lower in the intervention group (3% versus 5% in the control group) with a risk ratio (RR) of 0.55 (95% confidence interval (CI) 0.41 to 0.75; the evidence for this outcome was graded as of moderate quality). The incidence of preterm birth for infants with a weight equal to or below 2500 g (low birthweight) and infants with a weight equal to or below 1500 g (very low birthweight) were significantly lower in the intervention group than in the control group (RR 0.48, 95% CI 0.34 to 0.66 and RR 0.34; 95% CI 0.15 to 0.75, respectively; both graded as moderate quality evidence). Based on a subset of costs for preterm births of < 1900 g, the authors reported that for each of those preterm births averted, EUR 60,262 would be saved.
Authors' conclusions
There is evidence from one trial that infection screening and treatment programs for pregnant women before 20 weeks' gestation reduce preterm birth and preterm low birthweight. Infection screening and treatment programs are associated with cost savings when used for the prevention of preterm birth. Future trials should evaluate the effects of different types of infection screening programs.
Plain language summary
Antenatal lower genital tract infection screening and treatment programs for preventing preterm delivery
A genital tract infection during pregnancy can cross into the amniotic fluid and result in prelabour rupture of the membranes and preterm labour. Preterm birth (before 37 weeks of gestation) is associated with poor infant health, death, admission of the newborn to neonatal intensive care in the first few weeks of life, prolonged hospital stay and long‐term neurologic disability including cerebral palsy. In this review, only one study of moderate quality evidence was included. The study reported on 4155 women randomly assigned either to an intervention group (2058 women received infection screening and treatment for bacterial vaginosis, trichomonas vaginalis and candidiasis) or a control group (2097 women received screening, but the results of the screening program were not revealed). The present systematic review found that a simple infection screening and treatment program during routine antenatal care may reduce preterm births and preterm low (below 2500 g) and very low (below 1500 g) birthweight. The simple infection screening reduced preterm births from 5% of women in the control group to 3% in the intervention group. The number of low birthweight preterm infants and very low birthweight preterm infants were significantly lower in the intervention group than in the control group. Moreover, an infection screening and treatment program during routine antenatal care is likely to save over EUR 60,000 for each preterm birth averted.
Summary of findings
Summary of findings for the main comparison. Lower genital tract infection screening versus no screening for preventing preterm delivery.
| Lower genital tract infection screening versus no screening for preventing preterm delivery | ||||||
| Patient or population: pregnant women presenting for routine prenatal care Settings: Vienna, Austria Intervention: lower genital tract infection screening versus no screening | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No screening | Lower genital tract infection screening | |||||
| Preterm birth less than 37 weeks | Study population | RR 0.55 (0.41 to 0.75) | 4155 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 53 per 1000 | 29 per 1000 (22 to 40) | |||||
| Preterm low birthweight (below or equal 2500 g) | Study population | RR 0.48 (0.34 to 0.66) | 4155 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 51 per 1000 | 24 per 1000 (17 to 34) | |||||
| Preterm very low birthweight (below or equal 1500 g) | Study population | RR 0.34 (0.15 to 0.75) | 4155 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 11 per 1000 | 4 per 1000 (2 to 9) | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One study with design limitations.
Background
Description of the condition
Preterm birth, defined as birth occurring prior to 37 weeks' gestation, occurs in 5% to 10% of all pregnancies and is the most common cause of perinatal morbidity and mortality in the world. Preterm birth is implicated in at least two‐thirds of early infant deaths (Cunningham 1997) and 60% of perinatal mortality including long‐term neurologic disability such as cerebral palsy. It is associated with admission to neonatal intensive care, severe morbidity in the first weeks of life, prolonged hospital stay after birth, and readmission to hospital in the first year of life (Cunningham 2001; Goldenberg 1998; Roberts 2000; Wood 2000). Surviving infants, especially those born before 32 weeks, have a substantially increased risk of chronic lung disease, and major and minor impairments (Doyle 1996; Saigal 2000). Whatever the result, the emotional impact on the family can be enormous.
A wide spectrum of causes and demographic factors have been implicated in preterm birth. These can be categorized into four groups.
Medical and obstetric complications: there are associations with placental hemorrhage and hypertensive disorders in about one‐third of cases (Meis 1995).
Lifestyle factors: there is an association with alcohol abuse, low maternal age, and occupational factors (Henriksen 1995; Holzman 1995; Satin 1994).
Amniotic fluid infection caused by a variety of micro‐organisms located in the genital tract: approximately one‐third of preterm births are associated with chorioamniotic infection (Lettieri 1993).
Asymptomatic cervical dilatation (Papiernik 1986).
Many micro‐organisms cause both symptomatic and asymptomatic infection and may result in preterm prelabour rupture of membranes, preterm labour, or both. For example, bacterial vaginosis (including Gardnerella vaginalis, Bacteroides species, Mobiluncus species, Ureaplasma urealyticum, and Mycoplasma hominis) (Hillier 1995; McDonald 1994; McGregor 1990; Meis 1995), Chlamydia trachomatis (Gravett 1986), Trichomonas vaginalis (Cotch 1997), Neisseria gonorrhoeae (Elliott 1990), Group B Streptococci (GBS; Regan 1981), Staphylococcus aureus (McGregor 1990), syphilis (McFarlin 1995), HIV (Temmerman 1994), enteropharyngeal bacteria and Peptostreptococcus species (McDonald 1994) have been associated with an increased risk of preterm birth. Candida species, however, have been associated with an unclear risk of preterm birth (Roberts 2011).
A possible mechanism for the link between infection and preterm birth is the bacterial stimulation of the biosynthesis of prostaglandins. This may occur either directly via phospholipase A2 and C (Bejar 1981) or as a result of bacterial endotoxin introduced into the amniotic fluid which stimulates decidual cells to produce cytokines and prostaglandins that initiate labour (Cox 1989). Indirect links via substances such as interleukin‐1, tumour necrosis factor and platelet activating factor, all of which may be found in infected amniotic fluid, have also been identified (Romero 1992; Yoon 2000).
Description of the intervention
By identifying and treating vaginal infections, screening programs may be able to reduce the rate of preterm birth. Different screening methods are used for different types of organisms, however there is scant evidence to inform the optimal screening regimen for detecting these organisms during pregnancy. Therefore, it is unclear whether all women should be routinely screened, how often the screening should occur, and which tests should be used.
How the intervention might work
Chlamydia trachomatis has been identified by multiple tests from different specimen sources. The samples may be analysed by three types of DNA‐based test: ligase chain reaction, polymerase chain reaction (PCR) and enzyme immuno‐assay (Watson 2002). DNA amplification techniques allow for highly sensitive and specific tests (Black 1997) that are more sensitive than cell culture (Jespersen 2005). These screening tests can detect Chlamydia in genital secretions, urine specimens, and endocervical, vaginal or urethral samples (Domeika 1999; Shrier 2004).
Trichomoniasis may be asymptomatic in up to 50% of infected women (Wolner‐Hanssen 1989). The diagnosis is usually made on clinical findings and laboratory procedures (Petrin 1998). Most frequently, the saline wet‐mount preparation is used for observation of motile organisms under the light microscope. Wet‐mount smear is a cheap and quick method but more sensitive techniques are culture, immunofluorescence and enzyme immunoassay (Lossick 1991; Borchardt 1991). Different staining techniques include Gram stain, Giemsa stain, Papanicolaou smear, acridine orange (Borchardt 1991; Rein 1990); diverse molecularly‐based diagnostic methods, such as hybridization assay and PCR, may also be used. These tests vary widely in sensitivities and specificities for screening trichomoniasis (DeMeo 1996; Madico 1998; Mayta 2000; Muresu 1994).
Bacterial vaginosis is a clinical syndrome; the microbiology of bacterial vaginosis is complex and is composed of Gardnerella vaginalis, Mycoplasma hominis and anaerobic bacteria (Amsel 1983). The diagnosis is usually made on clinical Amsel criteria findings (Amsel 1983) and laboratory tests. Vaginal pH testing may be a valuable screening tool as it is a quick and inexpensive test (Gjerdingen 2000). Vaginal swab Gram stain with quantification of the microbial flora has high sensitivity and specificity and is accepted as an alternative method (Nugent 1991).
Multiple screening tests exist for other organisms including syphilis. Screening tests such as Treponema pallidum hemagglutination assay, Treponema pallidum particle agglutination assay, and enzyme‐linked immunosorbent assays (ELISAs) are more reliable than Venereal Disease Research Laboratory testing, the fluorescent treponemal antibody absorption test, and immunoblot assays (Muller 2006). The screening test for Neisseria gonorrhoeae, usually from a culture, remains accurate when transport conditions are suitable; this tests can be used with cervical, urine and vaginal swabs. Diagnosis of HIV infection can be obtained from enzyme‐linked immunosorbent assay (ELISA), Western blot, and RNA PCR testing (Kleinman 1998). The HIV‐p24 Ag is effective for early diagnosis of an acute HIV infection (Thies 1994). Strategies for the diagnosis of GBS include obtaining vaginal or both vaginal and anorectal GBS cultures (Quinlan 2000) and a rapid enrichment cum antigen detection test (Das 2003).
Why it is important to do this review
Other Cochrane reviews have addressed a number of issues regarding treatment of infection in pregnancy. Antibiotic treatment of chlamydia, trichomoniasis, bacterial vaginosis and gonorrhoeal infection in pregnancy appear to be effective to clear organisms (Brocklehurst 1998; Brocklehurst 2002; Gülmezoglu 2002; Brocklehurst 2013) but it is not known whether treatment of trichomonas will have any effect on pregnancy outcomes (Gülmezoglu 2002). There is also little evidence to show that screening and treatment in all asymptomatic pregnant women for bacterial vaginosis can prevent preterm birth (Brocklehurst 2013), although antibiotic prophylaxis in pregnancies with a previous preterm birth associated with bacterial vaginosis can reduce preterm delivery (Thinkhamrop 2002). There is insufficient evidence regarding the treatment of ureaplasmas to reduce preterm birth (Raynes‐Greenow 2004), and there is no evidence that antiretrovirals and the treatment of syphilis influence the incidence of preterm birth (Volmink 2007; Walker 2001). None of the aforementioned reviews are concerned primarily with screening programs for antenatal lower genital tract infection, thus a review of the effects of screening programs for lower genital tract infection to prevent preterm birth is required.
Objectives
To assess the effects of antenatal lower genital tract infection screening and treatment programs in reducing preterm birth and subsequent morbidity.
Methods
Criteria for considering studies for this review
Types of studies
We included all published and unpublished randomised controlled trials evaluating any described method of antenatal lower genital tract infection screening in pregnancy.
Types of participants
Women, at 37 or fewer weeks' gestation, who are not in labour, have no vaginal bleeding and are without symptoms of lower genital tract infection.
Types of interventions
Any lower genital tract infection screening and treatment program compared with no screening. The infection screening programs are defined as screening tests (such as wet mount, Gram stain and culture of vaginal secretions) followed by appropriate treatment after a positive screening test, or no treatment after a negative screening test. No screening is defined as routine antenatal care without screening for lower genital tract infections.
Types of outcome measures
Primary outcomes
Preterm birth (less than 37 weeks' gestation)
Secondary outcomes
Low birthweight (LBW) less than 2500 g
Very LBW less than 1500 g (not prespecified)
Neonatal morbidity: sepsis, respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, seizures
Duration of admission to neonatal intensive care unit or hospital
Death: stillbirth, neonatal mortality, infant mortality
Side‐effects of treatment including drug resistance
Persistent infection
Recurrent infection
Failure of treatment
Economic analysis (cost effectiveness, cost utility)
False positive/negative result of the screening program
Women's satisfaction
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 November 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched CENTRAL (The Cochrane Library 2014, Issue 7) using the search strategy detailed in Appendix 1.
Searching other resources
We did not identify any additional or ongoing trials from personal communication. We searched the reference lists of trials and review articles identified.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeSangkomkamhang 2008.
For this update, the following methods were used for assessing the three reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as having a(n):
low risk of bias (any truly random process, for example random number table or computer random number generator);
high risk of bias (any non‐random process, for example odd or even date of birth or hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as having a(n):
low risk of bias (for example telephone or central randomisation or consecutively numbered sealed opaque envelopes);
high risk of bias (for example open random allocation, unsealed or non‐opaque envelopes, alternation, based on date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as having a(n):
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as having a(n):
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed the methods as having a(n):
low risk of bias (for example no missing outcome data or missing outcome data balanced across groups);
high risk of bias (for example numbers or reasons for missing data imbalanced across groups or ‘as treated’ analysis done with substantial departure from the treatment assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as having a(n):
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (all of the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at a high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis).
For this update the quality of the evidence was assessed using the GRADE approach (Schunemann 2009). We assessed the quality of the body of evidence relating to the following outcomes for the comparison of lower genital tract infection screening versus no screening.
Preterm birth (less than 37 weeks).
Preterm low birthweight (below or equal to 2500 g).
Preterm very low birthweight (below or equal to 1500 g).
Outcomes number two and three are subsets of outcome number one.
GRADEprofiler (GRADE 2014) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials (and in this case, since there was only one included trial). We would have used the standardised mean difference to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
Cluster‐randomised trials
For future updates, we will include cluster‐randomised trials in the analyses along with individually randomised trials. We will adjust their sample sizes or standard errors using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of the intervention and the choice of the randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform sensitivity or subgroup analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For the included study, levels of attrition were noted. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as much as possible, on an intention‐to‐treat basis. In other words, we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used a fixed‐effect meta‐analysis in this case, since only one study was included. In future updates, we will also used a fixed‐effect meta‐analysis to combine data where it is reasonable to assume that studies are estimating the same underlying treatment effect, i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged to be sufficiently similar.
In future updates, ff there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity was detected, we will use a random‐effects meta‐analysis to produce an overall summary (if an average treatment effect across trials is considered clinically meaningful). The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
For future updates if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, we will use a random‐effects analysis to produce it.
We will carry out the following subgroup analyses.
Early versus late trimester at screening (defined by author).
Low risk versus high risk of preterm birth, for example multiple pregnancy or previous history of preterm birth.
We plan to restrict subgroup analyses to the primary outcome.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Also see Characteristics of included studies table.
Results of the search
A new search identified three reports assessed for possible inclusion in the review. Two reports related to the same trial; this trial was excluded (Sungkar 2012). The third report was an additional report for the included trial Kiss 2004, with additional outcome data and economic analysis.
Included studies
One included article (Kiss 2004) reported a randomised controlled trial designed to evaluate a vaginal infection screening strategy for the prevention of preterm delivery in a general population of pregnant women. A total of 4155 pregnant women presenting for a routine prenatal visit without subjective complaints were randomised to either the intervention group (n = 2058) or the control group (n = 2097). All women were screened by Gram stain for asymptomatic vaginal infection. For the intervention group, women found to have vaginal infection received standard treatment. For the control group, vaginal smear test results were not revealed so the standard antenatal care program could not be influenced.
Additionally, cost effectiveness of a screen‐and‐treat program for asymptomatic vaginal infections in pregnancy (the direct medical costs of preterm delivery of infants with a birthweight below 1900 g and the costs of the screen‐and‐treat program) was reported.
Excluded studies
Three trials (Gjerdingen 2000; McGregor 1995; Sungkar 2012) were excluded because the participants did not meet the inclusion criteria or the study was not a randomised controlled trial. For further details, please see the Characteristics of excluded studies table.
Risk of bias in included studies
For Kiss 2004, sequence generation was by computer which was judged to be at low risk of bias. See 'Risk of bias' table in Characteristics of included studies and Figure 1.
1.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
There were no details about the random assignment process, therefore the risk of selection bias was unclear.
Blinding
Blinding of the intervention was not possible. In the intervention group, women and their obstetricians were aware of the results of the screening tests, as women were treated for any detected infections. Thus, the risk of detection bias was present; the obstetricians may have provided a different level of care to women in the intervention group in whom an infection had been identified. Blinding of outcome assessors was not described, however this is unlikely to affect the outcome of birthweight.
Incomplete outcome data
Of the 4429 pregnant women who were randomised, 274 were excluded (140 lost to follow up; 68 did not fulfill all the inclusion criteria; 66 had multiple pregnancies). The overall attrition was less than 10%, however, it was not specified how many patients were lost to follow‐up in each arm.
Selective reporting
We did not have the protocol for the included study, therefore we assessed the risk of bias for selective reporting as unclear. We have requested that the authors provide us with additional data but have received no reply.
Other potential sources of bias
None identified.
Effects of interventions
See: Table 1
Lower genital tract infection screening versus no screening
Primary outcomes
We identified a single randomised controlled trial (Kiss 2004) comparing antenatal lower genital tract infection screening and treatment programs for preventing preterm delivery with no screening program. A total of 4429 women were randomised with 274 women excluded from the analysis. In the intervention group (2058 women), the results of infection screening and treatment for bacterial vaginosis, Trichomonas vaginalis and candidiasis were reported; in the control group (2097 women), the results of the screening tests for the women allocated to receive routine antenatal care were not reported. There was a statistically significant difference in number of preterm births before 37 weeks between the two groups (risk ratio (RR) 0.55, 95% confidence interval (CI) 0.41 to 0.75, Analysis 1.1).
1.1. Analysis.
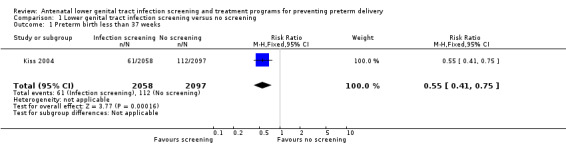
Comparison 1 Lower genital tract infection screening versus no screening, Outcome 1 Preterm birth less than 37 weeks.
Secondary outcomes
Numbers of preterm low birthweight infants (weight equal to or below 2500 g) and preterm very low birthweight infants (weight equal to or below 1500 g) were significantly lower in the intervention group than in the control group (RR 0.48, 95% CI 0.34 to 0.66 and RR 0.34, 95% CI 0.15 to 0.75, respectively) (Kiss 2004). None of the women reported adverse effects during the treatment period. Neonatal morbidity and mortality were not reported.
For a subset of preterm infants with a birthweight less than 1900 g in the Kiss 2004 trial, hospital costs for mother and baby and the costs of the screening program were assessed to be EUR 60,692 (with screening and treatment contributing only 7% of costs). Overall cost savings per prevented preterm birth for this subset therefore amounted to EUR 60,692. In this trial, the screening program halved the number of preterm infants with a birthweight < 1900 g. This threshold was chosen as these babies were all transferred to the neonatal intensive care unit.
Discussion
Summary of main results
In a single trial, an antenatal lower genital tract infection screening and treatment program was shown to significantly reduce preterm birth, low birthweight preterm births (below 2500 g) and very low birthweight preterm births (below 1500 g). This intervention led to savings in direct costs associated with prematurity. The quality of the evidence was rated as moderate.
Overall completeness and applicability of evidence
The included trial was conducted in a developed country (Austria) where characteristics of the population, such as incidence and pattern of lower genital tract infections and socioeconomic status, might differ compared to other countries. Therefore, the results of this review might not be globally generalisable. There was also economic evaluation of this intervention.
Quality of the evidence
Using the Cochrane Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach, the evidence for preterm birth outcomes was graded as moderate.The strength of this review was that the included trial was a large multi‐centre prospective, randomised controlled trial. There was a clear sample‐size calculation and an adequate number of participants were available for the analysis. However, around 3.2% of all randomised women (140/4429) were lost to follow up, and the study authors did not report whether the loss rate was balance between the two groups. Further, there was no blinding of group assignment or of screening results in the intervention group. The differences between the care received in the treatment and control arms may have introduced bias, depending upon the outcome measure in question.
The cost‐benefit analysis showed a substantial savings of more than EUR 60,000 per preterm birth averted for a subset of low birthweight babies routinely admitted to the neonatal intensive care unit. Costs for the 75% of preterm babies with birthweights > 1900 g in this trial were not reported but are assumed to be lower due to lower rates of hospitalisation.
Potential biases in the review process
We followed the methods set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). A comprehensive search was performed, all studies were examined, and the data were independently extracted by at least two review authors. We restricted the included studies to RCTs as they provide the strongest level of evidence. Therefore, we have attempted to reduce bias in the review process.
Agreements and disagreements with other studies or reviews
The results agree with another Cochrane systematic review (Brocklehurst 2013), which reported that antibiotic treatment of bacterial vaginosis in pregnant women with abnormal vaginal flora may reduce preterm birth at less than 37 weeks' gestation. Our results differed from Gülmezoglu 2011, which reported that metronidazole for the treatment of asymptomatic pregnant women with trichomoniasis led to an increase in preterm birth at less than 37 weeks' gestation compared with no treatment.
Authors' conclusions
Implications for practice.
Integrating a simple infection screening and treatment program into routine antenatal care may reduce preterm births in a general population of pregnant women. However, based on the evidence reviewed, we are not able to determine the effects of recurrent or persistent infection on preterm birth. Healthcare providers should discuss the potential benefits and harms of infection screening and tailor care to meet the specific needs of each care setting and healthcare system, or both.
Implications for research.
Further randomised controlled trials are needed to determine the effects of antenatal infection screening and treatment programs in different contexts, for example, different gestational ages, different types of infection screening, and in different populations, especially in developing countries with high rates of preterm birth.
Feedback
Nallendran, 16 February 2009
Summary
The Cochrane Review on antibiotic treatment of bacterial vaginosis in pregnancy1 suggests that there is no improvement in preterm birth rate with treatment, whereas your results suggest an improvement. Could you explain these apparently different conclusions?
References
1McDonald HM, Brocklehurst P, Gordon A. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No.: CD000262. DOI: 10.1002/14651858.CD000262.pub3.
(Summary of feedback received from Vijaianitha Nallendran)
Reply
McDonald’s review of antibiotic treatment for bacterial vaginosis compares "antibiotic treatment with placebo or no treatment". This is different to our review which compares "any lower genital tract infection screening and treatment programs with no screening". In the McDonald review, the subgroup analysis for the small number of women who received treatment before 20 weeks' gestation shows a promising reduction in the risk of preterm birth less than 37 weeks (odds ratio 0.72, 95% confidence interval (CI) 0.55 to 0.95; five trials, 4155 women). Although this is not strong evidence, it is consistent with the reduction in preterm birth before 37 weeks in our review (risk ratio 0.55, 95% CI 0.41 to 0.75; one trial, 4155 women).
Contributors
Ussanee S Sangkomkamhang on behalf of the review team.
What's new
| Date | Event | Description |
|---|---|---|
| 31 July 2014 | New citation required but conclusions have not changed | Review updated. No new trials included. |
| 31 July 2014 | New search has been performed | Search updated and three new reports assessed for inclusion. Two reports related to the same trial; this trial was excluded. The third report was an additional report for an included trial. Methods updated and 'Summary of findings' table added. |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 6 August 2009 | Feedback has been incorporated | Authors replied to feedback from Vijaianitha Nallendran. |
| 28 July 2009 | New search has been performed | Search updated. No new reports identified. |
| 26 March 2009 | Feedback has been incorporated | Feedback from Vijaianitha Nallendran added. |
| 10 November 2008 | Amended | Contact details updated. |
| 15 February 2008 | Amended | Converted to new review format. |
Acknowledgements
We, the authors, acknowledge the support we have received from the SEA ORCHID project. We thank Professor James P Neilson (Co‐ordinating Editor) and Sonja Henderson (Review Group Co‐ordinator) for advice and support in the preparation of Sangkomkamhang 2008. We also thank Lynn Hampson (Trials Search Co‐ordinator) for her contribution to the search strategy, Gill Gyte for her consumer advice and Janet Wale for preparing the plain language summary.
As part of the pre‐publication editorial process of this review, Sangkomkamhang 2008 was commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
We thank Nancy Medley for her support in the creation of the 'Summary of findings' table for this update. Nancy Medley's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Pregnancy explode all trees in MeSH products #2 MeSH descriptor Pregnancy Complications explode all trees in MeSH products #3 pregnan* in All Fields in all products #4 (preterm or premature) near (labour or labor) in All Fields in all products #5 MeSH descriptor Infection explode all trees in MeSH products #6 MeSH descriptor Mass Screening explode all trees in MeSH products #7 screen* in All Fields in all products #8 infect* in All Fields in all products #9 (#1 OR #2 OR #3 OR #4) #10 (#5 OR #8) #11 (#6 OR #7) #12 (#9 AND #10 AND #11)
Data and analyses
Comparison 1. Lower genital tract infection screening versus no screening.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth less than 37 weeks | 1 | 4155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.41, 0.75] |
| 2 Preterm very low birthweight (below or equal 1500 g) | 1 | 4155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.75] |
| 3 Preterm low birthweight (below or equal 2500 g) | 1 | 4155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.66] |
| 4 Neonatal morbidity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Duration of admission to neonatal intensive care unit/hospital | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal death | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Side‐effects of treatment (including drug resistance) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Persistent infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Recurrent infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Women's satisfaction | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.2. Analysis.
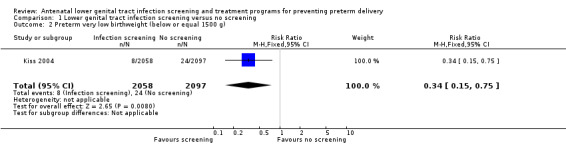
Comparison 1 Lower genital tract infection screening versus no screening, Outcome 2 Preterm very low birthweight (below or equal 1500 g).
1.3. Analysis.
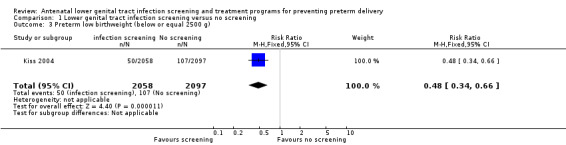
Comparison 1 Lower genital tract infection screening versus no screening, Outcome 3 Preterm low birthweight (below or equal 2500 g).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kiss 2004.
| Methods | Randomised trial with computer‐generated randomisation sequence. All pregnant women presenting for antenatal care were screened, with smear samples sent to the central laboratory where they were randomly assigned to the intervention or control group. Women in the intervention arm with a positive screening result received treatment. Women in the control group were blinded to screening results and received routine antenatal care. Description of withdrawals: yes. Intention‐to‐treat analysis: not used. | |
| Participants | 4429 pregnant women (mean age 28.9, SD 5.6) presenting for routine prenatal visits between 15 and 19 weeks' gestation (mean 17, SD 1.6). Intervention group: n = 2058 ; control group: n = 2097. Inclusion criteria: gestational age 15‐19 weeks without subjective complaints (e.g. contractions and vaginal bleeding). Exclusion criteria: clinical symptoms of vaginal infection, multiple pregnancies. Location: Vienna, Austria. | |
| Interventions | Intervention group: vaginal smears (Gram stain and evaluated by the scoring criteria proposed by Nugent 1991) screening for bacterial vaginosis, Trichomonas vaginalis and Candida species and received standard antibiotic treatment if positive screening test, i.e. 2% for 6 days local clindamycin for bacterial vaginosis, 300 mg twice daily for seven days oral clindamycin for recurrent bacterial vaginosis, 0.1 g for 6 days local clotrimazole for candidiasis, and 500 mg for 7 days local metronidazole for trichomoniasis (including treatment of the partner). Control group: were smeared, but the results of testing were not made available to the women's care providers and did not have any effect on the standard clinical antenatal care program routine antenatal examination. | |
| Outcomes | Primary outcome: spontaneous preterm delivery at less than 37 weeks' gestation. Secondary outcomes:
|
|
| Notes | 4429 randomised, 274 excluded from analysis, 140 lost to follow up, 68 did not fulfill all inclusion criteria, 66 multiple pregnancies. We have contacted the author and are waiting for a reply for our request for additional data (secondary outcomes e.g. neonatal necrotizing enterocolitis, neonatal sepsis, neonatal death, duration of neonatal admission to NICU/hospital). We will incorporate these additional data in an update to the review, should they be forthcoming. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A pre‐established computer generated randomisation list was used to allocate patients to the treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All obstetricians and women in the intervention group received their smear results and different treatment regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described, but assessors would not have influenced the objective outcome of birthweight. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4429 pregnant women: 2058 in the treatment group, 2097 in the control group. There were 274 patients excluded from the study with group allocation not stated (140 lost to follow up, 68 did not fulfill all inclusion criteria, 66 multiple pregnancies). Intention to treat analysis was not described. |
| Selective reporting (reporting bias) | Unclear risk | No information available because protocol is not accessible; we have contacted authors for additional outcome data. |
| Other bias | Low risk | The study seems to be free of other types of bias. |
NICU: neonatal intensive care unit SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gjerdingen 2000 | Participants did not meet inclusion criteria. Study compared standard prenatal care including routine inquiry about vaginal symptoms versus standard care supplemented by vaginal pH testing. Both arms had pregnant women who were diagnosed with lower genital tract infection and all participants received vaginal pH screening. Participants: 121 pregnant women with or without vaginal infection symptoms. Intervention: vaginal pH testing. Outcomes: bacterial vaginosis detection rate, preterm deliveries. |
| Gupta 2013 | Participants did not meet inclusion criteria. Study compared screening for and treatment of abnormal vaginal flora versus no treatment. Participants: 242 pregnant women with abnormal vaginal flora. Intervention: screening by vaginal swab for Gram stained and examined for budding yeast cells, pseudohyphae and bacteria of various morphotypes, and scored using the Nugent criteria. Outcomes: preterm birth. |
| McGregor 1995 | Methods not clearly described, but seems likely that this was not a randomised controlled trial. Described as a prospective observational trial. Participants: 1260 women. Intervention: lower genital tract micro‐organism screening (vaginal fluid enzyme; nonspecific protease, sialidase, phospholipase C, phospholipase A2). Outcomes: preterm birth, early pregnancy loss. |
| Sungkar 2012 | Participants did not meet inclusion criteria. Study compared self‐examination of vaginal acidity, and microbiologic testing for BV (Gram staining) versus usual prenatal care standard care. Participants: 176 singleton pregnant women with or without vaginal infection symptoms. Intervention: education about preterm birth and its risk factors, self‐examination of vaginal acidity, and microbiologic testing for BV (Gram staining). Outcomes: preterm birth. |
Contributions of authors
US and PL developed the title and question. US, PL and WP developed the protocol; ML commented on drafts of the protocol. US wrote the first draft of the original review; PL, ML, and WP reviewed and gave comments on the drafts of the review. US and ML wrote the draft of the 2014 updated review, WP gave comments on the drafts of the review, PL commented on and supervised the development of the updated review.
Sources of support
Internal sources
Khon Kaen Hospital, Khon Kaen, Ministry of Public Health, Thailand.
Khon Kaen University, Faculty of Medicine, Khon Kaen, Thailand.
Khon Kaen University, Faculty of Public Health, Thailand.
External sources
Thai Cochrane Network, Thailand.
Thailand Research Fund (Senior Research Scholar), Thailand.
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Kiss 2004 {published data only}
- Kiss H, Petricevic L, Husslein P. Prospective randomised controlled trial of an infection screening programme to reduce the rate of preterm delivery. BMJ 2004;329:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss H, Pichler E, Petricevic L, Husslein P. Cost effectiveness of a screen‐and‐treat program for asymptomatic vaginal infections in pregnancy: towards a significant reduction in the costs of prematurity. European Journal of Obstetrics & Gynecology and Reproductive Biology 2006;127(2):198‐203. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gjerdingen 2000 {published data only}
- Gjerdinjen D, Fontaine P, Bixby M, Santilli J, Welsh J. The impact of regular vaginal pH screening on the diagnosis of bacterial vaginosis in pregnancy. Journal of Family Practice 2000;49:39‐43. [PubMed] [Google Scholar]
Gupta 2013 {published data only}
- Gupta S, Tripathi R, Singh N, Bhalla P, Ramji S, Mala YM. Pregnancy outcome in asymptomatic women with abnormal vaginal flora without any treatment and after treatment with vaginal clindamycin and clotrimazole: a randomised controlled trial. South African Journal of Obstetrics and Gynaecology 2013;19(2):35‐8. [Google Scholar]
McGregor 1995 {published data only}
- McGregor JA, French JI, Parker R, Draper D, Patterson E, Jones W, et al. Prevention of premature birth by screening and treatment for common genital tract infections: results of a prospective controlled evaluation. American Journal of Obstetrics and Gynecology 1995;173(1):157‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sungkar 2012 {published data only}
- Sungkar A. Early self‐diagnosis and treatment of bacterial vaginosis to prevent preterm premature rupture of membranes. Journal of Perinatal Medicine 2013;41(Suppl 1):171. [Google Scholar]
- Sungkar A, Purwosunu Y, Aziz MF, Pratomo H, Sutrisna B, Sekizawa A. Influence of early self‐diagnosis and treatment of bacterial vaginosis on preterm birth rate. International Journal of Gynecology and Obstetrics 2012;117(3):264‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Amsel 1983
- Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. American Journal of Medicine 1983;74:14‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bejar 1981
- Bejar R, Curbelo V, Davi SC, Gluck L. Premature labour bacterial sources of phospholipase. Obstetrics & Gynecology 1981;57(4):479‐82. [MEDLINE: ] [PubMed] [Google Scholar]
Black 1997
- Black CM. Current methods of laboratory diagnosis of Chlamydia trachomatis infections. Clinical Microbiology Reviews 1997;10:160–84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borchardt 1991
- Borchardt KA, Smith RF. An evaluation of an InPouchTMTV culture method for diagnosing Trichomonas vaginalis infection. Genitourinary Medicine 1991;67(2):149‐52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brocklehurst 1998
- Brocklehurst P, Rooney G. Interventions for treating genital chlamydia trachomatis infection in pregnancy. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD000054] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brocklehurst 2002
- Brocklehurst P. Antibiotics for gonorrhoea in pregnancy. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD000098] [DOI] [PubMed] [Google Scholar]
Brocklehurst 2013
- Brocklehurst P, Gordon A, Heatley E, Milan SJ. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD000262.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cotch 1997
- Cotch MF, Pastorek JG 2nd, Nugent RP, Hillier SL, Gibbs RS, Martin DH, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sexually Transmitted Diseases 1997;24(6):361‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cox 1989
- Cox SM, MacDonald PC, Casey ML. Cytokines and prostaglandins in amniotic fluid of preterm labor pregnancies: decidual origin in response to bacterial toxins(lipopolysaccharide{LPS} and lipotechnoic acid {LTA}. 36th Annual Meeting of the Society for Gynecologic Investigation; 1989 March 16‐16; San Diego, CA. 1989.
Cunningham 1997
- Cunningham FG, MacDonald PC, Gant NF, Leveno KJ, Gilstrap LC, Hankins GDV, et al. Williams obstetrics. 20th Edition. Connecticut: Appleton & Lange, 1997. [Google Scholar]
Cunningham 2001
- Cunningham FG, MacDonald PC, Gant NF, Leveno KJ, Gilstrap LC, Hankins GDV, et al. Williams obstetrics. 21st Edition. Connecticut: Appleton & Lange, 2001. [Google Scholar]
Das 2003
- Das A, Ray P, Sharma M, Gopalan S. Rapid diagnosis of vaginal carriage of group B beta haemolytic streptococcus by an enrichment cum antigen detection test. Indian Journal of Medical Research 2003;117:247‐52. [MEDLINE: ] [PubMed] [Google Scholar]
DeMeo 1996
- DeMeo LR, Draper DL, McGregor JA, Moore DF, Peter CR, Kapernick PS, et al. Evaluation of a deoxyribonucleic acid probe for the detection of Trichomonas vaginalis in vaginal secretions. American Journal of Obstetrics and Gynecology 1996;174(4):1339‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Domeika 1999
- Domeika M, Bassiri M, Butrimiene I, Venalis A, Ranceva J, Vasjanova V. Evaluation of vaginal introital sampling as an alternative approach for the detection of genital Chlamydia trachomatis infection in women. Acta Obstetricia et Gynecologica Scandinavica 1999;78(2):131‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Doyle 1996
- Doyle LW, Ford GW, Olinsky A, Knoches AM, Callanan C. Bronchopulmonary dysplasia and very low birth weight: lung function at 11 years of age. Journal of Pediatrics and Child Health 1996;32:339‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Elliott 1990
- Elliott B, Brunham RC, Laga M, Piot P, Ndinya‐Achola JO, Maitha G, et al. Maternal gonococcal infection as a preventable risk factor for low birth weight. Journal of Infectious Diseases 1990;161(3):531‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goldenberg 1998
- Goldenberg RL, Rouse DJ. Prevention of premature birth. New England Journal of Medicine 1998;339(5):313‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2014 [Computer program]
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2014. McMaster University, 2014.
Gravett 1986
- Gravett MG, Nelson HP, DeRouen T, Critchlow C, Eschenbach DA, Holmes KK. Independent associations of bacterial vaginosis and chlamydia trachomatis infection with adverse pregnancy outcome. JAMA 1986;256:1899‐905. [MEDLINE: ] [PubMed] [Google Scholar]
Gülmezoglu 2002
- Gülmezoglu AM. Interventions for trichomoniasis in pregnancy. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD000220] [DOI] [PubMed] [Google Scholar]
Gülmezoglu 2011
- Gülmezoglu AM, Azhar M. Interventions for trichomoniasis in pregnancy. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD000220.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henriksen 1995
- Henriksen TB, Hedegaard M, Secher NS, Wilcox AJ. Standing at work and preterm delivery. British Journal of Obstetrics and Gynaecology 1995;102(3):198. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hillier 1995
- Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH. Association between bacterial vaginosis and preterm delivery of a low‐birth‐weight infant. New England Journal of Medicine 1995;333:1737‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Holzman 1995
- Holzman C, Paneth N, Little R, Pinto‐Martin J. Perinatal brain injury in premature infants born to mothers using alcohol in pregnancy. Pediatrics 1995;95(1):66. [MEDLINE: ] [PubMed] [Google Scholar]
Jespersen 2005
- Jespersen DJ, Flatten KS, Jones MF, Smith TF. Prospective comparison of cell cultures and nucleic acid amplification tests for laboratory diagnosis of Chlamydia trachomatis infections. Journal of Clinical Microbiology 2005;43(10):5324‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleinman 1998
- Kleinman S, Busch MP, Hall L, Thomson R, Glynn S, Gallahan D, et al. False‐positive HIV‐1 test results in a low‐risk screening setting of voluntary blood donation. Retrovirus Epidemiology Donor Study. JAMA 1998;280(12):1080‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lettieri 1993
- Lettieri L, Vintzileos AM, Rodis JF, Albini SM, Saladia CM. Does idiopathic preterm labor resulting in preterm birth exist?. American Journal of Obstetrics and Gynecology 1993;168(5):1480‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lossick 1991
- Lossick JG, Kent HL. Trichomoniasis: trends in diagnosis and management. American Journal of Obstetrics and Gynecology 1991;165(4 Pt 2):1217‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Madico 1998
- Madico G, Quinn TC, Rompalo A, McKee KT Jr, Gaydos CA. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swab samples. Journal of Clinical Microbiology 1998;36(11):3205‐10. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayta 2000
- Mayta H, Gilman RH, Calderon MM, Gottlieb A, Soto G, Tuero I, Sanchez S, et al. 18S ribosomal DNA‐based PCR for diagnosis of Trichomonas vaginalis. Journal of Clinical Microbiology 2000;38(7):2683‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 1994
- McDonald HM, O'Loughin JA, Jolley PT, Vigneswaran R, McDonald PJ. Changes in vaginal flora during pregnancy and association with preterm birth. Journal of Infectious Diseases 1994;170(3):724‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McFarlin 1995
- McFarlin BL, Bottoms SF. Maternal syphilis in Michigan: the challenge to prevent congenital syphilis. Midwifery 1995;11(2):55‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McGregor 1990
- McGregor JA, French JI, Richter R, Franco‐Buff A, Johnson A, Hillier S. Antenatal microbiological maternal risk factors associated with prematurity. American Journal of Obstetrics and Gynecology 1990;163(5 Pt 1):1465‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meis 1995
- Meis PJ, Goldenberg RL, Mercer B, Moawad A, Das A, McNellis D, et al. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal‐Fetal Medicine Units Network. American Journal of Obstetrics and Gynecology 1995;173(4):1231‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Muller 2006
- Muller I, Brade V, Hagedorn HJ, Straube E, Schorner C, Frosch M, et al. Is serological testing a reliable tool in laboratory diagnosis of syphilis? Meta‐analysis of eight external quality control surveys performed by the german infection serology proficiency testing program. Journal of Clinical Microbiology 2006;44(4):1335‐41. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muresu 1994
- Muresu R, Rubino S, Rizzu P, Baldini A, Colombo M, Cappuccinelli P. A new method for identification of Trichomonas vaginalis by fluorescent DNA in situ hybridization. Journal of Clinical Microbiology 1994;32(4):1018‐22. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nugent 1991
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. Journal of Clinical Microbiology 1991;29(2):297‐301. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papiernik 1986
- Papiernik E, Bouyer J, Collin D, Winisdoerffer G, Dreyfus J. Precocious cervical ripening and preterm labor. Obstetrics & Gynecology 1986;67(2):238. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Petrin 1998
- Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clinical Microbiology Reviews 1998;11(2):300‐17. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinlan 2000
- Quinlan JD, Hill DA, Maxwell BD, Boone S, Hoover F, Lense JJ. The necessity of both anorectal and vaginal cultures for group B streptococcus screening during pregnancy. Journal of Family Practice 2000;49(5):447‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Raynes‐Greenow 2004
- Raynes‐Greenow CH, Roberts CL, Bell JC, Peat B, Gilbert GL. Antibiotics for ureaplasma in the vagina in pregnancy. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003767.pub2] [DOI] [PubMed] [Google Scholar]
Regan 1981
- Regan JA, Chao S, James SL. Premature rupture of membranes, preterm delivery, and group B streptococcal colonization of mothers. American Journal of Obstetrics and Gynecology 1981;141(2):184‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rein 1990
- Rein MF, Muller M. Trichomonas vaginalis and trichomoniasis. In: Holmes KK, Mardh PA, Sparling PF, Wiesner PJ editor(s). Sexually Transmitted Diseases. McGraw‐Hill, 1990:481‐92. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2000
- Roberts JM. Recent advances: obstetrics. BMJ 2000;321(7252):33‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2011
- Roberts CL, Rickard K, Kotsiou G, Morris JM. Treatment of asymptomatic vaginal candidiasis in pregnancy to prevent preterm birth: an open‐label pilot randomized controlled trial. BMC Pregnancy and Childbirth 2011;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romero 1992
- Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. American Journal of Obstetrics and Gynecology 1992;166(5):1576‐87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saigal 2000
- Saigal S, Hoult LA, Streiner DL, Stoskopf BL, Rosenbaum PL. School difficulties at adolescence in a regional cohort of children who were extremely low birth weight. Pediatrics 2000;105(2):325‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Satin 1994
- Satin AJ, Leveno KJ, Sherman ML, Reedy NJ, Lowe TW. Maternal youth and pregnancy outcomes: middle school versus high school age groups compared to women beyond the teen years. American Journal of Obstetrics and Gynecology 1994;171(1):184. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Shrier 2004
- Shrier LA, Dean D, Klein E, Harter K, Rice PA. Limitations of screening tests for the detection of Chlamydia trachomatis in asymptomatic adolescent and young adult women. American Journal of Obstetrics and Gynecology 2004;190(3):654‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Temmerman 1994
- Temmerman M, Chomba EN, Ndinya‐Achola J, Plummer FA, Coppens M, Piot P. Maternal human immunodeficiency virus‐1 infection and pregnancy outcome. Obstetrics & Gynecology 1994;83(4):495‐501. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thies 1994
- Thies K, Anders C, Baldus M, Schleiffer T, Weber B, Rabenau H, et al. Detection of primary HIV infection by a second‐generation HIV(p24) antigen test. Infusionstherapie und Transfusionsmedizin 1994;21(5):333‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thinkhamrop 2002
- Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P. Prophylactic antibiotic administration in pregnancy to prevent infectious morbidity and mortality. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD002250] [DOI] [PubMed] [Google Scholar]
Volmink 2007
- Volmink J, Siegfried NL, Merwe L, Brocklehurst P. Antiretrovirals for reducing the risk of mother‐to‐child transmission of HIV infection. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD003510.pub2] [DOI] [PubMed] [Google Scholar]
Walker 2001
- Walker GJA. Antibiotics for syphilis diagnosed during pregnancy. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 2002
- Watson EJ, Templeton A, Russell I, Paavonen J, Mardh PA, Stary A, et al. The accuracy and efficacy of screening tests for Chlamydia trachomatis: a systematic review. Journal of Medical Microbiology 2002;51(12):1021‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wolner‐Hanssen 1989
- Wolner‐Hanssen P, Krieger JN, Stevens CE, Kiviat NB, Koutsky L, Critchlow C, et al. Clinical manifestations of vaginal trichomoniasis. JAMA 1989;261(4):571‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wood 2000
- Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. New England Journal of Medicine 2000;343(6):378‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yoon 2000
- Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kin CJ. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. American Journal of Obstetrics and Gynecology 2000;183(5):1124‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sangkomkamhang 2008
- Sangkomkamhang US, Lumbiganon P, Prasertcharoensook W, Laopaiboon M. Antenatal lower genital tract infection screening and treatment programs for preventing preterm delivery. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD006178.pub2] [DOI] [PubMed] [Google Scholar]


