Abstract
Background
Retained placenta affects 0.5% to 3% of women following delivery, with considerable morbidity if left untreated. Use of nitroglycerin (NTG), either alone or in combination with uterotonics, may be of value to minimise the need for manual removal of the placenta in theatre under anaesthesia.
Objectives
To evaluate the benefits and harms of NTG as a tocolytic, either alone or in addition to uterotonics, in the management of retained placenta.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (14 January 2015), reference lists of retrieved studies and contacted experts in the field.
Selection criteria
Any adequately randomised controlled trial (RCT) comparing the use of NTG, either alone or in combination with uterotonics, with no intervention or with other interventions in the management of retained placenta. All women having a vaginal delivery with a retained placenta, regardless of the management of the third stage of labour (expectant or active). We included all trials with haemodynamically stable women in whom the placenta was not delivered at least within 15 minutes after delivery of the baby.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
Main results
We included three randomised controlled trials (RCTs) with 175 women. The three published RCTs compared NTG alone versus placebo. The detachment status of retained placenta was unknown in all three RCTs. Collectively, among the three included trials, two were judged to be at low risk of bias and the third trial was judged to be at high risk of bias for two domains: incomplete outcome data and selective reporting. The three trials reported seven out of 23 of the review's pre‐specified outcomes.
The primary outcome "manual removal of the placenta" was reported in all three studies. No differences were seen between NTG and placebo for manual removal of the placenta (average risk ratio (RR) 0.83, 95% confidence interval (CI) 0.47 to 1.46; women = 175; I² = 81%). A random‐effects model was used because of evidence of substantial heterogeneity in the analysis. There were also no differences between groups for risk of severe postpartum haemorrhage (RR 0.93, 95% CI 0.62 to 1.39; women = 150; studies = two; I² = 0%). Blood transfusion was only reported in one study (40 women) and again there was no difference between groups (RR 1.00, 95% CI 0.07 to 14.90; women = 40; I² = 0%). Mean blood loss (mL) was reported in the three studies and no differences were observed (mean difference (MD) ‐115.31, 95% CI ‐306.25 to 75.63; women = 169; I² = 83%). Nitroglycerin administration was not associated with an increase in headaches (RR 1.09, 95% CI 0.80 to 1.47; women = 174; studies = three; I² = 0%). However, nitroglycerin administration was associated with a significant, though mild, decrease in systolic and diastolic blood pressure and a significant increase in pulse rate (MD ‐3.75, 95% CI ‐7.47 to ‐0.03) for systolic blood pressure, and (MD 6.00, 95% CI 3.07 to 8.93) for pulse rate (beats per minute) respectively (reported by only one study including 24 participants). Maternal mortality and addition of therapeutic uterotonics were not reported in any study.
Authors' conclusions
In cases of retained placenta, currently available data showed that the use of NTG alone did not reduce the need for manual removal of placenta. This intervention did not increase the incidence of severe postpartum haemorrhage nor the need for blood transfusion. Haemodynamically, NTG had a significant though mild effect on both pulse rate and blood pressure.
Plain language summary
Use of nitroglycerin to deliver a retained placenta
Failure to deliver the placenta after a vaginal birth is an uncommon event that can be associated with significant bleeding and even death if left untreated. The retained placenta may be detached from the uterine wall but is trapped so that it is not expelled through the cervix or non‐detached as the placenta fails to separate because of placental inhibition of uterine contractions. Conventional management involves spinal or general anaesthesia to enable introducing a hand inside the uterus to manually remove the placenta, which carries risks of infection and from the anaesthetic, and also requires special facilities. The use of uterine relaxing drugs (tocolytics), either alone or with other drugs to stimulate contractions of the uterus (uterotonics), may bring on the delivery of the placenta and avoid the need for this invasive procedure. This review included three trials that randomly assigned 175 women with the placenta remaining undelivered more than 15 minutes after delivery to either a placebo or the tocolytic nitroglycerin. Both groups received oxytocin to stimulate contractions of the uterus. Combined administration of nitroglycerin and oxytocin did not reduce the need for manual removal of placenta, blood loss, nor the incidence of severe postpartum haemorrhage. Nitroglycerin administration did not cause headache but resulted in a mild drop in blood pressure and a related increase in heart rate. Two out of the three trials had low risk of bias but this result needs confirmation in larger trials with adequate sample sizes to verify the role of nitroglycerin and other tocolytic drugs in managing different subtypes of retained placenta. The trials in this review did not specify the type of retained placenta. We have included an explanation of some of the scientific terms that are used in this review in a glossary (see Appendix 1).
Background
Description of the condition
Retained placenta (failure to deliver the placenta within 30 minutes after delivery of the baby) (NCCWCH 2007), affects 0.5% to 3% of women following delivery. The mortality rate of this condition is up to 10% if left untreated in low‐resource areas (Weeks 2001). Although the recurrence rate of retained placenta leading to manual removal of placenta is high, the incidence of associated major haemorrhage is low, especially in women who did not have postpartum haemorrhage in the index pregnancy (Ghag 2014). The cut‐off point of 30 minutes is regarded as a prolonged third stage, because complications arise when this interval is exceeded (Combs 1991). The most common treatment for retained placenta is manual removal under spinal or general anaesthetic. This procedure exposes the mother to the risks of anaesthesia as well as to the risk of infection associated with inserting a hand through the vagina into the uterus. Dynamic ultrasonographic imaging of the third stage of labour has demonstrated that retro‐placental myometrial contractions are necessary for placental separation and that lack of retro‐placental contractions results in retained placenta (Herman 1993). It was demonstrated that placental anchoring villi are able to contract and relax, and that this ability is under the control of nitric oxide (Farley 2004). An interaction between oxytocin and nitric oxide could play a role in promoting placental separation (Ticconi 2004).
Retained placenta can present in different clinical scenarios, which depends on the state of placenta: whether detached (trapped; separated from uterine wall but failed to be expelled through the cervix) or non‐detached (failure of the placenta to separate from the uterine wall). Excessive postpartum blood loss occurs, usually in the detached but not in the non‐detached form. Uterine atony is a common cause in both scenarios. The source of bleeding is the placental sinuses in the uterine wall after separation of the placenta (partial or total) and failure of the myometrium to contract. If the placenta is not completely detached, there will be no bleeding (Percival 1980). Constriction ring is a cause of retained detached placenta, while persistent placental inhibition of myometrial contractions explains some cases in the second scenario (non‐detached). Both types can be differentiated both clinically and sonographically (Herman 1993; Weeks 2005). Clinically, if the fundus of the uterus feels small and contracted or if the edge of the placenta is palpable through a tight cervical os, this a retained detached placenta. On the other hand, if the uterus is lax and no part of the placenta is felt through the cervix, this is a retained non‐detached placenta. Sonographically, the distinction is more obvious. With a retained detached placenta, the myometrium is seen to be thickened all around the uterus and a clear demarcation is often seen between the placenta and the myometrium. In contrast, with retained non‐detached placenta, the myometrium will be thickened in all areas except where the placenta is attached, where it will be very thin or even invisible (Herman 1993). Additionally, the use of colour Doppler sonography in the third stage of labour can differentiate between normal and abnormal placentation. In cases with normal placental separation, cessation of blood flow between placenta and myometrium occurs immediately after birth. Persistent blood flow from the myometrium deep into the placenta demonstrated by colour Doppler ultrasound is suggestive of placenta accreta (Krapp 2000). Ultrasound machines are readily available in well‐equipped delivery units and should be used in the third stage of labour complicated by retention of the placenta. Ultrasound imaging may help to judge the separation of the placenta from the uterine muscle and could be a useful tool in the management of pathologic third stage of labour (Urner 2014).
Description of the intervention
Options for management of retained placenta include: manual removal of placenta; use of uterotonic drugs plus controlled cord traction; the use of tocolytic drugs including nitroglycerin (NTG) to facilitate manual removal of the placenta (Bullarbo 2012; Chedraui 2003; Ross 1994), or to relax a constriction ring (hour glass constriction) preventing the expulsion of the placenta (retained but detached placenta) (Barnes 1881). NTG has been used together with uterotonics or sequentially after failure of uterotonics to obviate the need for manual removal of the placenta (Bullarbo 2005; Ekerhovd 2008). All interventions should be adjusted to a time frame to avoid maternal morbidity such as severe postpartum haemorrhage or even death (Urner 2014).
NTG is a nitric oxide donor with an effective smooth muscle relaxant and a potent and short‐lived tocolytic effect (Peng 1989). It is administered via both intravenous or sublingual routes. It is metabolised at the site of its action to its active compound nitric oxide that in turn exerts its relaxing effect using cyclic guanosine monophosphate as a second messenger (Dufour 1997b). It has been used in obstetric emergencies for over 100 years, and was first described by Barnes 1881 for use in a patient with an entrapped placenta behind an ergot‐induced contraction ring. When NTG is injected intravenously, uterine relaxation occurs within 45 to 60 seconds and normally lasts no longer than two minutes (Axemo 1998). However, when it is given sublingually, it reaches a maximum plasma concentration within five minutes, with a half‐life of three minutes (Jensen 1994). A prospective non‐controlled trial showed the effectiveness of 1 mg sublingual NTG in the delivery of retained placenta sequentially after oxytocin (Ekerhovd 2008). The authors reported a success rate in 21 out of 24 patients (87.5%) in placental delivery without the need for manual removal of the placenta.
NTG has also been used for replacement of a contracted inverted uterus (Altabef 1992) in caesarean delivery of twins performed under spinal anaesthesia (Mayer 1992), in both internal and external version during labour (Belfort 1993; Wessen 1995) and for delivery of entrapped after‐coming head during vaginal breech delivery (Dufour 1997a; Rolbin 1991).
How the intervention might work
NTG works in the treatment of a retained detached placenta by causing relaxation of uterine muscle constriction in the constriction ring. However, in instances of retained non‐detached placenta, its role is less clear. Farley 2004 demonstrated that human chorionic villi are able to contract and relax along their longitudinal axes and this ability is controlled by nitric oxide (Farley 2004). This mechanism could play a role in placental separation. Other research (Ticconi 2004), has demonstrated that oxytocin stimulates nitric oxide release by fetal membranes at term and that this stimulating effect is more marked after labour than before labour. An interaction between oxytocin and NTG (nitric oxide donor) could play a role in placental separation.
Why it is important to do this review
The only current standard treatment for retained placenta is manual removal of placenta. However, facilities for manual removal of the placenta are not immediately available in low‐income settings where the majority of deliveries occur outside hospitals. An effective medical treatment could have major implications for the reduction of the need for manual removal of the placenta and reduction of morbidity and mortality due to retained placenta (Weeks 2002). Also, the role of NTG has not been evaluated systematically, especially in relation to different types of retained placenta (detached or not detached).
Objectives
To evaluate the benefits and harms of NTG alone or in addition to uterotonics in the management of retained placenta.
Methods
Criteria for considering studies for this review
Types of studies
Any adequately randomised controlled trial (RCT) comparing the use of NTG (either alone or in combination with uterotonics) versus surgical management, and placebo/expectant management, for the management of retained placenta. Quasi‐randomised trials, cluster‐randomised trials and trials with a cross‐over design were not eligible for inclusion.
Types of participants
All women having a vaginal delivery with a retained placenta, regardless of the management of the third stage of labour (expectant or active). For this review, we chose all trials including women in whom the placenta was not delivered at least within 15 minutes after delivery of the baby. Women had to be haemodynamically stable. We have not included cases of placenta accreta.
Types of interventions
Regarding the use of NTG in the management of retained placenta, we considered the following comparisons.
NTG alone for management of retained (but detached) placenta versus placebo/expectant management.
NTG plus uterotonic drugs (different types, doses and routes of administration) for management of retained (but detached) placenta versus placebo/expectant management.
NTG alone for management of retained (but detached) placenta versus surgical management.
NTG plus uterotonic drugs for management of retained (but detached) placenta versus surgical management.
NTG alone for management of retained (non‐detached or adherens) placenta versus placebo/expectant management.
NTG plus uterotonic drugs for management of retained (non‐detached or adherens) placenta versus placebo/ expectant management.
NTG alone for management of retained (non‐detached or adherens) placenta versus surgical management.
NTG plus uterotonic drugs for management of retained (non‐detached or adherens) placenta versus surgical management.
NTG alone for management of retained (status unknown) placenta versus placebo/expectant management.
NTG plus uterotonic drugs for management of retained (status unknown) placenta versus placebo/expectant management.
NTG alone for management of retained (status unknown) placenta versus surgical management.
NTG plus uterotonic drugs for management of retained (status unknown) placenta versus surgical management.
a) NTG plus uterotonic drugs refer to the use of NTG simultaneously (combined) with uterotonic drugs.
b) Surgical management includes; manual removal of the placenta, ring removal or dilatation and curettage.
c) 'Status unknown' refers to trials which do not report whether the retained placenta is detached or not (whether clinically or by ultrasonographic diagnosis).
Types of outcome measures
Primary outcomes
Manual removal of the placenta.
Maternal mortality.
Severe postpartum haemorrhage (S‐PPH) (defined as clinically estimated or measured blood loss greater than or equal to 1000 mL) (not pre‐specified).
Blood transfusion.
Addition of therapeutic uterotonics (not pre‐specified).
Secondary outcomes
Serious maternal morbidity (hysterectomy, admission to intensive care, renal or respiratory failure, and other additional surgical procedures to treat PPH other than manual removal of placenta (not pre‐specified).
PPH (defined as clinically estimated or measured blood loss greater than or equal to 500 mL) (not pre‐specified).
Maternal postpartum anaemia (defined by the haemoglobin concentration according to the international standards) or fall in haemoglobin levels (defined as decrease in previous haemoglobin concentration levels by at least 10%) (not pre‐specified).
Mean blood loss (mL).
Mean time from injection to placental removal (minutes).
Iron tablets (therapeutic indication ) during the puerperium (not pre‐specified).
Subsequent surgical evacuation of retained products of conception.
Changes in pulse rate and blood pressure between administration of NTG and discharge from the labour ward.
Vomiting between administration of NTG and discharge from the labour ward.
Shivering between administration of NTG and discharge from the labour ward.
Nausea between administration of NTG and discharge from the labour ward.
Headache between administration of NTG and discharge from the labour ward.
Maternal pain between administration of NTG and discharge from the labour ward (not pre‐specified).
Maternal dissatisfaction with third stage management (not pre‐specified).
Secondary PPH (after 24 hours and before six weeks) (not pre‐specified).
Need for treatment with antibiotics (not pre‐specified).
Maternal fatigue (not pre‐specified).
Breastfeeding at discharge from hospital (not pre‐specified).
Secondary PPH (postpartum haemorrhage) is any excessive bleeding occurring from the genital tract in the period between 24 hours and six weeks after delivery.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (14 January 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of all articles retrieved and contacted experts in the field.
We did not apply any language or date restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAbdel‐Aleem 2011.
For this update we used the following methods when assessing the reports identified by the updated search. These methods are based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies that were identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third person.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resorted to the judgement of the first author to resolve any disagreement.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We described methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We described the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
Cluster‐randomised trials are not eligible for inclusion in this review.
Cross‐over trials
Cross‐over trials are not eligible for inclusion in this review.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we planned to carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
We planned to use funnel plots to investigate reporting biases (such as publication bias) if there were 10 or more studies in the meta‐analysis. In future updates, if more studies are included, we will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and we judged the trials’ populations and methods to be sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or where substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we have used random‐effects analyses, the results are presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Had we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. If we considered that an overall summary was meaningful, we would have used random‐effects analysis to produce it.
There were insufficient included trials in this update to explore heterogeneity with subgroup analysis.
In future updates, we plan to carry out the following subgroup analyses.
Compare the different groups according to management of third stage of labour: active versus expectant management.
Compare the different groups according to exposure to oxytocin before randomisation.
Subgroup analysis will be restricted to primary outcomes.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2014). In future updates of this review, we plan to report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to perform sensitivity analyses for aspects of the review that might affect the results, for example, where there was risk of bias associated with the quality of some of the included trials. Also, we planned sensitivity analysis to explore the effects of fixed‐effect or random‐effects analyses for outcomes with statistical heterogeneity. There were not enough included trials in this update in order to carry out sensitivity analysis. In future updates, sensitivity analysis will be restricted to the primary outcomes.
Results
Description of studies
Results of the search
See: Figure 1.
1.
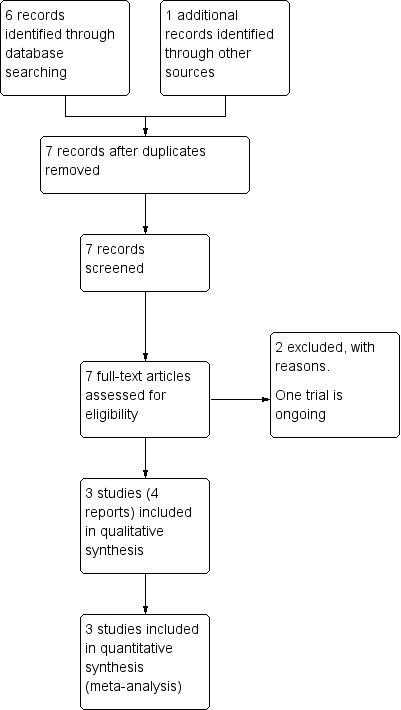
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register retrieved six reports of five trials. We identified one additional report through personal communication (Farrag 2009). We included three trials (four reports) (Bullarbo 2012; Bullarbo 2005; Visalyaputra 2011) and excluded two trials (Farrag 2009; Ross 1994). Denison 2014 is an ongoing study; estimated completion date January 2018.
Included studies
We included three randomised controlled trials (RCTs). The first study was conducted in a single hospital in Sweden (Bullarbo 2005). It included 24 women with placenta remaining undelivered 30 minutes after delivery. Women with the placenta undelivered at 40 minutes were randomised to receive either (two 0.5 mg nitroglycerin (NTG) tablets taken sublingually), or placebo. The authors did not specify the type of retained placenta. All cases had an initial active management of the third stage of labour.
The second study was conducted in a tertiary hospital in Thailand (Visalyaputra 2011). The study included 40 women with retained placenta (status not specified). The study compared intravenous administration of NTG 200 μg with normal saline in a 10 mL syringe in a double‐blind RCT. All cases had an initial active management of the third stage of labour.
The third study, was a multicentre study (including 111 women) conducted in Sweden (Bullarbo 2012). It compared the use 1 mg of sublingual NTG tablets, with placebo tablets administered 50 minutes after childbirth without delivery of the placenta. The status of the placenta was not specified. All cases had an initial active management of the third stage of labour.
Excluded studies
We excluded two studies; the first because it was a quasi‐randomised trial (Farrag 2009), and the other because the study was designed to facilitate manual removal of placenta, which is not within the scope of this review (Ross 1994). SeeCharacteristics of excluded studies.
Risk of bias in included studies
Collectively, among the three included trials, two were judged to be at low risk of bias (Bullarbo 2005; Visalyaputra 2011) and the third trial was judged to be at high risk of bias for two domains: incomplete outcome data and selective reporting (Bullarbo 2012). See Figure 2 and Figure 3 for a summary of the 'Risk of bias' assessments.
2.
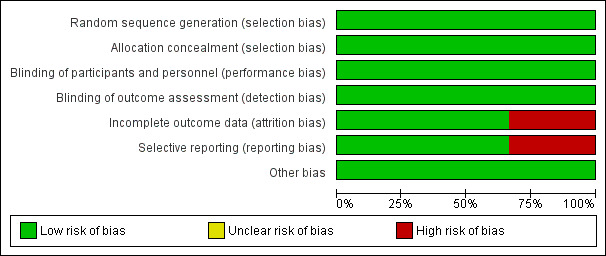
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
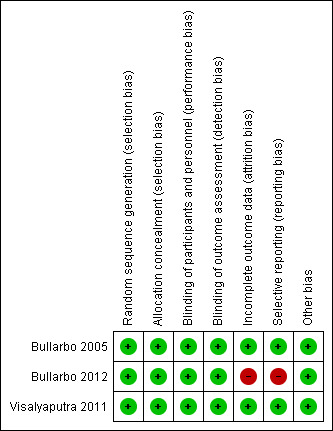
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included studies used a computer random number generator as a method for randomisation and were assessed as being at low risk of bias. Sealed opaque envelopes were used in all studies to conceal allocation.
Blinding
All studies were at low risk of both performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment).
Incomplete outcome data
Only one trial (Bullarbo 2012) showed incomplete reporting of some of the outcomes (mean blood loss, headache and palpitation).
Selective reporting
Only one study (Bullarbo 2012) reported outcomes that were not pre‐specified in the protocol. For example: the outcome "palpitations" and “blood loss > 1000mL” were reported in the paper but were not defined in advance during the protocol stage. The outcome “hypotension” was stated in advance during the protocol stage, but the paper mentioned the outcome "change in the SBP form baseline” instead of “hypotension”.
Other potential sources of bias
All included studies were at low risk of other potential source of bias as there was no premature termination of the study, baseline imbalance or differential diagnosis.
Effects of interventions
We planned to include 12 comparisons encompassing all possible clinical scenarios for the use of nitroglycerin (NTG) in the management of retained placenta. The three RCTs included in this updated review were analysed together under one comparison because all these studies tested the use of NTG versus placebo in the management of retained placenta. There are no data for any of the other pre‐specified comparisons.
Nitroglycerin (NTG) for management of retained (status unknown) placenta versus placebo/expectant management
Primary outcomes
Out of five primary outcomes, data were available for only three of them (manual removal of the placenta, severe postpartum haemorrhage, blood transfusion). The primary outcome "manual removal of the placenta" was reported in the three studies. There was no significant differences between NTG and placebo in reducing the need for manual removal of placenta (risk ratio (RR) 0.83, 95% confidence interval (CI) 0.47 to 1.46; women = 175; I² = 81%) Analysis 1.1.
1.1. Analysis.
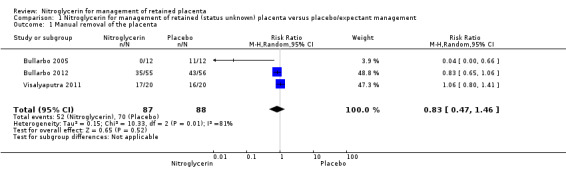
Comparison 1 Nitroglycerin for management of retained (status unknown) placenta versus placebo/expectant management, Outcome 1 Manual removal of the placenta.
Risk of severe postpartum haemorrhage was reported in two studies (Bullarbo 2012; Visalyaputra 2011). There was no increased risk of severe postpartum haemorrhage between both groups (RR 0.93, 95% CI 0.62 to 1.39; women = 150; I² = 0%). Analysis 1.2.
1.2. Analysis.
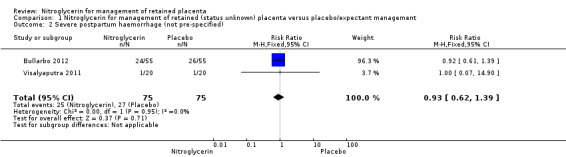
Comparison 1 Nitroglycerin for management of retained (status unknown) placenta versus placebo/expectant management, Outcome 2 Severe postpartum haemorrhage (not pre‐specified).
Blood transfusion was reported by one study (Visalyaputra 2011), with no difference between women who received NTG or placebo (RR 1.00, 95% CI 0.07 to 14.90; women = 40; I² = 0%) Analysis 1.6.
1.6. Analysis.
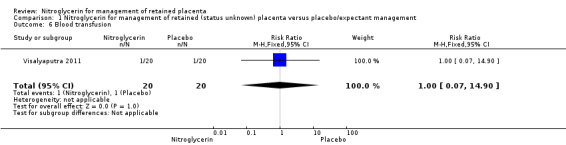
Comparison 1 Nitroglycerin for management of retained (status unknown) placenta versus placebo/expectant management, Outcome 6 Blood transfusion.
Other primary outcomes such as maternal mortality and addition of therapeutic uterotonics were not reported in the studies.
Secondary outcomes
We only found data for four out of the 18 secondary outcomes pre‐specified in this review (mean blood loss, subsequent surgical evacuation of retained products of conception, headache between administration of NTG injection and discharge from the labour ward, changes in pulse rate and blood pressure between administration of NTG and discharge from the labour ward).
Mean blood loss (mL) was reported in the three studies. It was lower in women receiving NTG versus placebo (MD ‐115.31, 95% CI ‐306.25 to 75.63; women = 169; I² = 83%), Analysis 1.3.
1.3. Analysis.
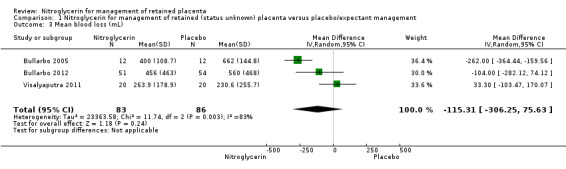
Comparison 1 Nitroglycerin for management of retained (status unknown) placenta versus placebo/expectant management, Outcome 3 Mean blood loss (mL).
One study (Visalyaputra 2011, 40 women) reported the need for subsequent surgical evacuation; there were three events in the NTG arm compared with none in the placebo arm Analysis 1.7.
1.7. Analysis.
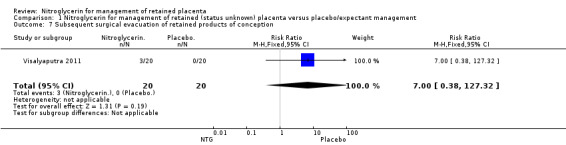
Comparison 1 Nitroglycerin for management of retained (status unknown) placenta versus placebo/expectant management, Outcome 7 Subsequent surgical evacuation of retained products of conception.
Headache was reported by all three studies with no significant differences between women receiving NTG or placebo (RR 1.09, 95% CI 0.80 to 1.47; women = 174; I² = 0%) Analysis 1.4.
1.4. Analysis.
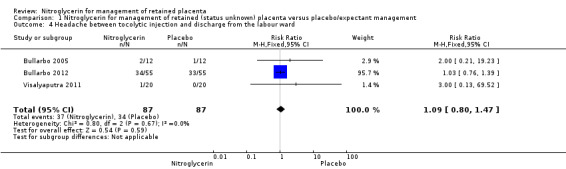
Comparison 1 Nitroglycerin for management of retained (status unknown) placenta versus placebo/expectant management, Outcome 4 Headache between tocolytic injection and discharge from the labour ward.
The three studies reported haemodynamic changes after NTG administration, however only one study gave data suitable for inclusion in our analysis (Bullarbo 2005). The mean systolic and diastolic blood pressures decreased ∼4 mmHg and the difference was statistically significant in comparison to the placebo group (systolic blood pressure (mean difference (MD) ‐3.75; 95% CI ‐7.47 to ‐0.03; women = 24;), and diastolic blood pressure (MD ‐3.75, 95% CI ‐6.48 to ‐1.02; women = 24;); Analysis 1.5. There was also an increase in the pulse rate in women who received NTG in comparison to those who received placebo (MD 6.00; 95% CI 3.07 to 8.93; women = 24;).
1.5. Analysis.
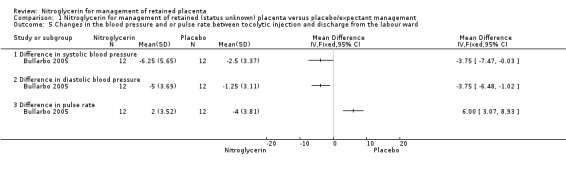
Comparison 1 Nitroglycerin for management of retained (status unknown) placenta versus placebo/expectant management, Outcome 5 Changes in the blood pressure and or pulse rate between tocolytic injection and discharge from the labour ward.
Discussion
Retained placenta is a potentially serious complication of labour. The gold standard of management is manual removal of the placenta under anaesthesia with its inherent risks due to its invasive nature. Alternative lines include the use of uterotonics and uterine‐relaxing drugs either in a combined or sequential mode.
Summary of main results
This review included three eligible clinical trials which compared the use of nitroglycerin (NTG) (sublingual or intravenous) with placebo in cases of retained placenta (status unknown). The studies reported seven out of 23 designated outcomes in this update. For the primary outcomes (manual removal of the placenta, severe postpartum haemorrhage (PPH) and blood transfusion), there were no significant differences between women who received NTG versus those who received placebo.
NTG was used in a variety of routes and doses. Both sublingual and intravenous routes of NTG have the same pharmacokinetic properties. Its action starts within two to three minutes and reaches mean peak NTG plasma concentrations at a mean time of approximately six to seven minutes post‐dose (Laufen 1987). The difference in the dose of NTG between different studies could explain the difference in direction of effect and heterogeneity between the studies included in the analysis of manual removal of the placenta (Bullarbo 2005; Bullarbo 2012; Visalyaputra 2011). Visalyaputra 2011 reported that 200 μg of intravenous NTG was ineffective in facilitating the delivery of a retained placenta (success rates of 15% in the study group and 20% in the control group). On the other hand, using 1 mg sublingual in the other studies may explain the success (statistically insignificant) in their series (Bullarbo 2005; Bullarbo 2012).
Severe PPH was reported by two studies (150 women), with no difference between women treated by NTG or placebo. The need for blood transfusion was reported by one study (40 women) with no differences between groups. This is reassuring because NTG is known to cause vasodilatation and relaxation of the uterus which could lead to atonic PPH. However, the small numbers of women in the included studies and the relatively rare incidence of severe PPH could explain the findings.
Among the pre‐specified 18 secondary outcomes, only four outcomes were reported in the included trials. The mean blood loss was reported by the three included studies (169 women) with no significant differences between women given NTG or placebo. All the studies documented no significant effect of medical importance on the haemodynamic parameters (Bullarbo 2005; Bullarbo 2012; Visalyaputra 2011). Nitroglycerin administration was associated with a significant mild hypotension and significant increase in pulse rate.This finding is supported by the study conducted by Ekerhovad and colleagues (Ekerhovd 2008). They reported statistical, but not clinical decline in both systolic blood pressure and diastolic blood pressure; from 120 to 112 mmHg in the former, and from 74.8 to 70.4 mmHg for the latter.These haemodynamic changes can be explained by the effect of NTG on the vascular smooth muscles causing vasodilatation, hypotension and compensatory tachycardia.
Overall completeness and applicability of evidence
This review assessed the value of the use of NTG either alone or in combination with oxytocin in the management of retained placenta (status unknown). This was addressed in only three included randomised controlled trials (RCTs) involving a total of 175 women. The studies were conducted in both high‐income settings (Sweden) and low‐income settings (Thailand). Not all pre‐specified outcomes could be analysed as they were not reported. Currently available data show no beneficial effect in using NTG alone or in reducing the need for manual removal of placenta. NTG has a significant, although mild, effect on both pulse rate and blood pressure. Using NTG alone in cases of retained placenta does not increase the incidence of severe PPH or the need for blood transfusion. The small sample size of the included studies, including only one comparison of the possible scenarios encountered in practice, and the limited number of outcomes reported, reduce the external validity of the review.
Quality of the evidence
The review included three RCTs involving a total of 175 women. Two out of the three studies are judged to be at low risk of bias. Not all pre‐specified outcomes could be analysed as they were not reported. There was heterogeneity among the studies in the primary outcome (manual removal of the placenta), possibly due to differences in the dose of NTG used in one of the studies and possibly the difference in the route of administration (sublingual in two studies and intravenous in one study). Though the evidence drawn from three RCTs suggests that there is no beneficial effect in using NTG alone in reducing the need for manual removal of placenta, we have to be cautious because of heterogeneity. For safety issues, NTG has a significant, although mild effect on both pulse rate and blood pressure.
Potential biases in the review process
The search identified all relevant studies. However, not all data pre‐specified in the review were reported in the studies (three out of five for the primary outcomes, and four out of 18 for the secondary outcomes).
Agreements and disagreements with other studies or reviews
There were no other RCTs or systematic reviews addressing this issue. A prospective non‐controlled trial showed the effectiveness of 1 mg sublingual NTG in the delivery of retained placenta sequentially after oxytocin (Ekerhovd 2008). The authors reported a success rate in 21 out of 24 patients (87.5%) in placental delivery without the need of manual removal of placenta. Three case series addressed the use of NTG to facilitate the manual removal of placenta without the need of endotracheal intubation in patients with constriction ring (retained but detached placenta) (Desimone 1990; Peng 1989; Ross 1994).They used intravenous NTG; from 50 to 500 μg. the authors have reported a 100% success rate in this context with no significant haemodynamic changes or uterine atony. An interesting case report addressing the safety of NTG in women with rheumatic valvular heart disease was reported in one study (Jha 2003). The authors described the use of two different protocols for management of retained placenta in one woman who had retained placenta in two consecutive deliveries. This parturient woman with rheumatic valvular heart disease was managed at one time by general anaesthesia and on the second occasion using only intravenous fentanyl and NTG. The authors reported on the efficacy of this last combination in this setting with remarkable safety in this woman with co‐morbidities. A recent case report described successful use of intracervical NTG to deliver a trapped retained placenta (Rodgers 2013). A 34‐year‐old gravida two, para one woman delivered a viable male baby with the placenta retained detached but entrapped. The NTG tablet was placed in the cervix and held in place by the delivering physician as it dissolved. Soon after administration, the intact placenta delivered with no reported side effects.
Authors' conclusions
Implications for practice.
In cases of retained placenta, currently available data show no beneficial effect for nitroglycerin (NTG) when used alone in reducing the need for manual removal of placenta.
Implications for research.
There is a need to conduct well‐designed randomised controlled trials to test the role of NTG, in different clinical scenarios of retained placenta (detached versus non‐detached). Addition of ultrasonography in the diagnosis of subtype of retained placenta may add much in the context of research to verify the role of NTG in the management of different subtypes of retained placenta.
What's new
| Date | Event | Description |
|---|---|---|
| 14 January 2015 | New citation required and conclusions have changed | Two studies have been added. With the addition of the new data, there are now no significant differences in the rate of manual removal of placenta or mean blood loss between groups (nitroglycerin plus uterotonics versus placebo plus uterotonics). |
| 14 January 2015 | New search has been performed | Search updated. The review now includes three trials. There is also one ongoing trial. The title of the review has changed from, 'Tocolytics for management of retained placenta', to 'Nitrogylcerin for management of retained placenta'. Pulse rate was added as a secondary outcome. |
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. GLOSSARY
Accreta: placenta abnormally invading the muscle layer of the uterus.
Chorionic villi: microscopic description of a part of placenta that is responsible for nutrition of the fetus.
Constriction ring: localised contraction of a part of the myometrium.
Cyclic guanosine monophosphate: a chemical substance used in cell‐to cell messaging and actions
Diastolic: measures the pressure on the blood vessel walls when the heart is at rest.
Dynamic ultrasonographic imaging: an investigation uses ultrasound waves to visualise changes that happen in the uterus during delivery of the placenta.
Ergot‐induced: caused by a drug commonly used to induce uterine contractions.
Haemodynamically: blood pressure and blood circulation.
Halothane: an anaesthetic used through the nose and trachea.
Internal and external version: obstetric maneuvers done by experienced obstetricians to change the lie of the fetus either internally through the vagina or externally through the abdomen.
Manual: by hand.
Mortality: death.
Myometrium: uterine muscle wall.
Nitroglycerin tablets: medicine to dilate the arteries to reduce the workload on the heart e.g. for angina.
Postpartum: after birth.
Retained placenta: placenta remains in the womb after delivery.
Retro‐placental myometrial contractions: contractions of the part of the uterine muscle that lies behind the placenta.
Sonographically: using ultrasound waves.
Sublingual: placed under the tongue.
Systolic: pumping phase of the heart where the ventricles are contracting.
Tachycardia: increased heart rate.
Tocolytics: drugs that are usually decrease contractions of the womb.
Uterotonics: substances that stimulate contractions of the womb; e.g. oxytocin, ergometrine, syntometrine.
Data and analyses
Comparison 1. Nitroglycerin for management of retained (status unknown) placenta versus placebo/expectant management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Manual removal of the placenta | 3 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.47, 1.46] |
| 2 Severe postpartum haemorrhage (not pre‐specified) | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.62, 1.39] |
| 3 Mean blood loss (mL) | 3 | 169 | Mean Difference (IV, Random, 95% CI) | ‐115.31 [‐306.25, 75.63] |
| 4 Headache between tocolytic injection and discharge from the labour ward | 3 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.80, 1.47] |
| 5 Changes in the blood pressure and or pulse rate between tocolytic injection and discharge from the labour ward | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Difference in systolic blood pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Difference in diastolic blood pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Difference in pulse rate | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Blood transfusion | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.90] |
| 7 Subsequent surgical evacuation of retained products of conception | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 127.32] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bullarbo 2005.
| Methods | Randomised controlled trial. Figure 11. | |
| Participants | Women (n = 24) failed to deliver the placenta 40 minutes after completion of second stage of labour despite additional administration of 10 IU oxytocin followed by controlled cord traction. All women were managed actively in the third stage of labour. Inclusion criteria: uncomplicated singleton pregnancy, spontaneous vertex delivery, healthy child. Exclusion criteria: pregnancy < 37 weeks, postpartum haemorrhage requiring immediate intervention, uterine malformation, intolerance to NTG, maternal age < 18 years, suspected placenta accreta, serious maternal disease. |
|
| Interventions |
Experimental Intervention: use of sublingual NTG tablets in a dose of 1 mg (2 tablets each is 0.5 mg) given 40 minutes after failure to deliver the placenta. Control intervention: 2 placebo tablets of similar design as the NTG tablets. Both groups received in addition oxytocin. |
|
| Outcomes |
Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Low risk for performance bias: the participating women were not aware of the agent administered. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for detection bias: the obstetrician on duty was aware of the agent administered. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. No excluded women. Analysis was by intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported in the results section. However, not all outcomes pre‐specified to be included in this review were reported in the trial. The study included the following outcomes; need for manual removal of placenta, haemodynamic changes of NTG (systolic blood pressure, diastolic blood pressure, pulse rate), mean blood loss during third stage of labour (although the method of estimation was not mentioned), side effects of NTG. |
| Other bias | Low risk | There is no premature termination of the study, baseline imbalances or differential diagnosis. |
4.
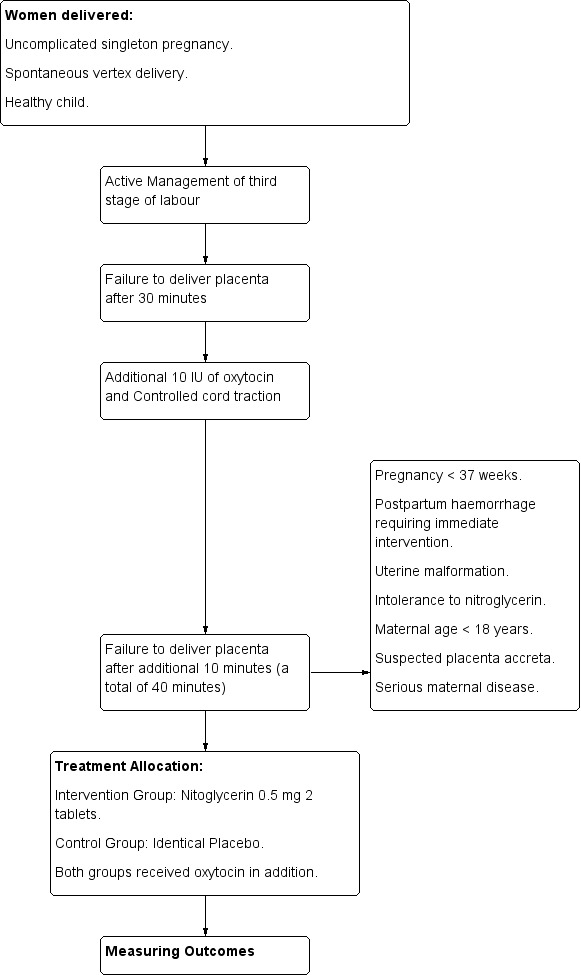
Bullarbo 2012.
| Methods | Multicentre randomised controlled trial. Figure 12. | |
| Participants | Women (n =111) with retained placenta (remained undelivered for 40 minutes after delivery of the baby). Inclusion criteria: women with uncomplicated singleton pregnancy with spontaneous vertex delivery of a healthy child at term. Exclusion criteria: women with serious maternal disease, less than 18 years of age, those with massive blood loss (blood loss more than 600 mL), those with any uterine malformation or those with suspected placental accreta were excluded. |
|
| Interventions |
Experimental intervention: 10 IU oxytocin + 1 mg of NTG tablets (2 x 0.5 mg tablets).The tablets were administered sublingually approximately 50 minutes after childbirth. Control intervention: 10 IU oxytocin + 2 placebo tablets. The tablets were administered sublingually approximately 50 minutes after childbirth. |
|
| Outcomes | 1.The need for manual removal of placenta: the procedure was regarded as successful if the placenta was delivered in the delivery room without the need of operative manual removal under either regional (epidural or spinal) or general anaesthesia. 2. Maternal blood pressure and pulse rate. These were measured before administration of NTG or placebo tablets and then repeated five minutes as well as 15 minutes after administration of the tablets. 3. Total blood loss during the third stage of delivery. 4. Possible side effects of NTG (by completion of a questionnaire). |
|
| Notes | Although the sample size was 120 women, 9 women were excluded because surgical removal of placenta was performed initially due to the preference of the obstetrician on duty despite the fact that the women had given their informed consent to participate. The intervention group included 55 women and the control group included 56 women. The missing 6 women comprise 4 women who did not receive study medication and 2 women who only received 0.5 mg NTG. Data are available only for some patients in some of the outcomes. Intervention group: 55 patients: 1. for blood loss outcome data are available for 51 patients; 2. for headache: data are available for 34 patients; 3. for palpitation data are available for 29 patients. Control group: 56 patients: 1. for blood loss outcome data are available for 54 patients; 2. for headache: data are available for 33 patients; 3. for palpitation: data are available for 32 patients. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by numbered opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A nurse who did not participate in the remainder of the study handed out the study medication as needed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neither the obstetrician nor the participating women were aware of the agent administered. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There are missing data in some of the outcomes. |
| Selective reporting (reporting bias) | High risk | There are some differences between the proposed (in the protocol) and the reported (in the report) outcomes (for example: the outcome "palpitations" and “blood loss > 1000mL” were reported into the paper but were not defined in advance during the protocol stage). The outcome “hypotension” was stated in advance during the protocol stage but the paper mentioned the outcome” change in the SBP form baseline” instead of “hypotension”. |
| Other bias | Low risk | There was no premature termination of the study,or baseline imbalances. |
5.
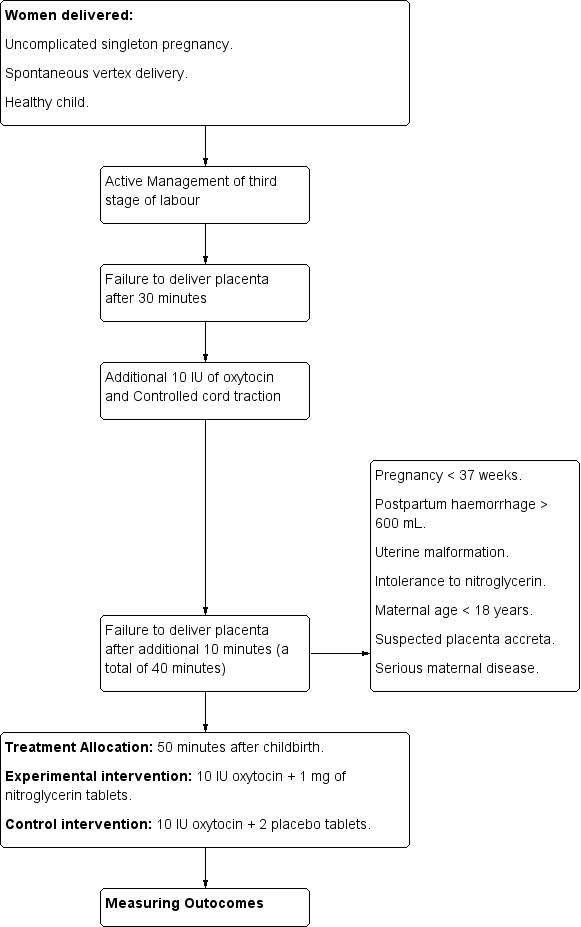
Visalyaputra 2011.
| Methods | Randomised controlled trial. Figure 13. | |
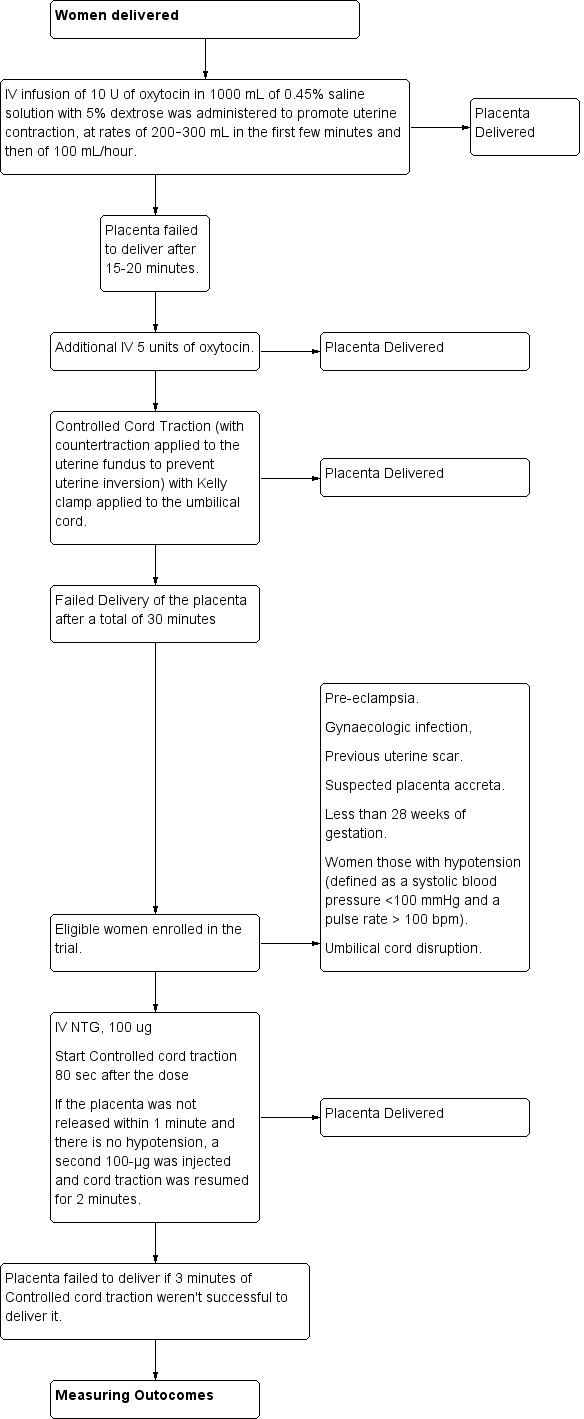
| Participants | Women (n = 40) with retained placenta (defined as a placenta not delivered within 30 minutes after fetal delivery). Inclusion criteria: singleton pregnancy with no cardiac, pulmonary, or other form of disease requiring treatment. Exclusion criteria: women with pre‐eclampsia, gynaecologic infection, previous uterine scar or suspected placenta accreta, women less than 28 weeks of gestation, those with hypotension (defined as a systolic blood pressure <100 mmHg and a pulse rate > 100 bpm) or umbilical cord disruption. |
|
| Interventions |
According to study protocol, placenta is considered retained if it was failed to deliver despite administration of 15 IU oxytocin intravenous drip combined with controlled cord traction. Intervention group: 200 μg of NTG intravenously, 100 μg at a time, in an amount of a normal saline solution sufficient to fill a10‐mL syringe. 80 seconds after the first 100μg of NTG (or 2 mL of the solution) was injected, the obstetrician began to gently pull on the cord. If the placenta was not released within 1 minute and the participant was not in a state of hypotension, a second 100‐μg bolus from the same syringe was injected and cord traction was resumed for no longer than 2 more minutes. If the placenta was not released after 3 minutes of controlled cord traction, the procedure was considered to have failed. Control group: 10 mL of normal saline solution, also in 10‐mL syringes. Additional medications: 50‐100 μg of fentanyl citrate was given to all participants for analgesia and norepinephrine bitartrate was administered intravenously to those with hypotension. Oxytocin or ergometrine was administered to the participants in whom the procedure succeeded. |
|
| Outcomes |
Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using the Research Randomizer program (made freely available by the Social Psychology Network at http://www.Randomizer.org), 40 successive eligible women with a retained placenta were randomised to a study or control group by the block‐of‐4 method. |
| Allocation concealment (selection bias) | Low risk | Group assignment was enclosed in a numbered envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A nurse who did not participate in the remainder of the study opened the envelopes as needed and filled the syringes with the appropriate solutions outside the operating room. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neither the investigators who gave the injections and managed the participants’ blood pressure, nor the obstetricians who performed controlled cord traction, nor the study participants were aware of the syringe content. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. No excluded women. Analysis was by intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported in the results section. |
| Other bias | Low risk | There is no premature termination of the study, baseline imbalances or differential diagnosis. |
bpm: beats per minute IU: international units NTG: nitroglycerin
6.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Farrag 2009 | Quasi‐randomised controlled trial. The study compared sublingual versus intravenous NTG when oxytocin plus controlled cord traction failed to deliver the placenta. |
| Ross 1994 | Study included patients who were eligible to have manual removal of placenta. Objective was to look for the role of nitroglycerin to facilitate manual removal of placenta. It was a double‐blinded study where intravenous 250 μg versus 500 μg nitroglycerin was used. |
NTG: nitroglycerin
Characteristics of ongoing studies [ordered by study ID]
Denison 2014.
| Trial name or title | GOT‐IT Trial: Glyceryl Trinitrate for Retained Placenta |
| Methods | This study will try to prove the clinical and cost effectiveness of GTN used to treat retained placenta. The investigators will compare GTN against a placebo (dummy treatment) in a randomised controlled blinded trial (GOT‐IT). The GOT‐IT Trial will be conducted in two phases. The first phase will involve an internal pilot study where the aim will be to test out and refine trial procedures in a small number of hospital sites. The second phase will be the main trial where recruitment will be extended to a larger number of hospitals in order to determine clinical and cost effectiveness. |
| Participants | Patients with placenta not delivered for 15 minutes or more after birth. |
| Interventions | Experimental: GTN Nitrolingual Pump Spray [Coro‐Nitro] A liquid within non‐pressurised, red plastic‐coated glass bottle fitted with a pump capable of delivering a metered dose containing 400 μg of GTN. Excipients: the formulation contains fractionated coconut oil, absolute ethanol, medium chain partial glycerides and peppermint oil. The treatment will be self administered (2 puffs) as a single intervention. No second intervention will be given. |
| Outcomes |
Primary outcome measures 1. Need for manual removal of placenta. 2. Blood loss. 3. Net incremental costs (or cost savings) to the National Health Service. Secondary outcome measures 1. Fall in haemoglobin. 2. Time from randomisation to delivery of placenta. 3. Need for manual removal of placenta in theatre. 4. Need for earlier than planned manual removal of placenta on the basis of the clinical condition. 5. Systolic and diastolic blood pressure. 6. Need for blood transfusion. 7. Need for general anaesthesia. 8. Maternal pyrexia. 9. Sustained uterine relaxation. 10. Mean costs for each treatment allocation group. |
| Starting date | September 2014. |
| Contact information | Fiona C Denison, fiona.denison@ed.ac.uk |
| Notes |
NCT02085213 Allocation: randomised. Endpoint classification: intervention model: single group assignment; masking: double blind (participant, investigator, outcomes assessor). |
GTN: glyceryl trinitrate
Differences between protocol and review
The title of the review has changed from, 'Tocolytics for management of retained placenta', to 'Nitrogylcerin for management of retained placenta'. Pulse rate was added as a secondary outcome.
The methods have been updated in accordance with the latest Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The outcomes for this update (2015) have been refined and the following outcomes are not included in this update: Duration of third stage of labour; haemoglobin level, hospital stay, postpartum infection, other medical interventions, initiation of lactation.
The following are new outcomes for this update.
Primary outcomes
Severe postpartum haemorrhage (S‐PPH) (defined as clinically estimated or measured blood loss greater than or equal to 1000 mL).
Addition of therapeutic uterotonics.
Secondary outcomes
Serious maternal morbidity (hysterectomy, admission to intensive care, renal or respiratory failure, and other additional surgical procedures to treat PPH other than manual removal of placenta.
PPH (defined as clinically estimated or measured blood loss greater than or equal to 500 mL).
Maternal postpartum anaemia (defined by the haemoglobin concentration according to the international standards) or fall in haemoglobin levels (defined as decrease in previous haemoglobin concentration levels by at least 10%).
Iron tablets (therapeutic indication) during the puerperium.
Maternal pain between administration of nitroglycerin (NTG) and discharge from the labour ward.
Maternal dissatisfaction with third stage management.
Secondary PPH (after 24 hours and before six weeks).
Need for treatment with antibiotics.
Maternal fatigue.
Breastfeeding at discharge from hospital.
Maternal mortality and blood transfusion, previously secondary outcomes are now primary outcomes of the review.
The methods for subgroup analysis have changed since the last update from:
We planned to carry out the following subgroup analyses.
Compare the different tocolytic drug.
Compare the different groups according to management of third stage of labour: active versus expectant management
to:
Compare the different groups according to management of third stage of labour: active versus expectant management.
Compare the different groups according to exposure to oxytocin before randomisation.
Contributions of authors
Hany Abdel‐Aleem is the guarantor of the review. He is responsible for conceiving the review; designing the review and co‐ordinating the review. In this updated version of the review, M Abdel‐Aleem (MAA) and O Shaaban (OS) assessed data quality and carried out the data extraction. OS performed the analysis. HAA reviewed both data extraction and analysis. MAA updated the text. HAA and OS reviewed the text.
Sources of support
Internal sources
Department of Obstetrics and Gynecology, Women's Health Centre, Faculty of Medicine, Assiut University, Egypt.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bullarbo 2005 {published data only}
- Bullarbo M, Tjugum J, Ekerhovd E. Sublingual nitroglycerin for management of retained placenta. International Journal of Gynecology & Obstetrics 2005;91:228‐32. [DOI] [PubMed] [Google Scholar]
Bullarbo 2012 {published data only}
- Bularbo M, Bokstrom H, Lilja H, Almstrom E, Lassenius N, Hansson A. Nitroglycerin for the management of retained placenta: a multicenter study. Obstetrics and Gynecology International 2012;2012:Article ID: 321207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullarbo M. Medical treatment with nitroglycerin for management of retained placenta: a multicentre trial. http://www.controlled‐trials.com/ISRCTN34755982 [accessed 11.05.2012] 2008.
Visalyaputra 2011 {published data only}
- Visalyaputra S, Prechapanich J, Suwanvichai S, Yimyam S, Permpolprasert L, Suksopee P. Intravenous nitroglycerin for controlled cord traction in the management of retained placenta. International Journal of Gynecology & Obstetrics 2011;112(2):103‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Farrag 2009 {published data only}
- Farrag MA. Sublingual versus intravenous nitroglycerin for management of retained placenta. Benha Medical Journal 2009;26(2):221‐30. [Google Scholar]
Ross 1994 {published data only}
- Ross A. Nitroglycerin and uterine relaxation. Anaesthesia and Intensive Care 1994;22:494. [Google Scholar]
References to ongoing studies
Denison 2014 {published data only}
- Denison FC. GOT‐IT Trial: Glyceryl trinitrate for retained placenta. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 20 March 2014] 2014.
Additional references
Altabef 1992
- Altabef KM, Spencer JT, Zinberg S. Intravenous nitroglycerin for uterine relaxation for an inverted uterus. American Journal of Obstetrics and Gynecology 1992;166:1237‐8. [DOI] [PubMed] [Google Scholar]
Axemo 1998
- Axemo P, Fu X, Lindberg B, Ulmsten U, Wessen A. Intravenous nitroglycerin for rapid uterine relaxation. Acta Obstetricia et Gynecologica Scandinavica 1998;77:503. [DOI] [PubMed] [Google Scholar]
Barnes 1881
- Barnes F. Hour‐glass contraction of the uterus treated with nitrite of amyl. British Medical Journal 1881;1:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Belfort 1993
- Belfort MA. Intravenous nitroglycerin as a tocolytic agent for intrapartum external cephalic version. South African Medical Journal 1993;83:656. [PubMed] [Google Scholar]
Chedraui 2003
- Chedraui PA, Insuasti DF. Intravenous nitroglycerine in the management of retained placenta. Gynecologic and Obstetric Investigation 2003;56:61‐4. [DOI] [PubMed] [Google Scholar]
Combs 1991
- Combs CA, Laros RK. Prolonged third stage of labour:morbidity and risk factors. Obstetrics and Gynecology 1991;77:863‐7. [PubMed] [Google Scholar]
Desimone 1990
- Desimone CA, Norris MC, Leighton BL. Intravenous nitroglycerin aids manual extraction of a retained placenta. Anesthesiology 1990;73:787. [DOI] [PubMed] [Google Scholar]
Dufour 1997a
- Dufour P, Vinatier D, Orazi G, Ducloy AS. The use of intravenous nitroglycerin for emergency cervico‐uterine relaxation. Acta Obstetricia et Gynecologica Scandinavica 1997;76:287‐8. [PubMed] [Google Scholar]
Dufour 1997b
- Dufour P, Vinatier D, Puech F. The use of intravenous nitroglycerin for cervico‐uterine relaxation: a review of the literature. Archives of Gynecology and Obstetrics 1997;261(1):1‐7. [DOI] [PubMed] [Google Scholar]
Ekerhovd 2008
- Ekerhovd E, Bullarbo M. Sublingual nitroglycerin seems to be effective in the management of retained placenta. Acta Obstetricia et Gynecologica Scandinavica 2008;87(2):222‐5. [DOI] [PubMed] [Google Scholar]
Farley 2004
- Farley AE, Graham CH, Smith GN. Contractile properties of human anchoring villi. American Journal of Physiology 2004;287(3):R680‐R684. [DOI] [PubMed] [Google Scholar]
Ghag 2014
- Ghag K, Bahl R. Recurrence rate of manual removal of placenta and associated postpartum haemorrhage. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(Suppl 1):A111. [Google Scholar]
Herman 1993
- Herman A, Weinraub Z, Bukovsky I, Arieli S, Zabow P, Caspi E. Dynamic ultrasonographic imaging of the third stage of labor: new perspectives into third‐stage mechanisms. American Journal of Obstetrics and Gynecology 1993;168:1496‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jensen 1994
- Jensen KM, Dahl JB. Plasma concentrations of glyceryl trinitrate and its dinitrate metabolites after sublingual administration to volunteers. Simultaneous determination of glyceryl trinitrate and its dinitrate metabolites. Arzneimittel‐Forschung 1994;44:9514. [PubMed] [Google Scholar]
Jha 2003
- Jha S, Chiu JW, Yeo IS. Intravenous nitro‐glycerine versus general anaesthesia for placental extraction‐‐a sequential comparison. Medical Science Monitor 2003;9(7):CS63‐CS66. [PubMed] [Google Scholar]
Krapp 2000
- Krapp M, Baschat AA, Hankeln M, Gembruch U. Grayscale and color Doppler sonography in the third stage of labor for early detection of failed placental separation. Ultrasound in Obstetrics and Gynecology 2000;15(2):138‐42. [DOI] [PubMed] [Google Scholar]
Laufen 1987
- Laufen H, Leitold M. Glyceryl‐1‐nitrate pharmacokinetics in healthy volunteers. British Journal of Clinical Pharmacology 1987;23(3):287‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayer 1992
- Mayer DC, Weeks SK. Antepartum uterine relaxation with nitroglycerin at caesarean delivery. Canadian Journal of Anaesthesia 1992;39:166‐9. [DOI] [PubMed] [Google Scholar]
NCCWCH 2007
- National Collaborating Centre for Women’s and Children’s Health (NCCWCH). Intrapartum Care. Care of Healthy Women and Their Babies During Childbirth. London: RCOG Press, 2007. [PubMed] [Google Scholar]
Peng 1989
- Peng ATC, Gorman RS, Shulman SM, Demarchis E, Nyunt K, Blancato LS. Intravenous nitroglycerin for uterine relaxation in the postpartum patient with retained placenta. Anesthesiology 1989;71:172‐3. [DOI] [PubMed] [Google Scholar]
Percival 1980
- Percival R. Holland and Brews Manual of Obstetrics. 14th Edition. English Language Book Society and Churchill Livingstone,Edinburgh, 1980. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodgers 2013
- Rodgers BC, Pasternak A, Gray R. A novel treatment for management of a trapped placenta using intracervical nitroglycerin tablets. BMJ Case Reports 2013;14(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rolbin 1991
- Rolbin SH, Hew EM, Bernstein A. Uterine relaxation can be life saving. Canadian Journal of Anaesthesia 1991;38(7):939‐40. [DOI] [PubMed] [Google Scholar]
Ticconi 2004
- Ticconi C, Zicari A, Realacci M, Vito M, Denora P, Narcisi M. Oxytocin modulates nitric oxide generation by human fetal membranes at term pregnancy. American Journal of Reproductive Immunology 2004;52(3):185‐91. [DOI] [PubMed] [Google Scholar]
Urner 2014
- Urner F, Zimmermann R, Krafft A. Manual removal of the placenta after vaginal delivery: an unsolved problem in obstetrics. Journal of Pregnancy 2014;2014:274651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weeks 2001
- Weeks AD. The retained placenta. African Health Sciences 2001;1(1):36‐41. [PMC free article] [PubMed] [Google Scholar]
Weeks 2002
- Weeks AD, Mirembe FM. The retained placenta‐new insights into an old problem. European Journal of Obstetrics & Gynecology and Reproductive Biology 2002;102(2):109‐10. [DOI] [PubMed] [Google Scholar]
Weeks 2005
- Weeks AD. The retained placenta. Progress in Obstetrics and Gynaecology 2005;16:133‐54. [Google Scholar]
Wessen 1995
- Wessen A, Elowsson P, Axemo P, Lindberg B. The use of intravenous nitroglycerin for emergency cervico‐uterine relaxation. Acta Anaesthesiologica Scandinavica 1995;39:847‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Abdel‐Aleem 2011
- Abdel‐Aleem H, Abdel‐Aleem MA, Shaaban OM. Tocolysis for management of retained placenta. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD007708.pub2] [DOI] [PubMed] [Google Scholar]


