Abstract
Stunting among children under five years old is still a problem in many developing countries including Kenya. However, there is little information linking stunting with mycotoxin contamination of complementary foods. The aim of this study was to assess knowledge about aflatoxin and fumonisin contamination in sorghum alongside postharvest handling and storage practices among caregivers of children under five years old in Kerio Valley, Kenya. A cross‐sectional study was conducted to obtain data from 353 randomly selected caregivers of children aged 6–59 months. Qualitative data were obtained through Focus Group Discussions and Key Informant Interviews. Overall, majority of the caregivers of young children had poor knowledge (61.8%) about mycotoxin contamination of food, and poor postharvest handling and storage practices (74.5%). The caregiver's knowledge about mycotoxins was significantly associated with age [(AOR=4.629, (95% Cl: 2.530–8.472), p < .001], education level [(AOR=0.275, (95% Cl: 0.088–0.434), p = .001], marital status [(AOR=15.187, (95% Cl: 1.830–126.007), p = .012], and household monthly income [(AOR=2.623, (95% Cl: 1.550–4.439), p < 0,001]. Furthermore, the caregiver's age [(AOR=3.845, (95% Cl: 1.558–9.490), p = .003], education level [(AOR=0.196, (95% Cl: 0.088–0.434), p < .001], monthly income [(AOR=3.291, (95% Cl: 1.550–6.986), p = .002], and knowledge on mycotoxin contamination of sorghum [AOR, 5.428 (95% Cl: 2.855–10.319), p < .001] were significantly associated with postharvest handling and storage practices except for marital status [AOR, 3.579 (95% Cl: 0.403–31.775), p = .252]. In conclusion, caregivers of young children had poor knowledge about mycotoxin contamination of complementary foods and suboptimal postharvest handling and storage practices of sorghum. This increases the risk of mycotoxin exposure to young children and necessitates mitigation measures including sensitization campaigns and social behavior change communication.
Keywords: aflatoxins, children's caregivers, fumonisins, knowledge, postharvest practices, sorghum‐based complementary foods
The caregivers of young children had poor knowledge about mycotoxin contamination of complementary foods prepared from sorghum.
Inadequate knowledge on mycotoxin contamination of food is significantly associated with poor post‐harvest handling and storage practices of sorghum.
The main determinants of caregivers’ knowledge and post‐harvest handling and storage practices of sorghum include age, education level and household monthly income.
1. INTRODUCTION
Mycotoxins are harmful substances produced by some species of fungi that contaminate food material when stored in conditions that favor fungal growth (Obonyo & Salano, 2018). The most significant mycotoxins found in cereals are aflatoxins and fumonisins due to their ubiquitous nature as well as their acute and chronic effects causing death (Probst et al., 2007) or stunting (Knipstein et al., 2015), respectively. There are studies that reported contamination of cereal‐based complementary foods by diverse mycotoxins (Kimanya et al., 2014; Ojuri et al., 2018). This could explain why dietary exposure to mycotoxins is identified as a major cause of undernutrition, morbidity and mortality among children (Paudyal et al., 2017). Children are more sensitive to mycotoxin exposure than the rest of the population due to their underdeveloped immune system, lower body weight, and less acidic stomachs among other factors (Magoha et al., 2014).
In Kenya, sorghum is an important staple food crop for many households especially in the arid and semi‐arid lands (ASALs) (Orr et al., 2016) due to its adaptability to harsh environmental conditions. In this regard, the Kenyan government has continued to develop and promote the production of improved sorghum varieties as a means to improving income and food security (Government of Kenya (GoK), 2018). The Kerio Valley, a semi‐arid area, with unreliable rainfall, chronically experiences food and nutrition insecurity (Elgeyo Marakwet County, 2018) and is currently leading or ranks poorly in the country as far as child nutrition is concerned. A recent study (Kipyego & Mugalavai, 2019) reported a high prevalence of underweight (27%), stunting (67%), and wasting (9%) among children under five years from the small holder farmer households. These rates have remained high despite intervention programs by the county government and other development partners, which included the USAID Feed the Future Accelerated Value Chain Development (AVCD). The AVCD program promoted the production and utilization of sorghum and other drought‐tolerant crops by integrating agricultural and nutrition interventions between 2016 and 2019 (Kiome et al., 2016). It was anticipated that the child nutrition outcomes would improve after the interventions. Therefore, the persistent undernutrition rates necessitated the assessment of underlying factors that contribute to stunting. This is especially important because caregivers of young children from Kerio Valley use sorghum as a sole or major component of complementary foods (in form of porridge) as a common practice (Kiome et al., 2016).
In addition, adequate evidence from neighboring counties (e.g., Nandi County, Kenya) indicates that sorghum is susceptible to aflatoxin and fumonisin contamination at levels above regulatory limits (Sirma et al., 2015). It is thought that co‐occurrence of aflatoxins and fumonisins in complementary foods contributes to stunting in an additive manner (World Health Organization (WHO), 2018) possibly through the toxin‐induced liver and intestinal injury (Knipstein et al., 2015), or impaired immune system function (Leroy et al., 2015). Therefore, this study investigated the factors that determine knowledge about aflatoxin and fumonisin contamination in sorghum and postharvest practices such as handling and storage practices of sorghum among children's caregivers as contributors to possible contamination of complementary foods. There is limited information on the contribution of occurrence and exposure to aflatoxin and fumonisin in sorghum‐based complementary foods to the persistent undernutrition among children under five years in Kerio Valley, which is considered an ultimate goal of this study.
2. METHODS
2.1. Study area
This study was undertaken in major sorghum growing areas (Emsoo, Endo, and Arror Wards) of Kerio Valley in Elgeyo Marakwet County. The Kerio Valley is semiarid with an annual rainfall of 850‐1000mm and temperature of 40℃‐ 17℃ (Elgeyo Marakwet County, 2018).
2.2. Survey design, population, and sampling
A cross‐sectional study was conducted in Kerio Valley, three months (June‐July, 2020) after government lockdown due to the COVID‐19 global pandemic. To obtain the study participants, multistage sampling procedures were adopted. First, Elgeyo Marakwet County was purposively selected being one of the counties where production and utilization of sorghum has been promoted in the AVCD program. Kerio valley was further purposively selected due to the high malnutrition rate reported among children under five years and being the major sorghum‐growing zone in the County, especially in Emsoo ward in Keiyo North, Endo (Marakwet East), and Arror (Marakwet West). The list of the villages growing sorghum in the three wards was obtained from the Ward Agriculture Office, and thirty villages were selected randomly based on probability proportional to population size (PPS) technique. A second sampling frame was generated from the randomly selected villages by enlisting all sorghum growing households with children aged 6–59 months, with the help of the Ward Agriculture Officer, village elders, and lead farmers. Lastly, using Microsoft Excel, simple random sampling was used to select 353 households (Emsoo, = 118; Endo, = 117 and Arror, =118) from the sampling frame generated. The study respondents were the mothers/caregivers of children 6–59 months of age.
2.3. Data collection tools
2.3.1. Structured Household Questionnaires
Structured household questionnaires were used to obtain information about respondents’ socio‐demographic information, knowledge about aflatoxin and fumonisin contamination in foods, the health effects of ingesting these toxins in food, postharvest handling, and storage practices of sorghum.
2.3.2. Qualitative data collection
Focus Group Discussion (FGD) and Key Informant Interviews (KII) guides were used to collect qualitative data. One FGD was conducted from each ward among mothers/caregivers of children 6–23 months of age to understand how they handled sorghum and if mycotoxin contamination was of concern when handling children's food. Children aged 6–23 months are nutritionally vulnerable and the introduction of complementary foods that are contaminated with mycotoxins could further impair their growth and development (Alamu et al., 2020). Therefore, it was of importance to have a focus group discussion with the mothers/caregivers of children 6–23 months of age. The FGD participants were selected from villages that were excluded from the household survey. For the key informant interviews, 14 participants including chairpersons for aggregation centers, Ward Agricultural Officers (WAOs), lead farmers, and community health volunteers (CHVs) were selected since they are the frontline agents of change at the community level. They act as intermediaries between researchers and farmers by training and motivating farmers to adapt the good agricultural practices as well as providing free health education training on essential healthcare for mothers and children. The thematic areas of discussion during the in‐depth interviews included food safety issues, aflatoxin and fumonisin awareness, and their management strategies.
2.4. Data analyses
All quantitative data were analyzed using Statistical Package for Social Software (SPSS) version 26. Data on caregivers' sociodemographic characteristics, knowledge about mycotoxins, and sorghum postharvest handling and storage practices were analyzed using the chi‐square test. To assess knowledge and practices, every correct answer to the questions was assigned a score of 1 while a score of 0 was given to a wrong answer and where the respondent did not know. Scores on knowledge and practices for each respondent were calculated by summing up the scores attained on each question and the overall score ranked as good or poor. Twelve knowledge questions were asked and thus the scores ranged from 0 to 12. Individuals scoring 7 and below (score below 60%) were categorized as having poor knowledge and scores above 7 as good knowledge (ul Haq et al., 2012; Wang et al., 2015). Regarding the caregivers’ postharvest practice, 18 questions were asked and a total score per individual of less than or equal to 10 (score below 60%) was categorized as poor practices and scores above 10 as good practices (Papagiannis et al., 2020; Wang et al., 2015). Binary logistic regression was used to determine the association between variables of interest. p <.05 was considered statistically significant. The qualitative data were analyzed using the thematic content analysis method by identifying similarities, differences, and trends between the individuals and group responses. Common themes that emerged from each individual and throughout the interviews were identified as well as any differences in responses based on the demographics. The findings from the FDGs and KII were then triangulated onto the household survey data to provide in‐depth understanding of the concepts studied.
3. RESULTS
3.1. Sociodemographic characteristics of the study population
The sociodemographic characteristics of the study participants are shown in Table 1. The household heads for majority of the farmer household were male (93%). More than half (52.1%) of the caregivers were aged 31–45 years and were in monogamous marriage (87.3%). About 45% of farmers had at least primary school education, indicating some level of literacy while more than a third (38%) had a monthly income of less than Ksh. 5,000. All sociodemographic characteristics (sex of the household head (p =.034), age of the respondent (p =.025), education level (p =.001), and monthly income (p =.001)) were significantly different in the three administrative wards except for marital status (p =.232).
TABLE 1.
Socio‐demographic characteristics of caregivers’ of children aged 6–59 months in Kerio Valley, Elgeyo Marakwet County
| Administrative wards | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Socio‐demographic factors |
Total (n = 353) |
Emsoo (n = 118) |
Endo (n = 117) |
Arror (n = 118) |
χ2 value | p‐value* | ||||
| n | % | n | % | n | % | n | % | |||
| Household head sex | 6.760 | 0.034* | ||||||||
| Male | 328 | 92.9 | 104 | 88.1 | 110 | 94.9 | 114 | 96.6 | ||
| Female | 25 | 7.1 | 14 | 11.9 | 7 | 6.0 | 4 | 3.4 | ||
| Marital status | 10.501 | 0.232 | ||||||||
| Married monogamous | 308 | 87.3 | 100 | 84.7 | 101 | 86.3 | 107 | 90.7 | ||
| Married polygamous | 20 | 5.7 | 4 | 3.4 | 9 | 7.7 | 7 | 5.9 | ||
| windowed | 9 | 2.5 | 5 | 4.2 | 2 | 1.7 | 2 | 1.7 | ||
| separated | 12 | 3.4 | 7 | 5.9 | 4 | 3.5 | 1 | 0.8 | ||
| single | 4 | 1.1 | 2 | 1.9 | 1 | 0.9 | 1 | 0.8 | ||
| Age of the respondents | 14.445 | 0.025* | ||||||||
| 18–30 | 110 | 31.2 | 51 | 43.2 | 31 | 26.5 | 28 | 23.7 | ||
| 31–45 | 184 | 52.1 | 51 | 43.2 | 62 | 53.0 | 71 | 60.2 | ||
| 46–60 | 39 | 11.0 | 12 | 10.2 | 16 | 13.7 | 11 | 9.3 | ||
| Above 60 | 20 | 5.7 | 4 | 3.4 | 8 | 6.8 | 8 | 6.8 | ||
| Education level | 30.198 | 0.001* | ||||||||
| None | 47 | 13.3 | 1 | 0.8 | 26 | 22.2 | 20 | 16.9 | ||
| Primary | 159 | 45.0 | 67 | 56.8 | 44 | 37.6 | 48 | 40.7 | ||
| Secondary | 113 | 32.0 | 37 | 31.4 | 40 | 34.2 | 36 | 30.5 | ||
| Tertiary | 34 | 9.6 | 13 | 11.0 | 7 | 6.0 | 14 | 11.9 | ||
| Monthly income | 91.990 | 0.001* | ||||||||
| Less than 5,000 | 134 | 38.0 | 67 | 56.8 | 4 | 3.4 | 63 | 53.4 | ||
| 5,001–10, 000 | 107 | 30.3 | 31 | 26.3 | 52 | 44.4 | 24 | 20.3 | ||
| Above 10, 000 | 112 | 31.7 | 20 | 16.9 | 61 | 52.1 | 31 | 26.3 | ||
p < 0 0.05 significant by chi‐square test
3.2. Knowledge about mycotoxin contamination in foods
The average score for knowledge about mycotoxins among the study respondents was 6.48 ± 2.94, out of a possible 12 score, indicating low level of knowledge among caregivers (Figure 1). Of the 353 respondents, 218 (61.8%) were within the poor knowledge score, with more caregivers from Emsoo (71.2%) having poor knowledge scores compared to those from Arror (57.6%), and Endo (56.4%) (Table 2). Although more than half (60.9%) of the respondents had heard about aflatoxins, only 25.2% and 19.8% were familiar with the terms fumonisin and mycotoxins, respectively. Furthermore, out of the caregivers who heard about aflatoxin and fumonisin, 49(13.9%) and 27(33.8%) knew that sorghum grain can be contaminated by aflatoxin and fumonisin respectively. Majority (67.4%) of them knew that consumption of mycotoxin‐contaminated food could lead to adverse health effects such as abdominal pain (55.5%) and diarrhea (22.0%), while 40.8% and 45.9% thought it could also lead to childhood stunting and immune suppression, respectively. The caregivers from Arror (81.4%) were more knowledgeable (χ2 =28.939, p =.001) of the health effects of mycotoxins consumption in foods was as compared to Endo (63.2%) and Emsoo (57.6%) ward.
FIGURE 1.
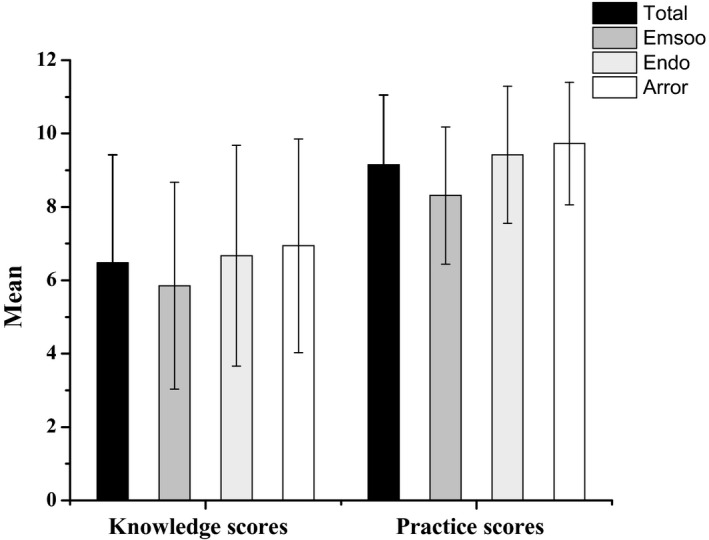
Mean scores of young children caregivers’ knowledge about aflatoxin and fumonisin and postharvest handling and storage practices of sorghum in Kerio valley, Elgeyo Marakwet County. Data are mean ±standard deviations, p <.05 significant by analysis of variance (ANOVA)
TABLE 2.
Knowledge of caregivers’ of children under five years old about mycotoxins in Kerio Valley, Elgeyo Marakwet County
| Administrative wards | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Questions regarding mycotoxins knowledge | Total (n = 353) | Emsoo (n = 118) | Endo (n = 117) | Arror (n = 118) | χ2 value | p‐value | ||||
| n | % | n | % | n | % | n | % | |||
| 1. Heard of mycotoxins? Yes | 70 | 19.8 | 23 | 19.5 | 30 | 25.6 | 17 | 14.4 | 4.677 | 0.096 |
| 2. Heard of aflatoxin? Yes | 215 | 60.9 | 66 | 55.9 | 80 | 68.4 | 69 | 58.5 | 4.261 | 0.119 |
| 3. Heard of fumonisin? Yes | 89 | 25.2 | 24 | 20.3 | 36 | 30.8 | 29 | 24.6 | 3.428 | 0.180 |
| 4. Can mention foods that can be contaminated by aflatoxin? Yes | 195 | 90.7 | 60 | 90.9 | 72 | 90.0 | 63 | 1.3 | 0.080 | 0.961 |
| 5. Can mention foods that can be contaminated by fumonisin? Yes | 57 | 64.0 | 17 | 70.8 | 20 | 69.0 | 20 | 55.6 | 1.912 | 0.384 |
| 6. Able to identify spoilt sorghum grains? Yes | 327 | 92.6 | 108 | 91.5 | 111 | 94.9 | 108 | 91.5 | 1.284 | 0.526 |
| 7. Do you know the causes of mold growth in foods? Yes | 321 | 90.9 | 109 | 92.4 | 103 | 88.0 | 109 | 92.4 | 1.786 | 0.409 |
| 8. Does consumption of mycotoxins contaminated food has any negative health effects? Yes | 238 | 67.4 | 68 | 57.6 | 74 | 63.2 | 96 | 81.4 | 28.939 | 0.001* |
| 9. Do aflatoxins and fumonisins in complementary foods cause: | ||||||||||
| 1. Stunting? Yes | 144 | 40.8 | 38 | 32.2 | 43 | 36.8 | 63 | 53.4 | 15.207 | 0.004* |
| 2. Impaired immunity? Yes | 162 | 45.9 | 40 | 33.9 | 51 | 43.6 | 71 | 60.7 | 22.974 | 0.001* |
| 3. Abdominal pain? Yes | 230 | 65.2 | 68 | 57.6 | 74 | 63.2 | 88 | 74.6 | 11.756 | 0.019* |
| 4. Diarrhea? Yes | 239 | 67.7 | 70 | 59.3 | 84 | 71.8 | 85 | 72.0 | 7.657 | 0.105 |
| Source of information about mycotoxins | 16.308 | 0.178 | ||||||||
| Mass media (e.g., radio) | 86 | 39.4 | 24 | 36.9 | 34 | 42.0 | 28 | 38.9 | ||
| Agricultural extension officers | 41 | 18.8 | 11 | 16.9 | 17 | 21.0 | 13 | 18.1 | ||
| Friends/neighbors | 41 | 18.8 | 10 | 15.4 | 17 | 21.0 | 14 | 19.4 | ||
| Reading | 35 | 16.1 | 18 | 27.7 | 8 | 9.9 | 9 | 12.5 | ||
| Seminars/experts | 12 | 5.5 | 2 | 3.1 | 5 | 6.2 | 5 | 6.9 | ||
| Women group | 1 | 0.5 | ‐ | ‐ | ‐ | ‐ | 1 | 1.4 | ||
| AVCD‐DT Project | 2 | 0.9 | ‐ | ‐ | ‐ | ‐ | 2 | 2.8 | ||
| Knowledge score | ||||||||||
| Poor | 218 | 61.8 | 84 | 71.2 | 66 | 56.4 | 68 | 57.6 | 6.711 | 0.035* |
| Good | 135 | 38.2 | 34 | 28.8 | 51 | 43.6 | 50 | 42.4 | ||
p <.05 significant by chi‐squaretest.
3.3. Postharvest practices favoring mycotoxin contamination in foods
Overall, score of the average practices among caregivers was 9.15 ± 1.90, out of 18 total scores (Figure 1). The postharvest practices for majority (75.4%) of the caregivers were poor and diverse (χ2 =12.237, p =.002) across the three wards. Caregivers from Emsoo ward recorded the highest number of respondents (86.4%) with poor postharvest practices followed by Endo (71.8%) and Arror (70.8%), respectively (Table 3). Overall, less than half of the respondents (35.1% and 31.2%) knew that inadequate drying and poor storage of grains contribute to mold growth resulting in food spoilage (Figure 2). The caregivers from Emsoo (37.8%) and Arror (36.3%) considered poor drying as the main cause of spoilage of sorghum while those from Endo ward (34.1%) pointed out storage as the critical point of contamination. Majority of caregivers dried sorghum after harvest; however, 12.2% left their crops to dry while standing in the field. Among those who dried their grains after harvest (87.8%), duration of drying (χ2 =20.807, p =.001) and materials used for drying (χ2 =15.157, p =.004) were significantly different across the wards. Drying sorghum for more than a week was popular in Emsoo while drying grains on bare ground was mainly practiced in Arror ward. To confirm grains dryness, none of the caregivers used a moisture meter but instead, they used traditional methods such as biting (51.0%), looking (24.6%), and touching/squeezing (24.1%).
TABLE 3.
Practices of the caregivers toward mycotoxins management in Kerio Valley, Elgeyo Marakwet County
| Administrative wards | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Households postharvest practices | Total (n = 353) |
Emsoo (n = 118) |
Endo (n = 117) |
Arror (n = 118) |
χ2 value | P‐value | ||||
| n | % | n | % | n | % | n | % | |||
| 1. Dried sorghum after harvest? Yes | 310 | 87.8 | 100 | 84.7 | 102 | 87.2 | 108 | 91.5 | 2.602 | 0.272 |
| 2. Drying duration | ||||||||||
| <7 days | 36 | 11.6 | 4 | 4.0 | 8 | 7.8 | 24 | 22.2 | 20.807 | 0.001* |
| 7 days | 243 | 78.4 | 82 | 82.0 | 86 | 84.3 | 75 | 69.4 | ||
| >7days | 31 | 10.0 | 14 | 14.0 | 8 | 7.8 | 9 | 8.3 | ||
| 3. Drying on; | ||||||||||
| Bare grounds | 199 | 64.0 | 67 | 67.0 | 57 | 55.9 | 75 | 68.8 | 15.157 | 0.004* |
| Canvas | 103 | 33.1 | 33 | 33.0 | 37 | 36.3 | 33 | 30.3 | ||
| Others (Concrete asphalt, rock surface) | 9 | 2.9 | 0 | ‐ | 8 | 7.8 | 1 | 0.9 | ||
| 4. Methods of checking grains dryness | 3.053 | 0.802 | ||||||||
| Traditional methods | ||||||||||
| Looking at it | 87 | 24.6 | 27 | 22.9 | 27 | 23.1 | 33 | 28.0 | ||
| Squeezing/touching | 85 | 24.1 | 29 | 24.6 | 29 | 24.8 | 27 | 22.9 | ||
| Biting | 180 | 51.0 | 62 | 52.5 | 60 | 51.3 | 58 | 49.2 | ||
| Listening to the sound when winnowing | 1 | 0.3 | 0 | 0.0 | 1 | 0.9 | 0 | 0.0 | ||
| Modern method | ||||||||||
| Moisture meter | 0 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| 5. Threshing method used | ||||||||||
| Hand threshing | 340 | 96.3 | 116 | 98.3 | 114 | 97.4 | 110 | 93.0 | 4.918 | 0.086 |
| Use machine/thresher | 13 | 3.7 | 2 | 1.7 | 3 | 2.6 | 8 | 6.8 | ||
| 6. Removed spoilt panicles before threshing? Yes | 353 | 100 | 118 | 100 | 117 | 100 | 118 | 100 | ‐ | ‐ |
| 7. Suspect fungal contamination in grains based on; | ||||||||||
| Grains discoloration | 265 | 81.8 | 85 | 79.4 | 80 | 73.4 | 100 | 92.6 | ‐ | ‐ |
| Off‐smell | 152 | 46.9 | 56 | 52.3 | 38 | 34.9 | 58 | 53.7 | ||
| Bitter taste | 3 | 0.9 | 3 | 2.8 | 1 | 0.9 | 0 | 0 | ||
| I do not know | 4 | 1.2 | 2 | 1.9 | 1 | 0.9 | 0 | 0 | ||
| 8. Use of spoilt grains | ||||||||||
| Thrown away | 114 | 32.3 | 32 | 27.1 | 47 | 40.2 | 35 | 29.7 | 18.357 | 0.019* |
| Feed livestock | 183 | 51.8 | 61 | 51.7 | 55 | 47.0 | 67 | 56.8 | ||
| Making local brew | 48 | 13.6 | 19 | 16.1 | 14 | 12.0 | 15 | 12.7 | ||
| Re‐dry and consume | 6 | 1.7 | 6 | 5.1 | 0 | 0.0 | 0 | 0.0 | ||
| Sell | 2 | 0.6 | 0 | 0.0 | 1 | 0.9 | 1 | 0.9 | ||
| 9. Other practices before storage of sorghum grains? Yes | ||||||||||
| Grains sorting | 111 | 41.0 | 33 | 33.3 | 37 | 40.2 | 42 | 50.6 | ‐ | ‐ |
| Winnowing after threshing | 227 | 83.8 | 74 | 77.1 | 78 | 84.8 | 75 | 90.4 | ‐ | ‐ |
| Cleaning of storage area | 59 | 21.8 | 16 | 16.7 | 20 | 21.7 | 23 | 27.7 | ‐ | ‐ |
| Fumigation of storage area | 19 | 7.0 | 6 | 6.3 | 6 | 6.5 | 7 | 8.4 | ‐ | ‐ |
| 10. Type of store used | ||||||||||
| Traditional store | 285 | 52.4 | 53 | 44.9 | 61 | 52.1 | 71 | 60.2 | 11.267 | 0.080 |
| Modern store | 18 | 5.1 | 6 | 5.1 | 3 | 2.6 | 9 | 7.6 | ||
| Living room with improved structure | 37 | 10.5 | 17 | 14.4 | 12 | 10.3 | 8 | 6.8 | ||
| Living room without improved structure | 131 | 32.0 | 42 | 35.6 | 41 | 35.0 | 30 | 25.4 | ||
| 11. Type of storage bags used | ||||||||||
| Gunny bags | 94 | 26.6 | 47 | 39.8 | 28 | 23.9 | 19 | 16.1 | 40.828 | 0.001* |
| Hermetic bags | 227 | 64.3 | 51 | 43.2 | 80 | 68.4 | 96 | 81.41 | ||
| Sisal bags | 23 | 6.5 | 14 | 11.9 | 7 | 6.0 | 2 | 0.7 | ||
| Placed bundle of grains on the bare floor (No use of bags) | 9 | 2.5 | 6 | 5.1 | 2 | 1.7 | 1 | 0.8 | ||
| 12. Stacking of storage bags | ||||||||||
| On wood pallets | 207 | 58.6 | 59 | 50.0 | 64 | 54.7 | 84 | 71.2 | 16.396 | 0.012* |
| On the floor | 131 | 37.1 | 52 | 44.1 | 48 | 41.0 | 31 | 26.3 | ||
| On the stones | 6 | 1.7 | 1 | 0.8 | 3 | 2.6 | 2 | 1.7 | ||
| 13. Duration of grains storage | ||||||||||
| 0–6 months | 235 | 66.6 | 71 | 65.3 | 84 | 71.8 | 74 | 62.7 | 3.316 | 0.314 |
| More than 6 months | 118 | 33.4 | 41 | 34.7 | 32 | 28.2 | 44 | 37.3 | ||
| 14. Sorghum flour storage material | ||||||||||
| Plastic bucket | 265 | 75.1 | 66 | 55.9 | 99 | 84.6 | 100 | 84.7 | 36.830 | 0.001* |
| Gunny bag | 63 | 17.8 | 39 | 33.1 | 10 | 11.9 | 14 | 11.9 | ||
| Others (Khaki bag, animal skin bag) | 25 | 7.1 | 13 | 11.0 | 8 | 6.8 | 4 | 3.4 | ||
| 15. Duration of flour storage | ||||||||||
| Less than 7 days | 319 | 90.4 | 100 | 84.7 | 104 | 88.9 | 115 | 97.5 | 11.393 | 0.003* |
| More than 7 days | 34 | 9.6 | 18 | 15.3 | 13 | 11.6 | 3 | 2.5 | ||
| 16. Ferment sorghum flour (Yes) | 140 | 39.7 | 52 | 44.1 | 41 | 35.0 | 47 | 39.8 | 2.002 | 0.368 |
| 17. Attended training on IYC (Yes) | 36 | 10.2 | 12 | 10.2 | 7 | 6.0 | 17 | 14.4 | 4.552 | 0.103 |
| 18. Mycotoxins contamination was part of the training (Yes) | 9 | 25.7 | ‐ | ‐ | 6 | 85.7 | 3 | 18.8 | 17.752 | 0.001* |
| Practices score | ||||||||||
| Poor | 266 | 75.4 | 102 | 86.4 | 84 | 71.8 | 80 | 70.8 | 12.237 | 0.002* |
| Good | 87 | 24.6 | 16 | 13.6 | 33 | 28.2 | 38 | 32.2 | ||
p <.05 significant by chi‐square test.
FIGURE 2.
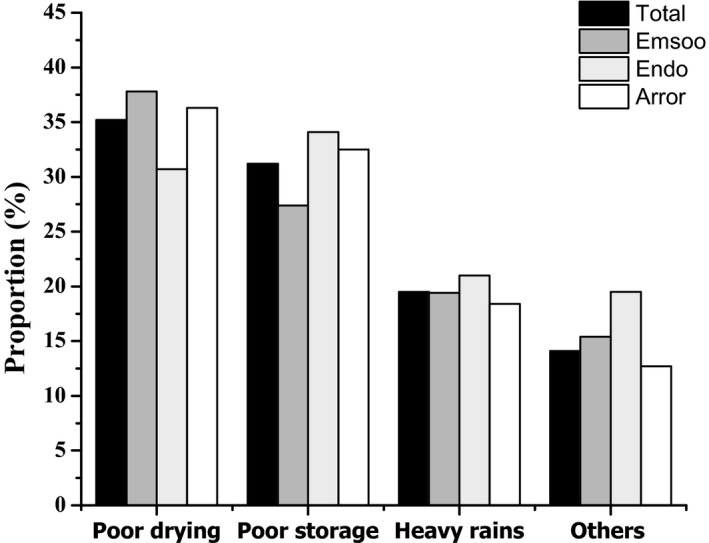
Responses on causes of mold spoilage in sorghum among caregivers’ of young children in Kerio Valley, Elgeyo Marakwet County
Nearly, all the caregivers (96.3%) used manual threshing methods to shell sorghum, and before shelling, they separated spoilt sorghum panicles from the clean ones. Although more than a third (32.3%) of the caregivers threw away the spoilt grains, majority (51.6%) used them as animal feed, prepared local brews (13.6%) while some from Emsoo ward (5.1%) reported using such grains for human consumption. More than half (52.4%) of the caregivers stored their grains in traditional granaries and before grains storage, less than half sorted (41%) their grains, cleaned (21.8%), or fumigated storage area (7%). Majority of the caregivers from Arror (81.4%) used hermetic bags to store sorghum grains compared with those from Emsoo and Endo (χ2 =40.828, p =.001). Additionally, a large proportion of the caregivers from Arror (84.7%) and Endo (84.6%) wards used plastic buckets to store sorghum flour for preparing children's porridge with majority of them storing the flour for less than a week (χ2 =11.393, p =.003). Among the small proportion of caregivers (10.2%) who attended training on Infant and Young Child Feeding (IYCF), only nine of them were trained on mycotoxin contamination of complementary foods. More of the caregivers from Endo ward (χ2 =17.752, p =.001) were trained about mycotoxins during IYCF trainings while none of the IYCF training content included mycotoxin contamination of foods in Emsoo ward.
3.4. Factors associated with caregivers’ knowledge about mycotoxin contamination in sorghum and their postharvest practices
The caregivers’ knowledge scores about mycotoxins contamination in sorghum were significantly associated with age [(AOR=4.629, (95% Cl: 2.530–8.472), p <.001], education level [(AOR=0.275, (95% Cl: 0.088–0.434), p =.001], marital status [(AOR=15.187, (95% Cl: 1.830–126.007), p =.12], and household monthly income [(AOR=2.623, (95% Cl: 1.550–4.439), p <.001] (Table 4). The postharvest handling and practices of sorghum (Table 5) were also significantly associated with caregivers’ age [(AOR=3.845, (95% Cl: 1.558–9.490), p =.003], education level [(AOR=0.196, (95% Cl: 0.088–0.434), p <.001], monthly income [(AOR=3.291, (95% Cl: 1.550–6.986), p =.002], and knowledge about mycotoxins [(AOR=5.428, (95% Cl: 2.855–10.319), p <.001]. Marital status [(AOR=3.579, (95% Cl: 0.403–31.775, p =.252)] was not associated with caregivers’ postharvest handling practices.
TABLE 4.
Factors associated with caregivers’ knowledge about mycotoxin contamination of sorghum in Kerio Valley, Elgeyo Marakwet County
| Demographic variables | Knowledge scores (≤7 and >7) | P‐value | |
|---|---|---|---|
| AOR | 95% Cl | ||
| Age‐group (years) | |||
| 18–30 (reference) | 1 | ||
| >30 | 4.629 | 2.530–8.472 | <0.001* |
| Level of education | |||
| No formal education (reference) | 1 | 0.001* | |
| Formal education | 0.275 | 0.129–0.588 | |
| Marital status | |||
| Not married (reference) | 1 | 0.012* | |
| Married | 15.187 | 1.830–126.007 | |
| Monthly average income (in Ksh) | |||
| Less than 5, 000 (reference) | 1 | <0.001* | |
| More than 5, 000 | 2.623 | 1.550–4.439 | |
Abbreviation: AOR, adjusted odds ratio.
p <.05 significant using binary logistic regression.
TABLE 5.
Factors associated with postharvesting handling and storage practices of sorghum among caregivers of children aged 6–59 months in Kerio Valley, Elgeyo Marakwet County
| Factors | Practices scores (≤10 and >10) | P‐value | |
|---|---|---|---|
| AOR | 95% Cl | ||
| Age‐group (years) | |||
| 18–30 (reference) | 1 | 0.003* | |
| >30 | 3.845 | 1.558–9.490 | |
| Level of education | |||
| No formal education (reference) | 1 | <0.001* | |
| Formal education | 0.196 | 0.088–0.435 | |
| Marital status | |||
| Not married(Reference ) | 1 | 0.252 | |
| Married | 3.579 | 0.403–31.775 | |
| Monthly average income (Ksh) | |||
| Less than 5, 000 (Reference) | 1 | 0.002* | |
| More than 5, 000 | 3.291 | 1.550–6.986 | |
| Mycotoxins knowledge | |||
| Poor (Reference) | 1 | <0.001* | |
| Good | 5.428 | 2.855–10.319 | |
Abbreviation: AOR, adjusted odds ratio.
p <.05 significant using binary logistic regression.
4. DISCUSSION
The findings of the current study demonstrated that majority of children's caregivers had poor knowledge about aflatoxin and fumonisin contamination of sorghum‐based foods. Poor knowledge may have contributed to poor postharvest handling and storage practices. During focus group discussions (FGDs), caregivers revealed that mycotoxin contamination was not their concern when preparing sorghum‐based complementary foods. Majority of them thought that aflatoxin and fumonisin solely affect maize, another major staple crop in Kenya. These findings corroborate several other studies that have reported that large rural populations in Kenya are only knowledgeable about aflatoxins contamination of maize‐based foods (Njeru et al., 2019; Obonyo & Salano, 2018; Walker & Davies, 2013). Aflatoxin in maize may have received considerable attention in Kenya because of severe aflatoxin contamination of maize that has caused human deaths repeatedly over the years (Probst et al., 2011). Comparable with the findings reported by Njeru et al., (2019), majority of caregivers in this study were not familiar with fumonisins. This could be explained by the current national efforts that are solely focused on aflatoxins, a situation that is similar in most African countries (Matumba et al., 2014).
Sorting and removing visibly defective panicles and grains is an important critical control point for reducing mycotoxin contamination in farm produce. However, visible grain mold may indicate mycotoxins presence, but still very high levels are possible without any noticeable effect on appearance or smell (Ayo et al., 2018). In the present study, caregivers suspected the presence of fungal toxins in sorghum if the grains were discolored, had an off‐smell or bitter taste, and they used these features during panicles or grains sorting. The spoilt sorghum however still ends up in the food chain for majority of caregivers because they mainly used it as animal feed and/or for human consumption. This practice was notable in Emsoo ward, and one of the caregivers said "Last year, all of my neighbour's produce got spoilt but she refused to dispose off; they consumed all of it." Lack of knowledge on the health effects associated with mycotoxins (Mboya & Kolanisi, 2014) and food shortage are among the contributing factors for the use of spoilt grains for human consumption and as animals feed (Anitha et al., 2019; Matumba et al., 2016). The study participants showed a general lack of awareness on the health effects associated with mycotoxins on humans. Notably, like their counterparts from Tanzania (Ngoma et al., 2017), a large number of children's caregivers in the present study associated mycotoxins consumption mainly with acute health effects (abdominal pain and diarrhea). For example, a key informant said, “These women just know about diarrhoea as the only side effect of consuming contaminated foods. Taking Flagyl® stops it”—this means that farmers mainly associate mycotoxins with diarrhea and they thought the use of Flagyl® (an antibiotic‐nitroimidazole) automatically resolves this effect. On the other hand, majority of them could not link chronic health effects such as stunting with aflatoxins or any other toxins possibly because they are long‐term effects.
Poor harvesting and postharvest handling practices are the major factors that promote mycotoxin contamination (Magembe et al., 2016). In the current study, the caregivers had inadequate knowledge on the contribution of these practices on mycotoxin production in grains. For example, majority of them both in FGDs and household survey pointed out inadequate drying and poor storage as the main factors for the development of molds or fungi although other critical factors like length of storage contributed significantly (Gnonlonfin et al., 2013). While a few of the farmers allowed their produce to dry while standing in the field, majority of them sun‐dried sorghum on the bare ground due to the cost of drying materials. These inappropriate practices increase the risk for mycotoxin contamination of the grains. To confirm grain dryness before storage, all farmers used traditional methods such as biting, touch/squeezing, and a combination of visualization and sound tests. Such traditional practices are inadequate and might lead to the storage of grains while still having high residual moisture content (Hell et al., 2008). In the current study, nearly all caregivers threshed/shelled sorghum using manual methods (beating sorghum panicles in a sack or using a traditional pestle (stone) and mortar (hollow tree trunk), with some of them threshing sorghum on bare ground. Manual threshing exposes grains to fungi through kernel breakage and eventually, mycotoxin production (Taye et al., 2018).
Storing properly dried grains in a clean and dry environment as well as controlling storage pests by use of insecticides and hermetic bags can reduce the build‐up of mycotoxins in the store (Abass et al., 2018). In the present study, more than half of the caregivers from Endo and Arror ward used hermetic bags to store sorghum grains attributable to the intervention by different community development projects. Some caregivers, however, highlighted placing sorghum bundles directly on the floor. Worse still, a good number of them placed the storage bags directly on the floor, which may increase fungal growth in stored food commodities due to moisture absorption (Suleiman & Rosentrater, 2015). Storage structures also play an important role in determining the quality of the stored food. Traditional storage structures, such as traditional granaries and living rooms increase the deterioration of grains by mycotoxins due to rodent attacks, insect damage, high temperatures, and humidity (Maina et al., 2016). In this study, over two‐thirds of caregivers stored sorghum grains in traditional granaries and in dwelling houses. Additionally, the material for keeping flour is of critical importance to maintain the quality of the flour (Hemery et al., 2020). Furthermore, the flour for preparing children's porridge was mainly stored in plastic buckets, which are known to retain heat and moisture and easily promote fungal growth and mycotoxin contamination (Mutegi et al., 2013). Sacco et al., (2020) and Selemani (2018) suggested that paper bags could be a suitable packaging material for flours as they found lower fungal count in flour stored in paper bags compared with flour stored in plastic buckets and low‐density polyethylene bags.
Poor mycotoxins knowledge could lead to poor postharvest practices which in turn contributes to mycotoxin production in susceptible food commodities (Makun., 2013). Poor mycotoxins knowledge observed in this study could be attributed to the low number of mycotoxins awareness campaigns in Kerio valley since very few of the study participants have been trained on issues concerning mycotoxins contamination of complementary foods. In addition, the low number of trainings on infant and young children complementary feeding (IYCF), where more than three‐quarters of caregivers did not attend, possibly contributed to the low mycotoxin knowledge, and eventually poor postharvest handling of ingredients for preparing children's food. In general, caregivers in this study received fewer trainings as compared to their counterparts from Tanzania (Katengesya, 2018). Inadequate awareness on issues related to mycotoxins poses a risk of producing and feeding young children with contaminated complementary foods (Katengesya, 2018). For instance, mixing sorghum flour for preparing child's food with more than two other flours such as maize, millet, groundnuts, or cassava was a common practice in the study area. Apart from this practice interfering with the absorption of important nutrients, it could also increase children's exposure to several mycotoxins (Ojuri et al., 2018). Furthermore, it was revealed during FGDs that traditional tools that were used for grains threshing and grinding were not cleaned between each batch of grains. One participant explained that the reason for not washing some of these tools, for example, the hollow tree trunk (wero konee) for threshing, was that it would crack. This indicates that caregivers of young children do not have adequate knowledge on the risks of cross‐contamination of children's food.
A remarkable finding from the study is that the older caregivers (≥30 years) were four times more likely to be aware of aflatoxin and/or fumonisin and had good postharvest practices compared to the younger ones. It is possible that the older caregivers have traditional knowledge or may have learned about these toxins through local mass media during aflatoxin outbreaks in Eastern Kenya. For instance, one of the key informants mentioned having heard about aflatoxin from “the Machakos‐outbreak” a part of the Eastern Kenya hotspot (Obonyo & Salano, 2018). The experience caregivers gained over time could also improve their knowledge on mycotoxins and their management practices (Udomkun et al., 2018). Interestingly, the caregivers who attended a formal system of education were less likely to know about mycotoxins or to have good postharvest handling practices. This may imply that knowledge on mycotoxins and management practices are more of transferrable skills (Gichohi‐Wainaina et al., 2020) than what is learnt in school. In the study, very few caregivers obtained information about mycotoxins through reading despite some level of literacy reported which probably indicates the scarcity of written resources, low reading motivation on the side of farmers, or else the materials are too technical for them to understand (Logrieco et al., 2018). Hence, the findings suggest the need to possibly include topics on mycotoxin and food safety in the available infant and young child feeding guidelines as well as in the primary and secondary school curricula. However, finding on the education level contradict previous studies that reported that the literate population had more knowledge on aflatoxin and other mycotoxins than those who have not attended school (Magembe et al., 2016; Matumba et al., 2016; Udomkun et al., 2018). Therefore, more studies are needed to better inform behavior change communication strategies targeted at mitigating mycotoxin contamination of complementary foods.
Another important determinant of knowledge about mycotoxin contamination of foods was the marital status of the caregiver. Similar to findings reported by Magembe et al., (2016), the current study clearly showed that caregivers who are married and living together with their spouse were more likely to be aware of aflatoxin and/or fumonisin than those who are not (single, separated, or widowed). Living together with a spouse might help in sharing information on mycotoxin issues and their management thus contribute to better mycotoxin knowledge. Income is also one of the most salient factors that influence farmers’ perception and awareness of mycotoxins (Redzwan et al., 2012). In this study, a positive relationship was observed between household monthly income and knowledge about mycotoxins and postharvest handling practices of sorghum. The discussions with the caregivers and interviews with key informants suggested the reluctance by sorghum farmers to invest their limited income to learn about or pay for aflatoxin or fumonisin control. This implies that caregivers with less monthly income are less likely to purchase nonfood items such as drying materials, hermetic bags, or radio that will enhance good postharvest practices and mycotoxins’ awareness. Additionally, people with high incomes are likely to be more careful about food and are more willing to pay for food safety than those with lower incomes (Redzwan et al., 2012). This suggests that poverty and lack of sufficient income might contribute to low levels of awareness and knowledge about mycotoxin, leading to high mycotoxin exposure (Leroy et al., 2015) through poor food handling and preparation.
5. CONCLUSION
The study demonstrated that caregivers of young children implement suboptimal postharvest handling and storage practices due to poor knowledge about mycotoxin contamination of sorghum‐based foods and their effects on health. Knowledge and postharvest handling and storage practices were associated with the caregiver's age, educational level, and household monthly income. Therefore, to mitigate the occurrence of mycotoxins in complementary foods and across the food value chain; there is a need to increase awareness about aflatoxins and fumonisins among smallholder farmers and young mothers/caregivers.
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest.
ETHICAL APPROVAL
This study protocols and procedures were reviewed and approved by the Egerton University Research Ethics Committee (ethical code, EUREC/APP/130/2021). Permission to conduct the study was obtained from Kenya National Commission for Science, Technology, and Innovation, relevant authorities at County, Sub‐County, location, and sub‐location level. All study protocols were conducted in accordance with the Declaration of Helsinki recommendations of 1975, revised 2000. Informed consent was obtained from all participants before the interviews. The Kenya Government guidelines and measures on the prevention of COVID‐19 were followed during interviews.
ACKNOWLEDGMENTS
The National Research Fund (NRF), Kenya, supported this work. We acknowledge postgraduate students from the Department of Human Nutrition (Sharon Kemboi and Grace Khatenya) for their support during data cleaning and analysis, Elgeyo Marakwet County government, Ward Agriculture Officers, Enumerators, Administrators, and the sorghum farmers who participated in the study.
Lesuuda, L. , Obonyo, M. A. , & Cheserek, M. J. (2021). Determinants of knowledge about aflatoxin and fumonisin contamination in sorghum and postharvest practices among caregivers of children aged 6–59 months in Kerio Valley, Kenya. Food Science & Nutrition, 9, 5435–5447. 10.1002/fsn3.2502
DATA AVAILABILITY STATEMENT
Due to privacy/ethical restrictions, the data that support the findings of this study are available on request from corresponding author.
REFERENCES
- Abass, A. B. , Fischler, M. , Schneider, K. , Daudi, S. , Gaspar, A. , Rüst, J. , Kabula, E. , Ndunguru, G. , Madulu, D. , & Msola, D. (2018). On‐farm comparison of different post‐harvest storage technologies in a maize farming system of Tanzania Central Corridor. Journal of Stored Products Research, 77, 55–65. 10.1016/j.jspr.2018.03.002 [DOI] [Google Scholar]
- Alamu, E. O. , Gondwe, T. , Akello, J. , Maziya‐Dixon, B. , & Mukanga, M. (2020). Relationship between serum aflatoxin concentrations and the nutritional status of children aged 6–24 months from Zambia. International Journal of Food Sciences and Nutrition, 71(5), 593–603. [DOI] [PubMed] [Google Scholar]
- Anitha, S. , Tsusaka, T. , Njoroge, S. , Kumwenda, N. , Kachulu, L. , Maruwo, J. , Machinjiri, N. , Botha, R. , Msere, H. , Masumba, J. , Tavares, A. , Heinrich, G. , Siambi, M. , & Okori, P. (2019). Knowledge, attitude and practice of Malawian farmers on pre‐and post‐harvest crop management to mitigate aflatoxin contamination in groundnut, maize and sorghum implication for behavioral change. Toxins, 11(12), 716. 10.3390/toxins11120716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayo, E. M. , Matemu, A. , Laswai, G. H. , & Kimanya, M. E. (2018). Socioeconomic characteristics influencing level of awareness of aflatoxin contamination of feeds among livestock farmers in Meru District of Tanzania. Scientifica. 10.1155/2018/3485967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgeyo Marakwet County (2018). Elgeyo Marakwet County Integrated Development Plan. Available at: http://www.elgeyomarakwet.go.ke/Publications/EMC_CIDP.pdf. [Google Scholar]
- Gichohi‐Wainaina, W. N. , Kumwenda, N. , Zulu, R. , Munthali, J. , & Okori, P. (2020). Aflatoxin contamination: Knowledge disparities among agriculture extension officers, frontline health workers and small holder farming households in Malawi. Food Control, 12, 107672. 10.1016/j.foodcont.2020.107672 [DOI] [Google Scholar]
- Gnonlonfin, G. J. B. , Hell, K. , Adjovi, Y. , Fandohan, P. , Koudande, D. O. , Mensah, G. A. , Sanni, A. , & Brimer, L. (2013). A review on aflatoxin contamination and its implications in the developing world: A sub‐Saharan African perspective. Critical Reviews in Food Science and Nutrition, 53(4), 349–365. 10.1080/10408398.2010.535718 [DOI] [PubMed] [Google Scholar]
- Government of Kenya (GoK) (2018). National crop variety list. Kenya Plant Health Inspectorate Service (KEPHIS). http://www.kephis.org [Google Scholar]
- Hell, K. , Fandohan, P. , Bandyopadhyay, R. , Kiewnick, S. , Sikora, R. , & Cotty, P. J. (2008). Pre‐ and post‐harvest management of aflatoxin in maize: an African perspective. In Leslie J. F., Bandyopadhyay R., & Viscont A. (Eds.), Mycotoxins: detection methods, management, public health and agricultural trade (pp. 219–229). CAB International. [Google Scholar]
- Hemery, Y. M. , Fontan, L. , Laillou, A. , Jallier, V. , Moench‐Pfanner, R. , Avallone, S. , & Berger, J. (2020). Influence of storage conditions and packaging of fortified wheat flour on microbial load and stability of folate and vitamin B12. Food Chemistry, 5, 100076. 10.1016/j.fochx.2019.100076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katengesya, T. P. (2018). Aflatoxin and fumonisin contamination in homemade and commercial cereal based complementary foods with formula in Morogoro Municipality. Sokoine University of Agriculture. (Doctoral dissertation). [Google Scholar]
- Kimanya, M. E. , Shirima, C. P. , Magoha, H. , Shewiyo, D. H. , De Meulenaer, B. , Kolsteren, P. , & Gong, Y. Y. (2014). Co‐exposures of aflatoxins with deoxynivalenol and fumonisins from maize based complementary foods in Rombo, Northern Tanzania. Food Control, 41, 76–81. 10.1016/j.foodcont.2013.12.034 [DOI] [Google Scholar]
- Kiome, R. M. , Rao, E. J. O. , & Wamwere‐Njoroge, G. J. (2016). Kenya Accelerated Value Chain Development (AVCD) program scorecard 2015–18. ILRI. https://hdl.handle.net/10568/101622 [Google Scholar]
- Kipyego, R. , & Mugalavai, V. K. (2019). The nexus of drought tolerant crops and food and nutrition security among smallholder farmers in Kerio Valley, Elgeyo Marakwet County, Kenya. International Journal of Scientific and Research Publications, 9(9). ISSN: 2250–3153. 10.29322/IJSRP.9.09.2019.p9354 [DOI] [Google Scholar]
- Knipstein, B. , Huang, J. , Barr, E. , Sossenheimer, P. , Dietzen, D. , Egner, P. A. , Groopman, J. D. , & Rudnick, D. A. (2015). Dietary aflatoxin‐induced stunting in a novel rat model: Evidence for toxin‐induced liver injury and hepatic growth hormone resistance. Pediatric Research, 78(2), 120–127. 10.1038/pr.2015.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy, J. , Wang, J. S. , & Jones, K. (2015). Serum aflatoxin B1‐lysine adduct level in adult women from Eastern Province in Kenya depends on household socio‐economic status: A cross sectional study. Social Science & Medicine, 146, 104–110. [DOI] [PubMed] [Google Scholar]
- Logrieco, A. , Miller, J. , Eskola, M. , Krska, R. , Ayalew, A. , Bandyopadhyay, R. , Battilani, P. , Bhatnagar, D. , Chulze, S. , De Saeger, S. , Li, P. , Perrone, G. , Poapolathep, A. , Rahayu, E. , Shephard, G. , Stepman, F. , Zhang, H. , & Leslie, J. (2018). The Mycotox Charter: Increasing awareness of, and concerted action for, minimizing mycotoxin exposure worldwide. Toxins, 10(4), 149. 10.3390/toxins10040149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magembe, K. S. , Mwatawala, M. W. , Mamiro, D. P. , & Chingonikaya, E. E. (2016). Assessment of awareness of mycotoxins infections in stored maize (Zea mays L.) and groundnut (arachis hypogea L.) in Kilosa District, Tanzania. International Journal of Food Contamination, 3(1), 12. 10.1186/s40550-016-0035-5 [DOI] [Google Scholar]
- Magoha, H. , Kimanya, M. , De Meulenaer, B. , Roberfroid, D. , Lachat, C. , & Kolsteren, P. (2016). Risk of dietary exposure to aflatoxins and fumonisins in infants less than 6 months of age in Rombo, Northern Tanzania. Maternal & Child Nutrition, 12(3), 516–527. 10.1111/mcn.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina, A. W. , Wagacha, J. M. , Mwaura, F. B. , Muthomi, J. W. , & Woloshuk, C. P. (2016). Post‐harvest practices of maize farmers in Kaiti district, Kenya and the impact of hermetic storage on populations of Aspergillus spp. and aflatoxin contamination. Journal of Food Research, 5(6), 53. 10.5539/jfr.v5n6p53 [DOI] [Google Scholar]
- Makun, H. , (Eds)., (2013). Mycotoxin and food safety in developing countries. BoD–Books on Demand. [Google Scholar]
- Matumba, L. , Monjerezi, M. , Biswick, T. , Mwatseteza, J. , Makumba, W. , Kamangira, D. , & Mtukuso, A. (2014). A survey of the incidence and level of aflatoxin contamination in a range of locally and imported processed foods on Malawian retail market. Food Control, 39, 87–91. 10.1016/j.foodcont.2013.09.068 [DOI] [Google Scholar]
- Matumba, L. , Monjerezi, M. , Kankwamba, H. , Njoroge, S. M. C. , Ndilowe, P. , Kabuli, H. , Kambewa, D. , & Njapau, H. (2016). Knowledge, attitude, and practices concerning presence of molds in foods among members of the general public in Malawi. Mycotoxin Research, 32(1), 27–36. 10.1007/s12550-015-0237-3 [DOI] [PubMed] [Google Scholar]
- Mboya, R. M. , & Kolanisi, U. (2014). Subsistence farmers’ mycotoxin contamination awareness in the SADC region: Implications on Millennium Development Goal 1, 4 and 6. Journal of Human Ecology, 46(1), 21–31. 10.1080/09709274.2014.11906702 [DOI] [Google Scholar]
- Mutegi, C. K. , Wagacha, J. M. , Christie, M. E. , Kimani, J. , & Karanja, L. (2013). Effect of storage conditions on quality and aflatoxin contamination of peanuts (Arachis hypogaea L.). International Journal of AgriScience, 3(310), 746–758. http://oar.icrisat.org/id/eprint/7288 [Google Scholar]
- Ngoma, S. J. , Kimanya, M. , & Tiisekwa, B. (2017). Perception and attitude of parents towards aflatoxins contamination in complementary foods and its management in Central Tanzania. The Journal of Middle East and North Africa Sciences, 10(4086), 1–16. 10.12816/0034793 [DOI] [Google Scholar]
- Njeru, N. K. , Midega, C. A. O. , Muthomi, J. W. , Wagacha, J. M. , & Khan, Z. R. (2019). Influence of socio‐economic and agronomic factors on aflatoxin and fumonisin contamination of maize in western Kenya. Food Science & Nutrition, 7(7), 2291–2301. 10.1002/fsn3.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obonyo, M. A. , & Salano, E. N. (2018). Perennial and seasonal contamination of maize by aflatoxins in eastern Kenya. International Journal of Food Contamination, 5(1), 1–5. 10.1186/s40550-018-0069-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojuri, O. T. , Ezekiel, C. N. , Sulyok, M. , Ezeokoli, O. T. , Oyedele, O. A. , Ayeni, K. I. , Eskola, M. K. , Šarkanj, B. , Hajšlová, J. , Adeleke, R. A. , Nwangburuka, C. C. , Elliott, C. T. , & Krska, R. (2018). Assessing the mycotoxicological risk from consumption of complementary foods by infants and young children in Nigeria. Food and Chemical Toxicology, 121, 37–50. 10.1016/j.fct.2018.08.025 [DOI] [PubMed] [Google Scholar]
- Orr, A. , Mwema, C. , Gierend, A. & Nedumaran, S. (2016). Sorghum and millets in Eastern and Southern Africa: Facts, trends, and outlook. 10.13140/RG.2.1.5154.5205 [DOI] [PMC free article] [PubMed]
- Papagiannis, D. , Malli, F. , Raptis, D. G. , Papathanasiou, I. V. , Fradelos, E. C. , Daniil, Z. , Rachiotis, G. , & Gourgoulianis, K. I. (2020). Assessment of knowledge, attitudes, and practices towards new coronavirus (SARS‐CoV‐2) of health care professionals in Greece before the outbreak period. International Journal of Environmental Research and Public Health, 17(14), 4925. 10.3390/ijerph17144925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudyal, N. , Anihouvi, V. , Hounhouigan, J. , Matsheka, M. I. , Sekwati‐Monang, B. , Amoa‐Awua, W. , Atter, A. , Ackah, N. B. , Mbugua, S. , Asagbra, A. , Abdelgadir, W. , Nakavuma, J. , Jakobsen, M. , & Fang, W. (2017). Prevalence of foodborne pathogens in food from selected African countries – A meta‐analysis. International Journal of Food Microbiology, 249, 35–43. 10.1016/j.ijfoodmicro.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Probst, C. , Bandyopadhyay, R. , Price, L. E. , & Cotty, P. J. (2011). Identification of atoxigenic Aspergillus flavus isolates to reduce aflatoxin contamination of maize in Kenya. Plant Disease, 95(2), 212–218. [DOI] [PubMed] [Google Scholar]
- Probst, C. , Njapau, H. , & Cotty, P. J. (2007). Outbreak of an acute aflatoxicosis in Kenya in 2004: Identification of the causal agent. Applied and Environmental Microbiology, 73(8), 2762–2764. 10.1128/AEM.02370-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redzwan, M. , Mutalib, A. , Sokhini, M. , & Rahman, A. (2012). Socio–demographic and socio–economic determinants of adults' knowledge on fungal and aflatoxin contamination in the diets. Asian Pacific Journal of Tropical Biomedicine, 2(3), S1835–S1841. [Google Scholar]
- Sacco, C. , Donato, R. , Zanella, B. , Pini, G. , Pettini, L. , Marino, M. F. , Rookmin, A. D. , & Marvasi, M. (2020). Mycotoxins and flours: Effect of type of crop, organic production, packaging type on the recovery of fungal genus and mycotoxins. International Journal of Food Microbiology, 334, 108808. 10.1016/j.ijfoodmicro.2020.108808 [DOI] [PubMed] [Google Scholar]
- Selemani, F. A. (2018). Fumonisin contamination in cereal‐legume complementary flour marketed in Dar‐Es‐Salaam (Doctoral dissertation). Sokoine University of Agriculture. http://www.suaire.sua.ac.tz/handle/123456789/2800 [Google Scholar]
- Sirma, A. J. , Ouko, E. O. , Murithi, G. , Mburugu, C. , Mapenay, I. , Ombui, J. N. , Kang’ethe, E. K. , & Korhonen, H. (2015). Prevalence of aflatoxin contamination in cereals from Nandi County, Kenya. International Journal of Agricultural Sciences and Veterinary Medicine, 3(3), 55–63. https://hdl.handle.net/10568/68311 [Google Scholar]
- Suleiman, R. , & Rosentrater, K. (2015). Current maize production, post‐harvest losses and the risk of mycotoxins contamination in Tanzania In: Proceedings of ASABE Annual International Meeting (p. 1). American Society of Agricultural and Biological Engineers. [Google Scholar]
- Taye, W. , Ayalew, A. , Dejene, M. , & Chala, A. (2018). Fungal invasion and mycotoxin contamination of stored sorghum grain as influenced by threshing methods. International Journal of Pest Management, 64(1), 66–76. 10.1080/09670874.2017.1327681 [DOI] [Google Scholar]
- Udomkun, P. , Wossen, T. , Nabahungu, N. L. , Mutegi, C. , Vanlauwe, B. , & Bandyopadhya, R. (2018). Incidence and farmers’ knowledge of aflatoxin contamination and control in Eastern Democratic Republic of Congo. Food Science & Nutrition, 6(6), 1607–1620. 10.1002/fsn3.735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ul Haq, N. , Hassali, M. A. , Shafie, A. A. , Saleem, F. , Farooqui, M. & Aljadhey, H. (2012). A cross sectional assessment of knowledge, attitude and practice towards Hepatitis B among healthy population of Quetta, Pakistan. BMC Public Health, 12(1), 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, S. , & Davies, B. (2013). Farmer perceptions of aflatoxins: Implications for intervention in Kenya (No. 20 (7)). International Food Policy Research Institute (IFPRI). http://doi/ifpri.org/utils/getfile/collection/p15738coll2/id/127877/filename/128088.pdf [Google Scholar]
- Wang, R. , Yang, Y. , Chen, R. , Kan, H. , Wu, J. , Wang, K. , Maddock, J. , & Lu, Y. (2015). Knowledge, attitudes, and practices (KAP) of the relationship between air pollution and children’s respiratory health in Shanghai, China. International Journal of Environmental Research and Public Health, 12(2), 1834–1848. 10.3390/ijerph120201834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2018). Co‐exposure of fumonisins with aflatoxins. Food Safety Digest. http://www.who.int/foodsafety/Food_Safety_Digest_Fumonisins_aflatoxins_EN.pdf?ua=1 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to privacy/ethical restrictions, the data that support the findings of this study are available on request from corresponding author.


