Abstract
Oxidative stress (OS), the absence of equilibrium between prooxidants and antioxidants in the body, has been shown to play a pivotal role in the initiation and progression of many diseases. Saffron has been noted for its antioxidant capacity and can be used to improve OS parameters in unhealthy patients. Our aim was to evaluate the efficacy of saffron supplementation on OS parameters in unhealthy patients in randomized controlled trials (RCTs). We searched Medline, EMBASE, Cochrane CENTRAL, Scopus, and Web of Science without language restrictions for RCTs up until April 2021. Studies were included if they compared any form of saffron supplementation to placebo or no supplementation on OS parameters in unhealthy patients. Using a random‐effects model with calculated standardized mean difference (SMD) and 95% confidence intervals (CI), we quantitatively synthesized the data. Heterogeneity was assessed using Cochrane's I 2 values. Ten randomized controlled trials were eligible for this review. Seven were included in the meta‐analysis and indicated an association between saffron intake and a statistically significant decrease in malondialdehyde (MDA) levels (SMD: −0.40; 95% CI: −0.63, −0.17; I 2 = 32.6%) and a significant increase in total antioxidant capacity (TAC, SMD: 0.24; 95% CI: 0.05, 0.42; I 2 = 00.0%). Saffron intake was shown to significantly impact MDA and TAC, indicating its beneficial properties in improving OS in unhealthy patients. However, additional RCTs are required to evaluate the effect on other OS parameters.
Keywords: malondialdehyde, oxidative stress, saffron, total antioxidant capacity
Saffron intake was shown to significantly impact MDA and TAC, indicating its beneficial properties in improving OS in unhealthy patients. However, additional RCTs are required to evaluate the effect on other OS parameters.
Abbreviations
- 4‐HNE
4‐hydroxynonenal
- AGE
advanced glycation end product
- BMI
body mass index
- CAT
catalase
- CI
confidence interval
- DPPH
diphenyl pycryl hydrazyl
- FBG
fasting blood glucose
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GSH
glutathione
- HbA1c
Hemoglobin A1C
- IQR
interquartile range
- MAPK
mitogen‐activated protein kinase
- MDA
malondialdehyde
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- Nrf2
the nuclear factor erythroid 2–related factor 2
- OS
oxidative stress
- ox‐LDL
oxidized low‐density lipoprotein
- RCTs
randomized clinical trials
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SD
standard deviations
- SE
standard errors
- SMD
standard mean difference
- SOD
superoxide dismutase
- TAC
total antioxidant capacity
- XO
xanthine oxidase
1. INTRODUCTION
Oxidative stress (OS) describes a microenvironment manifested by an absence of equilibrium between prooxidants and antioxidants (Liguori et al., 2018). The most important prooxidant enzymes comprise xanthine oxidase (XO), nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and nitric oxide synthase (NOS), whereas the most important antioxidant enzymes are glutathione reductase (GR), glutathione peroxidase (GPx), superoxide dismutase‐1 (SOD‐1), and catalase (CAT, Akbari et al., 2020; Maciejczyk et al., 2018). The repercussion of OS is the development of a biologically destructive microenvironment enriched with free radicals, namely reactive oxygen species (ROS) and reactive nitrogen species (RNS, Phaniendra et al., 2015). These free radicals can be generated in response to various endogenous sources, such as XO, NADPH, and NOS enzymes, and exogenous sources, such as pollution, medication, and radiation (Phaniendra et al., 2015). These free radicals play critical physiological roles in cell signaling, immune defense, and energy extraction, and their deleterious effects are adequately counterbalanced by anti‐oxidant machineries (Genestra, 2007). However, unneutralized excessive free radicals culminate in OS, and consequently bring about tissue injury and cell death (Cooke et al., 2003). Biochemically, the most important OS damage products comprise malondialdehyde (MDA), 4‐hydroxynonenal (4‐HNE), and advanced glycation end product (AGE, Maciejczyk et al., 2018). Total antioxidant capacity (TAC) and total oxidant status (TOS) are the two most frequent biochemical parameters employed to gauge the overall oxidant/anti‐oxidant profile (Maciejczyk et al., 2018).
Current research highlights the pivotal, pathophysiologic role of OS in the initiation and progression of a wide array of disorders, such as hypertension (Rodrigo et al., 2011), diabetes mellitus (Asmat et al., 2016), obesity (Manna & Jain, 2015), metabolic syndrome (Mahjoub & Masrour‐Roudsari, 2012), neurodegenerative disorders (Yaribeygi et al., 2018), cardiovascular disorders (Taleb et al., 2018), and malignancies (Hayes et al., 2020). Many factors, namely genetic, environmental, and dietary, can modulate the interplay between OS and the aforementioned disorders (Morvaridzadeh et al., 2020). Indeed, diet composition can considerably influence the OS microenvironment through the intrinsic prooxidant or antioxidant properties of such dietary elements (Namazi et al., 2018; Vetrani et al., 2013). Several dietary constituents have been greenlighted as naturally occurring antioxidants, such as cranberries (Blumberg et al., 2013) and a variety of spices, including garlic, ginger, and turmeric (Yashin et al., 2017).
Saffron, also known as Crocus sativus, is a common Mediterranean spice plant. It is composed of four key ingredients, namely crocin, crocetin, picrocrocin, and safranal (Roshanravan et al., 2020). Previous meta‐analyses have illustrated the beneficial effects of saffron on select blood pressure, glycemic, lipid, metabolic, and anthropometric indices (Asbaghi et al., 2019; Giannoulaki et al., 2020; Pourmasoumi et al., 2019; Rahmani et al., 2019; Roshanravan et al., 2020). A key property of saffron is its antioxidant capacity (Boskabady & Farkhondeh, 2016; Mashmoul et al., 2013). Several randomized controlled trials were carried out to examine the effect of saffron supplementation on mitigating OS (Ebrahimi et al., 2019; Hamidi et al., 2020; Karimi‐Nazari et al., 2019). However, the OS outcomes were inconsistent, as well as methodologically limited by small sample size, variable study duration, and heterogeneous underlying disorder. To the best of our knowledge, to date, no meta‐analysis has been performed to pool evidence from randomized controlled trials (RCTs) to inform concrete dietary recommendations concerning saffron supplementation and OS mitigation.
Thus, the goal of this research was to carry out a systematic review and meta‐analysis of all RCTs that evaluated the impact of saffron supplementation on OS indices in unhealthy individuals.
2. METHODS
2.1. Data sources and search strategy
This systematic review and meta‐analysis was performed according to The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (Moher et al., 2009). Searches were run in Medline, EMBASE, Cochrane CENTRAL, Scopus, and Web of Science without language restrictions for RCTs up until April 2021. Crosschecking references of relevant reviews was also performed. The main terms searched in the databases included: “Saffron OR Crocus sativus OR Safranal OR Crocin OR Crocetin OR Picrocrocin AND Oxidative Stress OR Total Antioxidant Capacity OR antioxidant OR Oxidant OR reactive oxygen species OR Catalase OR Oxygen Radical Absorbance OR reactive nitrogen species OR lipid peroxide OR Malondialdehyde OR Nitric oxide”. Details on the search strategy and syntaxes for individual databases can be found in Appendix 1.
2.2. Selection criteria
We included randomized, placebo‐controlled or crossover design trials that compared any form of saffron supplementation versus no supplementation on OS parameters, namely TAC, MDA, GSH, GPx, SOD, NO, CAT, and isoprostanes, in unhealthy patients. Papers reporting sufficiently on outcomes comprised the difference in means with 95% confidence intervals (95% CI). Non‐randomized studies (cross‐sectional, case series, case studies, case–controls, and cohort studies) were excluded. We also excluded nonrandomized articles, abstracts, letters to the editor, in vitro and animal studies, and studies with less than one week of follow up after intervention. Table 1 indicates the Cochrane's evidence‐based PICO search criteria for this meta‐analysis.
TABLE 1.
PICO inclusion criteria
| Domain | Selection criteria |
|---|---|
| Participants | Unhealthy adults >18 years old with any disease |
| Interventions | Supplementation with any kind of saffron (Saffron OR Crocus sativus OR Safranal OR Crocin OR Crocetin OR Picrocrocin) |
| Comparators |
Placebo No intervention |
| Outcomes | Oxidative stress parameters, including TAC, MDA, GSH, GPx, SOD, NO, CAT, and isoprostanes |
| Study design | Randomized controlled trials (including parallel or crossover studies) |
Abbreviations: CAT, catalase activity; GPx, glutathione peroxidase activity; GSH, glutathione; MDA, malondialdehyde; NO, nitric oxide; SOD, superoxide dismutase; TAC, total antioxidant capacity.
2.3. Data extraction and statistical analysis
We extracted author details, general population characteristics, the dose of saffron, the kind of saffron supplement given, and main outcomes. Results were extracted in mean and standard deviation (SD). In order to calculate standard mean difference (SMD), results reported as standard error (SE), confidence interval, interquartile range (IQR), and the minimum and maximum value of each variable were converted to SD. We used STATA software version 11 (STATA Corp) and applied the random‐effects model. Statistical significance was defined at p < .05.
Heterogeneity was assessed using chi‐squared tests with significant heterogeneity considered at a p‐value <0.1 and I 2 statistic around 25% (I 2 = 25), 50% (I2 = 50), and 75% (I 2 = 75), which would mean low, medium, and high heterogeneity, respectively(Huedo‐Medina et al., 2006). Sensitivity analysis and funnel plots were used to evaluate the overall effect of each study. Funnel plots were used to visualize and evaluate publication error, and symmetry was examined using Egger's regression asymmetry test and Begg's rank correlation. Two researchers (JH and MM) independently performed risk of bias assessment using the Cochrane risk of bias tool (Higgins et al., 2011).
3. RESULTS
3.1. Search results
The initial search of electronic databases yielded a total of 183 studies. After removing duplicates, 124 unique records remained. After analyzing the titles and the abstracts, we concluded that 84 additional articles did not fulfil our inclusion criteria. After full text screening, we excluded further 20 studies as they failed to report on variables of interest. Thus, 10 trials met the established criteria to be included in this systematic review and meta‐analysis (Abedimanesh et al., 2020; Azimi et al., 2014; Ebrahimi et al., 2019; Ghaderi et al., 2019; Ghiasian et al., 2019; Hamidi et al., 2020; Karimi‐Nazari et al., 2019; Pour et al., 2020; Shahbazian et al., 2019; Tahvilian et al., 2020). The flow diagram of study selection is presented in Figure 1.
FIGURE 1.
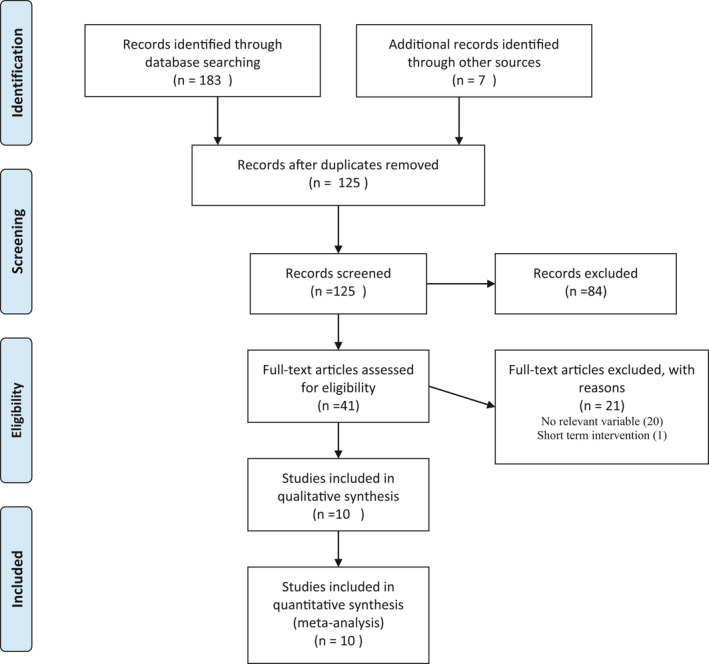
PRIMSA flow diagram
3.2. Characteristics of the included studies
Characteristics of the included trials are presented in Table 2. In total, 651 subjects were recruited in primary studies. Included articles were published between 2014 and 2020. The duration of supplementation with saffron ranged between four and twelve weeks. Saffron dosage ranged from 30 to 1,000 mg/day. The sample size of the included trials ranged from 40 to 80 subjects. All included trials were performed in Iran. The age of participants in included trials ranged from 31 to 57 years. The studies enrolled participants with type 2 diabetes (Azimi et al., 2014; Ebrahimi et al., 2019; Shahbazian et al., 2019), coronary artery disease (Abedimanesh et al., 2020), ulcerative sclerosis (Tahvilian et al., 2020), multiple sclerosis (Ghiasian et al., 2019), nonalcoholic fatty liver (Pour et al., 2020), rheumatoid arthritis (Hamidi et al., 2020), overweight/obese prediabetic patients (Karimi‐Nazari et al., 2019), and methadone maintenance treatment patients (Ghaderi et al., 2019). In addition, articles were performed on subjects with various baseline body mass index (BMI) values; nine studies (Abedimanesh et al., 2020; Azimi et al., 2014; Ebrahimi et al., 2019; Ghaderi et al., 2019; Giannoulaki et al., 2020; Hamidi et al., 2020; Karimi‐Nazari et al., 2019; Milne et al., 2015; Pour et al., 2020; Shahbazian et al., 2019; Tahvilian et al., 2020) were conducted on participants with a BMI over 25 kg/m2 up to 29.9 kg/m2, and one study did not report BMI (Ghiasian et al., 2019).
TABLE 2.
Main characteristics of included studies
| Study, (reference) | Country | Condition | Duration (weeks) | n | Group | Dose | Female gender, n (%) | Age, years, Mean ± SD | BMI, kg/m2, Mean ± SD | Main outcomes a |
|---|---|---|---|---|---|---|---|---|---|---|
| Abedimanesh et al. (2020) | Iran | Coronary artery disease | 8 |
22 20 |
Saffron Placebo |
30 mg/day 30 mg/day |
NR NR |
54.83 ± 5.99 56.00 ± 5.67 |
28.77 ± 3.75 27.91 ± 2.69 |
↓ Ox‐LDL |
| Azimi et al. (2014) | Iran | Type 2 diabetes mellitus | 8 |
42 39 |
Saffron Placebo |
1,000 mg/day |
26(61.9) 24 (61.5) |
57.02 ± 1.0 53.64 ± 1.3 |
28.86 ± 0.2 28.40 ± 0.2 |
↔ F2‐isoprostan |
| Ebrahimi et al. (2019) | Iran | Type 2 diabetes mellitus | 12 | 40 | Saffron | 100 mg/day | 20 (50) | 55.2 ± 7.3 | 29.3 ± 4.9 | ↓MDA, ↔TAC |
| 40 | Placebo | 100 mg/day | 24 (60) | 53 ± 10.6 | 30.5 ± 4.7 | |||||
| Karimi‐Nazari et al. (2019) | Iran | Overweight/obese prediabetic | 8 |
36 39 |
Saffron Placebo |
30 mg/day 30 mg/day |
35 (64.9) 35 (64.4) |
57.95 ± 8.12 57.9 ± 8.7 |
29.35 ± 1.50 28.78 ± 2.02 |
↑ DPPH |
| Tahvilian et al. (2020) | Iran | Ulcerative sclerosis | 8 | 40 | Saffron | 100 mg/day | 19 (47.5) | 40.55 ± 12.71 | 26.95 ± 10.68 | ↔MDA, ↑TAC, ↑SOD, ↑GPX |
| 35 | Placebo | 100 mg/day | 17 (48.6) | 40.97 ± 11.34 | 24.80 ± 3.46 | |||||
| Ghiasian et al. (2019) | Iran | Multiple sclerosis | 4 | 20 | Saffron | 30 mg/day | 17 (85) | 29 ± 4.99 | NR | ↑TAC, ↓MDA, ↔TTG |
| 20 | Placebo | 30 mg/day | 18 (90) | 31.47 ± 5.31 | NR | |||||
| Ghaderi et al. (2019) | Iran | Methadone maintenance treatment | 8 | 26 | Saffron | 30 mg/day | NR | 44.5 ± 9.4 | 24.5 ± 4.4 | ↑TAC, ↓MDA, ↔GSH |
| 27 | Placebo | 30 mg/day | NR | 45.6 ± 9.9 | 25.2 ± 4.2 | |||||
| Pour et al. (2020) | Iran | Nonalcoholic fatty liver disease | 12 | 38 | Saffron | 100 mg/day | 17 (44.7) | 43.42 ± 10.62 | 28.85 (27.05, 32.68) b | ↑TAC, ↓MDA |
| 38 | Placebo | 100 mg/day | 16 (42.1) | 42.05 ± 8.27 | 29.60 (27.99, 32.59) b | |||||
| Hamidi et al. (2020) | Iran | Rheumatoid arthritis | 12 | 33 | Saffron | 100 mg/day | 33 (100) | 51.55 ± 8.26 | 28.17 ± 3.74 | ↔TAC, ↔MDA |
| 32 | Placebo | 100 mg/day | 32 (100) | 51.80 ± 9.62 | 28.39 ± 3.70 | |||||
| Shahbazian et al. (2019) | Iran | Type 2 diabetes mellitus | 12 | 32 | Saffron | 30 mg/day | 24 (75) | 53.5 ± 9.9 | 28.8 ± 4.0 | ↔TAC, ↔MDA |
| 32 | Placebo | 30 mg/day | 21 (65.5) | 52.4 ± 13 | 27.5 ± 4.2 |
Abbreviations: GPX, glutathione peroxidase; GSH, total glutathione; MDA, malondialdehyde; SD, standard deviation; SOD, superoxide dismutase; T2D, type 2 diabetes mellitus; TAC, total antioxidant capacity; TTG, total thiol group; UC, ulcerative colitis.
Main outcomes were expressed in terms of statistical significance (p <.05) as either increased (↑), decreased (↓), or no difference (↔) between saffron versus placebo group.
Data were reported as median (interquartile range)
3.3. Qualitative results
Several OS‐related variables were assessed in the included trials, however, due to the small number of studies, they were not included in the meta‐analysis. The main results are summarized narratively. One study evaluated the effect of saffron intake on serum levels of oxidized low‐density cholesterol (ox‐LDL) as an OS indicator (Abedimanesh et al., 2020). Oxidized LDL is a marker of lipoprotein‐related OS and a risk factor for cardiovascular diseases (Holvoet et al., 2008). Abedimanesh et al. indicated that saffron intake significantly reduces ox‐LDL levels (Abedimanesh et al., 2020).
F2‐isoprostanes from arachidonic acid are the most primary substances of lipid peroxidation and are one of the most reliable variables for assessing OS according to recent investigations (Milne et al., 2015). One of the included studies investigated the effect of saffron intake on F2‐isoprostanes levels, and they reported that saffron intake had no significant effect on F2‐isoprostanes concentration (Azimi et al., 2014). Another finding of our primary studies was elevated levels of diphenyl pycryl hydrazyl (DPPH) radical scavenging activity after saffron intake, which can be explained by the fact that saffron can function as an antioxidant factor by donating a hydrogen atom to the DPPH radical anion (Karimi‐Nazari et al., 2019).
3.4. Meta‐analysis
This meta‐analysis included seven studies and indicated that the saffron intake was associated with a statistically significant decrease in MDA levels (SMD: −0.40; 95% CI: −0.63, −0.17; I 2 = 32.6%, Figure 2) and a significant increase in TAC (SMD: 0.24; 95% CI: 0.05, 0.42; I 2 = 00.0%, Figure 3). We performed a subgroup analysis based on disease type (metabolic vs. nonmetabolic), duration and dose of saffron intake, and age of participants. For patients to be considered as having a metabolic disease, one of the indicators of metabolic syndrome needed to be met: hyperlipidemia, hyperglycemia, hypertension, and obesity. The results of the subgroup analysis showed that MDA decreased significantly in patients with nonmetabolic diseases and after a dose of over 50 mg/day compared to patients with metabolic diseases and doses of <50 mg/day. The subgroup analysis also revealed that saffron intake significantly increased TAC in nonmetabolic disease patients compared to metabolic patients. TAC was also significantly increased in patients in interventions of fewer than 10 weeks duration compared to longer interventions. Further, saffron intake also significantly increased TAC in middle‐aged adults compared to senior adults. Results of the subgroup analysis are presented in Table 3.
FIGURE 2.

Forest plot showing the summary effect size for MDA levels between saffron and placebo groups
FIGURE 3.
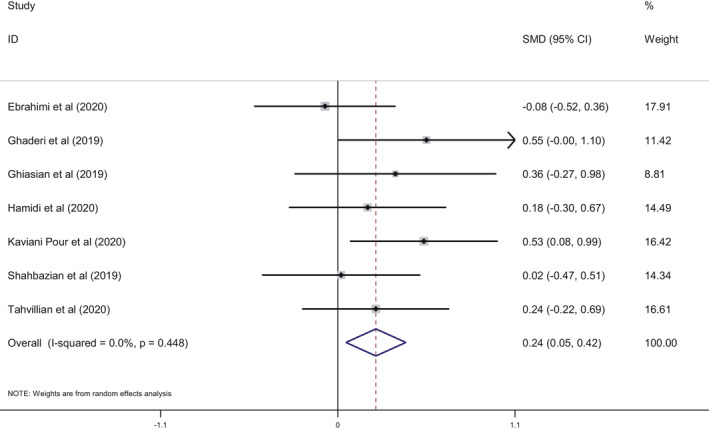
Forest plot showing the summary effect size for TAC levels between saffron and placebo groups
TABLE 3.
Subgroup analysis assessing the effect of saffron intake on MDA and TAC
| Variable | Sub‐grouped by | No. of arms | Effect size (SMD) | 95% CI | I 2 (%) | p for heterogeneity | |
|---|---|---|---|---|---|---|---|
| MDA | Disease type | Metabolic b | 3 | 0.25 | −0.52, 0.02 | 00.0 | 0.637 |
| Non‐Metabolic | 4 | −0.55 | −0.94, −0.17 a | 51.3 | 0.104 | ||
| Duration | ≥10 weeks | 4 | −0.28 | −0.51, −0.04 a | 00.0 | 0.787 | |
| <10 weeks | 3 | 0.64 | −0.18, −0.10 a | 64.8 | 0.059 | ||
| Saffron dosage | ≥50 mg/day | 4 | −0.34 | −0.56, −0.11 a | 00.0 | 0.980 | |
| <50 mg/day | 3 | −0.57 | −1.24, 0.10 | 75.8 | 0.016 | ||
| Age | Senior adults | 3 | −0.28 | −0.56, −0.01 a | 00.0 | 0.592 | |
| Middle‐age adults | 4 | −0.52 | −0.92, −0.13 a | 56.5 | 0.075 | ||
| TAC | Disease type | Metabolic b | 3 | 0.16 | −0.22, 0.54 | 50.1 | 0.135 |
| Non‐Metabolic | 4 | 0.31 | 0.05, 0.57 a | 00.0 | 0.776 | ||
| Duration | ≥10 weeks | 4 | 0.16 | −0.11, 0.43 | 25.3 | 0.260 | |
| <10 weeks | 3 | 0.36 | 0.06, 0.67 a | 00.0 | 0.690 | ||
| Saffron dosage | ≥50 mg/day | 4 | 0.21 | −0.04, 0.47 | 16.9 | 0.307 | |
| <50 mg/day | 3 | 0.28 | −0.04, 0.60 | 02.5 | 0.359 | ||
| Age | Senior adults | 3 | 0.03 | −0.24, 0.30 | 00.0 | 0.730 | |
| Middle‐age adults | 4 | 0.41 | 0.16, 0.67 a | 00.0 | 0.774 | ||
Abbreviations: CI, confidence interval; MDA, malondialdehyde; SMD, standard mean difference; TAC, total antioxidant capacity.
Statistically significant.
According to metabolic syndrome components.
3.5. Quality appraisal
The results of the risk of bias assessment are presented in Appendix 2. Almost all included trials were rated at a low risk of bias for randomization, concealment, and blinding of participants and personnel. Further, five included studies were evaluated at an unclear risk for incomplete outcome data, and six of trials were rated at unclear risk for reporting bias. Three of included studies were rated unclear for other potential sources of biases.
4. DISCUSSION
To the best of my knowledge, this is the first comprehensive systematic review and meta‐analysis that investigated the impact of saffron supplementation on OS parameters. Saffron is widely used for many purposes, including medicinal applications, due to its complex composition of flavoring, aromatic, and colorful substances that are associated with health‐promoting benefits. Saffron compounds with higher biological activity include crocin, crocetin, picrocrocin, and safranal (Melnyk et al., 2010). In addition to the broad ancient uses of saffron for medicinal purposes, several studies have demonstrated that saffron has antioxidant, anti‐inflammatory, anticancer, antidepressant, and antiplatelet effects which suggest that saffron may be a potential therapeutic agent in many diseases, such as neurodegenerative diseases, diabetes mellitus, atherosclerosis, cancer, and sexual dysfunction, among others (Christodoulou et al., 2015; Leone et al., 2018; Razavi & Hosseinzadeh, 2017; Schmidt et al., 2007; Shakeri et al., 2020).
The major components of saffron have been studied in animal and cellular models and have been shown to have positive effects on the level and activity of enzymes involved in cellular antioxidant activity, such as CAT and SOD. Meanwhile, the levels of recognized markers of OS, such as MDA (an indicator of the peroxidation of lipids), were shown to be decreased after treatment with saffron constituents (reviewed in Lopresti & Drummond, 2014; Yousefi et al., 2020).
The total antioxidant status of an individual may also be evaluated by measuring the TAC, as initially proposed by Miller et al.,1993. This parameter is easily determined and can be applied in many clinical contexts to evaluate the TAC of foods, as many nutrients have been recognized by their role as antioxidants (reviewed in Kaliora & Dedoussis, 2007; Kusano & Ferrari, 2008). Our study evaluated the clinical context of the effect of saffron consumption on OS markers, and mainly focused on the results obtained for MDA and TAC levels. The seven trials included in the meta‐analysis indicated that the intake of saffron significantly decreased serum MDA and increased TAC (Figures 2 and 3), which highlights the positive effect that saffron appears to exhibit on OS.
Oxidative stress is associated with a wide range of diseases, such as diabetes mellitus and metabolic syndrome (including hyperlipidemia, hyperglycemia, hypertension, and obesity), which all of which are risk factors for cardiovascular diseases (Denisenko et al., 2020; Gil‐del Valle et al., 2005). To date, several studies have been carried out to evaluate the effect of saffron on different OS markers in these aforementioned diseases and others (Razavi & Hosseinzadeh, 2017; Pour et al., 2020). For example, in patients with nonalcoholic fatty liver disease, the ingestion of 100 mg saffron per day for 12 weeks induced a significant increase in TAC and decrease in MDA levels (Pour et al., 2020) while the intake of 15 mg of crocin twice a day for 8 weeks induced a significant reduction of the pro‐oxidant‐anti‐oxidant balance, in patients with metabolic syndrome (Nikbakht‐Jam et al., 2016).
Recent systematic reviews and meta‐analyses have highlighted a possible positive effect of saffron against cardiovascular diseases and as a modulator of serum lipid profile, showing that saffron can lead to decreases in serum total cholesterol and triglyceride concentrations, as well as an increase in high‐density lipoprotein levels. However, these results were based on a limited number of trials and should be interpreted with caution (Asbaghi et al., 2019; Pourmasoumi et al., 2019; Rahmani et al., 2019; Roshanravan et al., 2020).
Several other studies have shown that saffron or its biologically active components may have a positive impact on several parameters related to glucose metabolism, in patients with different non‐communicable diseases, and in patients undergoing hypoglycemic treatment, despite some discrepancies in the obtained conclusions. For example, saffron intake was shown to decrease fasting blood glucose (FBG), fasting insulin, and, for longer interventions, decrease HbA1c in one study (Sohaei et al., 2019). However, in another analysis, the effect on FBG was similar but more significant for interventions carried out for periods of 12 weeks or more, while no significant effect was observed in HbA1c (Rahmani et al., 2020). The intake of crocin was also evaluated and may also reduce FBG (Naserizadeh et al., 2020). In addition, another recent meta‐analysis focusing solely on patients with diabetes mellitus or metabolic syndrome assessed the effects of either saffron or its component, crocin, on several outcomes related to the metabolic profile of the patients. The authors concluded that although saffron appears to be an important regulator of FBG, however, most trials carried out thus far are not robust enough to clearly state the impact of saffron on these patients (Giannoulaki et al., 2020). These contradictory results may be due to the fact that saffron components are difficult to absorb by the human body and act only in the gastrointestinal tract (Naserizadeh et al., 2020). It is clear in all reported results that all the findings need to be carefully interpreted as the different trials included present high levels of heterogeneity at several levels.
The results obtained after the subgroup analysis depicted statistically significant positive effects of saffron for both MDA and TAC levels in patients with non‐metabolic diseases. Additionally, when elevated doses of saffron were used (50 mg/day or more) or in shorter interventions (fewer than 10 weeks), similar results were observed for MDA and TAC levels, respectively (Table 3). In this analysis, the age of the participants was determined to be another important factor to consider when assessing the health benefits of saffron, as TAC levels increased for younger patients (middle age vs. senior adults, Table 3). Several possible mechanisms could be responsible for the beneficial effect of saffron on improving OS parameters. It has recently been shown that saffron may affect cardiometabolic outcomes through its effect on non‐coding RNAs, such as microRNAs (miRNAs, Ahmadi Khatir et al., 2018). MiRNAs are endogenous ∼23‐nucleotide RNAs that can bind to different locations on mRNAs of protein‐coding genes to modulate expression of these genes (Emami, Akbari, et al., 2019, Emami, Nekouian, et al., 2019; Talebi et al., 2020). MicroRNAs are also known to impact the stability and evolution of several mRNAs (Akbari et al., 2016; Zamani et al., 2019). It has also been demonstrated that saffron may affect up‐stream genes related to OS, such as Nrf2 and MAPK, through changes in miRNAs (Ashrafizadeh et al., 2020; Hassani et al., 2017).
Although the results on the effects of saffron against OS are promising, several factors should also be considered when interpreting the available data. Plants’ composition and quality vary with the methods used for cultivation, production, and extraction, affecting the possible outcomes and their reproducibility (Moratalla‐Lopez et al., 2021; Schmidt et al., 2007). Additionally, the means of saffron or its components consumption may also affect bioavailability, which may also affect the health benefits being evaluated. More studies are needed to fully understand how saffron and its constituents are absorbed and reach the cells to exert their effect (Giannoulaki et al., 2020).
Additional parameters that need to be taken in consideration include the amount of saffron ingested, the duration of the intervention, and the number of patients enrolled in each trial. The studies available and included in this work were all carried out with small groups of patients, weakening the statistical value of the results. As discussed by Kaliora and Dedoussis (Kaliora & Dedoussis, 2007), the characteristics of the studied patients (e.g., age, sex, ethnicity, body mass index, initial total antioxidant status, and diet consumed) should also be considered carefully. For example, this meta‐analysis only includes studies that were carried out in Iran, which limits the possible extrapolation of the results for other populations. Another limitation of this review is that we didn't register the protocol in advance. Further, when studying unhealthy subjects, different trials may be needed for different diseases. The stage of the disease, the medication administered, and additional factors that may contribute to improving the outcomes evaluated need to be identified so they do not interfere with the final results. More studies should be carried out addressing these questions to increase the current knowledge base about the health benefits of saffron on different illnesses.
5. COMPETING INTERESTS
The authors have no conflict of interest to declare.
6. ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
7. CONSENT FOR PUBLICATION
All authors consent for publication of article in this journal.
AUTHOR'S CONTRIBUTION
Javad Heshmati: Conceptualization, methodology, manuscript preparation Mojgan Morvaridzadeh and Gholamreza Rezamand: Data extraction, writing, original draft preparation. Shahram Agah and Shima abdollahi: Data analysis, investigation. M. Dulce Estêvão: Writing, original draft preparation, reviewing, and editing. Emma Persad: Writing, reviewing, and editing, final draft validation. Ahmed Abu‐Zaid and Omid Toupchian: Writing original draft. Ava sadat Hosseini and Hafez Heydari: Editing and revising the manuscript.
ACKNOWLEDGMENT
None.
Morvaridzadeh, M. , Agah, S. , Dulce Estêvão, M. , Hosseini, A. S. , Heydari, H. , Toupchian, O. , Abdollahi, S. , Persad, E. , Abu‐Zaid, A. , Rezamand, G. , & Heshmati, J. (2021). Effect of saffron supplementation on oxidative stress parameters: A systematic review and meta‐analysis of randomized placebo‐controlled trials. Food Science & Nutrition, 9, 5809–5819. 10.1002/fsn3.2463
Funding information
Authors receive no funding for this article
Contributor Information
Gholamreza Rezamand, Email: Rezamandreza@yahoo.com.
Javad Heshmati, Email: javad.heshmati@gmail.com.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- Abedimanesh, N. , Motlagh, B. , Abedimanesh, S. , Bathaie, S. Z. , Separham, A. , & Ostadrahimi, A. (2020). Effects of crocin and saffron aqueous extract on gene expression of SIRT1, AMPK, LOX1, NF‐κB, and MCP‐1 in patients with coronary artery disease: A randomized placebo‐controlled clinical trial. Phytotherapy Research, 34(5), 1114–1122. [DOI] [PubMed] [Google Scholar]
- Ahmadi Khatir, S. , Bayatian, A. , Barzegari, A. , Roshanravan, N. , Safaiyan, A. , Pavon‐Djavid, G. , & Ostadrahimi, A. (2018). Saffron (Crocus sativus L.) supplements modulate circulating MicroRNA (miR‐21) in atherosclerosis patients; a randomized, double‐blind, placebo‐controlled trial. Iranian Red Crescent Medical Journal, 20(10). 10.5812/ircmj.80260 [DOI] [Google Scholar]
- Akbari, A. , Farahnejad, Z. , Akhtari, J. , Abastabar, M. , Mobini, G. R. , & Mehbod, A. S. A. (2016). Staphylococcus aureus enterotoxin B down‐regulates the expression of transforming growth factor‐beta (TGF‐β) signaling transducers in human glioblastoma. Jundishapur Journal of Microbiology, 9(5). 10.5812/jjm.27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari, A. , Mobini, G. R. , Agah, S. , Morvaridzadeh, M. , Omidi, A. , Potter, E. , Fazelian, S. , Ardehali, S. H. , Daneshzad, E. , & Dehghani, S. (2020). Coenzyme Q10 supplementation and oxidative stress parameters: A systematic review and meta‐analysis of clinical trials. European Journal of Clinical Pharmacology, 76(11), 1483–1499. 10.1007/s00228-020-02919-8 [DOI] [PubMed] [Google Scholar]
- Asbaghi, O. , Soltani, S. , Norouzi, N. , Milajerdi, A. , Choobkar, S. , & Asemi, Z. (2019). The effect of saffron supplementation on blood glucose and lipid profile: A systematic review and meta‐analysis of randomized controlled trials. Complementary Therapies in Medicine, 47, 102158. 10.1016/j.ctim.2019.07.017 [DOI] [PubMed] [Google Scholar]
- Ashrafizadeh, M. , Ahmadi, Z. , Samarghandian, S. , Mohammadinejad, R. , Yaribeygi, H. , Sathyapalan, T. , & Sahebkar, A. (2020). MicroRNA‐mediated regulation of Nrf2 signaling pathway: Implications in disease therapy and protection against oxidative stress. Life Sciences, 244, 117329. [DOI] [PubMed] [Google Scholar]
- Asmat, U. , Abad, K. , & Ismail, K. (2016). Diabetes mellitus and oxidative stress‐A concise review. Saudi Pharmaceutical Journal, 24(5), 547–553. 10.1016/j.jsps.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimi, P. , Ghiasvand, R. , Feizi, A. , Hariri, M. , & Abbasi, B. (2014). Effects of cinnamon, cardamom, saffron, and ginger consumption on markers of glycemic control, lipid profile, oxidative stress, and inflammation in type 2 diabetes patients. The Review of Diabetic Studies: RDS, 11(3–4), 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg, J. B. , Camesano, T. A. , Cassidy, A. , Kris‐Etherton, P. , Howell, A. , Manach, C. , Ostertag, L. M. , Sies, H. , Skulas‐Ray, A. , & Vita, J. A. (2013). Cranberries and their bioactive constituents in human health. Advances in Nutrition, 4(6), 618–632. 10.3945/an.113.004473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskabady, M. H. , & Farkhondeh, T. (2016). Antiinflammatory, antioxidant, and immunomodulatory effects of Crocus sativus L. and its main constituents. Phytotherapy Research, 30(7), 1072–1094. [DOI] [PubMed] [Google Scholar]
- Christodoulou, E. , Kadoglou, N. P. , Kostomitsopoulos, N. , & Valsami, G. (2015). Saffron: A natural product with potential pharmaceutical applications. Journal of Pharmacy and Pharmacology, 67(12), 1634–1649. 10.1111/jphp.12456 [DOI] [PubMed] [Google Scholar]
- Cooke, M. S. , Evans, M. D. , Dizdaroglu, M. , & Lunec, J. (2003). Oxidative DNA damage: Mechanisms, mutation, and disease. The FASEB Journal, 17(10), 1195–1214. 10.1096/fj.02-0752rev [DOI] [PubMed] [Google Scholar]
- Denisenko, Y. K. , Kytikova, O. Y. , Novgorodtseva, T. P. , Antonyuk, M. V. , Gvozdenko, T. A. , & Kantur, T. A. (2020). Lipid‐induced mechanisms of metabolic syndrome. Journal of Obesity, 2020, 5762395. 10.1155/2020/5762395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi, F. , Sahebkar, A. , Aryaeian, N. , Pahlavani, N. , Fallah, S. , Moradi, N. , Abbasi, D. , & Hosseini, A. F. (2019). Effects of saffron supplementation on inflammation and metabolic responses in type 2 diabetic patients: A randomized, double‐blind, placebo‐controlled trial. Diabetes Metabolic Syndrome and Obesity, 12, 2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami, S. S. , Akbari, A. , Zare, A.‐A. , Agah, S. , Masoodi, M. , Talebi, A. , Minaeian, S. , Fattahi, A. , & Moghadamnia, F. (2019). MicroRNA expression levels and histopathological features of colorectal cancer. Journal of Gastrointestinal Cancer, 50(2), 276–284. [DOI] [PubMed] [Google Scholar]
- Emami, S. , Nekouian, R. , Akbari, A. , Faraji, A. , Abbasi, V. , & Agah, S. (2019). Evaluation of circulating miR‐21 and miR‐222 as diagnostic biomarkers for gastric cancer. Journal of Cancer Research and Therapeutics, 15(1), 115–119. [DOI] [PubMed] [Google Scholar]
- Genestra, M. (2007). Oxyl radicals, redox‐sensitive signalling cascades and antioxidants. Cellular Signalling, 19(9), 1807–1819. [DOI] [PubMed] [Google Scholar]
- Ghaderi, A. , Rasouli‐Azad, M. , Vahed, N. , Banafshe, H. R. , Soleimani, A. , Omidi, A. , Ghoreishi, F. S. , & Asemi, Z. (2019). Clinical and metabolic responses to crocin in patients under methadone maintenance treatment: A randomized clinical trial. Phytotherapy Research, 33(10), 2714–2725. 10.1002/ptr.6445 [DOI] [PubMed] [Google Scholar]
- Ghiasian, M. , Khamisabadi, F. , Kheiripour, N. , Karami, M. , Haddadi, R. , Ghaleiha, A. , Taghvaei, B. , Oliaie, S. S. , Salehi, M. , Samadi, P. , & Ranjbar, A. (2019). Effects of crocin in reducing DNA damage, inflammation, and oxidative stress in multiple sclerosis patients: A double‐blind, randomized, and placebo‐controlled trial. Journal of Biochemical and Molecular Toxicology, 33(12), e22410. 10.1002/jbt.22410 [DOI] [PubMed] [Google Scholar]
- Giannoulaki, P. , Kotzakioulafi, E. , Chourdakis, M. , Hatzitolios, A. , & Didangelos, T. (2020). Impact of Crocus sativus L. on metabolic profile in patients with diabetes mellitus or metabolic syndrome: A systematic review. Nutrients, 12(5), 1424. 10.3390/nu12051424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil‐del Valle, L. , Milian, L. C. , Toledo, A. , Vilaró, N. , Tápanes, R. , & Otero, M. A. (2005). Altered redox status in patients with diabetes mellitus type I. Pharmacological Research, 51(4), 375–380. 10.1016/j.phrs.2004.10.012 [DOI] [PubMed] [Google Scholar]
- Hamidi, Z. , Aryaeian, N. , Abolghasemi, J. , Shirani, F. , Hadidi, M. , Fallah, S. , & Moradi, N. (2020). The effect of saffron supplement on clinical outcomes and metabolic profiles in patients with active rheumatoid arthritis: A randomized, double‐blind, placebo‐controlled clinical trial. Phytotherapy Research, 34(7), 1650–1658. [DOI] [PubMed] [Google Scholar]
- Hassani, F. V. , Mehri, S. , Abnous, K. , Birner‐Gruenberger, R. , & Hosseinzadeh, H. (2017). Protective effect of crocin on BPA‐induced liver toxicity in rats through inhibition of oxidative stress and downregulation of MAPK and MAPKAP signaling pathway and miRNA‐122 expression. Food and Chemical Toxicology, 107, 395–405. 10.1016/j.fct.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Hayes, J. D. , Dinkova‐Kostova, A. T. , & Tew, K. D. (2020). Oxidative stress in cancer. Cancer Cell, 38(2), 167–197. 10.1016/j.ccell.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. T. , Altman, D. G. , Gotzsche, P. C. , Juni, P. , Moher, D. , Oxman, A. D. , Savovic, J. , Schulz, K. F. , Weeks, L. , & Sterne, J. A. C. (2011). The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 343, d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holvoet, P. , Keyzer, D. D. , & Jacobs Jr., D. (2008). Oxidized LDL and the metabolic syndrome. Future Lipidology, 3(6), 637–649. 10.2217/17460875.3.6.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo‐Medina, T. B. , Sánchez‐Meca, J. , Marín‐Martínez, F. , & Botella, J. (2006). Assessing heterogeneity in meta‐analysis: Q statistic or I² index? Psychological Methods, 11(2), 193. 10.1037/1082-989X.11.2.193 [DOI] [PubMed] [Google Scholar]
- Kaliora, A. C. , & Dedoussis, G. V. (2007). Natural antioxidant compounds in risk factors for CVD. Pharmacological Research, 56(2), 99–109. 10.1016/j.phrs.2007.04.018 [DOI] [PubMed] [Google Scholar]
- Karimi‐Nazari, E. , Nadjarzadeh, A. , Masoumi, R. , Marzban, A. , Mohajeri, S. A. , Ramezani‐Jolfaie, N. , & Salehi‐Abargouei, A. (2019). Effect of saffron (Crocus sativus L.) on lipid profile, glycemic indices and antioxidant status among overweight/obese prediabetic individuals: A double‐blinded, randomized controlled trial. Clinical Nutrition ESPEN, 34, 130–136. 10.1016/j.clnesp.2019.07.012 [DOI] [PubMed] [Google Scholar]
- Kusano, C. & Ferrari, B. (2008). Total antioxidant capacity: A biomarker in biomedical and nutritional studies. Journal of cell and molecular biology, 7(1), 1–15. [Google Scholar]
- Leone, S. , Recinella, L. , Chiavaroli, A. , Orlando, G. , Ferrante, C. , Leporini, L. , Brunetti, L. , & Menghini, L. (2018). Phytotherapic use of the Crocus sativus L. (Saffron) and its potential applications: A brief overview. Phytotherapy Research, 32(12), 2364–2375. [DOI] [PubMed] [Google Scholar]
- Liguori, I. , Russo, G. , Curcio, F. , Bulli, G. , Aran, L. , Della‐Morte, D. , Gargiulo, G. , Testa, G. , Cacciatore, F. , Bonaduce, D. , & Abete, P. (2018). Oxidative stress, aging, and diseases. Clinical Interventions in Aging, 13, 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresti, A. L. , & Drummond, P. D. (2014). Saffron (Crocus sativus) for depression: A systematic review of clinical studies and examination of underlying antidepressant mechanisms of action. Human Psychopharmacology, 29(6), 517–527. [DOI] [PubMed] [Google Scholar]
- Maciejczyk, M. , Żebrowska, E. , Zalewska, A. , & Chabowski, A. (2018). Redox balance, antioxidant defense, and oxidative damage in the hypothalamus and cerebral cortex of rats with high fat diet‐induced insulin resistance. Oxidative Medicine and Cellular Longevity, 2018, 6940515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjoub, S. , & Masrour‐Roudsari, J. (2012). Role of oxidative stress in pathogenesis of metabolic syndrome. Caspian Journal of Internal Medicine, 3(1), 386–396. [PMC free article] [PubMed] [Google Scholar]
- Manna, P. , & Jain, S. K. (2015). Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metabolic Syndrome and Related Disorders, 13(10), 423–444. 10.1089/met.2015.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashmoul, M. , Azlan, A. , Khaza'ai, H. , Yusof, B. N. , & Noor, S. M. (2013). Saffron: A natural potent antioxidant as a promising anti‐obesity drug. Antioxidants (Basel), 2(4), 293–308. 10.3390/antiox2040293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, N. J. , Rice‐Evans, C. , Davies, M. J. , Gopinathan, V. , & Milner, A. (1993). A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates.. Clinical Science. 84, 407–412. 10.1042/cs0840407 [DOI] [PubMed] [Google Scholar]
- Melnyk, J. P. , Wang, S. , & Marcone, M. F. (2010). Chemical and biological properties of the world's most expensive spice: Saffron. Food Research International, 43(8), 1981–1989. 10.1016/j.foodres.2010.07.033 [DOI] [Google Scholar]
- Milne, G. L. , Dai, Q. , & Roberts, L. J. II (2015). The isoprostanes—25 years later. Biochimica et Biophysica Acta (BBA)‐Molecular and Cell Biology of Lipids, 1851(4), 433–445. 10.1016/j.bbalip.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Med, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla‐Lopez, N. , Parizad, S. , Habibi, M. K. , Winter, S. , Kalantari, S. , Bera, S. , Lorenzo, C. , García‐Rodríguez, M. V. , Dizadji, A. , & Alonso, G. L. (2021). Impact of two different dehydration methods on saffron quality, concerning the prevalence of Saffron latent virus (SaLV) in Iran. Food Chemistry, 337, 127786. [DOI] [PubMed] [Google Scholar]
- Morvaridzadeh, M. , Nachvak, S. M. , Agah, S. , Sepidarkish, M. , Dehghani, F. , Rahimlou, M. , Pizarro, A. B. , & Heshmati, J. (2020). Effect of soy products and isoflavones on oxidative stress parameters: A systematic review and meta‐analysis of randomized controlled trials. Food Research International, 137, 109578. 10.1016/j.foodres.2020.109578 [DOI] [PubMed] [Google Scholar]
- Namazi, N. , Larijani, B. , & Azadbakht, L. (2018). Association between the dietary inflammatory index and the incidence of cancer: A systematic review and meta‐analysis of prospective studies. Public Health, 164, 148–156. 10.1016/j.puhe.2018.04.015 [DOI] [PubMed] [Google Scholar]
- naserizadeh, S. K. , Taherifard, M. H. , Shekari, M. , Mesrkanlou, H. A. , Asbaghi, O. , Nazarian, B. , Khosroshahi, M. Z. , & Heydarpour, F. (2020). The effect of crocin supplementation on lipid concentrations and fasting blood glucose: A systematic review and meta‐analysis and meta‐regression of randomized controlled trials. Complement Ther Med, 52, 102500. 10.1016/j.ctim.2020.102500 [DOI] [PubMed] [Google Scholar]
- Nikbakht‐Jam, I. , Khademi, M. , Nosrati, M. , Eslami, S. , Foroutan‐Tanha, M. , Sahebkar, A. , Tavalaie, S. , Ghayour‐Mobarhan, M. , Ferns, G. , Hadizadeh, F. , Tabassi, S. , Mohajeri, S. A. , & Emamian, M. (2016). Effect of crocin extracted from saffron on pro‐oxidant–anti‐oxidant balance in subjects with metabolic syndrome: A randomized, placebo‐controlled clinical trial. European Journal of Integrative Medicine, 8(3), 307–312. 10.1016/j.eujim.2015.12.008 [DOI] [Google Scholar]
- Phaniendra, A. , Jestadi, D. B. , & Periyasamy, L. (2015). Free radicals: Properties, sources, targets, and their implication in various diseases. Indian Journal of Clinical Biochemistry, 30(1), 11–26. 10.1007/s12291-014-0446-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pour, F. K. , Aryaeian, N. , Mokhtare, M. , Mirnasrollahi Parsa, R. S. , Jannani, L. , Agah, S. , Fallah, S. , & Moradi, N. (2020). The effect of saffron supplementation on some inflammatory and oxidative markers, leptin, adiponectin, and body composition in patients with nonalcoholic fatty liver disease: A double‐blind randomized clinical trial. Phytotherapy Research, 34(12):3367–3378. 10.1002/ptr.6791 [DOI] [PubMed] [Google Scholar]
- Pourmasoumi, M. , Hadi, A. , Najafgholizadeh, A. , Kafeshani, M. , & Sahebkar, A. (2019). Clinical evidence on the effects of saffron (Crocus sativus L.) on cardiovascular risk factors: A systematic review meta‐analysis. Pharmacological Research, 139, 348–359. 10.1016/j.phrs.2018.11.038 [DOI] [PubMed] [Google Scholar]
- Rahmani, J. , Bazmi, E. , Clark, C. , & Hashemi Nazari, S. S. (2020). The effect of Saffron supplementation on waist circumference, HA1C, and glucose metabolism: A systematic review and meta‐analysis of randomized clinical trials. Complementary Therapies in Medicine, 49, 102298. [DOI] [PubMed] [Google Scholar]
- Rahmani, J. , Manzari, N. , Thompson, J. , Clark, C. C. T. , Villanueva, G. , Varkaneh, H. K. , & Mirmiran, P. (2019). The effect of saffron on weight and lipid profile: A systematic review, meta‐analysis, and dose‐response of randomized clinical trials. Phytotherapy Research, 33(9), 2244–2255. 10.1002/ptr.6420 [DOI] [PubMed] [Google Scholar]
- Razavi, B. M. , & Hosseinzadeh, H. (2017). Saffron: A promising natural medicine in the treatment of metabolic syndrome. Journal of the Science of Food and Agriculture, 97(6), 1679–1685. 10.1002/jsfa.8134 [DOI] [PubMed] [Google Scholar]
- Rodrigo, R. , González, J. , & Paoletto, F. (2011). The role of oxidative stress in the pathophysiology of hypertension. Hypertension Research, 34(4), 431–440. 10.1038/hr.2010.264 [DOI] [PubMed] [Google Scholar]
- Roshanravan, B. , Samarghandian, S. , Ashrafizadeh, M. , Amirabadizadeh, A. , Saeedi, F. , & Farkhondeh, T. (2020). Metabolic impact of saffron and crocin: An updated systematic and meta‐analysis of randomised clinical trials. Archives of Physiology and Biochemistry, 1–13. 10.1080/13813455.2020.1716020 [DOI] [PubMed] [Google Scholar]
- Schmidt, M. , Betti, G. , & Hensel, A. (2007). Saffron in phytotherapy: Pharmacology and clinical uses. Wiener Medizinische Wochenschrift, 157(13–14), 315–319. 10.1007/s10354-007-0428-4 [DOI] [PubMed] [Google Scholar]
- Shahbazian, H. , Aleali, A. M. , Amani, R. , Namjooyan, F. , Cheraghian, B. , Latifi, S. M. , Bahrainian, S. , & Ghadiri, A. (2019). Effects of saffron on homocysteine, and antioxidant and inflammatory biomarkers levels in patients with type 2 diabetes mellitus: A randomized double‐blind clinical trial. Avicenna Journal of Phytomedicine, 9(5), 436–445. [PMC free article] [PubMed] [Google Scholar]
- Shakeri, M. , Hashemi Tayer, A. , Shakeri, H. , Sotoodeh Jahromi, A. , Moradzadeh, M. , & Hojjat‐Farsangi, M. (2020). Toxicity of saffron extracts on cancer and normal cells: A review article. Asian Pacific Journal of Cancer Prevention, 21(7), 1867–1875. 10.31557/APJCP.2020.21.7.1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohaei, S. , Amani, R. , Tarrahi, M. J. , & Ghasemi‐Tehrani, H. (2019). The effects of curcumin supplementation on glycemic status, lipid profile and hs‐CRP levels in overweight/obese women with polycystic ovary syndrome: A randomized, double‐blind, placebo‐controlled clinical trial. Complementary Therapies in Medicine, 47, 102201. 10.1016/j.ctim.2019.102201 [DOI] [PubMed] [Google Scholar]
- Tahvilian, N. , Masoodi, M. , Faghihi Kashani, A. , Vafa, M. , Aryaeian, N. , Heydarian, A. , Hosseini, A. , Moradi, N. , & Farsi, F. (2020). Effects of saffron supplementation on oxidative/antioxidant status and severity of disease in ulcerative colitis patients: A randomized, double‐blind, placebo‐controlled study. Phytotherapy Research, 35(2):946–953. 10.1002/ptr.6848 [DOI] [PubMed] [Google Scholar]
- Taleb, A. , Ahmad, K. A. , Ihsan, A. U. , Qu, J. , Lin, N. A. , Hezam, K. , Koju, N. , Hui, L. , & Qilong, D. (2018). Antioxidant effects and mechanism of silymarin in oxidative stress induced cardiovascular diseases. Biomedicine & Pharmacotherapy, 102, 689–698. 10.1016/j.biopha.2018.03.140 [DOI] [PubMed] [Google Scholar]
- Talebi, A. , Masoodi, M. , Mirzaei, A. , Mehrad‐Majd, H. , Azizpour, M. , & Akbari, A. (2020). Biological and clinical relevance of metastasis‐associated long noncoding RNAs in esophageal squamous cell carcinoma: A systematic review. Journal of Cellular Physiology, 235(2), 848–868. 10.1002/jcp.29083 [DOI] [PubMed] [Google Scholar]
- Vetrani, C. , Costabile, G. , Di Marino, L. , & Rivellese, A. A. (2013). Nutrition and oxidative stress: A systematic review of human studies. International Journal of Food Sciences and Nutrition, 64(3), 312–326. 10.3109/09637486.2012.738651 [DOI] [PubMed] [Google Scholar]
- Yaribeygi, H. , Panahi, Y. , Javadi, B. , & Sahebkar, A. (2018). The underlying role of oxidative stress in neurodegeneration: A mechanistic review. CNS & Neurological Disorders: Drug Targets, 17(3), 207–215. 10.2174/1871527317666180425122557 [DOI] [PubMed] [Google Scholar]
- Yashin, A. , Yashin, Y. , Xia, X. , & Nemzer, B. (2017). Antioxidant activity of spices and their impact on human health: A review. Antioxidants (Basel), 6(3). 10.3390/antiox6030070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi, F. , Arab, F. L. , Rastin, M. , Tabasi, N. S. , Nikkhah, K. , & Mahmoudi, M. (2020). Comparative assessment of immunomodulatory, proliferative, and antioxidant activities of crocin and crocetin on mesenchymal stem cells. Journal of Cellular Biochemistry. 10.1002/jcb.29826 [DOI] [PubMed] [Google Scholar]
- Zamani, S. , Sohrabi, A. , Hosseini, S. M. , Rahnamaye‐Farzami, M. , & Akbari, A. (2019). Deregulation of miR‐21 and miR‐29a in cervical cancer related to HPV infection. MicroRNA, 8(2), 110–115. 10.2174/2211536607666181017124349 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


