Abstract
Eggplant is a popular vegetable in Asia; however, it has a short storage life and considerable economic losses have resulted from eggplant browning. Calcium has been reported to play a key role in the postharvest storage of plants. Here, we found that exogenous calcium application could delay eggplant fruit browning and maintain higher storage quality. The increased browning index (BI), relative electrolytic leakage (REL), and water loss were suppressed by calcium treatment during storage. Delayed browning with calcium treatment might result from a higher phenolic level and suppressed the activity of polyphenol oxidase (PPO). Less H2O2 and O2 ‐ but more activated reactive oxygen species (ROS) scavenging enzymes accumulated in calcium‐treated fruits than in H2O‐treated fruits. Moreover, the nonenzymatic antioxidant, ascorbic acid (AsA), was accumulated more in calcium‐treated eggplant fruits. Taken together, our data demonstrated that exogenous calcium application delayed eggplant fruit browning by regulating phenol metabolism and enhancing antioxidant systems.
Keywords: browning, calcium, eggplant, phenolic metabolism, reactive oxygen species (ROS), ROS metabolism
1. Calcium treatment delayed browning of eggplant fruits during storage.2. Calcium treatment maintained ROS scavenging enzyme activities in eggplant fruits during storage.

1. INTRODUCTION
Eggplant (Solanum melongena L.) is a native plant from Southeast Asia that was domesticated more than 4,000 years ago (Das et al., 2011). The world production of eggplant in 2019 was approximately 55.2 million tons, with China being the main producer (http://www.fao.org/). Eggplant fruit is rich in vitamins, dietary fiber, and phytonutrients, especially phenolic compounds, such as caffeic acid and chlorogenic acid, and flavonoids. Eggplant has highly beneficial effects on human health due to its high content of phenolic acids (Salerno et al., 2014; Toppino et al., 2016). These phenolic acids are important due to their various health‐promoting effects (Kaushik et al., 2015).
Fresh eggplant fruits deteriorate rapidly after harvesting and have a very limited shelf life at ambient temperature. In addition, postharvest problems include softening, browning, flavor loss, and disease infections that negatively affect the quality of eggplant fruits during storage or transportation. Many reports have demonstrated that elevated phenolic acid levels in fruit flesh increase the risk of eggplant browning (Taranto et al., 2017). Depending on the mechanism, browning reactions in food products are generally divided into enzymatic and nonenzymatic browning. Enzymatic browning is the main form that occurs during harvesting, transportation, storage, and processing of eggplant fruit (Concellón, CAñón, & RChaves, 2004). Due to tissue damage, phenolic compounds and polyphenol oxidase (PPO) are exposed to oxygen, which triggers the oxidation of phenols into quinones. Subsequently, these quinones and their derivatives polymerize through alternating reactions to form a relatively insoluble brown pigment called melanin (Moon et al., 2020; Taranto et al., 2017). Additionally, changes in antioxidant and nonenzymatic systems have been reported to play a role in the browning of fruits (Hodges et al., 2004; Maioli et al., 2020; Zhang et al., 2015). Therefore, extending the storage life and delaying the decrease in storage quality, especially suppressing browning in eggplant fruits, has become a research hot spot.
As a second messenger, calcium (Ca2+) was reported to have a positive function in response to abiotic stresses, including drought, cold, heat, heavy metal, and oxidative stresses (Aldon et al., 2018; Nasir Khan et al., 2009). Recently, postharvest application of calcium was reported to maintain the quality of fresh fruits and vegetables (Li et al., 2020; Xiong et al., 2021). Postharvest application of Ca2+ reduced the severity of chilling damage by increasing the calcium in the pulp, thereby delaying browning of the fruit after cold storage (Manganaris et al., 2007). Wang et al. found that exogenous calcium treatment increased cherry firmness and reduced pitting (Wang et al., 2014). 4% Ca2+ can improve the postharvest quality and shelf life of bananas, indicating that coating bananas with calcium improves the postharvest quality and shelf life of fruits (Elbagoury et al., 2021). However, little is known about the function of calcium in the eggplant fruit. The objective of this work was, first, to explore the effect of calcium on the browning of eggplant fruit and, second, to investigate the effects of calcium on ROS, phenolics, and antioxidants in eggplant fruits under storage.
2. MATERIALS AND METHODS
2.1. Fruit materials and treatments
Eggplant fruits (Solanum melongena L. cv. “Heilong”) were harvested at a commercially ripe stage (physiologically immature), when the length of the eggplant fruits reached 20 cm, in an orchard in Nanjing, Jiangsu, China. Fruits with uniform size and color and nonvisible damage spots were selected. After removal from the filed heat, the fruits were immersed in 0.05% Tween‐20 solutions containing 0%, 1%, 2%, 3%, and 4% CaCl2 for 20 min and then naturally air‐dried for 2 hr at 25°C. In total, 60 eggplant fruits were used for each treatment. All samples were then subjected to room temperature (25°C with 80%–85% relative humidity) storage. Fifteen fruits were sampled randomly on each sampling day.
2.2. Browning index detection
Fruit flesh browning was measured as previously described (Kaushik, 2019). The parameters L*, a*, and b* were measured using a Cr‐400 Chroma Meter (Konica Minolta, Japan). The parameters L*, a*, and b* were measured 10 min after the fruit was cut, with 5 fruits per treatment. The value of the browning index (BI) was determined as previously described by using the values of L*, a*, and b* (Palou et al., 1999).
2.3. Total calcium content detection
The total calcium content was measured as described previously (Codling et al., 2007; Sun et al., 2020). Over dried fruit tissue (1 g) from 5 fruits was used to determine the calcium content using an Optima 4,300 DV Inductively Coupled Plasma Optical Emission Spectrometer (PerkinElmer) with strontium as an internal standard.
2.4. Determinations of storage quality
Fruits from each treatment of 15 fruits per replicate were weighed at each point. For relative electrolytic leakage detection, 15 disks from the pulp tissues of 15 fruits were obtained with a 1 cm‐diameter puncher and incubated in 50 ml ddH2O. The electrolytic leakage was first measured at 25°C. Then, the solutions were transported to boiling water for 20 min, and the electrolytic leakage was measured after quick cooling.
After removing the 2 mm‐thick peel, a pressure tester (Effegi Model FT32, Italy) with a 12 mm tapered probe was used to measure the firmness of 15 fruits in each replicate. The maximum force was recorded and expressed in newtons (N).
2.5. Determinations of total phenolic and antioxidant metabolite contents
The total phenolic content was detected as previously described (Habibi & Ramezanian, 2017; Shao et al., 2020). One gram of pulp tissue from 5 fruits was ground in liquid nitrogen. Phenolic compounds were extracted in 50 ml of methanol containing 1% (V/V) HCl for 20 min at 4°C in darkness. After centrifugation at 12,000 g at 4°C for 15 min, the absorbance of the supernatant at 280 nm was detected using a spectrophotometer (UV‐1800, MAPADA). Gallic acid was used to construct a standard curve.
H2O2, O2 ‐, CAT, PPO, POD, SOD, and AsA detection assays were performed according to the manufacturer's instructions (Comin).
2.6. Statistical analysis
Three biological replicates were performed in each experiment. The experimental data are presented as the means ± standard deviations of three independent replicates. Data were analyzed via analysis of variance (ANOVA), and mean values were compared by Tukey's multiple range test (p < .05). All statistical analyses were performed using SPSS18 statistical software package (IBM SPSS Statistics).
3. RESULTS
3.1. Effect of different calcium concentrations on eggplant under storage
“Heilong” eggplant (Solanum melongena L.) fruits were treated with different concentrations of CaCl2. As shown in Figure 1, treatment with 1%–3% CaCl2 significantly decreased the values of L*, b*, and the browning index (BI) and increased the value of a* . As the Ca2+ concentration increased (2%–4%), the values of L*, b*, and BI increased (Figure 1). At these concentrations, the effect of 2% Ca2+ treatment was the most significant. The BI of 2% Ca2+‐treated eggplant fruits was 53.38% that of H2O‐treated fruits. However, the BI of 4% Ca2+‐treated fruits was not significantly higher than that of H2O‐treated fruits.
FIGURE 1.
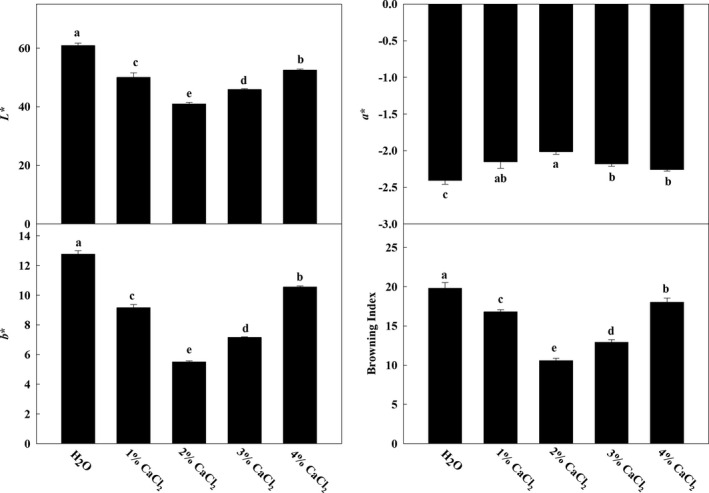
The L*, a*, b*, and browning index (BI) of eggplant fruits with different concentrations of calcium. Data are means of three replicates with SD. Different letter indicated significant differences, according to one‐way ANOVA and Tukey's multiple range tests (p < .05)
3.2. Phenotype of 2% calcium‐treated fruits and endogenous calcium content
Because the effect of the 2% calcium treatment was the most significant among the different calcium concentrations, we chose 2% Ca2+ for further investigation. As shown in Figure 2 2% Ca2+ significantly delayed the browning and softening of the fruits (Figure 2a). The L*, b*, and BI values of calcium‐treated fruits were significantly lower than those of H2O‐treated fruits (Figure 2c,e,f) at 4 and 6 days post‐treatment (dpt). To examine whether exogenous Ca2+ application increased the fruit total calcium content, we also detected the total calcium content in eggplant fruits during storage. As shown in Figure 2b, the calcium content in the treated fruits was significantly higher than that in the H2O‐treated fruits during storage. The calcium content of treated fruits was more than 65% higher than that of H2O‐treated fruits.
FIGURE 2.
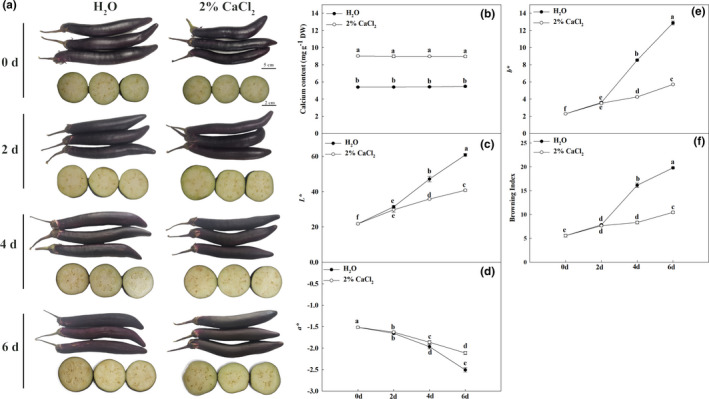
Calcium treatment delayed the browning of eggplant during storage. (a) Phenotypes of H2O‐ and calcium‐treated fruits. Upper part: the appearance of H2O‐ and calcium‐treated fruits, bar = 5 cm. Lower part: the browning phenotypes of transection of H2O‐ and calcium‐treated fruits, bar = 2 cm. (b) The calcium content in H2O‐ and calcium‐treated fruits. (c) The L* in H2O‐ and calcium‐treated fruits. (d) The a* in H2O‐ and calcium‐treated fruits. (e) The b* in H2O‐ and calcium‐treated fruits. (f) The BI in H2O‐ and calcium‐treated fruits. Data are means of three replicates with SD. Asterisks denote statistically significant differences between calcium‐ and H2O‐treated fruits (p < .05, ANOVA)
3.3. Effect of calcium on storage quality in eggplant fruits
It has been well established that weight loss is an important marker of the storage quality of horticultural products (Gao et al., 2015). Here, our data showed that weight loss in all treated eggplant fruits increased during storage (Figure 3). However, calcium treatment significantly suppressed this increase. The water loss from calcium‐treated fruits was 76.27% that of H2O‐treated fruits at 6 dpt (Figure 3). The cell membrane is damaged first during storage. Relative electrolytic leakage (REL) is an important indicator of integrality of the cell membrane. In the present study, the REL gradually increased during storage. However, calcium significantly delayed the increase in REL during storage. At 6 dpt, the REL of H2O‐treated fruits was 37.49% higher than that of calcium‐treated fruits (Figure 3). Moreover, calcium treatment delayed the reduction of the firmness of eggplant fruits during storage (Figure 3). These results suggested that calcium treatment maintains the storage quality of eggplant fruits during storage.
FIGURE 3.
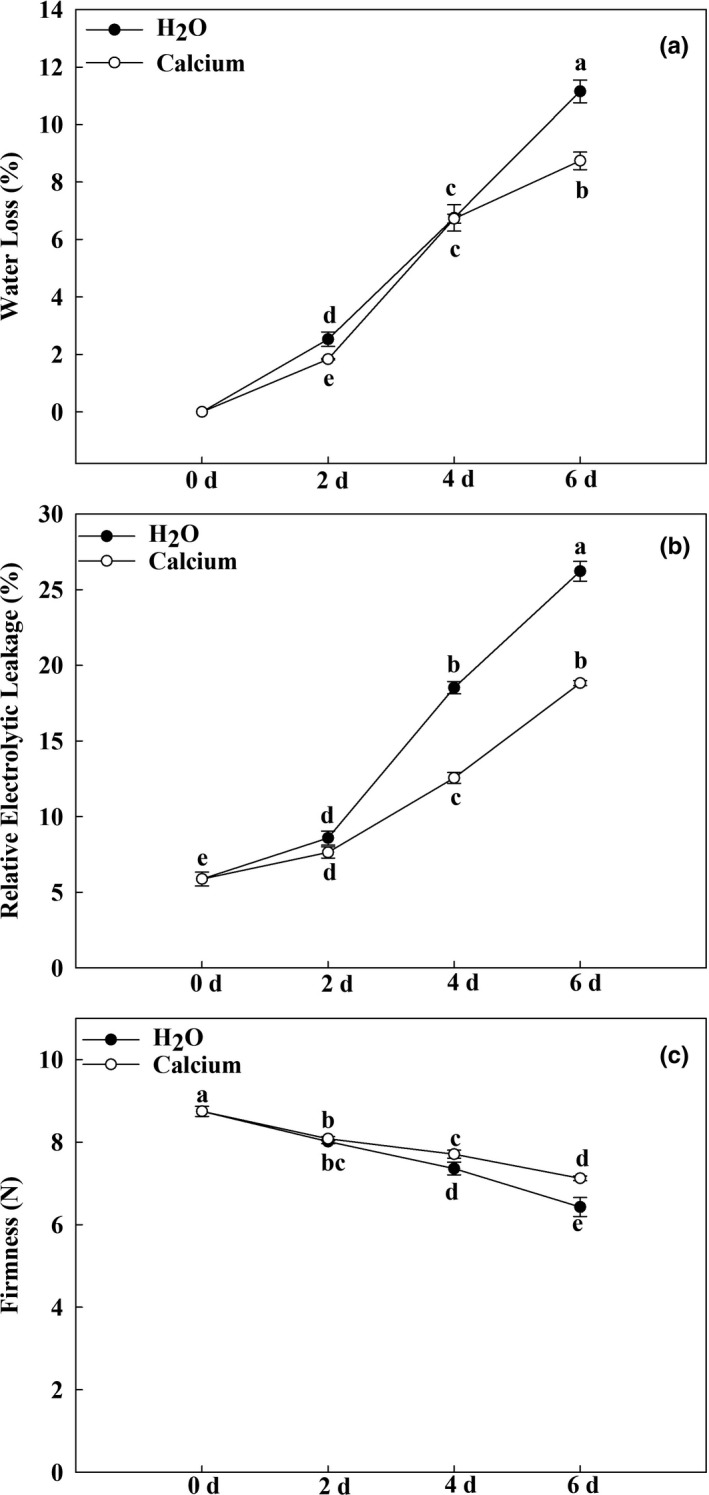
Changes in the storage quality of eggplant fruits during storage. Changes in water loss (a), relative electrolytic leakage (b), and firmness (c) during storage. Data are means of three replicates with SD. Different letter indicated significant differences, according to one‐way ANOVA and Tukey's multiple range tests (p < .05)
3.4. Effect of calcium on the activity of PPO and the phenolic content in eggplant fruits
As shown in Figure 2, calcium significantly delayed the browning of fruits. Many studies have reported that PPO and phenolics play an important role in the browning of fruits (Concellón et al., 2004; Maioli et al., 2020). Here, we detected the activity of PPO and the level of total phenolics after calcium treatment during eggplant fruit storage. As shown in Figure 4, the PPO activity of calcium‐treated fruits was lower than that of H2O‐treated fruits. Decreased total phenolic contents were detected in all treatments during storage. However, calcium treatment delayed the decrease in total phenolics, especially at 6 dpt (Figure 4). These results indicated that calcium delayed the increase in PPO activity and decrease in total phenolic production, which resulted in a reduction in browning.
FIGURE 4.
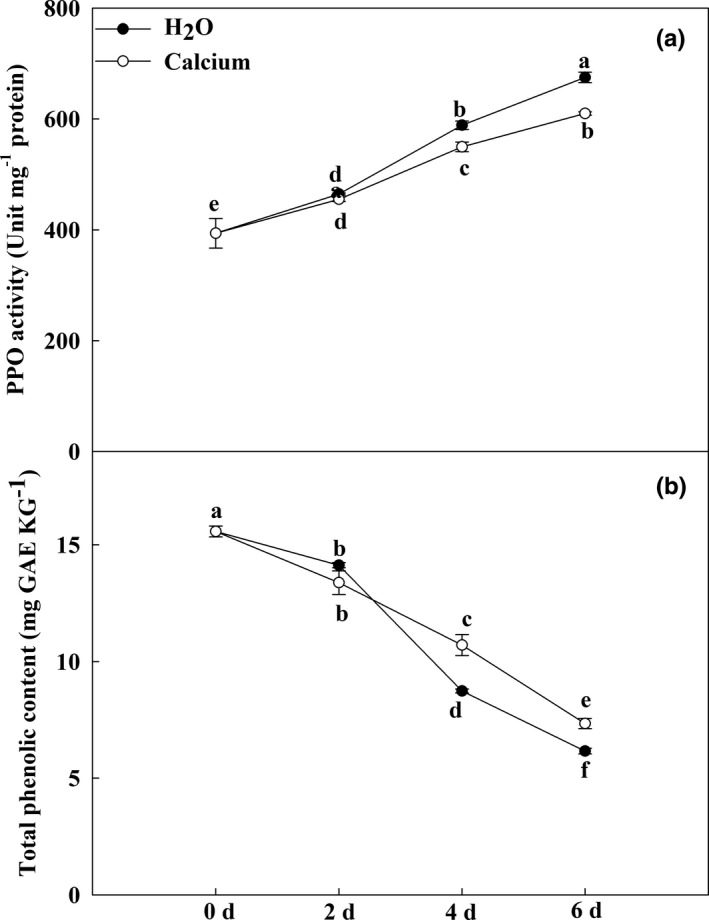
Changes in the total phenolic content and PPO activity during storage. (a) Changes in PPO activity in H2O‐ and calcium‐treated fruits during storage. (b) Changes in total phenolic content in H2O‐ and calcium‐treated fruits during storage. Data are means of three replicates with SD. Different letter indicated significant differences, according to one‐way ANOVA and Tukey's multiple range tests (p < .05)
3.5. Effect of calcium on antioxidant system activity in eggplant fruits
ROS scavenging systems play a key role in reducing ROS and stabilizing the cell membrane structure during fruit storage (Hodges et al., 2004; Shao et al., 2020). To analyze the oxidation status during storage, we examined the contents of H2O2 and O2 −, which are two major stable ROS. As shown in Figure 5, the H2O2 and O2 − levels gradually increased during storage. However, calcium treatment obviously delayed this increase. At 6 dpt, the H2O2 and O2 − contents of calcium‐treated fruits were significantly lower than those of H2O‐treated fruits (Figure 5). We also detected the activities of peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD). After calcium treatment, the activities of POD, CAT, and SOD were significantly higher than those under H2O treatment. For example, the activity of CAT in calcium‐treated fruits was 1.28‐fold that in H2O‐treated fruits at 6 dpt. In addition, ascorbic acid (AsA) plays a key role in the nonenzymatic antioxidant system (Gallie, 2013; Sun et al., 2018). We also analyzed the change in AsA content during eggplant fruit storage. The AsA level was elevated under all treatments during storage. The AsA content under calcium treatment was significantly higher than that under H2O treatment at 6 dpt. These results suggest that calcium treatment improves the ability to produce and maintain higher levels of beneficial antioxidants during storage.
FIGURE 5.
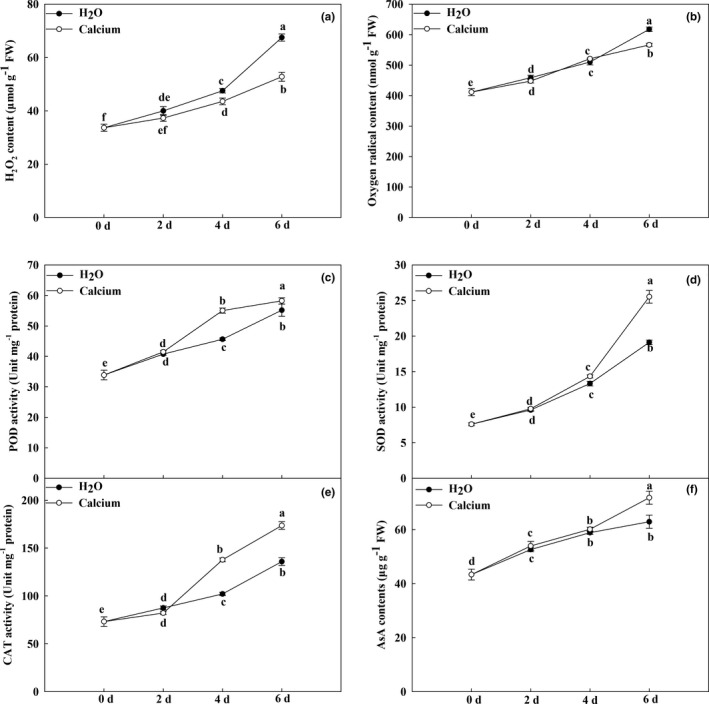
Changes in ROS levels and antioxidant activities during storage. Changes in H2O2 (a), O2 − (b), and AsA (f) content during storage. Change in POD (c), SOD (d), and CAT (e) activities during storage. Data are means of three replicates with SD. Different letter indicated significant differences, according to one‐way ANOVA and Tukey's multiple range tests (p < .05)
4. DISCUSSION
Calcium was established to play a key role in horticultural fruit storage (Elbagoury et al., 2021; X. Kou et al., 2015). However, there are few studies about the effects of calcium application on eggplant fruits. In the present research, we sprayed 0%–4% CaCl2 on eggplant fruits to detect the effects of calcium on fruit storage quality. We found that 2% CaCl2 application significantly alleviated the browning of eggplant fruits. The calcium‐treated fruits had significantly higher calcium content and lower BI, water loss, and REL values during storage. The lower BI in calcium‐treated fruits may result from a higher phenolic content and lower POD activity. Fewer ROS and enhanced antioxidative activity were detected in the calcium‐treated fruits during storage. These results indicated that exogenous calcium application maintained higher storage quality of eggplant fruits.
Previous studies have shown that an appropriate calcium concentration is beneficial to plant development and adaptation to stress, but excessive calcium application may disrupt the normal metabolism of plants (L. Kou et al., 2014; Sun et al., 2020). A consistent phenotype was observed in our research. The 3%–4% CaCl2 treatment showed a decreased effect on the BI of eggplant fruits at 6 dpt (Figure 1), indicating that the effect of exogenous calcium on fruit browning is dose‐dependent. As a secondary messenger, calcium transmits signals received from the cell surface to the cell interior by changing the cytoplasmic concentration, thereby participating in multiple cellular processes, which are decoded by a series of Ca2+ sensors (Ranty et al., 2016; Yang & Poovaiah, 2003). Under normal conditions, the intracellular calcium concentration can be well controlled by the mechanism of calcium inflow and outflow in the cell membrane, but a high dose of calcium affects the balance of calcium inflow and outflow, leading to intracellular calcium disorder (Kudla et al., 2010; Steinhorst & Kudla, 2014). These uncontrolled calcium disorders ultimately lead to cell damage.
Phenolics are localized in vacuoles and participate in the browning of eggplant (Holderbaum et al., 2010; Mishra et al., 2012). In a previous study, exogenous calcium alleviated pericarp browning of pears in cold storage (Li et al., 2020). This delayed browning may result from increased endogenous γ‐aminobutyrate (GABA) content, GABA‐related gene expression, and enzyme activity (Li et al., 2020). In addition, calcium treatment reduced the brown spots of pear fruits under cold storage by inhibiting PPO and POD activities and delaying phenolic compound losses (X. Kou et al., 2015). These results indicated that calcium could delay fruit browning by inhibiting PPO activity and phenolic decreases. In our study, the content of phenolics gradually decreased during storage, and calcium treatment significantly delayed this decrease. This suggested that calcium treatment suppressed the decrease in phenolic compounds and fruit browning. This delayed browning may be due to the lower activity of PPO (Figure 4), which could catalyze the phenolic compounds into highly reactive quinones by its oxidizability (González et al., 2019; Plazas et al., 2013).
During fruit storage, ROS were stimulated when the plant cells suffered stress. Extremely high levels of intracellular ROS can damage various components of the cell or activate specific signaling pathways that remove ROS before they can cause cell damage (Asensio et al., 2012). Kou et al. found that the activities of CAT and SOD in exogenous calcium‐treated pear fruits were significantly higher than those in the control treated pear fruits (X. Kou et al., 2015). In fresh fruits and vegetables, the higher activities of enzymes may inhibit the accumulation of ROS, stabilize the cell membrane, and reduce phenolic oxidation by ROS (Li et al., 2020; Moon et al., 2020). Thus, the resulting lower level of ROS may result in delayed browning of pear fruits. Here, less H2O2 and O2 − accumulated in the calcium‐treated fruits than in other fruits during storage. Moreover, the activities of the enzymatic ROS scavenging antioxidants CAT, POD, and SOD were significantly higher in calcium‐treated fruits than in H2O‐treated fruits. Moreover, the level of the nonenzymatic ROS scavenging antioxidant AsA was also significantly higher in calcium‐treated fruits than in H2O‐treated fruits. These results indicated that calcium may elevate the ROS scavenging system to protect cells from oxidative damage.
5. CONCLUSION
Exogenous calcium application delayed browning and maintained the quality of eggplant fruits during storage. The lower BI may have resulted from a higher phenolic content and lower POD activity. Higher calcium contents and firmness were detected after calcium treatment. The REL and water loss were suppressed by calcium treatment. Moreover, the calcium‐treated fruits accumulated lower levels of ROS and showed higher SOD, POD, and CAT activities. Additionally, the AsA level was higher in calcium‐treated fruits than in H2O‐treated fruits. These results provide further insight into the function of calcium in eggplant fruits storage. Thus, spray application of exogenous calcium onto eggplant fruits can be used to maintain storage quality.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTION
Qiuyan Ban: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Funding acquisition (lead); Investigation (lead); Software (lead); Writing‐original draft (lead); Writing‐review & editing (lead). Tongjin Liu: Data curation (supporting); Formal analysis (supporting). Kun Ning: Data curation (supporting); Formal analysis (supporting). Junjun Fan: Data curation (supporting); Formal analysis (supporting); Writing‐original draft (supporting). Qunxiang Cui: Data curation (supporting); Formal analysis (supporting); Investigation (supporting). Yanle Guo: Data curation (supporting); Formal analysis (supporting). Xueming Zai: Data curation (supporting); Formal analysis (supporting).
ETHICAL APPROVAL
Ethics approval was not required for this research.
ACKNOWLEDGMENT
This work was supported by the Scientific Research Programs for High‐level Talents Start‐up Fund of Jinling Institute of Technology (jit‐b‐202002) and the Research Foundation Incubation Project of Jinling Institute of Technology (jit‐fhxm‐202022).
Ban, Q. , Liu, T. , Ning, K. , Fan, J. , Cui, Q. , Guo, Y. , & Zai, X. (2021). Effect of calcium treatment on the browning of harvested eggplant fruits and its relation to the metabolisms of reactive oxygen species (ROS) and phenolics. Food Science & Nutrition, 9, 5567–5574. 10.1002/fsn3.2517
REFERENCES
- Aldon, D. , Mbengue, M. , Mazars, C. , & Galaud, J. P. (2018). Calcium signalling in plant biotic interactions. International Journal of Molecular Sciences, 19(3), 665. 10.3390/ijms19030665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio, A. C. , Gil‐Monreal, M. , Pires, L. , Gogorcena, Y. , Aparicio‐Tejo, P. M. , & Moran, J. F. (2012). Two Fe‐superoxide dismutase families respond differently to stress and senescence in legumes. Journal of Plant Physiology, 169(13), 1253–1260. 10.1016/j.jplph.2012.04.019 [DOI] [PubMed] [Google Scholar]
- Codling, E. E. , Mulchi, C. L. , & Chaney, R. L. (2007). Grain yield and mineral element composition of maize grown on high phosphorus soils amended with water treatment residual. Journal of Plant Nutrition, 30(2), 225–240. 10.1080/01904160601117937 [DOI] [Google Scholar]
- Concellón A., CAñón M., & Chaves A. R. (2004). Characterization and changes in polyphenol oxidase from eggplant fruit (Solanum melongena L.) during storage at low temperature. Food Chemistry, 88(1), 17–24. [Google Scholar]
- Das, S. , Raychaudhuri, U. , Falchi, M. , Bertelli, A. , Braga, P. C. , & Das, D. K. (2011). Cardioprotective properties of raw and cooked eggplant (Solanum melongena L). Food Funct, 2(7), 395–399. 10.1039/c1fo10048c [DOI] [PubMed] [Google Scholar]
- Elbagoury, M. M. , Turoop, L. , Runo, S. , & Sila, D. N. (2021). Regulatory influences of methyl jasmonate and calcium chloride on chilling injury of banana fruit during cold storage and ripening. Food Sciences and Nutrition, 9(2), 929–942. 10.1002/fsn3.2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie, D. R. (2013). The role of L‐ascorbic acid recycling in responding to environmental stress and in promoting plant growth. Journal of Experimental Botany, 64(2), 433–443. 10.1093/jxb/ers330 [DOI] [PubMed] [Google Scholar]
- Gao, H. , Kang, L. , Liu, Q. , Cheng, N. , Wang, B. , & Cao, W. (2015). Effect of 24‐epibrassinolide treatment on the metabolism of eggplant fruits in relation to development of pulp browning under chilling stress. Journal of Food Science and Technology, 52(6), 3394–3401. 10.1007/s13197-014-1402-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- González, M. N. , Massa, G. A. , Andersson, M. , Turesson, H. , Olsson, N. , Fält, A.‐S. , Storani, L. , Décima Oneto, C. A. , Hofvander, P. , & Feingold, S. E. (2019). Reduced enzymatic browning in potato tubers by specific editing of a polyphenol oxidase gene via ribonucleoprotein complexes delivery of the CRISPR/Cas9 system. Frontiers in Plant Science, 10, 1649. 10.3389/fpls.2019.01649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi, F. , & Ramezanian, A. (2017). Vacuum infiltration of putrescine enhances bioactive compounds and maintains quality of blood orange during cold storage. Food Chemistry, 227, 1–8. 10.1016/j.foodchem.2017.01.057 [DOI] [PubMed] [Google Scholar]
- Hodges, D. M. , Lester, G. E. , Munro, K. D. , & Toivonen, P. M. A. (2004). Oxidative stress: Importance for postharvest quality. Horticultural Science, 39(5), 924–929. [Google Scholar]
- Holderbaum, D. , Kon, T. , Kudo, T. , & Guerra, M. (2010). Enzymatic browning, polyphenol oxidase activity, and polyphenols in four apple cultivars: Dynamics during fruit development. Horticultural Science, 45, 1150–1154. 10.21273/HORTSCI.45.8.1150 [DOI] [Google Scholar]
- Kaushik, P. (2019). Genetic analysis for fruit phenolics content, flesh color, and browning related traits in eggplant (Solanum melongena). International Journal of Molecular Sciences, 20(12), 2990. 10.3390/ijms20122990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik, P. , Andújar, I. , Vilanova, S. , Plazas, M. , Gramazio, P. , Herraiz, F. , Brar, N. , & Prohens, J. (2015). Breeding vegetables with increased content in bioactive phenolic acids. Molecules, 20(10), 18464–18481. 10.3390/molecules201018464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou, L. , Yang, T. , Luo, Y. , Liu, X. , Huang, L. , & Codling, E. (2014). Pre‐harvest calcium application increases biomass and delays senescence of broccoli microgreens. Postharvest Biology and Technology, 87, 70–78. 10.1016/j.postharvbio.2013.08.004 [DOI] [Google Scholar]
- Kou, X. , Wu, M. , Li, L. , Wang, S. , Xue, Z. , Liu, B. , & Fei, Y. (2015). Effects of CaCl2 dipping and pullulan coating on the development of brown spot on ‘Huangguan’ pears during cold storage. Postharvest Biology and Technology, 99, 63–72. 10.1016/j.postharvbio.2014.08.001 [DOI] [Google Scholar]
- Kudla, J. , Batistic, O. , & Hashimoto, K. (2010). Calcium signals: The lead currency of plant information processing. The Plant Cell, 22(3), 541–563. 10.1105/tpc.109.072686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Zhou, Q. , Zhou, X. , Wei, B. , Zhao, Y. , & Ji, S. (2020). Calcium treatment alleviates pericarp browning of 'Nanguo' pears by regulating the GABA shunt after cold storage. Frontiers in Plant Science, 11, 580986. 10.3389/fpls.2020.580986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maioli, A. , Gianoglio, S. , Moglia, A. , Acquadro, A. , Valentino, D. , Milani, A. M. , Prohens, J. , Orzaez, D. , Granell, A. , Lanteri, S. , & Comino, C. (2020). Simultaneous CRISPR/Cas9 editing of three PPO genes reduces fruit flesh browning in Solanum melongena L. Frontiers in Plant Science, 11, 607161. 10.3389/fpls.2020.607161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganaris, G. A. , Vasilakakis, M. , Diamantidis, G. , & Mignani, I. (2007). The effect of postharvest calcium application on tissue calcium concentration, quality attributes, incidence of flesh browning and cell wall physicochemical aspects of peach fruits. Food Chemistry, 100(4), 1385–1392. 10.1016/j.foodchem.2005.11.036 [DOI] [Google Scholar]
- Mishra, B. B. , Gautam, S. , & Sharma, A. (2012). Purification and characterisation of polyphenol oxidase (PPO) from eggplant (Solanum melongena). Food Chemistry, 134(4), 1855–1861. 10.1016/j.foodchem.2012.03.098 [DOI] [PubMed] [Google Scholar]
- Moon, K. M. , Kwon, E. B. , Lee, B. , & Kim, C. Y. (2020). Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules, 25(12), 2754. 10.3390/molecules25122754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir Khan, M. , Siddiqui, M. H. , Mohammad, F. , Naeem, M. , & Khan, M. M. A. (2009). Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiologiae Plantarum, 32(1), 121. 10.1007/s11738-009-0387-z [DOI] [Google Scholar]
- Palou, E. , López‐Malo, A. , Barbosa‐Cánovas, G. V. , Welti‐Chanes, J. , & Swanson, B. G. (1999). Polyphenoloxidase activity and color of blanched and high hydrostatic pressure treated banana puree. Journal of Food Science, 64(1), 42–45. 10.1111/j.1365-2621.1999.tb09857.x [DOI] [Google Scholar]
- Plazas, M. , López‐Gresa, M. P. , Vilanova, S. , Torres, C. , Hurtado, M. , Gramazio, P. , Andújar, I. , Herráiz, F. J. , Bellés, J. M. , & Prohens, J. (2013). Diversity and relationships in key traits for functional and apparent quality in a collection of eggplant: Fruit phenolics content, antioxidant activity, polyphenol oxidase activity, and browning. Journal of Agricultural and Food Chemistry, 61(37), 8871–8879. 10.1021/jf402429k [DOI] [PubMed] [Google Scholar]
- Ranty, B. , Aldon, D. , Cotelle, V. , Galaud, J. P. , Thuleau, P. , & Mazars, C. (2016). Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Frontiers in Plant Science, 7, 327. 10.3389/fpls.2016.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno, L. , Modica, M. N. , Pittalà, V. , Romeo, G. , Siracusa, M. A. , Di Giacomo, C. , & Acquaviva, R. (2014). Antioxidant activity and phenolic content of microwave‐assisted Solanum melongena extracts. Scientific World Journal, 2014, 719486, 10.1155/2014/719486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, Y. , Jiang, Z. , Zeng, J. , Li, W. , & Dong, Y. (2020). Effect of ethanol fumigation on pericarp browning associated with phenol metabolism, storage quality, and antioxidant systems of wampee fruit during cold storage. Food Sciences and Nutrition, 8(7), 3380–3388. 10.1002/fsn3.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhorst, L. , & Kudla, J. (2014). Signaling in cells and organisms‐calcium holds the line. Current Opinion in Plant Biology, 22, 14–21. 10.1016/j.pbi.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Sun, X. , Pan, B. , Wang, Y. , Xu, W. , & Zhang, S. (2020). Exogenous calcium improved resistance to Botryosphaeria dothidea by increasing autophagy activity and salicylic acid level in pear. Molecular Plant‐Microbe Interactions, 33(9), 1150–1160. 10.1094/mpmi-04-20-0101-r [DOI] [PubMed] [Google Scholar]
- Sun, X. , Wang, P. , Jia, X. , Huo, L. , Che, R. , & Ma, F. (2018). Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnology Journal, 16(2), 545–557. 10.1111/pbi.12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranto, F. , Pasqualone, A. , Mangini, G. , Tripodi, P. , Miazzi, M. M. , Pavan, S. , & Montemurro, C. (2017). Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. International Journal of Molecular Sciences, 18(2), 377. 10.3390/ijms18020377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppino, L. , Barchi, L. , Lo Scalzo, R. , Palazzolo, E. , Francese, G. , Fibiani, M. , D'Alessandro, A. , Papa, V. , Laudicina, V. A. , Sabatino, L. , Pulcini, L. , Sala, T. , Acciarri, N. , Portis, E. , Lanteri, S. , Mennella, G. , & Rotino, G. L. (2016). Mapping quantitative trait loci affecting biochemical and morphological fruit properties in eggplant (Solanum melongena L.). Frontiers . Plant Science, 7, 256. 10.3389/fpls.2016.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Xie, X. , & Long, L. E. (2014). The effect of postharvest calcium application in hydro‐cooling water on tissue calcium content, biochemical changes, and quality attributes of sweet cherry fruit. Food Chemistry, 160, 22–30. 10.1016/j.foodchem.2014.03.073 [DOI] [PubMed] [Google Scholar]
- Xiong, T. , Tan, Q. , Li, S. , Mazars, C. , Galaud, J. P. , & Zhu, X. (2021). Interactions between calcium and ABA signaling pathways in the regulation of fruit ripening. Journal of Plant Physiology, 256, 153309. 10.1016/j.jplph.2020.153309 [DOI] [PubMed] [Google Scholar]
- Yang, T. , & Poovaiah, B. W. (2003). Calcium/calmodulin‐mediated signal network in plants. Trends in Plant Science, 8(10), 505–512. 10.1016/j.tplants.2003.09.004 [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Huber, D. J. , Qu, H. , Yun, Z. E. , Wang, H. , Huang, Z. , Huang, H. , & Jiang, Y. (2015). Enzymatic browning and antioxidant activities in harvested litchi fruit as influenced by apple polyphenols. Food Chemistry, 171, 191–199. 10.1016/j.foodchem.2014.09.001 [DOI] [PubMed] [Google Scholar]


