Abstract
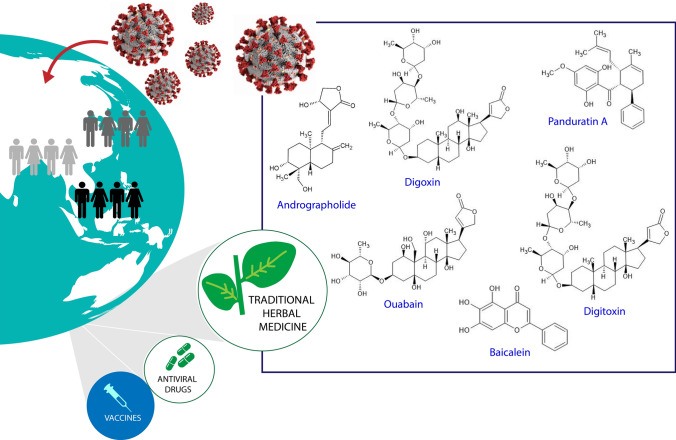
The outbreak of COVID-19 disease has led to a search for effective vaccines or drugs. However, insufficient vaccine supplies to meet global demand and no effective approved prescribed drugs for COVID-19 have led some people to consider the use of alternative or complementary medicines, such as traditional herbal medicine. Medicinal plants have various therapeutic properties that depend on the active compounds they contain. Obviously, herbal medicine has had an essential role in treatment and prevention during COVID-19 outbreak, especially in Asian cultures. Hence, we reviewed the uses of herbal medicine in Asian cultures and described the prominent families and species that are sources of antiviral agents against COVID-19 on the basis of case reports, community surveys, and guidelines available in the literature databases. Antiviral efficacy as determined in laboratory testing was assessed, and several promising active compounds with their molecular targets in cell models against SARS-CoV-2 viral infection will be discussed. Our review findings revealed the highly frequent use of Lamiaceae family members, Zingiber officinale, and Glycyrrhiza spp. as medicinal sources for treatment of COVID-19. In addition, several plant bioactive compounds derived from traditional herbal medicine, including andrographolide, panduratin A, baicalein, digoxin, and digitoxin, have shown potent SARS-CoV-2 antiviral activity as compared with some repurposed FDA-approved drugs. These commonly used plants and promising compounds are recommended for further exploration of their safety and efficacy against COVID-19.
Graphic abstract
Keywords: COVID-19, Asian, Herbal medicine, Plant bioactive compounds, Treatment, Prevention
Background
An outbreak of coronavirus 19 (COVID-19) disease caused by the beta-coronavirus, severe acute respiratory syndrome 2 (SARS-CoV-2), was first reported in late December 2019 in Wuhan, China and continued to rapidly spread worldwide. The angiotensin-converting enzyme 2 (ACE2) receptor, which is known to be an entry point of SARS-CoV-2, is widely presented on lung alveolar cells but also can be found in other organs’ cells, such as the upper esophagus, small intestine, colon, bile, heart, kidney, and bladder; hence, this virus can attack various organs and cause damage [1, 2]. Penetration by SARS-CoV-2 stimulates the immune response leading to production of various cytokines, which generates a cytokines storm that triggers disease symptoms. Depending on the cytokines produced, COVID-19 symptoms can be mild, moderate, or severe [2]. Severe disease is characterized by progressive lung damage, over-inflammation, pulmonary tissue edema, vascular leakage, coagulation, and endotheliitis (vascular inflammation). The cytokine storms in the most severely affected COVID-19 patients are very destructive and cause endothelial dysfunction, inflammation, and vasodilatation of pulmonary capillaries. Furthermore, this process triggers alveolar disfunction, acute respiratory distress syndrome with hypoxic respiratory, and multiple-organ failure, resulting in death [3, 4].
A global effort to develop effective vaccines or therapeutic drugs for COVID-19 disease is urgently needed for combating the pandemic. Hence, several approaches have been adopted, including drug repurposing and vaccine development. In drug repurposing, various existing drugs, such as chloroquine, hydroxychloroquine, ivermectin, camostat mesylate, lopinavir, ritonavir, remdesivir, and favipiravir, have been tested in clinical trials [5]. In vaccine development, numerous pharmaceutical/academic developers have developed COVID-19 vaccine candidates using various platforms, such as live-attenuated virus (e.g., Codagenix [6]), inactivated virus (e.g., Sinovac [7], Sinopharm [8]); mRNA (e.g., Pfizer [9] Moderna [10], ChulaCov19 [11], and Curevac [12]); DNA (e.g., COVIGEN [13], Inovio [14]); non-replicating viral vectors (e.g., AstraZeneca [15], Johnson & Johnson [16]); and protein sub-units (e.g., Novavax[17]), and conducted clinical trials [18]. The World Health Organization (WHO) has approved several vaccines for emergency use, including AZD1222/AstraZeneca, Janssen/Johnson & Johnson mRNA 1273/Moderna, Sinopharm, and Sinovac-CoronaVac, since 2020 [19]. The rapid transmission of COVID-19 disease along with insufficient vaccine supplies for health care workers and the absence of specific and effective prescribed drug treatments for COVID-19 patients [20] have led to some people taking herbal medicines for prevention or treatment of this disease.
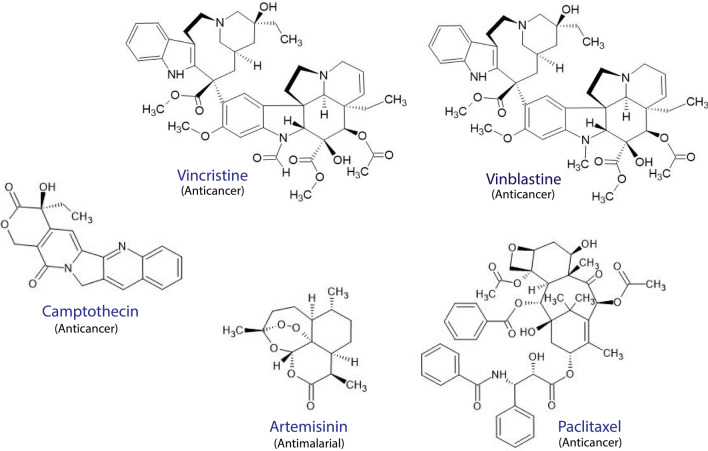
Herbal medicine has been used in complementary medicine in various cultures for at least 1000 years and still has an important role in health care systems currently. The extensive use of plant sources of medicines with proven therapeutic ability for treating various diseases has led to more plant-based drug discoveries of numerous effective drugs such as vincristine, vinblastine, paclitaxel, and camptothecin (Fig. 1) [21]. Promising compounds, such as psoralens, guggulsterons, piperidines, phyllanthins, picrosides, curcuminoids, withanolides, steroidal lactones, and glycosides, have been discovered from traditional Ayuverdic medicine [22]. In addition, the discovery of the potent antimalarial drug, artemisinin (Fig. 1), has been attributed to the historical use of the plant Artemisia annua in Traditional Chinese Medicine (TCM) [23].
Fig. 1.
Several effective modern drugs derived from traditional herbal medicine
The perception that many herbal medicines have shown promising efficacy, safety, accessibility, environmental friendliness, and affordability has led to increased attention on traditional medicines in recent years. The efficacy of traditional herbal medicines for treating various diseases is due to the pharmacological activity of the active compounds in the plants [24]. Obviously, herbal medicine also plays essentially for prevention and treatment of COVID-19 disease in various cultures. Herbal medicines may act directly or indirectly by attacking the virus, viral–host interaction, signaling pathways, host receptors, molecular targets, immune system, and microenvironments. In TCM, herbal medicines alone or in combination with modern synthetic drugs are prescribed for COVID-19 patients [4]. The Qingfei Paidu decoction, which contains several herbs, has been used for treatment of COVID-19 patients in China [24]. Lianhua–Qingwen, Shufeng Jiedu, Huoxiang Zhengqi, and Jinhua Qinggan herbal formulas also have been used in COVID-19 treatment in TCM [25]. Herbal medicines are widely used, mostly in China and other Asian countries, such as Japan, Korea, Thailand, Vietnam, India, Bangladesh, and Indonesia. Accordingly, the aim of this review article was to describe various medicinal plants used in COVID-19 prevention and treatment from various countries in Asia as obtained from a literature search and discuss the efficacy results from laboratory testing. Additionally, the antiviral activities of natural products isolated from medicinal plants against SARS-CoV-2 are discussed.
Use of traditional herbal medicine during the COVID-19 pandemic and its antiviral activity against SARS-CoV-2
The plants identified as having been used for treatment and prevention of COVID-19 diseases were extracted from literature searches of the ScienceDirect, PubMed, Scopus, and Google Scholar databases from 28th May 2021 until 20th August 2021. Plant data were retrieved from published articles about community surveys, case reports, and relevant articles that described the use of herbal medicines in Asian communities during the COVID-19 pandemic for both prevention and treatment (Tables 1, 2). The species and families of plant taxa were confirmed and verified using World Flora Online database (http://www.worldfloraonline.org/), a specified plant species database maintained by the Royal Botanic Gardens and Kew and Missouri Botanical Gardens. A number of 91 plant taxa were obtained after extracting the data and then further analyzed to determine the most frequently used families and species for treatment and prevention of COVID-19.
Table 1.
Herbal medicines used during the COVID-19 pandemic based on guidelines, community surveys, and case reports
| Family | Species | Country origin | References |
|---|---|---|---|
| Amaryllidaceae | Allium sativum | Vietnam | [51] |
| Apiaceae | Centella asiatica | Vietnam | [51] |
| Apiaceae | Saposhnikovia divaricata | China | [52] |
| Apiaceae | Glehnia spp. | China | [52] |
| Araliaceae | Panax ginseng | Vietnam | [51] |
| Asparagaceae | Ophiopogon spp. | China | [52] |
| Brassicaceae | Isatis indigotica | China | [52] |
| Campanulaceae | Platycodon grandiflorus | China | [52] |
| Caprifoliaceae | Lonicera japonicae | China | [52] |
| Compositae | Artemisia vulgaris | Vietnam | [51] |
| Compositae | Cynara cardunculus | Vietnam | [51] |
| Compositae | Atractylodes macrocephalae | China | [52] |
| Compositae | Atractylodes spp. | China | [52] |
| Compositae | Eupatorium spp. | China | [52] |
| Cucurbitaceae | Citrullus lanatus | Bangladesh | [53] |
| Dryopteridaceae | Cyrtomium fortune | China | [52] |
| Lamiaceae | Perilla frutescens | Vietnam | [51] |
| Lamiaceae | Plectranthus amboinicus | Vietnam | [51] |
| Lamiaceae | Elsholtzia ciliata | Vietnam | [51] |
| Lamiaceae | Ocimum tenuiflorum | India | [54] |
| Lamiaceae | Vitex negundo | Bangladesh | [53] |
| Lamiaceae | Pogostemon cablin | China | [52] |
| Lamiaceae | Perilla spp. | China | [52] |
| Leguminosae | Glycyrrhiza spp. | Vietnam | [51] |
| Leguminosae | Astragalus spp. | China | [52] |
| Leguminosae | Glycyrrhiza spp. | China | [52] |
| Menispermaceae | Tinospora cordifolia | India | [54] |
| Moraceae | Morus alba | China | [52] |
| Oleaceae | Forsythia suspensa | China | [52] |
| Phyllanthaceae | Emblica officinalis | India | [54] |
| Piperaceae | Piper lolot | Vietnam | [51] |
| Poaceae | Phragmites communis | China | [52] |
| Poaceae | Coix lacryma-jobi | China | [52] |
| Ranunculaceae | Nigella sativa | Bangladesh | [53] |
| Rutaceae | Citrus reticulata | China | [52] |
| Saururaceae | Houttuynia cordata | Vietnam | [51] |
| Xanthorrhoeaceae | Aloe vera | Vietnam | [51] |
| Zingiberaceae | Zingiber officinale | Vietnam | [51] |
| Zingiberaceae | Curcuma longa | India | [54] |
Table 2.
Use of formulated herbal medicines for prevention and treatment during the COVID-19 pandemic from community case reports and its antiviral activity against SARS-CoV-2
| Formula | Country origin | Family | Species | Usage report in community | References | Antiviral activity against SARS-CoV-2 | References |
|---|---|---|---|---|---|---|---|
| Lianhua-Qingwen | China | Oleaceae | Forsythia suspensa | Yes | [1] | In an in vitro model using infected Vero E6 cell lines with SARS-CoV-2, an herbal formula inactivated virus replication, altered virus morphology, and reduced pro-inflammatory cytokines | [1, 55, 56] |
| Caprifoliaceae | Lonicera japonica | ||||||
| Ephedraceae | Ephedra sinica | ||||||
| Dryopteridaceae | Dryopteris crassirhizoma | ||||||
| Rosaceae | Prunus armeniaca | ||||||
| Crassulaceae | Rhodiola rosea | ||||||
| Polygonaceae | Rheum palmatum | ||||||
| Saururaceae | Houttuynia cordata | ||||||
| Brassicaceae | Isatis indigotica | ||||||
| Lamiaceae | Pogostemon cablin | ||||||
| Lamiaceae | Mentha haplocalyx | ||||||
| Leguminosae | Glycyrrhiza uralensis | ||||||
| Pudilan Xiaoyan Oral Liquid (PDL) | China | Brassicaceae | Isatis indigotica | N/D | – | Inhibit SARS-CoV-2 replication in Vero E6 cells with EC50 1.078 mg/ml and in vivo study with hACE2 mice model infected SARS-CoV-2 revealed that this formula is able to relieve symptoms of pneumonia, chronic obstructive pulmonary disease, and asthma | [1, 57] |
| Compositae | Taraxacum mongolicum | ||||||
| Lamiaceae | Scutellaria baicalensis | ||||||
| Papaveraceae | Corydalis bungeana | ||||||
| Shuanghuanglian | China | Caprifoliaceae | Lonicera japonica | N/D | – | Inhibit SARS-CoV-2 in Vero E6 cells, with an EC50 0.93 µl/ml | [45] |
| Lamiaceae | Scutellaria baicalensis | ||||||
| Oleaceae | Forsythia suspense | ||||||
| Ma Xing Shi Gan | China | Rosaceae | Prunus armeniaca | Yes | [58] | N/D | – |
| Leguminosae | Glycyrrhiza spp. | ||||||
| Ephedraceae | Ephedra sp. | ||||||
| Da Yuan Yin | China | Magnoliaceae | Magnolia officinalis | Yes | [58] | N/D | – |
| Arecaceae | Areca catechu | ||||||
| Zingiberaceae | Amomum tsao-ko | ||||||
| Lamiaceae | Scutellaria baicalensis | ||||||
| Leguminosae | Glycyrrhiza uralensis | ||||||
| Asparagaceae | Anemarrhena asphodeloides | ||||||
| Dioscoreaceae | Dioscorea polystachya | ||||||
| Qing Fei Pai Du | China | Ephedraceae | Ephedra sp. | Yes | [1, 58] | N/D | – |
| Araceae | Pinellia ternata | ||||||
| Zingiberaceae | Zingiber officinale | ||||||
| Rutaceae | Citrus aurantium | ||||||
| Yu Ping Feng San | China | Leguminosae | Astragalus membranaceus | Yes | [1, 58] | N/D | – |
| Compositae | Atractylodes macrocephala | ||||||
| Apiaceae | Saposhnikovia divaricata | ||||||
| Ayush Kwath | India | Lamiaceae | Ocimum sanctum | Yes | [59] | N/D | – |
| Lauraceae | Cinnamomum zeylanicum | ||||||
| Zingiberaceae | Zingiber officinale | ||||||
| Piperaceae | Piper nigrum | ||||||
| N/D | Bangladesh | Zingiberaceae | Zingiber officinale | Yes | [60] | N/D | – |
| Myrtaceae | Syzygium aromaticum | ||||||
| Lauraceae | Cinnamomum verum | ||||||
| N/D | Bangladesh | Zingiberaceae | Zingiber officinale | Yes | [60] | N/D | – |
| Myrtaceae | Syzygium aromaticum | ||||||
| Lauraceae | Cinnamomum verum | ||||||
| Lamiaceae | Ocimum sanctum | ||||||
| Theaceae | Camellia sinensis | ||||||
| N/D | Bangladesh | Zingiberaceae | Zingiber officinale | Yes | [60] | N/D | – |
| Theaceae | Camellia sinensis | ||||||
| N/D | Bangladesh | Lamiaceae | Ocimum sanctum | Yes | [53] | N/D | – |
| Piperaceae | Piper nigrum | ||||||
| N/D | Bangladesh | Zingiberaceae | Zingiber officinale | Yes | [53] | N/D | – |
| Rutaceae | Citrus limon | ||||||
| N/D | Bangladesh | Lamiaceae | Ocimum sanctum | Yes | [53] | N/D | – |
| Lamiaceae | Vitex negundo | ||||||
| Rutaceae | Citrus limon | ||||||
| Zingberaceae | Zingiber officinale | ||||||
| Ranunculaceae | Nigella sativa |
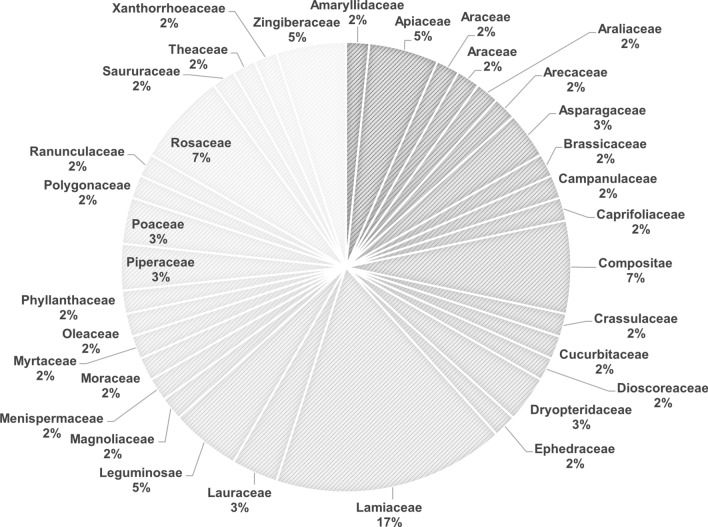
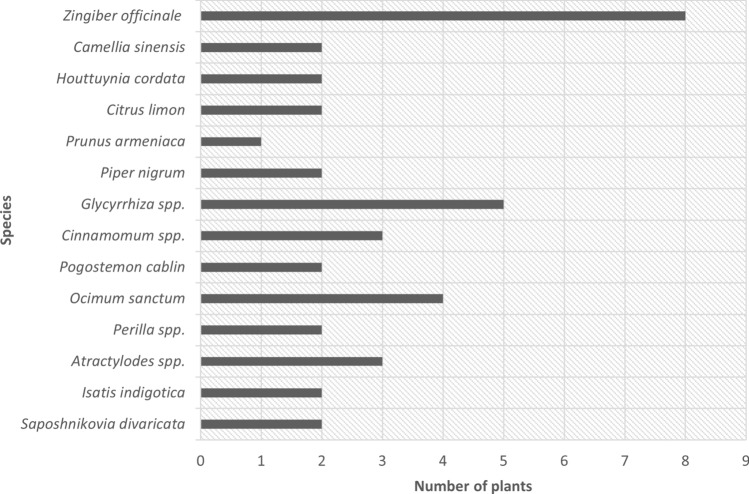
Based on the analysis of medicinal plants used in the communities across several countries in Asia, our findings showed that the Lamiaceae family of medicinal plants was the most frequently used for prevention and treatment of COVID-19 during pandemic (Fig. 2). In addition, our findings revealed that Zingiber officinale was the most frequently used species followed by Glycyrrhiza spp. and Ocimum sanctum (Fig. 3).
Fig. 2.
Frequently used families of medicinal herbs for prevention and treatment of COVID-19
Fig. 3.
Frequently used species in herbal medicines against COVID-19
Z. officinale (ginger) has been shown to be the most frequently used species in herbal medicine in communities across the countries studied. The crude extract of this herb reportedly exhibited antiviral activity against SARS-CoV-2 in a Vero E6 cell model, with IC50 of 29.19 µM. The main phenolic compound in ginger, 6-gingerol, only showed an IC50 of ≤ 100 µM [26], which suggests that the efficacy of Z. officinale may depend on mechanisms that do not involve 6-gingerol for suppressing COVID-19 symptoms, do not directly attack the virus, or that is synergistic with other herbs to achieve a better therapeutic effect. Z. officinale is a spice in which gingerols and shogaol are the active compounds thought to provide health benefits. This herb possesses a range of medicinal benefits, including antiviral activity against feline calicivirus, human respiratory syncytial virus, influenza A, and H9N2, and its activity for stimulating tumor necrosis factor alpha (TNF-α) is postulated as a first-line defense for virus infection [27].
Along with ginger, Glycyrrhiza spp. is a frequently used medicinal plant for COVID-19 treatment. This herb is mostly included in various formulas in TCM for COVID-19 treatment, such as Ma Xing Shi Gan, Da Yuan Yin, and Lianhua–Qingwen. The Lianhua–Qingwen formula contains various herbs, including Glycyrrhiza uralensis, and reportedly exhibits inhibitory activity against SARS-CoV-2 (Table 2). On the other hand, the aqueous extract of Glycyrrhiza glabra root reportedly exhibits inhibitory activity against SARS-CoV-2 infected Vero E6 cells. The extract has shown antiviral effects at 2 mg/ml, which is lower than its toxic concentration. Glycyrrhizin, as a triterpenoid glycoside, has also shown viral blocking ability at 0.5 mg/ml and 1 mg/ml in the pre-entry and post-entry conditions, respectively. In addition, no cytotoxic effect has been found at ≤ 4 mg/ml. The half-maximal effective concentration (EC50) of this compound reportedly is 0.44 mg/ml. Glycyrrhizin has also been shown to significantly reduce the SARS-CoV-2 RNA level [28]. An in silico study showed that this compound binds to the ACE2 receptor, which possibly blocks viral entry [29].
Molecular drug targets for COVID-19
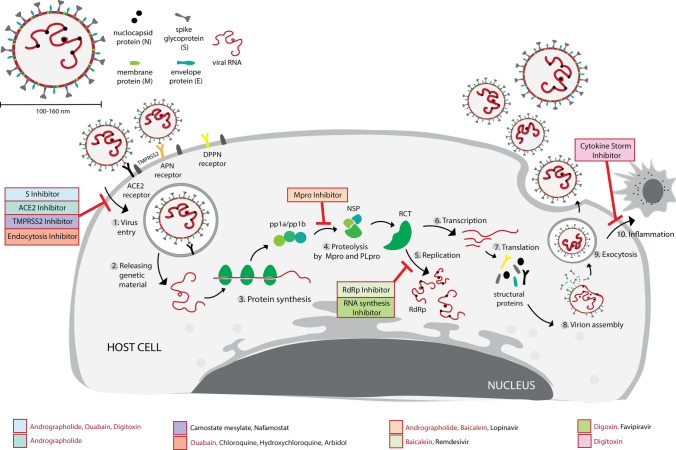
SARS-CoV-2 is a novel coronavirus that belongs to the Coronavirinae family. Coronaviruses are RNA viruses that have a spherically shaped envelope 100–160 nm in diameter. The genome size is 27–32 kb. The 3′ end of the genome encodes structural proteins, such as envelope (E), spike glycoprotein (S), nucleocapsid (N), and membrane glycoprotein (M), and the 5′ end encodes polyproteins involved in replication and transcription [20]. SARS-CoV-2 can enter the host cell via various receptors, such as ACE2, aminopeptidase N (APN), and dipeptidyl peptidase 4 (DPP4). Hence, these three receptors are promising targets for treatment of COVID-19 infection. ACE2 is known to be prevalent in alveolar cells. In addition, males are known to express higher levels of ACE2 than females. The expression of ACE2 can be increased by binding of the S protein of SARS-CoV-2 to ACE2. On the other hand, DPP4 mediates entry of the virus into the host cell via directed cell–cell fusion, whereas APN promotes cross-species transmission of SARS-CoV-2, which is involved in receptor binding [30].
The ACE2 receptor, S protein, RNA-dependent RNA polymerase (RdRp), papain-like protease (PLpro), and 3-chymotrypsin-like protease (3CLpro or main protease (Mpro)) are known to be potential targets for COVID-19 drugs because these biomaterials are essential for viral invasion of the host cell [4]. SARS-CoV-2 invades the host cell by binding of its S protein to the ACE2 receptor of the host cell followed by S protein cleavage by transmembrane serine protease 2 (TMPRSS2). Next, genomic RNA is released into the host cell cytoplasm and undergoes translation of polyprotein (ppa1/ab), which is cleaved further by viral Mpro and PLpro into non-structural protein (nsp). The nsp protein then interacts with RdRp for building the replication–transcription complex. After entering the cell, with RdRp, the virus takes over genetic reproduction to produce new viral RNA. Cleaved glycoproteins by virus proteases (PLpro and Mpro) are then produced, and the viral material is assembled so that it can synthesize new viral particles to further infect other host cells [4, 31, 32].
ACE2 is also the proposed drug target for preventing SARS-CoV-2 infection. This enzyme is a transmembrane protein, localized in alveolar epithelial cells, vascular endothelial cells, small intestine epithelial cells, and renal tubular epithelial cells. ACE2 functions by cleaving the C-terminal amino acid residue of angiotensin II (Ang II), which maintains the balance of generated Ang II. Furthermore, ACE2 is known to be the receptor for S proteins of SARS-Cov-2; hence, inhibition of this enzyme may be a drug target for preventing SARS-CoV-2 infection [2, 33]. The expressed S protein of SARS-CoV-2 functions by binding to the ACE2 receptor. Infection by this virus can be through two pathways: endocytic and direct fusion. In the endocytic pathway, cleavage of S proteins is mediated by cathepsin B/L in lysosomes. On the other hand, virus entry via direct fusion is mediated by TMPRSS2 for cleavage of S proteins. The cleavage of S viral protein by these two mediators is a critical factor that enables RNA viruses to enter the cytosol of host cells. Hence, these two proteases and the ACE2 receptor determine the susceptibility to SARS-CoV-2 infection [31, 34]. On the other hand, Mpro/3CLpro have essential roles in the viral polyprotein maturation process [1]. 3CLpro is known to be the main drug target of coronaviruses, mainly inhibits replication of SARS-CoV-2, and is required for viral replication through cleavage of viral polyproteins to undergo the life cycle. Hence, 3CLpro is a target of antiviral drugs [35].
Identified medicinal plants and their active compounds against SARS-CoV-2
Various medicinal plants and isolated compounds have been tested against SARS-CoV-2 and revealed some promising activities based on in vitro and in cell studies (Tables 3, 4). Several crude drugs derived from mostly Asian herbs have been tested for anti-SARS-CoV-2 activity in some infected cells and protein targets.
Table 3.
Anti-SARS-CoV-2 activity of medicinal plants (crude drugs)
| Origin | Species | Family | IC50 (µg/ml) | EC50 (µg/ml) | Experimental result | References |
|---|---|---|---|---|---|---|
| Thailand | Andrographis paniculata | Acanthaceae | 0.036 | – | SARS-CoV-2 infected Calu-3 cells | [37] |
| Thailand | Andrographis paniculata | Acanthaceae | 68.06 | – | SARS-CoV-2 infected Vero E6 cells | [52] |
| China | Scutellaria baicalensis | Lamiaceae | – | 0.74 | SARS-CoV-2 infected Vero cells | [43] |
| Korea | Platycodon grandiflorum | Campanulaceae | 5,010 | – | In vitro study using ACE2+ cells using H1299 cell | [34] |
| Thailand | Boesenbergia rotunda | Zingiberaceae | 3.62 | – | SARS-CoV-2 infected Vero E6 cells | [26] |
| India | Camellia sinensis | Theaceae | 8.9 ± 0.5 | – | SARS-CoV-2 Mpro/3CLpro | [61] |
| India | Terminalia chebula | Combretaceae | 8.8 ± 0.5 | – | SARS- CoV-2 Mpro/3CLpro | [61] |
| Thailand | Zingiber officinale | Zingiberaceae | 29.19 | – | SARS-CoV-2 infected Vero E6 cells | [26] |
| Germany | Glycyrrhiza glabra | Fabaceae | – | – | Blocking SARS-CoV-2 replication at pre-entry stage in infected Vero E6 cells at 0.5 mg/ml | [28] |
| China | Reynoutria sachalinensis | Polygonaceae | 4.013 | – | SARS-CoV-2 Mpro/3CLpro | [62] |
| China | Reynoutria japonica | Polygonaceae | 7.877 | – | SARS-CoV-2 Mpro/3CLpro | [62] |
| China | Lycoris radiata | Amaryllidaceae | – | 2.4 ± 0.2 | SARS-CoV infected Vero E6 cells | [63] |
| China | Artemisia annua | Compositae | – | 34.5 ± 2.6 | SARS-CoV infected Vero E6 cells | [63] |
| China | Pyrrosia lingua | Polypodiaceae | – | 43.2 ± 14.1 | SARS-CoV infected Vero E6 cells | [63] |
| China | Lindera aggregata | Lauraceae | – | 88.2 ± 7.7 | SARS-CoV infected Vero E6 cells | [63] |
Table 4.
Anti-SARS-CoV-2 activity of active compounds isolated from medicinal plants
| Plant origin | Compound | Plant | Family | IC50 (μM) | EC50 (μM) | Model | References |
|---|---|---|---|---|---|---|---|
| Thailand | 6-Gingerol | Zingiber officinale | Zingiberaceae | 1.38 | – | SARS-CoV-2 NP mAb Plaque reduction assay in Vero cells | [26] |
| Thailand | 6-Gingerol | Zingiber officinale | Zingiberaceae | > 100 | – | SARS-CoV-2 infected Vero E6 cells | [26] |
| Thailand | Andrographolide | Andrographis paniculata | Acanthaceae | 0.034 | – | SARS-CoV-2 in Calu-3 cells | [37] |
| Thailand | Andrographolide | Andrographis paniculata | Acanthaceae | 6.58 | – | SARS-CoV-2 infected Vero E6 cells | [52] |
| Thailand | Andrographolide | Andrographis paniculata | Acanthaceae | 0.28 | – | NP mAb SARS-CoV-2 | [26] |
| Asia | Artemisinin | Artemisia annua | Compositae | – | 64.45 ± 2.58 | SARS-CoV-2 infected Vero E6 cells | [64] |
| China | Baicalein | Scutellaria baicalensis | Lamiaceae | 0.39 | – | SARS-CoV-2 infected Vero cells | [43] |
| China | Baicalein | Scutellaria baicalensis | Lamiaceae | 0.94 ± 0.20 (in vitro 3CLpro) | 2.94 ± 1.19 (in Vero E6 cells) | SARS-CoV-2 infected Vero E6 and in vitro assay against SARS-CoV-2 Mpro/3CLpro | [45] |
| Baicalin | 6.41 ± 0.95 (in vitro 3CLpro) | 27.87 ± 0.04 (in Vero E6 cells) | SARS-CoV-2 infected Vero E6 and in vitro assay against SARS-CoV-2 Mpro/3CLpro | ||||
| Korea | Cannabidiol | Cannabis sativa | Cannabaceae | 7.91 | – | SARS-CoV-2 infected Vero cells | [65] |
| Asia | Cepharanthine | Stephania cephalanta | Menispermaceae | 4.47 | – | SARS-CoV-2 infected Vero cells | [47] |
| China | Chlorogenic acid | Lonicera japonica | Caprifoliaceae | 39.48 ± 5.51 | – | SARS-CoV-2 Mpro/3CLpro | [45] |
| N/D | Digitoxin | Digitalis purpurea | Plantaginaceae | 0.23 | – | SARS-CoV-2 infected Vero cells based on cytopathic effect | [47] |
| N/D | Digoxin | Digitalis purpurea | Plantaginaceae | 0.19 | – | SARS-CoV-2 infected Vero cells based on cytopathic effect | [47] |
| Asia | Epigallocatechin gallate (EGCG) | Camellia sinensis | Theaceae | 16.53 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [35] |
| Germany | Glycyrrhizin | Glycyrrhiza glabra | Fabaceae | – | 53.46 | SARS-CoV-2 infected Vero E6 cells | [28] |
| Asia | Myricetin | Myrica rubra | Myricaceae | 0.22 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [33] |
| N/D | Osajin | Maclura pomifera | Moraceae | 3.87 | – | SARS-CoV-2 infected Vero cells | [47] |
| N/D | Ouabain | Acokanthera ouabaio | Apocynaceae | 0.024 | – | SARS-CoV-2 infected Vero E6 cells | [48] |
| Thailand | Panduratin A | Boesenbergia rotunda | Zingiberaceae | 0.81 | – | SARS-CoV-2 infected Vero E6 cells by IFA assay | [26] |
| 2.04 | – | SARS-CoV-2 infected Calu-3 cells by IFA assay | |||||
| 0.53 | – | SARS-CoV-2 NP mAb plaque reduction assay in Calu-3 cells | |||||
| 0.078 | – | SARS-CoV-2 NP mAb plaque reduction assay in Vero cells | |||||
| China | Phillyrin | Forsythia suspensa | Oleaceae | 1.13 | – | SARS-CoV-2 infected Vero E6 cells | [66] |
| Korea | Platycodin D | Platycodon grandiflorum | Campanulaceae | 0.69 | – | In vitro study using ACE2+ cells using H1299 cells | [34] |
| N/D | Quercetin | N/D | 4.48 | – | Inhibition of rhACE2 (recombinant human) in vitro | [33] | |
| China | Scutellarein | Erigeron karvinskianus | Compositae | 5.80 | – | SARS-CoV-2 in vitro | [1] |
| Asia | Tetandrine | Stephania tetrandra | Menispermaceae | 3 | – | SARS-CoV-2 infected Vero cells | [47] |
| Korea | Tetrahydrocannabinol | Cannabis sativa | Cannabaceae | 10.25 | – | SARS-CoV-2 infected Vero cells | [65] |
| Asia | Theaflavin | Camellia sinensis | Theaceae | 14.95 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [35] |
| Asia | Allicin | Allium sativum | Amaryllidaceae | – | – | Sub-lethal effect at 50–75 μM with SARS-CoV-2 infected Vero E6 cell | [67] |
| N/D | Betulinic acid | Olea europaea | Oleaceae | 10 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [68] |
| N/D | Betulin | Olea europaea | Oleaceae | 89.67 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [68] |
| N/D | Ursolic acid | Olea europaea | Oleaceae | 12.57 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [68] |
| N/D | Maslinic acid | Olea europaea | Oleaceae | 3.22 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [68] |
| Egypt | Cnicin | Cnicus benedictus | Compositae | 3.12 | – | SARS-CoV-2 infected Vero E6 cells | [69] |
| Egypt | Arctiin | Cnicus benedictus | Compositae | > 150 | – | SARS-CoV-2 infected Vero E6 cells | [69] |
| Egypt | Sitogluside | Cnicus benedictus | Compositae | > 150 | – | SARS-CoV-2 infected Vero E6 cells | [69] |
| Egypt | Nortracheloside | Cnicus benedictus | Compositae | > 150 | – | SARS-CoV-2 infected Vero E6 cells | [69] |
| Egypt | Apigenin 7-O-glucoside | Cnicus benedictus | Compositae | > 200 | – | SARS-CoV-2 infected Vero E6 cells | [69] |
| Egypt | Luteolin | Cnicus benedictus | Compositae | > 300 | – | SARS-CoV-2 infected Vero E6 cells | [69] |
| Egypt | Astragalin | Cnicus benedictus | Compositae | > 200 | – | SARS-CoV-2 infected Vero E6 cells | [69] |
| China | Vanicoside A | Reynoutria sachalinensis | Polygonaceae | 1.364 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [62] |
| China | Vanicoside B | Reynoutria sachalinensis | Polygonaceae | 1.639 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [62] |
| China | Kobophenol A | Caragana sinica | Leguminosae | – | 71.6 | SARS-CoV-2 infected Vero E6 cells | [70] |
| China | Dihydromyricetin | Ampelopsis grossedentata | Vitaceae | 4.91 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [71] |
| China | Isodihydromyricetin | Ampelopsis grossedentata | Vitaceae | 3.73 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [71] |
| China | Taxifolin | Ampelopsis grossedentata | Vitaceae | 72.27 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [71] |
| China | Ebselen | Ampelopsis grossedentata | Vitaceae | 2.62 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [71] |
| China | Resveratrol | N/D | N/D | – | 4.48 | SARS-CoV-2 infected Vero cells | [72] |
| N/D | Hopeaphenol | N/D | N/D | 42.5 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [73] |
| N/D | Vaticanol B | N/D | N/D | 47.6 | – | In vitro assay against SARS-CoV-2 Mpro/3CLpro | [73] |
Antiviral drugs or therapy for SARS-CoV-2 can be achieved by targeting the virus itself or enhancing immunity, which may help to suppress viral replication. Inhibition of viral replication may be through a mechanism that blocks the virus from entering human cells (preventing binding of the virus’ S protein to the ACE2 receptor) or by attacking the viral enzymes that are essential for its replication. In addition, the drug can act as an inhibitor of various structural proteins, such as Mpro, PLpro, helicase, serine protease, and RdRp [2]. Severe COVID-19 patients also may be treated by blocking the cytokine storm through suppression of pro-inflammatory cytokines. In severe COVID-19 patients, the cytokine storm occurs in response to significantly elevated levels of the inflammatory cytokines, such as interleukin (IL)-6, IL-7, IL8, IL9, IL-10, IL-1B, IL-1Ra, granulocyte macrophage colony-stimulating factor, fibroblast growth factor, IFN-γ, TNF-α, platelet-derived growth factor, monocyte chemoattractant protein, macrophage inflammatory protein 1-α, and vascular endothelial growth factor [4].
Numerous existing drugs have been repurposed for treating COVID-19 disease, such as chloroquine, remdesivir, favipiravir, and umifenovir [30]. However, currently there is no effective treatment that has been approved by regulators [36]. Chloroquine, an antimalarial drug, possesses antiviral activity, including against coronaviruses, and has been considered as a possible antiviral drug for SARS-CoV-2 and been tested against COVID-19 in China. Chloroquine can block cathepsin by increasing lysosomal pH and modulating pro-inflammatory cytokines, including TNF-α and IL-6. However, the failure of this drug in a clinical trial was attributed to its inability to block TMPRSS2-mediated viral entry [30, 34]. Remdesivir, an antiviral drug for Ebola and Marburg virus also has been tested in a clinical trial due to its ability to attack coronaviruses by inhibiting RdRp. The influenza drug, favipiravir, can terminate incorporation of viral RNA into the host cell. Umifemovir/Arbidol, which are influenza A and B drugs, also have been reported to block viral fusion into host cell membranes [30]. Additionally, lopinavir reportedly blocks 3CLpro of the virus, whereas both camostat mesylate and nafamostat reportedly inhibit viral entry and act as TMPRSS2 inhibitors. Chloroquine and hydroxychloroquine have also shown inhibition of viral entry [32].
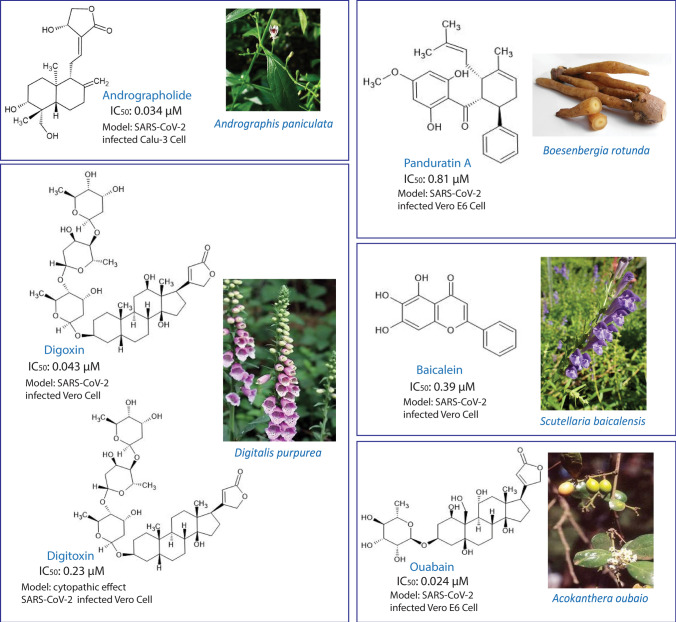
The present review focuses on compounds isolated from medicinal plants against SARS-CoV-2 shown to be active in vitro and in cell studies (Table 4), and several compounds show promising antiviral activity as compared with several FDA-approved drugs as mentioned above (Figs. 4, 5). Among the other tested compounds, andrographolide has shown promising potency (IC50 of 0.034 µM, against SARS-CoV-2-infected Calu-3 cells). Andrographolide is a major active compound isolated from Andrographis paniculata that has been used for a long time in Thai traditional medicine for diarrhea, common cold, fever, and viral infections. This bicyclic diterpene lactone exhibits various pharmacological properties, such as antioxidant, anticancer, anti-inflammatory, antimicrobial, cardiovascular protection, hepatoprotection, and immunomodulatory. Andrographolide has shown broad-spectrum antiviral activity against various viral infections, such as influenza, hepatitis, HIV, chikungunya, herpes, and HPV. Based on an in vitro assay, the extract and its active compound exhibited anti-SARS-CoV-2 activity. In an enzyme-based assay, andrographolide inhibited Mpro (the SARS-CoV-2 main protease), with an IC50 of 15 µM, possibly through formation of a covalent bond with the active site at the Cys145 amino acid residue. It is postulated that this compound would attack the virus through multiple pathways, including viral entry, replication, protein synthesis, and protein expression. Andrographolide has also shown binding affinity with the S protein and the ACE2 receptor, hence, it may inhibit viral entry. This compound is thought to be more potent in the late phase of the SARS-CoV-2 life cycle than in genome replication and protein expression [37–40]. The 50% cytotoxic concentration (CC50) of this compound to various normal cell lines from various organs, including liver, kidney, intestine, lung, and brain (HepG-2/imHC, HK-2, Caco-2, Calu-3, and SH-SY5Y, respectively), ranges from 13.2 to 81.5 µM [37].
Fig. 4.
Promising natural product isolated from medicinal plants as an antiviral drug against SARS-CoV-2 in a cell model
Fig. 5.
Summary of the antiviral activity in cell targets of selected isolated active compounds from medicinal plants (highlighted in red) and FDA-approved drugs/antiviral agents against SARS-CoV-2 (highlighted in black)
A prenylated cyclohexenyl chalcone, panduratin A, exhibited potent antiviral activity against SARS-CoV-2 in both pre-entry and post-infection. This compound has been isolated from a fingerroot of Boesenbergia rotunda that is commonly used in Southeast Asia and China as a culinarily spice. The rhizome has various pharmacological properties, such as antibacterial, antitumor, anti-allergic, and antioxidant. Panduratin A, as a major active compound, has shown antiviral activity against HIV [41] and Dengue virus [42]. The study showed that the antiviral activity against SARS-CoV-2 was superior to the FDA-approved drug, hydroxychloroquine. The value of cytotoxicity against the normal Vero E6 cell line was 14.71 µM [26]. However, molecular targets for this compound have not been well explored.
Baicalin and baicalein, active compounds isolated from Scutellaria baicalensis, are used in TCM for treatment of respiratory disorders, heat clearing, detoxification, fire purging, and viral diseases, including hepatitis. The herb has shown antitumor, antimicrobial, anti-inflammatory, and broad-spectrum antiviral activity against various viruses, such as Zika, HIV, DENV, and H1N1. Baicalin and baicalein have shown inhibitory activity against the 3CLpro main protease of SARS-CoV-2, with IC50 values of 6.41 µM and 0.94 µM, respectively. The anti-SARS-CoV-2 activity of baicalein has been shown to be superior to that of baicalin [43, 44]. In a cell assay model using SARS-Co-2-infected Vero E6 cells, baicalein exhibited IC50 and EC50 values of 0.39 and 2.94 µM, respectively. The CC50 of baicalein against Vero E6 cells was > 200 µM, which is categorized as low cytotoxicity. Baicalein reportedly has closed activity with chloroquine, with an EC50 of 2.71 µM [43, 45]. This compound reportedly inhibited viral replication by blocking 3CLpro via in an in vitro study, and a molecular docking study showed that the 6-OH and 7-OH of its structure interacted with a carbonyl group of Leu141 and an amide group of Gly143 of 3CLpro, respectively [43]. In addition, baicalein also has been reported to inhibit the RdRp SARS-CoV-2 in an in vitro assay. In the subsequent molecular docking study, inhibition was not predicted on the active site of the enzyme since it is not an analog of a nucleoside. An in silico study showed that the compound had binding affinities with the Asn705 and His133 residues of RdRp on the palm subdomain and nucleotidyltransferase, respectively [46].
Furthermore, cardiac glycosides, including digoxin, digitoxin, and ouabain, also have shown good properties for treating COVID-19 disease. Cardiac glycosides, molecules that contain a steroid moiety, a 5–6C lactone ring, and a sugar moiety, have been suggested as promising treatments for COVID-19 by targeting NA+/K+-ATPase (NKA). Some cardiac glycosides have shown antiviral activity through inhibition of NKA and activation of tyrosine kinase (Src). Src regulates nuclear factor kappa B (NFkB), which is the important transcription factor for SARS-CoV-2 [36].
Digoxin and digitoxin, FDA-approved drugs for cardiovascular diseases, are isolated from Digitalis purpurea and reported to have activity against SARS-CoV-2-infected Vero cells based on their cytopathic effect, with IC50 values of 0.19 µM and 0.23 µM, respectively. The evaluation of cytotoxicity is measured at a CC50 > 50 µM for both compounds [47]. Another report with a different assay showed inhibitory activity of digoxin against SARS-CoV-2-infected Vero cells (human isolate BetaCoV/Korea), with an IC50 of 0.043 µM and a CC50 > 10 µM. The IC50 of digoxin has been shown to be tenfold higher than those of chloroquine and remdesivir, which have IC50 values of 0.526 µM and 1.57 µM, respectively. Digoxin also inhibited viral mRNA expression, protein expression, and viral copy number. Furthermore, the inhibition of mRNA expression of digoxin was superior to those of remdesivir and chloroquine. However, digoxin reportedly is not effective in the viral entry stage. Digoxin appears to act as a viral RNA synthesis inhibitor [48]. On the other hand, digitoxin can suppress the levels of cytokines, including TNFα, M1P2, IFNγ, MCP1, and GRO/KC in an influenza-infected rat lung model. This finding implied that digitoxin may be able to block the cytokine storm caused by the elevated levels of pro-inflammatory cytokines during coronavirus infection. Digitoxin was reported as one of the top-ten pro-inflammatory cytokine inhibitors among 2800 FDA-approved drugs by suppressing TNFα-activated NFkB. Furthermore, compared with digoxin, digitoxin has an affinity to the SARS-CoV-2 spike pseudo-typed VSV in human lung cells and hence is able to block ACE2-S binding for viral entry. Digoxin is postulated to act as an inhibitor at the intracellular level of the host cell rather than at the entry stage [49, 50].
On the other hand, ouabain reportedly showed the antiviral activity against SARS-CoV-2-infected Vero cells, with an IC50 of 0.024 µM and a CC50 > 416.66 µM. The IC50 of ouabain is reported to be superior to those of chloroquine and remdesivir. This compound also exhibited inhibition of viral copy number, mRNA, and protein expression. It has been postulated that ouabain inhibits at the viral entry stage by blocking Src-mediated endocytosis [48]. Furthermore, another study reported that the blocking ability of ouabain occurred via binding to the S protein of SARS-CoV-2, so it also may block viral penetration into human lung cells [50].
Conclusion
Herbal medicine has been applied in the treatment of COVID-19 disease in various Asian cultures. Several medicinal plants from both single- and multiple-component herbal medicines have been found to have antiviral properties against SARS-CoV-2 in cell-based assays and in vitro studies against various molecular targets, which implies some degree of efficacy for these traditional medicines. Our review showed that the Lamiaceae family was the most frequently used plant family in the treatment and prevention of COVID-19, which suggests that it is a promising source of antiviral agents. In addition, a direct approach using testing of isolated compounds from medicinal plants against SARS-CoV-2 also revealed some promising antiviral activity when compared with repurposed FDA-approved drugs (e.g., digoxin, digitoxin, panduratin A, and andrographolide). These Lamiaceae family members and the isolated compounds discussed in the review warrant further investigations for their activities against coronaviruses, including SAR-CoV-2.
Acknowledgements
We would like to thank the College of Public Health Science, Chulalongkorn University.
Author contributions
AP and DL designed the study. AP initiated the concept and idea, supervised it, provided input, and revised the manuscript. DL collected, curated, analyzed the data, and wrote the draft of the manuscript. All authors made equal contributions, have read the manuscript, and agreed on the final form for submission.
Funding
No funding was received to assist with the preparation of this manuscript.
Availability of data and materials
All data are obtained from published literatures which are presented in the references.
Declarations
Conflict of interest
Authors declared there is no competing of interest.
Ethic approval and consent to participate
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/23/2021
A Correction to this paper has been published: 10.1007/s11418-021-01580-4
References
- 1.Wang Z, Yang L. Chinese herbal medicine: fighting SARS-CoV-2 infection on all fronts. J Ethnopharmacol. 2021;270:113869. doi: 10.1016/j.jep.2021.113869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Illian DN, Siregar ES, Sumaiyah S, Utomo AR, Nuryawan A, Basyuni M. Potential compounds from several Indonesian plants to prevent SARS-CoV-2 infection: a mini-review of SARS-CoV-2 therapeutic targets. Heliyon. 2021;7(1):e06001. doi: 10.1016/j.heliyon.2021.e06001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teuwen L-A, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung EL-H, Pan H-D, Huang Y-F, Fan X-X, Wang W-Y, He F, Cai J, Zhou H, Liu L. The scientific foundation of Chinese herbal medicine against COVID-19. Engineering. 2020;6(10):1099–1107. doi: 10.1016/j.eng.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akindele AJ, Agunbiade FO, Sofidiya MO, Awodele O, Sowemimo A, Ade-Ademilua O, Akinleye MO, Ishola IO, Orabueze I. COVID-19 Pandemic: a case for phytomedicines. Nat Prod Commun. 2020;15(8):1–9. doi: 10.1177/1934578X20945086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Codagenix I (2019) Codagenix and Serum Institute of India Initiate Co-Development of a Scalable, Live-Attenuated Vaccine Against the 2019 Novel Coronavirus, COVID-19. https://www.prnewswire.com/news-releases/codagenix-and-serum-institute-of-india-initiate-co-development-of-a-scalable-live-attenuated-vaccine-against-the-2019-novel-coronavirus-covid-19-301004654.html. Accessed 25 July 2021
- 7.Wu Z, Hu Y, Xu M, Chen Z, Yang W, Jiang Z, Li M, Jin H, Cui G, Chen P. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803–812. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baraniuk C. What do we know about China’s covid-19 vaccines? BMJ. 2021;373:n912. doi: 10.1136/bmj.n912. [DOI] [PubMed] [Google Scholar]
- 9.Britton A, Slifka KMJ, Edens C, Nanduri SA, Bart SM, Shang N, Harizaj A, Armstrong J, Xu K, Ehrlich HY. Effectiveness of the Pfizer-BioNTech COVID-19 vaccine among residents of two skilled nursing facilities experiencing COVID-19 outbreaks—Connecticut, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(11):396–401. doi: 10.15585/mmwr.mm7011e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliver SE. The advisory committee on immunization practices’ interim recommendation for use of moderna COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1653–1656. doi: 10.15585/mmwr.mm695152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ClinicalTrials.gov. ChulaCov19 mRNA vaccine in healthy adults. https://clinicaltrials.gov/ct2/show/NCT04566276. Accessed 25 July 2021
- 12.Cohen J. What went wrong with CureVac's mRNA vaccine? Accessed 20 July 2021
- 13.ClinicalTrials.gov. The safety and immunogenicity of a DNA-based Vaccine (COVIGEN) in Healthy Volunteers (COVALIA). https://clinicaltrials.gov/ct2/show/NCT04742842. Accessed 25 July 2021
- 14.ClinicalTrials.gov. Safety, tolerability and immunogenicity of INO-4800 followed by electroporation in healthy volunteers for COVID19. https://clinicaltrials.gov/ct2/show/NCT04447781. Accessed 25 July 2021
- 15.Knoll MD, Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet Inf Dis. 2021;397(10269):72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livingston EH, Malani PN, Creech CB. The Johnson & Johnson Vaccine for COVID-19. JAMA. 2021;325(15):1575–1575. doi: 10.1001/jama.2021.2927. [DOI] [PubMed] [Google Scholar]
- 17.Mahase E. Covid-19: Novavax vaccine efficacy is 86% against UK variant and 60% against South African variant. BMJ. 2021;372:n296. doi: 10.1136/bmj.n296. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Tenchov R, Smoot J, Liu C, Watkins S, Zhou Q. A comprehensive review of the global efforts on COVID-19 vaccine development. ACS Cent Sci. 2021;7(4):512–533. doi: 10.1021/acscentsci.1c00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Coronavirus disease (COVID-19): vaccines. https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines?adgroupsurvey. Accessed 11 July 2021
- 20.Zhao J, Zhao S, Ou J, Zhang J, Lan W, Guan W, Wu X, Yan Y, Zhao W, Wu J. COVID-19: vaccine development updates. Front Immunol. 2020;11:3435. doi: 10.3389/fimmu.2020.602256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cragg GM, Newman DJ. Natural product drug discovery in the next millennium. Pharm Biol. 2001;39(sup1):8–17. doi: 10.1076/phbi.39.s1.8.0009. [DOI] [PubMed] [Google Scholar]
- 22.Patwardhan B, Mashelkar RA. Traditional medicine-inspired approaches to drug discovery: can Ayurveda show the way forward? Drug Discov Today. 2009;14(15):804–811. doi: 10.1016/j.drudis.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Sucher NJ. The application of Chinese medicine to novel drug discovery. Expert Opin Drug Disc. 2013;8(1):21–34. doi: 10.1517/17460441.2013.739602. [DOI] [PubMed] [Google Scholar]
- 24.Shahrajabian MH, Sun W, Cheng Q. Traditional herbal medicine for the prevention and treatment of cold and flu in the autumn of 2020, overlapped with COVID-19. Nat Prod Commun. 2020;15(8):1–10. doi: 10.1177/1934578X20951431. [DOI] [Google Scholar]
- 25.Shahrajabian MH, Sun W, Shen H, Cheng Q. Chinese herbal medicine for SARS and SARS-CoV-2 treatment and prevention, encouraging using herbal medicine for COVID-19 outbreak. Acta Agric Scand Soil Plant Sci. 2020;70(5):437–443. doi: 10.1080/09064710.2020.1763448. [DOI] [Google Scholar]
- 26.Kanjanasirirat P, Suksatu A, Manopwisedjaroen S, Munyoo B, Tuchinda P, Jearawuttanakul K, Seemakhan S, Charoensutthivarakul S, Wongtrakoongate P, Rangkasenee N. High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents. Sci Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-77003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahzad F, Anderson D, Najafzadeh M. The antiviral, anti-inflammatory effects of natural medicinal herbs and mushrooms and SARS-CoV-2 infection. Nutrients. 2020;12(9):2573. doi: 10.3390/nu12092573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Sand L, Bormann M, Alt M, Schipper L, Heilingloh CS, Steinmann E, Todt D, Dittmer U, Elsner C, Witzke O. Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease. Viruses. 2021;13(4):609. doi: 10.3390/v13040609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Du Q (2020) Potential natural compounds for preventing SARS-CoV-2 (2019-nCoV) infection. Preprints. 10.20944/preprints202001.0358.v3
- 30.Asai A, Konno M, Ozaki M, Otsuka C, Vecchione A, Arai T, Kitagawa T, Ofusa K, Yabumoto M, Hirotsu T. COVID-19 drug discovery using intensive approaches. Int J Mol Sci. 2020;21(8):2839. doi: 10.3390/ijms21082839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong GU, Song H, Yoon GY, Kim D, Kwon Y-C. Therapeutic strategies against COVID-19 and structural characterization of SARS-CoV-2: a review. Front Microbiol. 2020;11:1723. doi: 10.3389/fmicb.2020.01723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez-Fernandez B, D’marco L, Górriz JL, Jacobs-Cachá C, Kanbay M, Luis-Lima S, Porrini E, Sarafidis P, Soler MJ, Ortiz A. Exploring sodium glucose co-transporter-2 (SGLT2) inhibitors for organ protection in COVID-19. J Clin Med. 2020;9(7):2030. doi: 10.3390/jcm9072030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Raghuvanshi R, Ceylan FD, Bolling BW. Quercetin and its metabolites inhibit recombinant human angiotensin-converting enzyme 2 (ACE2) activity. J Agric Food Chem. 2020;68(47):13982–13989. doi: 10.1021/acs.jafc.0c05064. [DOI] [PubMed] [Google Scholar]
- 34.Kim TY, Jeon S, Jang Y, Gotina L, Won J, Ju YH, Kim S, Jang MW, Won W, Park MG, Pae AN, Han S, Kim S, Lee CJ. Platycodin D, a natural component of Platycodon grandiflorum, prevents both lysosome- and TMPRSS2-driven SARS-CoV-2 infection by hindering membrane fusion. Exp Mol Med. 2021;53(5):956–972. doi: 10.1038/s12276-021-00624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jang M, Park YI, Cha YE, Park R, Namkoong S, Lee JI, Park J. Tea polyphenols EGCG and theaflavin inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Evid Based Complement Altern Med. 2020 doi: 10.1155/2020/5630838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Souza KFC, Souza BPTM, de Palmer Paixão ICN, Burth P, Silva AR, Gonçalves-de-Albuquerque CF. Na+/K+-ATPase as a target of cardiac glycosides for the treatment of SARS-CoV-2 infection. Front Pharmacol. 2021;12:624704. doi: 10.3389/fphar.2021.624704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sa-Ngiamsuntorn K, Suksatu A, Pewkliang Y, Thongsri P, Kanjanasirirat P, Manopwisedjaroen S, Charoensutthivarakul S, Wongtrakoongate P, Pitiporn S, Chaopreecha J. Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component Andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J Nat Prod. 2021;84(4):1261–1270. doi: 10.1021/acs.jnatprod.0c01324. [DOI] [PubMed] [Google Scholar]
- 38.Gupta S, Mishra KP, Ganju L. Broad-spectrum antiviral properties of andrographolide. Arch Virol. 2017;162(3):611–623. doi: 10.1007/s00705-016-3166-3. [DOI] [PubMed] [Google Scholar]
- 39.Maurya VK, Kumar S, Prasad AK, Bhatt MLB, Saxena SK. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. Virus Dis. 2020;31(2):179–193. doi: 10.1007/s13337-020-00598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi T-H, Huang Y-L, Chen C-C, Pi W-C, Hsu Y-L, Lo L-C, Chen W-Y, Fu S-L, Lin C-H. Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage. Biochem Biophys Res Commun. 2020;533(3):467–473. doi: 10.1016/j.bbrc.2020.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheenpracha S, Karalai C, Ponglimanont C, Subhadhirasakul S, Tewtrakul S. Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata. Bioorg Med Chem. 2006;14(6):1710–1714. doi: 10.1016/j.bmc.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Kiat TS, Pippen R, Yusof R, Ibrahim H, Khalid N, Rahman NA. Inhibitory activity of cyclohexenyl chalcone derivatives and flavonoids of fingerroot, Boesenbergia rotunda (L.), towards dengue-2 virus NS3 protease. Bioorg Med Chem Lett. 2006;16(12):3337–3340. doi: 10.1016/j.bmcl.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 43.Liu H, Ye F, Sun Q, Liang H, Li C, Lu R, Huang B, Tan W, Lai L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J Enzyme Inhib Med Chem. 2020;36(1):497–503. doi: 10.1101/2020.04.10.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su H, Yao S, Zhao W, Li M, Liu J, Shang W, Xie H, Ke C, Gao M, Yu K. Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. BioRxiv. 2020 doi: 10.1101/2020.04.13.038687. [DOI] [Google Scholar]
- 45.Su H-x, Yao S, Zhao W-f, Li M-j, Liu J, Shang W-j, Xie H, Ke C-q, Hu H-c, Gao M-n, Yu K-q, Liu H, Shen J-s, Tang W, Zhang L-k, Xiao G-f, Ni L, Wang D-w, Zuo J-p, Jiang H-l, Bai F, Wu Y, Ye Y, Xu Y-c. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol Sin. 2020;41(9):1167–1177. doi: 10.1038/s41401-020-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zandi K, Musall K, Oo A, Cao D, Liang B, Hassandarvish P, Lan S, Slack RL, Kirby KA, Bassit L, Amblard F, Kim B, AbuBakar S, Sarafianos SG, Schinazi RF. Baicalein and baicalin inhibit SARS-CoV-2 RNA-dependent-RNA polymerase. Microorganisms. 2021;9(5):893. doi: 10.3390/microorganisms9050893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeon S, Ko M, Lee J, Choi I, Byun Soo Y, Park S, Shum D, Kim S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents Chemother. 2020;64(7):e00819–00820. doi: 10.1128/AAC.00819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho J, Lee YJ, Kim JH, Kim Si, Kim SS, Choi B-S, Choi J-H. Antiviral activity of digoxin and ouabain against SARS-CoV-2 infection and its implication for COVID-19. Sci Reports. 2020;10(1):16200. doi: 10.1038/s41598-020-72879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollard BS, Blanco JC, Pollard JR. Classical drug digitoxin inhibits influenza cytokine storm, with implications for COVID-19 therapy. In Vivo. 2020;34(6):3723–3730. doi: 10.21873/invivo.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caohuy H, Eidelman O, Chen T, Yang Q, Bera A, Walton N, Pollard HB. Common cardiac medications potently inhibit ACE2 binding to the SARS-CoV-2 Spike, and block virus penetration into human lung cells. BioRxiv. 2021 doi: 10.1101/2021.06.02.446343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen PH, Tran VD, Pham DT, Dao TNP, Dewey RS. Use of and attitudes towards herbal medicine during the COVID-19 pandemic: a cross-sectional study in Vietnam. Euro J Integr Med. 2021;44:101328. doi: 10.1016/j.eujim.2021.101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo H, Tang Q-L, Shang Y-X, Liang S-B, Yang M, Robinson N, Liu J-P. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin J Integr Med. 2020;26(4):243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azam MNK, Al Mahamud R, Hasan A, Jahan R, Rahmatullah M. Some home remedies used for treatment of COVID-19 in Bangladesh. J Med Plant Stud. 2020;8(4):27–32. [Google Scholar]
- 54.MOA. Guidelines for Ayuverda Practitioners for COVID19. https://www.ayush.gov.in/docs/ayurved-guidlines.pdf. Accessed 28 May 2021
- 55.Panyod S, Ho C-T, Sheen L-Y. Dietary therapy and herbal medicine for COVID-19 prevention: a review and perspective. J Tradit Complement Med. 2020;10(4):420–427. doi: 10.1016/j.jtcme.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S, Chufang L, Jin Z, Zhenhua J, Haiming J. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng W, Xu Y, Kong Q, Xue J, Yu P, Liu J, Lv Q, Li F, Wei Q, Bao L. Therapeutic efficacy of pudilan xiaoyan oral liquid (PDL) for COVID-19 in vitro and in vivo. Signal Transduct Target Ther. 2020;5(1):66. doi: 10.1038/s41392-020-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mirzaie A, Halaji M, Dehkordi FS, Ranjbar R, Noorbazargan H. A narrative literature review on traditional medicine options for treatment of corona virus disease 2019 (COVID-19) Complement Ther Clin Pract. 2020;40:101214. doi: 10.1016/j.ctcp.2020.101214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sen B. Potentiality and possibility of medicinal plants on ayurvedic principle in prevention and treatment of COVID-19. J Ayurvedic Herb Med. 2020;6(2):100–107. doi: 10.31254/jahm.2020.6216. [DOI] [Google Scholar]
- 60.Anam E, Swachho RB, Jannat K, Rahmatullah M. Home remedies for COVID-19 treatment in Gazipur district, Bangladesh. J Med Plant. 2021;9(1):25–28. [Google Scholar]
- 61.Upadhyay S, Tripathi PK, Singh M, Raghavendhar S, Bhardwaj M, Patel AK. Evaluation of medicinal herbs as a potential therapeutic option against SARS-CoV-2 targeting its main protease. Phytother Res. 2020;34(12):3411–3419. doi: 10.1002/ptr.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nawrot-Hadzik I, Zmudzinski M, Matkowski A, Preissner R, Kęsik-Brodacka M, Hadzik J, Drag M, Abel R. Reynoutria rhizomes as a natural source of SARS-CoV-2 Mpro inhibitors-molecular docking and in vitro study. Pharmaceuticals. 2021;14(8):742. doi: 10.3390/ph14080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li S-y, Chen C, Zhang H-q, Guo H-y, Wang H, Wang L, Zhang X, Hua S-n, Yu J, Xiao P-g, Li R-s, Tan X. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao R, Hu H, Li Y, Wang X, Xu M, Liu J, Zhang H, Yan Y, Zhao L, Li W, Zhang T, Xiao D, Guo X, Li Y, Yang J, Hu Z, Wang M, Zhong W. Anti-SARS-CoV-2 potential of artemisinins in vitro. ACS Infect Dis. 2020;6(9):2524–2531. doi: 10.1021/acsinfecdis.0c00522. [DOI] [PubMed] [Google Scholar]
- 65.Raj V, Park JG, Cho K-H, Choi P, Kim T, Ham J, Lee J. Assessment of antiviral potencies of cannabinoids against SARS-CoV-2 using computational and in vitro approaches. Int J Biol Macromol. 2021;168:474–485. doi: 10.1016/j.ijbiomac.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma Q, Li R, Pan W, Huang W, Liu B, Xie Y, Wang Z, Li C, Jiang H, Huang J, Shi Y, Dai J, Zheng K, Li X, Hui M, Fu L, Yang Z. Phillyrin (KD-1) exerts anti-viral and anti-inflammatory activities against novel coronavirus (SARS-CoV-2) and human coronavirus 229E (HCoV-229E) by suppressing the nuclear factor kappa B (NF-κB) signaling pathway. Phytomedicine. 2020;78:153296. doi: 10.1016/j.phymed.2020.153296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mösbauer K, Fritsch VN, Adrian L, Bernhardt J, Gruhlke MCH, Slusarenko AJ, Niemeyer D, Antelmann H (2021) Allicin inhibits SARS-CoV-2 replication and abrogates the antiviral host response in the Calu-3 proteome. bioRxiv. 10.1101/2021.05.15.444275
- 68.Alhadrami HA, Sayed AM, Sharif AM, Azhar EI, Rateb ME. Olive-derived triterpenes suppress SARS COV-2 main protease: a promising scaffold for future therapeutics. Molecules. 2021;26(9):2654. doi: 10.3390/molecules26092654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alhadrami HA, Sayed AM, Hassan HM, Youssif KA, Gaber Y, Moatasim Y, Kutkat O, Mostafa A, Ali MA, Rateb ME, Abdelmohsen UR, Gamaleldin NM. Cnicin as an anti-SARS-CoV-2: an integrated in silico and in vitro approach for the rapid identification of potential COVID-19 therapeutics. Antibiotics. 2021;10(5):542. doi: 10.3390/antibiotics10050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gangadevi S, Badavath VN, Thakur A, Yin N, De Jonghe S, Acevedo O, Jochmans D, Leyssen P, Wang K, Neyts J, Yujie T, Blum G. Kobophenol A inhibits binding of host ACE2 receptor with spike RBD domain of SARS-CoV-2, a lead compound for blocking COVID-19. J Phys Chem Lett. 2021;12(7):1793–1802. doi: 10.1021/acs.jpclett.0c03119. [DOI] [PubMed] [Google Scholar]
- 71.Xiong Y, Zhu G-H, Zhang Y-N, Hu Q, Wang H-N, Yu H-N, Qin X-Y, Guan X-Q, Xiang Y-W, Tang H, Ge G-B. Flavonoids in Ampelopsis grossedentata as covalent inhibitors of SARS-CoV-2 3CLpro: inhibition potentials, covalent binding sites and inhibitory mechanisms. Int J Biol Macromol. 2021;187:976–987. doi: 10.1016/j.ijbiomac.2021.07.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang M, Wei J, Huang T, Lei L, Shen C, Lai J, Yang M, Liu L, Yang Y, Liu G. Resveratrol inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cultured Vero cells. Phytother Res. 2020;2020:1–3. doi: 10.1002/ptr.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tietjen I, Cassel J, Register ET, Zhou XY, Messick TE, Keeney F, Lu LD, Beattie KD, Rali T, Ertl HCJ, Salvino JM, Davis RA, Montaner LJ (2021) The natural stilbenoid (–)-hopeaphenol inhibits cellular entry of SARS-CoV-2 USA-WA1/2020, B.1.1.7 and B.1.351 variants. bioRxiv. 10.1101/2021.04.29.442010 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are obtained from published literatures which are presented in the references.