Abstract
Elevated serum chitotriosidase (CHITO) is an indication of macrophage activation, and its capacity have been explored as a marker of inflammation in a number of disease states. For over a decade, CHITO plasma levels have been used by clinicians as a biomarker of inflammation in the lysosomal disease, Gaucher disease, including monitoring response to therapies in patients with Gaucher disease type I. Although it is becoming increasingly recognized that inflammation is a prominent component of many lysosomal diseases, the relation of CHITO levels to disease burden has not been well-characterized in the large majority of lysosomal diseases. Moreover, the role of CHITO in lysosomal diseases that affect the central nervous system (CNS) has not been systematically studied. In this study, one hundred and thirty-four specimens of CSF and serum were collected from 34 patients with lysosomal diseases affecting the CNS. This study included patients with GM1-gangliosidosis, GM2-gangliosidosis, mucopolysaccharidoses (MPS), multiple sulfatase deficiency and Gaucher disease. CHITO levels in the CSF were significantly higher in patients with more rapidly progressing severe neurological impairment: GM1-gangliosidosis vs MPS (p < 0.0001); GM2-gangliosidosis vs MPS (p < 0.0001). CHITO levels were higher in patients with the more severe phenotypes compared to milder phenotypes in GM1-gangliosidosis and GM2-gangliosidosis (serum CHITO in GM1-gangliosidosis infantile vs juvenile p = 0.025; CSF CHITO in Tay-Sachs infantile vs Tay-Sachs late-onset p < 0.0001). Moreover, higher CHITO levels in the CSF were significantly associated with lower cognitive test scores in patients with GM1-gangliosidosis, GM2-gangliosidosis, and MPS (p = 1.12*10−5, R2 = 0.72). Patients with infantile GM1-gangliosidosis showed increasing CSF CHITO over time, suggesting that CSF CHITO reflects disease progression and a possible surrogate endpoint for future clinical trials with infantile GM1-gangliosidosis. In summary, these results support the use of CSF CHITO to diagnose between different disease phenotypes and as a valuable tool for monitoring disease progression in patients. These results necessitate the inclusion of CHITO as an exploratory biomarker for clinical trials.
Keywords: Lysosomal diseases, Chitotriosidase, GM1-gangliosidosis, GM2-gangliosidosis, Mucopolysaccharidosis, Gaucher
1. Introduction
Chitotriosidase enzyme (CHITO) (EC: 3.2.1.14) is exclusively produced by activated macrophages in humans [1], [2], [3]. It is considered part of the innate immunity's response to chitin-containing pathogens [1], [2], [3]. CHITO is a marker of macrophage activation and has also been explored as a marker of inflammation in a number of disease states, including some lysosomal diseases [1], [4], [5], [6], [7], [8]. For over a decade, CHITO plasma levels have been used by clinicians as a biomarker of inflammation in the lysosomal disease, Gaucher disease, including monitoring response to therapies in patients with Gaucher disease type I [9], [10], [11], [12], [13].
Progressive neurodegeneration and associated neuro-inflammation are becoming increasingly recognized as important contributors towards morbidity and mortality in several lysosomal diseases, including GM1-gangliosidosis, GM2-gangliosidosis, and mucopolysaccharidoses (MPS) diseases [14], [15], [16], [17], [18], [19], [20]. The relation of CHITO levels to disease burden and disease-associated inflammation, however, has not been well-characterized in the majority of lysosomal diseases.
As part of an ongoing natural history study of the gangliosidoses and mucopolysaccharidoses, nearly 200 analytes in the cerebrospinal fluid (CSF) and serum from patients with GM1-gangliosidosis, GM2-gangliosidosis, and MPS were screened and the results reported by Jarnes, et al. (2014) [17]. In this previous work, five inflammatory mediators (ENA-78, MCP-1, MIP-1α, MIP-1β, and TNFR2) were identified to be persistently elevated in the CSF in the most severe phenotypes of the gangliosidoses [17]. CHITO was not assessed in the initial screening. Subsequent work by the same researchers yielded additional, albeit preliminary data, which suggested that levels of CHITO levels were persistently elevated in the CSF in the severe phenotypes of GM1- and GM2-gangliosidosis. This was reported at WORLDSymposium annual meeting, Orlando, Florida, 2019 [21].
A biomarker that reliably reflects neuro-inflammation, especially that of the CNS, would have the potential for being a valuable tool for diagnosing disease phenotypes and assessing response to lysosomal diseases therapies. The objectives of the study herein reported were to develop a validated chitotriosidase assay in a clinical laboratory and to determine if chitotriosidase levels in the serum or CSF were related to important clinical parameters in patients with lysosomal diseases. More specifically, this study aimed to determine if chitotriosidase levels could be diagnostic for different lysosomal diseases or phenotypes, useful in monitoring for disease progression, or associated with neurocognitive impairment in patients with lysosomal diseases.
2. Methods
2.1. Participant specimens
This study was conducted under clinical trials (NCT00668187 and NCT02030015) of the Lysosomal Disease Network (U54NS065768) which is a part of the National Institutes of Health (NIH) Rare Diseases Clinical Research Network (RDCRN). This study was conducted with IRB approval and IRB-approved consent of the patients' parents or legal guardians.
CSF specimens were collected by lumbar puncture with a 22-gauge (1.5- or 3.5-in.) Quincke spinal tap needle while the patient was under general anesthesia for MRI imaging. CSF was collected in 1 mL aliquots directly into cryovials, immediately labeled, placed on dry ice, and then transported to a laboratory for distribution or stored in a freezer at −80 °C. Blood specimens were obtained concurrently by phlebotomy, taken to a laboratory for centrifugation, aliquoted, frozen on dry ice, and then transported to the laboratory for distribution or stored in a freezer at −80 °C.
2.2. Chitotriosidase activity assay
Chitotriosidase activity assays were performed at two different labs. The original lab eventually discontinued its services for CSF specimens. At that time, the Gene Therapy and Diagnostics (GTD) Laboratory then developed its own chitotriosidase activity assay for CSF and serum specimens. Both labs used an artificial substrate for chitotriosidase and measured the fluorescent product. The GTD Lab performed the chitotriosidase assay for patient specimens as previously described with minor modifications [22]. Briefly, 5 μL of CSF or serum specimens was mixed with 100 μL of 26 μM of 4-methylumbelliferyl-β-D-N,N′,N″-triacetylchitotrioside (Sigma M5639) in 0.1 M/0.2 M citrate-phosphate buffer. This mixture was incubated at 37 °C for 15 min. To stop the reaction, 210 μL of 0.5 M glycine-NaOH buffer, pH 10.6, was added. Fluorescence was measured at 360 nm excitation and 455 nm emission using a Biotek plate reader. The lower limit of detection at the GTD Lab was 1.0 nmol/h/mL. The reference range for serum specimens was <80 nmol/h/mL. The reference range for CSF specimens was 5 nmol/h/mL.
2.3. Statistics
Statistics were performed using R and Graphpad Prism 8. To compare chitotriosidase levels reported between labs, a Pearson's correlation test was performed. To compare the independent disease profiles, showing changes in chitotriosidase levels at different ages (and therefore different times in disease progression) in the CSF between different lysosomal diseases, a one-way ANOVA type III test was performed with disease as the independent variable and chitotriosidase levels in the CSF as the dependent variable. Chitotriosidase levels in the CSF were natural log transformed, and outliers were excluded based on a pre-determined, studentized residual cut-off of ≥2.5. Multiple comparisons were adjusted using Tukey's method. These statistical steps were repeated for CSF specimens for different disease phenotypes, serum specimens for different diseases, and serum specimens for different disease phenotypes.
To analyze the relationship between chitotriosidase levels in the CSF and serum and neuropsychological testing done in patients, neuropsychological scores from the Bayley Scales of Infant and Toddler Development®, Third Edition (Bayley-III®) were used. All CSF and serum specimens were taken within one week of neuropsychological evaluations. A multiple linear regression was calculated to predict the raw score of the cognitive domain in the Bayley-IIII test based on the disease and chitotriosidase levels in the CSF. A Pearson's correlation test was performed with natural log transformed serum chitotriosidase levels and the raw score of the cognitive domain in the Bayley-III test. Chitotriosidase levels were natural log transformed, and outliers were excluded based on a pre-determined, studentized residual cut-off of ≥2.5.
3. Results
3.1. Validation of gene therapy and diagnostics lab's chitotriosidase activity assay to the original lab's assay
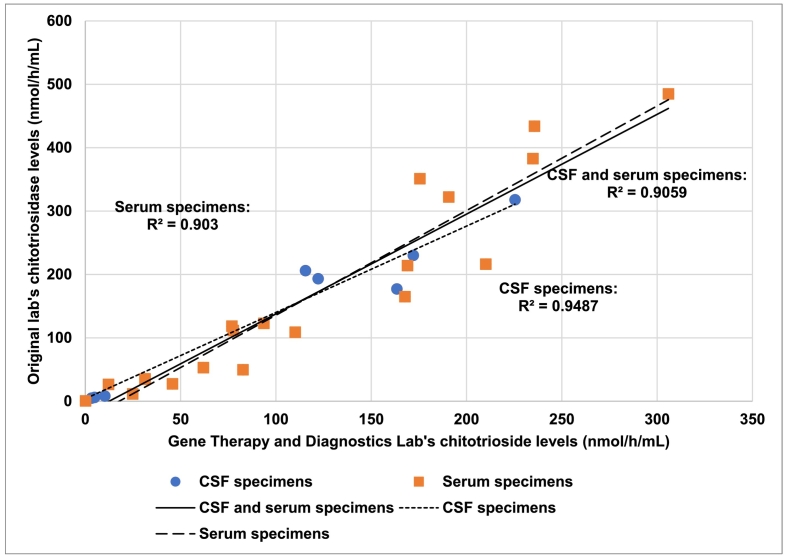
Since two different labs performed chitotriosidase assays for patient specimens, it was possible that chitotriosidase levels could differ between labs, which could confound further analysis. Therefore, a Pearson's correlation test was performed to see if there was a correlation in chitotriosidase levels obtained between the two labs. Twenty-eight specimens were processed at both labs. Of these specimens, eight were CSF specimens and 20 were serum specimens. Chitotriosidase levels between the two labs were highly correlated (R2 = 0.91 for CSF and serum specimens) (Fig. 1). Levels of chitotriosidase were highly correlated between the two labs, regardless of the specimen type (R2 = 0.95 for CSF specimens, R2 = 0.90 for serum specimens) (Fig. 1). Therefore, chitotriosidase levels reported by the two different labs were unlikely to confound analysis.
Fig. 1.
Comparison of chitotriosidase levels between labs.
Eight cerebrospinal fluid (CSF) specimens and 20 serum specimens were assayed at both the Gene Therapy and Diagnostics Lab and the original lab. Three Pearson's correlation tests were performed to see if there was a correlation between chitotriosidase levels reported at the Gene Therapy and Diagnostics Lab and the original lab. Correlation tests were performed for serum specimens alone, CSF specimens alone, and CSF and serum specimens together.
3.2. Summary of chitotriosidase levels in the CSF and serum specimens from patients
A total of 134 specimens from 34 patients were reported: 52 CSF specimens from 30 patients and 82 serum specimens from 33 patients. Patients with Gaucher disease, GM1-gangliosidosis, GM2-gangliosidosis, MPS, and multiple sulfatase deficiency (MSD) were included in this study.
3.3. Chitotriosidase levels in the CSF from patients
Patients with GM1- or GM2-gangliosidosis had higher levels of chitotriosidase and higher variations in levels than patients with Gaucher disease or MPS. To determine if there were significant differences in chitotriosidase levels in the CSF between diseases, a one-way ANOVA was performed. There was a statistically significant difference in chitotriosidase levels in the CSF between Gaucher disease and GM1-gangliosidosis (p = 0.0349); GM1-gangliosidosis and MPS (p < 0.0001); and GM2-gangliosidosis and MPS (p < 0.0001).
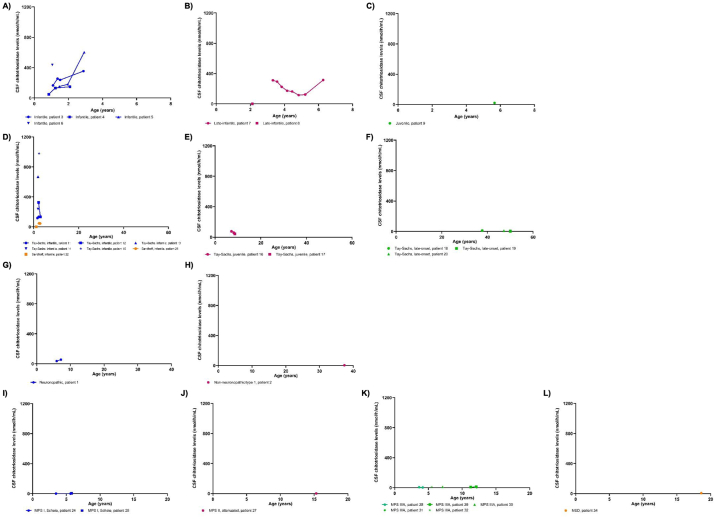
Chitotriosidase levels in the CSF within each lysosomal disease are shown in Fig. 2. In GM1-gangliosidosis, patients with infantile phenotype had increasing levels of chitotriosidase in the CSF over time (Fig. 2A). One patient with late-infantile GM1 had eight sequential levels that showed a steady decrease over time, with exception of the last level (Fig. 2B). CSF chitotriosidase appeared to be higher in the infantile and late-infantile phenotypes than the juvenile phenotype (Fig. 2A through 2C).
Fig. 2.
Chitotriosidase levels in the CSF in multiple lysosomal diseases.
Chitotriosidase levels in the cerebrospinal fluid (CSF) are shown for patients with lysosomal diseases. Sequential points from patients are connected by lines. A-C) GM1-gangliosidoses. Infantile: n = 11 CSF specimens, late-infantile: n = 9, juvenile: n = 1. D-F) GM2-gangliosidoses. Tay-Sachs infantile: n = 7 CSF specimens, Tay-Sachs juvenile: n = 3, Tay-Sachs adult: n = 3, and Sandhoff infantile: n = 3. G-H) Gaucher disease. Non-neuronopathic/type 1: n = 1 CSF specimen, neuronopathic phenotype: n = 2. I-L) Mucopolysaccharidoses (MPS) and multiple sulfatase deficiency (MSD). MPS IS: n = 3 CSF specimens, MPS II attenuated: n = 1, MPS IIIA: n = 7, and MSD: n = 1.
In GM2-gangliosidosis, two patients with infantile Sandhoff disease had lower levels of chitotriosidase levels than patients with infantile Tay-Sachs disease (Fig. 2D). Patients with infantile Tay-Sachs had higher chitotriosidase levels in the CSF than patients with juvenile or late-onset Tay-Sachs (Fig. 2D through 2F).
In patients with Gaucher disease, chitotriosidase levels decreased over time in the patient with known Gaucher disease type I phenotype, which may have been a treatment effect (Fig. 2G through 2H). It is unknown if these patients were receiving treatment for Gaucher disease.
In MPS, patients with MPS III type A (Sanfilippo syndrome type A, or MPS IIIA), an MPS condition associated with severe CNS disease, had higher chitotriosidase levels in the CSF than patients with MPS I Scheie (MPS IS), a phenotype not associated with severe CNS disease (Fig. 2I through 2L). Chitotriosidase levels were similar between patients with MPS IS, attenuated MPS II, and multiple sulfatase deficiency.
To determine if there were significant differences in chitotriosidase levels in the CSF between disease phenotypes, a one-way ANOVA was performed (Appendix Table 1). CSF chitotriosidase levels were significantly higher in most GM1-gangliosidosis phenotypes analyzed compared to Gaucher type I. Infantile GM1- and late-infantile GM1-gangliosidosis had significantly higher CSF chitotriosidase levels than nearly all MPS subtypes analyzed. Furthermore, infantile Tay-Sachs and juvenile Tay-Sachs had significantly higher CSF chitotriosidase levels compared to nearly all MPS subtypes analyzed. There was no significant difference between CSF chitotriosidase levels between the infantile and late-infantile GM1-gangliosidosis (p = 1) (Appendix Table 1), between the infantile GM1- and juvenile GM1-gangliosidosis (p = 0.17), and between the late-infantile GM1- and juvenile GM1-gangliosidosis (p = 0.19).
Infantile Tay-Sachs had a statistically significantly higher CSF chitotriosidase levels than late-onset Tay-Sachs (p < 0.0001). There was no significant difference between the infantile Tay-Sachs and juvenile Tay-Sachs (p = 0.16). However, juvenile Tay-Sachs had higher CSF chitotriosidase levels than late-onset Tay-Sachs, which was near statistical significance (p = 0.06).
3.4. Chitotriosidase levels in the serum from patients
Patients with Gaucher disease or GM1-gangliosidosis had higher CHITO serum levels than patients with GM2-gangliosidosis or MPS. A one-way ANOVA determined a significant difference in CHITO levels in the serum between all diseases analyzed, except GM2-gangliosidosis and MPS, which was near statistical significance.
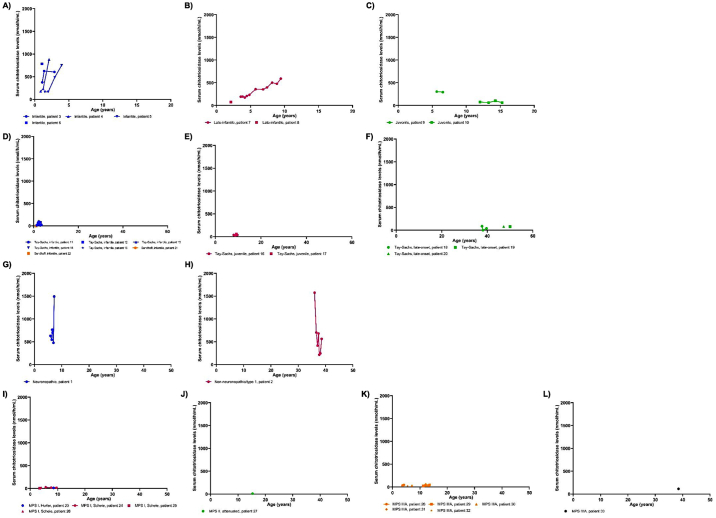
CHITO levels in the serum within each lysosomal disease group are shown in Fig. 3. In GM1-gangliosidosis, chitotriosidase levels increased over time for patients with infantile and late-infantile phenotypes but remained stable for patients with the juvenile phenotype (Fig. 3A through 3C). Serum chitotriosidase levels were the highest in the infantile, followed by the late-infantile, then juvenile phenotype.
Fig. 3.
Chitotriosidase levels in the serum in multiple lysosomal diseases.
Chitotriosidase levels in the serum are shown for patients with lysosomal diseases. Sequential points from patients are connected by lines. A-C) GM1-gangliosidoses. Infantile: n = 11 serum specimens, late-infantile: n = 12, juvenile: n = 6. D-F) GM2-gangliosidoses. Tay-Sachs infantile: n = 10 serum specimens, Tay-Sachs juvenile: n = 4, Tay-Sachs adult: n = 5, and Sandhoff infantile: n = 3. G-H) Gaucher disease. Non-neuronopathic/type 1: n = 7 serum specimens, neuronopathic phenotype: n = 6. I-L) Mucopolysaccharidoses (MPS). MPS IH: n = 1 serum specimen, MPS IS: n = 5, MPS II attenuated: n = 1, MPS IIIA: n = 9, and MPS IVA: n = 1.
In GM2-gangliosidosis, there were no apparent increases or decreases in serum CHITO over time across the GM2-gangliosidosis phenotypes (Fig. 3D through 3F). Serum CHITO levels were similar between infantile Tay-Sachs, juvenile Tay-Sachs, and late-onset Tay-Sachs, suggesting that chitotriosidase levels in the serum may not reflect disease severity in Tay-Sachs.
Serum CHITO levels were elevated in patients with Gaucher disease, independent of age (Fig. 3G through 3H). CHITO levels were similar between for the pediatric and adult patients, suggesting that serum CHITO is not influenced by age in Gaucher disease. The use of enzyme replacement therapy may have stabilized serum chitotriosidase levels between different age groups in Gaucher disease.
In MPS, patients with MPS IIIIA had levels that increased over time, but one patient with MPS IS subtype had levels that decreased over time (Fig. 3I through 3L), possibly a treatment effect. MPS IV type A (MPS IVA) had higher serum chitotriosidase levels than other MPS diseases, although more data is needed to confirm this. Patients with MPS IIIA had slightly higher chitotriosidase levels than patients with MPS IH, MPS IS, or MPS II attenuated. There were no apparent differences between the Hurler and Scheie subtypes of MPS I.
There was a statistically significant difference in chitotriosidase levels between different phenotypes of lysosomal diseases (Appendix Table 2). Infantile GM1- and late-infantile GM1-gangliosidosis had significantly higher serum chitotriosidase levels than infantile GM2- and juvenile GM2-gangliosidosis, and most MPS subtypes analyzed. There were no significant differences between infantile Tay-Sachs or juvenile Tay-Sachs compared to the MPS subtypes.
There was no difference between serum CHITO levels between the infantile and late-infantile GM1-gangliosidosis (p = 0.97) (Appendix Table 2), but infantile GM1-gangliosidosis had significantly higher serum CHITO levels than juvenile GM1-gangliosidosis (p = 0.03).
3.5. Chitotriosidase levels and cognitive performance in patients
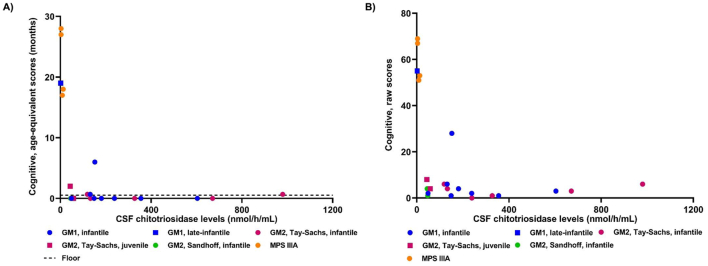
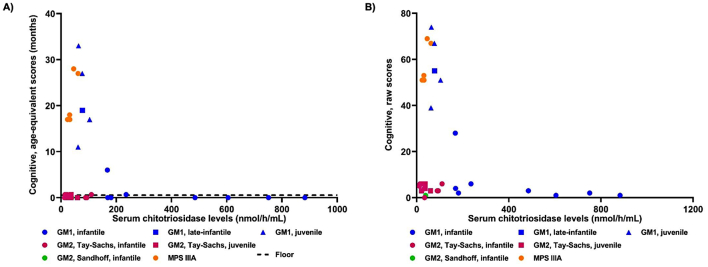
Bailey-III cognitive raw scores and age-equivalent scores were higher in patients with the lowest CHITO levels in the CSF (Fig. 4). The majority of the patients with GM1- or GM2-gangliosidosis scored at the lowest possible score of age-equivalent scores, which was <16 days. Therefore, the age-equivalent scores could not capture changes in cognitive function for most patients with GM1- or GM2-gangliosidosis. Patients with different diseases were tested at different ages, making it difficult to compare them to each other. Therefore, a multiple linear regression model was created to determine if there was a significant relationship between CSF CHITO levels and the Bayley-III cognitive domain's raw score, accounting for disease. CHITO levels in the CSF were a significant predictor of Bayley-III cognitive raw scores (p = 1.12*10−5). A significant regression equation was found with an adjusted R2 of 0.72 (p = 9.96*10−7). Bayley-III cognitive domain's raw scores were equal to 60.79-[9.92*ln(CHITO level in the CSF as nmol/h/mL)]-(7.17*disease), where disease was coded as 1 = GM1, 0 = GM2, and − 1 = MPS. Moreover, every increase in the natural log of CSF CHITO levels was associated with a decrease of 9.92 in the raw scores from the Bayley-III cognitive domain, adjusting for disease.
Fig. 4.
Bayley cognitive domain and chitotriosidase levels in the CSF.
Chitotriosidase levels in the CSF were plotted against the cognitive domain in the Bayley-III test measured as either age-equivalent scores (3A) or raw scores (3B). A) The floor (dotted line) designates the lowest possible score of the age-equivalent score, which is <16 days or 0.53 month.
Serum CHITO levels showed a strong association with raw scores from the Bayley-III cognitive domain for GM1-gangliosidosis but not for GM2-gangliosidosis or MPS (Fig. 5). A Pearson's correlation test showed that there was a statistically significant relationship between serum CHITO levels and raw scores from the Bayley-III cognitive domain (p = 0.0002, R2 of 0.69).
Fig. 5.
Bayley cognitive domain and chitotriosidase levels in the serum.
Chitotriosidase levels in the serum were plotted against the cognitive domain in the Bayley-III test measured as either age-equivalent scores (4A) or raw scores (4B). A) The floor (dotted line) designates the lowest possible score of the age-equivalent score, which is <16 days or 0.53 month.
4. Discussion
This is the first study to report CHITO levels in the CSF of patients with lysosomal diseases. In this study, CHITO levels were found to be elevated in the CSF of several lysosomal diseases with a more rapidly progressing CNS pathology, suggesting CSF CHITO has potential as a biomarker in lysosomal diseases that affect the CNS, with important applications in the diagnosing of disease phenotypes and monitoring disease progression. Higher CHITO levels in the CSF were associated with worsened cognitive function for GM1-gangliosidosis, GM2-gangliosidosis, and MPS.
4.1. Diagnosing disease phenotypes
The ability to predict phenotype (e.g. infantile vs juvenile vs late-onset) is becoming increasingly important in the era of newborn screening and will be critical for making treatment decisions for newly diagnosed infants. Currently, diagnosis of phenotypes is based on onset of signs and symptoms. This poses a problem because symptoms begin after neurological damage has already occurred [14]. Genotype-phenotype relationships have been helpful in predicting disease phenotypes of gangliosidoses but are not comprehensive, and there are discrepancies with some genotype-phenotype that have yet to be better understood [23], [24]. In this study, average CHITO levels were higher in more severe phenotypes of GM1-gangliosidosis (infantile vs juvenile) in both CSF and serum specimens. These results are similar to another study which also saw elevated serum chitotriosidase levels in patients with the more severe phenotypes of GM1 gangliosidosis [25]. CSF CHITO levels were higher in more severe phenotypes of GM2-gangliosidosis (infantile vs late-onset/adult onset). Thus, CHITO serum and CSF levels may aid in predicting gangliosidoses disease phenotypes, and thereby help guide treatment decisions and lead to earlier treatment.
4.2. Changes in CHITO levels over time
Measurement of serum CHITO levels in patients with Gaucher disease type I, is often used as an aid in determining response to therapy. In the untreated adult patient with Gaucher disease type I, CHITO levels are typically elevated, relatively stable. In contrast, CHITO levels in the blood and CSF showed marked increases over time in the severe infantile phenotype of GM1-gangliosidosis. Better understanding how a biomarker will fluctuate over time, if at all, as part of a disease natural history or assessment of response to therapy, will improve appropriate application and accurate interpretation of biomarker values.
Increases in the CHITO levels in the CSF over time were associated with progressive decreases in cognitive function. For every increase of the natural log CSF CHITO, there was a decrease of 9.92 raw scores in the cognitive domain of Bayley-III, adjusting for disease. CSF and serum chitotriosidase levels increased after the onset of seizures in one patient with infantile GM1-gangliosidosis and one patient with infantile Tay-Sachs disease. In this study, a broader comparison of CHITO levels before and after onset of initial seizures could not be performed as most of the initial chitotriosidase levels were drawn after the patients were experiencing seizures. Future studies could examine if CHITO is associated with other neurological outcomes in patients, such as seizures or changes in MRI.
4.3. Serum CHITO and bone health
In humans, CHITO is produced exclusively by activated macrophages. Osteoclasts are the resident macrophages in bones and play important roles in bone resorption. As such, increases in serum CHITO has been shown to be associated with increase bone resorption and is a biomarker in osteolytic diseases [26], [27]. Elevated serum chitotriosidase is a known indicator of bone health in Gaucher disease type I [28], [29]. This study demonstrated that serum CHITO is higher in MPS IVA than other MPS diseases, consistent with MPS IVA having more severe bone pathology and osteolytic pathology. In like fashion, serum CHITO was also elevated in GM1-gangliosidosis, a condition sharing numerous bone pathology with MPS conditions. Therefore, the elevated chitotriosidase levels in the serum may reflect the skeletal pathogenesis in numerous lysosomal diseases.
4.4. Additional considerations
Approximately 5% of the general population have homozygous mutations in the chit1 gene resulting in significantly reduced production or no production of CHITO (i.e. CHITO non-producers) [30]. In this study, one patient with infantile Sandhoff (patient 22) was a CHITO nonproducer and was excluded from the analysis. It is unknown if the other subjects were heterozygotes in terms of their chit1 genotype.
Recent studies have shown that chitotriosidase may induce pathogenic inflammation and tissue remodeling [31], [32]. Evidence of microglial activation and elevated chitotriosidase levels were seen in the brain tissue of a patient with Sandhoff disease [33]. Serum CHITO levels can be elevated from various non-lysosomal conditions or diseases. Elevations in serum CHITO levels has been reported in athlerosclerosis, brucellosis, and active tuberculosis [34], [35], [36]. Elevations in CHITO levels in the CSF has been reported in Alzheimer's disease and cerebral adrenoleukodystrophy [37], [38]. The elevations in CHITO levels from such conditions, however, are not as high as the elevations typically seen in untreated Gaucher disease type I, which commonly exceed levels greater than 1000 nmol/h/mL. Likewise, serum CHITO elevations found in subjects in this study who had infantile GM1-gangliosidosis and MPS IVA, exceeded that reported in non-lysosomal conditions.
4.5. Conclusions
This study has shown that CSF and serum CHITO may serve as a candidate biomarker for select lysosomal diseases with CNS, as well as peripheral disease pathology. Specifically, CSF CHITO levels may distinguish the most severe infantile forms of the gangliosidoses from more attenuated juvenile and adult/late-onset forms and may be a marker of disease progression and for therapy outcomes.
Acknowledgments
Acknowledgement
Dr. Sarah Kim is a fellow of the Lysosomal Disease Network. The LDN (2U54NS065768-06) is a part of the NCATS Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Diseases Research (ORDR), NCATS, funded through a collaboration between the NCATS, the NINDS and the NIDDK.
Appendix
Appendix Table 1.
Comparison of average chitotriosidase levels in the CSF between different lysosomal diseases and disease phenotypes.
| Disease Phenotype Comparisons | p-value | |
|---|---|---|
| Gaucher I | GM1, infantile | 0.0003*** |
| Gaucher I | GM1, juvenile | 0.8167 |
| Gaucher I | GM1, late-infantile | 0.0004*** |
| Gaucher I | MPS IS | 0.8759 |
| Gaucher I | MPS II, attenuated | 1 |
| Gaucher I | MPS IIIA | 0.9981 |
| Gaucher I | MSD | 0.9998 |
| Gaucher I | Sandhoff, infantile | 0.1616 |
| Gaucher I | Tay-Sachs, late-onset | 0.9969 |
| Gaucher I | Tay-Sachs, infantile | <0.0001**** |
| Gaucher I | Tay-Sachs, juvenile | 0.0622 |
| GM1, infantile | GM1, juvenile | 0.1704 |
| GM1, infantile | GM1, late-infantile | 1 |
| GM1, infantile | MPS IS | <0.0001**** |
| GM1, infantile | MPS II, attenuated | 0.0001**** |
| GM1, infantile | MPS IIIA | <0.0001**** |
| GM1, infantile | MSD | 0.005** |
| GM1, infantile | Sandhoff, infantile | 0.3447 |
| GM1, infantile | Tay-Sachs, late-onset | <0.0001**** |
| GM1, infantile | Tay-Sachs, infantile | 0.9996 |
| GM1, infantile | Tay-Sachs, juvenile | 0.341 |
| GM1, juvenile | GM1, late-infantile | 0.1944 |
| GM1, juvenile | MPS IS | 0.0209* |
| GM1, juvenile | MPS II, attenuated | 0.6151 |
| GM1, juvenile | MPS IIIA | 0.9515 |
| GM1, juvenile | MSD | 0.9948 |
| GM1, juvenile | Sandhoff, infantile | 0.9986 |
| GM1, juvenile | Tay-Sachs, late-onset | 0.9865 |
| GM1, juvenile | Tay-Sachs, infantile | 0.0925 |
| GM1, juvenile | Tay-Sachs, juvenile | 0.984 |
| GM1, late-infantile | MPS IS | <0.0001**** |
| GM1, late-infantile | MPS II, attenuated | 0.0001**** |
| GM1, late-infantile | MPS IIIA | <0.0001**** |
| GM1, late-infantile | MSD | 0.0064** |
| GM1, late-infantile | Sandhoff, infantile | 0.4022 |
| GM1, late-infantile | Tay-Sachs, late-onset | <0.0001**** |
| GM1, late-infantile | Tay-Sachs, infantile | 0.9997 |
| GM1, late-infantile | Tay-Sachs, juvenile | 0.417 |
| MPS IS | MPS II, attenuated | 0.9808 |
| MPS IS | MPS IIIA | 0.0075** |
| MPS IS | MSD | 0.3465 |
| MPS IS | Sandhoff, infantile | <0.0001**** |
| MPS IS | Tay-Sachs, late-onset | 0.0265* |
| MPS IS | Tay-Sachs, infantile | <0.0001**** |
| MPS IS | Tay-Sachs, juvenile | <0.0001**** |
| MPS II, attenuated | MPS IIIA | 0.9603 |
| MPS II, attenuated | MSD | 0.9947 |
| MPS II, attenuated | Sandhoff, infantile | 0.0697 |
| MPS II, attenuated | Tay-Sachs, late-onset | 0.9557 |
| MPS II, attenuated | Tay-Sachs, infantile | <0.0001**** |
| MPS II, attenuated | Tay-Sachs, juvenile | 0.0226* |
| MPS IIIA | MSD | 1 |
| MPS IIIA | Sandhoff, infantile | 0.0822 |
| MPS IIIA | Tay-Sachs, late-onset | 1 |
| MPS IIIA | Tay-Sachs, infantile | <0.0001**** |
| MPS IIIA | Tay-Sachs, juvenile | 0.0072** |
| MSD | Sandhoff, infantile | 0.6089 |
| MSD | Tay-Sachs, late-onset | 1 |
| MSD | Tay-Sachs, infantile | 0.0024** |
| MSD | Tay-Sachs, juvenile | 0.366 |
| Sandhoff, infantile | Tay-Sachs, late-onset | 0.2568 |
| Sandhoff, infantile | Tay-Sachs, infantile | 0.1776 |
| Sandhoff, infantile | Tay-Sachs, juvenile | 1 |
| Tay-Sachs, late-onset | Tay-Sachs, infantile | <0.0001**** |
| Tay-Sachs, late-onset | Tay-Sachs, juvenile | 0.0615 |
| Tay-Sachs, infantile | Tay-Sachs, juvenile | 0.1622 |
A one-way ANOVA was performed with disease phenotype and chitotriosidase levels in the CSF. Neuronopathic phenotype of Gaucher disease was excluded from analysis because the exact phenotype of Gaucher was uncertain (i.e. type 2 vs type 3). Multiple comparisons were adjusted using Tukey's method. Gaucher I = Gaucher disease type I. MPS = mucopolysaccharidosis. MPS IS = MPS I Scheie. MPS IIIA = MPS III type A. MSD = multiple sulfatase deficiency. * p < 0.5. ** p ≤ 0.01. *** p ≤ 0.001. ****p ≤ 0.0001.
Appendix Table 2.
Comparison of average chitotriosidase levels in the serum between different lysosomal diseases and phenotypes.
| Disease Phenotype Comparisons | p-value | |
|---|---|---|
| Gaucher I | GM1, infantile | 0.9868 |
| Gaucher I | GM1, juvenile | 0.0016*** |
| Gaucher I | GM1, late-infantile | 0.3498 |
| Gaucher I | MPS IH | <0.0001**** |
| Gaucher I | MPS IS | <0.0001**** |
| Gaucher I | MPS II, attenuated | <0.0001**** |
| Gaucher I | MPS IIIA | <0.0001**** |
| Gaucher I | MPS IVA | 0.4582 |
| Gaucher I | Sandhoff, infantile | 0.0001**** |
| Gaucher I | Tay-Sachs, late-onset | 0.0001**** |
| Gaucher I | Tay-Sachs, infantile | <0.0001**** |
| Gaucher I | Tay-Sachs, juvenile | <0.0001**** |
| GM1, infantile | GM1, juvenile | 0.0252* |
| GM1, infantile | GM1, late-infantile | 0.9696 |
| GM1, infantile | MPS IH | 0.0002*** |
| GM1, infantile | MPS IS | <0.0001**** |
| GM1, infantile | MPS II, attenuated | <0.0001**** |
| GM1, infantile | MPS IIIA | <0.0001**** |
| GM1, infantile | MPS IVA | 0.8031 |
| GM1, infantile | Sandhoff, infantile | 0.0007*** |
| GM1, infantile | Tay-Sachs, late-onset | 0.0012** |
| GM1, infantile | Tay-Sachs, infantile | <0.0001**** |
| GM1, infantile | Tay-Sachs, juvenile | <0.0001**** |
| GM1, juvenile | GM1, late-infantile | 0.3462 |
| GM1, juvenile | MPS IH | 0.0876 |
| GM1, juvenile | MPS IS | <0.0001**** |
| GM1, juvenile | MPS II, attenuated | 0.0273* |
| GM1, juvenile | MPS IIIA | 0.0393* |
| GM1, juvenile | MPS IVA | 1 |
| GM1, juvenile | Sandhoff, infantile | 0.5838 |
| GM1, juvenile | Tay-Sachs, late-onset | 0.9842 |
| GM1, juvenile | Tay-Sachs, infantile | 0.0603 |
| GM1, juvenile | Tay-Sachs, juvenile | 0.207 |
| GM1, late-infantile | MPS IH | 0.0015** |
| GM1, late-infantile | MPS IS | <0.0001**** |
| GM1, late-infantile | MPS II, attenuated | 0.0003*** |
| GM1, late-infantile | MPS IIIA | <0.0001**** |
| GM1, late-infantile | MPS IVA | 0.9815 |
| GM1, late-infantile | Sandhoff, infantile | 0.0085** |
| GM1, late-infantile | Tay-Sachs, late-onset | 0.0264* |
| GM1, late-infantile | Tay-Sachs, infantile | <0.0001**** |
| GM1, late-infantile | Tay-Sachs, juvenile | 0.0001**** |
| MPS IH | MPS IS | 1 |
| MPS IH | MPS II, attenuated | 1 |
| MPS IH | MPS IIIA | 0.9404 |
| MPS IH | MPS IVA | 0.453 |
| MPS IH | Sandhoff, infantile | 0.9748 |
| MPS IH | Tay-Sachs, late-onset | 0.4759 |
| MPS IH | Tay-Sachs, infantile | 0.9013 |
| MPS IH | Tay-Sachs, juvenile | 0.9565 |
| MPS IS | MPS II, attenuated | 1 |
| MPS IS | MPS IIIA | 0.1424 |
| MPS IS | MPS IVA | 0.1025 |
| MPS IS | Sandhoff, infantile | 0.6765 |
| MPS IS | Tay-Sachs, late-onset | 0.0087** |
| MPS IS | Tay-Sachs, infantile | 0.0736 |
| MPS IS | Tay-Sachs, juvenile | 0.3625 |
| MPS II, attenuated | MPS IIIA | 0.736 |
| MPS II, attenuated | MPS IVA | 0.2572 |
| MPS II, attenuated | Sandhoff, infantile | 0.8632 |
| MPS II, attenuated | Tay-Sachs, late-onset | 0.2314 |
| MPS II, attenuated | Tay-Sachs, infantile | 0.6547 |
| MPS II, attenuated | Tay-Sachs, juvenile | 0.7912 |
| MPS IIIA | MPS IVA | 0.8914 |
| MPS IIIA | Sandhoff, infantile | 1 |
| MPS IIIA | Tay-Sachs, late-onset | 0.8819 |
| MPS IIIA | Tay-Sachs, infantile | 1 |
| MPS IIIA | Tay-Sachs, juvenile | 1 |
| MPS IVA | Sandhoff, infantile | 0.9687 |
| MPS IVA | Tay-Sachs, late-onset | 1 |
| MPS IVA | Tay-Sachs, infantile | 0.9284 |
| MPS IVA | Tay-Sachs, juvenile | 0.9319 |
| Sandhoff, infantile | Tay-Sachs, late-onset | 0.9952 |
| Sandhoff, infantile | Tay-Sachs, infantile | 1 |
| Sandhoff, infantile | Tay-Sachs, juvenile | 1 |
| Tay-Sachs, late-onset | Tay-Sachs, infantile | 0.9416 |
| Tay-Sachs, late-onset | Tay-Sachs, juvenile | 0.9667 |
| Tay-Sachs, infantile | Tay-Sachs, juvenile | 1 |
A one-way ANOVA was performed with disease phenotype and chitotriosidase levels in the serum. Neuronopathic phenotype of Gaucher disease was excluded from analysis because the exact phenotype of Gaucher was uncertain (i.e. type 2 vs type 3). Multiple comparisons were adjusted using Tukey's method. Gaucher I = Gaucher type I. MPS = mucopolysaccharidosis. MPS IH = MPS I Hurler. MPS IS = MPS I Scheie. MPS IIIA = MPS III type A. MPS IVA = MPS IV type A. * p < 0.5. ** p ≤ 0.01. *** p ≤ 0.001. ****p ≤ 0.0001.
References
- 1.Hollak C.E., van Weely S., van Oers M.H., Aerts J.M. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J. Clin. Invest. 1994;93:1288–1292. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renkema G.H., Boot R.G., Strijland A., Donker-Koopman W.E., van den Berg M., Muijsers A.O., Aerts J.M. Synthesis, sorting, and processing into distinct isoforms of human macrophage chitotriosidase. Eur. J. Biochem. 1997;244:279–285. doi: 10.1111/j.1432-1033.1997.00279.x. [DOI] [PubMed] [Google Scholar]
- 3.van Eijk M., van Roomen C.P., Renkema G.H., Bussink A.P., Andrews L., Blommaart E.F., Sugar A., Verhoeven A.J., Boot R.G., Aerts J.M. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int. Immunol. 2005;17:1505–1512. doi: 10.1093/intimm/dxh328. [DOI] [PubMed] [Google Scholar]
- 4.Guo Y., He W., Boer A.M., Wevers R.A., de Bruijn A.M., Groener J.E., Hollak C.E., Aerts J.M., Galjaard H., van Diggelen O.P. Elevated plasma chitotriosidase activity in various lysosomal storage disorders. J. Inherit. Metab. Dis. 1995;18:717–722. doi: 10.1007/BF02436762. [DOI] [PubMed] [Google Scholar]
- 5.Michelakakis H., Dimitriou E., Labadaridis I. The expanding spectrum of disorders with elevated plasma chitotriosidase activity: an update. J. Inherit. Metab. Dis. 2004;27:705–706. doi: 10.1023/b:boli.0000043025.17721.fc. [DOI] [PubMed] [Google Scholar]
- 6.Isman F., Hobert J.A., Thompson J.N., Natowicz M.R. Plasma chitotriosidase in lysosomal storage diseases. Clin. Chim. Acta<font color="#000000"/>. 2008;387:165–167. doi: 10.1016/j.cca.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Elmonem M.A., Ramadan D.I., Issac M.S., Selim L.A., Elkateb S.M. Blood spot versus plasma chitotriosidase: a systematic clinical comparison. Clin. Biochem. 2014;47:38–43. doi: 10.1016/j.clinbiochem.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Sheth J.J., Sheth F.J., Oza N.J., Gambhir P.S., Dave U.P., Shah R.C. Plasma chitotriosidase activity in children with lysosomal storage disorders. Indian J. Pediatr. 2010;77:203–205. doi: 10.1007/s12098-009-0249-0. [DOI] [PubMed] [Google Scholar]
- 9.Aerts J.M., Kallemeijn W.W., Wegdam W., Joao Ferraz M., van Breemen M.J., Dekker N., Kramer G., Poorthuis B.J., Groener J.E., Cox-Brinkman J., Rombach S.M., Hollak C.E., Linthorst G.E., Witte M.D., Gold H., van der Marel G.A., Overkleeft H.S., Boot R.G. Biomarkers in the diagnosis of lysosomal storage disorders: proteins, lipids, and inhibodies. J. Inherit. Metab. Dis. 2011;34:605–619. doi: 10.1007/s10545-011-9308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollak C.E., Maas M., Aerts J.M. Clinically relevant therapeutic endpoints in type I Gaucher disease. J. Inherit. Metab. Dis. 2001;24 Suppl 2:97–105. doi: 10.1023/a:1012492429191. [DOI] [PubMed] [Google Scholar]
- 11.Genzyme Corporation Cerezyme-imiglucerase injection, powder, lyophilized, for solution. Human prescription drug label. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=df60f030-866b-4374-a31f-8ae3f6b45c38
- 12.Takeda Pharmaceuticals America, Inc. VPRIV- velaglucerase alfa injection, powder, lyophilized, for solution. Human prescription drug label. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ca02f7a4-ae4f-43c1-a06a-259fe4fcf9cf
- 13.Pfizer Elelyso- taliglucerase alfa injection, powder, lyophilized, for solution. Human prescription drug label. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fa3cbd5d-677c-4b19-9032-d9182cb69a83
- 14.Jarnes Utz J.R., Kim S., King K., Ziegler R., Schema L., Redtree E.S., Whitley C.B. Infantile gangliosidoses: mapping a timeline of clinical changes. Mol. Genet. Metab. 2017;121:170–179. doi: 10.1016/j.ymgme.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro E.G., Nestrasil I., Rudser K., Delaney K., Kovac V., Ahmed A., Yund B., Orchard P.J., Eisengart J., Niklason G.R., Raiman J., Mamak E., Cowan M.J., Bailey-Olson M., Harmatz P., Shankar S.P., Cagle S., Ali N., Steiner R.D., Wozniak J., Lim K.O., Whitley C.B. Neurocognition across the spectrum of mucopolysaccharidosis type I: age, severity, and treatment. Mol. Genet. Metab. 2015;116:61–68. doi: 10.1016/j.ymgme.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeyakumar M., Butters T.D., Dwek R.A., Platt F.M. Glycosphingolipid lysosomal storage diseases: therapy and pathogenesis. Neuropathol. Appl. Neurobiol. 2002;28:343–357. doi: 10.1046/j.1365-2990.2002.00422.x. [DOI] [PubMed] [Google Scholar]
- 17.Utz J.R., Crutcher T., Schneider J., Sorgen P., Whitley C.B. Biomarkers of central nervous system inflammation in infantile and juvenile gangliosidoses. Mol. Genet. Metab. 2015;114:274–280. doi: 10.1016/j.ymgme.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Archer L.D., Langford-Smith K.J., Bigger B.W., Fildes J.E. Mucopolysaccharide diseases: a complex interplay between neuroinflammation, microglial activation and adaptive immunity. J. Inherit. Metab. Dis. 2014;37:1–12. doi: 10.1007/s10545-013-9613-3. [DOI] [PubMed] [Google Scholar]
- 19.Ou L., Przybilla M.J., Whitley C.B. Proteomic analysis of mucopolysaccharidosis I mouse brain with two-dimensional polyacrylamide gel electrophoresis. Mol. Genet. Metab. 2017;120:101–110. doi: 10.1016/j.ymgme.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ou L., Przybilla M.J., Whitley C.B. Metabolomics profiling reveals profound metabolic impairments in mice and patients with sandhoff disease. Mol. Genet. Metab. 2019;126:151–156. doi: 10.1016/j.ymgme.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitley C.B., Jarnes J.R. February 2019. Chitotriosidase as a biomarker for central nervous system inflammation in the gangliosidosis diseases. [Google Scholar]
- 22.Wajner A., Michelin K., Burin M.G., Pires R.F., Pereira M.L., Giugliani R., Coelho J.C. Comparison between the biochemical properties of plasma chitotriosidase from normal individuals and from patients with Gaucher disease, GM1-gangliosidosis, Krabbe disease and heterozygotes for Gaucher disease. Clin. Biochem. 2007;40:365–369. doi: 10.1016/j.clinbiochem.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Ou L., Kim S., Whitley C.B., Jarnes-Utz J.R. Genotype-phenotype correlation of gangliosidosis mutations using in silico tools and homology modeling. Mol. Genet. Metabol. Rep. 2019;20 doi: 10.1016/j.ymgmr.2019.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ou L., Przybilla M.J., Whitley C.B. SAAMP 2.0: analgorithm to predict genotype-phenotype correlation of lysosomal storage diseases. Clin. Genet. 2018;93:1008–1014. doi: 10.1111/cge.13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arash-Kaps L., Komlosi K., Seegräber M., Diederich S., Paschke E., Amraoui Y., Beblo S., Dieckmann A., Smitka M., Hennermann J.B. The clinical and molecular spectrum of GM1 gangliosidosis. J. Pediatr. 2019;215:152–157.e153. doi: 10.1016/j.jpeds.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Di Rosa M., Tibullo D., Vecchio M., Nunnari G., Saccone S., Di Raimondo F., Malaguarnera L. Determination of chitinases family during osteoclastogenesis. Bone. 2014;61:55–63. doi: 10.1016/j.bone.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Rosa M.Di, Malaguarnera L. In: in Biomarkers in Bone Disease. Patel V.B., Preedy V.R., editors. Springer; Netherlands, Dordrecht: 2017. pp. 301–327. [Google Scholar]
- 28.Stirnemann J., Belmatoug N., Vincent C., Fain O., Fantin B., Mentre F. Bone events and evolution of biologic markers in Gaucher disease before and during treatment. Arthritis Res. Therapy. 2010;12:R156. doi: 10.1186/ar3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raskovalova T., Deegan P.B., Mistry P.K., Pavlova E., Yang R., Zimran A., Berger J., Bourgne C., Pereira B., Labarere J., Berger M.G. Accuracy of chitotriosidase activity and CCL18 concentration in assessing type I Gaucher disease severity. A systematic review with meta-analysis of individual participant data. Haematologica. 2020 doi: 10.3324/haematol.2019.236083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elmonem M.A., van den Heuvel L.P., Levtchenko E.N. Immunomodulatory effects of chitotriosidase enzyme. Enzyme Res. 2016;2016:2682680. doi: 10.1155/2016/2682680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varghese A.M., Sharma A., Mishra P., Vijayalakshmi K., Harsha H.C., Sathyaprabha T.N., Bharath S.M., Nalini A., Alladi P.A., Raju T.R. Chitotriosidase - a putative biomarker for sporadic amyotrophic lateral sclerosis. Clin. Proteomics. 2013;10:19. doi: 10.1186/1559-0275-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malaguarnera L., Di Rosa M., Zambito A.M., dell'Ombra N., Di Marco R., Malaguarnera M. Potential role of chitotriosidase gene in nonalcoholic fatty liver disease evolution. Am. J. Gastroenterol. 2006;101:2060–2069. doi: 10.1111/j.1572-0241.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 33.Wada R., Tifft C.J., Proia R.L. Microglial activation precedes acute neurodegeneration in Sandhoff disease and is suppressed by bone marrow transplantation. Vol. 97. 2000. pp. 10954–10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coskun O., Oter S., Yaman H., Kilic S., Kurt I., Eyigun C.P. Evaluating the validity of serum neopterin and chitotriosidase levels in follow-up brucellosis patients. Intern. Med. 2010;49:1111–1118. doi: 10.2169/internalmedicine.49.3113. [DOI] [PubMed] [Google Scholar]
- 35.Tasci C., Tapan S., Ozkaya S., Demirer E., Deniz O., Balkan A., Ozkan M., Inan I., Kurt I., Bilgic H. Efficacy of serum chitotriosidase activity in early treatment of patients with active tuberculosis and a negative sputum smear. Ther. Clin. Risk Manag. 2012;8:369–372. doi: 10.2147/TCRM.S31752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Artieda M., Cenarro A., Ganan A., Jerico I., Gonzalvo C., Casado J.M., Vitoria I., Puzo J., Pocovi M., Civeira F. Serum chitotriosidase activity is increased in subjects with atherosclerosis disease. Arterioscler. Thromb. Vasc. Biol. 2003;23:1645–1652. doi: 10.1161/01.ATV.0000089329.09061.07. [DOI] [PubMed] [Google Scholar]
- 37.Orchard P.J., Lund T., Miller W., Rothman S.M., Raymond G., Nascene D., Basso L., Cloyd J., Tolar J. Chitotriosidase as a biomarker of cerebral adrenoleukodystrophy. J. Neuroinflammation. 2011;8:144. doi: 10.1186/1742-2094-8-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattsson N., Tabatabaei S., Johansson P., Hansson O., Andreasson U., Mansson J.E., Johansson J.O., Olsson B., Wallin A., Svensson J., Blennow K., Zetterberg H. Cerebrospinal fluid microglial markers in Alzheimer's disease: elevated chitotriosidase activity but lack of diagnostic utility. Neuromol. Med. 2011;13:151–159. doi: 10.1007/s12017-011-8147-9. [DOI] [PubMed] [Google Scholar]