Highlights
-
•
This review summarizes the current state of cardiac radioablation (CRA) for ventricular tachycardia.
-
•
Clinical data and radiobiologic principles supporting CRA are reviewed.
-
•
An example workflow, including guidance for interpreting cardiac mapping, is provided.
Keywords: Cardiac radioablation, SBRT, Ventricular tachycardia, Cardiac mapping, Cardiac arrhythmias
Abstract
Cardiac radioablation with SBRT is a very promising non-invasive modality for the treatment of refractory VT and potentially other cardiac arrhythmias. Initial reports indicate that it is relatively safe and associated with excellent responses, particularly in reduction of ICD-related events, need for anti-arrhythmic medications, and resulting in significantly improved quality of life for patients. Establishment of objective criteria for candidates for cardiac radioablation will accelerate the adoption of this important radiation therapy modality in the treatment of refractory VT and other cardiac arrhythmias in the coming years. In addition, in order to develop more prospective safety and efficacy data, treatment of patients should ideally be performed in the context of clinical trials or prospective registries at, or in collaboration with, experienced centers. Taken together, the future of cardiac radioablation is rich and worthy of further investigation to become a standard treatment in the armamentarium against refractory VT.
1. Introduction
In 2020, over 14 million Americans are living with a heart rhythm disorder whose incidence increases with age and cardiovascular comorbidities [1]. Covering a wide spectrum of disorders, cardiac arrhythmias are commonly divided into supraventricular arrhythmias such as atrial fibrillation, Wolff-Parkinson-White Syndrome and ventricular tachyarrhythmias including ventricular tachycardia (VT) and ventricular fibrillation (VF), which can lead to sudden cardiac death (SCD) if not managed appropriately. Significant progress has been made in the treatment of ventricular tachycardia with individually-tailored medical management, implantable cardioverter-defibrillators (ICD) placement, and catheter ablations. However, these cardiac arrhythmias remain a debilitating problem for millions of patients. Terminal cases with frequent ICD discharges are associated with severe anxiety and drastically impact patient’s quality of life [2]. Despite clear advances in the pharmacologic and non-pharmacologic management of VT and VF, more research and additional therapeutic modalities need to be explored to treat and improve outcomes in this vulnerable patient population.
2. Pathophysiology
Ventricular tachycardia is defined as three or more consecutive beats from the ventricles occurring at a rate of >100 beats per minute. VT commonly arises from heterogeneous myocardial fibrosis causing abnormal automaticity leading to electrical reentry [3]. VT is generally categorized as sustained (lasting >30 s) or non-sustained (<30 s). Sustained VT is most commonly a result of ischemic heart disease leading to potassium leakage, abnormal conduction, and may ultimately culminate in polymorphic VT/VF and SCD. Among patients with acute myocardial infarct (AMI), 5–10% experience sustained VT or VF prior to hospital presentation with the majority experiencing ventricular dysrhythmias within 48 h of admission [4]. Beyond ischemic heart disease, structural and congenital heart disease, non-ischemic cardiomyopathy, ion channel abnormalities, and other hereditary cardiac conditions can contribute to the development of ventricular tachycardia.
3. Standard treatment paradigm
The treatment of VT ranges from observation to medical management to cardioversion and complex surgical interventions like heart transplant. Non-sustained VT in asymptomatic patients without underlying cardiac comorbidities can be observed safely. Stable, yet symptomatic patients can be medically-managed with antiarrhythmics until VT termination is achieved. Hemodynamically unstable patients should immediately undergo external defibrillation and advanced cardiovascular life support in the hospital setting. Chronic VT treatment consists of pharmacological, catheter-based, and surgical interventions [5]. First-line pharmacologic treatment generally consists of Vaughan-Williams Class II (Beta blockers) and class III antiarrhythmic drugs. Beta-blockers are widely used given their safety profile and have been proven to decrease mortality secondary to heart failure and SCD [6]. Class III antiarrhythmics (Amiodarone, Sotalol, Dofetilide) are generally added for patients with VT refractory to beta-blockers alone [7]. Additionally, mexiletine is a Vaughan-Williams Class Ib agent and has similar electrophysiological properties to lidocaine. Mexiletine has been used alone and more commonly in conjunction with class III drugs. In cases where VT is refractory to pharmacological therapy, catheter ablation can be utilized to identify and ablate areas where electrical reentry is occurring. Survivors of a SCD event (secondary prevention) and those who are deemed at risk of a SCD event due to severe cardiomyopathy (primary prevention) should undergo ICD placement. Lastly, when VT fails to respond to pharmacological and catheter-based interventions, surgical ablation, cardiac sympathetic denervation, and even heart transplant may be performed [8], [9], [10]. In the past, ablation and other surgical interventions were commonly used as a last line of therapy. Guidelines and current trends are advocating for earlier implementation of ablation and other surgical intervention rather than exhausting pharmacologic options upfront [11]. Despite this, these treatments are expensive, limited by available resources (e.g. expertise of surgeon, availability of orthotopic transplant hearts, etc.), and can result in significant morbidity leading to changes in quality of life. Thus, there is a need for novel therapies for VT that are readily available and are associated with preservation of quality of life for patients who fail conventional therapies.
4. History of cardiac radioablation
Stereotactic body radiation therapy (SBRT) is a widely used technique within radiation oncology capable of delivering high doses of ablative radiation with sub-millimeter precision. First developed in Sweden in the early 1990s for abdominal malignancies, it has since become a standard of care in the treatment of prostate, lung, hepatocellular, breast, and many other metastatic cancers to multiple sites in the body (e.g. lungs, liver, adrenal, pancreas, bone) [12]. In 2010 Sharma et al. published their pioneering work demonstrating SBRT was a novel and effective treatment for cardiac arrhythmias using a pre-clinical porcine model. Using a CyberKnife platform (CyberHeart) with motion management software his team demonstrated 25 Gy in a single fraction could produce an electrophysiologic effect on 16 Hanford-Sinclair mini swine. Pathology performed on the mini-swine model 90 days following the single fraction treatment found abnormal foci in the targeted area with essentially no damage outside the treatment area [13]. In 2016, a German group treated another porcine model and found heavy ion radiation doses of 40 and 55 Gy led to a strong fibrotic response on histologic analysis, while sparing adjacent normal tissue [14]. Taken together, these two pre-clinical models demonstrated cardiac radioablation was feasible, induced altered electrophysiologic effects through scar formation, and could be accurately delivered in the pre-clinical setting, laying the ground work for human clinical trials in the coming years.
The first in-human treatment of cardiac arrhythmia using SBRT occurred in 2014 when a group at Stanford University successfully treated a 71 year-old male with drug refractory VT who was not a candidate for catheter ablation. A radiation treatment plan was created targeting the proposed ablation volume using cardiac mapping, and 25 Gy in a single fraction was successfully delivered over 90 min. Overall the patient tolerated the procedure well with no complications and did not require sedation. Follow-up ICD interrogations revealed a decrease in average episodes from 562 prior to treatment to 52 in the 9 months following SBRT [15]. In July 2014, a group in the Czech Republic reported the second in-human treatment when they successfully treated a 72 year-old female with recurrent VT. Again, using a single fraction of 25 Gy delivered with the CyberKnife platform, the treatment was well-tolerated and no episodes of malignant arrhythmia were detected by ICD in the 120 days following treatment [16].
5. Subsequent clinical studies
Building on these pre-clinical and first in-human studies, a team at Washington University in St. Louis, MO published their sentinel work in the New England Journal of Medicine in December 2017 reporting on their experience of treating five patients in a seven month window in 2015. All five patients had VT refractory to conventional treatment modalities and were treated with SBRT, 25 Gy in 1 fraction, similar to the pre-clinical models and case studies outlined above. The treating radiation oncology and electrophysiology team used single photon emission CT (SPECT) or contrast-enhanced cardiac magnetic resonance imaging (CMR) to localize the cardiac regions of anatomical scarring, non-invasive electrocardiographic mapping, and a four-dimensional CT simulation to identify the target volume and develop a radiation treatment plan. Patients were immobilized in vacuum-assisted immobilization devices and treatment was delivered using on-board imaging. Each treatment lasted on average for fifteen minutes. In the three months prior to radioablation, these five patients had 6577 episodes of VT in aggregate. Following a 6 week “blanking period” only 4 episodes of VT were identified over the next 46 months, a remarkable 99.9% reduction in ICD events from baseline [17]. Equally impressive was how well-tolerated the procedures were in this exploratory cohort. No complications occurred during the radioablations or index hospitalizations and patients were discharged within 3 days of treatment on average. Baseline and post-treatment echocardiograms demonstrated mean left ventricular ejection fraction rose 6% from a baseline score of 23% at the last follow-up visit (range −2 to + 22%). No adverse effects were observed in ICD performance and there were no acute heart failure exacerbations recorded in the treated patients [17]. Of note, one patient did suffer a fatal stroke three weeks after treatment. The patient, an 83-year old woman who had a history of atrial fibrillation and severe cardiomyopathy, was not on anticoagulation therapy during or after her radioablation. After post-mortem work-up, it remained unclear whether the patient’s stroke was associated with her SBRT or from a pre-existing medical condition.
Building upon the above-mentioned study, the ENCORE-VT Trial (Electrophysiology-Guided Noninvasive Cardiac Radioablation for Ventricular Tachycardia) was a prospective phase I/II of noninvasive cardiac radioablation in adults with treatment-refractory episodes of VT or cardiomyopathy. Accruing nineteen patients over a two-year period from 2016 to 2018 at Washington University, ENCORE-VT continued the promising work outlined in the exploratory case series. With a median follow-up of 13 months, similar outcomes were observed. Using the same treatment workflow and cardiac mapping methodology, 94% (17/18) of patients met the primary endpoint of “Reduction in VT episodes or PVC burden” along with a concurrent reduction in antiarrhythmic medication. In the 16 patients with ICD-treated VT, there were 1778 episodes in aggregate in the 6 months prior to treatment, followed by 111 episodes after a 6-week “blanking” period, for an overall reduction of 94%. Coinciding with less ICD episodes was a reduction in the distribution of antiarrhythmic medication in the six months before and after radioablation. Use of dual antiarrhythmic medication decreased 47% (p = 0.008) and high-dose Amiodarone (>300 mg per day) decreased from 47% to 12% (p = 0.03). Treatments were overall well tolerated. There were no acute procedural toxicities or damage to the indwelling ICD systems, and the only isolated treatment-related toxicity (pericarditis) was successfully managed with medication. Taken together, Robinson et al. reasonably concluded their results support cardiac radioablation as a safe, effective, and well-tolerated treatment option of intractable VT worthy of further investigation.
The second largest case series was reported in July 2019 from the Czech Republic consisting of ten patients treated with 25 Gy single fraction SBRT using CyberKnife for recurrent VT. Mirroring the results from the two prior case series, Neuwirth et al. demonstrated a 87.6% reduction in VT burden, with a median decrease of ICD episodes from 18 to 0.93 (p = 0.012). To date, this case series represents the longest follow-up on safety and efficacy for SBRT in patient with recurrent VT with a median follow-up of 28 months (range 16–54 months) [18]. Treatments were again well tolerated. Grade 1 nausea was the only acute treatment-related toxicity corresponding to lesions treated along the inferior wall of the left ventricle in close proximity to the stomach. One possible late grade 3 toxicity was identified when a patient with known mitral regurgitation presented with progression of regurgitation and changes to valvular morphology 17 months after their treatment. No other late radiation-related toxicities were noted. Overall, the authors concluded 3D electroanatomical mapping guided SBRT for ventricular tachycardia was safe and resulted in a significant reduction in VT burden. However, it should be noted that long-term results are not available at this time. These studies utilizing cardiac radioablation for refractory VT are summarized in Table 1.
Table 1.
Reduction in ICD events in refractory VTACH patients treated with cardiac radioablation.
| Institution: | Patients: | Median Follow-up: | Mean Age (Range): | # of ICD Events before RA (3 months) | # of ICD Events after Blanking Period | Percent Reduction ICD Events |
|---|---|---|---|---|---|---|
| Exploratory Analysis (Wash U) | 5 | 12 months | 66 (60–83) | 6577 | 4 | 99.90% |
| ENCORE-RT (Wash U) | 16 | 13 months | 66 (49–81) | 1778 | 111 | 94% |
| Czech Republic | 10 | 28 months | 66 (61–78) | 212 (Per Patient) | 26 (Per Patient) | 87.56% |
The most recent study from Emory University published in March 2020 mirrored the results of ENCORE-VT and the Czech Republic. 10 patients with advanced heart failure (HF) underwent treatment and among 8 patients with available ICD data, the total reduction in seconds of detected VT was 69% (pretreatment 1065 s/month versus post-treatment 332 s/month). The reduction in total ICD shocks after SBRT was 68% (2.9 shocks/month pretreatment versus 0.9 shocks/month post-treatment). When the researchers excluded a single nonresponding patient, there was a significant reduction in VT seconds (94%; P = 0.04) with a trend toward ICD shock reduction (90%; P = 0.07) post-SBRT. In a similar fashion to the preceding two trials, the work at Emory demonstrated cardiac radioablation with SBRT was feasible and effective at reducing VT burden in critically ill patients [19].
6. Radiation biology of cardiac radioablation
External beam and stereotactic radiation therapy capitalize on the ability of ionizing radiation to cause double-stranded DNA breaks within cancer cells leading to mitotic catastrophe and ultimately cell death through apoptosis [20]. DNA damage caused by ionizing radiation also occurs through both direct and indirect mechanisms. Direct DNA damage occurs with the transfer of energy to the DNA molecule causing formation of single and double stranded DNA breaks. Indirect damage occurs when ionizing radiation interacts with water within the cellular environment, forming hydroxyl radicals which in turn damages nearby DNA. Both direct and indirect damage occurs from conventional doses of radiation commonly administered over weeks in definitive cases. Animal models have shown increasing doses used with stereotactic body radiation therapy (>3Gy per fraction) are associated with cell hypoxia and tissue necrosis [21]. High doses (20 Gy or higher) used in stereotactic radiosurgery are associated with reduced capillary density, myocardial degeneration, and fibrosis in animal models [13], [22], [23]. Regarding cardiac fibrosis specifically, perivascular and interstitial collagen deposition caused by ionization radiation leads to decreased myocardial perfusion driving cardiac injury. While the underlying biology in VT radioablation remains predominately based on animal models, myocyte necrosis, microvascular injury, and radiation-induced fibrosis may be crucial for the underlying mechanism regarding its effectiveness [23]. These changes have been validated in animal models where atrioventricular nodal and pulmonary vein ablation were achieved through SBRT. These studies evaluated single fraction doses of 25 Gy and found them effective at creating myocardial fibrosis and ultimately conduction block within months of treatment [7]. These studies shed light on the possible biologic mechanism of CRA, but further work is needed.
7. Imaging and electrophysiologic mapping modalities in cardiac radioablation
There are a multitude of cardiac imaging and techniques available to aid radiation oncologists in the localization of an arrhythmogenic focus and subsequent target delineation for cardiac radioablation. Broadly speaking, these techniques can be broken down into structural mapping (ECG-gated computed tomography (CT) imaging), CMR, metabolic mapping (nuclear imaging SPECT and Positron Emission Tomography (PET)) and invasive as well as non-invasive electrocardiographic mapping. All of these modalities can be used complementarily with one another when the treating physician team is creating their target volume.
Contrast-enhanced ECG-gated Cardiac CT: To overcome the motion artifact associated with a beating heart, an ECG can be recorded during CT imaging acquisition [24]. Using IV contrast, cardiac CT imaging provides excellent spatial resolution on static imaging and can provide four-dimensional (4D) imaging encompassing systole and diastole when using ECG-gating. The 4D component and excellent resolution allows for visualization of ventricular wall thinning corresponding to scarred/damaged myocardium (VIDEO) [25]. During CT scanning, post-processing algorithms allow for reconstruction of CT images in specific phases of the cardiac cycle, much like a respiratory-gated CT used for target delineation in thoracic radiation oncology cases [26].
Cardiovascular Magnetic Resonance (CMR): CMR with and without gadolinium contrast is the gold standard for non-invasive assessment of myocardial tissue. Late gadolinium enhancement (LGE) imaging characteristics are able to accurately differentiate between healthy and scarred myocardium based on washout kinetics and is highly predictive of malignant ventricular arrhythmias in ischemic and non-ischemic cardiomyopathies (Fig. 1) [27], [28]. Technological advances like wideband MR, 3D LGE sequences, and low-field scanners are reducing the long scan times and improving image quality for patients undergoing cardiac radioablation and have become integral in the treatment planning process, especially in patients with ICD or pacemaker devices.
Fig. 1.
Comparison of necrosis stained autopsy slides (left) and magnetic resonance (right). Adapted from Kim et al. Circulation. 1999 Nov 9;100 [19]:1992–2002.
Nuclear Imaging: Single photon emission computed tomography (SPECT) employs 99mTc-sestamibi and 99mTc-tetrofosmin radiotracers which localize along scarred myocardium in a Na+/K+ ATPase-dependent manner. Alternatively, 18-fluorodeoxyglucose positron emission tomography (FDG-PET) functions in a similar mechanism when its glucose radiotracer congregates in highly metabolic cells while being relatively absent in non-functional/scarred myocytes. Use of both SPECT and PET together improves their accuracy as it can distinguish between an arrhythmogenic scar (low perfusion/low metabolism) and hibernating myocardium (normal perfusion/low metabolism) [29]. Both modalities have a high correlation to voltage-defined scar as determined by invasive electro-anatomical mapping and PET/CT may even be able to identify successful ablation sites with greater sensitivity than voltage mapping. Both SPECT/PET imaging can be fused with the planning CT to easily aid with target contouring.
Invasive Cardiac Mapping: Cardiac mapping encompasses three-dimensional (3D) electro-anatomical mapping (EAM) and advanced image-guidance platforms that allow electrophysiologists to non-fluoroscopically localize intra-cardiac catheters, create a 3D map of the cardiac chamber of interest, and automatically measure and display electrophysiological data (scar versus healthy tissue based on bipolar voltage amplitude) at precise locations (Fig. 2). The coordinates of the catheter tip are indexed in real time to a 3D model created from a previously acquired CT or CMR, allowing for the construction of a detailed location specific record of the relevant electrophysiological data [30]. Different mapping techniques such as pace, activation/entrainment, and substrate all utilize an EAM system and can be used in combination with one another to identify “candidate” sites for an ablation, while minimizing nearby bystander tissue [31], [32].
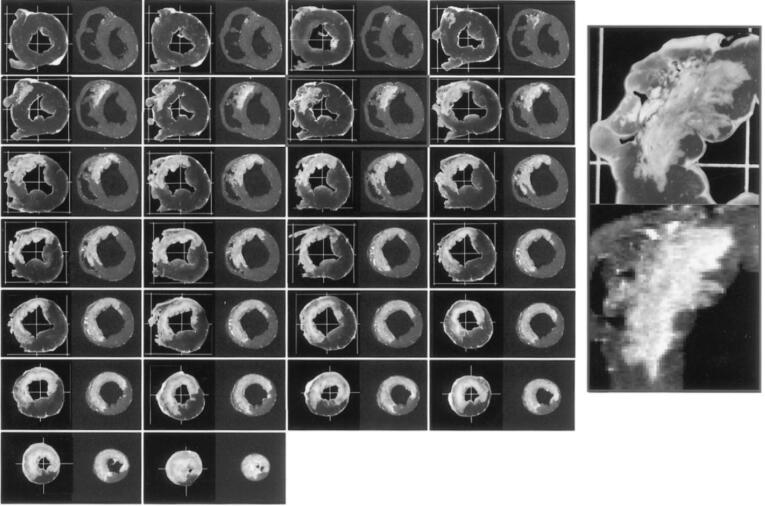
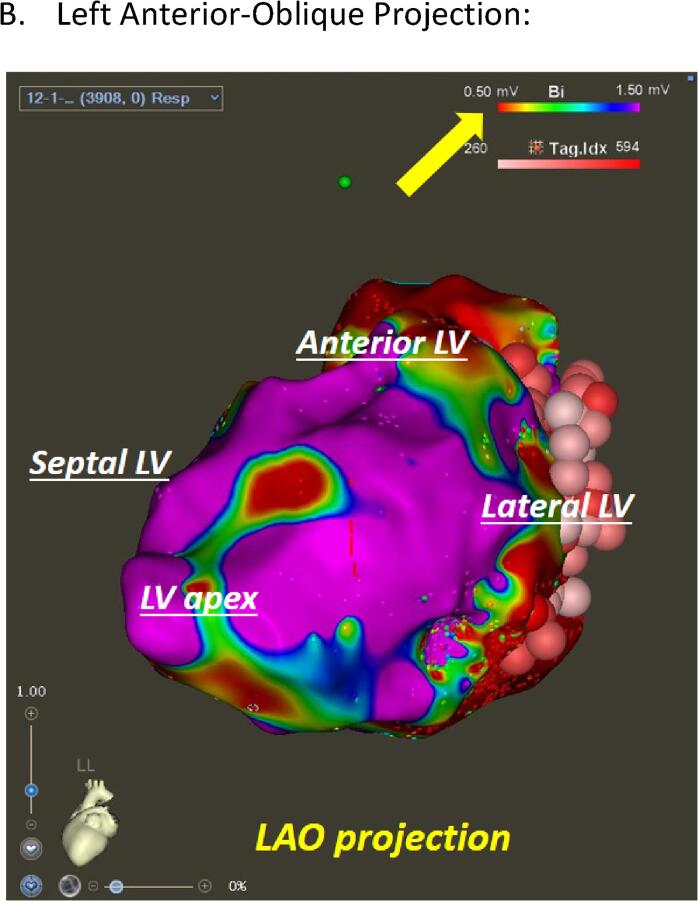
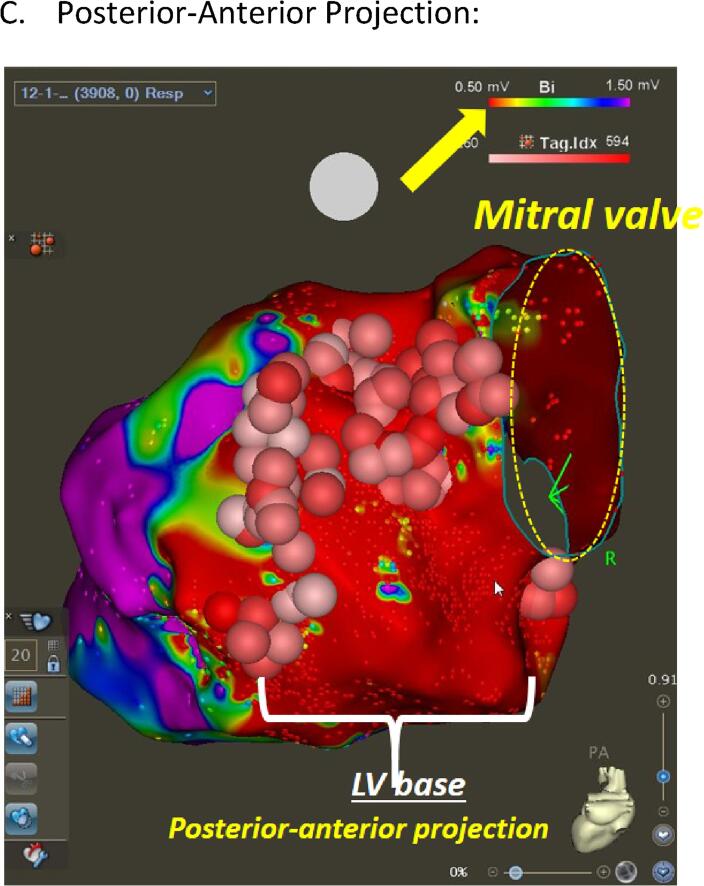
Fig. 2.
CRT Substrate Mapping. (A) Right Anterior-Oblique Projection. (B) Left Anterior-Oblique Projection:. (C) Posterior-Anterior Projection: Left ventricular endocardial bipolar voltage mapping in a patient with prior myocardial infarction (MI). Three maps in different projections (RAO = right anterior oblique (A), LAO = left anterior oblique (B), and posterior-anterior projection (C)) are displayed with color coding based on local voltage measurements. Areas with voltage less than 0.5 mV (dense scar) are color coded as red whereas the healthy tissues (>1.5 mV) are displayed as purple. This patient demonstrated extensive basal anterior, basal lateral, and basal inferior scar segments, consistent with prior MI due to the large left circumflex artery occlusion. Surviving tissues within the dense scar were identified and extensive ablation was carried out to “homogenize” the scar zone (scar mapping and ablation). Pink and red circles indicate ablation points. The rainbow color bar in the top right corner in each figure (yellow arrow) indicates the color coding scheme used in this map and cut-off values for dense scar (less than0.5 mV) and healthy tissues (>1.5 mV). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Non-Invasive Cardiac Mapping: External vests using multiple skin surface electrodes used in conjunction with patient-specific measurements gleaned from CT imaging are used to create activation and voltage maps similar to invasive cardiac maps. One difference between non-invasive and invasive techniques is the source of the “mapped” potential. Non-invasive techniques localize epicardial potentials whereas invasive mapping identifies endocardial abnormalities. Some critics have noted significant discrepancies between the data collected from non-invasive mapping systems and invasive electroanatomical mapping, and the latter remains the gold standard. Despite this, non-invasive cardiac mapping has been regularly used in cardiac radioablation target delineation [33], [34].
8. Radiation simulation & target delineation
CT simulation is typically conducted with full body immobilization, arms up, abdominal compression (if possible), and both free breathing CT with IV contrast and 4DCT to capture respiratory and cardiac motion [17]. An alternate to abdominal compression is motion management with respiratory gating. The gross target volume (GTV) is defined by EP through co-registration of DICOM compatible anatomic imaging (CT, SPECT, PET, CMR) and side by side comparison using incompatible electrical imaging (EKG, ECHO, electrocardiographic imaging). Fusing a contrast-enhanced cardiac CT to the free-breathing and 4DCTs accounts for respiratory motion while providing a high resolution cardiac imaging specific to diastole or another cardiac phase allowing for anatomical delineation of the arrhythmic ventricular scar by identifying regions with > 50% wall thinning [35]. The American Heart Association (AHA) 17-segment left ventricular segmentation using two left ventricular “long” and one “short” axes has been proposed to characterize SBRT targets within the left ventricle. The left ventricle is split into four regions that are further subdivided into segments [basal segments (1–6), middle (7–12), apical (13–16), and true apex (17)]. Because this model is derived from a cardiac coordinate system, the CT simulation imaging must be carefully rotated to transition from an axial, sagittal, and coronal based system to ventricular coordinate system. First, the left ventricular apex is defined as the rotational axis after being identified in the treatment planning software as the most lateral, anterior, and inferior location of the LV. Using coronal and then sagittal views, the image is rotated around the apex until the sagittal and coronal axis respectively evenly divide the mitral valve annulus (base). If oriented correctly, axial images will correlate to the “LV short-axis” view used by cardiologists.
Additional noninvasive imaging can be fused to the planning CT to further aid in anatomic scar delineation. CMR LGE myocardial scar correlates with CT at the tissue level (Fig. 1) [27]. SPECT in conjunction with PET can reveal scar at regions of reduced radiotracer uptake and reduced perfusion [29], [36]. DICOM incompatible electrical data pulled up “side by side” is also used to fully define the target volume (GTV), including echocardiography identifying areas of akinesis and wall thinning, and invasive cardiac testing such as pace mapping of induced VT [35]. Next, an anisotropic internal target volume (ITV or iGTV) is applied by assessing the largest extent of respiratory/cardiac motion superior/inferior, medial/lateral, and anterior/posterior. To account for setup uncertainty, a planning target volume (PTV) expansion ranging from 3 to 7 mm is applied depending on each patient’s setup reproducibility. An anisotropic expansion can be considered based on extent of respiratory motion and toxicity to nearby organs-at-risk (OARs). OARs are drawn on the CT planning average scan and include the left ventricle, right ventricle, heart, lungs, esophagus, spinal cord, ICD, and any bronchus, great vessels, stomach, small bowel and large bowel in proximity to the PTV.
9. Treatment planning and delivery
The following summarizes the recommended methodology at our institution, recognizing that institutions can vary practices. Twenty-five Gy in a single fraction is prescribed to the PTV with at least 95% of the PTV expected to receive prescription dose unless necessary to lower dose or prescription to meet OAR constraints [17], with a target conformality index (prescription volume/PTV) of less than 1.2. At this time, although contouring occurs using cardiac segmentation through extensive manual adjustments as described above, treatment planning software remains based upon conventional axial, sagittal, and coronal axes. Treatment planning utilizes volumetric modulated arc therapy (VMAT) with non-coplanar arcs and 6X energy. Flattening filter free delivery is preferred for increased dose rate, and using less than 10X energy avoids excess neutron production (especially important for ICD). Standard single fraction SBRT dose constraints are used to limit dose to OARs as per TG101, with critical organs taking priority over the PTV [37]. Both the treating radiation oncologist and EP evaluate and approve the treatment plan prior to implementation.
On the day of treatment delivery, the patient undergoes a history and physical with EP, IV placement, and ICD interrogation 1–2 h prior to radiation therapy. The patient’s ICD remains on for treatment. If amiodarone is prescribed, delivery is conducted and monitored by EP. Both the radiation oncologist and EP are present for treatment set up, delivery, and until the patient is deemed stable post procedure and monitoring is discontinued. The EP physician determines the appropriate cardiac monitoring on a per patient basis and is responsible for deciding to halt procedures in the event of an acute cardiac event.
First, radiation therapists reproduce the CT simulation setup, apply CT origin to isocenter, and shifts as instructed in the source-to-surface distance (SSD) document. MR-guided linear accelerators have proven to be effective and feasible in this space as well [38]. Next, the radiation oncologist performs the timeout procedure and administers abdominal compression as per CT simulation. A cone-beam CT is performed, and therapists align the patient to the vertebral bodies before further aligning to the ITV and PTV targets. After the radiation oncologist confirms alignment, approves shifts, and shifts are applied, a verification CBCT is performed and treatment delivery can occur if no further shifts are necessary. If an excessive number of CBCTs are performed without confidently aligning the patient, re-planning should be considered by the multidisciplinary team. Once shifts and imaging are approved, a medical physicist confirms treatment parameters prior to each field with the therapist. After all fields are delivered, vital signs are monitored for up to 2–3 h and the ICD is re-interrogated prior to discharge home if there are no noted abnormalities. Regularly scheduled follow up with EP and radiation oncology occurs at 1, 3, 6, and 12 months after treatment with ICD interrogation and EKG at each visit and CT chest at 3 and 12 months [7]. Echocardiogram is performed at 3 months and yearly. Of note, it is common for arrhythmic events to continue to occur in the blanking period before subsiding thereafter.
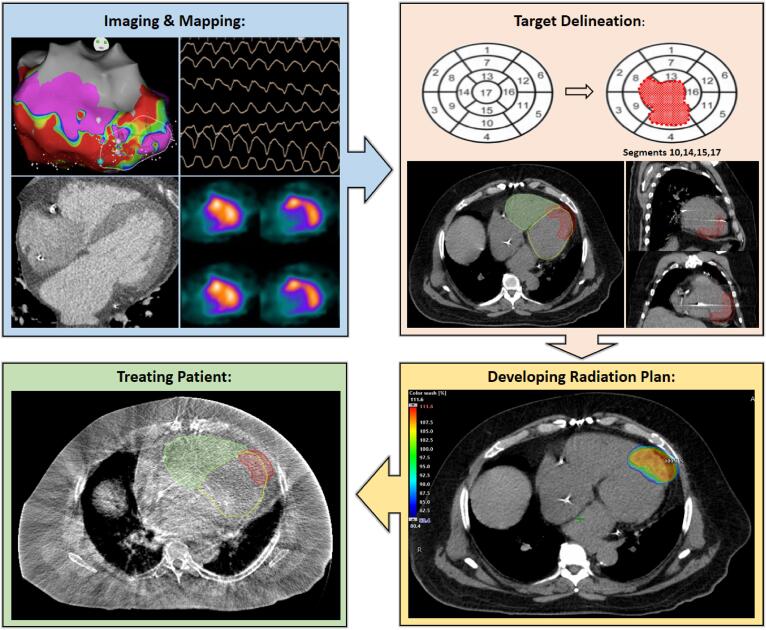
CRA is unique within radiation oncology and truly requires a team-based approach to contouring as well as treatment planning and delivery. This modality presents a unique opportunity for researchers to develop novel methodologies, treatment algorithms, and quality assurance platforms which will likely benefit the CRA field as a whole. A common cardiac radioablation workflow is shown in Fig. 3.
Fig. 3.
Cardiac Radioablation Treatment Workflow. Blue Panel: Identifying and Mapping the CRA Target. In clockwise order, non-invasive EKG, SPECT, Cardiac CT w/ contrast, and endocardial catheter-based voltage map used to localize the ventricular scar prior to radiation planning. Orange Panel: Target Delineation. Top panel shows 17-segment left ventricular model targeting 4 segments identified during mapping. Bottom panel with radiation therapy planning software showing PTV (red) contoured within the left and right ventricle. Yellow Panel: Developing a Radiation Plan. External beam planning software showing dose color wash of PTV (red) from 80 to 110% of prescription dose. Green Panel: Treating the Patient. Aligning PTV using cone beam CT on the day of treatment. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
10. Future directions
Non-invasive cardiac radioablation has shown to be a valid new therapeutic option for treatment-resistant VT. In the coming years, we will begin to see cardiac radioablation spread to additional academic and community centers as contouring guidelines and radiation therapy workflows become adopted and standardized. In the next decade we will see pilot studies explore important unanswered questions about radiation dose (is 25 Gy above dose–response curve?), volume of target needed to be treated, fractionation (e.g. 1 vs 2–3 fractions), re-treatment, and whether radiation therapy can effectively treat other atrial arrhythmias like atrial fibrillation and flutter [39]. The future is bright for cardiac radioablation and these important clinical questions warrant further investigation. Constantly improving linear accelerator technology, image guided radiation, and improved motion management/gating along with high definition cardiac imaging could ultimately allow cardiac radioablation to be considered a frontline alternative therapy in VT patients.
Like many other disease sites treated with photon radiation, proton beam therapy (PBT) appears to be a promising modality for the delivery of cardiac radioablation. Compared with traditional photon-based therapies, protons exhibit minimal entrance dose, sharp lateral fall-off, and a nearly non-existent exit dose due to the charged particle’s inherent physical properties and interaction with the surrounding tissue. Using a proton’s sharp dose falloff to their advantage, a clinician may successfully treat a lesion along the inferior segment of the ventricle while minimizing dose to the rest of the chamber, and adjacent lung/stomach/esophagus. In the same spirit as the work done twenty years ago with SBRT, PBT has proven to be effective in porcine models at generating cardiac myocyte fibrosis in targeted areas while sparing adjacent normal tissue [40]. However, for patients with pacemakers/ICDs, there are concerns that neutron exposure from proton beam scattering may cause device failure. While neutron production is significantly lower in newer active scanning systems (as opposed to older passive-scattering systems), the neutrons produced from scatter within tissues may still pose a risk (TG-203), thus further investigation is needed [40]. In the coming years we will undoubtedly see the beginning of the next chapter in the story of cardiac radioablation as the first case reports are published of refractory VT patients being successfully treated with PBT.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge Dr. John Hummel, Dr. Emile Daoud, Dr. Doug Martin, and Dr. Nilendu Gupta for support in this review.
References
- 1.Khurshid S., Choi S.H., Weng L.C. Frequency of cardiac rhythm abnormalities in a half million adults. Circ Arrhythm Electrophysiol. 2018;11:e006273. doi: 10.1161/CIRCEP.118.006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motté G., Laine J.F., Slama M. Physiopathology of ventricular tachyarrhythmias. Pacing Clin Electrophysiol. 1984;7:1129–1136. doi: 10.1111/j.1540-8159.1984.tb05672.x. [DOI] [PubMed] [Google Scholar]
- 3.Al-Khatib S.M., Stevenson W.G., Ackerman M.J. 2017 aha/acc/hrs guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. Circulation. 2018;138:e272–e391. doi: 10.1161/CIR.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 4.Breithardt G, Borggrefe M, Martinez-Rubio A, et al. Pathophysiological mechanisms of ventricular tachyarrhythmias. Eur Heart J 1989;10 Suppl E:9–18. [DOI] [PubMed]
- 5.Foth C, Gangwani MK, Alvey H. Ventricular tachycardia (vt, v tach) Statpearls. Treasure Island (FL); 2020. [PubMed]
- 6.Koplan B.A., Stevenson W.G. Ventricular tachycardia and sudden cardiac death. Mayo Clin Proc. 2009;84:289–297. doi: 10.4065/84.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson C.G., Samson P.P., Moore K.M.S. Phase i/ii trial of electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation. 2019;139:313–321. doi: 10.1161/CIRCULATIONAHA.118.038261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shivkumar K. Catheter ablation of ventricular arrhythmias. N Engl J Med. 2019;380:1555–1564. doi: 10.1056/NEJMra1615244. [DOI] [PubMed] [Google Scholar]
- 9.Vaseghi M., Barwad P., Malavassi Corrales F.J. Cardiac sympathetic denervation for refractory ventricular arrhythmias. J Am Coll Cardiol. 2017;69:3070–3080. doi: 10.1016/j.jacc.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivari M.T., Windle J.R. Cardiac transplantation in patients with refractory ventricular arrhythmias. J Heart Lung Transplant. 2000;19:S38–S42. doi: 10.1016/s1053-2498(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 11.Wei C., Qian P., Tedrow U. Non-invasive stereotactic radioablation: a new option for the treatment of ventricular arrhythmias. Arrhythm Electrophysiol Rev. 2020;8:285–293. doi: 10.15420/aer.2019.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lax I., Blomgren H., Näslund I. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol. 1994;33:677–683. doi: 10.3109/02841869409121782. [DOI] [PubMed] [Google Scholar]
- 13.Sharma A., Wong D., Weidlich G. Noninvasive stereotactic radiosurgery (cyberheart) for creation of ablation lesions in the atrium. Heart Rhythm. 2010;7:802–810. doi: 10.1016/j.hrthm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Lehmann H.I., Graeff C., Simoniello P. Feasibility study on cardiac arrhythmia ablation using high-energy heavy ion beams. Sci Rep. 2016;6:38895. doi: 10.1038/srep38895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loo B.W., Jr., Soltys S.G., Wang L. Stereotactic ablative radiotherapy for the treatment of refractory cardiac ventricular arrhythmia. Circ Arrhythm Electrophysiol. 2015;8:748–750. doi: 10.1161/CIRCEP.115.002765. [DOI] [PubMed] [Google Scholar]
- 16.Cvek J.N.R., Knybel L. Cardiac radiosurgery for malignant ventricular tachycardia. Crues. 2014 [Google Scholar]
- 17.Cuculich P.S., Schill M.R., Kashani R. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med. 2017;377:2325–2336. doi: 10.1056/NEJMoa1613773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuwirth R., Cvek J., Knybel L. Stereotactic radiosurgery for ablation of ventricular tachycardia. Europace. 2019;21:1088–1095. doi: 10.1093/europace/euz133. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd M.S., Wight J., Schneider F. Clinical experience of stereotactic body radiation for refractory ventricular tachycardia in advanced heart failure patients. Heart Rhythm. 2020;17:415–422. doi: 10.1016/j.hrthm.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Kiani S., Kutob L., Schneider F. Histopathologic and ultrastructural findings in human myocardium after stereotactic body radiation therapy for recalcitrant ventricular tachycardia. Circ Arrhythm Electrophysiol. 2020 doi: 10.1161/CIRCEP.120.008753. [DOI] [PubMed] [Google Scholar]
- 21.Lauk S., Kiszel Z., Buschmann J. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 1985;11:801–808. doi: 10.1016/0360-3016(85)90314-1. [DOI] [PubMed] [Google Scholar]
- 22.Krüse J.J., Zurcher C., Strootman E.G. Structural changes in the auricles of the rat heart after local ionizing irradiation. Radiother Oncol. 2001;58:303–311. doi: 10.1016/s0167-8140(00)00327-3. [DOI] [PubMed] [Google Scholar]
- 23.Boerma M., Sridharan V., Mao X.W. Effects of ionizing radiation on the heart. Mutat Res. 2016;770:319–327. doi: 10.1016/j.mrrev.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbara S., Blanke P., Maroules C.D. Scct guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography guidelines committee: Endorsed by the north american society for cardiovascular imaging (nasci) J Cardiovasc Comput Tomogr. 2016;10:435–449. doi: 10.1016/j.jcct.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Rizvi A., Deaño R.C., Bachman D.P. Analysis of ventricular function by ct. J Cardiovasc Comput Tomogr. 2015;9:1–12. doi: 10.1016/j.jcct.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandner E.D., Chetty I.J., Giaddui T.G. Motion management strategies and technical issues associated with stereotactic body radiotherapy of thoracic and upper abdominal tumors: A review from nrg oncology. Med Phys. 2017;44:2595–2612. doi: 10.1002/mp.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim R.J., Fieno D.S., Parrish T.B. Relationship of mri delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 28.Bello D., Fieno D.S., Kim R.J. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45:1104–1108. doi: 10.1016/j.jacc.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 29.Duell J., Dilsizian V., Smith M. Nuclear imaging guidance for ablation of ventricular arrhythmias. Curr Cardiol Rep. 2016;18:19. doi: 10.1007/s11886-015-0697-2. [DOI] [PubMed] [Google Scholar]
- 30.Chowdhary A., Garg P., Das A. Cardiovascular magnetic resonance imaging: Emerging techniques and applications. Heart. 2021 doi: 10.1136/heartjnl-2019-315669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitamura T., Martin C.A., Vlachos K. Substrate mapping and ablation for ventricular tachycardia in patients with structural heart disease: How to identify ventricular tachycardia substrate. J Innov Card Rhythm Manag. 2019;10:3565–3580. doi: 10.19102/icrm.2019.100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhakta D., Miller J.M. Principles of electroanatomic mapping. Indian Pacing Electrophysiol J. 2008;8:32–50. [PMC free article] [PubMed] [Google Scholar]
- 33.Singh A., Kawaji K., Goyal N. Feasibility of cardiac magnetic resonance wideband protocol in patients with implantable cardioverter defibrillators and its utility for defining scar. Am J Cardiol. 2019;123:1329–1335. doi: 10.1016/j.amjcard.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham A.J., Orini M., Zacur E. Evaluation of ecg imaging to map hemodynamically stable and unstable ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2020;13:e007377. doi: 10.1161/CIRCEP.119.007377. [DOI] [PubMed] [Google Scholar]
- 35.Mahida S., Sacher F., Dubois R. Cardiac imaging in patients with ventricular tachycardia. Circulation. 2017;136:2491–2507. doi: 10.1161/CIRCULATIONAHA.117.029349. [DOI] [PubMed] [Google Scholar]
- 36.Slart R.H., Bax J.J., van Veldhuisen D.J. Imaging techniques in nuclear cardiology for the assessment of myocardial viability. Int J Cardiovasc Imaging. 2006;22:63–80. doi: 10.1007/s10554-005-7514-8. [DOI] [PubMed] [Google Scholar]
- 37.Bissonnette J.P., Balter P.A., Dong L. Quality assurance for image-guided radiation therapy utilizing ct-based technologies: a report of the aapm tg-179. Med Phys. 2012;39:1946–1963. doi: 10.1118/1.3690466. [DOI] [PubMed] [Google Scholar]
- 38.Mayinger M., Kovacs B., Tanadini-Lang S. First magnetic resonance imaging-guided cardiac radioablation of sustained ventricular tachycardia. Radiother Oncol. 2020;152:203–207. doi: 10.1016/j.radonc.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Pierre Qian MS, Koichiro Kumagai, Jose R Azpiri, Jose Assad, Jun Itami, Koji Inaba, Hiroyuki Okamoto, Abe Yoshihisa, Carlos Erick Cardona Ibarra, Cuauhtemoc de la Pena, Miguel Hinojosa, Edward Gardener, Douglas Wong, Alice B Jack, Thomas J Fogarty, Patrick Maguire, Paul Zei. Abstract 12078: Stereotactic radioablation for atrial fibrillation: A first in man series. Circulation 2019;140.
- 40.Hohmann S., Deisher A.J., Konishi H. Catheter-free ablation of infarct scar through proton beam therapy: tissue effects in a porcine model. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.07.011. [DOI] [PubMed] [Google Scholar]