Abstract
Background
Alterations in the brain cortical structures of patients with chronic kidney disease (CKD) have been reported; however, the cause has not been determined yet. Herein, we used Mendelian randomization (MR) to reveal the causal effect of kidney damage on brain cortical structure.
Methods
Genome-wide association studies summary data of estimated glomerular filtration rate (eGFR) in 480,698 participants from the CKDGen Consortium were used to identify genetically predicted eGFR. Data from 567,460 individuals from the CKDGen Consortium were used to assess genetically determined CKD; 302,687 participants from the UK Biobank were used to evaluate genetically predicted albuminuria. Further, data from 51,665 patients from the ENIGMA Consortium were used to assess the relationship between genetic predisposition and reduced eGFR, CKD, and progressive albuminuria with alterations in cortical thickness (TH) or surficial area (SA) of the brain. Magnetic resonance imaging was used to measure the SA and TH globally and in 34 functional regions. Inverse-variance weighted was used as the primary estimate whereas MR Pleiotropy RESidual Sum and Outlier, MR-Egger and weighted median were used to detect heterogeneity and pleiotropy.
Findings
At the global level, albuminuria decreased TH (β = −0.07 mm, 95% CI: −0.12 mm to −0.02 mm, P = 0.004); at the functional level, albuminuria reduced TH of pars opercularis gyrus without global weighted (β = −0.11 mm, 95% CI: −0.16 mm to −0.07 mm, P = 3.74×10−6). No pleiotropy was detected.
Interpretation
Kidney damage causally influences the cortex structure which suggests the existence of a kidney-brain axis.
Funding
This study was supported by the Science and Technology Planning Project of Guangdong Province (Grant No. 2020A1515111119 and 2017B020227007), the National Key Research and Development Program of China (Grant No. 2018YFA0902803), the National Natural Science Foundation of China (Grant No. 81825016, 81961128027, 81772719, 81772728), the Key Areas Research and Development Program of Guangdong (Grant No. 2018B010109006), Guangdong Special Support Program (2017TX04R246), Grant KLB09001 from the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes, and Grants from the Guangdong Science and Technology Department (2020B1212060018).
Keywords: Chronic kidney disease, Brain cortical structure, Mendelian randomization, Causal effect
Research in context.
Evidence before this study
Previous observational studies have reported that brain cortical structure alterations occurred in patients with chronic kidney disease (CKD). However, avoiding bias proved to be difficult in retrospective observation studies and previous observational studies fail to report the detailed alteration of the specific brain functional regions.
Added value of this study
Using a Mendelian randomization study, we reveal the causal effect of CKD on the brain cortical structure in what is referred to, magnetic resonance imaging (MRI)-measure surficial areas and cortical thickness. We found that genetic predicted albuminuria decreased the whole brain cortical thickness (β = −0.07 mm, 95% CI: −0.12 mm to −0.02 mm, P = 0.004); at the functional region level, genetic predicted albuminuria reduced thickness of pars opercularis gyrus without global weighted (β = −0.11 mm, 95% CI: −0.16 mm to −0.07 mm, P = 3.74×10−6).
Implications of all the available evidence
Kidney damage causally influences the brain cortical structure which suggests the existence of a kidney-brain axis. Based on our results, a brain MRI could potentially be used for patients with CKD for the early diagnosis of neuropsychiatric disorders. Neurologists should be cautious about patients’ kidney function when patients exhibit alterations of the pars opercularis.
Alt-text: Unlabelled box
1. Introduction
Chronic kidney disease (CKD) affects approximately 10% of the global population and has a high mortality rate [1], [2], [3], thereby imposing a great burden on global health [4,5]. The alteration of the brain cortical structure in patients with CKD is concerning and suggests the presence of a kidney–brain axis [6,7]. However, CKD is most common among the elderly whose brain cortical atrophy can be caused by normal degeneration due to ageing; hence, a causal relation between CKD and cortical structure remains unclear and difficult to establish [8].
Brain cortical structure alteration in patients with CKD was first described by Passer et al. in 1977, who reported a high prevalence of cerebral atrophy identified using computed tomography in end-stage uraemia [9]; prominent alterations were observed in the frontal lobes. Magnetic resonance imaging (MRI) is a sensitive tool used to examine the brain cortical structure and detect related alteration in patients with CKD. Approximately, half of the patients with CKD have silent brain infarcts, whereas the prevalence of this condition among the general populations ranges from 8 to 28% [10]. This finding was emphasized by several other studies [11]. However, previous studies exhibit limitations including insufficient statistical robustness owing to the small size of populations, varied interpretation of findings, confounding factors such as sociocultural demographic characteristics, presence of the depressive syndrome, and pre-existing cerebrovascular events.
Mendelian randomization (MR), using genetic variants indexing of exposure to the inter-causality of risk factors related to diseases, can overcome confounding biases inherent in observational studies [12,13]. Using MR analysis, several risk factors of CKD, and the causal relationship between CKD and other diseases have been reported [14], [15], [16]; however, the causal relation between CKD and the brain cortical structure has not been demonstrated yet [16]. As an extension of the MR method, two-sample MR analysis allows for the use of summary statistics of genome-wide association studies (GWASs) for MR studies, without directly analysing individual-level data. Based on the publicly available GWAS data from large population, we used the two-sample MR analysis to illustrate the effect of CKD on the brain cortex structure.
We utilised human genetic data within the MR framework to reveal the effect of CKD on the brain cortex structure, defined as human brain cortical surface area (SA) and cortical thickness (TH), as detected using MRI. Three sets of parameters: CKD, eGFR, and albuminuria, were used to provide the MR estimates. We also carried out subgroup analyses based on distinct functional regions. Our results provide new insights into the possible existence of a kidney-brain axis.
2. Methods
2.1. Data sources for chronic kidney disease, estimated glomerular filtration rate, and albuminuria
The summary-level GWAS data correlated with CKD and eGFR were obtained from a meta-analysis of the GWAS of European-ancestry participants from the CKDGen Consortium. The meta-analysis of the GWAS of CKD included 23 cohorts of European ancestry (n = 480,698; 41,395 cases and 439,303 controls, enrolled cohorts were listed in Supplementary Table S4). CKD was reported to have an eGFR of < 60 mL/min/1.73 m2. The meta-analysis of the GWAS of eGFR included 54 cohorts of European ancestry (n = 567,460). eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration equation for adults (> 18 years) [17] and the Schwartz formula (< 18 years) [18]. The details of the participant characteristics of the CKDGen Consortium studies have been reported by Wuttke et al. [19]. Albuminuria may be associated with hypertension. In addition, hypertension and anti-hypertensive drugs could alter the brain cortical structure. Therefore, to avoid violations from the use of anti-hypertensive drugs [20,21], albuminuria index SNPs were identified in 302,687 individuals from the UK Biobank who were not on hypertensive medication and who had albuminuria estimated according to the study of Haas et al. [22].
2.2. Data source for brain cortex surficial area and cortex thickness
The brain cortical structure-related GWAS data were obtained from the ENIGMA Consortium [23]. The brain cortical TH and SA were measured in 51,665 individuals, primarily (∼94%) of European descent across 60 cohorts around the world, using MRI. Meta results including only European-ancestry participants were used in our study (detailed cohort information was listed in Supplementary Table S5). The 34 regions were defined based on the Desikan-Killiany atlas, establishing coarse partitions of the cortex, and the regional boundaries were determined according to the gyral anatomy labelled between the depths of the sulci [24]. The regions were averaged between both hemispheres. We performed MR analysis from CKD, eGFR, and albuminuria on TH and SA of the entire cortex, as well as TH and SA for 34 brain cortical regions with known functional specialisations with or without the weighted estimates of the entire brain, yielding 138 outcomes. Data comprising global weighted estimates indicated the SA and TH of specific regions across the SA and TH of the entire brain, while those without global weighted estimates indicated the SA and TH measure of specific regions, regardless of the total brain SA and TH.
2.3. Selection of genetic instruments
To identify the causal relationship between kidney function and the brain cortical structure, we used three sets of genetic instruments indicating different aspects of renal pathophysiology, including i) index SNPs representing eGFR (listed in Supplementary Table S1), ii) index SNPs representing CKD which was defined as eGFR < 60 mL/min/1.73 m2 (listed in Supplementary Table S2), and iii) index SNPs representing albuminuria (listed in Supplementary Table S3). CKD is diagnosed by doctors as, a chronic illness related to kidney impairment. eGFR is regarded as a measure of the renal clearance function and can be impaired by different incursions to different areas of the nephron whereas albuminuria is a subclinical marker of the pathologic damage that specifically affects glomeruli and endothelia and is associated with microvascular dysfunction [25,26]. The accurate measurement of kidney function is difficult and generally, estimations are made using biomarkers [27]. Both measurements are necessary for proper evaluation of kidney function.
Genetic instruments were selected via the following criteria: i) a GWAS-correlated P-value of 5×10−8, and ii) a linkage disequilibrium [LD] r2 of < 0.001, and < 1 MB from the index variant.
Because some loci of eGFR are violated by the creatine metabolism, we used the GWAS of blood urea nitrogen (BUN) from the CKDGen Consortium, an alternative marker of kidney function, to remove SNPs that were unlikely to be related to kidney function [19]. Finally, SNPs inverse, significantly associated with BUN (P < 0.05) were selected as genetic instruments for eGFR.
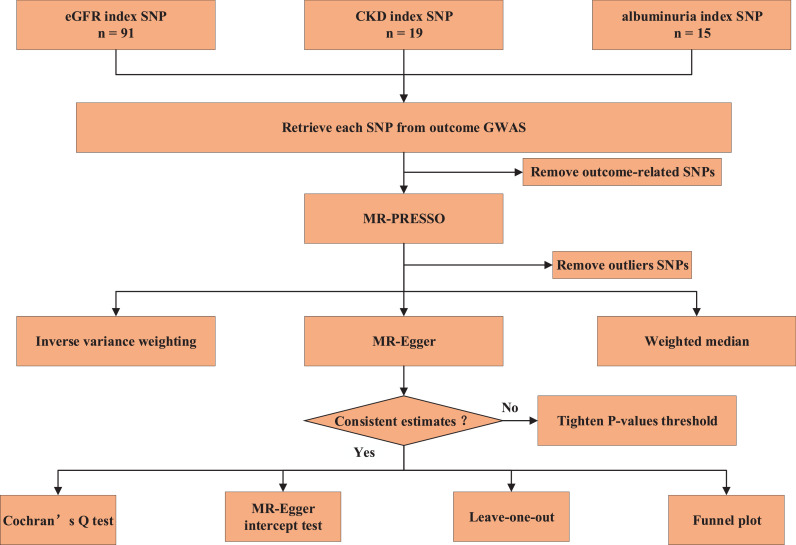
After removing the cortical structure-related SNPs with a threshold of 5×10−8, MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO), tests that are optimally applicable when a horizontal pleiotropy is found in < 50% of the instruments [28], were applied to remove the underlying outliers before each MR analysis. The study frame chart is presented in Fig. 1.
Fig. 1.
Study flame chart of the Mendelian randomization study revealing the causal relationship between albuminuria, chronic kidney disease, and eGFR and the brain cortical structure as defined using magnetic resonance imaging-measured brain cortical surficial area and thickness.
SA: Surficial area; TH: thickness.
2.4. Mendelian randomization analyses
Three different methods of MR [random-effect inverse-variance weighted (IVW), MR Egger, and weighted median] were performed to address variant heterogeneity and the pleiotropy effect. The brain cortical structure-related SNPs and outliers identified with MR-PRESSO were removed. IVW was used as the major outcome, whereas MR-Egger and weighted median were used to improve the IVW estimates as they could provide more robust estimates in a broader set of scenarios, despite being less efficient (wider CIs). MR-Egger allows all genetic variants to have pleiotropic effect but requires that the pleiotropic effects be independent of the variant-exposure association [29]. Weighted median allows for the use of invalid instruments under the assumption that at least half of the instruments used in the MR analysis are valid. In the IVW analysis, the slope of the weighted regression of the SNP-outcome effects on the SNP-exposure effects, where the intercept constrained to zero, represented the resulting estimate. Similar to our previous MR study, for significant estimates of IVW, if the estimates of these approaches were inconsistent, a tighten instrument P-value threshold was used and then the MR analysis was re-performed (Supplementary Table S6) [13]. For significant estimates, we further assessed horizontal pleiotropy using the MR-Egger intercept test and leave-one-out analyses. The Cochran's Q test was also used to identify heterogeneity. A funnel plot was used to assess the probable directional pleiotropy, similar to that being used to assess publication bias in meta-analysis.
We also checked in PhenoScanner (www.phenoscanner.medschl.cam.ac.uk), a platform with comprehensive information on the association of genotype and phenotype, to see whether these SNPs were associated with the potential risk factors, including body mass index, obesity, smoking, drinking, neuropsychiatric disease, hypertension, and hyperlipemia, and remove SNPs associated with any of these potential confounders at genome-wide significance [30].
3. Ethics
This study used publicly available de-identified data from participant studies that were approved by an ethical standards committee with respect to human experimentation. No separate ethical approval was required in this study.
4. Statistics
All analyses were performed using the packages TwoSampleMR (version 0.4.25) and MRPRESSO (version 1.0) in R (version 3.6.1) packages. For a global-level test, a significant two-sided P-value was set as 0.05. For region-level analyses, given the 408 MR estimates, a Bonferroni-corrected P-value was set as 0.05/408 (1.22×10−4), and meanwhile P < 0.05 was regarded as nominally significant.
5. Role of funding source
The funders played no role in the study design, data collection, data analyses, interpretation, or writing of the report. The corresponding author had full access to all the data in this study and was ultimately responsible for the decision to submit them for publication.
6. Results
In total, 91 index SNPs were selected to genetically predict eGFR, 19 index SNPs were used to genetically predict CKD and 15 SNPs predict albuminuria. F statistics for these genetic instruments were all larger than the normally selected value of 10, indicating strong instruments [31]. SNP rs4410790 was overlapped in eGFR and albuminuria, 4 SNPs (rs10224002, rs1458038, rs3925584 and rs77924615) were overlapped in CKD and eGFR. There was no overlapping between albuminuria and CKD.
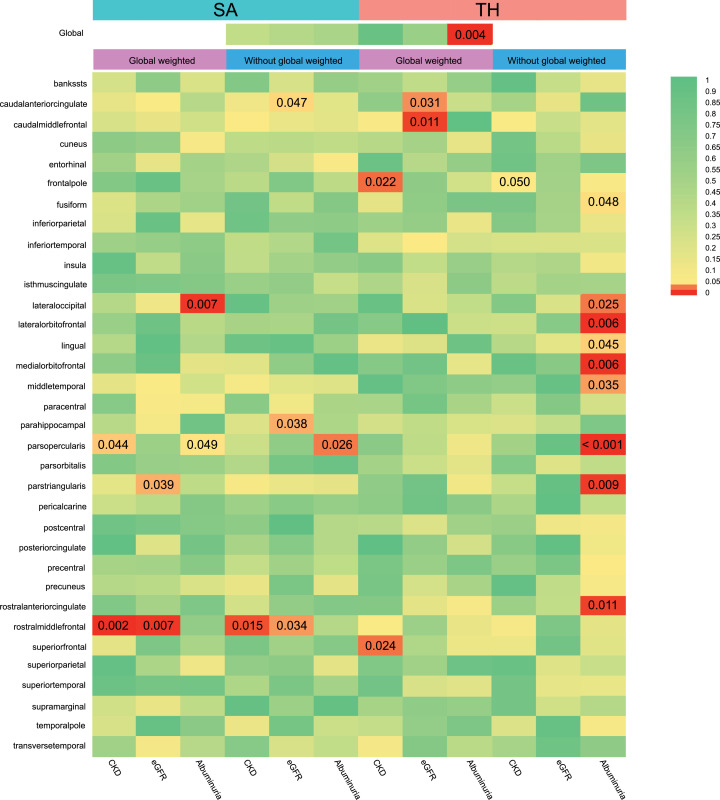
We conducted a comprehensive MR study from genetic predicted eGFR, CKD and albuminuria on global SA/TH as well as 34 functional gyrus with and without global weighted (Fig. 2) and identified several significant or nominal significant gyrus influenced by kidney function (Fig. 3). At the global level, albuminuria was found to decrease TH (β = −0.07 mm, 95% CI: −0.12 mm to −0.02 mm, P = 0.004) but had no causal relationship with SA (βSA = 2319.17mm2, SESA = 3431.60, P = 0.499) (Table 1). Heterogeneity was observed with a Cochran Q-derived P value < 0.05. As we used the random-effects IVW as main result, heterogeneity is acceptable [32]. The P value for MR-Egger intercept is > 0.05. No outliers were identified with MR-PRESSSO and the leave-one-out plot as well as funnel plots (Supplementary Figure S1). CKD had no causal relationship with the global SA and TH (βSA = 327.66 mm2, SESA = 355.04, PSA = 0.36; βTH = 2.41×10−4 mm, SETH = 0.002, PTH = 0.92). Genetic predicted eGFR had no causal relationship with the global SA and TH (βSA = -3238.39 mm2, SESA = 4228.318, PSA = 0.44; βTH = -0.017 mm, SETH = 0.032, PTH = 0.59).
Fig. 2.
IVW estimates from albuminuria, chronic kidney disease, and eGFR on brain cortical structure as defined using magnetic resonance imaging-measured brain cortical surficial area and thickness. The colour of each block represents the IVW-derived P-values of every MR analysis. P-values of < 0.05 were shown in red and P-values of > 0.05 were shown in yellow or green. P value < 0.05 is set as nominal significant, whereas < 1.22×10−4 is set as significant.
SA: Surficial area; TH: thickness; CKD: chronic kidney disease.
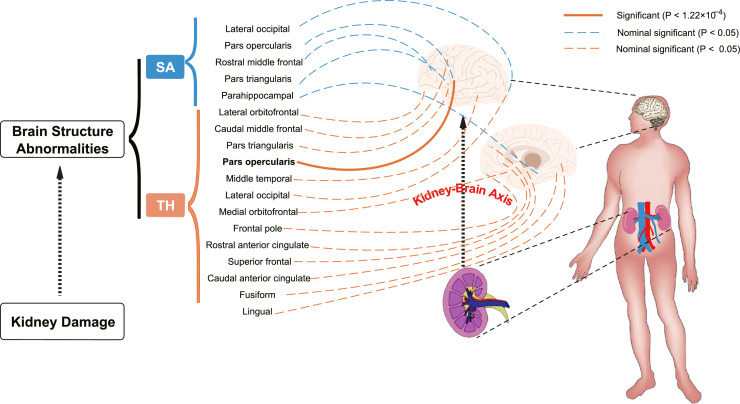
Fig. 3.
Using two-sample Mendelian randomization framework, we reveal that kidney damage causally influences brain cortical structure alteration, supporting the existence of kidney-brain axis. P value of IVW estimate < 1.22×10−4 is set as significant, represented in solid line whereas < 0.05 is set as nominal significant, represented in dotted line.
SA: Surficial area; TH: thickness.
Table 1.
Significant and nominal significant Mendelian randomization estimates from albuminuria, CKD and, eGFR on genetically predicted cortical structure.
| Exposure | Outcome | IVW-derived P value | β (95% Confidence intervals) | Cochran's Q-derived P value | MR-Egger intercept-derived P value | |
|---|---|---|---|---|---|---|
| Significant estimates*** | Albuminuria | Global TH | 0.004 | -0.07 mm (-0.12 mm to -0.02 mm) | 0.02 | 0.23 |
| TH of pars opercularis gyrus without global weighted | 3.74×10−06 | -0.12 mm(-0.16 mm to -0.07 mm) | 0.76 | 0.64 | ||
| Nominal significant Estimates* | Albuminuria | SA of lateral occipital with global weighted | 0.007 | -207.18 mm2 (-358.93 mm2 to -55.43 mm2) | 0.26 | 0.06 |
| SA of pars opercularis with global weighted | 0.049 | 62.35 mm2 (0.25 mm2 to 124.45 mm2) | 0.62 | 0.56 | ||
| SA of pars opercularis without global weighted | 0.026 | 93.95 mm2 (11.32 mm2 to 176.57 mm2) | 0.28 | 0.67 | ||
| TH of fusiform without global weighted | 0.048 | -0.05mm (-0.10mm to -0.0004mm) | 0.61 | 0.33 | ||
| TH of lateral occipital without global weighted | 0.025 | -0.07mm (-0.13 mm to -0.008mm) | 0.02 | 0.05 | ||
| TH of lateral orbitafrontal without global weighted | 0.006 | -0.07mm (-0.12 mm to -0.02mm) | 0.60 | 0.74 | ||
| TH of lingual without global weighted | 0.045 | -0.05mm (-0.09mm to -0.001mm) | 0.33 | 0.02 | ||
| TH of medial orbitofrontal without global weighted | 0.006 | -0.08mm (-0.14 mm to -0.02 mm) | 0.35 | 0.95 | ||
| TH of middle temporal without global weighted | 0.035 | -0.06mm (-0.11mm to -0.004mm) | 0.48 | 0.93 | ||
| TH of pars triangularis without global weighted | 0.009 | -0.09mm (-0.16mm to -0.02mm) | 0.02 | 0.85 | ||
| TH of rostral anterior cingulate without global weighted | 0.011 | -0.11mm (-0.18mm to -0.02mm) | 0.18 | 0.29 | ||
| CKD | SA of pars opercularis gyrus with global weighted | 0.04 | -9.48 mm2 (-18.69 mm2 to -0.27 mm2) | 0.16 | 0.81 | |
| SA of rostral middle frontal gyrus with global weighted | 0.002 | 34.32 mm2 (12.70 mm2 to 55.93 mm2) | 0.12 | 0.19 | ||
| TH of frontal pole gyrus with global weighted | 0.02 | -0.01 mm (-0.02 mm to -0.002 mm) | 0.92 | 0.32 | ||
| TH of superior frontal gyrus with global weighted | 0.02 | -0.005 mm (-0.009 mm to -0.0006 mm) | 0.56 | 0.25 | ||
| TH of frontal pole gyrus without global weighted | 0.05 | -0.01 mm (-0.02 mm to -6.98×10−06 mm) | 0.23 | 0.33 | ||
| SA of rostral middle frontal gyrus without global weighted | 0.02 | 43.35 mm2 (8.40 mm2 to 78.31 mm2) | 0.23 | 0.07 | ||
| eGFR | SA of pars triangularis with global weighted | 0.039 | 97.92 mm2 (5.03 mm2 to 190.81 mm2) | 0.02 | 0.88 | |
| SA of rostral middle frontal with global weighted | 0.007 | -274.57 mm2 (-475.74 mm2 to -73.30 mm2) | 0.40 | 0.94 | ||
| SA of rostral middle frontal without global weighted | 0.033 | -402.57 mm2 (-774.02 mm2 to -31.12 mm2) | 0.14 | 0.70 | ||
| SA of parahippocampal without global weighted | 0.038 | -56.24 mm2 (-109.45 mm2 to -3.02 mm2) | 0.07 | 0.43 | ||
| TH of caudal anterior cingulate with global weighted | 0.031 | 0.11 mm (0.01 mm to 0.22 mm) | 0.36 | 0.62 | ||
| TH of caudal middle frontal with global weighted | 0.011 | 0.07 mm (0.02mm to 0.13 mm) | 0.02 | 0.68 |
***Significant estimate is defined as IVW-derived P < 1.22 ×10-4, *nominal significant estimate is defined as IVW-derived P < 0.05. Cochran's Q-derived P value and MR-Egger intercept-derived P value < 0.05 is significant. IVW, Inverse-variance weighted; MR, Mendelian randomization; TH, cortical thickness; SA, cortical surficial area; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate
At the functional region level analysis, albuminuria significantly decreased the TH of the pars opercularis without global weighted (β = −0.11 mm, 95% CI: −0.16 mm to −0.07 mm, P = 3.74×10−6). No pleiotropy or heterogeneity was detected. There were also several suggestive gyrus, including lateral occipital, caudal anterior cingulate, caudal middle frontal, frontal pole, fusiform, lateral occipital, lateral orbitofrontal, lingual, medial orbitofrontal, middle temporal, parahippocampal, pars triangularis, rostral anterior cingulate, rostral middle frontal and superior frontal gyrus, potentially influenced by kidney function. Details are presented in Supplementary Figure S2.
To test whether the significant estimate was violated by risk factors, we conducted SNPs lookup in Phenoscanner. SNPs rs1047891, rs4665972 and rs838142 were associated with cholesterol, rs2236295 associated with self-reported hypertension, and rs2470893 and rs4410790 were related with BMI as well as caffeine. After removing these SNPs, estimates were consistent with the previous result (β = −0.11 mm, 95% CI: −0.17 mm to −0.04 mm, P = 0.0024), indicating that the causal relationship between albuminuria on the TH of the pars opercularis without global weighted was not violated by potential risk factors.
For both significant and nominal significant estimates, Cochran's Q test, MR-Egger intercept test, leave-one-out analyses, and funnel plot were used to assess horizontal pleiotropy All P-values of MR-Egger intercept tests were > 0.05 except for estimates of albuminuria on TH of lingual without weighted, indicating that no horizontal pleiotropy existed. Leave-one-out analyses and funnel plots are shown in Supplementary Figure S3-11. The estimates were not biased by single SNP, indicating that estimates were not violated. Cochran's Q derived P-values were all > 0.05 except for estimates of eGFR on TH of caudal middle frontal with global weighted and SA of the parstriangularis gyrus with global weighted, and albuminuria on TH of pars triangularis without global weighted.
7. Discussion
To the best of our knowledge, this is the first large-scale MR analysis that comprehensively determines the causal relationship between CKD, eGFR, and albuminuria with the brain cortical structure. In the present MR study, we systematically assessed the casual relation of genetically predicted kidney function and the brain cortex structure. Our results show that CKD, eGFR, and albuminuria could affect the brain cortex, and endorse the findings of earlier observational studies indicating the pathophysiologic interactions between renal damage and brain functions, thereby highlighting the existence of the kidney-brain axis.
CKD patients face relatively high risks of cognitive impairment and neuropsychiatric disorders. However, the causal relationship between CKD and the brain cortical structure or series of neuropsychiatric disorders have not yet been comprehensively illustrated. Cerebral cortical structures, especially cortical thickness, are regarded as neuroimaging biomarkers to predict cognitive decline. Therefore, scholars hypothesise the existence of a kidney-brain axis, through which kidney damage influences brain health [26,33]. These effects comprise cortical structure alteration and brain functional alteration/neuropsychiatric disorders. Brain functional alteration and neuropsychiatric disorders should be measured using MRI and scale assessments. When using MR to reveal the causal effect between CKD and neuropsychiatric disorders, the heterogeneity and severity of the disease must be adequately addressed. Furthermore, revealing the causal effect between CKD and structure alteration is more objective. Besides, the alteration of brain structures can somehow also indicate functional alteration and pathogenesis of neuropsychiatric disorders [25]. Inspired by Grasby KL, we conducted a comprehensive MR to investigate the causal effect between CKD and brain cortical structures. We intend to study the mechanism of the alterations on brain function as well as neuropsychiatric disorders in future. Further association studies will pave the way to the early identification of patients at higher risk, which is essential to achieving more optimized surveillance and earlier diagnosis to conduct effective preventive and treatment strategies for cognitive impairment and other neuropsychiatric disorders in patients with CKD.
The main finding of our study is that the pars opercularis is significantly influenced by kidney function. The pars opercularis is an essential language site [34] and its alteration has been reported in patients with multiple sclerosis [35]. Orsolya Inhóf also reported that the volume of the pars opercularis is associated with internet addiction [36]. Lauren B Curley found that the pars opercularis is related to motor-inhibitory performance in observational study, though their findings suggests that SA rather than TH of the pars opercularis may be more associated with motor-inhibitory performance [37]. The underlying mechanism of alterations of the pars opercularis and other neuropsychiatric disorders warrants further investigations. Whether CKD will lead to these functional changes or neuropsychiatric disorders mediated the alteration of TH of the pars opercularis could also be expected in the future studies.
Cho et al. retrospectively analysed 1,215 normal elderly individuals and found that albuminuria was associated with brain cortical thinning, whereas frontal and occipital regions were the most related regions, implying that albuminuria may directly cause neuronal toxicity and eventually result in TH alterations [38]. Alterations of TH have also been reported in dystonia, voice tremor, Parkinson's disease, depression, schizophrenia, attention deficit hyperactivity disorder, and insomnia [39], [40], [41], [42]. Our estimates indicate that albuminuria causally reduces global TH. Whether albuminuria leads to these neuropsychiatric disorders by affecting TH remains yet to be investigated.
Though only one estimate passed Bonferroni correction, other estimates with IVW-derived P value < 0.05 should also be treated cautiously. In our study, the nominally significant results of TH and SA of the lateral occipital were in opposing directions, which were consistent with the results of a previous study [28]. A significant negative genetic correlation between the total SA and average TH (rG = −0.32, SE = 0.05, z-score P = 6.50×10−12) was also observed. Bioinformatic analyses show that SA was influenced by genetic variants that altered the gene regulatory activity in the neural progenitor cells during foetal development, whereas TH was influenced by active regulatory elements in the adult brain sample, which may reflect processes whose onset occurs after mid-foetal development, such as myelination, branching, or pruning. Thus, the contrasting results related to SA and TH in our study could be explained.
Notably, certain estimates varied from logical expectation. A higher eGFR indicates more optimized kidney function and hence should lead to a larger SA. However, in our study, genetically predicted higher eGFR leads to a smaller SA of lateral occipital, rostral middle frontal and parahippocampal gyrus. Similarly, diagnosed CKD or albuminuria correlated with a larger SA of lateral occipital and rostral middle frontal gyrus. Herein, estimates using different indicators for kidney function were consistent and no pleiotropy was detected. Our findings are also consistent with those of previous reports. Hartung et al. reported that in CKD patients, lower eGFR was associated with higher white matter in whole brain (P = 0.05) and frontal (P = 0.04) [43]. This may possibly be a compensatory hypertrophy or encephaledema. Further studies are required to elucidate the underlying mechanism.
A robust MR estimate is built on three MR assumptions: (i) the instrumental variables are strongly associated with exposure; (ii) the instrumental variables are independent of confounders; (iii) the instrumental variables affect outcomes only through their effect on exposure and not through an alternative causal pathway. In our study, selected genetic instruments were robust instrumental variables with F statistics that were larger than the normally used value of 10. In addition, we have checked the Phenoscanncer to ensure that our findings are not violated by potential risk factors. We also carefully evaluated the directional pleiotropy based on the intercept obtained from the MR-Egger analysis and performed a leave-one-out analysis to evaluate whether the MR estimates was biased by a single SNP.
To yield a robust MR estimate, genetic instruments must be carefully selected. In addition to selecting robust genetic instruments (F > 10), excluding SNPs correlated with outcomes, performing clumping, we need to be cautious about traits related with every SNP and avoid violations from potential confounders. In our study, we used three sets of genetic instruments as opposed to using only one to symbolize kidney function from different aspects, thus increasing our chances of identifying significant estimates. During the selection of significant loci for eGFR, we also used GWAS of BUN to prioritize eGFR loci, which are more likely to be related to kidney function. When selecting genetic instruments for albuminuria, violations from hypertension as well as anti-hypertensive drugs were avoided.
To the best of our knowledge, this is the first study that has implemented an MR analysis to address the causal relation between kidney damage and the brain cortical structure. Our study supports the causal relationship between kidney damage and brain dysfunction and provides novel information on the regional alterations ascribed to kidney function markers.
This study has several limitations. First, the enrolled patients were all European, and hence, the causal relationship between kidney functions and the brain cortical structure in other populations remains unknown. Second, our findings only report the alteration of the brain cortical structure in CKD patients but the underlying mechanisms warrant further investigation. Third, there are 3 cohorts that overlap between CKDGen consortium and ENIGMA, and total number of overlapped participants is less than 2263. The ideal situation for two sample MR is if there is no overlap between exposure and outcome. However, using publicly available summary data is difficult to achieve full non-overlap. In our study, the proportion of overlapped participants is less than 0.4%. Based on Burgess's simulation, the expected bias is less than 0.00068 and type I error is less than 0.0503 whereas ideal type I error is less than 0.05 [44].
Our results provide a platform for researchers to explore the relationship between kidney functions and other neuropsychiatric disorders, especially focusing on the specific gyrus of CKD patients. Future studies should elucidate the mechanism underlying the association between CKD and neuropsychiatric disorders or explore novel treatment modalities to prevent or treat neuropsychiatric disorders in patients with CKD.
8. Conclusion
This is the first comprehensive MR analysis that reveals associations among eGFR, CKD, albuminuria, and the brain cortex structure. Our estimates illustrate that albuminuria causally decreases global TH as well as the TH of the pars opercularis gyrus. A brain MRI could potentially be used for patients with CKD for early diagnosis of neuropsychiatric disorders. Neurologists must also be cautious with respect to patients’ kidney function when patients exhibit alterations of the the pars opercularis. The mechanisms of the association between kidney damage and brain function alterations should be studied further.
9. Declarations
9.1. Contributors
XC and JQK designed the study, wrote the first draft of the manuscript and verified the underlying data. XC, JQK, JXP, and KH conducted statistical analyses. Wenhao Zhou, Xiayao Diao, Jiahao Cai, Junjiong Zheng, Xuefan Yang, Weibin Xie and Hao Yu played roles in acquisition of the data and analyses. Jiande Li, Lu Pei, Wen Dong, Haide Qin, Jian Huang and Tianxin Lin participated in data interpretation. All authors revised and approved the final manuscript. The guarantor (TXL) confirms that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted."
Data Sharing Statement
All data are publicly available.
Declaration of Competing Interest
All authors declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgments
This study was supported by the Science and Technology Planning Project of Guangdong Province (Grant No. 2020A1515111119 and 2017B020227007), the National Key Research and Development Program of China (Grant No. 2018YFA0902803), the National Natural Science Foundation of China (Grant No. 81825016, 81961128027, 81772719, 81772728), the Key Areas Research and Development Program of Guangdong (Grant No. 2018B010109006), Guangdong Special Support Program (2017TX04R246), Grant KLB09001 from the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes, and Grants from the Guangdong Science and Technology Department (2020B1212060018). We thank CKDGen Consortium, the UK Biobank, and the ENIGMA Consortium for providing GWAS data.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103592.
Contributor Information
Jian Huang, Email: huangj8@mail.sysu.edu.cn.
Tianxin Lin, Email: lintx@mail.sysu.edu.cn.
Appendix. Supplementary materials
References
- 1.Collaborators GBDCoD Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151-210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Disease G.B.D., Injury I., Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990−2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo K.T., Choong H.L., Wong K.S., Tan H.B., Chan C.M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2012;81(10):1044–1045. doi: 10.1038/ki.2012.39. [DOI] [PubMed] [Google Scholar]
- 4.Ene-Iordache B., Perico N., Bikbov B., Carminati S., Remuzzi A., Perna A. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health. 2016;4(5):e307–e319. doi: 10.1016/S2214-109X(16)00071-1. [DOI] [PubMed] [Google Scholar]
- 5.Eckardt K.U., Coresh J., Devuyst O., Johnson R.J., Kottgen A., Levey A.S. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382(9887):158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 6.Moodalbail D.G., Reiser K.A., Detre J.A., Schultz R.T., Herrington J.D., Davatzikos C. Systematic review of structural and functional neuroimaging findings in children and adults with CKD. Clin J Am Soc Nephrol. 2013;8(8):1429–1448. doi: 10.2215/CJN.11601112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuruya K., Yoshida H., Kuroki Y., Nagata M., Mizumasa T., Mitsuiki K. Brain atrophy in peritoneal dialysis and CKD stages 3-5: a cross-sectional and longitudinal study. Am J Kidney Dis. 2015;65(2):312–321. doi: 10.1053/j.ajkd.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Collins A.J., Kasiske B., Herzog C., Chavers B., Foley R., Gilbertson D. Excerpts from the United States Renal Data System 2006 Annual Data Report. Am J Kidney Dis. 2007;49(1):S1–296. doi: 10.1053/j.ajkd.2006.11.019. 1 SupplA6-7. [DOI] [PubMed] [Google Scholar]
- 9.Passer J.A. Cerebral atrophy in end-stage uremia. Proc Clin Dial Transplant Forum. 1977;7:91–94. [PubMed] [Google Scholar]
- 10.Vermeer S.E., Prins N.D., den Heijer T., Hofman A., Koudstaal P.J., Breteler M.M. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 11.Cusmano F., Savazzi G.M. Cerebral computed tomography in uremic and hemodialyzed patients. J Comput Assist Tomogr. 1986;10(4):567–570. doi: 10.1097/00004728-198607000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Davey Smith G., Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X., Kong J., Diao X., Cai J., Zheng J., Xie W. Depression and prostate cancer risk: a Mendelian randomization study. Cancer Med. 2020;9(23):9160–9167. doi: 10.1002/cam4.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng T., Smith C.E., Li C., Huang T. Childhood BMI and Adult Type 2 Diabetes, Coronary Artery Diseases, Chronic Kidney Disease, and Cardiometabolic Traits: a Mendelian Randomization Analysis. Diabetes Care. 2018;41(5):1089–1096. doi: 10.2337/dc17-2141. [DOI] [PubMed] [Google Scholar]
- 15.Jordan D.M., Choi H.K., Verbanck M., Topless R., Won H.H., Nadkarni G. No causal effects of serum urate levels on the risk of chronic kidney disease: a Mendelian randomization study. PLoS Med. 2019;16(1) doi: 10.1371/journal.pmed.1002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Z., Coresh J., Qi G., Grams M., Boerwinkle E., Snieder H. A bidirectional Mendelian randomization study supports causal effects of kidney function on blood pressure. Kidney Int. 2020;98(3):708–716. doi: 10.1016/j.kint.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., Feldman H.I., 3rd A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz G.J., Schneider M.F., Maier P.S., Moxey-Mims M., Dharnidharka V.R., Warady B.A. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82(4):445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wuttke M., Li Y., Li M., Sieber K.B., Feitosa M.F., Gorski M. A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet. 2019;51(6):957–972. doi: 10.1038/s41588-019-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanholder R., De Deyn P.P., Van Biesen W., Lameire N. Marconi revisited: from kidney to brain-two organ systems communicating at long distance. J Am Soc Nephrol. 2008;19(7):1253–1255. doi: 10.1681/ASN.2008040404. [DOI] [PubMed] [Google Scholar]
- 21.Sedaghat S., Sorond F., Yaffe K., Sidney S., Kramer H.J., Jacobs D.R., Jr. Decline in kidney function over the course of adulthood and cognitive function in midlife. Neurology. 2020;95(17):e2389–e2e97. doi: 10.1212/WNL.0000000000010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas M.E., Aragam K.G., Emdin C.A., Bick A.G. International Consortium for Blood P, Hemani G, et al. Genetic Association of Albuminuria with Cardiometabolic Disease and Blood Pressure. Am J Hum Genet. 2018;103(4):461–473. doi: 10.1016/j.ajhg.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grasby K.L., Jahanshad N., Painter J.N., Colodro-Conde L., Bralten J., Hibar D.P. The genetic architecture of the human cerebral cortex. Science. 2020;367(6484) doi: 10.1126/science.aay6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Marini S., Georgakis M.K., Chung J., Henry J.Q.A., Dichgans M., Rosand J. Genetic overlap and causal inferences between kidney function and cerebrovascular disease. Neurology. 2020;94(24):e2581–e2e91. doi: 10.1212/WNL.0000000000009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ariton D.M., Jimenez-Balado J., Maisterra O., Pujadas F., Soler M.J., Delgado P. Diabetes, Albuminuria and the Kidney-Brain Axis. J Clin Med. 2021;10(11) doi: 10.3390/jcm10112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey A.S., Inker L.A. GFR as the "Gold Standard": Estimated, Measured, and True. Am J Kidney Dis. 2016;67(1):9–12. doi: 10.1053/j.ajkd.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Verbanck M., Chen C.Y., Neale B., Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staley J.R., Blackshaw J., Kamat M.A., Ellis S., Surendran P., Sun B.B. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. 2016;32(20):3207–3209. doi: 10.1093/bioinformatics/btw373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierce B.L., Ahsan H., Vanderweele T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740–752. doi: 10.1093/ije/dyq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgess S., Davey Smith G., Davies N.M., Dudbridge F., Gill D., Glymour M.M. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019;4:186. doi: 10.12688/wellcomeopenres.15555.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miranda A.S., Cordeiro T.M., Santos Lacerda D.os, Soares T.M., Ferreira R.N., Simoes E.S.A.C. Kidney-brain axis inflammatory cross-talk: from bench to bedside. Clin Sci (Lond) 2017;131(11):1093–1105. doi: 10.1042/CS20160927. [DOI] [PubMed] [Google Scholar]
- 34.Lin Y., Zhang K., Li S., Li S., Jin J., Jin F. [Hodotopical research on neural pathway of Chinese language in posterior inferior frontal gyrus] Zhonghua Yi Xue Za Zhi. 2016;96(17):1364–1367. doi: 10.3760/cma.j.issn.0376-2491.2016.17.013. [DOI] [PubMed] [Google Scholar]
- 35.Cohen-Adad J., Benner T., Greve D., Kinkel R.P., Radding A., Fischl B. In vivo evidence of disseminated subpial T2* signal changes in multiple sclerosis at 7 T: a surface-based analysis. Neuroimage. 2011;57(1):55–62. doi: 10.1016/j.neuroimage.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inhof O., Zsido A.N., Perlaki G., Orsi G., Labadi B., Kovacs N. Internet addiction associated with right pars opercularis in females. J Behav Addict. 2019;8(1):162–168. doi: 10.1556/2006.7.2018.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curley L.B., Newman E., Thompson W.K., Brown T.T., Hagler D.J., Jr., Akshoomoff N. Cortical morphology of the pars opercularis and its relationship to motor-inhibitory performance in a longitudinal, developing cohort. Brain Struct Funct. 2018;223(1):211–220. doi: 10.1007/s00429-017-1480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho E.B., Shin H.Y., Park S.E., Chun P., Jang H.R., Yang J.J. Albuminuria, cerebrovascular disease and cortical atrophy: among cognitively normal elderly individuals. Sci Rep. 2016;6:20692. doi: 10.1038/srep20692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomic A., Agosta F., Sarasso E., Svetel M., Kresojevic N., Fontana A. Brain structural changes in focal dystonia-what about task specificity? A Multimodal MRI Study. Mov Disord. 2021;36(1):196–205. doi: 10.1002/mds.28304. [DOI] [PubMed] [Google Scholar]
- 40.de Lima Xavi.er L., Simonyan K. Neural representations of the voice tremor spectrum. Mov Disord. 2020;35(12):2290–2300. doi: 10.1002/mds.28259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filippi M., Canu E., Donzuso G., Stojkovic T., Basaia S., Stankovic I. Tracking cortical changes throughout cognitive decline in Parkinson's disease. Mov Disord. 2020;35(11):1987–1998. doi: 10.1002/mds.28228. [DOI] [PubMed] [Google Scholar]
- 42.Writing Committee for the Attention-Deficit/Hyperactivity D. Autism Spectrum D., Bipolar D., Major Depressive D., Obsessive-Compulsive D., Schizophrenia E.W.G. Virtual histology of cortical thickness and shared neurobiology in 6 psychiatric disorders. JAMA Psychiatry. 2021;78(1):47–63. doi: 10.1001/jamapsychiatry.2020.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartung E.A., Erus G., Jawad A.F., Laney N., Doshi J.J., Hooper S.R. Brain magnetic resonance imaging findings in children and young adults with CKD. Am J Kidney Dis. 2018;72(3):349–359. doi: 10.1053/j.ajkd.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burgess Ste.phen, Davies N.M., Thompson S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40(7):597–608. doi: 10.1002/gepi.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.