Abstract
Background
In the Preterm Erythropoietin (Epo) NeUroproTection (PENUT) Trial, potential biomarkers of neurological injury were measured to determine their association with outcomes at two years of age and whether Epo treatment decreased markers of inflammation in extremely preterm (<28 weeks’ gestation) infants.
Methods
Plasma Epo was measured (n=391 Epo, n=384 placebo) within 24h after birth (baseline), 30min after study drug administration (day 7), 30min before study drug (day 9), and on day 14. A subset of infants (n=113 Epo, n=107 placebo) had interferon-gamma (IFN-γ), Interleukin (IL)-6, IL-8, IL-10, Tau, and tumour necrosis factor-α (TNF-α) levels evaluated at baseline, day 7 and 14. Infants were then evaluated at 2 years using the Bayley Scales of Infant and Toddler Development, 3rd Edition (BSID-III).
Findings
Elevated baseline Epo was associated with increased risk of death or severe disability (BSID-III Motor and Cognitive subscales <70 or severe cerebral palsy). No difference in other biomarkers were seen between treatment groups at any time, though Epo appeared to mitigate the association between elevated baseline IL-6 and lower BSID-III scores in survivors. Elevated baseline, day 7 and 14 Tau concentrations were associated with worse BSID-III Cognitive, Motor, and Language skills at two years.
Interpretation
Elevated Epo at baseline and elevated Tau in the first two weeks after birth predict poor outcomes in infants born extremely preterm. However, no clear prognostic cut-off values are apparent, and further work is required before these biomarkers can be widely implemented in clinical practice.
Funding
PENUT was funded by the National Institute of Neurological Disorders and Stroke (U01NS077955 and U01NS077953).
Keywords: Premature, Inflammation, Neurodevelopment, Biomarker, Erythropoietin
Research in context.
Evidence before this study
Around 50% of infants born extremely preterm (<28 weeks’ gestation) will experience death or severe long-term disability. Preterm birth is often associated with infection, growth restriction, hypoxia, and ischaemia, which motivates the exploration of mechanistic biomarkers of injury that could aid in long-term patient identification and prognostication. Previously, the Extremely Low Gestational Age Newborns (ELGAN) study found that persistent elevation of a number of inflammatory markers 14-28 days after birth was associated with an increased risk of neurodevelopmental impairment, attention deficit disorders, and poorer reading, mathematics, and executive function skills. These included elevated levels of erythropoietin (Epo), a haematopoietic cytokine induced by chronic fetal hypoxia that is also a putative anti-inflammatory and neuroprotective agent when administered exogenously in high doses. In the Preterm Erythropoietin NeUroproTection (PENUT) Trial, extremely preterm infants were randomised to receive high dose Epo or placebo until the subjects were 32-6/7 weeks postmenstrual age. A secondary aim of the PENUT Trial was to evaluate plasma Epo concentrations and potential biomarkers of preterm neurological injury at baseline (prior to first study drug administration within 24h of birth) as well as on days 7 and 14. Long-term outcomes associated with inflammatory biomarkers immediately and within two weeks after birth had not previously been documented. The effect of exogenous Epo on inflammatory biomarkers in human neonates was also not known.
Added value of this study
We show that elevated endogenous Epo at baseline is associated with a significantly increased risk of death or severe neurodevelopmental impairment. In surviving infants, elevated circulating levels of Tau at baseline as well as on days 7 and 14 were associated with worse cognitive, motor, and language skills at two years of age. Exogenous high-dose Epo did not reduce the burden of inflammatory cytokines in the Epo group, but Epo did display a potential benefit in infants with high levels of IL-6 at baseline.
Implications of all the available evidence
When considered in conjunction with previous data such as that from the ELGAN study, elevations in endogenous Epo throughout the first month after birth are associated with worse long-term outcomes in extremely preterm infants, likely due to Epo's association with chronic hypoxia or inflammation. Circulating Tau appears to be an important potential prognostic marker of long-term outcome in survivors. Importantly, despite the anti-inflammatory effect of Epo seen in preclinical studies, Epo does not appear to be significantly anti-inflammatory in preterm human neonates; however, it may have an isolated benefit in those exposed to insults around birth that result in increased IL-6.
Alt-text: Unlabelled box
1. INTRODUCTION
More than 10% of infants in the United States are born prematurely, [1] and for those born extremely preterm (EP, <28 weeks’ gestation), around 50% will have a poor outcome [[2], [3], [4]]. As no therapeutics currently exist that specifically target improved neurodevelopmental outcome in preterm infants, neuroprotective therapies for infants born preterm remain an unmet need. In addition, biomarkers of brain injury in preterm infants are needed to aid prognostication and monitor response to these putative therapies.
Preterm birth is commonly initiated by maternal infection (chorioamnionitis) and is often associated with perinatal insults such as growth restriction, hypoxia, and ischaemia [5]. Downstream mechanistic processes of injury include both local and systemic inflammation, oxidative and nitrosative stress, and multiple types of cell death, with the developing brain naturally primed to activate cellular apoptosis [6,7]. These exposures and downstream injury mechanisms motivate the exploration of biomarkers of injury that could aid in patient identification and long-term prognostication. For instance, the Extremely Low Gestational Age Newborns (ELGAN) study examined a number of inflammatory cytokines at 14-28 days after birth in infants born <28 weeks’ gestation, as well as their relationship with both short- and long-term neurological and neurodevelopmental outcomes. They found that infants with persisting levels (top quartile) of inflammation-associated proteins such as interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α and Intercellular Adhesion Molecule (ICAM)-1 had an increased risk of white matter lesions on cranial ultrasound, neurodevelopmental impairment (NDI) at two years of age, attention deficit disorders, and poorer reading, mathematics, and executive function skills at 10 years of age [[8], [9], [10], [11], [12], [13]].
Erythropoietin (Epo) is a haematopoietic cytokine induced by chronic fetal hypoxia and/or as part of a broad inflammatory response, [14,15] and elevated baseline Epo concentrations are associated with long-term NDI in extremely preterm infants [16]. However, high dose Epo has well-described neurotrophic properties and neuroreparative effects in the brain after experimental injury [[17], [18], [19]]. In the Preterm Erythropoietin NeUroproTection (PENUT) Trial, infants 24-0/7 to 27-6/7 weeks’ gestation were randomised to receive high dose Epo or placebo until the subjects were 32-6/7 weeks postmenstrual age (PMA). A secondary aim of the PENUT Trial was to evaluate Epo concentrations and potential biomarkers of preterm neurological injury at baseline as well as days 7, 9, and 14 after birth. We hypothesised that: 1) Epo treatment would result in lower circulating pro-inflammatory cytokines and markers of neurological injury compared to placebo, and 2) elevated Epo at baseline would act as a biomarker of prolonged in utero hypoxia and be associated with worse outcomes at 24 months corrected age (CA).
2. METHODS
2.1. Inclusion criteria
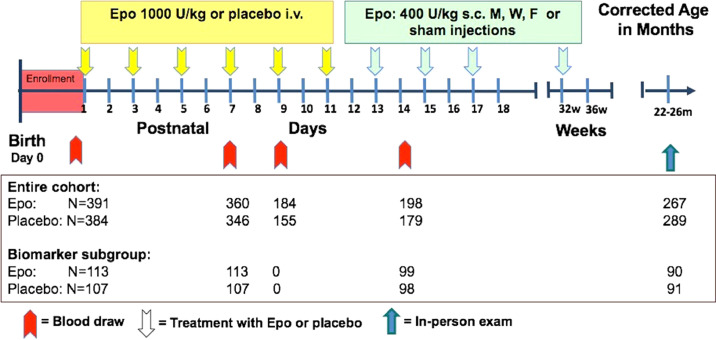
Patients enrolled in the PENUT Trial [20] were eligible for this analysis if they had baseline Epo levels measured. Also included were a subset of infants from 9 pre-selected PENUT sites who had plasma biomarkers evaluated at baseline and on days 7 and 14 after birth (Figure 1). Infants with plasma biomarker measurements were included if they survived through 36 weeks PMA and had neurodevelopmental testing with a Bayley Scales of Infant Development, 3 rd edition (BSID-III) between 19 and 33 months (90% were assessed between 22 and 26 months). Briefly, PENUT subjects were born between 24-0/7 and 27-6/7 weeks’ gestation and after parental consent, were enrolled at 19 US sites between December 2013 and September 2017. Enrolment and initial treatment with study drug occurred within 24 h after birth. Baseline samples were obtained after enrolment, but prior to study drug administration. Subjects received Epo 1,000 U/kg/dose or placebo intravenously every 48 h for 6 doses, followed by maintenance subcutaneous injections of 400 U/kg/dose Epo or sham injections three times a week through 32-6/7 weeks PMA. The primary outcome of the PENUT Trial was death or severe NDI at 22-26 months corrected age (CA).
Figure 1.
Treatment, sample collection, and measurement protocol. Diagram depicting when samples were taken in relation to dosing of study drug in both the entire cohort (Epo measurements) and the biomarker subgroup, as well as the number of infants at each time point for which samples were available for analysis.
2.2. Ethics
This study was institutional review board (IRB) approved at all participating sites; All Childrens Hospital, St. Petersburg, FL (Johns Hopkins Office of Human Subjects Research Institutional Review Boards IRB00060341); Beth Israel Deaconess Medical Center, Boston, MA (Boston Children's Office of Clinical Investigation IRB-P00008415); Childrens Hospitals and Clinics of Minnesota, Minneapolis, MN (Children's Hospitals and Clinicals of Minnesota IRB# 1308-079); Childrens Hospitals and Clinics of Minnesota, St. Paul, MN (Children's Hospitals and Clinicals of Minnesota IRB# 1308-079); Florida Hospital Orlando, Orlando, FL (Florida Hospital Office of Research Administration IRB-6055); Johns Hopkins University, Baltimore, MD (Johns Hopkins Office of Human Subjects Research Institutional Review Boards IRB00026419); Methodist Childrens Hospital, San Antonio, TX (Methodist Healthcare IRB 440455-3); Maria Fareri Childrens Hospital, Westchester, NY (New York Medical College Office of Research Administration IRB# 10265); South Miami Hospital, Miami, FL (Baptist Health South Florida IRB# 15-037); Prentice Womens Hospital of Northwestern Memorial Hospital, Chicago, IL (Ann and Robert H Lurie Children's Hospital of Chicago 2013-1543); University of Florida, Gainsville, FL (University of Florida #142-2013); Childrens Hospital of the University of Illinois (University of Illinois Chicago Office for the Protection of Research Subjects #2013-0835); University of Minnesota Childrens Hospital, Minneapolis, MN (University of Minnesota 1308M41201); University of New Mexico, Albuquerque, NM (University of New Mexico Health Sciences Center Human Research Protections Office #13-464, Presbyterian Healthcare Services Institutional Review Board 662482-1); University of Arkansas, Fayetteville, AR (University of Arkansas for Medical Sciences IRB #202449); Kosair Childrens Hospital, Louisville, KY (Norton Healthcare Office of Research Administration NHORA#13-N0095); University of Utah, Salt Lake City, UT (University of Utah IRB #00063749, Intermountain Healthcare IRB #1024761); University of Washington, Seattle, WA (University of Washington Human Subjects Division STUDY00007467); Wake Forest University, Wake Forest, NC (Wake Forest University Health Sciences IRB #00023079). All participants’ parents in the PENUT study at all sites provided written informed consent. Additional protocol details and results of the primary outcome are published elsewhere [17,20]. With the exception of the data coordination centre, the site pharmacist, and the individuals administering the maintenance injections, all personnel in the study were blinded to treatment groups. This study was registered with ClinicalTrials.gov (NCT01378273) and the FDA (IND#12656) [17].
2.3. Neurodevelopmental Outcome
Severe NDI was defined as the presence of severe cerebral palsy (CP) or BSID-III Motor or Cognitive Composite Score < 70. For completeness, BSID-III outcomes from examinations collected outside the 22-26 month primary outcome window (range 19-33 months) were included in this analysis. CP was classified as hemiplegia, diplegia, or quadriplegia, with severity determined by Gross Motor Function Classification System (GMFCS). A GMFCS level of 2 was defined as moderate CP, and 3 or greater was defined as severe CP [ [21], [22], [23], [24]]. Individuals performing the BSID-III assessments and standardised neurologic exams were centrally certified. To minimise bias, examiners were blinded to the child's medical history and brain imaging studies.
2.4. Epo and biomarker analysis
Plasma Epo concentrations were measured in both groups within the first 24 h after birth before study drug administration (baseline), 30 minutes after study drug administration on day 7 (peak Epo concentration) and 30 minutes before study drug on day 9 (trough Epo concentration). On day 14, a random blood sample was drawn after the child had transitioned to subcutaneous dosing (Figure 1). Blood samples (0·5 mL in EDTA tubes) were spun down at the clinical site at 1,000 x g for 10 minutes, plasma separated, and stored at -70°C until shipping on dry ice to the University of Washington. After thawing, plasma biomarker concentrations from baseline and days 7 and 14 after birth were measured using Meso Scale Discovery (MSD) electrochemiluminescence-based assays. Brain-specific Tau levels were quantified using the MSD V-PLEX Human Total Tau assay (Meso Scale Discovery, Rockville, MD). Inflammatory cytokines (IFN-γ, TNFα, IL-6, IL-8, IL-10) were determined with the Meso Scale Discovery V-PLEX Proinflammatory Panel 1 (Meso Scale Discovery, Rockville, MD). Epo was measured with the Human Epo Base Kit (Meso Scale Discovery, Rockville, MD). For a few samples, Epo levels were determined using the Meso Scale Discovery U-Plex assay since the Human EPO Base Kit was no longer available. Details of assay characteristics are summarized in Supplemental Table 1. All samples were assayed in duplicate, and the duplicate measurements were averaged. All assays were performed according to the manufacturer's recommended protocol.
2.5. Statistical analysis
Simple descriptive statistics were used to describe the characteristics of the Epo cohort as well as the biomarker sub-cohort. Due to skewness in the Epo and biomarker measurements, these data were log-transformed, using the natural log, in all statistical analyses and medians and interquartile ranges were used to characterise the distributions by treatment group. For statistical inference, we utilized generalised estimating equations (GEE) with robust standard errors to appropriately account for potential correlation of biomarkers or outcomes for same-birth siblings [25]. Each respective GEE model adjusted for gestational age at birth and treatment assignment as fixed factors associated with the original study design. Biomarker levels at each follow-up time point were analysed using separate GEE models. Statistical significance was evaluated using a Wald's test, and this and all other statistical tests were declared significant if p < 0·05.
The primary outcome measure of death or severe NDI was tabulated by baseline Epo quartile and analysed with a GEE model appropriate for binary data. The Motor, Cognitive, and Language composite summary scores of the BSID-III examination were evaluated with separate GEE models for evidence of association with each (log-transformed) baseline plasma marker. In a second model, we assessed the evidence for treatment effect modification of the association between BSID-III scores and each marker by also including a statistical interaction term between treatment assignment and the marker. Finally, we sought to examine whether multiple biomarkers or linear combinations of biomarkers (mean or difference) at baseline, day 7 or day 14 predicted BSID-III scores at age two using a stepwise-AIC (Akaike Information Criteria) variable selection procedure in a multivariable linear regression model. Although we used a “working independence” AIC procedure due to the relatively small number of multiples, we followed this with GEE to estimate valid standard errors. All statistical analyses were conducted using the R statistical package (version 3·6·1) [26].
2.6. Role of the funding source
The PENUT Trial was funded by the National Institute of Neurological Disorders and Stroke (NINDS). The funding body had no active role in study design, data collection, data analyses, interpretation, or writing of this report.
3. RESULTS
3.1. Demographics and data collection
Baseline Epo concentrations were available from 775/936 (82·8%) of infants in the PENUT Trial (n=391 Epo treated, n=384 placebo treated). Maternal and neonatal demographics were similar in the cohort that had Epo measurements done and the biomarker subgroup (Table 1). Epo concentrations on days 7, 9, and 14 were available from n=360, n=184, and n=198 infants in the Epo group, and n=346, n=155, and n=179 infants in the placebo group, respectively (Figure 1). Biomarker data was available from n=113 infants in the Epo group and n=107 infants in the placebo group at baseline and day 7, with n=99 and n=98 samples available on day 14 (Figure 1). Median (IQR) age in hours at time of baseline biomarker measurement – taken immediately prior to first study drug dose administration – was 20 hours (15-23 hours) in both the Epo and biomarker cohorts. Overall outcomes including mortality and presence of severe NDI were available for n=676 (n=332 Epo treated, n=334 placebo treated) infants with baseline Epo values, and n=185 (n=92 Epo treated, n=93 placebo treated) infants with biomarker data. Developmental outcomes at 22-26 months CA were available for n=579 (n=278 Epo treated, n=301 placebo treated) infants with Epo data, and n=183 (n=90 Epo treated, n=93 placebo treated) infants with biomarker data.
Table 1.
Baseline characteristics by biomarker cohort.
| Epo cohort | Biomarker subgroup | |
|---|---|---|
| N | 775 | 220 |
| Maternal demographics, n (%) | ||
| Age, mean (SD) | 29.0 (6.3) | 29.0 (6.3) |
| Hispanic | 170 (22%) | 74 (34%) |
| Race | ||
| White | 505 (65%) | 148 (67%) |
| Black | 199 (26%) | 55 (25%) |
| Other | 71 (9.2%) | 17 (7.7%) |
| Education* | ||
| High School or less | 260 (34%) | 79 (36%) |
| Some college | 227 (29%) | 57 (26%) |
| College degree or greater | 192 (25%) | 56 (25%) |
| Neonatal data at enrollment, n (%) | ||
| Complications – maternal indications for delivery | 118 (15%) | 32 (15%) |
| Complications – risk of infection† | 564 (73%) | 167 (76%) |
| Complications – pregnancy induced hypertension | 61 (7.9%) | 19 (8.6%) |
| Chorioamnionitis | 97 (13%) | 36 (16%) |
| Prenatal steroids | 694 (90%) | 206 (94%) |
| Prenatal magnesium sulphate | 620 (80%) | 176 (80%) |
| Delivery complications‡ | 127 (16%) | 28 (13%) |
| Caesarean delivery | 550 (71%) | 147 (67%) |
| Delayed cord clamping§ | 266 (47%) | 100 (59%) |
| Female, n (%) | 410 (53%) | 111 (50%) |
| Gestational age, n (%) | ||
| 24w | 196 (25%) | 45 (20%) |
| 25w | 210 (27%) | 51 (23%) |
| 26w | 172 (22%) | 61 (28%) |
| 27w | 197 (25%) | 63 (29%) |
| Mean (SD) | 25.9 (1.2) | 26.0 (1.1) |
| Multiple gestation, n (%) | 214 (28%) | 58 (26%) |
| Infant weight (grams), mean (SD) | 799 (186) | 830 (180) |
| Weight < 10th percentile, n (%) | 120 (16%) | 32 (15%) |
| Occipital front circumference <10th percentile, n (%) | 129 (17%) | 43 (20%) |
| Apgar score at 5 minutes, median (25th, 75th percentile) | 7 (5, 8) | 7 (6, 8) |
| Apgar score at 5 minutes < 5, n (%) | 165 (21%) | 35 (16 %) |
| Intracranial haemorrhage prior to 1st dose | 165 (21%) | 47 (21%) |
| Age at first dose, median (25th, 75th percentile) hours | 20.4 (14.8, 23.3) | 20.1 (14.9, 23.1) |
Education unknown or not reported for 96 (12%) and 28 (13%) in the Epo cohort and biomarker subgroup respectively.
Defined as the presence of pyrexia, chorioamnionitis, prolonged rupture, antibiotics, or pre-term labor.
Defined as the presence of one or more of the following complications during delivery: prolapsed cord, true knot, tear or rupture of cord, placental abruption, twin-twin transfusion, feto-maternal bleeding, ruptured uterus, or traumatic instrument delivery.
Unknown for n=212 and n=51 participants and percentages reflect denominators of n=563 and n=169 in the Epo and placebo groups respectively.
3.2. Epo concentrations over time by treatment group
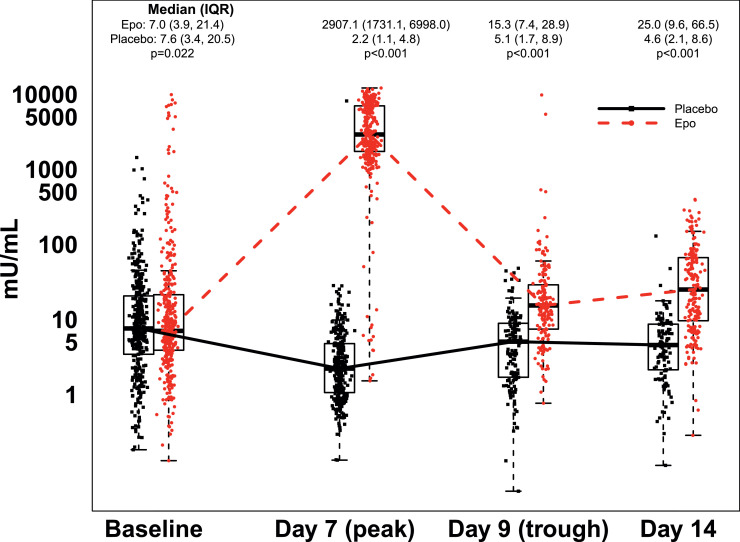
At baseline, median Epo concentration was 7.0 mU/mL (IQR: 3·9-21·4, range: 0·1-9,892 mU/mL) in the Epo group, and 7·6 mU/ml (IQR: 3·4–20·5, range: 0·2-1,436 mU/mL) in the placebo group (p=0·022, GEE linear model; Figure 2). Peak (day 7) Epo values were significantly higher in the Epo group (median: 2,907 mU/mL, IQR: 1,731-6,998 mU/mL, range: 1·5-12,120 mU/mL) than in the placebo group (median: 2·2 mU/mL, IQR:1·1-4·8 mU/mL, range: 0·1-8,144; p<0·001, GEE linear model). Trough (day 9) Epo levels were also significantly higher in the Epo group (median: 15·3 mU/mL, IQR: 7·4-28·9 mU/mL, range: 0·8-9,887 mU/mL) than in the placebo group (median: 5·1 mU/mL, IQR: 1·7-8·9 mU/mL, range: 0·1-48 mU/mL; p<0·001, GEE linear model). After transition to maintenance Epo (400 mU/kg administered subcutaneously), on day 14, random Epo concentrations remained significantly elevated in the Epo group (median: 25·0 mU/mL, IQR: 9·6-66·5 mU/mL, range: 0·3-397 mU/mL) compared to the placebo group (median:4.6, IQR: 2·1-8·6 mU/mL, range: 0·1-129 mU/mL; p<0·001, GEE linear model). On day 7, one sample in the placebo group and 15 samples in the Epo group were outside of the expected range for that group (Figure 2). Similarly, on day 9, two samples in the Epo group were above the range expected for an Epo trough measurement.
Figure 2.
Serum Epo concentration over time by treatment group. Box plots (median, IQR) with all data points overlaid, depicting Epo levels in both treatment groups for the first 14 days of the PENUT study. Numbers above each box indicate median and IQR Epo levels in each group at each time point, with p-value for the difference based on a GEE linear model adjusting for gestational age. Epo levels were significantly greater in the Epo group compared to the placebo group.
3.3. Biomarker concentrations over time by treatment group
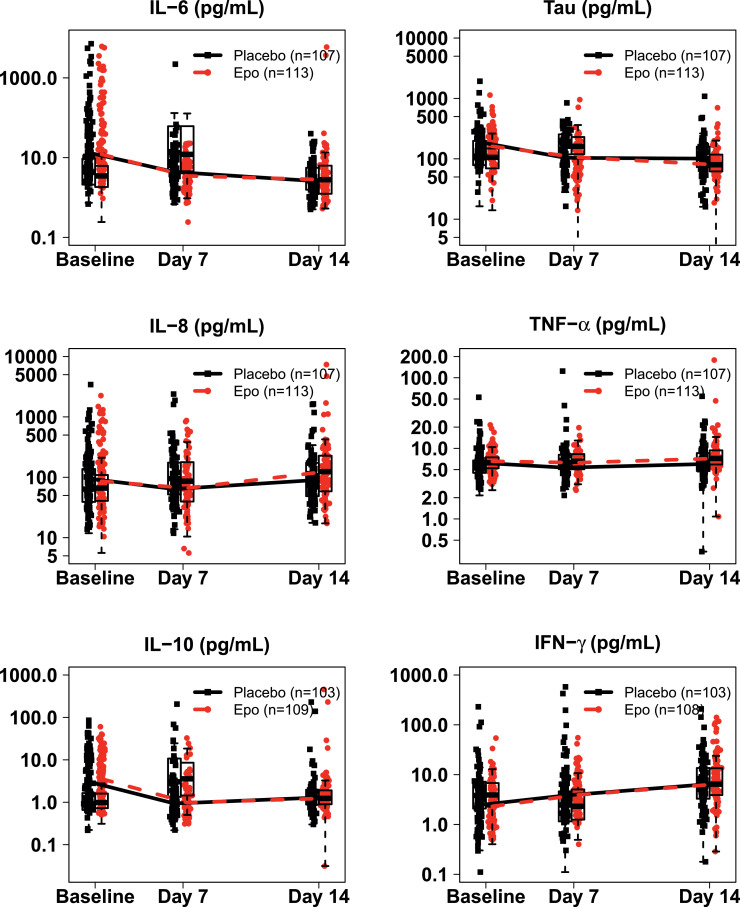
Baseline plasma concentrations of IL-6, IL-8, IL-10, TNF-α, IFN-γ, and Tau were similar in both groups (Figure 3). IL-6 tended to decrease over time, and IFN-γ tended to increase over time; however, there was no difference in any marker at any timepoint between the two groups.
Figure 3.
Serum biomarker concentrations over time by treatment group. Box plots (median, IQR) with all data points overlaid, depicting IL-6, Tau, IL-8, TNF-α, IL-10, and IFN-γ levels in both the Epo and placebo group of the biomarker cohort at baseline, and day 7 and 14 after birth. There was no difference in any biomarker level at any time point between the two groups based on GEE linear models adjusting for gestational age.
3.4. Baseline Epo concentration and neurodevelopmental outcome at two years corrected age
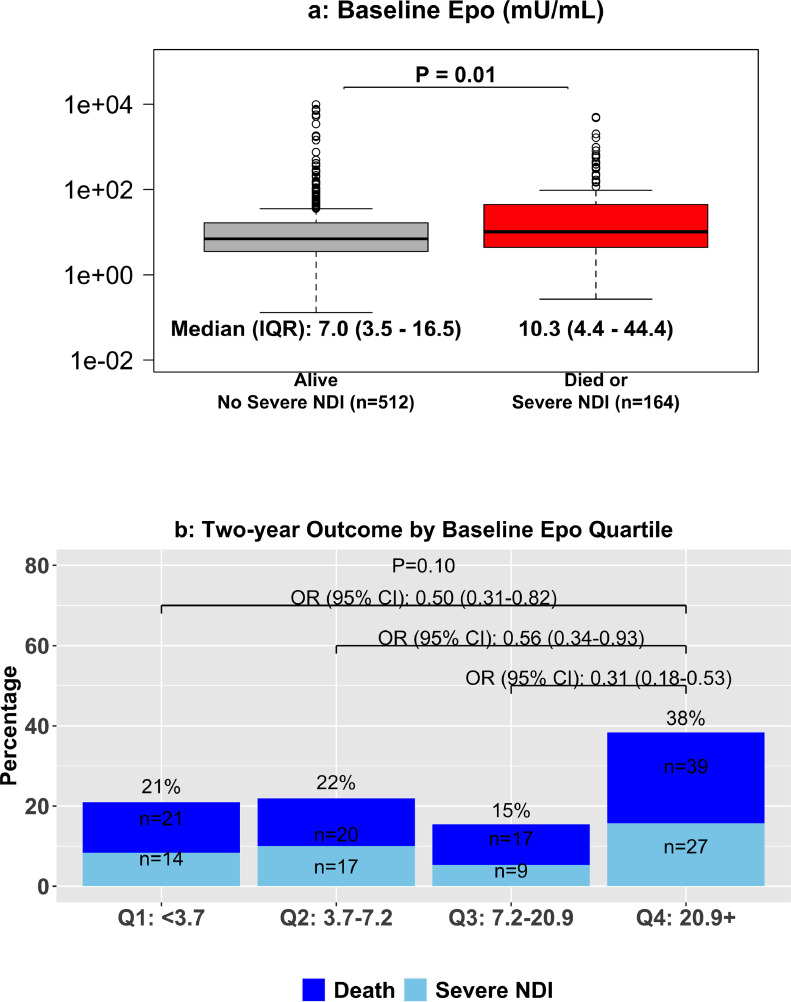
Of the infants for whom baseline Epo concentrations were available, 54 (16·3%) died and 29 (8·7%) had severe NDI in the Epo group, and 43 (12·5%) died and 38 (11·1%) had severe NDI in the placebo group. No significant association between baseline Epo levels and BSID-III Cognitive, Motor, or Language scores was seen at two years CA in either group (Figure 4). In all infants, median (IQR) baseline Epo in those with a good outcome was 7·0 mU/mL (3·5-16·5, mU/mL), which was significantly lower than in infants that died or had severe NDI (10·3 mU/mL; 4·4-44·4 mU/mL; p=0·01, GEE linear model; Figure 5A). When separated by Epo quartile at baseline, 21%, 22%, 15%, and 38% of infants experienced death or severe NDI in the first, second, third, and fourth quartiles, respectively, with risk of death and severe NDI significantly lower in all quartiles relative to the fourth quartile (Figure 5B).
Figure 4.
BSID III scores at age 2 by baseline Epo. Linear regression of log Epo concentration at baseline and BSID-III Cognitive, Motor, and Language scores in survivors at two years of age. P-values are for evidence of an overall trend (groups combined) or for a difference in slope between the two groups (interaction term in GEE linear model). Baseline Epo levels were not associated with any of the BSID-III subscales in either treatment group.
Figure 5.
Baseline serum Epo levels by death or severe NDI at age 2 and death or severe NDI by Epo quartile. Upper panel: box plot depicting baseline Epo levels by long-term outcome with median and IQR Epo levels at baseline. In infants who died or survived with severe NDI, Epo at baseline was significantly higher (GEE linear model). Lower panel: comparison of odds of death or severe NDI by quartiles of baseline Epo. Infants who were in the top quartile of Epo at baseline had significantly greater risk of death or severe NDI compared to each other quartile (GEE logistic regression model).
3.5. Baseline biomarker concentrations and death or severe NDI at 22-26 months corrected age
As no differences were seen between treatment groups in any of the biomarkers at any time point, data from the two groups were combined for assessment of the dichotomous death or severe NDI outcome. In infants who died or survived with severe NDI, baseline levels of IL-6, Tau, IL-8, TNF-α, IL-10, and IFN-γ were all higher compared to infants who survived without severe NDI (Figure 6), though none of the differences reached statistical significance. When separated by quartiles, the trend between baseline IL-8 levels and risk of death or severe NDI was significantly different between the treatment groups (Supplemental Table 2).
Figure 6.
Baseline serum biomarker levels by death or severe NDI at two years. Box plots depicting baseline median and IQR Epo levels at baseline of IL-6, Tau, IL-8, TNF-α, IL-10, and IFN-γ levels by long-term outcome. Infants who died or survived with severe NDI did not have significantly different baseline biomarker levels compared to those who survived without severe NDI (GEE linear model).
3.6. Baseline biomarker concentrations and BSID-III outcomes at 22-26 months corrected age
No association between baseline IL-8, IL-10, TNF-α, or IFN-γ and Cognitive, Motor, or Language BSID-III scores was seen at 22-26 months CA in either treatment group (Supplemental Figure 1). Log baseline Tau concentration was negatively associated with BSID-III Cognitive, Motor, and Language scores at two years CA, with no difference in association by treatment group (Figure 7). Overall (combined) beta, which is the average change (with 95% CI) in BSID-III subscale score for each unit increase in log biomarker concentration, for baseline Tau was -3·67 (-6·50, -0·83, p=0·01, GEE linear model) for Cognitive, -3·90 (-7·11, -0·68, p=0·02, GEE linear model) for Motor, and -4·94 (-8·17, -1·71, p<0·01, GEE linear model) for Language. By comparison, overall log baseline IL-6 was not significantly associated with BSID-III subscale outcomes; however, a significant difference between the slope of the line in the Epo and Placebo groups was seen (Figure 7). In general, increasing baseline IL-6 levels were associated with decreasing BSID-III subscale scores in survivors from the placebo group, which was significant for the Motor subscale (beta = -1·93; -3·12, -0·73). This association was mitigated in the Epo group, with significant differences in the slope of the line for both the Cognitive (p=0·02, GEE linear model) and Motor (p=0·01, GEE linear model) scores. In infants with documented exposure to chorioamnionitis, median (IQR) IL-6 was 7·7 pg/mL (3·7-28·7 pg/mL, n=36) compared to 13·7 pg/mL (4·7-68·6 pg/mL, n=184) in those without chorioamnionitis, which was not significantly different (p=0·06, GEE linear model). A multivariable stepwise-AIC model including all baseline biomarker levels is shown in Supplemental Table 3 (Model 1). The final model included IL-6, IL-10, Tau, and IFN-γ for predicting BSID-III Cognitive; IL-10, Tau, and IFN-γ for Motor; and Tau only for Language. Of these, only IL-6 and Tau predicting Cognitive score, and Tau predicting Language score, reached individual statistical significance.
Figure 7.
BSID III composite scores at two years of age by baseline Tau and IL-6. Linear regressions of log Tau (upper panels) and IL-6 (lower panels) concentration at baseline and BSID-III Cognitive, Motor, and Language scores in survivors at two years of age. P-values are for evidence of an overall trend (groups combined) or for a difference in slope between the two groups (GEE linear model interaction term between the biomarker and treatment). Overall, Tau was significantly and negatively associated with all three BSID-III subscales in survivors at two years of age. By comparison, baseline IL-6 was negatively associated with both Cognitive and Motor scores in the placebo group only, with an apparent reversal of the association in infants treated with Epo.
3.7. Day 7-14 Epo concentration and outcome at two years corrected age
Epo levels on day 7, day 9, and day 14 of treatment were not associated with a greater risk of death or severe NDI in either treatment group (Supplemental Table 2). Multivariable stepwise-AIC models including baseline biomarker level, average biomarker level across days 0-14, and change in biomarker level from day 0 to day 7 or day 14 also did not result in any significant associations between Epo levels and the BSID-III subscales (Supplemental Table 3).
3.8. Day 0-7 biomarker concentrations and multivariate models to predict outcomes at two years corrected age
When separated into quartiles as individual predictors and adjusted for treatment group (Supplemental Table 2), day 7 IL-10 was significantly and positively associated with increased risk of death or severe NDI (p=0·003, GEE linear model), and the association between day 7 IL-8 and risk of death or severe NDI was different based on treatment group (p<0.0001, GEE linear model). A multivariable stepwise-AIC model predicting BSD-III subscale scores using baseline and day 7 biomarker levels is shown in Supplemental Table 3 (Model 2). Higher average levels of log IL-10 concentration across day 0 and day 7 were significantly associated with lower Cognitive and Motor scores. Higher average levels of log Tau concentration across baseline and day 7 were significantly associated with lower scores in all BSID-III subscales. When examining dynamic changes in biomarker concentrations between baseline and day 7, increases in log IL-6 concentration were significantly associated with lower scores across all BSID-III subscale scores. Conversely, increases in log TNF-α concentration were associated with increases in Cognitive and Motor scores.
3.9. Day 0-14 biomarker concentrations and multivariate models to predict outcomes at two years corrected age
When separated into quartiles as individual predictors and adjusted for treatment group, no individual marker level at day 14 was associated with death or severe NDI (Supplemental Table 2). A multivariable stepwise-AIC model predicting BSD-III subscale scores using baseline, day 7, and day 14 biomarker levels is shown in Supplemental Table 3 (Model 3). When log biomarker concentrations were averaged across baseline and days 7 and 14, higher IL-10 was significantly associated with lower Motor scores, and higher Tau was significantly associated with lower Language scores. When examining dynamic changes in biomarker concentrations between baseline and day 14, greater increases in log IL-6 concentration were associated with significantly lower Cognitive and Motor scores. Greater increases in log IL-10 and TNF-α concentrations were associated with a significant increase in Cognitive score.
4. DISCUSSION
Here we present a secondary analysis from the PENUT Trial, a multicentre, placebo-controlled, randomised trial of the use of Epo in EP infants, which provides the opportunity to further explore the hypotheses that 1) elevated Epo at baseline would act as a biomarker of prolonged in utero hypoxia and be associated with worse outcome at 24 months CA, and 2) exogenous supraphysiological Epo treatment would result in lower circulating pro-inflammatory cytokines and markers of neurological injury compared to placebo. We found that elevated baseline Epo was significantly associated with increased risk of poor outcome (death or severe NDI), but contrary to our hypothesis, it was not associated with a decrease in circulating cytokines at any time point. Though Epo treatment did not affect final outcome in the PENUT Trial, [20] based on results from the biomarker subgroup it may have mitigated the detrimental effects of certain acute inflammatory insults that resulted in elevated IL-6.
Longitudinal Epo measurements were used to assess the effects of exogenous Epo administration on circulating Epo levels. Though median baseline endogenous Epo was significantly higher in the Epo group, this difference (7·6 mU/mL vs 7·0 mU/mL) is unlikely to be clinically meaningful. However, Epo levels in the treatment group were well below the expected range of 10,000-15,000 mU/mL previously seen after an intravenous dose of 1,000 U/kg in preterm infants [17,27]. In fact, only 11 (3·1%) infants in the Epo group achieved a peak Epo level >10,000 mU/mL on day 7. The reason for this discrepancy is not known, though it may be partly due to the fact that the Phase I/II trial measured Epo levels via enzyme-linked immunosorbent assay (ELISA), [27] whereas the PENUT Trial used MSD. As Epo is a glycoprotein that has multiple analogues and glycans, not all of which can be determined by antibody-based methods, this may also explain some of the discrepancy. Importantly, however, as quartiles of day 7 Epo levels in the Epo group were not associated with later outcomes, it is unlikely that lower than expected circulating Epo levels in the PENUT Trial explain the lack of overall therapeutic benefit. On day nine of treatment, Epo levels did decrease as expected to a trough level, but this remained significantly greater than the level seen in the placebo group (median 15·3 vs 5·1 mU/mL). This suggests that, at least during the early high-dose phase (1,000 U/kg every 48h) of Epo administration, a small amount of Epo was still present at the time of the next dose. Repeat Epo measurements also confirmed that the large majority of infants were treated and tested as per the study protocol, though 18 outlier measurements were identified across days 7 and 9. In the placebo group, one infant had Epo levels similar to the Epo group on day 7, which may have been due to accidental Epo administration. In the Epo group, 15 infants had Epo levels lower than expected on day 7 (peak levels), and two infants had higher than expected levels on day 9 (trough levels). These are most likely due to accidental measurement of trough levels on day 7 and peak levels on day 9 and are not expected to alter the results of our analysis.
Elevated fetal endogenous Epo levels have been associated with a variety of high-risk fetal conditions, including maternal diabetes, fetal distress in Rh-immunised pregnancies, and intrauterine hypoxia [[28], [29], [30], [31], [32]]. Measurements of Epo in amniotic fluid have also been associated with biomarkers of oxidative and nitrosative stress [33]. As upregulation of Epo production is part of the coordinated response to hypoxia, and Epo requires several hours of hypoxia before levels increase, [14] circulating Epo at baseline is thought to reflect the degree of significant hypoxia in utero, which is likely to vary significantly between patients. In agreement with this, we found that baseline Epo levels in both groups were highly-variable across several orders of magnitude. When combining baseline data from both groups, being in the top quartile of Epo (>20·9 mU/mL) was associated with an approximately two-fold increase in risk of death or severe NDI compared to the other quartiles. In survivors, however, baseline Epo was not significantly associated with any of the BSID-III subscales as a continuous outcome variable. Epo levels on day 7-14 in the placebo group were also not predictive of later outcome. In contrast, the ELGAN study demonstrated that infants born <28 weeks’ gestation whose endogenous Epo was in the top quartile on day 14 had a significantly increased risk of NDI and microcephaly at two years of age. 16 Part of the discrepancy may be the larger sample size of the ELGAN study, or additional evidence to support the idea that circulating biomarkers of long-term outcome in neonates generally have heterogeneous utility across sites and studies. From the PENUT cohort, our data suggest that significantly elevated Epo at baseline is a potentially useful proxy of prolonged intra-uterine hypoxia and a biomarker of significant injury, but it is not able to predict more subtle differences in developmental outcome among survivors. If available early on in the clinical course, measurement of Epo levels shortly after birth remains a promising potential addition to the prognostic toolkit.
In contrast to baseline Epo levels, which were partially predictive of outcome, none of the cytokines measured were significantly associated with either dichotomous poor outcome or continuous BSID-III subscales at two years of age. Circulating levels of the microtubule-associated protein Tau at baseline were negatively associated with all three BSID-III subscales at two years of age in infants who survived; however, Tau was not significantly greater in those who died or had severe NDI compared to those that did not, and also did not predict death or severe NDI when infants were separated by quartiles of Tau levels. In multivariable models, Tau remained one of the strongest predictors of BSID-III subscales both at baseline as well as when persistently elevated for 7-14 days after birth. Tau proteins, produced by alternative splicing from the microtubule-associated protein tau (MAPT) gene, are abundant in the central nervous system due to their role in stabilising the axonal cytoskeleton [34,35]. Measurement of circulating total Tau protein levels has been suggested as a prognostic marker in adult acute brain injuries and neurodegenerative conditions including Alzheimer's disease and traumatic brain injury, [36,37] as well as being a promising biomarker of outcome after hypoxic-ischemic encaphalopathy in term neonates [38]. Despite the expanding body of evidence in other neurological conditions, less is curently known about tau as a prognostic marker in EP infants, though preclinical studies suggest that exposing the developing rodent brain to inflammatory and other noxious stimuli results in altered tau expression and associated behavioural and pathologcial changes later in life [[39], [40], [41]]. In humans, tau pathology has been described even earlier in the brain than previously thought, and remains a critical aspect of the biochemical cascades associated with Alzheimer's disease [42]. Overall, the data presented in this manuscript as well as multiple other lines of evidence suggest that measurement of Tau in EP infants may provide some useful prognostic information for infants who survive and could be particularly important when examining whether EP infants are at greater risk of neurodegenerative disease as adults, as appears to be the case for many chronic health conditions [[43], [44], [45], [46]]. Though we are unable to provide specific prognostic cut-offs with the available data, we remain cautiously optimistic of the future utility of Tau as a biomarker in this group.
When the inflammatory biomarkers were compared over time, one notable finding was that levels of IFN-γ, IL-6, L-8, IL-10, and TNF-α, as well as Tau, did not differ between the groups at any time point. This is in contrast to the preclinical literature, in which Epo has been shown to significantly decrease circulating cytokines following brain injury when administered in doses equivalent to those used in the PENUT Trial. For instance, after unilateral hypoxia-ischemia (HI) in postnatal day (P) 7 rat pups (late preterm equivalent), Epo suppresses the post-injury increase in IL-1β which is associated with significant neuroprotection [47]. In a similar model in the P10 mouse, post-HI increases in expression of a number of pro-inflammatory cytokines are seen, including IL-1β and IL-6, which are largely normalised by Epo treatment [48]. The dose and timing of Epo may also play a critical role in the outcomes seen, with the important caveats that i) not all the preclinical data has found a neuroprotective effect of Epo in the developing brain, and ii) most preclinical studies involve a single injurious event rather than the confluence of heterogeneous factors associated with preterm brain injury [49]. Indeed, the difference between expected and measured outcomes may be due to a different magnitude of anti-inflammatory effect of Epo in extremely preterm human infants, a population that is difficult to model in the laboratory [50,51]. Epo may also have a greater effect on cytokines not measured in the Epo cohort such as IL-1β. However, as Epo did not provide significant neuroprotection in the PENUT cohort, any effect of Epo on the levels of inflammatory cytokines or other pathways, such as the production of neurotrophic factors, [52] is unlikely to have been clinically significant.
Though Epo did not appear to be uniformly anti-inflammatory in the PENUT cohort, one potential exception to this is in surviving infants with significantly elevated IL-6 at baseline, in whom Epo treatment was associated with a reversal in the negative association between IL-6 and the Cognitive and Motor BSID-III subscales at 2 years of age. In multivariable models, IL-6 remained significantly associated with BSID-III subscales, particularly when increases in IL-6 were seen between baseline and day 7 or 14. As IL-6 is a primary driver of the acute phase response and has a reported half-life in the range of 10 minutes to 1-2 hours, increases in IL-6 at baseline are likely to indicate either chorioamnionitis or early neonatal sepsis, [[53], [54], [55], [56], [57]] though in the PENUT biomarker cohort evidence of chorioamnionitis was not associated with increased levels of IL-6 at baseline. Hansen-Pupp et al. previously found that elevated IL-6 in the cord blood of very preterm (<32 weeks’ gestational age) was associated with increased risk of neurodevelopmental impairment at 2 years [58]. The ELGAN study found that early elevation of IL-6 (defined as within the first 14 days after birth) was not associated with Differential Ability Scales (DAS) intelligence quotient at 10 years, but was associated with increased odds of moderate or severe impairment after latent profile analysis to classify children into subgroups based on similarities in their outcome profiles [59]. In particular, having 4+ early elevations of proteins from a group of nine inflammatory biomarkers was particularly predictive of outcome in this study [59]. This again highlights the fact that multiple biomarkers are likely to be necessary for accurate long-term prediction, with no particular combination having been assessed in multiple studies. Despite this, considering the interaction between Epo treatment and IL-6 levels at baseline, it may yet be the case that Epo is a useful neuroprotective agent for a certain subset of extremely preterm infants who have experienced an acute inflammatory insult around the time of birth, with further investigation of this interaction warranted.
With respect to primary outcome, the overall absence of any strong predictive value of inflammatory cytokines at any time point in the PENUT cohort confirms the difficulty of predicting composite yet divergent outcomes (e.g. death or survival with disability) using a small grouping of circulating biomarkers. Similar to the findings of the ELGAN study, these biomarkers appear to have a slightly better ability to predict outcomes in survivors [[8], [9], [10], [11], [12], [13]]. Importantly, the ELGAN study showed that a number of neurotrophic factors were also associated with mitigation of some of the risks associated with elevated pro-inflammatory cytokines in extremely preterm infants, [12] with a large-scale systems biology approach including both epigenomic and metabolomic approaches perhaps required to truly apply biomarkers to the accurate prediction of outcomes in preterm neonates [60]. However, it is also important to highlight that the ELGAN study measured biomarker levels from dried whole blood spots collected on filter paper. Dried blood spots may not accurately represent circulating plasma levels of a number of biomarkers of neurological injury, [61] and the difference in collection methods could explain some of the differences seen between the ELGAN and PENUT cohorts.
This study does have several limitations. As we used Epo as a biomarker of injury in a trial of Epo neuroprotection, it is important to separate endogenous Epo as a biomarker of a pathological process from exogenous Epo at supraphysiological levels provided as a therapeutic agent. For instance, the upper end of the interquartile range of Epo at baseline in both groups was ∼20 mU/mL, whereas the median level in the Epo group on day 7 was two orders of magnitude greater (∼3,000 mU/mL). The biomarker subgroup of the PENUT study was recruited to examine serum biomarkers and MRI neuroimaging biomarkers in the same subgroup of infants that underwent MRI at 36 weeks. Therefore, we must acknowledge that the strength of the overall group randomisation in the PENUT trial was potentially lost in this subgroup and that power calculations performed to determine the size of the subgroup were based on estimated MRI rather than serum biomarker endpoints. Future work will examine whether serum and neuroimaging biomarkers improve prediction of 2-year outcome when used in combination. Assessment of long-term outcome prediction was therefore a secondary consideration in the biomarker group, and as such, the biomarkers measured encompass only a small fraction of the potential downstream mechanistic pathways that might be involved in determining neurodevelopmental outcomes in EP infants. This includes a number of anti-inflammatory cytokines and trophic growth factors examined in the ELGAN study, as well an increasing array of potential urinary and circulating biomarkers, particularly those produced in response to oxidative stress and lipid peroxidation [33,59,62]. We also acknowledge that developmental changes in regulation of the assessed biomarkers are an important confounder if directly comparing measured cytokine levels between gestational ages in EP infants, and for this reason gestational age was used as a covariate in all models examining the association between a given biomarker at a given timepoint and long-term outcome. In line with the study goals, we only examined associations between biomarkers and outcomes and did not examine infant or other factors associated with elevations of individual biomarkers, largely due to the complex time-varying nature of many of these potential factors such as occurrence of sepsis. For instance, a recent study has described sex-dependent alterations in pro-inflammatory cytokines in response to transfusions in preterm infants [63]. While the Epo group did require fewer transfusions, [64] the effect of transfusions or other variables on biomarker levels is beyond the scope of this study examining their potential for outcome prediction. The timing of biomarker level measurement was determined by timing of study drug – with baseline measurements immediately prior to the first dose being administered and day 7 and 9 measurements timed to determine peak and trough Epo levels, respectively. Though the timing of baseline measurements was variable relative to the time from birth and other associated exposures, the ∼8-hour baseline measurement window relative to birth more accurately mimics real-world clinical scenarios, which increases the translatability of the results. With respect to intra-uterine exposures in particular, cord blood sampling for potential biomarkers remains an important area that was not explored in the PENUT trial [[65], [66], [67], [68], [69]]. Our study was also not powered to examine multiple combinations of biomarkers at multiple timepoints and their associations with long-term outcomes, and we were unable to compare the prognostic accuracy of individual biomarkers against other tools such as the Score for Neonatal Acute Physiology (SNAP-II) and SNAP-Perinatal Extension-II (SNAPPE-II) scores as not all the necessary markers were routinely available [70]. However, a more holistic and systematic approach to primary and neurodevelopmental outcome prediction including a range of biomarker and clinical data from the PENUT cohort will be part of planned future work from this dataset.
Overall, our data suggest that significantly elevated baseline Epo may provide a biomarker of long-term poor outcome in infants born <28 weeks’ GA. Increased level of Tau at baseline and 7-14 days after birth were also predictive of worse neurodevelopment of survivors at two years of age. Epo levels in the treatment group were lower than expected based on initial pilot studies. Regardless of individual Epo level, there was little observable effect of Epo on circulating cytokines over the first two weeks after birth or on long-term outcomes, which is in agreement with the overall lack of neuroprotection by Epo in the PENUT cohort.
6. Contributors
T.R.W., P.P., B.A.C., J.B.L., T.K.B. D.E.M., P.J.H., and S.E.J. conceived the manuscript. T.K.B. oversaw the measurement of biomarkers from PENUT samples. B.A.C., P.J.H., and S.E.J. verified the data and B.A.C. and P.J.H. performed the statistical analyses. B.A.C. made the figures. T.R.W. and P.P. drafted the manuscript. All authors contributed to editing the manuscript and approved the final draft. D.E.M., P.J.H., and S.E.J. oversaw the methodology and administration of the PENUT Trial and resulting investigations. S.E.J. provided overall supervision and necessary material resources.
Declaration of Competing Interest
The authors declare that they have no relevant conflicts of interest.
Acknowledgments
Acknowledgements
The PENUT Trial was funded by the NINDS, U01NS077955 and U01NS077953.
Data sharing
De-identified individual participant data will be made available through the NINDS Data Archive: https://www.ninds.nih.gov/Current-Research/Research-Funded-NINDS/Clinical-Research/Archived-Clinical-Research-Datasets. The data will be de-identified and a limited access data set will be available in 2021 through a request form on that page. Data dictionaries, in addition to study protocol, the statistical analysis plan, and the informed consent form will be included. The data will be made available upon publication of all PENUT Trial related manuscripts to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103605.
Appendix. Supplementary materials
REFERENCES
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: Final data for 2018. National Vital Statistics Reports - National Center for Health Statistics. 2019;68(13) [PubMed] [Google Scholar]
- 2.Younge N, Goldstein RF, Bann CM, Hintz SR, Patel RM, Smith PB, Bell EF, Rysavy MA, Duncan AF, Vohr BR, Das A, Goldberg RN, Higgins RD, Cotten CM. Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Survival and Neurodevelopmental Outcomes among Periviable Infants. The New England journal of medicine. 2017;376(7):617–628. doi: 10.1056/NEJMoa1605566. doi:PubMed PMID: 28199816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson S, Marlow N. Early and long-term outcome of infants born extremely preterm. Archives of Disease in Childhood. 2017;102(1):97. doi: 10.1136/archdischild-2015-309581. [DOI] [PubMed] [Google Scholar]
- 4.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: Final Data for 2017. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2018;67(8):1–49. Epub 2018/05/19. PubMed PMID: 29775434. [PubMed] [Google Scholar]
- 5.Galinsky R, Polglase GR, Hooper SB, Black MJ, Moss TJM. The Consequences of Chorioamnionitis: Preterm Birth and Effects on Development. Journal of Pregnancy. 2013;2013:11. doi: 10.1155/2013/412831. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez FF, Ferriero DM. Neuroprotection in the newborn infant. Clin Perinatol. 2009;36(4):859–880. doi: 10.1016/j.clp.2009.07.013. vii. Epub 2009/12/01. doi:PubMed PMID: 19944839; PMCID: PMC2786822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kole AJ, Annis RP, Deshmukh M. Mature neurons: equipped for survival. Cell Death Dis. 2013;4:e689. doi: 10.1038/cddis.2013.220. Epub 2013/06/29. doi:PubMed PMID: 23807218; PMCID: PMC3702294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph RM, O'Shea TM, Allred EN, Heeren T, Hirtz D, Jara H, Leviton A, Kuban KC, Investigators ES. Neurocognitive and Academic Outcomes at Age 10 Years of Extremely Preterm Newborns. Pediatrics. 2016;137(4) doi: 10.1542/peds.2015-4343. Epub 2016/03/24. doi:PubMed PMID: 27006473; PMCID: PMC4811321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leviton A, Dammann O, Allred EN, Joseph RM, Fichorova RN, O'Shea TM, Kuban KCK. Neonatal systemic inflammation and the risk of low scores on measures of reading and mathematics achievement at age 10 years among children born extremely preterm. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2018;66:45-53. Epub 2018/02/08. doi: 10.1016/j.ijdevneu.2018.01.001. PubMed PMID: 29413878; PMCID: PMC5879009. [DOI] [PMC free article] [PubMed]
- 10.Leviton A, Joseph RM, Allred EN, Fichorova RN, O'Shea TM, Kuban KKC, Dammann O. The risk of neurodevelopmental disorders at age 10 years associated with blood concentrations of interleukins 4 and 10 during the first postnatal month of children born extremely preterm. Cytokine. 2018;110:181–188. doi: 10.1016/j.cyto.2018.05.004. Epub 2018/05/16. doi:PubMed PMID: 29763840; PMCID: PMC6668706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leviton A, Joseph RM, Fichorova RN, Allred EN, Gerry Taylor H, Michael O'Shea T, Dammann O. Executive Dysfunction Early Postnatal Biomarkers among Children Born Extremely Preterm. J Neuroimmune Pharmacol. 2019;14(2):188–199. doi: 10.1007/s11481-018-9804-7. Epub 2018/09/08. doi:PubMed PMID: 30191383; PMCID: PMC6401360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leviton A, Kuban K, O'Shea TM, Paneth N, Fichorova R, Allred EN, Dammann O. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J Pediatr. 2011;158(6):897–903. doi: 10.1016/j.jpeds.2010.11.059. .e1-5. Epub 2011/01/18. doi:PubMed PMID: 21238986. [DOI] [PubMed] [Google Scholar]
- 13.O'Shea TM, Allred EN, Kuban KC, Dammann O, Paneth N, Fichorova R, Hirtz D, Leviton A. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. The Journal of Pediatrics. 2012;160(3):395–401. doi: 10.1016/j.jpeds.2011.08.069. e4. Epub 2011/10/18. doi:PubMed PMID: 22000304; PMCID: 3279610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teramo KA, Klemetti MM, Widness JA. Robust increases in erythropoietin production by the hypoxic fetus is a response to protect the brain and other vital organs. Pediatric research. 2018;84(6):807–812. doi: 10.1038/s41390-018-0054-4. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan JW, Allred EN, Fichorova RN, Engelke S, Dammann O, Leviton A. Investigators ES. Endogenous erythropoietin varies significantly with inflammation-related proteins in extremely premature newborns. Cytokine. 2014;69(1):22–28. doi: 10.1016/j.cyto.2014.04.009. Epub 2014/07/16. doi:PubMed PMID: 25022958; PMCID: PMC4285695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korzeniewski SJ, Allred E, Logan JW, Fichorova RN, Engelke S, Kuban KCK, O'Shea TM, Paneth N, Holm M, Dammann O, Leviton A, Es investigators. Elevated endogenous erythropoietin concentrations are associated with increased risk of brain damage in extremely preterm neonates. PloS one. 2015;10(3) doi: 10.1371/journal.pone.0115083. -e. doi:PubMed PMID: 25793991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juul SE, Mayock DE, Comstock BA, Heagerty PJ. Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Maternal Health. Neonatology and Perinatology. 2015;1:27. doi: 10.1186/s40748-015-0028-z. doi: DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh P, Juul SE. Neuroprotective Strategies in Neonatal Brain Injury. The Journal of Pediatrics. 2018;192:22–32. doi: 10.1016/j.jpeds.2017.08.031. doi: [DOI] [PubMed] [Google Scholar]
- 19.Rangarajan V, Juul SE. Erythropoietin: emerging role of erythropoietin in neonatal neuroprotection. Pediatric neurology. 2014;51(4):481–488. doi: 10.1016/j.pediatrneurol.2014.06.008. Epub 06/24. doi:PubMed PMID: 25266611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juul SE, Comstock BA, Wadhawan R, Mayock DE, Courtney SE, Robinson T, Ahmad KA, Bendel-Stenzel E, Baserga M, LaGamma EF, Downey LC, Rao R, Fahim N, Lampland A, Frantz Iii ID, Khan JY, Weiss M, Gilmore MM, Ohls RK, Srinivasan N, Perez JE, McKay V, Vu PT, Lowe J, Kuban K, O'Shea TM, Hartman AL, Heagerty PJ, Consortium PT. A Randomized Trial of Erythropoietin for Neuroprotection in Preterm Infants. The New England journal of medicine. 2020;382(3):233–243. doi: 10.1056/NEJMoa1907423. Epub 2020/01/16. doi:PubMed PMID: 31940698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. Epub 1997/04/01. PubMed PMID: 9183258. [DOI] [PubMed] [Google Scholar]
- 22.Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the gross motor function classification system. Dev Med Child Neurol. 2006;48(6):424–428. doi: 10.1017/S0012162206000934. Epub 2006/05/17. doi:PubMed PMID: 16700931. [DOI] [PubMed] [Google Scholar]
- 23.Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol. 2008;50(10):744–750. doi: 10.1111/j.1469-8749.2008.03089.x. Epub 2008/10/07. doi:PubMed PMID: 18834387. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum PL, Palisano RJ, Bartlett DJ, Galuppi BE, Russell DJ. Development of the Gross Motor Function Classification System for cerebral palsy. Dev Med Child Neurol. 2008;50(4):249–253. doi: 10.1111/j.1469-8749.2008.02045.x. Epub 2008/03/06. doi:PubMed PMID: 18318732. [DOI] [PubMed] [Google Scholar]
- 25.Diggle P, Heagerty PJ, Liang K-Y, Zeger S. Analysis of Longitudinal Data: Oxford University Press; 2002.
- 26.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical computing; 2019.
- 27.Juul SE, McPherson RJ, Bauer LA, Ledbetter KJ, Gleason CA, Mayock DE. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics. 2008;122(2):383–391. doi: 10.1542/peds.2007-2711. Epub 2008/08/05. doi:PubMed PMID: 18676557. [DOI] [PubMed] [Google Scholar]
- 28.Philipps AF, Widness JA, Garcia JF, Raye JR, Schwartz R. Erythropoietin elevation in the chronically hyperglycemic fetal lamb. Proc Soc Exp Biol Med. 1982;170(1):42–47. doi: 10.3181/00379727-170-41394. PubMed PMID: 7043470. [DOI] [PubMed] [Google Scholar]
- 29.Voutilainen PE, Widness JA, Clemons GK, Schwartz R, Teramo KA. Amniotic fluid erythropoietin predicts fetal distress in Rh-immunized pregnancies. Am J Obstet Gynecol. 1989;160(2):429–434. doi: 10.1016/0002-9378(89)90466-3. [DOI] [PubMed] [Google Scholar]
- 30.Halmesmaki E, Teramo KA, Widness JA, Clemons GK, Ylikorkala O. Maternal alcohol abuse is associated with elevated fetal erythropoietin levels. Obstet Gynecol. 1990;76(2):219–222. [PubMed] [Google Scholar]
- 31.Brace RA, Cheung CY, Davis LE, Gagnon R, Harding R, Widness JA. Sources of amniotic fluid erythropoietin during normoxia and hypoxia in fetal sheep. Am J Obstet Gynecol. 2006;195(1):246–254. doi: 10.1016/j.ajog.2005.12.008. PubMed PMID: 16813755. [DOI] [PubMed] [Google Scholar]
- 32.Teramo KA, Widness JA. Increased fetal plasma and amniotic fluid erythropoietin concentrations: markers of intrauterine hypoxia. Neonatology. 2009;95(2):105–116. doi: 10.1159/000153094. Epub 2008/09/09. doi: 000153094 [pii]PubMed PMID: 18776724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Escobar J, Teramo K, Stefanovic V, Andersson S, Asensi MA, Arduini A, Cubells E, Sastre J, Vento M. Amniotic fluid oxidative and nitrosative stress biomarkers correlate with fetal chronic hypoxia in diabetic pregnancies. Neonatology. 2013;103(3):193–198. doi: 10.1159/000345194. Epub 2013/01/09. doi:PubMed PMID: 23295371. [DOI] [PubMed] [Google Scholar]
- 34.Lee G. Tau protein: an update on structure and function. Cell Motil Cytoskeleton. 1990;15(4):199–203. doi: 10.1002/cm.970150402. Epub 1990/01/01. doi:PubMed PMID: 2110865. [DOI] [PubMed] [Google Scholar]
- 35.Matus A. Microtubule-associated proteins. Curr Opin Cell Biol. 1990;2(1):10–14. doi: 10.1016/s0955-0674(05)80024-9. Epub 1990/02/01. doi:PubMed PMID: 2109620. [DOI] [PubMed] [Google Scholar]
- 36.Nam E, Lee Y-B, Moon C, Chang K-A. Serum Tau Proteins as Potential Biomarkers for the Assessment of Alzheimer's Disease Progression. International journal of molecular sciences. 2020;21(14):5007. doi: 10.3390/ijms21145007. PubMed PMID: 32679907. [DOI] [PMC free article] [PubMed]
- 37.Adrian H, Mårten K, Salla N, Lasse V. Biomarkers of Traumatic Brain Injury: Temporal Changes in Body Fluids. eNeuro. 2016;3(6) doi: 10.1523/eneuro.0294-16.2016. Epub 2016/12/30. doi:PubMed PMID: 28032118; PMCID: PMC5175263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massaro AN, Wu YW, Bammler TK, Comstock B, Mathur A, McKinstry RC, Chang T, Mayock DE, Mulkey SB, Van Meurs K, Juul S. Plasma Biomarkers of Brain Injury in Neonatal Hypoxic-Ischemic Encephalopathy. The Journal of Pediatrics. 2018;194:67–75.e1. doi: 10.1016/j.jpeds.2017.10.060. doi: [DOI] [PubMed] [Google Scholar]
- 39.Yu Y, Yang Y, Tan H, Boukhali M, Khatri A, Yu Y, Hua F, Liu L, Li M, Yang G, Dong Y, Zhang Y, Haas W, Xie Z. Tau Contributes to Sevoflurane-induced Neurocognitive Impairment in Neonatal Mice. Anesthesiology. 2020;133(3):595–610. doi: 10.1097/aln.0000000000003452. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buratovic S, Stenerlöw B, Fredriksson A, Sundell-Bergman S, Viberg H, Eriksson P. Neonatal exposure to a moderate dose of ionizing radiation causes behavioural defects and altered levels of tau protein in mice. Neurotoxicology. 2014;45:48–55. doi: 10.1016/j.neuro.2014.09.002. Epub 2014/09/30. doi:PubMed PMID: 25265567. [DOI] [PubMed] [Google Scholar]
- 41.Dias P, Freiberger V, Ventura L, Bragagnolo D, Dutra ML, Horewicz VV. Comim CM. Late Brain Involvement after Neonatal Immune Activation. Biomed Res Int. 2019;2019 doi: 10.1155/2019/9573248. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braak H, Del Tredici K. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta neuropathologica. 2011;121(2):171–181. doi: 10.1007/s00401-010-0789-4. doi: [DOI] [PubMed] [Google Scholar]
- 43.Raju TNK, Buist AS, Blaisdell CJ, Moxey-Mims M, Saigal S. Adults born preterm: a review of general health and system-specific outcomes. Acta paediatrica (Oslo, Norway: 1992) 2017;106(9):1409–1437. doi: 10.1111/apa.13880. Epub 2017/04/19. doi:PubMed PMID: 28419544. [DOI] [PubMed] [Google Scholar]
- 44.Crump C, Howell EA, Stroustrup A, McLaughlin MA, Sundquist J, Sundquist K. Association of Preterm Birth With Risk of Ischemic Heart Disease in Adulthood. JAMA Pediatr. 2019 doi: 10.1001/jamapediatrics.2019.1327. Epub 2019/06/04. doi:PubMed PMID: 31157896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crump C, Sundquist J, Winkleby MA, Sundquist K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ. 2019;365:l1346. doi: 10.1136/bmj.l1346. Epub 2019/05/03. doi:PubMed PMID: 31043374; PMCID: PMC6490674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and mortality in young adulthood. Jama. 2011;306(11):1233–1240. doi: 10.1001/jama.2011.1331. Epub 2011/09/22. doi:PubMed PMID: 21934056. [DOI] [PubMed] [Google Scholar]
- 47.Sun Y, Calvert John W, Zhang John H. Neonatal Hypoxia/Ischemia Is Associated With Decreased Inflammatory Mediators After Erythropoietin Administration. Stroke. 2005;36(8):1672–1678. doi: 10.1161/01.STR.0000173406.04891.8c. doi: [DOI] [PubMed] [Google Scholar]
- 48.Juul SE, Beyer RP, Bammler TK, McPherson RJ, Wilkerson J, Farin FM. Microarray analysis of high-dose recombinant erythropoietin treatment of unilateral brain injury in neonatal mouse hippocampus. Pediatric research. 2009;65(5):485–492. doi: 10.1203/PDR.0b013e31819d90c8. Epub 2009/02/05. doi:PubMed PMID: 19190543. [DOI] [PubMed] [Google Scholar]
- 49.Galinsky R, Lear CA, Dean JM, Wassink G, Dhillon SK, Fraser M, Davidson JO, Bennet L, Gunn AJ. Complex interactions between hypoxia-ischemia and inflammation in preterm brain injury. Developmental medicine and child neurology. 2018;60(2):126-33. Epub 2017/12/02. doi: 10.1111/dmcn.13629. PubMed PMID: 29194585. [DOI] [PubMed]
- 50.Gilles F, Gressens P, Dammann O, Leviton A. Hypoxia-ischemia is not an antecedent of most preterm brain damage: the illusion of validity. Developmental medicine and child neurology. 2018;60(2):120–125. doi: 10.1111/dmcn.13483. Epub 2017/06/28. doi:PubMed PMID: 28656697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wood T, Moralejo D, Corry K, Fisher C, Snyder JM, Acuna V, Holden-Hunt A, Virk S, White O, Law J, Parikh P, Juul SE. A Ferret Model of Inflammation-sensitized Late Preterm Hypoxic-ischemic Brain Injury. J Vis Exp. 2019;153 doi: 10.3791/60131. Epub 2019/12/10. doi:PubMed PMID: 31814608. [DOI] [PubMed] [Google Scholar]
- 52.Juul SE, Pet GC. Erythropoietin and Neonatal Neuroprotection. Clinics in perinatology. 2015;42(3):469–481. doi: 10.1016/j.clp.2015.04.004. Epub 2015/05/14. doi:PubMed PMID: 26250911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cobo T, Kacerovsky M, Andrys C, Drahosova M, Musilova I, Hornychova H, Jacobsson B. Umbilical cord blood IL-6 as predictor of early-onset neonatal sepsis in women with preterm prelabour rupture of membranes. PloS one. 2013;8(7):e69341. doi: 10.1371/journal.pone.0069341. Epub 2013/07/31. doi:PubMed PMID: 23894452; PMCID: PMC3722235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, Kusanovic JP, Chaiyasit N, Dong Z, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L, Kim YM. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J Perinat Med. 2016;44(1):53–76. doi: 10.1515/jpm-2015-0121. doi:PubMed PMID: 26360486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez-Portilla RJ, Hawkins-Villarreal A, Alvarez-Ponce P, Chinolla-Arellano ZL, Moreno-Espinosa AL, Sandoval-Mejia AL, Moreno-Uribe N. Maternal Serum Interleukin-6: A Non-Invasive Predictor of Histological Chorioamnionitis in Women with Preterm-Prelabor Rupture of Membranes. Fetal Diagn Ther. 2019;45(3):168–175. doi: 10.1159/000488080. Epub 2018/04/11. doi:PubMed PMID: 29635237. [DOI] [PubMed] [Google Scholar]
- 56.Smulian JC, Vintzileos AM, Lai YL, Santiago J, Shen-Schwarz S, Campbell WA. Maternal chorioamnionitis and umbilical vein interleukin-6 levels for identifying early neonatal sepsis. J Matern Fetal Med. 1999;8(3):88–94. doi: 10.1002/(sici)1520-6661(199905/06)8:3<88::Aid-mfm4>3.0.Co;2-#. Epub 1999/05/25. doi:PubMed PMID: 10338061. [DOI] [PubMed] [Google Scholar]
- 57.Panero A, Pacifico L, Rossi N, Mancuso G, Stegagno M, Chiesa C. Interleukin 6 in neonates with early and late onset infection. Pediatr Infect Dis J. 1997;16(4):370–375. doi: 10.1097/00006454-199704000-00007. Epub 1997/04/01. doi:PubMed PMID: 9109138. [DOI] [PubMed] [Google Scholar]
- 58.Hansen-Pupp I, Hallin A-L, Hellström-Westas L, Cilio C, Berg A-C, Stjernqvist K, Fellman V, Ley D. Inflammation at Birth is Associated With Subnormal Development in Very Preterm Infants. Pediatric research. 2008;64(2):183–188. doi: 10.1203/PDR.0b013e318176144d. doi: [DOI] [PubMed] [Google Scholar]
- 59.Kuban KCK, Joseph RM, O'Shea TM, Heeren T, Fichorova RN, Douglass L, Jara H, Frazier JA, Hirtz D, Rollins JV, Paneth N. Extremely Low Gestational Age Newborn Study I. Circulating Inflammatory-Associated Proteins in the First Month of Life and Cognitive Impairment at Age 10 Years in Children Born Extremely Preterm. The Journal of pediatrics. 2017;180:116. doi: 10.1016/j.jpeds.2016.09.054. -23.e1. Epub 2016/10/24. doi:PubMed PMID: 27788929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knijnenburg TA, Vockley JG, Chambwe N, Gibbs DL, Humphries C, Huddleston KC, Klein E, Kothiyal P, Tasseff R, Dhankani V, Bodian DL, Wong WSW, Glusman G, Mauldin DE, Miller M, Slagel J, Elasady S, Roach JC, Kramer R, Leinonen K, Linthorst J, Baveja R, Baker R, Solomon BD, Eley G, Iyer RK, Maxwell GL, Bernard B, Shmulevich I, Hood L, Niederhuber JE. Genomic and molecular characterization of preterm birth. Proceedings of the National Academy of Sciences. 2019;116(12):5819–5827. doi: 10.1073/pnas.1716314116. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Massaro AN, Wu YW, Bammler TK, MacDonald JW, Mathur A, Chang T, Mayock D, Mulkey SB, van Meurs K, Afsharinejad Z, Juul SE. Dried blood spot compared to plasma measurements of blood-based biomarkers of brain injury in neonatal encephalopathy. Pediatric research. 2019;85(5):655–661. doi: 10.1038/s41390-019-0298-7. doi: [DOI] [PubMed] [Google Scholar]
- 62.Sjöbom U, Hellström W, Löfqvist C, Nilsson AK, Holmström G, Pupp IH, Ley D, Blennow K, Zetterberg H, Sävman K, Hellström A. Analysis of Brain Injury Biomarker Neurofilament Light and Neurodevelopmental Outcomes and Retinopathy of Prematurity Among Preterm Infants. JAMA Netw Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.4138. Epub 2021/04/03. doi:PubMed PMID: 33797551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benavides A, Bell EF, Georgieff MK, Josephson CD, Stowell SR, Feldman HA, Nalbant D, Tereshchenko A, Sola-Visner M, Nopoulos P. Sex-specific cytokine responses and neurocognitive outcome after blood transfusions in preterm infants. Pediatric research. 2021 doi: 10.1038/s41390-021-01536-0. Epub 2021/04/30. doi:PubMed PMID: 33911194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Juul SE, Vu PT, Comstock BA, Wadhawan R, Mayock DE, Courtney SE, Robinson T, Ahmad KA, Bendel-Stenzel E, Baserga M, LaGamma EF, Downey LC, O'Shea M, Rao R, Fahim N, Lampland A, Frantz ID, 3rd Khan J, Weiss M, Gilmore MM, Ohls R, Srinivasan N, Perez JE, McKay V, Heagerty PJ. Preterm Erythropoietin Neuroprotection Trial C. Effect of High-Dose Erythropoietin on Blood Transfusions in Extremely Low Gestational Age Neonates: Post Hoc Analysis of a Randomized Clinical Trial. JAMA Pediatr. 2020;174(10):933–943. doi: 10.1001/jamapediatrics.2020.2271. Epub 2020/08/18. doi:PubMed PMID: 32804205; PMCID: PMC7432302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Letunica N, Cai T, Cheong JLY, Doyle LW, Monagle P, Ignjatovic V. The use of proteomics for blood biomarker research in premature infants: a scoping review. Clin Proteomics. 2021;18(1):13. doi: 10.1186/s12014-021-09316-y. Epub 2021/04/16. doi:PubMed PMID: 33853516; PMCID: PMC8048323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Otsubo Y, Hashimoto K, Kanbe T, Sumi M, Moriuchi H. Association of cord blood chemokines and other biomarkers with neonatal complications following intrauterine inflammation. PloS one. 2017;12(5) doi: 10.1371/journal.pone.0175082. Epub 2017/05/23. doi:PubMed PMID: 28531215; PMCID: PMC5439663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chaudhury S, Saqibuddin J, Birkett R, Falcon-Girard K, Kraus M, Ernst LM, Grobman W, Mestan KK. Variations in Umbilical Cord Hematopoietic and Mesenchymal Stem Cells With Bronchopulmonary Dysplasia. Front Pediatr. 2019;7:475. Epub 2019/12/05. doi: 10.3389/fped.2019.00475. PubMed PMID: 31799226; PMCID: PMC6867971. [DOI] [PMC free article] [PubMed]
- 68.Kandasamy J, Olave N, Ballinger SW, Ambalavanan N. Vascular Endothelial Mitochondrial Function Predicts Death or Pulmonary Outcomes in Preterm Infants. Am J Respir Crit Care Med. 2017;196(8):1040–1049. doi: 10.1164/rccm.201702-0353OC. Epub 2017/05/10. doi:PubMed PMID: 28485984; PMCID: PMC5649986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mestan K, Yu Y, Thorsen P, Skogstrand K, Matoba N, Liu X, Kumar R, Hougaard DM, Gupta M, Pearson C, Ortiz K, Bauchner H, Wang X. Cord blood biomarkers of the fetal inflammatory response. J Matern Fetal Neonatal Med. 2009;22(5):379–387. doi: 10.1080/14767050802609759. Epub 2009/06/17. doi:PubMed PMID: 19529994; PMCID: PMC5001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138(1):92–100. doi: 10.1067/mpd.2001.109608. Epub 2001/01/10. doi:PubMed PMID: 11148519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.