Abstract
Objectives
This study aimed to clarify the validity and long‐term outcomes of colorectal endoscopic submucosal dissection (ESD) of visible lesions (≥20 mm) in patients with ulcerative colitis (UC) and investigate the incidence of undetected lesions in surgical specimens.
Methods
This single‐center retrospective study included 11 lesions from nine patients with UC who underwent ESD and 19 lesions from nine patients with UC who underwent colectomy between March 2001 and January 2019. We evaluated the endoscopic findings of scarring, atrophy, and loss of haustra in the ESD group, and we determined the lesion visibility in the colectomy group. We investigated the clinicopathological features of all lesions and examined the follow‐up evaluations in the ESD group.
Results
The en bloc and curative resection rates of ESDs were 91% and 82%, respectively. Endoscopic findings of scarring, atrophic colitis, and loss of haustra were observed in two (18%), seven (64%), and one (9%) lesions, respectively. The two lesions with scarring showed severe submucosal fibrosis. Mortality and recurrence were not observed during the median follow‐up of 25 months. Metachronous lesions ≥20 mm were detected in two patients, which were successfully treated with ESDs. In the colectomy specimens, 21% of the lesions were undetected, 67% had multiple neoplasms, and 33% had multiple invasive cancers.
Conclusions
ESD is feasible and valid for large visible lesions in patients with UC; however, for lesions with endoscopic findings of scarring, technical difficulties in endoscopic resection must be considered. In addition, intensive surveillance colonoscopy is necessary to detect undetected lesions.
Keywords: colectomy, colitis‐associated neoplasms, endoscopic submucosal dissection, epithelial neoplasia, ulcerative colitis
Key summary
Summarise the estabished knowledge on this subject
The number of patients with ulcerative colitis (UC) has increased.
Clinical guidelines recommend that endoscopic resection for endoscopically visible dysplasia rather than colectomy in patients with UC.
-
Although some studies on the treatment outcomes of endoscopic submucosal dissection (ESD) for patients with UC have been conducted, the sample size was small and almost all reported lesions were relatively small (i.e., <20 mm); thus, the available data are limited.
What are the significance and/or new findins of this study?
Good outcomes of ESD for visible lesions (>20 mm) in patients with ulcerative colitis were demonstrated.
Technical difficulties are highly possible in lesions with endoscopic findings of scarring.
The prevalence of undetected lesions (approximately 20%) was the same between high (2‐3) and low (0‐1) Mayo endoscopic subscore groups based on the surgical specimens.
INTRODUCTION
In Japan, the number of patients with ulcerative colitis (UC) was 168,000 in 2016, which shows an increase of 1.8‐fold compared to the number 10 years ago, and the number of treatments for UC‐associated neoplasias (UCANs) are expected to increase. 1 In the clinical guidelines from Western countries and Japan, surgical interventions, including total colectomy, are recommended to improve the prognosis of patients with UC when high‐grade dysplasia or invasive carcinoma is detected by surveillance colonoscopy. 2 , 3 However, for local lesions, such as low‐grade dysplasia and sporadic epithelial neoplasia (SPEN), endoscopic treatment can be a therapeutic option.
Recently, the Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients International Consensus Recommendations (SCENIC) announced a consensus statement that after complete endoscopic resection of endoscopically visible lesions, surveillance colonoscopy, rather than colectomy, is recommended. 4 This recommendation has been adopted by the American Society for Gastrointestinal Endoscopy 3 and the European Crohn's and Colitis Organization. 2
Several studies have reported favorable long‐term outcomes of endoscopic mucosal resection and polypectomy for polypoid or small neoplastic lesions in patients with UC. 5 , 6 , 7 Although some studies on the treatment outcomes of ESD for patients with UC have been conducted, the sample size was small (i.e., 7–32 patients) and almost all reported lesion’s were relatively small (i.e., <20 mm); thus, the available data are limited. 8 , 9 , 10 , 11 , 12 Furthermore, the endoscopic management of visible lesions greater than 20 mm in size remains a concern because of the technical difficulties and the possibility of other synchronous undetected lesions. Moreover, pre‐ESD endoscopic findings that could predict technical difficulties during ESD procedure are unknown.
Thus, in this study, we aimed to clarify the validity and long‐term outcomes of colorectal ESD of visible lesions ≥20 mm in patients with UC and investigate the incidence of undetected lesions in surgical specimens.
METHODS
Study population
This study was approved by the ethical review board of our institution (approval number 2016‐245). All patients with UC who underwent ESD or colectomy for colorectal neoplasms at the National Cancer Center Hospital between March 2001 and January 2019 were included in this study. The patients had a disease duration of ≥8 years.
ESD indications were as follows: lesion diameter ≥20 mm, no submucosal (SM) deep invasive cancer (≥1000 μm) based on endoscopic examination, and visible dysplasia. The surgical indications were the following: invasive cancer and invisible dysplasia.
Endoscopic submucosal dissection procedure
Details of the ESD techniques have been described previously. 13 All procedures were performed using the following: an insulation‐tipped knife (IT Knife from 1998 to 2009 or IT knife nano from 2010; Olympus Medical), a bipolar needle knife (B‐knife from 2006 to 2011 or Jet B‐knife from 2012; Zeon Medical), carbon dioxide insufflation from 2005, a bipolar‐type hemostatic forceps from 2005 (Hemostat‐Y; Pentax), and a distal attachment from 2007 (ST hood short‐type; Fujifilm Medical). Three endoscopists performed all the ESD procedures. The endoscopists were experts in ESD; each of them had performed more than 500 colorectal ESD procedures.
Histopathology analysis
Endoscopically resected specimens were serially sectioned at 2–3 mm intervals, and surgically resected specimens were sectioned at 4–5 mm intervals. Histological classification was according to the World Health Organization criteria. 14 We identified low‐grade dysplasia and low‐grade adenoma by evaluating the proliferative zone. 12 The histopathological classifications were as follows: low‐grade dysplasia, low‐grade tubular adenoma, high‐grade tubular adenoma, sessile serrated lesion, traditional serrated adenoma, low‐grade adenocarcinoma, high‐grade adenocarcinoma, squamous cell carcinoma. 12 Based on a previous study, low‐grade dysplasia was classified as UCAN, and low‐grade tubular adenoma and high‐grade tubular adenoma were classified as SPEN. 12 Curative resection was confirmed when pathological findings showed R0 resection without any of the following features: submucosal deep invasion (≥1000 μm), lymphovascular involvement, or poorly differentiated adenocarcinoma component. 13 Histological classification was confirmed by a gastrointestinal pathologist (SS).
Follow‐up
Patients who underwent ESD and curative resection were followed up with colonoscopy within 1 year after the ESD and at 1‐ to 2‐year intervals thereafter. Abdominal computed tomography (CT) was performed as needed.
Data collection
Long‐term clinical outcome data between May and November 2020 were obtained. Information on age, sex, Mayo endoscopic subscore, UC disease duration, histopathological type, and tumor invasion depth were obtained from medical records. The cecum, ascending colon, and transverse colon were classified as right‐sided colon; the descending colon and sigmoid colon, as left‐sided colon. The rectosigmoid colon was classified as the rectum.
Endoscopic submucosal dissection data
Clinicopathological and treatment outcome data were analyzed using our prospectively stored database. To examine the clinicopathological characteristics of the lesions resected by ESD, we evaluated the following: UC disease extent, endoscopic lesion size, lesion location, endoscopic macroscopic type based on the Paris classification, Japan NBI Expert Team classification, pit pattern, en bloc resection rate, R0 resection rate, curative resection rate, excision time, perforation, postoperative bleeding, local recurrence, metachronous dysplasia, and additional surgery. 15 The extent of UC disease was estimated from endoscopic findings.
Endoscopic findings of scarring, atrophy, and loss of haustra were reviewed by two experienced endoscopists (KK, MY). Scarring was defined as a whitish and stretching fold in the background mucosa of the lesion; atrophy, as whitish coloration in the background mucosa of the lesion; and loss of haustra, as endoscopic loss of normal haustral markings 16 (Figure 1). Endoscopic submucosal fibrosis during ESD was assessed according to a three‐point score: F0, no fibrosis; F1, mild; and F2, severe. 17
FIGURE 1.
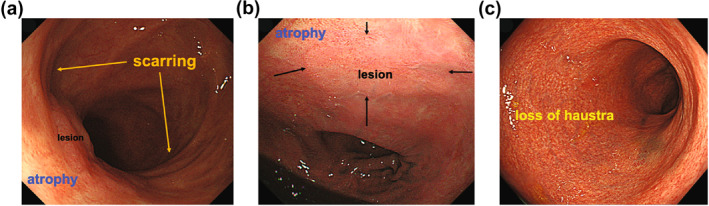
Representative images of endoscopic features. (a) Atrophy and scarring. (b) Atrophy. (c) Loss of haustra
Colectomy
To examine the clinicopathological characteristics of the lesions in the colectomy specimen, we evaluated the following: the lesion visibility, UC disease extent, endoscopic macroscopic type based on the Paris classification, surgical procedure, histopathological lesion size, lesion location, number of synchronous lesions, histopathological diagnosis of synchronous lesions, and tumor‐node‐metastasis (TNM) staging. Preoperative colonoscopy was performed with white‐light and, if necessary, using Indigo carmine to improve visualization of epithelial surface detail. We defined visible dysplasia as dysplasia identified on targeted biopsies from a lesion visualized at preoperative colonoscopy, 4 and we defined undetected dysplasia as dysplasia identified on resected surgical specimen without a visible lesion at preoperative colonoscopy. The extent of UC disease was estimated from both endoscopic and pathological findings. The macroscopic type of endoscopically undetected lesions was evaluated using the resected specimen. TNM staging was classified according to the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma (3d English Edition). 18 For synchronous multiple lesions, TNM staging was performed for the lesion with the highest tumor stage.
RESULTS
Patient characteristics
ESD was performed in 11 lesions from nine patients, including two patients with metachronous lesions detected by surveillance colonoscopy. Nine patients underwent colectomy, including one patient who had noncurative resection and total colectomy as additional surgeries (Figure 2).
FIGURE 2.
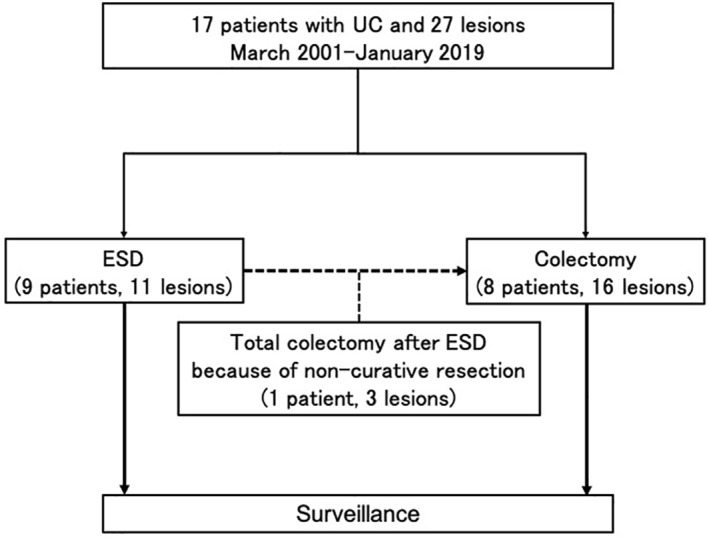
Study flowchart. ESD, endoscopic submucosal dissection; UC, ulcerative colitis
The characteristics of the patients who underwent ESD or colectomy are shown in Table 1. There were eight men and 10 women. The median age at treatment was higher in ESD patients (65 years; range, 35–85 years) than in colectomy patients (47 years; range, 35–62 years). The median disease duration was approximately 19 years (range, 10–33 years) in both the ESD and colectomy groups. There were 14 cases of pancolitis, one left‐sided colitis, and three proctitis. The median Mayo endoscopic subscore was 0 (range, 0–1) in the ESD group and 1 (range, 0–3) in the colectomy group.
TABLE 1.
Patients with UC who underwent ESD or colectomy
| Variables | ESD (n = 9) a | Colectomy (n = 9) a |
|---|---|---|
| Median age (years), median (range) b | 65 (35–85) | 47 (35–62) |
| Sex | ||
| Male/female | 4/5 | 4/5 |
| Disease duration (years), median (range) | 20 (10–33) | 19 (12–29) |
| Type of disease | ||
| Pancolitis | 7 | 7 |
| Left‐sided colitis | 0 | 1 |
| Proctitis | 2 | 1 |
| Mayo endoscopic subscore, median (range) | 0 (0–1) | 1 (0–3) |
One additional surgery case was duplicated.
Age at treatment.
Endoscopic characteristics of the lesions resected by ESD
Table 2 summarizes the endoscopic characteristics of the lesions resected by ESD. For the macroscopic type, three (27%) were sessile, eight (73%) were superficially elevated, and no depressed lesions were observed. Distinct margins of the lesion were detectable in all patients, and no ulcerations were noted. Scarring, atrophic colitis, and loss of haustra were observed in two (18%), seven (64%), and one (9%) lesion, respectively.
TABLE 2.
Characteristics of the lesions resected by ESD
| Variables | Total (n = 11) a |
|---|---|
| Location, n (%) | |
| Rectum b | 5 (45) |
| Left‐sided colon | 3 (27) |
| Right‐sided colon | 3 (27) |
| Paris classification, n (%) | |
| Type 0‐Is | 3 (27) |
| Type 0‐IIa | 8 (73) |
| Ulcers on the surface, n (%) | 0 |
| Border, n (%) | |
| Distinct border | 11 (100) |
| Indistinct border | 0 |
| JNET classification, n (%) | |
| 1 | 2 (18) |
| 2A | 8 (72) |
| NA | 1 (9) |
| Kudo's pit pattern, n (%) | |
| II | 2 (18) |
| IIIs | 1 (9) |
| IIIL | 2 (18) |
| Ⅳ | 3 (27) |
| IIIH | 2 (18) |
| ⅣH | 1 (9) |
| Scarring, n (%) | 2 (18) |
| Atrophic colitis, n (%) | 7 (64) |
| Loss of colonic haustra, n (%) | 1 (9) |
Abbreviations: JNET, Japan NBI Expert Team; NA, not assessed.
Two lesions were detected during surveillance colonoscopy.
Proctodeum area is included in the rectum area.
Treatment outcome of ESD
The treatment outcomes of ESD are shown in Table 3. En bloc resection, R0 resection, and curative resection rates were 91% (10 lesions), 82% (nine lesions), and 82% (nine lesions), respectively. The median excision time was 95 min (range, 60–200 min), and the median tumor size was 30 mm (range, 20–50 mm). Regarding endoscopic submucosal fibrosis, F0, F1, and F2 were observed in 0, 7 (63%), and 4 (36%) lesions, respectively. Postoperative bleeding occurred in three (27%) lesions. No perforation occurred during the procedure.
TABLE 3.
Treatment outcomes of ESD
| Variable | Total (n = 11) a | En bloc resection (n = 10) | Piecemeal resection (n = 1) |
|---|---|---|---|
| R0 resection, n (%) | 9 (82) | 9 (90) | NA |
| Curative resection, n (%) | 9 (82) | 9 (90) | NA |
| Size of resected specimen (mm), median (range) | 40 (20–55) | 40 (20–55) | 30 |
| Tumor size (mm), median (range) | 30 (20–50) | 31 (20–50) | 15 |
| Excision time (min), median (range) | 95 (60–200) | 90 (60–200) | 100 |
| Endoscopic submucosal fibrosis, n (%) F0 | 0 | 0 | 0 |
| F1 | 7 (63) | 7 (70) | 0 |
| F2 | 4 (36) | 3 (30) | 1 |
| Complications, n (%) Delayed bleeding | 3 (27) | 3 (30) | 0 |
| Perforation | 0 | 0 | 0 |
| Histology, n (%) | |||
| Low‐grade dysplasia | 6 (55) | 5 (50) | 1 |
| Tubular adenoma, low grade | 1 (9) | 1 (10) | 0 |
| Tubular adenoma, high grade | 1 (9) | 1 (10) | 0 |
| Sessile serrated lesion | 1 (9) | 1 (10) | 0 |
| Traditional serrated adenoma | 1 (9) | 1 (10) | 0 |
| Low‐grade adenocarcinoma | 1 (9) | 1 (10) | 0 |
Abbreviation: NA, not assessed.
Two lesions were detected during surveillance colonoscopy.
Moreover, noncurative resection was performed in two lesions. One was a piecemeal resection that was accompanied by scarring and atrophy, and the other noncurative resection did not exhibit such endoscopic findings but required additional surgery because of superficial tumor invasion (500 μm from the muscularis mucosa) associated with lymphatic invasion. 19
In addition, two lesions showed severe submucosal fibrosis, and these lesions had endoscopic findings of scarring and atrophic colitis. In the remaining nine lesions, severe submucosal fibrosis was not observed; of the nine lesions, one had endoscopic findings of loss of haustra and five had endoscopic findings of atrophic colitis.
The lesions were histologically classified as UCAN, SPEN, sessile serrated lesion, traditional serrated adenoma, and low‐grade adenocarcinoma in six (55%), two (18%), one (9%), one (9%), and one (9%) lesion, respectively.
Follow‐up
The median follow‐up period of nine patients whose lesions were resected by ESD was 25 months (range, 1–132 months) (Figure 1 in supplementary information material). During the follow‐up, metachronous lesions were detected in two patients by surveillance colonoscopy. Data of the two patients were as follows: age at UC onset was 51 and 52 years, age at diagnosis of second ESD lesion was 72 and 85 years, and the time after the first ESD was 11 and 1 year. Both patients underwent ESD, and curative resection was performed. Subsequent surveillance colonoscopy revealed no local recurrence. None of the patients died of colorectal cancer associated with ESD.
Clinicopathological characteristics of colectomy
The characteristics of the 19 lesions detected in the nine resected colectomy specimens are shown in Table 4. Most of the lesions were distributed in the left‐sided colon and rectum (84%). Total colectomy was performed in seven patients (17 lesions), total pelvic excision in one patient (one lesion), and lower anterior resection in one patient (one lesion). Moreover, seven patients (78%) had at least one invasive cancer, and six patients (67%) had multiple neoplasms, regardless of tumor depth (Table 5). Three of the nine patients (33%) had synchronous multiple invasive cancers, and the six patients with multiple neoplasms had pancolitis and underwent total colectomy. All nine patients had preoperative colonoscopy. Of the 19 lesions, 13 (68%) were visible and four (21%) were undetected. The colonoscope could not reach the remaining two lesions (11%) because of stenosis of the lumen.
TABLE 4.
Clinicopathological characteristics of the lesion resected by colectomy
| Outcomes | (n = 19) |
|---|---|
| Tumor size (mm), median (range) | 60 (10–140) |
| Paris classification, n (%) | |
| Type 0‐Is | 2 (11) |
| Type 0‐Ip | 1 (5) |
| Type 0‐IIb | 4 (21) |
| Type 0‐IIa | 5 (26) |
| Type 1 | 1 (5) |
| Type 2 | 3 (16) |
| Type 3 | 1 (5) |
| Type 4 | 1 (5) |
| Type 5 | 1 (5) |
| Location, n (%) | |
| Rectum | 9 (47) |
| Left‐sided colon | 7 (37) |
| Right‐sided colon | 3 (16) |
| Operative procedure, n (%) | |
| Total proctocolectomy | 17 (89) |
| Pelvic visceral resection | 1 (5) |
| Low anterior resection | 1 (5) |
| Histology, n (%) | |
| Low‐grade dysplasia | 6 (32) |
| Low‐grade adenocarcinoma | 7 (37) |
| High‐grade adenocarcinoma | 4 (21) |
| Sessile serrated lesion | 1 (5) |
| Squamous cell carcinoma | 1 (5) |
| T stage a , n (%) | |
| T0 (M) | 1 (5) |
| T1 (SM) | 1 (5) |
| T2 (MP) | 0 |
| T3 (SS) | 4 (21) |
| T4 (SE) | 3 (16) |
Abbreviations: M, mucosa; MP, muscularis propria; SS, subserosa; SE, serosa; SM, submucosa.
Tumor‐node‐metastasis staging is classified according to Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: The 3d English Edition. For synchronous multiple lesions, tumor‐node‐metastasis staging was performed for the lesion with the highest tumor stage.
TABLE 5.
Clinical data of 19 lesions of nine surgical patients
| Patient number | Lesion number | Mayo | Identification | Location | Lesion size (mm) | Tumor depth of invasion d | Disease duration (year) | Type of disease | Operative procedure |
|---|---|---|---|---|---|---|---|---|---|
| A | 1 b | 1 | Visible | R | 65 | SS | 30 | Left‐sided colitis | Low anterior resection |
| B | 2 b | 0 | Visible | R | 65 | SS | 29 | Proctitis | Pelvic visceral resection |
| C | 3 b | 0 | Visible | R | 42 | AI | 13 | Pancolitis | Total proctocolectomy |
| D | 4 b | 3 | Visible | D | 80 | SS | 19 | Pancolitis | Total proctocolectomy |
| 5 | 3 | Undetected | R | 40 | M | 19 | Pancolitis | Total proctocolectomy | |
| 6 | 3 | NA c | A | 70 | M | 19 | Pancolitis | Total proctocolectomy | |
| E | 7 b | 3 | Visible | R | 140 | SE | 12 | Pancolitis | Total proctocolectomy |
| 8 | 3 | NA c | S | 85 | SM | 12 | Pancolitis | Total proctocolectomy | |
| 9 | 3 | Visible | R | 75 | M | 12 | Pancolitis | Total proctocolectomy | |
| F | 10 b | 1 | Visible | R | 45 | AD | 16 | Pancolitis | Total proctocolectomy |
| 11 | 1 | Visible | R | 24 | SS | 16 | Pancolitis | Total proctocolectomy | |
| G | 12 b | 1 | Visible | S | 15 | SS | NA c | Pancolitis | Total proctocolectomy |
| 13 | 1 | Visible | S | 10 | SM | NA c | Pancolitis | Total proctocolectomy | |
| H | 14 b | 2 | Visible | R | 105 | M | 20 | Pancolitis | Total proctocolectomy |
| 15 | 2 | Visible | S | 45 | M | 20 | Pancolitis | Total proctocolectomy | |
| 16 | 2 | Undetected | D | 60 | M | 20 | Pancolitis | Total proctocolectomy | |
| I a | 17 b | 0 | Visible | S | 60 | SM | 21 | Pancolitis | Total proctocolectomy |
| 18 | 0 | Undetected | T | 20 | M | 21 | Pancolitis | Total proctocolectomy | |
| 19 | 0 | Undetected | T | 25 | M | 21 | Pancolitis | Total proctocolectomy |
Abbreviations: A, ascending colon; D, descending colon; M, mucosa; MP, muscularis propria; NA, not assessed; R, rectum; S, sigmoid colon; SE, serosa; SM, submucosa; SS, subserosa; T, transverse colon.
Additional surgery for non‐curative ESD case.
Main lesion for colectomy.
The colonoscope could not be inserted into the proximal lesion because of distal stenosis.
Depth of invasion was classified according to the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: The 3d English Edition.
DISCUSSION
This study demonstrated favorable treatment outcomes of ESD for large superficial lesions in patients with UC. ESD demonstrated high (80%–90%) en bloc, R0, and curative resection rates, and these results were consistent with those of previous studies. 8 , 9 , 10 , 11 , 12 However, our study differs from previous studies in that two metachronous superficial large nonpolypoid lesions (Paris classification type 0‐IIa) developed in two patients, which were detected by surveillance colonoscopy; the lesions were successfully treated by ESDs, and the two patients could continue receiving surveillance colonoscopy without colectomy. In addition, previous studies reported various metachronous lesion incidence rates: 4% (1/25), 11.5% (3/26), 14.3% (2/14), 33% (3/9), and 71.4% (5/17) during median follow‐up periods of 21, 33, 33, 24, and 180 months, respectively. Considering the metachronous cancer risk, Gastrointestinal Endoscopy editorial recommended ESD of nonpolypoid dysplasia only for 50 or more aged patients. 20 In our study, a high metachronous incidence rate was also observed, and the median age of ESD group was 65 years old. Because these metachronous lesions were completely resected by ESD and the patients could continue surveillance colonoscopy, ESD followed by surveillance colonoscopy was considered an optimized approach for the early detection of lesions if the UC patients are aged 50 or over.
The investigation of endoscopic findings to predict technical difficulties in resection is considered a strength of our study. In this study, two lesions had endoscopic findings of scarring and showed severe submucosal fibrosis. In the remaining nine lesions, endoscopic findings of scarring or severe submucosal fibrosis were not observed. We found that technical difficulties are highly possible in lesions with endoscopic findings of scarring, and ESD for such lesions should be performed by experts. To the best of our knowledge, no previous studies have investigated endoscopic findings to predict submucosal fibrosis in patients with UC. Nonetheless, further studies are required to clarify the predictive factors for the technical difficulty of ESD in patients with UC.
Regarding the treatment outcomes of ESD in individuals without UC, Saito et al. 13 investigated 1111 colorectal cases and reported an average lesion diameter of 35 mm, average treatment time of 116 min, and en bloc resection rate of 88%; moreover, for the complications, perforation and delayed bleeding rates were 4.9% and 1.5%, respectively. The en bloc resection rate was similar between their study and our study; however, the rate of delayed bleeding was higher (27%) in our study. Regarding the treatment outcomes of ESD in patients with UC, several studies have reported outcomes of ESD for colorectal neoplasms. The en bloc and R0 resection rates were 80%–100% and 67%–80%, respectively, 8 , 9 , 10 , 11 , 12 which were consistent with the en bloc and R0 resection rates (91% and 82%, respectively) in our study. Generally, the treatment outcomes in patients with UC were favorable.
Furthermore, UCAN could result in an accumulation of molecular changes due to persistent epithelial injury associated with inflammation. 21 , 22 SPEN, such as conventional adenoma and adenocarcinoma, can be seen in patients with and those without UC. 23 However, the histological distinction between UCAN and SPEN is typically limited to low‐grade dysplasia and low‐grade adenoma. 23 Matsumoto et al. 12 reported outcomes of colorectal ESD of nine low‐grade dysplasias and three high‐grade dysplasias that were classified as UCAN in seven patients with UC. En bloc and R0 resection rates were 83% and 67%, respectively. No complication occurred. However, nine of the 12 lesions were small (<20 mm). 12 For the treatment outcomes of ESD of visible lesions ≥20 mm in our study, the en bloc and R0 resection rates were 83% and 83%, respectively, in six low‐grade dysplasias. The ESD outcomes were limited to UCAN cases; nonetheless, the results are consistent with those of the previous reports. 12
Local recurrence has been reported to be low when curative resection is achieved. 8 , 9 , 10 , 11 , 24 No local recurrence was observed in any patient during the follow‐up period in our study. Thus, ESD techniques may have improved because of changes over time.
In addition, attention should also be paid to undetected lesions, particularly invisible dysplasia or cancer, during surveillance colonoscopy in patients with UC. In our study, three patients had four undetected lesions (21%) based on the surgical specimens. Four undetected lesions were evaluated the macroscopic type using the surgical specimens, three were flat and diagnosed as 0‐IIb, and one was diagnosed as 0‐Is. Using nowadays with better image‐enhanced endoscopy, the case that was 0‐IIb may have been found as nonpolypoid dysplasia, but it may be not easy to find. Since the case with 0‐Is had a Mayo endoscopic subscore of 3, it is difficult to detect even with better image‐enhanced endoscopy. Furthermore, the prevalence of undetected lesions (approximately 20%) was the same between high (2–3) and low (0–1) Mayo endoscopic subscore groups. Hence, attention should be paid to undetected lesions, which are usually flat morphology, even when the patient achieves mucosal healing. Moreover, six of nine patients (66.6%) had synchronous multiple neoplasms, and three (33%) had multiple invasive cancers. In a previous report, 37 of 238 patients (15.5%) who underwent surgical resection had multiple invasive cancers. 25 Therefore, if invasive cancer is present, proctocolectomy is recommended, even if there are other lesions that can be treated endoscopically. This recommendation is consistent with the American Journal of Gastroenterology guidelines, in which proctocolectomy is the suggested surgical procedure for invasive cancer. 3
This study has some limitations. First, this study employed a retrospective design, and biases inherent to the study design are possible. Second, this study used a single‐center database; thus, the results need external validation. Lastly, the number of patients was small, and the follow‐up period was relatively short. Therefore, our results must be verified in multicenter studies with a larger number of cases.
In conclusion, ESD is a feasible and valid approach for large neoplastic lesions in patients with UC; however, attention to technical difficulties in endoscopic resection is needed in lesions with endoscopic findings of scarring. In addition, attention should be paid to undetected lesions during surveillance colonoscopy, even in patients with mucosal healing.
CONFLICT OF INTERESTS
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTION
Kengo Kasuga and Masayoshi Yamada contributed to the study concept and design. Kengo Kasuga, Masayoshi Yamada, Dai Shida, Teppei Tagawa, Hiroyuki Takamaru, Masau Sekiguchi, Taku Sakamoto, Yukihide Kanemitsu, and Yutaka Saito collected and interpreted the data. Shigeki Sekine performed the histological analysis. Kengo Kasuga and Masayoshi Yamada wrote the manuscript, which was revised by Dai Shida, Toshio Uraoka, and Shigeki Sekine. All the authors approved the final version of the manuscript.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
The authors would like to thank Editage (www.editage.jp) for editing the draft of this manuscript. This study was supported by a grant from JSPS KAKENHI (no. 18K07925).
Kasuga K, Yamada M, Shida D, Tagawa T, Takamaru H, Sekiguchi M, et al. Treatment outcomes of endoscopic submucosal dissection and surgery for colorectal neoplasms in patients with ulcerative colitis. United European Gastroenterol J. 2021;9(8):964–72. 10.1002/ueg2.12118
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Matsuoka K, Kobayashi T, Ueno F, Matsui T, Hirai F, Inoue N, et al. Evidence‐based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53:305–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO‐ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohn's Colitis. 2018;13:144–64. [DOI] [PubMed] [Google Scholar]
- 3. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 4. Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R, et al. Scenic international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology. 2015;148:639–51. [DOI] [PubMed] [Google Scholar]
- 5. Engelsgjerd M, Farraye FA, Odze RD. Polypectomy may be adequate treatment for adenoma‐like dysplastic lesions in chronic ulcerative colitis. Gastroenterology. 1999;117:1288–94. [DOI] [PubMed] [Google Scholar]
- 6. Rubin PH, Friedman S, Harpaz N, Goldstein E, Weiser J, Schiller J, et al. Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology. 1999;117:1295–300. [DOI] [PubMed] [Google Scholar]
- 7. Hurlstone DP, Sanders DS, Atkinson R, Hunter MD, McAlindon ME, Lobo AJ, et al. Endoscopic mucosal resection for flat neoplasia in chronic ulcerative colitis: can we change the endoscopic management paradigm? Gut. 2007;56:838–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kinoshita S, Uraoka T, Nishizawa T, Naganuma M, Iwao Y, Ochiai Y, et al. The role of colorectal endoscopic submucosal dissection in patients with ulcerative colitis. Gastrointest Endosc. 2018;87:1079–84. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki N, Toyonaga T, East J. Endoscopic submucosal dissection of colitis‐related dysplasia. Endoscopy. 2017;49:1237–42. [DOI] [PubMed] [Google Scholar]
- 10. Yang DH, Kim J, Song EM, Chang K, Lee SH, Hwang SW, et al. Outcomes of ulcerative colitis‐associated dysplasia patients referred for potential endoscopic submucosal dissection. J Gastroenterol Hepatol. 2019;34:1581–9. [DOI] [PubMed] [Google Scholar]
- 11. Iacopini F, Saito Y, Yamada M, Grossi C, Rigato P, Costamagna G, et al. Curative endoscopic submucosal dissection of large nonpolypoid superficial neoplasms in ulcerative colitis (with videos). Gastrointest Endosc. 2015;82:734–8. [DOI] [PubMed] [Google Scholar]
- 12. Matsumoto K, Oka S, Tanaka S, Tanaka H, Boda K, Yamashita K, et al. Long‐term outcomes after endoscopic submucosal dissection for ulcerative colitis‐associated dysplasia. Digestion. 2019:1–11. 10.1159/000503341 [DOI] [PubMed] [Google Scholar]
- 13. Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72:1217–25. [DOI] [PubMed] [Google Scholar]
- 14. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 who classification of tumours of the digestive system. Histopathology. 2020;76:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kobayashi S, Yamada M, Takamaru H, Sakamoto T, Matsuda T, Sekine S, et al. Diagnostic yield of the Japan NBI expert team (JNET) classification for endoscopic diagnosis of superficial colorectal neoplasms in a large‐scale clinical practice database. United Eur Gastroenterol J. 2019;7:914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gore RM. Colonic contour changes in chronic ulcerative colitis: reappraisal of some old concepts. AJR Am J Roentgenol. 1992;158:59–61. [DOI] [PubMed] [Google Scholar]
- 17. Matsumoto A, Tanaka S, Oba S, Kanao H, Oka S, Yoshihara M, et al. Outcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosis. Scand J Gastroenterol. 2010;45:1329–37. [DOI] [PubMed] [Google Scholar]
- 18. Japanese Society for Cancer of the Colon and Rectum . Japanese classification of colorectal, appendiceal, and anal carcinoma: the 3d English edition [secondary publication]. J Anus, Rectum Colon 2019; 3:175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suzuki H, Saito Y, Moriya Y, Shimoda T, Saito D. A superficial early colitic cancer that resembled a laterally spreading tumor on chromoendoscopy. Endoscopy. 2008;40:E130–31. [DOI] [PubMed] [Google Scholar]
- 20. Soetikno R, East J, Suzuki N, Uedo N, Matsumoto T, Watanabe K, et al. Endoscopic submucosal dissection for nonpolypoid colorectal dysplasia in patients with inflammatory bowel disease: in medias res. Gastrointest Endosc. 2018;87:1085–94. [DOI] [PubMed] [Google Scholar]
- 21. Baker KT, Salk JJ, Brentnall TA, Risques RA. Precancer in ulcerative colitis: the role of the field effect and its clinical implications. Carcinogenesis. 2018;39:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Umetani N, Sasaki S, Watanabe T, Shinozaki M, Matsuda K, Ishigami H, et al. Genetic alterations in ulcerative colitis‐associated neoplasia focusing on apc, k‐ras gene and microsatellite instability. Jpn J Canc Res. 1999;90:1081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawachi H. Histopathological diagnosis of ulcerative colitis‐associated neoplasia. Dig Endosc. 2019;31:31–5. [DOI] [PubMed] [Google Scholar]
- 24. Yamada M, Saito Y, Takamaru H, Sasaki H, Yokota T, Matsuyama Y, et al. Long‐term clinical outcomes of endoscopic submucosal dissection for colorectal neoplasms in 423 cases: a retrospective study. Endoscopy. 2017;49:233–42. [DOI] [PubMed] [Google Scholar]
- 25. Hata K, Anzai H, Ikeuchi H, Futami K, Fukushima K, Sugita A, et al. Surveillance colonoscopy for ulcerative colitis‐associated colorectal cancer offers better overall survival in real‐world surgically resected cases. Am J Gastroenterol. 2019;114:483–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


