Abstract
Non‐alcoholic fatty liver disease (NAFLD) is an increasingly prevalent and potentially severe liver disease, emphasizing the need for implementation of widely supported care paths for patients at risk for advanced stages of NAFLD. In particular, the distinction of patients with a progressive and/or advanced, fibrotic NAFLD from those with simple steatosis requires improvement, as well as the awareness for NAFLD among health care professionals. Broad acceptance and implementation of interdisciplinary care paths in the near future will bring enhanced identification of those patients that benefit from surveillance, intensive lifestyle management, and empirical or investigational pharmacotherapy and enhance our epidemiological grasp of NAFLD in relation to lifestyle, genetic background, and cardiometabolic comorbidities related to NAFLD.
Keywords: care path, guideline, hepatology, liver fibrosis, non‐alcoholic fatty liver disease
BRIEF CLINICAL CASE
A 54 year‐old female with a body mass index (BMI) of 29 kg/m2 and 18 years of type 2 diabetes mellitus (T2DM) with oral treatment in primary care attends the emergency department for hematemesis. Her liver enzymes had shown a continuous mild elevation over the past years (aspartate aminotransferase 32 U/L, alanin aminotransferase 46 U/L, GGT 48 U/L, bilirubin 12 μmol/L). She refrained the use of alcohol. Viral hepatitis were previously excluded, and she has no family history of liver diseases. A gastroduodenoscopy was performed, and esophageal varices with bleeding stigmata were found. Additional imaging demonstrated a cirrhotic liver with an early‐stage hepatocellular carcinoma (HCC) in segment 4, see Figure 1.
FIGURE 1.
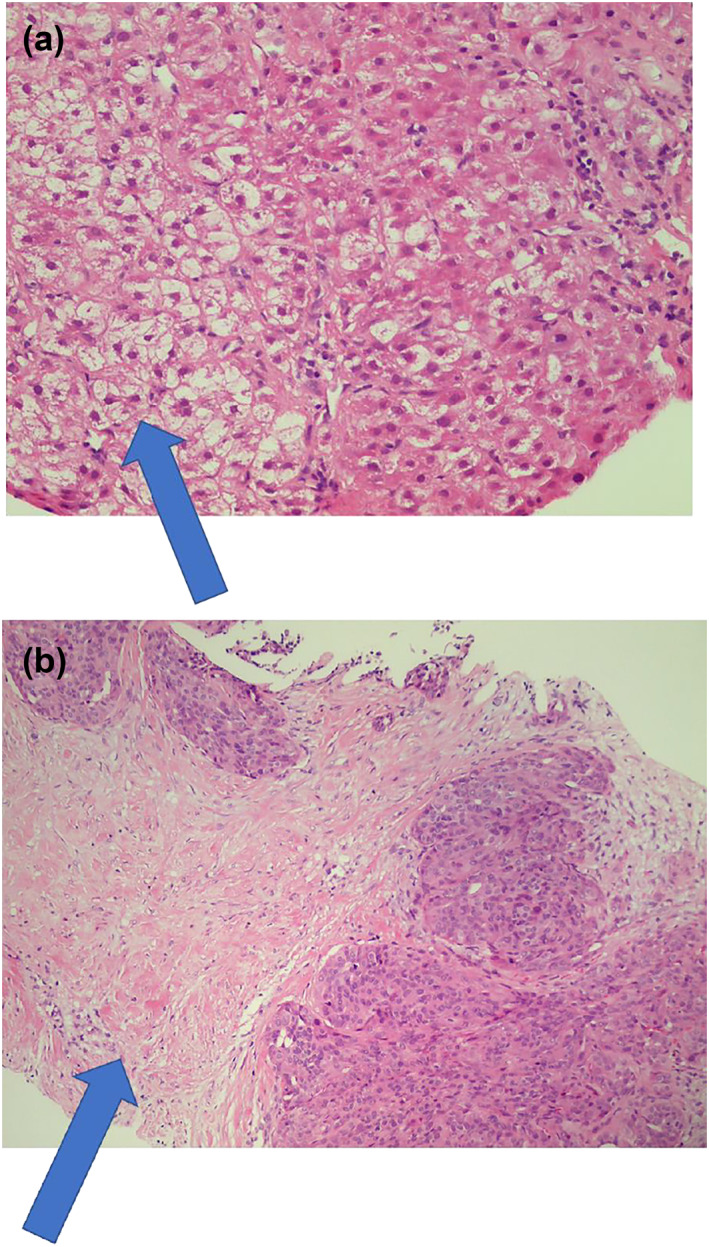
Histology images of the liver with (a) ballooned cells; (b) invasive grow of the hepatocellular carcinoma
NATURAL HISTORY, INCLUDING LIVER‐ AND NON‐LIVER–RELATED COMPLICATIONS
Non‐alcoholic fatty liver disease is an increasingly prevalent liver disease; however, it is still underdiagnosed. 1 It is defined as excessive fat accumulation in the liver in the absence of excessive alcohol consumption. 2 , 3 The disease spectrum of non‐alcoholic fatty liver disease (NAFLD) ranges from simple steatosis (or NAFL), to non‐alcoholic steatohepatitis (NASH), and NASH‐related fibrosis (F0–F3) and ultimately to cirrhosis (F4). Despite NAFLD being a prevalent disease in the general population, only a minority of patients with NAFLD will develop NASH and NAFLD‐related fibrosis. Due to the association of advanced stages of NAFLD with liver‐related complications, identifying patients at high risk of developing advanced stages of the NAFLD spectrum is vital to prevent and monitor these complications. Alarmingly, progression along the NAFLD disease spectrum often goes unnoticed by both patient and physician since it does not result in specific symptoms until advanced phases of cirrhosis develop. 4 The case above is an example of such a silent and unnoticed progress. This illustrates that despite overall relatively slow progression rate, timely diagnosis remains important to enable strategies to prevent severe stages and complications.
Current epidemiological data suggests that over 25% of the general European population have some stage of NAFLD. 2 In a Dutch study of the general population with the age greater than 45 (n = 3041), 5.6% of the participants had significant liver fibrosis (defined as transient elastography ≥8 kPa), likely mainly driven by NAFLD. 5 A recent meta‐analysis conducted to quantify the prognostic value of fibrosis in patients with NAFLD showed an unadjusted increased risk with increasing stage of fibrosis of liver‐related mortality of 11.1 and all‐cause mortality of 3.4. 6 Patients with liver cirrhosis are at high risk for liver decompensation and HCC. 7 According to a meta‐analytic assessment, the annual incidence of HCC in NAFLD patients is 0.44 per 1000 person‐years. 2
Since NAFLD strongly coincides with obesity and the metabolic syndrome, most notably insulin resistance and hyperglycemia (or even T2DM), it does not come as a surprise that atherosclerotic cardiovascular disease (asCVD) is an important cause of mortality in patients with NAFLD. 8 This can potentially be explained by overlapping risk factors between NAFLD and asCVD. Genetic studies support that the causal link between NAFLD and asCVD are atherogenic plasma lipids, that is the mixed hyperlipidemia of low‐density lipoproteins and triglycerides is often observed in these patients. 9 , 10
Non‐alcoholic fatty liver disease progression shows high interindividual variability. Despite overall slow progression in fibrosis stage, a subgroup of patients may rapidly progress three to four stages within 2–6 years. 11 , 12 This high interindividual variability can be attributed to multiple factors such as dietary behavior, like fructose intake and other environmental factors, comorbidities, genetic risk factors, and variation in the composition of the gut microbiome. 13 The importance of genetic factors in NAFLD progression has been observed in a family cohort in the US, where first‐degree relatives of patients with NAFLD cirrhosis have a 12 times higher risk of advanced fibrosis. 14 Genome‐wide association studies indicate that variations in genes have a role in the disease course of NAFLD. The best established example is patatin‐like phospholipase domain‐containing 3 (PNPLA3). 4 The rare allele of the T143M variant in PNPLA3, most prevalent in Hispanic Americans, was strongly associated with increased hepatic fat levels, whereas the common allele, most common in African‐Americans, was associated with lower hepatic fat content. 15
DIAGNOSTIC MODALITIES FOR NAFLD
Liver biopsy is the gold standard to diagnose and stage a patient with NAFLD, detecting both steatotic and fibrotic range, and scoring the activity and inflammatory component of the disease, that is NASH. Yet, it is an invasive procedure with some risk of complications and probability of sampling error. 16 Therefore, liver biopsy is not suitable for the highly necessary risk stratification in all patients with NAFLD. Several alternatives are discussed below.
Around 2009–2010, non‐invasive liver fibrosis tests (NITs) emerged. These can serve two main functions. First, to determine in primary care when a patient needs to be referred to a liver specialist. Second, NITs can predict which patients are at high risk for progression into advanced fibrosis. 17 Several non‐invasive tests exist, ranging from simpler scores such as Fatty Liver Index for steatosis measurement, Fibrosis‐4 score (FIB‐4) and NAFLD Fibrosis Score (NFS) to more intricate tools such as Enhanced Liver Fibrosis (ELF) test and FibroMeter all latter for fibrosis staging. 16 More information about use of these NITs can be found in the next section.
Ultrasonography can identify histological steatosis when it is higher than 20%–30% and indications of cirrhosis, but not NASH or degrees of fibrosis. In addition to limited sensitivity when steatosis is <20%, ultrasonography also has low sensitivity in patient with a BMI >40 kg/m2. 18 Despite these limitations, ultrasound can be an acceptable first‐line screening procedure for NAFLD in clinical practice. 19 Furthermore, it may provide additional diagnostic information. 18
The use of vibration controlled transient elastography (VCTE) is increasingly recommended in NAFLD guidelines. 16 , 18 In 2019, Eddowes et al. conducted prospective research to assess the accuracy of FibroScan VCTE. They found controlled attenuation parameter as indicator for steatosis as well as liver stiffness measurement for fibrosis, to be in an AUROC range of 0.70–0.89. 20 Advantage of VCTE is its simplicity to perform and to learn. A limitation is the applicability in severe obesity. 16 The European Association for Study of Liver ‐ Asociacion Latinoamericana para el Estudio del Higado guideline states that VCTE can be considered the non‐invasive standard for measurement of liver stiffness. However, it is recommended to interpret VCTE results in consideration with serum aminotransferase levels, BMI, absence of extra‐hepatic cholestasis, and absence of right heart failure. 16
DEVELOPMENT AND IMPLEMENTATION OF CLINICAL CARE PATHS AND GUIDELINES FOR NAFLD
Despite the rising prevalence and severity of NAFLD, there is still much room for improvement among health care professionals across the lines of care and also on the national guideline level. 21 Lazarus et al. 1 conducted a research including 29 European countries for assessment of their NAFLD guidelines and strategies. The United Kingdom scored highest mainly driven by a national guideline that focuses on early detection of NAFLD and associated comorbidities in primary care. None of the surveyed countries had written strategies for NAFLD assessment, 10 had clinical guidelines regarding NAFLD and 11 recommended screening in patients with T2DM, obesity, and/or metabolic syndrome. This paper maps the challenges in health policy, guidelines, epidemiological grasp, and care management for NAFLD. 1
The unnoticed progression of NAFLD and limited awareness among health care professionals both lead to over‐referrals and underdiagnoses. Most cases referred for assessment of NAFLD by the general practitioner or the internist to the hepatologist actually have mildly active and mildly progressive disease and could have been retained in primary care. On the other hand, obese patients with T2DM may progress to NAFLD with fibrosis stage 3 or 4 (F3–F4) while being under care of their general practitioner or internist, because the hepatic component of their metabolic syndrome is being overlooked. 22 The European Association for Study of Liver ‐ European Association for the Study of Diabetes ‐ European Association for the Study of Obesity (EASL‐EASD‐EASO) guideline states that NITs should aim to identify and assess NAFLD in individuals with increased metabolic risk in primary care and that NITs in secondary and tertiary care should identify those with worse liver prognosis. 18
For primary care, Srivastava et al. published the Camden & Islington care path, using a 2‐tier system with FIB‐4 and ELF plasma tests to detect advanced stages of NASH fibrosis in primary care practices. 23 This pathway led to a profound reduction of referrals to the hepatologist by 80%, whilst at the same time significantly improving the detection of advanced fibrosis or cirrhosis by 4‐fold. In this care path, patients with steatosis hepatitis on ultrasound and/or elevated alanine aminotransferase (ALT) levels were included when excessive alcohol use or other hepatic diseases were excluded. 23
In addition to screening patients with elevated ALT or steatosis hepatitis with ultrasound, several guidelines state that screening for advanced liver disease in patients with T2DM may be performed based on the high prevalence of NASH and liver fibrosis. 18 , 19 Additionally, according to the EASL‐EASD‐EASO guideline, this screening should be irrespective of liver enzyme levels, when risk factors for NAFLD such as T2DM and obesity are present. 18 A recent prospective study determined prevalence and severity of NAFLD by liver biopsy among patients with T2DM. The prevalence of significant (≥F2) and advanced fibrosis (F ≥ 3) were 29.5% and 29.5%, respectively. Based on these results, more aggressive screening for NAFLD and fibrosis in T2DM patients seems justified. 24 For the screening of this high‐risk group attending primary care the NFS, FIB‐4 and VCTE can be used to identify those at low or high risk of advanced fibrosis, 25 since increased liver enzymes alone are insensitive for advanced fibrosis. 26 FIB‐4 and NFS perform best at excluding severe fibrosis and cirrhosis, with negative predictive values of >90%. Because of this high negative predictive value, these tests could be used in primary care to identify patients at low risk of severe fibrosis. 16 Unfortunately, due to low performance in patients with T2DM non‐invasive tests including FIB‐4 and NFS seems unsuitable for evaluating liver fibrosis in this group. 27 , 28
In secondary and tertiary care, different guidelines recommend NITs to distinguish patients with NAFLD at low risk of advanced fibrosis from those at high risk. 16 , 22 To incorporate NITs into clinical practice, the simplest strategy is to start with a test with a high negative likelihood ratio in order to rule out high‐risk cases. 17 In the European 18 and American 25 guidelines for the management of NAFLD in secondary and tertiary care, NFS and FIB‐4 are mentioned as possible NITs with intensive external validation in ethnically diverse NAFLD populations, with consistent results. These fibrosis scores and other biomarkers, as well as VCTE, are acceptable non‐invasive procedures for the identification of patients at low risk of advanced fibrosis. 18 A recent meta‐analysis showed that ELF test is an option in high prevalence settings such as secondary and tertiary care. 29 However, ELF test consists of three relatively complex assays and is patented and therefore comes at a higher cost than FIB‐4. In a screening study with 1000 patients with NAFLD, ELF test was costlier than the combination of FIB‐4 and FibroScan for patients with indeterminate FIB‐4. Detection of advanced fibrosis was comparable between ELF test and FIB‐4/FibroScan. 30 Combination of a NIT with VCTE would increase diagnostic accuracy and might reduce the number of liver biopsies 17 , 18 ; yet comparative care path studies with large sample sizes are required to determine the optimal two‐tiered care path screening test combination.
In the evaluation of NAFLD, excessive alcohol consumption and other, more sporadic liver diseases should be excluded. In addition, upon suspicion of NAFLD, associated comorbidities should be assessed and treated, such as obesity, T2DM, dyslipidemia, hypothyroidism, polycystic ovary syndrome, sleep apnea, and hypogonadism. 25 Consideration of a liver biopsy is recommended when significant fibrosis is confirmed by screening with NITs and imaging with VCTE, only if it impacts the management. 18 In patients with indeterminate results upon screening (e.g., positive autoimmune hepatitis serology) or increased risk of having NASH and when other liver disease cannot be excluded, a liver biopsy should be considered. 19 , 25
HOW TO INCLUDE THERAPEUTIC OPTIONS IN THE FUTURE CARE PATHS?
In the management of NAFLD, therapy for liver disease should be included, as well as treating associated comorbidities. 25 It is fairly well accepted that a modest percentage of body weight loss reduces the metabolically active and responsive liver fat, mainly by reducing the hepatopetal free fatty acid flux from peripheral adipose tissue and improving hepatic insulin sensitivity. Loss of 7%–10% in body weight results in improvement of liver enzymes and histology. 18 , 25 Dietary recommendations should comprise energy restriction and exclusion of processed foods and foods enriched in fructose. 18 Some studies recommend a Mediterranean diet, which contains more monounsaturated fatty acids and less carbohydrates, with evidence supporting significant reduction of steatosis. 25
In patients with simple stages of NAFLD, no pharmacotherapy is currently recommended, since any drug treatment would be off‐label. 18 , 25 Yet as patients with NAFLD are at high risk for cardiovascular morbidity and mortality, treatment of cardiovascular risk factors is indicated for all patients with NAFLD. Statins are widely known for its positive effects on asCVD, but are also associated with reduced mortality and reduced levels of advanced fibrosis in patients with NAFLD. 31
In patients with (fibrotic) NASH, some pharmaca can be considered, such as vitamin E and pioglitazone. Recently, glucagon‐like peptide‐1 receptor agonists (GLP1RAs) gained interest: they increase satiety, reduce weight, and reduce hepatopetal free fatty acid flux. 32 A recent phase 2 trial in patients without T2DM showed a reduction of NASH and non‐invasive proxies of liver fibrosis, although histological fibrosis was not reduced. 7 Another interesting development after failure of single phase 3 drug trials to reduce NASH and fibrosis are the arrival of several combination trials: a combination of tropifexor, a farnesoid X receptor agonist with licogliflozin, a sodium‐glucose cotransporter 1/2 inhibitor and a combination of tropifexor with LYS006, a LTA4 hydrolase, inhibitor. 33
In the presence of severe obesity and when conservative measures prove unsuccessful, ample evidence supports that bariatric surgery can be effective to reverse NAFLD fibrosis. 18 , 25 , 34 A recent systematic review of Lee et al. showed that bariatric surgery in severely obese patients leads to a complete resolution of liver biopsy proven NAFLD fibrosis in around 40% of the patients. Yet, worsening or development of de novo NAFLD occurred in 12% of the patients. 35 Of note, all bariatric surgery studies in patients with NAFLD to date are observational. Studies are called for which offer comparison of different bariatric procedures and gauging NAFLD regression with weight loss rather than time after surgery. 35 , 36
The optimal follow‐up of patients with NAFLD is undetermined. Monitoring should include routine biochemistry, assessment of comorbidities, and non‐invasive monitoring of fibrosis. 18 Figure 2 shows a summary flowchart for NAFLD care paths, which is work in progress. It highlights the necessity of combination of tests, evaluation in secondary care when signs of advanced fibrosis are present, and the role for the diabetologist in a NAFLD care path.
FIGURE 2.
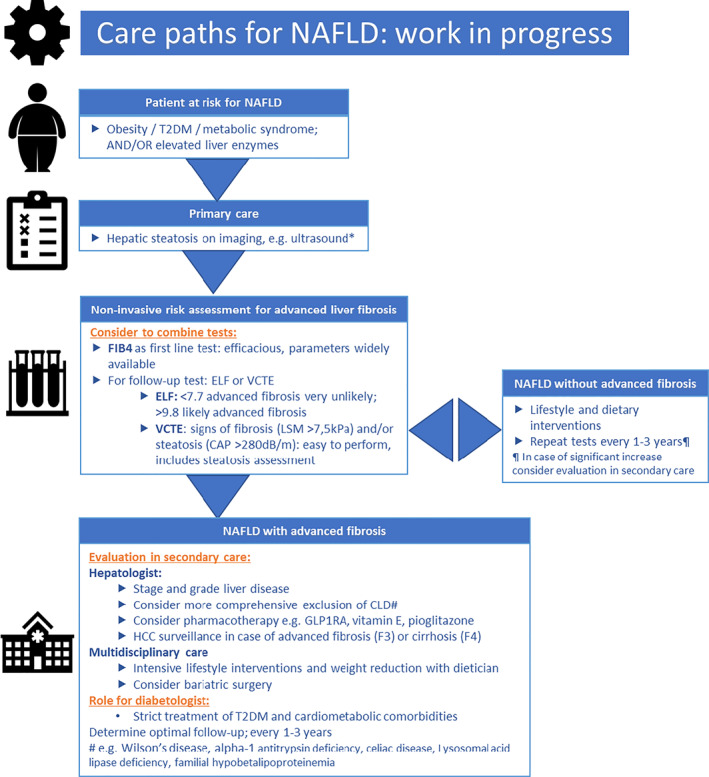
Care paths for non‐alcoholic fatty liver disease (NAFLD): work in progress. *Exclusion of excessive alcohol use and chronic liver diseases (CLD): hepatitis B, hepatitis C, autoimmune hepatitis, and hemochromatosis. CAP, continued attenuation parameter; ELF, Enhanced Liver Fibrosis; FIB‐4, Fibrosis‐4; GLP1RA, glucagon‐like peptide‐1 receptor agonists; LSM, liver stiffness measurement; T2DM, type 2 diabetes mellitus; VCTE, vibration controlled transient elastography
FINAL OUTCOME, AREAS OF UNCERTAINTY
The options for pharmacotherapy to treat patients with advanced NAFLD fibrosis are as of yet limited, but this is a field of enormous development. Therefore, it is deemed timely to identify patients with advanced fibrosis and/or a more rapidly progressive disease course, that is, those who will be likely benefit most from future pharmacotherapy in order to halt or even revert NAFLD progression. Because advanced stages of NAFLD are becoming more prevalent, there is a requirement for broadly validated and implemented care paths. It is necessary to generalize the guidelines for definitions and timing of risk assessment and follow‐up. Most of the patients with obesity and T2DM are in primary care, and therefore, these care paths and risk assessments should be well implemented in primary care. In particular, referral of primary to secondary care of only those patients at high risk of progression into severe NAFLD stages demands explicit and generally recognized guidelines, Figure 3. Lifestyle interventions are crucial in preventing development of severe NAFLD stages and should always have a central place in these care paths and guidelines. This is unlikely to change even after the future advent of approved pharmacotherapy.
FIGURE 3.
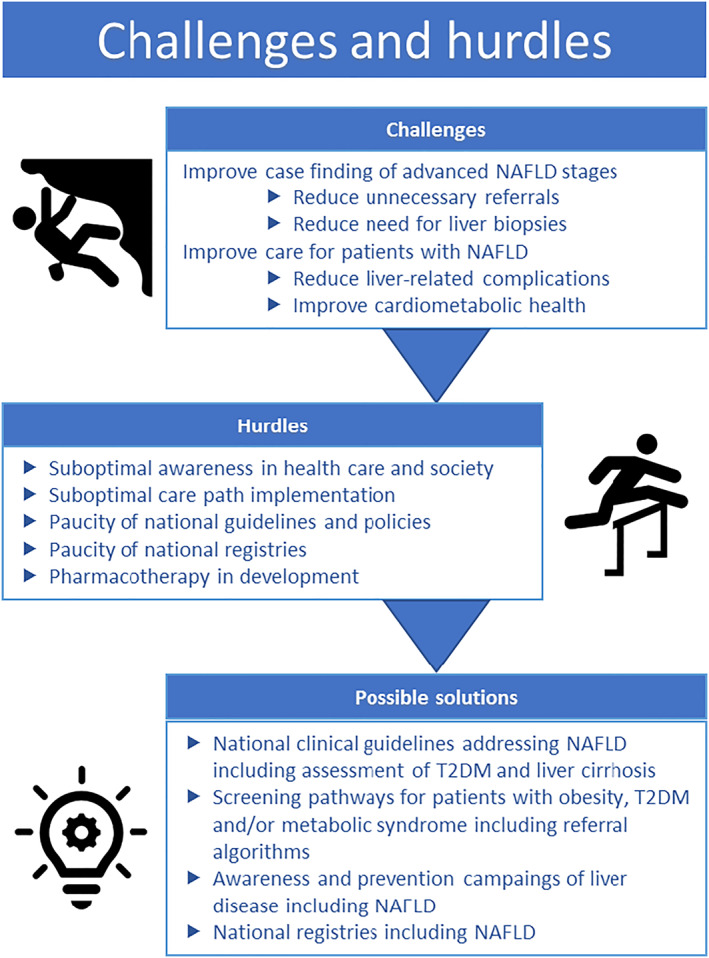
Challenges and hurdles in non‐alcoholic fatty liver disease (NAFLD) care. T2DM, type 2 diabetes mellitus
In our clinical case (FIB‐4 was 1.89), a timely risk assessment implemented in a care path would have encouraged strict observation and therapy adjustments. This would have provided a window for available lifestyle interventions and pharmacotherapy, such as a GLP1RA or in future even possible combination therapy. With better awareness for driving risk factors for NAFLD present in this patient, that is T2DM and obesity, earlier liver risk assessment might have taken place, and the worst‐case scenario which now occurred could have been prevented.
CONFLICT OF INTEREST
The authors have no conflict of interests related to this publication.
ACKNOWLEDGMENTS
We thank Stijn Crobach for providing the histology images. Adriaan G. Holleboom was supported by the Amsterdam UMC Fellowship grant, a Holland Health TKI‐PPP grant and by research grants from Gilead and Novo Nordisk.
van Dijk A‐M, Schattenberg JM, Holleboom AG, Tushuizen ME. Referral care paths for non‐alcoholic fatty liver disease—Gearing up for an ever more prevalent and severe liver disease. United European Gastroenterol J. 2021;9(8):903–9. 10.1002/ueg2.12150
Adriaan G. Holleboom and Maarten E. Tushuizen have equal contributions.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Lazarus JV, Palayew A, Carrieri P, Ekstedt M, Marchesini G, Novak K, et al. European ‘NAFLD Preparedness Index’—is Europe ready to meet the challenge of fatty liver disease? JHEP Rep. 2021;3:100234. 10.1016/j.jhepr.2021.100234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 3. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–33. 10.1002/hep.29466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vieira Barbosa J, Lai M. Nonalcoholic fatty liver disease screening in type 2 diabetes mellitus patients in the primary care setting. Hepatol Commun. 2021;5(2):158–67. 10.1002/hep4.1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koehler EM, Plompen EPC, Schouten JNL, Hansen BE, Darwish Murad S, Taimr P, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology. 2016;63(1):138–47. 10.1002/hep.27981 [DOI] [PubMed] [Google Scholar]
- 6. Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Gastroenterology. 2020;158:1611–25. 10.1053/j.gastro.2020.01.043 [DOI] [PubMed] [Google Scholar]
- 7. Ruissen MM, Mak AL, Beuers U, Tushuizen ME, Holleboom AG. Non‐alcoholic fatty liver disease: a multidisciplinary approach towards a cardiometabolic liver disease. Eur J Endocrinol. 2020;183:R57–R73. 10.1530/EJE-20-0065 [DOI] [PubMed] [Google Scholar]
- 8. Stols‐Goncalves D, Hovingh GK, Nieuwdorp M, Holleboom AG. NAFLD and atherosclerosis: two sides of the same dysmetabolic coin? Trends Endocrinol Metab. 2019;30(12):891–902. 10.1016/j.tem.2019.08.008 [DOI] [PubMed] [Google Scholar]
- 9. Lauridsen BK, Stender S, Kristensen TS, Kofoed KF, Køber L, Nordestgaard BG, et al. Liver fat content, non‐alcoholic fatty liver disease, and ischaemic heart disease: Mendelian randomization and meta‐analysis of 279 013 individuals. Eur Heart J. 2018;39(5):385–93. 10.1093/eurheartj/ehx662 [DOI] [PubMed] [Google Scholar]
- 10. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–50. 10.1056/nejmra0912063 [DOI] [PubMed] [Google Scholar]
- 11. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing‐steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148–55. 10.1016/j.jhep.2014.11.034 [DOI] [PubMed] [Google Scholar]
- 12. Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu Rev Pathol. 2016;11:451–96. 10.1146/annurev-pathol-012615-044224 [DOI] [PubMed] [Google Scholar]
- 13. Friedman SL, Neuschwander‐Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–22. 10.1038/s41591-018-0104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caussy C, Soni M, Cui J, Bettencourt R, Schork N, Chen C‐H, et al. Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J Clin Invest. 2017;127:2697–704. 10.1172/JCI93465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–5. 10.1038/ng.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castera L, Yuen Chan HL, Arrese M, Afdhal N, Bedossa P, Friedrich‐Rust M, et al. EASL‐ALEH Clinical Practice Guidelines: non‐invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–64. 10.1016/j.jhep.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 17. Tapper EB, Lok AS‐F. Use of liver imaging and biopsy in clinical practice. N Engl J Med. 2017;377:756–68. 10.1056/nejmra1610570 [DOI] [PubMed] [Google Scholar]
- 18. Marchesini G, Day CP, Dufour JF, Canbay A, Nobili V, Ratziu V, et al. EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. 10.1016/j.jhep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 19. Wedemeyer H, Thursz M, Cortez‐Pinto H, Day C, Marchesini G. The role of different EASL‐papers: clinical practice guidelines vs. position papers vs. conference summaries. J Hepatol. 2010;53(2):372–84. 10.1016/j.jhep.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 20. Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–30. 10.1053/j.gastro.2019.01.042 [DOI] [PubMed] [Google Scholar]
- 21. Lazarus JV, Anstee QM, Hagström H, Cusi K, Cortez‐Pinto H, Mark HE, et al. Defining comprehensive models of care for NAFLD. Nat Rev Gastroenterol Hepatol. 2021. Epub 2021 Jun 25. 10.1038/s41575-021-00477-7 [DOI] [PubMed] [Google Scholar]
- 22. Lazarus JV, Ekstedt M, Marchesini G, Mullen J, Novak K, Pericàs JM, et al. A cross‐sectional study of the public health response to non‐alcoholic fatty liver disease in Europe. J Hepatol. 2019;72(1):14–24. 10.1016/j.jhep.2019.08.027 [DOI] [PubMed] [Google Scholar]
- 23. Srivastava A, Gailer R, Tanwar S, Trembling P, Parkes J, Rodger A, et al. Prospective evaluation of a primary care referral pathway for patients with non‐alcoholic fatty liver disease. J Hepatol. 2019;71:371–8. 10.1016/j.jhep.2019.03.033 [DOI] [PubMed] [Google Scholar]
- 24. Mikolasevic I, Domislovic V, Turk Wensveen T, Delija B, Klapan M, Juric T, et al. Screening for nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus using transient elastography–a prospective, cross sectional study. Eur J Intern Med. 2020;82:68–75. 10.1016/j.ejim.2020.08.005 [DOI] [PubMed] [Google Scholar]
- 25. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 26. Verma S, Jensen D, Hart J, Mohanty SR. Predictive value of ALT levels for non‐alcoholic steatohepatitis (NASH) and advanced fibrosis in non‐alcoholic fatty liver disease (NAFLD). Liver Int. 2013;33:1398–405. 10.1111/liv.12226 [DOI] [PubMed] [Google Scholar]
- 27. Bril F, McPhaul MJ, Caulfield MP, Clark VC, Soldevilla‐Pico C, Firpi‐Morell RJ, et al. Performance of plasma biomarkers and diagnostic panels for nonalcoholic steatohepatitis and advanced fibrosis in patients with type 2 diabetes. Diabetes Care. 2020;43:290–7. 10.2337/dc19-1071 [DOI] [PubMed] [Google Scholar]
- 28. Blank V, Petroff D, Beer S, Böhlig A, Heni M, Berg T, et al. Current NAFLD guidelines for risk stratification in diabetic patients have poor diagnostic discrimination. Sci Rep. 2020;10:18345. 10.1038/s41598-020-75227-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vali Y, Lee J, Boursier J, Spijker R, Löffler J, Verheij J, et al. Enhanced liver fibrosis test for the non‐invasive diagnosis of fibrosis in patients with NAFLD: a systematic review and meta‐analysis. J Hepatol. 2020;73:252–62. 10.1016/j.jhep.2020.03.036 [DOI] [PubMed] [Google Scholar]
- 30. Srivastava A, Jong S, Gola A, Gailer R, Morgan S, Sennett K, et al. Cost‐comparison analysis of FIB‐4, ELF and FibroScan in community pathways for non‐alcoholic fatty liver disease. BMC Gastroenterol. 2019;19:122. 10.1186/s12876-019-1039-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thomson MJ, Serper M, Khungar V, Weiss LM, Trinh H, Firpi‐Morell R, et al. Prevalence and factors associated with statin use among patients with non‐alcoholic fatty liver disease in TARGET‐NASH. Clin Gastroenterol Hepatol. 2021. Epub 2021 Mar 26. 10.1016/j.cgh.2021.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Drucker DJ. Mechanisms of action and therapeutic application of glucagon‐like peptide‐1. Cell Metabol. 2018;27:740–56. 10.1016/j.cmet.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 33. Campbell P, Symonds A, Barritt AS. Therapy for nonalcoholic fatty liver disease: current options and future directions. Clin Therapeut. 2021;43:500–17. 10.1016/j.clinthera.2021.01.021 [DOI] [PubMed] [Google Scholar]
- 34. Lassailly G, Caiazzo R, Ntandja‐Wandji LC, Gnemmi V, Baud G, Verkindt H, et al. Bariatric surgery provides long‐term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159:1290–301. 10.1053/j.gastro.2020.06.006 [DOI] [PubMed] [Google Scholar]
- 35. Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, et al. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2019;17:1040–60. 10.1016/j.cgh.2018.10.017 [DOI] [PubMed] [Google Scholar]
- 36. Morales‐Maza J, Santes O. Do we need a randomized controlled trial on this issue? Complete resolution of nonalcoholic fatty liver disease after bariatric surgery. Clin Gastroenterol Hepatol. 2019;17:1006. 10.1016/j.cgh.2018.11.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


