Abstract
Background and Aims
Transient elastography (TE) to estimate liver stiffness has proved to be very useful in the diagnosis of chronic liver disease. Here, we intend to evaluate its use in a large Spanish cohort.
Method
Nested study within the PREVHEP‐ETHON (Epidemiological sTudy of Hepatic infectiONs; NCT02749864) population‐based, cross‐sectional study performed between July 2015 and April 2017. An epidemiological questionnaire, laboratory tests and TE and anthropometric measurements were obtained.
Results
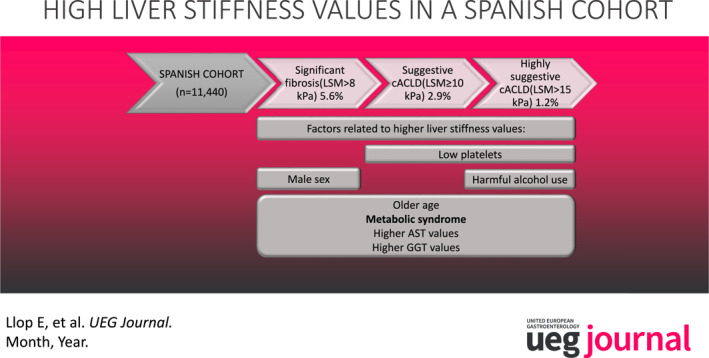
Data from 11,440 subjects were analyzed. Mean age was 50.3 (SD 12.4), of which 58.1% were women. 15.4% showed metabolic syndrome (NCEP ATP‐III), 1.3% were positive for hepatitis C antibodies, 0.8% positive for HBsAg, 9.1% reported harmful use of alcohol. The prevalence of significant fibrosis (LSM > 8 kPa), suggestive compensated advanced chronic liver disease (cACLD) (LSM ≥ 10 kPa) and highly suggestive cACLD (LSM > 15 kPa) was 5.6%, 2.9%, and 1.2% respectively. Risk factors associated with significant fibrosis were age (OR 1.03 [1.02–1.04; p < 0.001]), sex (OR 0.8 [0.6–0.95; p = 0.02]), AST (OR 1.01 [1.01–1.02; p < 0.001]), GGT (OR 1.005 [1.003–1.006; p < 0.001]) and metabolic syndrome (OR 2.1 [1.7–2.6; p < 0.001]); risk factors associated with suggestive cACLD were age (OR 1.04 [1.02–1.05; p < 0.001]), AST (OR 1.01 [1.01–1.02; p < 0.001]), GGT (OR 1.006 [1.004–1.008; p < 0.001]), low platelets (OR 0.997 [0.994–0.999; p = 0.02]) and metabolic syndrome (OR 2.2 [1.6–2.9; p < 0.001]); and risk factors associated with highly suggestive cACLD were age (OR 1.04 [1.02–1.06; p = 0.001]), AST (OR 1.02 [1.01–1.03; p < 0.001]), GGT (OR 1.005 [1.003–1.007; p < 0.001]), low platelets (OR 0.993 [0.989–0.997; p < 0.001]), metabolic syndrome (OR 2.1 [1.4–3.3; p = 0.001]) and alcohol consumption (OR 1.8 [1.05–3.1; p = 0.03]). A non‐negligible proportion of patients with normal transaminase levels, even with healthy transaminase levels, showed significant fibrosis and suggestive and highly suggestive cACLD 4.6% (95% CI 2.4–3.0), 2.1% (95% CI 1.9–2.5) and 1% (95% CI 0.7–1.1), respectively.
Conclusion
We found high proportion of significant fibrosis and cACLD measured by TE. The most relevant factor associated with significant fibrosis was metabolic syndrome, however TE is still an imperfect method since it overestimated the fibrosis stage in 50% of the histologically analyzed subjects.
Keywords: cirrhosis, liver stiffness, non‐alcoholic fatty liver disease, screening for chronic liver diseases, significant fibrosis, transient elastrography
INTRODUCTION
Chronic liver diseases are an important cause of overall morbidity and mortality worldwide, with about 1,000,000 deaths per year attributable to liver cirrhosis. 1 Despite the good expectations offered by recent therapy for hepatitis C virus infection and vaccination programs for hepatitis B, other causes are not predictably going to decrease. In fact, the average world alcohol consumption has increased since 2005 and this tendency is expected to continue through 2025. 2 Likewise, the estimated prevalence of non‐alcoholic fatty liver disease (NAFLD) is 25% worldwide and recent studies suggest that NAFLD is increasing significantly, along with obesity and DM. 3 , 4 , 5
The development of hepatic fibrosis is a complex process associated with multiple liver conditions. Most patients with chronic liver diseases at mild, moderate or even advanced stages of liver fibrosis remain asymptomatic for a long period. Therefore, liver cirrhosis is frequently diagnosed once hepatic decompensation occurs. At this point, the prognosis is already poor. 6 , 7 For this reason, early diagnosis is critical. Liver biopsy has been traditionally considered the gold standard for diagnosing liver fibrosis. However, it is an invasive technique that may have serious complications, as well as other drawbacks such as sampling error and inter‐ and intra‐observer variability. This is why non‐invasive methods such as serological tests or elastographic assessments have been developed in recent years. Transient elastography (TE) (Fibroscan®) has been widely validated for assessing liver stiffness for different conditions. 8 , 9 , 10 Liver stiffness has been correlated with the different stages of hepatic fibrosis and shows excellent diagnostic accuracy for cirrhosis. 11 , 12 A few studies have proposed using TE to screen for liver disease in some groups of the general population. 13 , 14 , 15 , 16 , 17 However, it is important to determine the population group that would benefit the most from this technique.
Therefore, the aim of our study was (a) to evaluate the usefulness of TE to predict significant fibrosis and cACLD in a large Spanish cohort, and (b) to determine risk factors associated with the presence of significant fibrosis and cACLD.
PATIENTS AND METHODS
This is a nested study within the PREVHEP‐ETHON Cohort, observational, cross‐sectional, population‐based study performed in Spain between July 2015 and April 2017 (NCT02749864). 18 We selected subjects between 20 and 79 years of age from the population of 18 primary care centers belonging to three university hospitals in Madrid, Santander, and Valencia. The total eligible population was 332,127 subjects. Participants were selected using two‐stage conglomerate sampling and stratified by age, with randomized subject selection. Those who accepted to participate underwent TE in one of the three reference hospitals. On the same day the subjects filled out an epidemiological questionnaire, had a fasting blood sample collected and received a physical examination. The following data were collected in the epidemiological questionnaire: age, sex and alcohol intake through the Alcohol Use Disorders Identification Test (AUDIT) (harmful use of alcohol ≥8). 19 , 20 Blood tests included the determination of ALT, AST, GGT, bilirubin, albumin, glucose, triglycerides, HDL, LDL and total cholesterol, platelets, HCV antibodies, and HBsAg. AST or ALT beyond 40 IU/L were considered abnormal transaminase levels (ALT above 30 IU/L in males and 19 IU/L in females for healthy transaminases). 21 GGT above 36 IU/L was considered abnormal. Metabolic syndrome was defined according to NCEP ATP III criteria 22 and metabolic phenotype was defined as described previously: metabolically healthy non‐obese (MHNO), metabolically healthy obese (MHO), metabolically unhealthy non‐obese (MUNO), metabolically unhealthy obese (MUO). 23 Also, a metabolically healthy status was defined as the lack of metabolic risk factors (diabetes mellitus, low HDL, hypertriglyceridemia, arterial hypertension). 23 Non‐invasive serological indices for liver fibrosis (FIB‐4 [age, ALT, AST, and platelets]) and APRI score (AST/platelet ratio index) were calculated. Physical evaluation included systolic and diastolic blood pressure, height, weight, BMI (obese if BMI ≥ 30 kg/m2) and waist circumference. TE measurements using Fibroscan® (Echosens®, Paris, France) were expressed in kilopascals (kPa). Only valid measurements were considered (median measurement/interquartile range <0.3). The XL probe was used when necessary. All measurements were made after overnight fasting. According to previous studies, for significant fibrosis we considered a cutoff value of 8 kPa, and for cACLD we considered cutoff values of 10 kPa (suggestive) and 15 kPa (highly suggestive). 24 , 25
This study was carried out in accordance with the declaration of Helsinki (2008), after approval by the Ethics Committee of each participating center. All the participants accepted and gave written informed consent.
Statistical analysis
Continuous variables were described as mean and standard deviation. Differences between means were analyzed using Student's t‐test. Categorical variables were described as numbers and percentages, and 95% CIs were given where necessary. Differences between proportions were analyzed using the χ 2 test or Fisher's exact test where appropriate. Multivariate logistic regression analysis was conducted to identify risk factors associated with significant fibrosis and cirrhosis. Non‐collinear variables with p < 0.05 were included in the multivariate analysis. Receiver operating characteristic curves (ROC) were performed to evaluate the accuracy of non‐invasive serological tests for diagnosing significant fibrosis. All statistical analyses were conducted using the statistics program R (R 4.0.0 GUI 1.71 Catalina build) and Stata/IC 16.1.
RESULTS
Between July 2015 and April 2017 12,246 subjects agreed to participate in the study, from which no valid results were obtained in 806 (6.6%) cases. Finally, a total of 11,440 subjects were included (Figure 1). Baseline subject characteristics are shown in Table 1. The distribution of TE values is shown in Figure S1.
FIGURE 1.
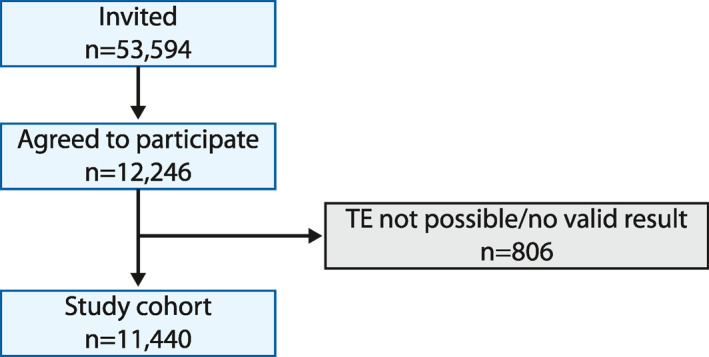
Flow‐chart of the study
TABLE 1.
Baseline subject characteristics
| Characteristics | n = 11,440 |
|---|---|
| Age, years (SD) (range) | 50.3 (12.5) (18–79) |
| Sex, M:F (%) | 4792:6648 (41.9:58.1) |
| BMI, kg/m2 (SD) (range) | 26.7 (4.8) (16–57) |
| Obese (BMI > 30 kg/m2), n (%) | 2075 (21.8) |
| Diabetes mellitus, n (%) | 1540 (13.5) |
| High blood pressure, n (%) | 5206 (53.6%) |
| Waist circumference, cm (SD) (range) | 90.4 (13.5) (55–178) |
| Systolic blood pressure, mm Hg (SD) (range) | 130.2 (16.8) (130–17) |
| Diastolic blood pressure, mm Hg (SD) (range) | 77.8 (9.8) (40–127) |
| TE, kPa (SD) | 5.2 (4.1) |
| TE, KPa (median) (range) | 4.5 (2–72) |
| ALT, IU/L (SD) (range) | 24.4 (17.4) (1–459) |
| AST, IU/L (SD) (range) | 24.4 (12.7) (2–402) |
| GGT, IU/L (SD) (range) | 31.7 (51.2) (19–2045) |
| Alkaline phosphatase, IU/L (SD) (range) | 71.9 (28.7) (2–790) |
| Bilirubin, mg/dl (SD) (range) | 1.4 (17.6) (0.03–8) |
| Abnormal transaminases (AST or ALT > 40 IU/L), n (%) | 1113 (9.7) |
| Abnormal healthy transaminase levels, n (%) | 4061 (35.5) |
| Platelets, x103/ml (SD) (range) | 246.5 (62.0) (21–725) |
| Albumin, g/dl (SD) (range) | 4.5 (0.3) (2.7–5.6) |
| HDL cholesterol, mg/dl (SD) (range) | 58.8 (16.1) (8–116) |
| Triglycerides, mg/dl (SD) (range) | 141.2 (98.4) (20–400) |
| Glucose, mg/dl (SD) (range) | 91.6 (29.1) (42–344) |
| Metabolic syndrome ç(NCEP‐ATP‐III), n (%) | 1765 (15.4) |
| Harmful use of alcohol, n (%) | 1049 (9.2) |
| Anti‐HCV Ab+, n (%) | 143 (1.3) |
| HBsAg+, n (%) | 90 (0.8) |
| FIB‐4 (SD) | 1.1 (0.7) |
| FIB‐4 ≥ 3.24, n (%) | 106 (0.9) |
| APRI (SD) | 0.3 (0.2) |
| APRI ≥ 1, n (%) | 79 (0.7) |
Note: Quantitative data are expressed as mean (standard deviation). Qualitative data are expressed as number and percentage. Healthy transaminase levels were defined as ALT > 30 IU/L for men and ALT > 19 IU/L for women.
Abbreviations: Anti‐HCV Ab+, positive for anti‐hepatitis C virus antibodies; APRI, ALT to Platelet Ratio Index; FIB‐4, Fibrosis‐4 score; HBsAg+, positive for hepatitis B surface antigen.
Prevalence of significant fibrosis and cACLD in the global cohort
Significant fibrosis was observed in 5.6% (95% CI, 5.2–6.0) and cACLD was suggestive and highly suggestive in 2.9% (95% CI, 2.6–3.2) and 1.2% (95% CI, 1.0–1.5) of the subjects respectively. Overall, risk factors independently associated with significant fibrosis were age, sex, AST, GGT, and metabolic syndrome. Risk factors associated with suggestive cACLD were age, AST, GGT, low platelets and metabolic syndrome and risk factors associated with highly suggestive cACLD were age, AST, GGT, low platelets, metabolic syndrome, and harmful alcohol consumption (Tables 2, 3, 4). As age was one of the main factors related to the presence of fibrosis and cACLD, we compared their according age > 40 (significant fibrosis, suggestive and highly suggestive cACLD in patients over 40: 6.3%, 3.4%, 1.5% resp.; p < 0.001, and below 40: 3.2%, 1%, and 0.3%; p < 0.001). On the other hand, we calculated risk for significant fibrosis, suggestive and highly suggestive cACLD related to the presence of DM (2.1 [95% CI 1.7–2.7], p < 0.001; 2.1 [95% CI 1.6–2.8], p < 0.001; and 2.0 [95% CI 1.3–3.2], p < 0.001, resp.) and to the presence of obesity (2.8 [95% CI 2.4–3.4], p < 0.001; 3.1 [95% CI 2.4–3.9], p < 0.001; and 2.5 [95% CI 1.7–3.6], p < 0.001, resp.).
TABLE 2.
Univariate and multivariate analysis
| Significant fibrosis (n = 641) | Absence of fibrosis (10,799) | p | Multivariate analysis | |
|---|---|---|---|---|
| Age (y) | 55.0 (10.9) | 50.1 (12.0) | <0.001 | OR 1.03 (1.02–1.04; p < 0.001) |
| Gender (M:F) | 347:294 (54.1:45.9) | 4445:6354 (41.2:58.8) | <0.001 | OR 0.8 (0.6–0.95; p = 0.02) |
| BMI (kg/m2) | 29.4 (6.1) | 26.6 (4.7) | <0.001 | |
| Waist circumference (cm) | 99.4 (16.3) | 90.0 (13.2) | <0.001 | |
| Systolic/diastolic blood pressure (mmHg) | 136.1 (17.6)/79.2 (10.2) | 129.9 (16.7)/77.7(9.8) | <0.001 | |
| Liver test | ||||
| ALT (IU/L) | 34.7 (30.3) | 23.8 (16.1) | <0.001 | OR 1.01 (1.01–1.02; p < 0.001)OR 1.005 (1.003–1.006; p < 0.001) |
| AST (IU/L) | 32.2 (22.6) | 24.0 (11.7) | <0.001 | |
| GGT (IU/L) | 63.4 (95.8) | 29.7 (46.6) | <0.001 | |
| Alkaline phosphatase (IU/L) | 79.2 (36.6) | 71.5 (28.1) | <0.001 | |
| Bilirubin (mg/dl) | 1.2 (15.1) | 1.4 (17.7) | 0.8 | |
| Abnormal transaminases (AST or ALT > 40 IU/L) | 172 (26.8) | 941 (8.7) | <0.001 | |
| Healthy transaminase cutoffs (ALT <30 male or <19 female) | 416 (64.9) | 5550 (51.4) | <0.001 | |
| Albumin (g/dl) | 4.5 (0.3) | 4.5 (0.3) | 0.5 | |
| Glucose (mg/dl) | 102.0 (47.6) | 91.0 (27.5) | <0.001 | |
| Platelets (x103) | 237.0 (77.6) | 247.1 (61.0) | <0.001 | |
| HDL cholesterol (mg/dl) | 54.6 (16.3) | 59.1 (16.1) | <0.001 | |
| Triglycerides (mg/dl) | 168.3 (108.5) | 139.6 (97.5) | <0.001 | |
| Metabolic syndrome (NCEP ATP III) | 171 (38.2) | 1594 (18.7) | <0.001 | OR 2.1 (1.7–2.6; p < 0.001) |
| HCV Ab+ | 12 (1.9) | 131 (1.2) | 0.1 | |
| HBsAg+ | 11 (1.7) | 79 (0.7) | 0.01 | |
| Harmful use of alcohol | 85 (13.3) | 964 (8.9) | <0.001 | |
Note: Significant fibrosis (LSM > 8 kPa) versus absence of fibrosis. Quantitative data are expressed as mean (standard deviation). Qualitative data are expressed as number and percentage. Variables included in the multivariate analysis: age, gender, ALT, AST, GGT, alkaline phosphatase, platelets, metabolic syndrome, HBsAg+, alcohol consumption.
TABLE 3.
Univariate and multivariate analysis
| Suggestive cACLD (n = 328) | Absence of cACLD (n = 11112) | p | Multivariate analysis | |
|---|---|---|---|---|
| Age (y) | 56.5 (10.6) | 50.2 (12.5) | <0.001 | OR 1.04 (1.02–1.05; p < 0.001) |
| Gender (M:F) | 176:152 (53.7:46.6) | 4616:6496 (41.5:58.5) | <0.001 | |
| BMI (kg/m2) | 29.7 (6.2) | 26.7 (4.7) | <0.001 | |
| Waist circumference (cm) | 99.4 (16.3) | 90.0 (13.2) | <0.001 | |
| Systolic/diastolic blood pressure (mmHg) | 136.8 (17.4)/80.2 (9.9) | 130.0 (16.8)/77.7 (9.8) | 0.02 | |
| Liver test | ||||
| ALT (IU/L) | 34.7 (30.3) | 23.8 (16.1) | <0.001 | OR 1.01 (1.01–1.02; p < 0.001) OR 1.006 (1.004–1.008; p < 0.001) |
| AST (IU/L) | 35.7 (26.4) | 24.1 (11.9) | <0.001 | |
| GGT (IU/L) | 85.6 (124.1) | 30.0 (46.4) | <0.001 | |
| Alkaline phosphatase (IU/L) | 87.6 (45.3) | 71.4 (27.9) | <0.001 | |
| Bilirubin (mg/dl) | 1.8 (21.0) | 1.4 (17.5) | 0.7 | |
| Abnormal transaminases (AST or ALT > 40 IU/L) | 110 (33.5) | 1003 (9.0) | <0.001 | |
| Healthy transaminase cutoffs (ALT < 30 male or < 19 female) | 229 (69.8) | 5737 (51.6) | <0.001 | |
| Albumin (g/dl) | 4.4 (0.3) | 4.5 (0.3) | 0.04 | |
| Glucose (mg/dl) | 101.0 (39.3) | 91.3 (28.7) | <0.001 | |
| Platelets (x103) | 228.9 (82.6) | 247.1 (61.2) | <0.001 | OR 0.997 (0.994–0.999; p = 0.02) |
| HDL cholesterol (mg/dl) | 54.3 (16.5) | 59 (16.1) | <0.001 | |
| Triglycerides (mg/dl) | 172.7 (107.3) | 140.2 (98.0) | <0.001 | |
| Metabolic syndrome (NCEP ATP III) | 94 (28.7) | 1671 (15.03) | <0.001 | OR 2.2 (1.6–2.9; p < 0.001) |
| HCV Ab+ | 3 (0.9) | 140 (1.3) | 0.6 | |
| HBsAg+ | 5 (1.5) | 85 (0.8) | 0.1 | |
| Harmful use of alcohol | 45 (13.7) | 1004 (9) | 0.004 | |
Note: Suggestive cACLD (LSM ≥ 10 kPa) versus absence of cACLD. Quantitative data are expressed as mean (standard deviation). Qualitative data are expressed as number and percentage. Variables included in the multivariate analysis: age, gender, ALT, AST, GGT, alkaline phosphatase, platelets, metabolic syndrome, alcohol consumption.
TABLE 4.
Univariate and multivariate analysis
| Highly suggestive cACLD (n = 141) | Absence of cACLD (n = 11299) | p | Multivariate analysis | |
|---|---|---|---|---|
| Age (Y) | 58 (9.6) | 50.3 (12.5) | <0.001 | OR 1.04 (1.02–1.06; p = 0.001) |
| Gender (M:F) | 113:28 (80.1:19.9) | 4692:6561 (41.7:58.3) | 0.002 | |
| BMI (kg/m2) | 29.1 (5.7) | 26.7 (4.7) | <0.001 | |
| Waist circumference (cm) | 100.5 (16.6) | 90.3 (13.5) | <0.001 | |
| Systolic/diastolic blood pressure (mmHg) | 137.0 (16.6)/80.5 (10) | 130.1 (16.8)/77.8 (9.8) | <0.001 | |
| Liver test | ||||
| ALT (IU/L) | 39.8 (37.9) | 24.2 (16.9) | <0.001 | OR 1.02 (1.01–1.03; p < 0.001) OR 1.005 (1.003–1.007; p < 0.001) |
| AST (IU/L) | 39.9 (30.9) | 24.2 (12.2) | <0.001 | |
| GGT (IU/L) | 102.0 (127.1) | 30.7 (48.8) | <0.001 | |
| Alkaline phosphatase (IU/L) | 87.3 (38.3) | 71.7 (28.5) | <0.001 | |
| Bilirubin (mg/dl) | 1.5 (0.6) | 1.4 (17.7) | 0.7 | |
| Abnormal transaminases (AST or ALT > 40 IU/L) | 54 (38.3) | 1059 (9.4) | <0.001 | |
| Healthy transaminase cutoffs (ALT <30 male or <19 female) | 103 (73) | 5863 (51.9) | <0.001 | |
| Albumin (g/dl) | 4.4 (0.4) | 4.5 (0.3) | 0.01 | |
| Glucose (mg/dl) | 103.8 (47.3) | 91.4 (28.8) | <0.001 | |
| Platelets (x103) | 219.7 (83.4) | 247 (61.6) | <0.001 | OR 0.993 (0.989–0.997; p < 0.001) |
| HDL cholesterol (mg/dl) | 57.4 (17.5) | 58.8 (16.1) | <0.001 | |
| Triglycerides (mg/dl) | 169.8 (113.2) | 140.8 (98.1) | <0.001 | |
| Metabolic syndrome (NCEP ATP III) | 36 (25.5) | 1729 (15.3) | <0.001 | OR 2.1 (1.4–3.3; p = 0.001) |
| HCV Ab+ | 2 (1.4) | 141 (1.2) | 0.9 | |
| HBsAg+ | 0 (0) | 90 (0.8) | 0.3 | |
| Harmful use of alcohol | 28 (19.9) | 1021 (9.0) | <0.001 | OR 1.8 (1.05–3.1; p = 0.03) |
Note: Highly suggestive cACLD (LSM > 15 kPa) vs. absence of cACLD. Quantitative data are expressed as mean (standard deviation). Qualitative data are expressed as number and percentage. Variables included in the multivariate analysis: age, gender, ALT, AST, GGT, alkaline phosphatase, platelets, metabolic syndrome, alcohol consumption.
Prevalence of significant fibrosis and cACLD according to harmful alcohol consumption
1049 (9.2%) subjects showed harmful alcohol consumption. To perform this analysis, we excluded concomitant anti‐HCV antibodies or HBsAg (resulting n = 1023). In subjects with harmful alcohol consumption the prevalence of significant fibrosis was 7.7% (95% CI, 6.2–9.5) and the prevalence of suggestive and highly suggestive cACLD was 4.3% (95% CI, 3.2–5.7) and 2.7% (95% CI, 1.9–3.9), respectively. Risk factors associated with significant fibrosis in this subset of patients were age, AST levels, and metabolic syndrome; the factor associated with suggestive cACLD was AST levels; and risk factors associated with highly suggestive cACLD were AST levels and metabolic syndrome (Tables S1–S3).
Prevalence of significant fibrosis and cACLD according to metabolic phenotype
Given that one of the main factors associated with significant fibrosis and cACLD was metabolic syndrome, we refined our analysis by considering metabolic phenotype. 22 To this end, we excluded subjects with positive anti‐HCV antibodies, positive HBsAg or harmful use of alcohol (resulting n = 10,190). Obese subjects (n = 1825, 21.5%) showed a higher prevalence of significant fibrosis (9.4% vs. 3.7%; p < 0.001), suggestive cACLD (5.3% vs. 1.8%; p < 0.001) and highly suggestive cACLD (1.7% vs. 0.9%; p = 0.003). Regarding metabolic risk factors, 30.7%, 38.5%, 21%, 8.1%, and 1.7% of the subjects showed none, one, two, three or four, respectively. The prevalence of significant fibrosis and suggestive and highly suggestive cACLD increased significantly with the number of risk factors (Figure 2a). Combining these data, we found that the prevalence of significant fibrosis, suggestive and highly suggestive cACLD was highest within metabolically unhealthy obese subjects (Figure 2b).
FIGURE 2.
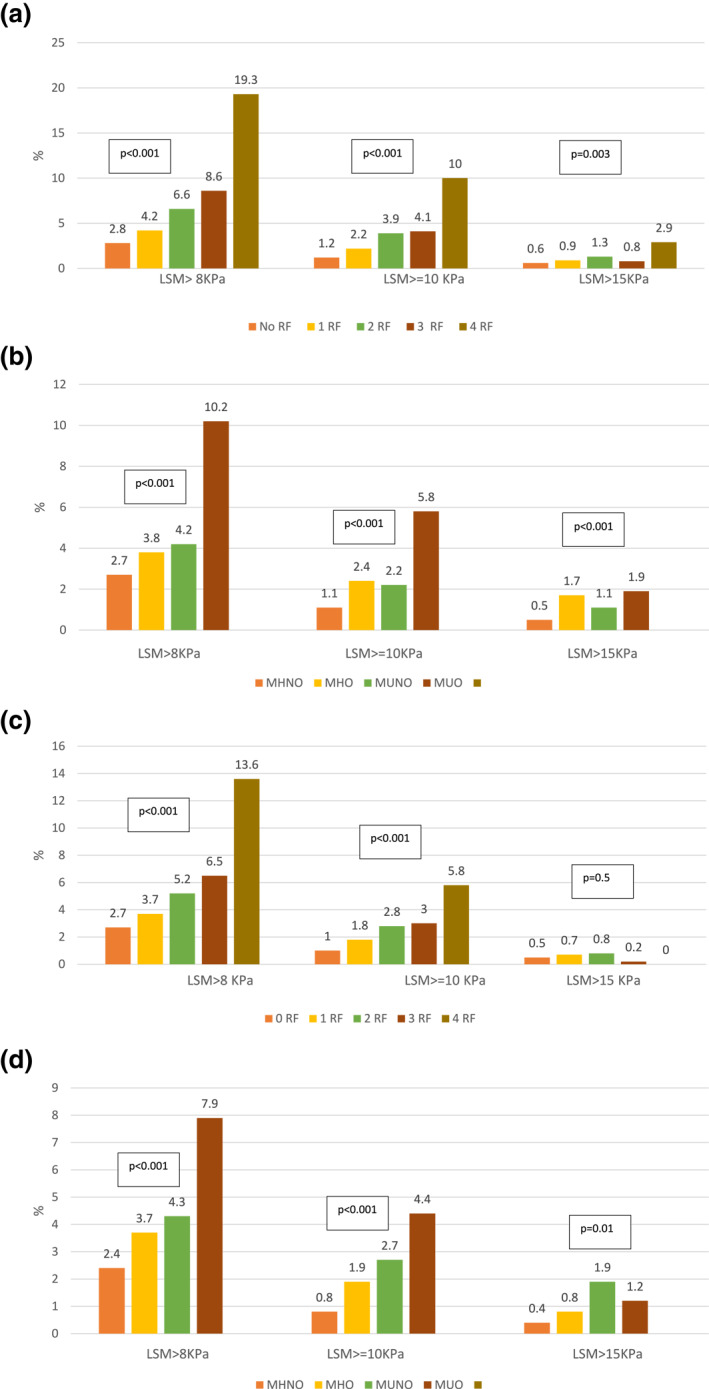
(a) Percentage of significant fibrosis, suggestive compensated advanced chronic liver disease (cACLD) and highly suggestive cACLD considering the number of metabolic risk factors in subjects without hepatitis B virus or hepatitis C virus infection, nor harmful use of alcohol (n = 10,190). (b) Percentage of significant fibrosis, suggestive cACLD, and highly suggestive cACLD considering metabolic phenotype in subjects without hepatitis B virus or hepatitis C virus infection, nor harmful use of alcohol (n = 10,190). (c) Percentage of significant fibrosis, suggestive cACLD and highly suggestive cACLD in subjects with normal transaminases considering the number of metabolic risk factors in subjects without hepatitis B virus or hepatitis C virus infection, nor harmful use of alcohol (n = 8861). (d) Percentage of significant fibrosis, suggestive cACLD and highly suggestive cACLD in subjects with normal transaminases considering metabolic phenotype in subjects without hepatitis B virus or hepatitis C virus infection, nor harmful use of alcohol (n = 8861) [Correction added on June 17th, 2021, after first Online publication: Figure 2 has been updated]
Prevalence of significant fibrosis and cACLD excluding HCV and HBV
To perform this analysis, we excluded patients with positive anti‐HCV antibodies and positive HBsAg (resulting n = 11,213). The prevalence of significant fibrosis in this subgroup was 5.5 (95% CI, 5.1–5.9) and the prevalence of suggestive and highly suggestive cACLD was 2.9 (95% CI, 2.6–3.2) and 1.2 (95% CI, 1.1–1.5). Risk factors associated with the presence of significant fibrosis were age, male sex, AST or GGT levels and metabolic syndrome. Risk factors associated with suggestive cACLD were age, AST or GGT levels, low platelets and metabolic syndrome and risk factors associated with highly suggestive cACLD were age, AST or GGT levels, low platelets, metabolic syndrome, and harmful alcohol consumption (Tables S4–S6).
Prevalence of significant fibrosis and cirrhosis in subjects with normal and healthy transaminases
We observed that a non‐negligible percentage of subjects with normal transaminases (n = 9877, 86.3%) presented significant fibrosis (4.6%, 95% CI 2.4–3.0) and suggestive and highly suggestive cACLD (2.1%, 95% CI 1.9–2.5 and 1%, 95% CI 0.7–1.1, resp.). In fact, only 27.7% of subjects with significant fibrosis, 34.2% of subjects with suggestive cACLD and 38.8% with highly suggestive cACLD showed abnormal transaminase levels. To enhance the sensitivity of our analysis, we focused on subjects with healthy transaminase levels (n = 7379, 64.5%). The prevalence of significant fibrosis, suggestive cACLD and highly suggestive cACLD in this group was 4.1% (95% CI, 3.6–4.7), 1.8% (95% CI, 1.5–2.2), and 0.7% (95% CI, 0.5–1), respectively.
We also analyzed the metabolic phenotype in this subgroup. The same factors previously excluded from the overall cohort (subjects with positive anti‐HCV antibodies, positive HBsAg or harmful use of alcohol) were also excluded for this analysis (resulting n = 8861). The prevalence of significant fibrosis and suggestive cACLD were greater for metabolically unhealthy obese subjects with normal transaminases. The prevalence of highly suggestive cACLD was significantly higher in metabolically unhealthy (Figure 2 c,d).
Serological test (FIB‐4 and APRI) to predict significant fibrosis and cACLD
To carry out this analysis, we excluded viral etiologies and harmful alcohol use (resulting n = 10,190). Mean FIB‐4 and APRI score were 1.1 (95% CI 1.1–1.2) and 0.27 (95% CI 0.26–0.27), respectively. APRI and FIB‐4 were greater in those who had significant fibrosis (APRI: 0.4 [0.37 vs. 0.45 vs. 0.26] [95% CI 0.25–0.26] [p < 0.001]; FIB‐4: 1.6 [95% CI 1.5–1.7] vs. 1.1 [95% CI 1.10–1.12]; p < 0.001), suggestive cACLD (APRI: 0.5 [95% CI 0.4–0.6] vs. 0.26 [95% CI 0.25–0.26] [p < 0.001]; FIB‐4: 1.8 [95% CI 1.6–2.0] vs. 1.12 [95% CI 1.10–1.13]; p < 0.001), and highly suggestive cACLD (APRI: 0.6 [95% CI 0.5–0.7] vs. 0.26 [95% CI 0.26–0.27] [p < 0.001]; FIB‐4: 2.2 [95% CI 1.9–2.6] vs. 1.12 [96% CI 1.10–1.14]; p < 0.001). We created receiving operating characteristic curves (ROC) to determine the accuracy of these scores to predict significant fibrosis and cACLD (Figure S2).
We created an algorithm using FIB‐4 as previously described. 24 Subjects with FIB‐4 ≥ 1.3 had a significantly higher proportion of significant fibrosis (p < 0.001) (Figure 3). With the application of this algorithm, 2.9% patients with significant fibrosis were not diagnosed. However, its use would have avoided performing TE in 68% of the subjects.
FIGURE 3.
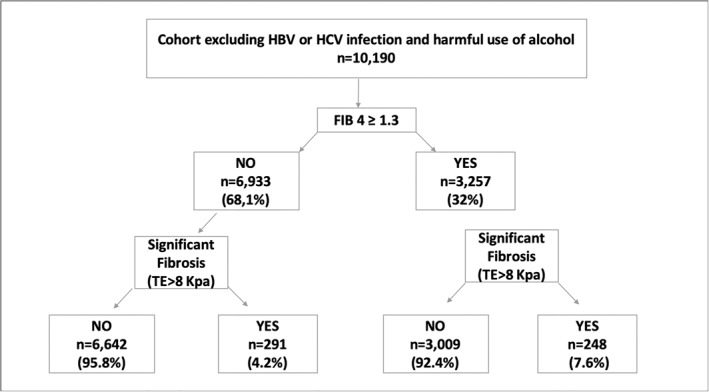
Application of the algorithm in our cohort using FIB‐4 ≥ 1.3
Histological data
By protocol, liver biopsy was not performed. However, all those patients with significant fibrosis in TE were referred to outpatient consultations for evaluation and were treated according to standard clinical practice. In the case of patients with positive serologies for HBV and HCV, treatment was considered if appropriate. In the case of harmful alcohol consumption, suspension of the alcohol habit was recommended and the patient was reevaluated at 6 months. None of these patients were biopsied. In the case of patients with suspected NAFLD, hygienic‐dietary measures were recommended for weight loss and TE was repeated at 6 months. If TE remained altered (ET > 8 KPa) and serological tests were also suggestive of advanced fibrosis, liver biopsy was recommended. We have histological data from 170 out of 539 subjects with significant fibrosis excluding those with viral hepatitis or harmful alcohol use. Liver fibrosis distribution in histological specimens was: F0‐1: 93 (54.7%), F2: 35 (20.7%), F3: 29 (17%), and F4: 13 (7.6%). NAFLD as the cause was confirmed in 146 (86.7%) subjects.
DISCUSSION
In our study, we found a relatively high prevalence of TE‐estimated significant fibrosis and suggestive and highly suggestive cACLD in a large Spanish cohort, up to 5.6%, 2.9%, and 1.2% respectively. These results confirm the findings of previous studies, which included fewer subjects and were performed in smaller areas, thus being more limited. In a study by Roulot et al. 15 including 1190 individuals older than 45 years with no previous liver disease who attended a primary care center for routine check‐up, 7.5% had liver stiffness greater than 8 kPa and 0.7% had liver stiffness greater than 13 kPa. Similar findings were reported in a population‐based study by Koheler et al. 16 which included 3041 subjects older than 45 years, of which 5.6% showed liver stiffness above 8 kPa and a 0.6% above 13 kPa. More recently, in a population‐based study including 3076 subjects, Caballeria et al. 14 reported a prevalence of increased liver stiffness of 9.0%, 5.8%, and 3.6% for ≥6.8, ≥8.0, and ≥ 9.0 kPa, respectively. The prevalence of increased liver stiffness seems higher when the studies are carried out in cohorts with known risk factors for liver fibrosis. In a study of 1918 patients with type 2 diabetes, the prevalence of increased liver stiffness (9.6 kPa) was 18%. 17 In another study on 378 UK patients with either type 2 diabetes or high alcohol consumption and abnormal ALT, 26 27% had liver stiffness greater than 8 kPa. These findings were also confirmed in our study given our considerable sample size, which allowed for extensive sub‐analysis.
One of the most important factors predicting the presence of fibrosis or cirrhosis was age. In this regard, the Rotterdam study, 16 conducted in an adult population ≥45 years, demonstrated that older age was associated with greater liver stiffness. In fact, when we analyzed the presence of cACLD in people below 40 it was very low (suggestive: 1% and highly suggestive: 0.3%). On the other hand, significant fibrosis seems to be significantly influenced by gender, with higher values found in men than in women, confirming previous results and suggesting that gender has to be taken into account when measuring liver stiffness. 27 , 28 However, this was not confirmed in the case of cACLD, possibly because there are other risk factors that have more weight than sex. Harmful use of alcohol was associated as an independent risk factor only with highly suggestive cACLD, perhaps because these subjects have higher liver stiffness values due to co‐existing inflammation. 27 In fact, the prevalence of significant fibrosis and cACLD was higher in this subgroup. We found metabolic syndrome to be another major risk factor. And, even when we analyzed the subgroup of subjects with harmful use of alcohol by itself, metabolic syndrome proved to be an important independent factor for significant fibrosis and cACLD, probably because metabolic syndrome plays a key role in the development of fibrosis as a co‐factor in these subjects. This points to NAFLD as an overlooked potential cause for liver fibrosis. In fact, we showed that obese patients had an increased prevalence of significant fibrosis and cACLD. In addition, the prevalence of significant fibrosis and cACLD increased with the number of metabolic risk factors. Recent work by Ampuero et al. 23 showed the importance of assessing the metabolic status of NAFLD patients, beyond obesity. Accordingly, when we analyzed metabolic phenotype, metabolically unhealthy and obese subjects showed a significantly higher prevalence of significant fibrosis and cACLD compared to metabolically healthy and non‐obese subjects. This was also confirmed in the group with normal transaminases, supporting the fact that metabolic status should alert us to the presence of significant fibrosis even in individuals with normal transaminases. Furthermore, we showed that GGT or AST elevations were independent risk factors of significant fibrosis and cACLD. This finding may also be mediated by metabolic syndrome or harmful alcohol use since previous studies have shown that AST or GGT elevations are associated with fibrosis in patients with NAFLD and harmful alcohol use. 29 , 30 , 31 , 32 , 33 Likewise, abnormal transaminase levels were independent risk factors for significant fibrosis and cACLD, which can be easily explained by the fact that liver inflammation is associated with higher TE values in the general population. 14 , 15 , 16 , 17 However, subjects with normal transaminases showed a considerable prevalence of significant fibrosis and cACLD.
We evaluated serological tests (FIB‐4 or APRI) to detect significant fibrosis and cACLD. These indicated moderate accuracy for detecting significant fibrosis, as shown previously. 34 We decided to apply to our cohort a recently proposed algorithm. 24 This algorithm would have avoided the performance of TE in 68% of the subjects, however the diagnosis was not reached in 2.6% of the subjects, suggesting that serological tests are still imperfect for the detection of significant fibrosis.
We could obtain histological data from 170 patients, of which 45.3% had ≥ F2 and 54.7% had F0‐1. TE overestimated the stage of fibrosis in a high percentage of cases suggesting that liver stiffness is still not accurate enough to detect significant fibrosis. However, biopsy allowed us to diagnose NAFLD in 86.7% of subjects.
A limitation of our study is that metabolic syndrome was probably underestimated due to information bias, since data were collected through a self‐reported questionnaire. Nevertheless, the results are unlikely to have been different had metabolic syndrome been better captured. On the other hand, our study was performed in a mainly Caucasian population, which limits its external validity.
In conclusion, a considerable percentage of apparently healthy population with no liver disease showed elastographic evidence of significant fibrosis and cACLD. TE may be a useful tool for screening for liver disease in the general population in subjects with risk factors, even with normal liver function test results. Yet, the presence of large population strata at risk for significant fibrosis and cACLD remains a concern, and more studies are needed regarding how to screen these subjects in a cost‐efficient manner.
CONFLICT OF INTEREST
Miguel Ángel Serra reports grant support and/or consultancy and lecture fees from AbbVie, Gilead Sciences, Bristol‐Myers Squibb, Janssen, and MSD. Javier Crespo reports grant support and/or consultancy and lecture fees from AbbVie, Gilead Sciences, Bristol‐Myers Squibb, Janssen, and MSD. José Luis Calleja reports consultancy and lecture fees from Abbvie, Gilead Sciences, MSD. The other authors have no conflicts of interest to declare.
ETHICS STATEMENT
The study protocol was approved in the ethical committee of each participating center. Participating subjects had to give written informed consent.
AUTHOR CONTRIBUTIONS
Study concept and design: Javier Crespo, José Luis Calleja. Data acquisition: Elba Llop, Paula Iruzubieta, Christie Perelló, Joaquín Cabezas, María Desamparados Escudero, Marta González, Marta Hernández Conde, Laura Puchades, María Teresa Arias‐Loste, Miguel Ángel Serra. Data analysis and interpretation: Elba Llop, Paula Iruzubieta, Javier Crespo, José Luis Calleja. Drafting of the manuscript: Elba Llop, Paula Iruzubieta, Carlos Fernández Carrillo. Critical revision of the manuscript for important intellectual content: All the authors. Statistical analysis: Elba Llop, Paula Iruzubieta. Obtained funding: Javier Crespo, José Luis Calleja.
Supporting information
Supplementary Material S1
ACKNOWLEDGMENTS
This research was partially supported by a grant from the Spanish Government (Integrated Projects of Excellence Call; PIE15/00079 and PI15/02138). This study was funded by MSD under the Investigator Studies Program (code: MISPIIS#52863).
Llop E, Iruzubieta P, Perelló C, Fernández Carrillo C, Cabezas J, Escudero MD, et al. High liver stiffness values by transient elastography related to metabolic syndrome and harmful alcohol use in a large Spanish cohort. United European Gastroenterol J. 2021;9(8):892–902. 10.1002/ueg2.12109
Elba Llop and Paula Iruzubieta contributed equally as first co‐authors.
Javier Crespo and José Luis Calleja contributed equally as senior co‐authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Byass P. The global burden of liver disease: a challenge for methods and for public health. BMC Med. 2014;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global status report on alcohol and health 2014. World Health Organization. 2014:1–392. https://www.who.int/substance_abuse/activities/gsrah/en/ [Google Scholar]
- 3. Araújo AR, Rosso N, Bedogni G, Tiribelli C, Bellentani S. Global epidemiology of non‐alcoholic fatty liver disease/non‐alcoholic steatohepatitis: what we need in the future. Liver Int. 2018;38 (Suppl 1):47–51. [DOI] [PubMed] [Google Scholar]
- 4. Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Harper AM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant. 2018;18 (Suppl 1):172–253. [DOI] [PubMed] [Google Scholar]
- 5. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–85. [DOI] [PubMed] [Google Scholar]
- 6. Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20(23):7312–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, et al. The epidemiology of cirrhosis in the United States: a population‐based study. J Clin Gastroenterol. 2015;49(8):690–6. [DOI] [PubMed] [Google Scholar]
- 8. Cardoso AC, Carvalho‐Filho RJ, Stern C, Dipumpo A, Giuily N, Ripault M‐P, et al. Direct comparison of diagnostic performance of TE in patients with chronic hepatitis B and chronic hepatitis C. Liver Int. 2012;32(4):612–21. [DOI] [PubMed] [Google Scholar]
- 9. Ziol M, Handra‐Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41(1):48–54. [DOI] [PubMed] [Google Scholar]
- 10. Jiang W, Huang S, Teng H, Wang P, Wu M, Zhou X, et al. Diagnostic accuracy of point shear wave elastography and transient elastography for staging hepatic fibrosis in patients with non‐alcoholic fatty liver disease: a meta‐analysis. BMJ Open. 2018;8(8):e021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cai C, Song X, Chen X, Zhou W, Jin Q, Chen S, et al. Transient elastography in alcoholic liver disease and nonalcoholic fatty liver disease: a systemic review and meta‐analysis. Chin J Gastroenterol Hepatol. 2021;2021:8859338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friedrich‐Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, et al. Performance of TE for the staging of liver fibrosis: a meta‐analysis. Gastroenterology. 2008;134(4):960–74. [DOI] [PubMed] [Google Scholar]
- 13. Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta‐analysis of diagnostic accuracy. J Hepatol. 2011;54(4):650–9. [DOI] [PubMed] [Google Scholar]
- 14. Caballería L, Pera G, Arteaga I, Rodríguez L, Alumà A, Morillas RM, et al. High prevalence of liver fibrosis among European adults with unknown liver disease: a population‐based study. Clin Gastroenterol Hepatol. 2018;16(7):1138–45. [DOI] [PubMed] [Google Scholar]
- 15. Roulot D, Costes JL, Buyck JF, Warzocha U, Gambier N, Czernichow S, et al. TE as a screening tool for liver fibrosis and cirrhosis in a community‐based population aged over 45 years. Gut. 2011;60(7):977–84. [DOI] [PubMed] [Google Scholar]
- 16. Koehler EM, Plompen EP, Schouten JN, Hansen BE, Darwish Murad S, Taimr P, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology. 2016;63(1):138–47. [DOI] [PubMed] [Google Scholar]
- 17. Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL‐Y, Luk AO‐Y, et al. Screening diabetic patients for non‐alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65(8):1359–68. [DOI] [PubMed] [Google Scholar]
- 18. Crespo J, Cuadrado A, Perelló C, Cabezas J, Llerena S, Llorca J, et al. Epidemiology of hepatitis C virus infection in a country with universal access to direct‐acting antiviral agents: data for designing a cost‐effective elimination policy in Spain. J Viral Hepat. 2020;27(4):360–70. [DOI] [PubMed] [Google Scholar]
- 19. Bradley KA, Boyd‐Wickizer J, Powell SH, Burman ML. Alcohol screening questionnaires in women: a critical review. JAMA. 1998;280(2):166–71. 10.1001/jama.280.2.166 [DOI] [PubMed] [Google Scholar]
- 20. Rubio Valladolid G, Bermejo Vicedo J, Caballero Sánchez‐Serrano MC, Santo‐Domingo Carrasco J. Validación de la prueba para la identificación de trastornos por uso de alcohol (AUDIT) en Atención Primaria (Validation of the Alcohol Use Disorders Identification Test (AUDIT) in primary care). Rev Clin Esp. 1998;198(1):11–4. [PubMed] [Google Scholar]
- 21. Prati D, Taioli E, Zanella A, Torre ED, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 22. National Cholesterol Education Program (NCEP) EXPERT Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 23. Ampuero J, Aller R, Gallego‐Durán R, Banales JM, Crespo J, García‐Monzón C, et al. HEPAmet Registry. The effects of metabolic status on non‐alcoholic fatty liver disease‐related outcomes, beyond the presence of obesity. Aliment Pharmacol Ther. 2018;48(11‐12):1260–70. [DOI] [PubMed] [Google Scholar]
- 24. Castera L, Friedrich‐Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–52. 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 26. Harman DJ, Ryder SD, James MW, Jelpke M, Ottey DS, Wilkes EA, et al. Direct targeting of risk factors significantly increases the detection of liver cirrhosis in primary care: a cross‐sectional diagnostic study utilising TE. BMJ Open. 2015;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corpechot C, El Naggar A, Poupon R. Gender and liver: is the liver stiffness weaker in weaker sex? Hepatology. 2006;44(2):513–4. [DOI] [PubMed] [Google Scholar]
- 28. Di Martino V, Lebray P, Myers RP, Pannier E, Paradis V, Charlotte F, et al. Progression of liver fibrosis in women infected with hepatitis C: long‐term benefit of estrogen exposure. Hepatology. 2004;40(6):1426–33. [DOI] [PubMed] [Google Scholar]
- 29. Mueller S, Millonig G, Sarovska L, Friedrich S, Reimann FM, Pritsch M, et al. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J Gastroenterol. 2010;16(8):966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roulot D, Roudot‐Thoraval F, NKontchou G, Kouacou N, Costes J‐L, Elourimi G, et al. Concomitant screening for liver fibrosis and steatosis in French type 2 diabetic patients using Fibroscan. Liver Int. 2017;37(12):1897–906. [DOI] [PubMed] [Google Scholar]
- 31. Labenz C, Huber Y, Kalliga E, Nagel M, Ruckes C, Straub BK, et al. Predictors of advanced fibrosis in non‐cirrhotic non‐alcoholic fatty liver disease in Germany. Aliment Pharmacol Ther. 2018;48(10):1109–16. 10.1111/apt.14976 [DOI] [PubMed] [Google Scholar]
- 32. Lupsor M, Badea R, Stefanescu H, Grigorescu M, Serban A, Radu C, et al. Performance of unidimensional TE in staging non‐alcoholic steatohepatitis. J Gastrointestin Liver Dis. 2010;19(1):53–60. [PubMed] [Google Scholar]
- 33. Kumar R, Rastogi A, Sharma MK, Bhatia V, Tyagi P, Sharma P, et al. Liver stiffness measurements in patients with different stages of nonalcoholic fatty liver disease: diagnostic performance and clinicopathological correlation. Dig Dis Sci. 2013;58(1):265–74. [DOI] [PubMed] [Google Scholar]
- 34. Salomone F, Micek A, Godos J. Simple scores of fibrosis and mortality in patients with NAFLD: a systematic review with meta‐analysis. J Clin Med. 2018;7(8):219. 10.3390/jcm7080219 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


