Abstract
Background
Graft‐versus‐host disease (GVHD), particularly acute digestive GVHD (aDGVHD), is a severe complication of allogeneic hematopoietic stem cell transplantation (allo‐HSCT). It is necessary to identify predictive factors of GVHD to adapt prophylactic treatment.
Objective
In this context, our pilot study aimed (i) to determine whether an early remodeling of the colonic mucosa occurred after allo‐HSCT and (ii) to identify potential predictive mucosal markers of aDGVHD after allo‐HSCT.
Methods
Between day 21 and day 28 after the allo‐HSCT, 19 allo‐HSCT patients were included and had a rectosigmoidoscopy with probe‐based confocal laser endomicroscopy (pCLE) recording and biopsies. Sixteen patients were included in the control group. Morphological (pCLE), functional (intestinal permeability), and inflammatory parameters (cytokine multiplex immunoassay) were assessed.
Results
Among allo‐HSCT patients, 11 patients developed GVHD, and 6 of them developed aDGVHD. Morphological and functional changes of the colonic mucosa occurred after allo‐HSCT. Indeed, the perimeter of colonic crypts was significantly increased in allo‐HSCT patients compared to controls as well as crypt lumen fluorescein leakage (53% vs. 9%), whereas crypts sphericity, roundness, Feret diameter, and mean vessel area were significantly decreased in allo‐HSCT patients compared to the control group. In addition, interleukin‐6 (IL‐6), IL‐33, and IL‐15 levels in the supernatants of 24 h explant cultures of colonic biopsies were significantly increased in allo‐HSCT patients compared to controls. Finally, there was no difference in pCLE parameters, intestinal permeability, and inflammatory cytokines between patients who developed aDGVHD and those who did not.
Conclusion
This pilot study identified early colonic mucosa remodeling after allo‐HSCT conditioning therapy, that is morphological and functional mucosal alterations as well as mucosal inflammation. As to whether these changes are first steps in GVHD initiation and could be considered as predictive biomarkers of aDGVHD need to be determined in a larger cohort of patients.
Keywords: allogeneic hematopoietic stem cell transplantation, confocal endomicroscopy, digestive barrier, endoscopic imaging, graft‐versus‐host disease, immunology, inflammation
INTRODUCTION
The main complication of allogeneic hematopoietic stem cell transplantation (allo‐HSCT) is graft‐versus‐host disease (GVHD), a potentially severe immunological disorder that affects several organ systems. Acute digestive GVHD (aDGVHD) occurs in 30%–60% of patients after allo‐HSCT. 1 , 2 , 3 , 4
Graft‐versus‐host disease prophylaxis relies on immunosuppressive therapies, which carry a significant risk of drug toxicity and infections and therefore has to be tailored to the estimated risk of GVHD, in order to spare low‐risk patients from the side effects of an undue prophylaxis and optimize the preventive treatment in high‐risk patients. In addition, GVHD curative treatment relies mainly on corticosteroids, which have a poor initial response rate of about 50% and a sustained response rate of about 30%. 3 , 5 Second‐line treatments do not achieve better success rates. It is therefore necessary to stratify the risk of GVHD in order to adapt patient management prior to GVHD onset. Risk factors before transplantation that are associated with an increased risk of GVHD have been identified. 3 , 6 , 7 However, algorithms based solely on clinical factors fail to completely predict GVHD, 8 , 9 and despite attempts to develop prognostic biomarkers, 10 no blood or morphological examination can currently predict the occurrence of aDGVHD after allo‐HSCT.
The intestinal mucosa has been identified as a key player in the pathophysiology of GVHD initiation and as such could be a source of prognostic markers and new therapeutic approaches. First, intestinal damages 11 , 12 have been shown to occur after the conditioning regimen, an intensive chemotherapy sometimes associated to radiation, which precedes allo‐HSCT. Murine models of GVHD have also exhibited impairment of intestinal barrier function, with an increased intestinal permeability and morphological changes through downregulation and localization shift of the tight junction protein occludin. 13 In addition, alterations of long myosin light chain kinase, a regulator of tight junction permeability, have been shown in human GVHD biopsies, while mice deficient in this enzyme had a limited propagation of GVHD, suggesting that the increase in permeability plays a role in GVHD progression. 14 In addition, alterations of other components of the intestinal barrier such as Paneth cells, goblet cells, and intestinal stem cells, also occur in the context of conditioning. 15 These digestive damages concur to enhance the passage of microbial products such as lipopolysaccharides through the lamina propria, resulting in antigen‐presenting cells activation. 16 During the following phase of T lymphocytes recruitment, the digestive mucosa is one of the sites of lymphocytes activation by antigen‐presenting cells and migration. 17 , 18 The tumor necrosing factor‐α (TNF‐α) plays a major role in the pathogenesis of GVHD, on one hand indirectly by promoting the differentiation and proliferation of donor T cells and on the other hand directly by inducing apoptosis in GVHD target tissues during the third phase of tissue destruction. 19 Other Th1 cytokines, such as interferon gamma (IFN‐γ), Interleukin‐1beta (IL‐1beta), and Th17 cytokines such as Interleukin‐17 (IL‐17) or Interleukin‐6 (IL‐6), take part to the cytokine storm described in digestive mucosa of patients with aDGVHD. 15 , 18
In this context, analysis of the digestive barrier before the onset of GVHD could be of interest to better understand mucosal remodeling and identify potential prognostic factors. Probe‐based confocal laser endomicroscopy (pCLE) is a high‐resolution imaging modality enabling in vivo histology at the subcellular level during ongoing endoscopy. pCLE has been able to detect early alterations of the colonic microarchitecture in aDGVHD; but to our knowledge, it has never been evaluated prior to GVHD onset. 20 , 21
The primary aim of our study was to determine whether an early remodeling of the colonic mucosa occurred after allo‐HSCT. The secondary aim was to identify morphological and functional predictive markers of aDGVHD.
METHODS
Study design and patients
This prospective controlled single‐center study resulted from a collaboration between the Digestive Disease Institute and the Hematology Unit of the University Hospital of Nantes. Consecutive adult patients, who underwent allo‐HSCT from June 2016 to August 2018 for hematologic malignancies were included. All patients had received a reduced‐intensity conditioning regimen. Exclusion criteria were history of previous allo‐HSCT, development of clinical GVHD before initial rectosigmoidoscopy, treatment by corticosteroids, thrombocytopenia<70 g/L or prothrombin ratio <50%, uncontrolled medical conditions, prior history of allergy to fluorescein, renal dysfunction, bowel obstruction, known inflammatory bowel disease, and a history of major abdominal surgery. All patients were enrolled in a clinical research protocol approved by the ethical committee of Tours in February 2016 (RC15_0327, ClinicalTrial number NCT02707354), and written informed consent was obtained in accordance with the Declaration of Helsinki.
Eleven subjects who underwent a colonoscopy for screening or surveillance of polyps/cancer and had a pCLE examination performed for research purposes, after written informed consent (BRD 08/6‐A), served as a control group in the pCLE part of our study. Probe‐based confocal laser endomicroscopy data from some of these patients had been used in a previous study. 22
Five subjects undergoing surgery for colon cancer at the CHU of Nantes were also included in this study. Normal colonic mucosa was taken at distance from the tumor and served as control for assessment of cytokine levels in the supernatants of mucosal explants. These samples are part of a registered tissue biocollection (DC‐2014‐2206) with approval from EC (CPP Ouest IV–Nantes).
Study protocol
A rectosigmoidoscopy with standard biopsies and pCLE was performed between day 21 and day 28 after the allo‐HSCT. Patients were subsequently followed until day 100. Clinical symptoms, laboratory values, and medications were recorded. Medical records were then reviewed for acute or chronic GVHD with a median follow‐up of 21 months (3–35). During the follow‐up, a rectosigmoidoscopy was performed in case of clinical suspicion of aDGVHD.
Diagnosis of GVHD
Diagnostic criteria and classifications of GVHD are defined in Supplementary Material. 23 , 24 , 25
Acute GVHD included both classic acute GVHD, occurring within 100 days, and recurrent acute GVHD, occurring beyond 100 days after allo‐HSCT. 6 Chronic GVHD was defined as any typical sign of chronic GVHD without notion of time of onset after allo‐HSCT. Acute digestive graft‐versus‐host disease group refers to patients who have declared an aDGVHD, during the follow‐up. No GVHD group refers to patients who did not declare a GVHD during the follow‐up.
Rectosigmoidoscopy and pCLE procedures
Rectosigmoidoscopy was performed by a trained endoscopist, in non‐sedated patients, after distal colon cleansing with enema, using a standard colonoscope (EC530, Fujinon). First, examination of colonic mucosa was carried out up to 35 cm from the anal margin. Then, two standard biopsies were collected for histology and six for laboratory analysis.
Probe‐based confocal laser endomicroscopy recording was performed using a dedicated CLE system composed of a portable laser station (Cellvizio; MaunaKea Technologies) and an endoscopic probe (Coloflex; MaunaKea Technologies) after injection of fluorescein, as previously published. 22
Analysis of pCLE parameters
Probe‐based confocal laser endomicroscopy films were read according to a semi‐automated and reproducible method 22 in order to measure the following pCLE parameters: Perimeter, Sphericity, Roundness, Maximal Feret Diameter defined by the maximal distance between two points of the perimeter, Elongator factor defined by the ratio between the minor diameter and the major diameter, Ma/ma ratio defined by the ratio between the width and the height of the box containing the crypt, Crypt density, Minimal and Mean inter crypt distance, Wall thickness, Mean vessel area, and Mean vessel diameter (Figure 1 and Supplementary Material). A subjective analysis of the crypt lumen fluorescein leakage in the pCLE recordings of allo‐HSCT patients and controls was also performed (Figure S1). The movies were anonymized by a third party. A trained reader (LQ) then reviewed the movies for crypt lumen fluorescein leakage. A leakage of fluorescein was considered to occur whenever the lumen of crypt was observed by the reader as being brighter than the surrounding cells of the crypt, independently on the number of crypts analyzed.
FIGURE 1.

Analysis of crypt parameters in a representative image as obtained after probe‐based confocal laser endomicroscopy film mosaicking. Display of measured architectural parameters of the crypts (a) Sphericity 1 defined by 4 × π × Area/Perimeter2, Perimeter, 2 Maximal Feret Diameter defined by the maximal distance between two points of the perimeter, 3 Ma/ma ratio defined by the ratio between the width and the height of the box containing the crypt, 4 Elongator factor defined by the ratio between the minor diameter and the major diameter, 5 Roundness defined by the normalized ratio between radii of the minimum and maximum circles written in the form. 6 Display of distribution measurements (b) Wall thickness defined by the distance between nearest neighbor crypt, 7 Minimal and mean distance between the geometrical centers of neighbor crypts. 8 Display of crypt density measurement (c) defined by the ratio of the crypt area and the area of the field of view. Display of vessel area measurement (d) Mean vessel area defined by the ratio between the vessel area and the area of the field of view. Scale bars: 100 µm
Measurement of paracellular permeability
Paracellular permeability was measured in allo‐HSCT patients in three biopsies mounted in Ussing chambers 26 (Supplementary Material) and determined by averaging the gradient of change in fluorescence intensity over time, using a linear regression fit model. No biopsies were performed in pCLE controls, which precluded comparison of permeability between allo‐HSCT patients and controls.
Cytokines assay in culture supernatants of biopsies and normal mucosa explants and in sera
Cytokines (IL‐1β, IFNγ, TNFα, IL‐33, IL‐6, IL‐17A, IL‐15, and IL‐18) were measured using a bead‐based multiplex immunoassay technique (Legendplex multianalyte flow assay kit, Ozyme) in supernatant of explants of colonic biopsies from allo‐HSCT patients and normal colonic mucosa from controls that were cultured for 24 h (Supplementary Material). 27 Data were acquired on a Canto HTS flow cytometer (BD Biosciences) and analyzed using the Legendplex data analysis software. Finally, serum concentration of cytokines was determined using the same approach.
Statistical analysis
All statistical analyses were performed with GraphPad Prism version 7.00 for Windows (GraphPad Software). Continuous variables were analyzed using non‐parametric tests (Mann–Whitney test and ANOVA). In the text, numerical variables are described either as mean (standard deviation) or median (range). Categorical variables were analyzed using a Chi‐square test. A value of p inferior to 0.05 was considered as significant.
RESULTS
Patients
Between June 2016 and August 2018, 19 patients who underwent allo‐HSCT were included. There was a majority of males (63%) and the mean age was 61.3 years old (38–69). Median white cells count at inclusion was 5.8 per mm3 (range: 3.8–10.1). All patients were treated with antibiotics and antiviral drugs for prophylaxis. Patients' characteristics between GVHD and aDGVHD groups were similar (Table 1).
TABLE 1.
Subjects' characteristics
| Acute digestive GVHD (n = 6) | No GVHD (n = 8) | Allo‐HSCT (n = 19) | |
|---|---|---|---|
| Age mean ± SD | 61.2 ± 8 | 59.2 ± 9.0 | 61.3 ± 8 |
| Sex | |||
| Male | 4 (67%) | 4 (50%) | 12 (63%) |
| Female | 2 (33%) | 4 (50%) | 7 (37%) |
| BMI mean ± SD (kg/cm2) | 25.5 ± 2.5 | 25.2 ± 2.8 | 25.5 ± 2.5 |
| Hematologic disease | |||
| Non‐Hodgkin lymphoma | 1 (17%) | 3 (37%) | 7 (37%) |
| Acute leukemia | 3 (50%) | 5 (63%) | 10 (53%) |
| AREB | 2 (33%) | 0 (0%) | 2 (10%) |
| Conditioning regimen | |||
| Fludarabine‐busulfan‐ATG | 4 (67%) | 6 (75%) | 13 (68%) |
| Clofarabine‐busulfan‐ATG | 2 (33%) | 2 (25%) | 6 (32%) |
| GVHD prophylaxis | |||
| Cyclosporin | 2 (33%) | 3 (38%) | 7 (37%) |
| Cyclosporin + MMF | 4 (67%) | 5 (62%) | 12 (63%) |
| Graft type | |||
| Peripheral blood stem cell | 6 (100%) | 8 (100%) | 19 (100%) |
| HLA matching | |||
| Matched related donor | 3 (50%) | 5 (62%) | 10 (53%) |
| Matched unrelated donor | 3 (50%) | 3 (38%) | 9 (47%) |
| CMV matching | 2 (33%) | 6 (75%) | 15 (79%) |
| Sex matching | 2 (33%) | 4 (50%) | 9 (47%) |
Note: Values are n (%) unless otherwise defined.
Abbreviations: Allo‐HSCT, allogeneic hematopoietic stem cell transplantation; ATG, Anti‐thymocyte globulin; BMI, body mass index; CMV, cytomegalovirus; GVHD, graft‐versus‐host disease; HLA, human leukocyte antigen system; MMF, Mycophenolate mofetil; RAEB, Refractory anemia with excess blasts; SD, standard deviation.
GVHD characteristics
A digestive GVHD occurred in six patients (32%), among which three classic aDGVHDs (16%) and three recurrent aDGVHDs (16%). In total, 11 patients (58%) declared a GVHD, whatever the type and location, during the follow‐up (Figure 2). According to Glucksberg classification, four aDGVHDs were classified grade II or III, and two aDGVHDs were classified as severe, grade IV. Based on morphological criteria, five aDGVHDs were classified grade 1, and one aDGVHD was classified grade 4. The mean time from allo‐HSCT to aDGVHD onset and to any GVHD onset were 126 days (50–178) and 121 days (49–247), respectively. The median delay from initial rectosigmoidoscopy to diagnostic rectosigmoidoscopy, performed for aDGVHD symptoms, was 102 days (range: 30–156).
FIGURE 2.

Flow chart. Allo‐HSCT, allogeneic hematopoietic stem cell transplantation; GVHD, graft‐versus‐host disease; pCLE, probe‐based confocal laser endomicroscopy
Rectosigmoidoscopy and pCLE
Rectosigmoidoscopy was performed in all patients. Histologic assessment of biopsies was normal in all patients but one who showed a normal colonic mucosa with rare apoptotic cells in colonic crypts. Probe‐based confocal laser endomicroscopy images were obtained in all patients, enabling to characterize pCLE parameters. The mean number of crypts analyzed for each patient was 64 ± 29.
Crypt architecture, density, distribution, and vessels in pCLE
The median perimeter of crypts was significantly increased in allo‐HSCT patients compared to controls (684 ± 178 vs. 526 ± 75; p = 0.007) (Table 2). In addition, the crypts median sphericity (41 ± 10 vs. 60 ± 9; p < 0.0001), median roundness (47 ± 15 vs. 59 ± 6; p = 0.009), median Feret (105 ± 13 vs. 123 ± 18; p = 0.012), and mean vessel area (0.160 ± 0.04 vs. 0.209 ± 0.06) were significantly decreased in the allo‐HSCT group compared to the control group. The other pCLE parameters were unchanged between control and allo‐HSCT groups. Within the allo‐HSCT group, there was no significant difference between no GVHD and aDGVHD groups for the crypt parameters (Table 2). Interestingly, 53% of allo‐HSCT patients had a crypt lumen fluorescein leakage, compared to 9% of controls (p = 0.017) (Supplementary material Figure S1). Fluorescein leakage was present in 50% of aDGVHD patients and in 63% patients in the no GVHD group (p = 0.64).
TABLE 2.
Crypt architecture, density, distribution, and vessels in grafted patients and controls
| Acute digestive GVHD (n = 6) | No GVHD (n = 8) | Allo‐HSCT (n = 19) | Controls (n = 11) | |
|---|---|---|---|---|
| Perimeter (µm) | 785 ± 192 | 679 ± 179 | 684 ± 178 a | 526 ± 75 |
| Sphericity (%) | 34 ± 8 | 43 ± 10 | 41 ± 10 a | 60 ± 9 |
| Roundness (%) | 40 ± 14 | 46 ± 18 | 47 ± 15 a | 59 ± 6 |
| Maximal Feret diameter (µm) | 104 ± 11 | 102 ± 13 | 105 ± 13 a | 123 ± 18 |
| Elongation factor (ratio) | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.2 |
| Ma/ma ratio | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 |
| Crypt density (ratio) | 0.239 ± 0.05 | 0.241 ± 0.04 | 0.238 ± 0.04 | NA |
| Minimal ICD (µm) | 156 ± 25 | 140 ± 14 | 152 ± 25 | NA |
| Mean ICD (µm) | 309 ± 44 | 257 ± 53 | 289 ± 56 | NA |
| Wall thickness (µm) | 217 ± 39 | 174 ± 45 | 202 ± 49 | NA |
| Mean vessel area (%) | 0.142 ± 0.03 | 0.170 ± 0.04 | 0.160 ± 0.04 a | 0.209 ± 0.06 |
| Mean vessel diameter (µm) | 11.78 ± 1.01 | 12.36 ± 0.72 | 12.17 ± 0.85 | 12.6 ± 1.35 |
Note: Data are expressed as mean ± standard deviation.
Abbreviations: Allo‐HSCT, allogeneic hematopoietic stem cell transplantation; GVHD, graft‐versus‐host disease; ICD, inter crypt distance.
Comparison with controls p < 0.05.
Mucosal paracellular permeability
No study of paracellular permeability was performed between allo‐HSCT and controls, as no permeability measurement was available for the control group. Paracellular permeability was not significantly different between aDGVHD and no GVHD groups (mean 0.17 ± 0.01 vs. 0.22 ± 0.1, p = 0.40) (Supplementary material Figure S2).
Pro‐inflammatory cytokines
IL‐6 and IL‐33 levels, low in the supernatants of controls, were high to very high in the supernatants of allo‐HSCT biopsies (Figure 3a,b). IL‐15 levels, although lower, were also significantly increased in the allo‐HSCT patients compared to the control group (Figure 3c). The pro‐inflammatory cytokines IL‐1β and IL‐18, whose levels were moderate to low, were decreased in the allo‐HSCT group compared to the control group (Figure 3d,e). In addition, the amounts of the Th1 cytokines IFNγ and TNFα or of the Th17 cytokine IL‐17A were low or almost undetectable in all groups (Figure 3f–h). No difference was observed between aDGVHD and no GVHD patients regarding the serum levels of the studied cytokines, which were low or almost undetectable in several patients (Supplementary Material).
FIGURE 3.
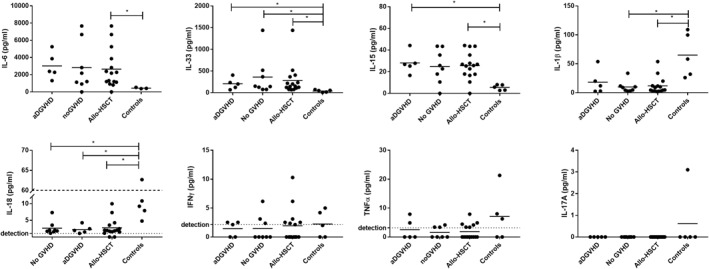
Pro‐inflammatory cytokines secreted in colonic biopsies and normal mucosa explant cultures. Comparison of (a) Interleukin‐6; (b) Interleukin‐33; (c) Interleukin‐15; (d) Interleukin‐1beta; (e) Interleukin‐18; (f) Interferon‐γ; (g) Tumor necrosing factor‐α; (h) Interleukin‐17A levels, between acute digestive graft‐versus‐host disease (aDGVHD), no graft‐versus‐host disease (GVHD) groups and controls as well as between allo‐grafted patients and controls. Cytokine levels are expressed as pg/ml. *p < 0.05. allo‐HSCT, allogeneic hematopoietic stem cell transplantation
DISCUSSION
Our study first aimed to determine if an early remodeling of the colonic mucosa occurred after allo‐HSCT. Interestingly, the perimeter of crypts was increased; the sphericity, the roundness, the Feret diameter, and the mean vessel area were decreased in the allo‐HSCT group compared to the control group. In addition, crypt lumen fluorescence leakage was significantly increased in allo‐HSCT patients compared to controls. Furthermore, the analysis of several cytokines of interest in biopsies explant cultures, albeit performed on a small number of patients and controls, showed that secretion of three pro‐inflammatory cytokines was increased in allo‐HSCT compared to the control group. The secondary aim of our study was to identify putative predictive factors among those studied that could be associated with aDGVHD onset. There was no statistical difference in pCLE parameters, paracellular permeability of colonic mucosa, and inflammatory cytokines between patients who had aDGVHD during follow‐up and patients who had no GVHD.
Our findings demonstrate that allo‐HSCT induces mucosal alterations, that is epithelial barrier remodeling, including changes in crypt architecture and epithelial barrier dysfunction assessed by the high‐resolution imaging pCLE. Indeed, pCLE analysis revealed an alteration of the shape of the crypts, which appeared distorted compared to controls crypts, suggesting that the overall architecture of the colonic mucosa was modified before any sign of GVHD. These changes in perimeter, sphericity, roundness, and Feret diameter were subtle and were not detected by standard pathology but could translate into functional changes. A decrease in the mucosal surface area occupied by vessels was also observed, with no change in vessel diameter, which may imply that allo‐HSCT patients showed a decrease in the number of colonic mucosal vessels. These preliminary data are of interest, as it has been shown that intestinal neovascularization during GvHD shares mechanisms with adaptive blood vessel growth as it occurs in response to ischemia after arterial occlusion. 28 Our results also suggest that changes in mucosal innate immunity occur after allo‐HSCT featured by an increase in the pro‐inflammatory cytokines involved in innate and adaptive immune responses, IL‐33, IL‐15, and IL‐6 occur. Studies exploring intestinal barrier functions before or immediately after allo‐HSCT in patients are scarce 29 , 30 ; indeed, studies in this field have mainly focused on the analysis of alterations of gut parameters after GVHD onset, and most mechanistic studies have been carried out in mice models of GVHD. 15 Nevertheless, there is evidence that recipient's intestinal barrier is altered by the transplant conditioning regimen 29 , 30 but also by the underlying disease, infections, and drug toxicity. 31 This change of the intestinal barrier is thought to play a role in digestive symptoms experienced by allo‐HSCT patients. This could also be the case in our study as eight patients (42%) had digestive symptoms early after allo‐HSCT and among them 63% had no digestive GVHD during follow‐up. An analysis of intestinal barrier remodeling prior conditioning, prior allo‐HSCT and at various times after allo‐HSCT would have been valuable to better understand its changes; however, repeated rectosigmoidoscopy in these altered patients was deemed excessive. It is worth noting that the main pCLE analysis was performed automatically as we believe that objective and reproducible results are valuable, especially when looking at very subtle changes. The fluorescein leakage was “manually” assessed by a reader blinded to the group of subjects, and we chose to limit this analysis to the crypt lumen because the assessment of inflammatory infiltrate is more subjective. 32
A major hypothesis is that the alteration of the intestinal barrier plays a role in the GVHD pathophysiology by promoting mucosal inflammation. 16 , 33 Our findings confirm mucosal alterations, in particular increased fluorescein leakage. In addition, we also showed increased secretion of some pro‐inflammatory cytokines involved in innate and acquired immunity (IL‐33, IL‐15, and IL‐6) from colonic mucosa explants of allo‐HSCT patients, even in the absence of subsequent GVHD. These findings suggest that this mucosal remodeling is not sufficient per se to induce GVHD. However, these alterations can be a facilitating factor to initiate GVHD. Thus, restoration of intestinal barrier integrity and/or modulation of the mucosal immune response could act as a prophylactic treatment of GVHD. Consistently, a previous study in a mouse model has shown that the mouse intestinal barrier can be protected from radiation injury and subsequent GVHD by systemic pretreatment with R‐spondin‐1, an intestinal epithelial stem cells growth factor. 34 In addition, after allo‐HSCT, the intestinal barrier and its microenvironment produced more IL‐6, a crucial cytokine in initiating a TH‐17 immune response. 15 , 35 Besides, it was demonstrated that patients with aDGVHD had higher levels of Th17 cells in the intestinal mucosa than those without aDGVHD. 18 After allo‐HSCT, the intestinal barrier and its microenvironment also produced IL‐33, an alarm signal cytokine released upon cell injury to activate immune cells expressing the ST2 36 and IL‐15, which stimulates activation of natural killer cells and memory CD8 T cells. 37 Interestingly, many second‐line treatments of GVHD, currently under evaluation, target these inflammatory cytokines, such as anti‐IL‐6 (tocilizumab) and anti‐TNFα (infliximab) therapies.
Our current pilot study failed to identify predictive factors of aDGVHD. A first possible explanation is that our study was performed too early after allo‐HSCT, that is first 3–4 weeks, suggesting that at this time point changes reported as compared to control are mainly driven by the pretreatment conditions. However, a previous study have highlighted crypt‐related biomarkers that, when measured 7 days after allo‐HSCT, can predict non‐relapse mortality, and consequently GVHD mortality, 10 suggesting that early alteration of the intestinal barrier could still predict GVHD onset. But in that study the median day of GVHD onset was 28 days, vs. 126 days in our series, suggesting that (1) our patients suffered from later forms of GVHD and (2) as a result, our pCLE analysis might be too early. Finally, the low number of patients is an important limitation of the study and this lack of power could explain the absence of a significant difference between the no GVHD group and aDGVHD group. Another potential issue is that the parameters used for the pCLE crypt analysis in our study lack sensitivity to measure subtle morphological changes enabling to discriminate between no GVHD group and aDGVHD group. Indeed, on one hand, the analysis could be too early after allo‐HSCT to detect any changes linked to GVHD onset. On the other hand, the use of complementary approaches, such as topical application of acriflavine to detect apoptotic cells, could have improved the sensitivity of pCLE to detect early GVHD. In addition, the few available studies suggest that pCLE allows to diagnose asymptomatic 20 and symptomatic 21 aDGVHD with a good sensitivity but is probably not sufficient on its own to predict the occurrence of aDGVHD.
In conclusion, this pilot study showed a modification of the colonic microarchitecture and epithelial integrity detectable in pCLE and an increase in mucosal inflammation in allo‐HSCT patients. Our study confirms the early remodeling of intestinal barrier in allo‐HSCT patients, which may represent a first step in GVHD initiation but does not appear to be sufficient to induce the disease by itself. Indeed, our pilot study failed to demonstrate any difference in colonic mucosa microarchitecture, permeability, and inflammation between patients who developed aDGVHD and patients who did not. Future studies on larger cohort should be performed to confirm our findings and identify novel putative predictive markers for GVHD.
CONFLICT OF INTEREST
The authors declare that no conflict of interest exists.
Supporting information
Supporting information S1
ACKNOWLEDGMENTS
The authors wish to thank the Hematology unit and the Clinical investigation center for screening and programing the patients, the endoscopy unit staff of the University Hospital of Nantes for performing the biopsies, as well as the Department of Pathology for its expertise in the processing of the biopsies, and for complying with the relevant regulation in terms of tissues availability for research. The authors also wish to thank Nicolas Jouand from the Cytocell Cytometry facility (SFR Bonamy, Nantes) for expert technical assistance. This work was supported by the DHU Oncogreffe.
Coron E, Esnaud E, Chevallier P, Bessard A, Perez Cuadrado‐Robles E, David G, et al. Early remodeling of the colonic mucosa after allogeneic hematopoietic stem cells transplantation: An open‐label controlled pilot study on 19 patients. United European Gastroenterol J. 2021;9(8):955–63. 10.1002/ueg2.12128
DATA AVAILABILITY STATEMENT
Data may be obtained from a third party and are not publicly available.
REFERENCES
- 1. Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft‐versus‐host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016. Jan 1;22(1):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jagasia M, Arora M, Flowers MED, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012. Jan 5;119(1):296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NKC, et al. Response of 443 patients to steroids as primary therapy for acute graft‐versus‐host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002. Jul 1;8(7):387–94. [DOI] [PubMed] [Google Scholar]
- 4. Bhatia S, Francisco L, Carter A, Sun C‐L, Baker KS, Gurney JG, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long‐term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007. Nov 15;110(10):3784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weisdorf D, Haake R, Blazar B, Miller W, McGlave P, Ramsay N, et al. Treatment of moderate/severe acute graft‐versus‐host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990. Feb 15;75(4):1024–30. [PubMed] [Google Scholar]
- 6. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: I. diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005. Dec 1;11(12):945–56. [DOI] [PubMed] [Google Scholar]
- 7. Flowers MED, Inamoto Y, Carpenter PA, Lee SJ, Kiem H‐P, Petersdorf EW, et al. Comparative analysis of risk factors for acute graft‐versus‐host disease and for chronic graft‐versus‐host disease according to National Institutes of Health consensus criteria. Blood. 2011. Mar 17;117(11):3214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee C, Haneuse S, Wang H‐L, Rose S, Spellman SR, Verneris M, et al. Prediction of absolute risk of acute graft‐versus‐host disease following hematopoietic cell transplantation. PLoS One. 2018;13(1):e0190610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sorror ML, Martin PJ, Storb RF, Bhatia S, Maziarz RT, Pulsipher MA, et al. Pretransplant comorbidities predict severity of acute graft‐versus‐host disease and subsequent mortality. Blood. 2014. Jul 10;124(2):287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hartwell MJ, Özbek U, Holler E, Renteria AS, Major‐Monfried H, Reddy P, et al. An early‐biomarker algorithm predicts lethal graft‐versus‐host disease and survival. JCI Insight. 2017;2(3):e89798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blijlevens NMA, Donnelly JP, de Pauw BE. Prospective evaluation of gut mucosal barrier injury following various myeloablative regimens for haematopoietic stem cell transplant. Bone Marrow Transplant. 2005. Apr;35(7):707–11. [DOI] [PubMed] [Google Scholar]
- 12. van der Velden WJFM, Herbers AHE, Feuth T, Schaap NPM, Donnelly JP, Blijlevens NMA. Intestinal damage determines the inflammatory response and early complications in patients receiving conditioning for a stem cell transplantation. PLoS One. 2010. Dec 20;5(12):e15156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Noth R, Lange‐Grumfeld J, Stüber E, Kruse M‐L, Ellrichmann M, Häsler R, et al. Increased intestinal permeability and tight junction disruption by altered expression and localization of occludin in a murine graft versus host disease model. BMC Gastroenterol. 2011. Oct 6;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nalle SC, Zuo L, Ong MLDM, Singh G, Worthylake AM, Choi W, et al. Graft‐versus‐host disease propagation depends on increased intestinal epithelial tight junction permeability. J Clin Invest. 2019. Feb 01;129(2):902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peled JU, Hanash AM, Jenq RR. Role of the intestinal mucosa in acute gastrointestinal GVHD. Blood. 2016. Nov 17;128(20):2395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft‐versus‐host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000. May 1;95(9):2754–9. [PubMed] [Google Scholar]
- 17. Piper C, Drobyski WR. Inflammatory cytokine networks in gastrointestinal tract graft vs. host disease. Front Immunol. 2019;10:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bossard C, Malard F, Arbez J, Chevallier P, Guillaume T, Delaunay J, et al. Plasmacytoid dendritic cells and Th17 immune response contribution in gastrointestinal acute graft‐versus‐host disease. Leukemia. 2012. Jul;26(7):1471–4. [DOI] [PubMed] [Google Scholar]
- 19. Levine JE. Implications of TNF‐α in the pathogenesis and management of GVHD. Int J Hematol. 2011. May;93(5):571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coron E, Laurent V, Malard F, Rhun ML, Chevallier P, Guillaume T, et al. Early detection of acute graft‐versus‐host disease by wireless capsule endoscopy and probe‐based confocal laser endomicroscopy: results of a pilot study. United European Gastroenterol J. 2014. Jun 1;2(3):206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bojarski C, Günther U, Rieger K, Heller F, Loddenkemper C, Grünbaum M, et al. In vivo diagnosis of acute intestinal graft‐versus‐host disease by confocal endomicroscopy. Endoscopy. 2009. May;41(5):433–8. [DOI] [PubMed] [Google Scholar]
- 22. Quénéhervé L, David G, Bourreille A, Hardouin JB, Rahmi G, Neunlist M, et al. Quantitative assessment of mucosal architecture using computer‐based analysis of confocal laser endomicroscopy in inflammatory bowel diseases. Gastrointest Endosc. 2019. Mar 1;89(3):626–36. [DOI] [PubMed] [Google Scholar]
- 23. Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft‐versus‐host disease in human recipients of marrow from HL‐A‐matched sibling donors. Transplantation. 1974. Oct;18(4):295–304. [DOI] [PubMed] [Google Scholar]
- 24. Sale GE, Shulman HM, McDonald GB, Thomas ED. Gastrointestinal graft‐versus‐host disease in man. A clinicopathologic study of the rectal biopsy. Am J Surg Pathol. 1979. Aug;3(4):291–300. [DOI] [PubMed] [Google Scholar]
- 25. Epstein RJ, McDonald GB, Sale GE, Shulman HM, Thomas ED. The diagnostic accuracy of the rectal biopsy in acute graft‐versus‐host disease: a prospective study of thirteen patients. Gastroenterology. 1980. Apr;78(4):764–71. [PubMed] [Google Scholar]
- 26. Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009. Feb;58(2):196–201. [DOI] [PubMed] [Google Scholar]
- 27. Jarry A, Bossard C, Bou‐Hanna C, Masson D, Espaze E, Denis MG, et al. Mucosal IL‐10 and TGF‐beta play crucial roles in preventing LPS‐driven, IFN‐gamma‐mediated epithelial damage in human colon explants. J Clin Invest. 2008. Mar;118(3):1132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leonhardt F, Grundmann S, Behe M, Bluhm F, Dumont RA, Braun F, et al. Inflammatory neovascularization during graft‐versus‐host disease is regulated by αv integrin and miR‐100. Blood. 2013. Apr 25;121(17):3307–18. [DOI] [PubMed] [Google Scholar]
- 29. Johansson JE, Brune M, Ekman T. The gut mucosa barrier is preserved during allogeneic, haemopoietic stem cell transplantation with reduced intensity conditioning. Bone Marrow Transplant. 2001. Oct;28(8):737–42. [DOI] [PubMed] [Google Scholar]
- 30. Johansson JE, Ekman T. Gastro‐intestinal toxicity related to bone marrow transplantation: disruption of the intestinal barrier precedes clinical findings. Bone Marrow Transplant. 1997. May;19(9):921–5. [DOI] [PubMed] [Google Scholar]
- 31. Ferrara JLM, Levine JE, Reddy P, Holler E. Graft‐versus‐host disease. Lancet. 2009. May 2;373(9674):1550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hundorfean G, Chiriac MT, Mihai S, Hartmann A, Mudter J, Neurath MF. Development and validation of a confocal laser endomicroscopy‐based score for in vivo assessment of mucosal healing in ulcerative colitis patients. Inflamm Bowel Dis. 2017. Dec 19;24(1):35–44. [DOI] [PubMed] [Google Scholar]
- 33. Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft‐versus‐host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997. Oct 15;90(8):3204–13. [PubMed] [Google Scholar]
- 34. Takashima S, Kadowaki M, Aoyama K, Koyama M, Oshima T, Tomizuka K, et al. The Wnt agonist R‐spondin1 regulates systemic graft‐versus‐host disease by protecting intestinal stem cells. J Exp Med. 2011. Feb 14;208(2):285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012. Oct;13(10):991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reichenbach DK, Schwarze V, Matta BM, Tkachev V, Lieberknecht E, Liu Q, et al. The IL‐33/ST2 axis augments effector T‐cell responses during acute GVHD. Blood. 2015. May 14;125(20):3183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Castillo EF, Schluns KS. Regulating the immune system via IL‐15 transpresentation. Cytokine. 2012. Sep;59(3):479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information S1
Data Availability Statement
Data may be obtained from a third party and are not publicly available.


