Abstract
Background and Aims
Barrett's esophagus (BE) is accompanied by an increased risk of developing esophageal cancer. Accurate risk‐stratification is warranted to improve endoscopic surveillance. Most data available on risk factors is derived from tertiary care centers or from cohorts with limited surveillance time or surveillance quality. The aim of this study was to assess endoscopic and clinical risk factors for progression to high‐grade dysplasia (HGD) or esophageal adenocarcinoma (EAC) in a large prospective cohort of BE patients from community hospitals supported by an overarching infrastructure to ensure optimal surveillance quality.
Methods
A well‐defined prospective multicenter cohort study was initiated in six community hospitals in the Amsterdam region in 2003. BE patients were identified by PALGA search and included in a prospective surveillance program with a single endoscopist performing all endoscopies at each hospital. Planning and data collection was performed by experienced research nurses who attended all endoscopies. Endpoint was progression to HGD/EAC.
Results
Nine hundred eighty‐five patients were included for analysis. During median follow‐up of 7.9 years (IQR 4.1–12.5) 67 patients were diagnosed with HGD (n = 28) or EAC (n = 39), progression rate 0.78% per patient‐year. As a clinical risk factor age at time of endoscopy was associated with neoplastic progression (HR 1.05; 95% CI 1.03–1.08). Maximum Barrett length and low‐grade dysplasia (LGD) at baseline were endoscopic predictors of progression (HR 1.15; 95% CI 1.09–1.21 and HR 2.36; 95% CI 1.29–4.33).
Conclusion
Risk of progression to HGD/EAC in a large, prospective, community‐based Barrett's cohort was low. Barrett's length, LGD and age were important risk factors for progression.
(www.trialregister.nl NTR1789)
Keywords: Barrett, Barrett's esophagus, esophageal adenocarcinoma, esophageal cancer, follow‐up, high‐grade dysplasia, malignancy, neoplastic progression, risk factors, surveillance

INTRODUCTION
Barrett's esophagus (BE) surveillance is common practice to detect esophageal adenocarcinoma at curable stage. Also, precursor lesions such as low‐grade dysplasia (LGD) and high‐grade dysplasia (HGD) may be found and treated before malignant progression. The effectiveness and efficiency of endoscopic surveillance, however, is questionable, given the low risk of malignant progression, 1 , 2 , 3 , 4 the high inter‐observer variability between pathologists in grading dysplasia, 5 , 6 , 7 possible biopsy sampling error and the costs of endoscopies and histopathological assessment.
Tools to stratify patients into a high‐risk group that benefits from endoscopic surveillance or prophylactic treatment, and a low‐risk group in which surveillance intervals can be prolonged or stopped, could reduce the clinical and economic burden of Barrett's surveillance.
Much research effort has been put in identifying endoscopic and clinical risk factors that can predict progression to HGD and cancer in BE patients and in assessing the progression risk. Most data, however have been derived from case‐control studies, 8 , 9 , 10 retrospective cohort studies or from prospective observational studies with limited surveillance history or no standardized endoscopic protocol. Other studies have included patients with prevalent HGD/cancer. 11 , 12 , 13 Another important limitation of most studies, however, is the tertiary referral center setting, where both the selected patient cohort and the setting are not representative for the majority of BE surveillance patients (Table S1). 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21
The aim of this project was to assess the risk of neoplastic progression in BE, to identify endoscopic and clinical risk factors for progression and to compose a biobank with biopsy samples to facilitate objective risk stratification using biomarkers. To overcome the previously mentioned drawback of other studies, we established a well‐defined prospective cohort of BE patients, in a multicenter community‐based setting, with long‐term and standardized surveillance, which was supervised by an over‐arching infrastructure to ensure surveillance quality (Table S2).
Key Summary.
Established knowledge
Barrett's esophagus is the most important risk factor for esophageal cancer.
Accurate risk‐stratification is warranted to improve endoscopic surveillance.
Most data available on risk factors is derived from tertiary care centers or from cohorts with limited surveillance time or surveillance quality.
New findings
Risk of progression to high‐grade dysplasia/esophageal adenocarcinoma in a large, prospective, community‐based Barrett's cohort is low.
Barrett's length, low‐grade dysplasia and age are important risk factors for progression.
METHODS
Study design and patient selection
In 2003, a prospective, multicenter cohort study was initiated and coordinated from the University Medical Centers Amsterdam, location AMC, including six community hospitals in the Amsterdam area. The Dutch nationwide database comprising all pathology reviews since 1971 (PALGA) 22 was searched for the identification of patients with known BE. Patient charts were reviewed to assess whether inclusion criteria, that is, endoscopic and histological evidence of BE, were met. Eligible patients were asked to participate in this prospective study. Patients who were newly diagnosed during the study period were asked informed consent for prospective inclusion.
Exclusion criteria were: no intestinal metaplasia (IM); history of HGD or esophageal cancer; prevalent HGD/cancer (i.e., at baseline or within 12 months after index endoscopy); unfit for endoscopic surveillance; history of endoscopic treatment; only one surveillance endoscopy during the study period.
Retrospective data collection at the start of the prospective registry
All endoscopy and pathology reports from examinations prior to the prospective surveillance, were retrieved from the patient charts and processed on standardized case record forms.
Prospective data collection
Data were prospectively collected from 2003 to July 2017. At each center, monthly endoscopy programs dedicated to surveillance of BE patients were scheduled. Patients were surveyed according to guidelines. 23 , 24 Patients with NDBE underwent endoscopy every 3 years and patients with LGD every 6–12 months. Adequate adherence to the advised surveillance intervals was ensured by supervised scheduling of patients by two research nurses operating from the coordinating center.
To further ensure protocol adherence, correct biopsy sampling, and data collection, surveillance programs were attended by one of these two research nurses, both with ample experience in Barrett's surveillance. At each center a single endoscopist was responsible for all surveillance endoscopies and all six endoscopists were uniformly trained. 25
Collected endoscopic data included Barrett length, signs of reflux, and presence of visible abnormalities. Questionnaires were used to collect data on length, weight, medication use (reflux, anti‐inflammatory and statins), smoking, alcohol use, family history of BE, and/or esophageal cancer.
Histological evaluation
Formalin‐fixed biopsies from four quadrants were taken every 2 cm of Barrett's mucosa according to the Seattle protocol, 26 and if present, from any visible abnormalities. All biopsies were assessed by local pathologists. Dysplasia was graded according to the Vienna classification. 27 If dysplasia was diagnosed, biopsies were reviewed by an expert pathologist at the coordinating center. 6
Database
All data were entered in a specially designed web‐based database (ProMISe software developed by the Leiden University Medical Center). Access to the database and coordination of the surveillance program was centrally managed at the coordinating center.
Outcomes and statistical analysis
Primary endpoint was progression to HGD/cancer, diagnosed by an expert pathologist. Total surveillance time was reported as the time from index endoscopy (first endoscopy with IM) to either last surveillance endoscopy, progression to HGD/cancer, or in until RFA treatment in a small subset of patients who developed LGD.
As secondary endpoints detection of progression through random or targeted biopsies and further treatment after progression were evaluated.
Endoscopic, histological, and demographic data were exported from the database to Statistical Package for the Social Sciences (SPSS 20.0, SPSS) and R (version 1.1.383, The R foundation for Statistical Computing) was used for statistical analysis.
Median and interquartile range (IQR) were used to describe variables with a skewed distribution. To assess the effect of each risk factor on progression cox proportional hazard models were used. Assumptions were tested and checked by visual inspection of plots. For each risk factor, the univariate association with the occurrence of neoplastic progression was calculated and presented as hazard ratio (HR) with 95% confidence interval (CI). Multivariable cox proportional hazard regression analysis was used to assess the adjusted associations between these risk factors and the occurrence of neoplastic progression. All risk factors that showed a significant association with neoplastic progression in the univariate analysis were included in the multivariable analysis, as well as sex. Missing data were assumed to be missing at random. Multiple imputation, using a multivariable model, was performed to adjust for missing values. 28 Analyses were performed using five imputed datasets.
Ethical considerations
The study was approved by the medical ethics committee of the Amsterdam UMC, location AMC and the medical ethic review board of each participating center (Dutch trial register NTR1789). Written informed consent was obtained from all patients. All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
Patient inclusion and characteristics
A total of 2710 patients were screened after being identified by the PALGA search or after being diagnosed with BE during the study period (Figure 1). Patients with no IM or gastric IM (n = 1053), prevalent HGD/cancer (n = 40) and with squamous‐cell carcinoma (n = 4) were excluded. Patient charts of 1613 patients were reviewed, after which 143 patients were excluded because they were unfit for surveillance, had died, had moved, declined surveillance or had undergone endoscopic treatment. In 318 cases, no informed consent could be obtained, or patients declined. One hundred sixty‐seven patients who were included during the study period, only had undergone one endoscopy by the time the study was closed in July 2017, these patients were also excluded.
FIGURE 1.
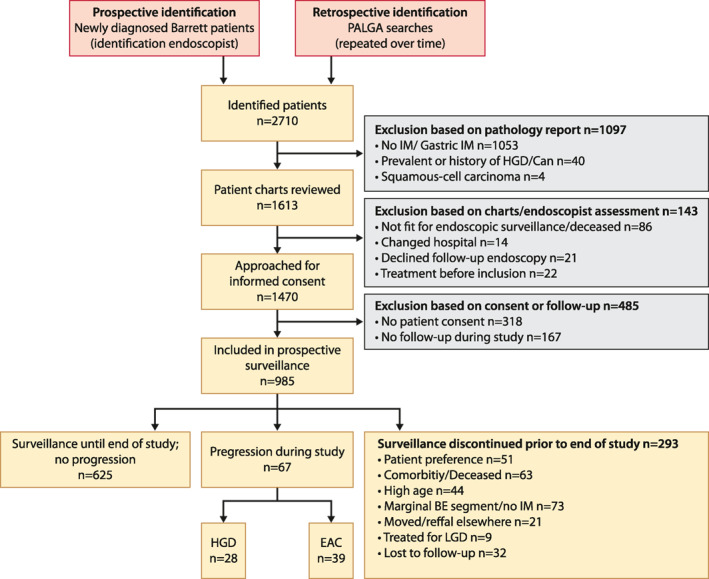
Flowchart
A total of 985 patients were included of which 485 patients had a IM diagnosis prior to inclusion. Table 1 shows baseline demographic, endoscopic, and histological characteristics. Mean age at first endoscopy was 57 (±11) years, 74% of patients were male, mean BMI was 27 (±4.3). A history of smoking was reported in 627 patients (64%), and alcohol use in 650 patients (66%). The majority of patients (90%) used PPI's. Patients had a median surveillance time of 7.9 years (IQR 4.1–12.5), with a median number of 4 endoscopies (IQR 3–6).
TABLE 1.
Baseline characteristics
| Baseline characteristics | |
|---|---|
| Total cohort size; n | 985 |
| Age at endoscopy; mean ± SD | 57 ± 11.4 |
| Male gender; n (%) | 727 (74%) |
| BMI (kg/m2); mean ± SD | 27 ± 4.3 |
| Smoking | |
| Former/current | 627 (63.7%) |
| No | 267 (27.1%) |
| Unknown | 91 (9.2%) |
| Alcohol use | |
| Former/current | 650 (66.0%) |
| No | 243 (24.7%) |
| Unknown | 92 (9.3%) |
| PPI use | |
| Yes | 882 (89.5%) |
| No | 19 (1.9%) |
| Unknown | 84 (8.5%) |
| Family members with Barrett's esophagus | |
| Yes | 87 (8.8%) |
| No | 679 (68.9%) |
| Unknown | 219 (22.2%) |
| Family members with esophageal cancer | |
| Yes | 73 (7.4%) |
| No | 702 (71.3%) |
| Unknown | 210 (21.3%) |
| Surveillance time; median (IQR) | 7.9 (4.1–12.5) |
| Number of endoscopies; median (IQR) | 4 (3–6) |
| Barrett length (maximum, cm); median (IQR) | 3 (2–5) |
| LGD at baseline; n (%) | 78 (7.9%) |
| Signs of reflux; n (%) | 306 (31.1%) |
Progressors
Progression to HGD (n = 28) or esophageal adenocarcinoma (n = 39) was diagnosed in 67/985 patients (6.8%), with a median time from index endoscopy to progression of 7.8 years (IQR 4.6–12.7; Table 2). Annual risk of progression to HGD/cancer was 0.78% per patient‐year with a total of 8642 patient‐years of follow‐up.
TABLE 2.
Characteristics progressors
| Progressors; n | 67 |
|---|---|
| Time to progression (years); median (IQR) | 7.8 (4.6–12.7) |
| Male; n (%) | 50 (74.6%) |
| Age at progression; median (IQR) | 69 (63–75) |
| Worst pathology | |
| HGD | 27 (40.3%) |
| EAC M1‐M3 | 30 (44.8%) |
| EAC ≥ SM1 | 10 (14.9%) |
| Treatment | |
| Conservative | 4 (6.0%) |
| Endoscopic | 54 (80.6%) |
| Surgery (± adjuvant therapy) | 6 (9.0%) |
| CRT | 2 (3.0%) |
| Palliative radiotherapy | 1 (1.5%) |
Abbreviations: CRT, chemoradiotherapy; EAC, esophageal adenocarcinoma; HGD, high grade dysplasia.
Of the 67 patients with progression to HGD or EAC 41 patients (61.2%) had a visible lesion. In 26 patients (38.8%) progression was detected by random biopsies. However, in 14/26 (53.8%) patients a lesion was detected at the next work up endoscopy at a Barrett expert center. All of the 12 patients without a visible lesion had a diagnosis of HGD.
The majority of progressors (81%) were treated endoscopically for HGD or T1 adenocarcinoma. Two patients with focal HGD diagnosed upon biopsy, confirmed after revision by an expert pathologist, had no more HGD at follow‐up endoscopy and were therefore not treated. Two patients declined treatment.
Three patients were treated with primary surgery (T1aN0M0, T1bN0M0, T2N0M0). Three patients received surgery with neo‐adjuvant chemoradiotherapy (T3N1M0, T2N0M0, T3N0M0). Two patients received definitive chemoradiotherapy (T1bN0M1, unknown). One patient with poor performance status with a T1bN0M0 tumor was deemed unfit for surgery and chemoradiotherapy and received palliative radiation therapy.
Predictors for progression
Clinical and endoscopic risk factors for progression to HGD or cancer in patients with BE are presented in Table 3. Patients with progression to HGD or cancer were older at their first endoscopy compared to patients with no progression (mean 59 years ± 10.2 vs. 57 years ± 11.5, resp.), and had a significantly longer maximum BE segment (median 6 cm [IQR 3–9] vs. 3 cm [IQR 2–5]). In the group of patients with progression, a baseline diagnosis of LGD was more common than in the non‐progressor group (19.4% vs. 7.1%). Univariate analyses showed that age at first endoscopy (HR 1.06, 95% CI 1.04–1.09, p < 0.05), maximum BE length in cm (HR 1.18, 95% CI 1.12–1.24, p < 0.05) and a diagnosis of LGD at baseline (HR 2.42, 95% CI 1.32–4.44, p < 0.05) were associated with an increased risk of progression. A family history with esophageal cancer was not significantly associated with progression (HR 1.72, 95% CI 0.88–3.34, p = 0.11). Multivariable analysis showed similar results.
TABLE 3.
Risk factors for neoplastic progression
| Non‐progressors n = 918 | Progressors n = 67 | Univariate HR (95% CI) | p value | Multivariable HR (95% CI) | p value | |
|---|---|---|---|---|---|---|
| Clinical factors | ||||||
| Age at first endoscopy ‐ years (mean ± SD) | 57 ± 11.5 | 59 ± 10.2 | 1.06 (1.04–1.09) | 0.00 | 1.05 (1.03–1.08) | 0.00 |
| Male (female reference) | 677 (73.7%) | 50 (74.6%) | 0.97 (0.56–1.68) | 0.90 | 1.05 (0.60–1.85) | 0.85 |
| BMI ‐ kg/m2 (mean ± SD) | 27.0 ± 4.3 | 27.6 ± 3.5 | 1.02 (0.97–1.08) | 0.37 | ||
| Smoking | 575 (62.6%) | 52 (77.6%) | 1.51 (0.84–2.01) | 0.17 | ||
| Alcohol | 599 (65.3%) | 51 (76.1%) | 1.19 (0.67–2.13) | 0.55 | ||
| PPI use | 817 (89.0%) | 66 (98.5%) | 1.08 (0.14–8.13) | 0.94 | ||
| Family history with Barrett's esophagus | 80 (8.7%) | 7 (10.4%) | 0.86 (0.37–2.01) | 0.73 | ||
| Family history with esophageal cancer | 63 (6.9%) | 10 (14.9%) | 1.72 (0.88–3.34) | 0.11 | ||
| Endoscopic and histology factors | ||||||
| Total endoscopies; median (IQR) | 4 (3–6) | 5 (4–8) | ||||
| Maximum BE segment length – median (IQR) | 3 (2–5) | 6.0 (3–9) | 1.18 (1.12–1.24) | 0.00 | 1.15 (1.09–1.22) | 0.00 |
| LGD at baseline | 65 (7.1%) | 13 (19.4%) | 2.42 (1.32–4.44) | 0.00 | 2.36 (1.29–4.33) | 0.01 |
| Signs of reflux | 280 (30.5%) | 26 (38.8%) | 1.37 (0.84–2.25) | 0.21 | ||
Notes: Data shown as n (%) or median + IQR unless otherwise stated. Predictors are measured at baseline, unless otherwise specified.
DISCUSSION
In this prospective, multicenter community‐based study in six Dutch hospitals with 985 BE patients undergoing standardized, high‐quality endoscopic surveillance for almost 8 years, we assessed the risk of developing HGD/cancer, and we identified associated risk factors. We found an HGD/cancer incidence rate of 0.78% per patient‐year. Higher age at first endoscopy, longer length of the maximum BE segment and LGD at baseline were significantly associated with progression. As listed in Supplement 1, most available studies on progression risk in BE have significant shortcomings, which may explain differences in incidence rate reported in literature, ranging from 0.22% to 1.02%. With our prospective study, we aimed to overcome these shortcomings. We only included patients from a community care setting, with histological and endoscopic evidence of BE, diagnosis of HGD/cancer was confirmed by an expert pathologist, endoscopies were performed by dedicated and uniformly trained endoscopists strictly adhering to guidelines, and data were collected on site by dedicated research nurses and by standardized questionnaires. Furthermore, we had a large patient cohort (n = 985) with surveillance of almost 8 years. We therefore think that our incidence risk of 0.78% per patient‐year is reliable and representative for progression to HGD and cancer in BE. Meta‐analyses on the incidence of HGD and esophageal adenocarcinoma in BE found incidence rates ranging from 0.93% to 1.02%. 4 , 29 This is slightly higher than our 0.78%, but this might be explained by the fact that most included studies came from tertiary care centers, resulting in possible selection bias by selecting high‐risk patients. Large population‐based studies with a reduced risk of selection bias of high‐risk patients found incidence rates ranging from 0.22% to 0.58%. A limitation of these studies, however, is the absence of endoscopic confirmation of BE, 1 , 2 , 30 which could have resulted in inclusion of patients with IM in the cardia, but without endoscopic BE, leading to an underestimation of the progression risk.
When looking at risk factors associated with progression, a recent systematic review and meta‐analysis including 20 cohort studies, found that older age, male sex, smoking, longer BE segment and LGD were associated with progression to HGD/cancer in BE. 31 In our study, we also found that older age at baseline endoscopy, maximum BE length and presence of LGD at baseline were significant risk factors for malignant progression. We did not, however, find a significant association between progression and male sex (HR 0.97 [0.56–1.68], p = 0.90) or smoking (HR 1.51 [0.84–2.01], p = 0.17). The categorization of smoking in “ever” versus “never,” without a distinction of “current” versus “former” or total years of smoking could have influenced this result.
LGD was the strongest predictor for malignant progression (HR 2.36; 95% CI 1.29–4.33) in our study. This is in line with previous studies that have demonstrated that patients with LGD confirmed by an expert pathologist have a significantly increased risk of malignant progression. 6 , 32
Increasing length of the BE segment has been found predictive of progression in multiple studies and has been incorporated in most recent guidelines that advise shorter surveillance intervals in patients with longer segments of BE. 33 , 34
A recent study by Parasa et al. also assessed risk factors for progression to HGD/cancer in BE in a multicenter cohort of 2697 patients from six tertiary referral centers. 21 When looking at the baseline characteristics of the patients included at the six participating centers, it is striking that there are remarkable differences in a number of relevant characteristics such as progression rate (ranging from 1.4% to 10.7%), number of female patients (1.7% to 26.8%), mean age (28.1–61.4), and LGD at baseline (2.3%–15%). Despite the baseline heterogeneity between the groups, data were pooled and 70% of the cohort was used to derive a model predicting risk of progression and 30% of the cohort was used to validate the developed scoring system. Progression to HGD/cancer occurred in 154 patients, with an annual progression rate of 0.95%. By using backward selection, Parasa et al. included four variables in their model that were significantly associated with progression: male sex, BE length, smoking, and confirmed LGD at baseline. The developed scoring system differentiated between low, intermediate, and high risk of progression to HGD/cancer. They found a total risk of progression at the end of study period to HGD/cancer of 0.5% in the low‐risk group, 4.6% in the intermediate group and 12.3% in the high‐risk group. When we applied this scoring system to our study population, however, we found a risk of progression of 3.6% in the low‐risk group, 5.3% in the intermediate group and 18.0% in the high‐risk group (Figure 2). Furthermore, Parasa et al. found a significant HR of 18.4 (95% CI 7.4–45.5) for the high‐risk group and HR 5.6 (95% CI 2.3–13.8) for the intermediate‐risk group at 7 years compared to the reference low‐risk group. In our cohort, the model was also able to show a significant difference between the low‐ and high‐risk group with a HR of 5.3 (95% CI 2.0–14.5), but the model could not distinguish between low and intermediate risk (HR 0.8 [95% CI 0.3–2.3]; Figure 3). The inability of the Parasa scoring system to differentiate between the intermediate‐risk group and the low‐risk group was also found when it was externally validated in the population‐based Northern Ireland Barrett's registry.
FIGURE 2.

Bar chart of risk scores and incidence of high‐grade dysplasia/esophageal adenocarcinoma
FIGURE 3.

Comparing the risk of progression in 7 years in our study with Parasa et al study
Since the scoring system developed by Parasa et al. does not adequately differentiate between the majority of the BE surveillance population, namely the intermediate and low risk BE patients, in two individual cohorts, its clinical value appears to be limited.
Despite the dedicated prospective surveillance protocol used in our study, risk of progression to HGD/cancer was low. Of the 67 patients that showed progression, all but one patient could be treated with a curative intent, and the majority of patients was successfully treated endoscopically. This implies that for the patients at risk of progression, current surveillance strategies are sufficient to prevent cancer‐related death in BE patients. The current problem in BE surveillance is thus not how to identify the small group of high‐risk patients, but how to stratify the remaining majority of BE patients into an intermediate‐risk and a low‐risk group. Optimization of BE surveillance will only happen if we can identify a true low‐risk group in whom surveillance is not beneficial. In addition, we have to remain critical on which patients to include in a surveillance program regarding comorbidity and life expectancy. As discussed, the currently known risk factors that are most consistently found in BE surveillance studies, o.a. BE length and LGD at baseline, appear to be not enough to optimize current Barrett surveillance strategies. Developments in the field of biomarkers that may be used to predict risk of malignant progression, however, hold the promise to make objective risk stratification of BE patients within reach. For all patients included, clinical data and biopsy specimens obtained during the prospective surveillance are centrally stored. Given the long follow‐up and number of events, this study population could be an ideal cohort to validate biomarker‐related risk stratification studies.
To our knowledge, this is the only large prospective multicenter study done in a completely community‐based setting, without inclusion of patients from an expert or tertiary care center. Results are therefore representative for the general Barrett surveillance population and risk of selection bias has been minimized. Another strength is the prospective setting with a standardized surveillance protocol. Patients were approached, planned, and actively followed by two research nurses, resulting in a high‐quality patient follow‐up with few patients lost to follow‐up. All endoscopies were performed by dedicated, trained endoscopists and dysplasia was revised by expert pathologists. Limitations of our study are the fact that although our cohort consisted of a large number of patients, progression to HGD/cancer does not occur frequently, leading to a limited number of events. Therefore, the possibility of type II errors must be taken into account. One of the strengths of our study, namely the standardized surveillance protocol on a dedicated program with dedicated endoscopists could also be a limitation, since in most community hospitals surveillance programs on BE will not be performed in these ideal circumstances. However, this will not affect the development of HGD or EAC, but could affect the stage of progression when detected.
Furthermore, the study may not be representative for a non‐Dutch population.
In conclusion, the results of our study demonstrate that the risk of malignant progression in patients with BE is low (0.78% per patient‐year). Maximum Barrett's length, presence of LGD at baseline and age at first endoscopy were identified as relevant risk factors for progression.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank B Elzer, H Verhulst, W Curvers, FJW ten Kate, GA Meijer, CA Seldenrijk, GJ Offerhaus and M Visser for their contribution to this study. This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Klaver E, Bureo Gonzalez A, Mostafavi N, Mallant‐Hent R, Duits LC, Baak B, et al. Barrett's esophagus surveillance in a prospective Dutch multi‐center community‐based cohort of 985 patients demonstrates low risk of neoplastic progression. United European Gastroenterol J. 2021;9(8):929–37. 10.1002/ueg2.12114
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hvid‐Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch‐Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375–83. 10.1056/NEJMoa1103042 [DOI] [PubMed] [Google Scholar]
- 2. Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population‐based study. J Natl Cancer Inst. 2011;103:1049–57. 10.1093/jnci/djr203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Desai TK, Krishnan K, Samala N, Singh J, Cluley J, Perla S, et al. The incidence of oesophageal adenocarcinoma in non‐dysplastic Barrett's oesophagus: a meta‐analysis. Gut. 2012;61:970–6. 10.1136/gutjnl-2011-300730 [DOI] [PubMed] [Google Scholar]
- 4. Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett's esophagus: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2010;8:235–44. 10.1016/j.cgh.2009.10.010 [DOI] [PubMed] [Google Scholar]
- 5. Montgomery E, Bronner MP, Goldblum JR, Greenson JK, Haber MM, Hart J, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Human Pathol. 2001;32:368–78. 10.1053/hupa.2001.23510 [DOI] [PubMed] [Google Scholar]
- 6. Curvers WL, ten Kate FJ, Krishnadath KK, Visser M, Elzer B, Baak LC, et al. Low‐grade dysplasia in Barrett's esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105:1523–30. 10.1038/ajg.2010.171 [DOI] [PubMed] [Google Scholar]
- 7. Kaye PV, Haider SA, Ilyas M, James PD, Soomro I, Faisal W, et al. Barrett's dysplasia and the Vienna classification: reproducibility, prediction of progression and impact of consensus reporting and p53 immunohistochemistry. Histopathology. 2009;54:699–712. 10.1111/j.1365-2559.2009.03288.x [DOI] [PubMed] [Google Scholar]
- 8. Pohl H, Pech O, Arash H, Stolte M, Manner H, May A, et al. Length of Barrett's oesophagus and cancer risk: implications from a large sample of patients with early oesophageal adenocarcinoma. Gut. 2016;65:196–201. 10.1136/gutjnl-2015-309220 [DOI] [PubMed] [Google Scholar]
- 9. Pohl H, Wrobel K, Bojarski C, Voderholzer W, Sonnenberg A, Rösch T, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol. 2013;108:200–7. 10.1038/ajg.2012.387 [DOI] [PubMed] [Google Scholar]
- 10. de Jonge PJ, Steyerberg EW, Kuipers EJ, Honkoop P, Wolters LMM, Kerkhof M, et al. Risk factors for the development of esophageal adenocarcinoma in Barrett's esophagus. Am J Gastroenterol. 2006;101:1421–9. 10.1111/j.1572-0241.2006.00626.x [DOI] [PubMed] [Google Scholar]
- 11. Anandasabapathy S, Jhamb J, Davila M, Wei C, Morris J, Bresalier R. Clinical and endoscopic factors predict higher pathologic grades of Barrett dysplasia. Cancer. 2007;109:668–74. 10.1002/cncr.22451 [DOI] [PubMed] [Google Scholar]
- 12. Bani‐Hani KE, Bani‐Hani BK, Martin IG. Characteristics of patients with columnar‐lined Barrett's esophagus and risk factors for progression to esophageal adenocarcinoma. World J Gastroenterol. 2005;11:6807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vaughan TL, Dong LM, Blount PL, Ayub K, Odze RD, Sanchez CA, et al. Non‐steroidal anti‐inflammatory drugs and risk of neoplastic progression in Barrett's oesophagus: a prospective study. Lancet Oncol. 2005;6:945–52. 10.1016/s1470-2045(05)70431-9 [DOI] [PubMed] [Google Scholar]
- 14. Kastelein F, Spaander MC, Steyerberg EW, Biermann K, Valkhoff VE, Kuipers EJ, et al. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett's esophagus. Clinical Gastroenterol Hepatol. 2013;11:382–8. 10.1016/j.cgh.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 15. Hardikar S, Onstad L, Blount PL, Odze RD, Reid BJ, Vaughan TL. The role of tobacco, alcohol, and obesity in neoplastic progression to esophageal adenocarcinoma: a prospective study of Barrett's esophagus. PloS One. 2013;8:e52192. 10.1371/journal.pone.0052192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rugge M, Zaninotto G, Parente P, Zanatta L, Cavallin F, Germanà B, et al. Barrett's esophagus and adenocarcinoma risk: the experience of the North‐Eastern Italian Registry (EBRA). Ann Surg. 2012;256:788–95. 10.1097/SLA.0b013e3182737a7e [DOI] [PubMed] [Google Scholar]
- 17. Jung KW, Talley NJ, Romero Y, Katzka DA, Schleck CD, Zinsmeister AR, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett's esophagus: a population‐based study. Am J Gastroenterol. 2011;106:1447–55. 10.1038/ajg.2011.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nguyen DM, El‐Serag HB, Henderson L, Stein D, Bhattacharyya A, Sampliner RE. Medication usage and the risk of neoplasia in patients with Barrett's esophagus. Clinical Gastroenterol Hepatol. 2009;7:1299–304. 10.1016/j.cgh.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wani S, Falk G, Hall M, Gaddam S, Wang A, Gupta N, et al. Patients with nondysplastic Barrett's esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clinical Gastroenterol Hepatol. 2011;9:220–7. 10.1016/j.cgh.2010.11.008 [DOI] [PubMed] [Google Scholar]
- 20. Solanky D, Krishnamoorthi R, Crews N, Johnson M, Wang K, Wolfsen H, et al. Barrett esophagus length, nodularity, and low‐grade dysplasia are predictive of progression to esophageal adenocarcinoma. J Clin Gastroenterol. 2019;53(5):361–5. 10.1097/mcg.0000000000001027 [DOI] [PubMed] [Google Scholar]
- 21. Parasa S, Vennalaganti S, Gaddam S, Vennalaganti P, Young P, Gupta N, et al. Development and validation of a model to determine risk of progression of Barrett's esophagus to neoplasia. Gastroenterology. 2018;154:1282–9. 10.1053/j.gastro.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 22. Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JHJM, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett's esophagus. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:1028–32. 10.1111/j.1572-0241.1998.00362.x [DOI] [PubMed] [Google Scholar]
- 24. Sampliner RE. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett's esophagus. Am J Gastroenterol. 2002;97:1888–95. 10.1111/j.1572-0241.2002.05910.x [DOI] [PubMed] [Google Scholar]
- 25. Curvers WL, van Vilsteren FG, Baak LC, Böhmer C, Mallant‐Hent RC, Naber AH, et al. Endoscopic trimodal imaging versus standard video endoscopy for detection of early Barrett's neoplasia: a multicenter, randomized, crossover study in general practice. Gastrointest Endosc. 2011;73:195–203. 10.1016/j.gie.2010.10.014 [DOI] [PubMed] [Google Scholar]
- 26. Levine DS, Haggitt RC, Blount PL, Rabinovitch PS, Rusch VW, Reid BJ. An endoscopic biopsy protocol can differentiate high‐grade dysplasia from early adenocarcinoma in Barrett's esophagus. Gastroenterology. 1993;105:40–50. [DOI] [PubMed] [Google Scholar]
- 27. Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Streiner DL. The case of the missing data: methods of dealing with dropouts and other research vagaries. Can J Psychiatry. 2002;47:68–75. [PubMed] [Google Scholar]
- 29. Yousef F, Cardwell C, Cantwell MM, Galway K, Johnston BT, Murray L. The incidence of esophageal cancer and high‐grade dysplasia in Barrett's esophagus: a systematic review and meta‐analysis. American J Epidemiol. 2008;168:237–49. 10.1093/aje/kwn121 [DOI] [PubMed] [Google Scholar]
- 30. de Jonge PJ, van Blankenstein M, Looman CW, Casparie MK, Meijer GA, Kuipers EJ. Risk of malignant progression in patients with Barrett's oesophagus: a Dutch nationwide cohort study. Gut. 2010;59:1030–6. 10.1136/gut.2009.176701 [DOI] [PubMed] [Google Scholar]
- 31. Krishnamoorthi R, Singh S, Ragunathan K, Visrodia K, Wang KK, Katzka DA, et al. Factors associated with progression of Barrett's esophagus: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2018;16(7):1046–55e8. 10.1016/j.cgh.2017.11.044 [DOI] [PubMed] [Google Scholar]
- 32. Duits LC, van der Wel MJ, Cotton CC, Phoa KN, ten Kate FJW, Seldenrijk CA, et al. Patients with Barrett's esophagus and confirmed persistent low‐grade dysplasia are at increased risk for progression to neoplasia. Gastroenterology. 2017;152:993–1001. 10.1053/j.gastro.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 33. Weusten B, Bisschops R, Coron E, Dinis‐Ribeiro M, Dumonceau JM, Esteban JM, et al. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2017;49:191–8. 10.1055/s-0042-122140 [DOI] [PubMed] [Google Scholar]
- 34. di Pietro M, Fitzgerald RC. Revised British Society of Gastroenterology recommendation on the diagnosis and management of Barrett's oesophagus with low‐grade dysplasia. Gut. 2017;67:392–93. 10.1136/gutjnl-2017-314135 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


