Abstract
Background
The 2020 postpolypectomy surveillance guideline update of European Society for Gastrointestinal Endoscopy defines a more restrictive group of individuals in need for surveillance 3 years after colonoscopy.
Aim
The aim of this cohort study was to validate the new guideline recommendation.
Methods
Based on a national quality assurance program, we compared the 2020 risk group definition with the previous 2013 recommendations for their strength of association with (1) colorectal cancer death, and (2) all‐cause death.
Results
A total of 265,608 screening colonoscopies were included in the study. Mean age was 61.1 years (SD ±9.0), and 50.6% were women. During a mean follow‐up of 59.3 months (SD ±35.0), 170 CRC deaths and 7723 deaths of any cause were identified. 62.4% of colonoscopies were negative and 4.9% were assigned to surveillance after 3 years according to the 2020 guidelines versus 10.4% following the 2013 guidelines, which corresponds to a relative reduction in colonoscopies by 47%. The strength of association with CRC mortality was markedly higher with the 2020 surveillance group as compared to the 2013 guidelines (HR 2.56, 95% CI 1.62–4.03 vs. HR 1.73, 95% CI 1.13–2.62), while the magnitude of association with CRC mortality for low risk individuals was lower (HR 1.17, 95% CI 0.83–1.63 vs. 1.25, 95% CI 0.88–1.76).
Conclusions
Adherence to the updated guidelines reduces the burden of surveillance colonoscopies by 47% while preserving the efficacy of surveillance in preventing CRC mortality.
Keywords: cancer, colonoscopy, colorectal cancer, CRC, mortality, polypectomy, risk‐stratification, screening, surveillance
Key summary
Summarize the established knowledge on this subject
Individuals who had colorectal polyps removed are advised to undergo surveillance; intervals are based on the number, size, and histopathological features of the resected lesions.
In 2020, the European Society for Gastrointestinal Endoscopy (ESGE) updated their postpolypectomy surveillance guideline.
What are the significant and/or new findings of this study?
The risk stratification introduced in the new guideline leads to a substantial reduction (by 47%) in the number of individuals in need for surveillance while preserving the efficacy of surveillance in preventing colorectal cancer mortality.
INTRODUCTION
Colorectal cancer (CRC) is the third most common malignant tumor and second leading cause of cancer deaths worldwide. 1 Screening colonoscopy aims to reduce CRC mortality through resection of early stage CRC or its precursor lesions polyps and adenomas. 2 Because of their increased risk for metachronous CRC lesions, individuals who had polyps or adenomas removed are advised to undergo surveillance. 3 , 4 , 5 , 6 The recommended time intervals are based on the number, size, and histopathological features of the resected lesions. 7 , 8 Due to increasing evidence on long‐term risk of CRC incidence and mortality after resection of premalignant lesions, the European Society for Gastrointestinal Endoscopy (ESGE) recently updated their postpolypectomy surveillance guidelines. 9 These recommendations are based on data suggesting that the criteria for individuals who are recommended surveillance after 3 years might have been too broadly defined and question the need of surveillance after 10 years. 3 , 4 , 10 , 11 , 12 However, data from randomized trials are still lacking and findings of ongoing trials are not anticipated before the mid‐2020. 13 , 14
Adequate surveillance strategies after polypectomy are crucial since shorter intervals increase both the burden of healthcare resources and the risk of serious complications for the screenees. If the surveillance interval is set too long, adenomas might have already progressed to invasive cancers, worsening the prognosis.
The aim of this study was to compare the performance of the 2020 to the 2013 postpolypectomy surveillance guideline. For validation of the new risk groups' stratification, we took advantage of an external screening colonoscopy data cohort, with well‐defined histopathological characterization of resected lesions and mortality data from the national death registry in all participants.
METHODS
This is a longtime follow‐up study of individuals who had undergone screening colonoscopy. Data derive from the Austrian quality assurance program of CRC screening. Details of the program have been described previously. 15 , 16 In brief, Austria implemented a colonoscopy‐based CRC screening program for average risk individuals starting by the age of 50 years in 2005. Because of the lack of obligatory quality control, a quality assurance program was implemented in 2007. Approximately half of all endoscopic centers, both hospitals and outpatient clinics, participate in this program. Screening colonoscopies and colonoscopies with screening character (individuals 30–49 years with family history of CRC or those who report fear of cancer) are included. Provided written informed consent by the patient, a standardized colonoscopy report form including patient demographics (age, sex) and details colonoscopy findings (bowel preparation quality, cecal intubation, number, size, localization and count of detected lesions, details on histology of the most advanced lesion) are transferred to the database of the quality assurance program.
Eligibility criteria
All colonoscopies performed within the quality assurance program between January 2007 and June 2018 were included in the study. In total, 265,608 of 287,649 colonoscopies were included in the analysis. Figure 1 depicts the study cohort flow‐chart.
FIGURE 1.
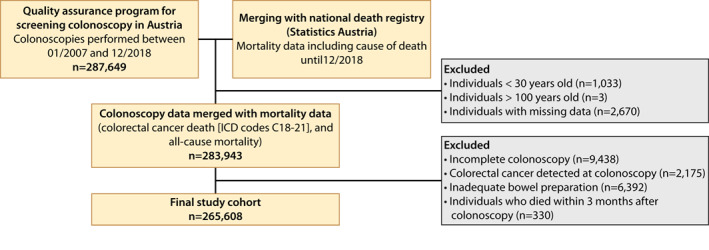
Patient flow
Postpolypectomy surveillance
The definition of surveillance groups and surveillance intervals according to the 2013 7 and 2020 9 guidelines are summarized in Table 1.
TABLE 1.
Stratification of postpolypectomy surveillance based on the Guidelines of 2020 and 2013
| Risk stratification after polypectomy | Cspy findings | Procedure | |
|---|---|---|---|
| 2020 Guideline | Surveillance | At least one adenoma ≥10 mm or with high‐grade dysplasia, or ≥5 adenomas, or any serrated polyp ≥10 mm or with dysplasia | Surveillance cspy after 3 years |
| No surveillance | 1–4 adenomas <10 mm with low‐grade dysplasia, or any serrated polyp <10 mm without dysplasia | No endoscopic surveillance, return to screening a | |
| 2013 Guideline | High‐risk | At least one adenoma ≥10 mm, or high‐grade dysplasia, or tubulovillous or villous histology, or serrated lesions ≥10 mm or with dysplasia | Surveillance cspy after 3 years |
| Low‐risk | 1–2 tubular adenomas <10 mm and low‐grade dysplasia, or serrated lesions <10 mm without dysplasia | Surveillance cspy after 10 years |
Abbreviation: Cspy, colonoscopy.
If organized screening is not available, repeat colonoscopy 10 years after the index procedure is recommended.
The histology of resected adenomas or polyps was classified according to the World Health Organization (WHO) classification. 17
Restrictive surveillance groups for systems with limited resources
The 2020 guideline update suggests considering surveillance only for adenomas ≥20 mm or with high‐grade dysplasia in context of a health system with limited capacity. 9 This recommendation is based on one single study 10 and the importance of future research on the impact of adenomas size is highlighted. We performed separate analyses for CRC mortality and overall mortality stratified by the restricted surveillance cohort for systems with limited resources to evaluate feasibility of this approach.
Ascertainment of CRC mortality and overall mortality
To identify deaths and causes of deaths, we used the national death registry (Statistics Austria), an institution with public rights established to provide federal statistics. All deaths, including cause of death based on the International Classification of Diseases (ICD), were recorded until end of December 2018. CRC deaths were defined as ICD‐10 codes C18‐21.
Statistical analysis
Demographic characteristics and colonoscopy findings are presented as mean and standard deviation (SD) as well as total numbers and percentage, as appropriate. Individuals were followed starting three month after colonoscopy, which corresponds to the time of the clinical work‐up. The primary endpoint was defined as CRC death, the secondary endpoint as all‐cause death. Administrative follow‐up was available until end of December 2018 in all participants.
CRC mortality and all‐cause mortality rates stratified by risk group according to the guidelines 7 , 9 were described by Kaplan–Meier estimators. We performed Cox regression analyses, adjusted for sex and age, to assess the association of the 2020 and the 2013 risk stratification with (1) CRC death and (2) all‐cause death. Risk stratification in both guidelines distinguishes negative colonoscopy from low‐risk and high‐risk groups. Adjusted hazard ratios (HR) and 95% confidence intervals (CI) are displayed with negative colonoscopy as being the reference group.
To compare the overall performance of both risk stratification groups (2020 and 2013 guidelines), we calculated and compared the area under the curve (ROC) to predict (1) CRC death and (2) all‐cause death. The level of significance was set to p < 0.05. All analyses were performed using dedicated software (StataCorp. 2017. Stata Statistical Software: Release 15: StataCorp LLC).
RESULTS
Cohort characteristics
A total of 265,608 individuals were included in the final cohort. Mean age was 61.1 years (SD ±9.0), and 50.6% were women (Table 2). Mean follow‐up time was 59.3 months (SD ±35.0) with a maximum follow‐up of 146 months.
TABLE 2.
Baseline characteristics
| All individuals 265,608 (100%) | |
|---|---|
| Female, n (%) | 134,412 (50.6%) |
| Age—years, mean ± SD | 61.1 ± 9.0 |
| <50 years, n (%) | 9296 (3.5%) |
| 59–59 years, n (%) | 124,836 (47.0%) |
| 60–69 years, n (%) | 82,119 (30.9%) |
| 70–79 years, n (%) | 43,443 (16.4%) |
| ≥80 years, n (%) | 5914 (2.2%) |
| 2020 Guideline | |
| No adenoma, n (%) | 165,788 (62.4%) |
| No surveillance, n (%) | 86,866 (32.7%) |
| Surveillance, n (%) | 12,954 (4.9%) |
| 2013 Guideline | |
| No adenoma, n (%) | 165,788 (62.4%) |
| Low‐risk, n (%) | 72,113 (27.2%) |
| High‐risk, n (%) | 27,707 (10.4%) |
A total of 170 CRC deaths (61 women, 109 men), and 7723 deaths of any cause (2812 women, 4011 men) were identified. The cumulative CRC mortality and all‐cause mortality rates were 0.06% (0.05% of all women and 0.08% of all men) and 2.91% (2.1% of all women, 3.7% of all men), respectively.
CRC mortality stratified by risk group
A total of 62.4% colonoscopies were negative. Following the 2020 guidelines, 4.9% of the screening population was assigned to surveillance after three years (surveillance group, 2020 guideline), versus 10.4% following the 2013 guidelines (high‐risk group, 2013 guideline).
The strength of association with CRC mortality was markedly higher for the surveillance group (2020 guideline) as compared to the high‐risk group (2013 guidelines) (HR 2.56, 95% CI 1.62–4.03 vs. HR 1.73, 95% CI 1.13–2.62). In contrast, the magnitude of association with CRC mortality was lower for the no‐surveillance group (2020 guidelines) compared to the low‐risk group (2013 Guidelines) (HR 1.17, 95% CI 0.83–1.63 and HR 1.25, 95% CI 0.88–1.76) (Figure 2), indicating improved risk stratification in the 2020 guidelines for both high‐risk and low‐risk individuals.
FIGURE 2.
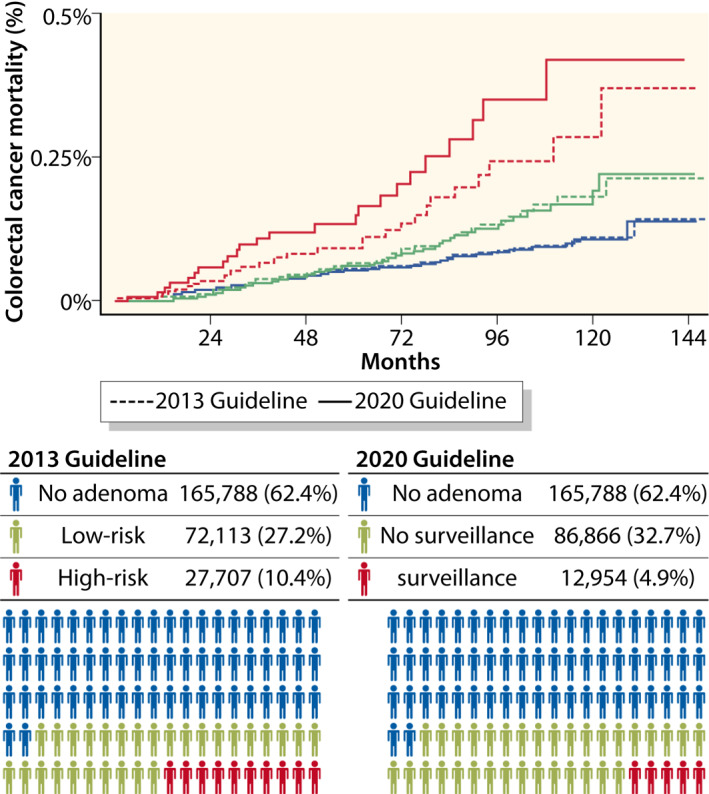
Colorectal cancer mortality after colonoscopy stratified by surveillance groups according to the 2020 Guidelines 9 compared to the 2013 Guidelines. 7 No adenoma: individuals with negative colonoscopy (no conventional adenoma or serrated polyp). Surveillance: individuals with at least one adenoma ≥10 mm or with high‐grade dysplasia, or ≥5 adenomas, or any serrated polyp ≥10 mm or with dysplasia. No surveillance: individuals with 1–4 adenomas <10 mm with low‐grade dysplasia, or any serrated polyp <10 mm without dysplasia. High‐risk: individuals with at least one adenoma ≥10 mm, or high‐grade dysplasia, or tubulovillous or villous histology, or serrated lesions ≥10 mm or with dysplasia. Low‐risk: individuals with 1–2 tubular adenomas <10 mm and low‐grade dysplasia, or serrated lesions <10 mm without dysplasia
Findings were consistent across all age groups with smaller impact in individuals of age 80 years and older (Figure 3).
FIGURE 3.
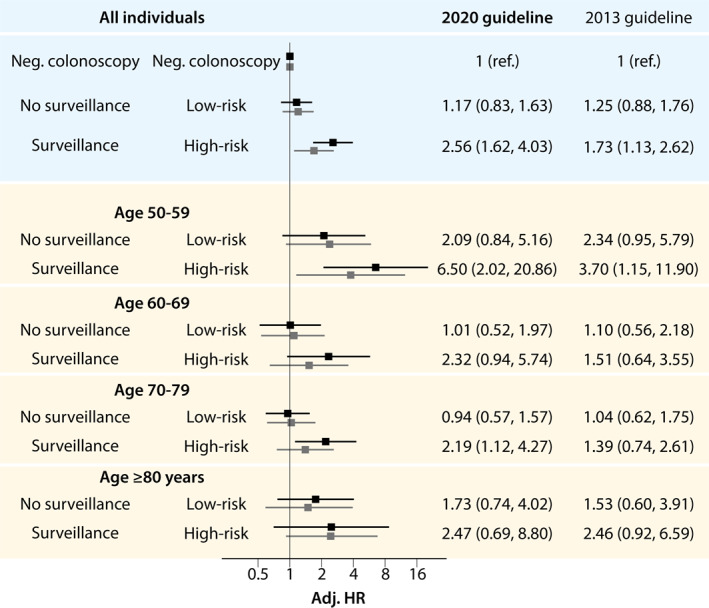
Hazard ratio for colorectal cancer mortality stratified by surveillance groups according to the 2020 Guidelines 9 compared to the 2013 Guidelines. 7 Age and sex adjusted for all individuals, and sex adjusted according to age group. Surveillance: individuals with at least one adenoma ≥10 mm or with high‐grade dysplasia, or ≥5 adenomas, or any serrated polyp ≥10 mm or with dysplasia. No surveillance: individuals with 1–4 adenomas <10 mm with low‐grade dysplasia, or any serrated polyp <10 mm without dysplasia. High‐risk: individuals with at least one adenoma ≥10 mm, or high‐grade dysplasia, or tubulovillous or villous histology, or serrated lesions ≥10 mm or with dysplasia. Low‐risk: individuals with 1–2 tubular adenomas <10 mm and low‐grade dysplasia, or serrated lesions <10 mm without dysplasia
All‐cause mortality
Similar to CRC death findings, the surveillance group (2020 guideline) showed a stronger association with all‐cause mortality as compared to the high‐risk group (2013 guideline) (HR 1.43, 95% CI 1.32–1.55 vs. HR 1.29, 95% CI 1.20–1.38). The association for the no‐surveillance group (2020 guideline) was almost identical to the low‐risk group (2013 guideline) (HR 1.06, 95% CI 1.01–1.11 vs. HR 1.05, 95% CI 0.99–1.10).
Restrictive surveillance group for systems with limited resources
Restricting the definition of the surveillance group to colonoscopies with an adenoma or any serrated polyp ≥20 mm or with high‐grade dysplasia, 1.3% of all colonoscopies would be assigned to the surveillance group as compared to 4.9% in the conventional 2020 guideline definition. The association of this restrictive surveillance group with CRC mortality was stronger as compared to the conventional 2020 surveillance group (HR 4.15, 95% CI 2.15–8.02 vs. 2.56, 95% CI 1.62–4.03) while the difference in the low‐risk group was small (HR 1.27, 95% CI 0.93–1.74 vs. 1.17, 95% CI 0.84–1.63, Figure 4). Similar trends were observed with all‐cause mortality (HR 1.65, 95% CI 1.43–1.90 vs. HR 1.43, 95% CI 1.32–1.55 and 1.09, 95% CI 1.04–1.45 vs. HR 1.06, 95% CI 1.01–1.11, respectively).
FIGURE 4.
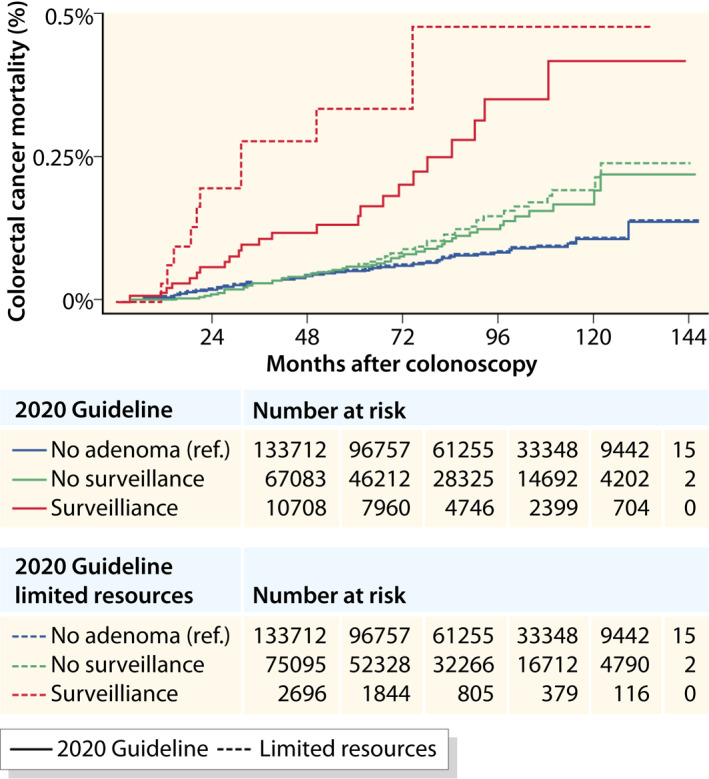
Colorectal cancer mortality after colonoscopy stratified by risk group according to the 2020 Guideline 9 compared to the proposed limited surveillance group for countries with limited resources. No adenoma: individuals with negative colonoscopy (no conventional adenoma or serrated polyp). Surveillance: individuals with at least one adenoma ≥10 mm or with high‐grade dysplasia, or ≥5 adenomas, or any serrated polyp ≥10 mm or with dysplasia. No surveillance: individuals with 1–4 adenomas <10 mm with low‐grade dysplasia, or any serrated polyp <10 mm without dysplasia. High‐risk: individuals with at least one adenoma ≥10 mm, or high‐grade dysplasia, or tubulovillous or villous histology, or serrated lesions ≥10 mm or with dysplasia. Low‐risk: individuals with 1–2 tubular adenomas <10 mm and low‐grade dysplasia, or serrated lesions <10 mm without dysplasia
Receiver operating characteristics
The area under the curve (AUC) for prediction of CRC mortality was 0.780 for the 2020 and 0.775 for the 2013 guidelines (p = 0.056). For all‐cause mortality, the AUC was 0.766 for the 2020 guidelines and 0.765 for the 2013 guidelines (p < 0.001). The prediction model of a surveillance group in systems with limited resources the area under the curve was 0.776 for CRC mortality and 0.765 for all‐cause mortality (p = 0.281 and p < 0.001 when compared with the conventional 2020 recommendations) (Figure S1a–d).
DISCUSSION
In this study, we provide the first external validation of the 2020 postpolypectomy guideline update of the ESGE. 9 Our findings show that the new recommendations lead to a substantial reduction in the burden of surveillance colonoscopies with an equally adequate selection of patients for surveillance in terms of CRC mortality. The number of patients in need of surveillance was reduced by 5.5% from 10.4% to 4.9%, which corresponds to a relive reduction in colonoscopies by 47%. The more restrictive surveillance group for systems with limited resources reduced the burden of surveillance colonoscopy by 9.1% to 1.3%.
The discovery that CRC does not arise de novo but from preexisting lesions and the growing knowledge on the pathophysiological background on polyps and adenomas opened door for the idea of early detection and screening. 18 Large randomized controlled trials showed a significant reduction in mortality, first for stool‐based screening and, as technology evolved, for endoscopic screening. 19 , 20 , 21 , 22 , 23 The subsequential implementation of large‐scale CRC screening programs came at the costs of the increasing need of surveillance, resulting in a major burden for healthcare systems. In the United States, surveillance after polyp or adenoma removal is the most common indication for colonoscopy for individuals aged more than 74 years. 24 Whether patients truly benefit from this high amount of surveillance effort or whether we are doing too much is controversial. In contrast to the basic concept of CRC screening, surveillance intervals are based on retrospective studies and expert opinions, 7 , 8 whereas randomized trials are still ongoing. 13 , 14 Nevertheless, there is growing evidence to question the need of intensive surveillance as currently performed. Recent large retrospective studies provide data on long‐term risk of CRC and death after adenoma removal, which affirm the assumption of a very low malignant potential of low‐risk adenomas or serrated polyps. 25 , 26 It was considered feasible to extend the surveillance interval to 17 years after a negative colonoscopy if the colonoscopy was performed with high quality. 27 Moreover, data suggest that fewer individuals benefit from an intensive surveillance of 3 years as currently assumed. 3 , 4 , 10 , 11
Based on this recent evidence, the group of individuals in need of surveillance after 3 years was restricted to those with adenomas ≥10 mm, adenomas with high‐grade dysplasia, ≥5 adenomas, or any serrated polyp ≥10 mm or with dysplasia in the new 2020 guidelines. According to the present study, this more restrictive definition of the group of patients in need for surveillance after three years led to a significant reduction in the burden of surveillance colonoscopies. While following the 2013 guidelines, 10.4% of the 265,608 screened individuals would be recommended surveillance after 3 years, this volume was reduced to 4.9%, which corresponds to a reduction of 14,753 colonoscopies in the present cohort. Importantly, this substantial reduction in costs and saving of resources is not at the expense of CRC mortality and overall mortality. Moreover, the 2020 guidelines provide efficient considerations for health care systems with limited resources. However, the proposed high‐risk group limited to adenomas ≥20 mm or with high‐grade dysplasia 10 has not been adopted as general recommendation because of limited evidence. Importantly, our study now confirms the benefit of this restrictive high‐risk group in terms of identifying individuals at highest risk, at the cost of a small increase of CRC and all‐cause death risk in the group who would not be assigned to surveillance. This approach reduces the percentage of colonoscopies further to 1.3%.
Strengths of the present study include a well‐defined primary screening colonoscopy cohort. In contrast to flexible sigmoidoscopy, which is limited to inspection of the left‐sided colon, colonoscopy investigates also the right‐sided colon, the predominate location of serrated lesions. 3 , 4 , 6 , 12 , 28 Another strength is the availability of outcome data of CRC mortality and all‐cause mortality rather than advanced adenomas or CRC since the goal of screening is ultimately the reduction of mortality rates. Moreover, the study design avoided potential bias of pre‐selected or unbalanced patient cohorts. 26 Finally, the large study cohort provides details on colonoscopy findings and assessment of quality parameters (e.g., bowel preparation quality, cecal intubation). The validity of data deriving from colonoscopies of the 1980s or 1990s might be limited due to the lack of awareness of the importance of a high quality baseline colonoscopy, 29 which might result in misleading colonoscopy findings, particularly for small and flat or serrated lesions. 5 , 26
Study limitations result from updates in colonoscopy report forms during the study period. Bowel preparation quality was not assessed before 2012, however since its implementation, preparation quality was adequate in more than 90% of colonoscopes. At the beginning of study period, polyp count was assessed categorically as “1,” “2–4,” and “>4.” Colonoscopies with “2–4” polyps detected were assigned to the low‐risk group (2013 guideline) because after implementation of the exact polyp size the majority of the of this group was procedures with two lesions. However, recent studies considered multiplicity adenomas relevant only for colonoscopies ≥5 adenomas. 3 , 4 , 10 , 30 Finally, we did not assess life style risk factors for CRC that is smoking or alcohol intake. However, risk‐based screening concepts, with the exception of demographic data, for which the results are adjusted for, are not implemented in current screening recommendations.
In conclusion, the new 2020 postpolypectomy guideline leads to a substantial reduction in the number of individuals in need for surveillance saving costs and resources. The burden of surveillance colonoscopies decreased by 47% while the efficacy of surveillance in preventing CRC mortality was preserved.
CONFLICT OF INTERESTS
The authors have no conflicts of interest to declare.
Supporting information
Supplementary Material
Waldmann E, Kammerlander A, Gessl I , Penz D, Majcher B, Hinterberger A, et al. New risk stratification after colorectal polypectomy reduces burden of surveillance without increasing mortality. United European Gastroenterol J. 2021;9(8):947–54. 10.1002/ueg2.12119
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68 (6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp‐Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long‐term prevention of colorectal‐cancer deaths. N Engl J Med. 2012;366 (8):687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atkin W, Wooldrage K, Brenner A, Martin J, Shah U, Perera S, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18 (6):823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Click B, Pinsky PF, Hickey T, Doroudi M, Schoen RE. Association of colonoscopy adenoma findings with long‐term colorectal cancer incidence. Jama. 2018;319(19):2021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cottet V, Jooste V, Fournel I, Bouvier AM, Faivre J, Bonithon‐Kopp C. Long‐term risk of colorectal cancer after adenoma removal: a population‐based cohort study. Gut. 2012;61(8):1180–6. [DOI] [PubMed] [Google Scholar]
- 6. Song M, Emilsson L, Bozorg SR, Nguyen LH, Joshi AD, Staller K, et al. Risk of colorectal cancer incidence and mortality after polypectomy: a Swedish record‐linkage study. Lancet. 2020;5:537‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hassan C, Quintero E, Dumonceau JM, Regula J, Brandão C, Chaussade S, et al. Post‐polypectomy colonoscopy surveillance: European Society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2013;45(10):842–64. [DOI] [PubMed] [Google Scholar]
- 8. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi‐Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844–57. [DOI] [PubMed] [Google Scholar]
- 9. Hassan C, Antonelli G, Dumonceau JM, Regula J, Bretthauer M, Chaussade S, et al. Post‐polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline – update 2020. Endoscopy. 2020;52(8):687–700. [DOI] [PubMed] [Google Scholar]
- 10. Wieszczy P, Kaminski MF, Franczyk R, Loberg M, Kobiela J, Rupinska M, et al. Colorectal cancer incidence and mortality after removal of adenomas during screening colonoscopies. Gastroenterology. 2020;158(4):875–83. [DOI] [PubMed] [Google Scholar]
- 11. Erichsen R, Baron JA, Hamilton‐Dutoit SJ, Snover DC, Torlakovic EE, Pedersen L, et al. Increased risk of colorectal cancer development among patients with serrated polyps. Gastroenterology. 2016;150(4):895–902. [DOI] [PubMed] [Google Scholar]
- 12. Holme O, Loberg M, Kalager M, Bretthauer M, Hernán MA, Aas E, et al. Long‐term effectiveness of sigmoidoscopy screening on colorectal cancer incidence and mortality in women and men: a randomized trial. Ann Intern Med. 2018;168(11):775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jover R, Bretthauer M, Dekker E, Holme O, Kaminski MF, Løberg M, et al. Rationale and design of the European polyp surveillance (EPoS) trials. Endoscopy. 2016;48(6):571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colonoscopy versus fecal immunochemical test in reducing mortality from colorectal cancer (CONFIRM). ClinicalTrials.gov: NCT01239082. https://clinicaltrials.gov/ct2/show/NCT01239082. Accessed 3 Jun 2015.
- 15. Ferlitsch M, Reinhart K, Pramhas S, Wiener C, Gal O, Bannert C, et al. Sex‐specific prevalence of adenomas, advanced adenomas, and colorectal cancer in individuals undergoing screening colonoscopy. Jama. 2011;306 (12):1352–8. [DOI] [PubMed] [Google Scholar]
- 16. Waldmann E, Gessl I, Sallinger D, Jeschek P, Britto‐Arias M, Heinze G, et al. Trends in quality of screening colonoscopy in Austria. Endoscopy. 2016;48 (12):1102–9. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organisation . Classification of tumors of the digestive tract. Lyon: IARC Press; 2019. [Google Scholar]
- 18. Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal‐tumor development. N Engl J Med. 1988;319 (9):525–32. [DOI] [PubMed] [Google Scholar]
- 19. Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328 (19):1365–71. [DOI] [PubMed] [Google Scholar]
- 20. Kronborg O, Fenger C, Worm J, Pedersen SA, Hem J, Bertelsen K, et al. Causes of death during the first 5 years of a randomized trial of mass screening for colorectal cancer with fecal occult blood test. Scand J Gastroenterol. 1992;27 (1):47–52. [DOI] [PubMed] [Google Scholar]
- 21. Hardcastle JD, Thomas WM, Chamberlain J, Balfour TW, Armitage NC, Thomas WM, et al. Randomised, controlled trial of faecal occult blood screening for colorectal cancer. Results for first 107,349 subjects. Lancet. 1989;1 (8648):1160–4. [DOI] [PubMed] [Google Scholar]
- 22. Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329 (27):1977–81. [DOI] [PubMed] [Google Scholar]
- 23. Atkin W, Wooldrage K, Parkin DM, Kralj‐Hans I, MacRae E, Shah U, et al. Long term effects of once‐only flexible sigmoidoscopy screening after 17 years of follow‐up: the UK Flexible Sigmoidoscopy Screening Randomised Controlled Trial. Lancet. 2017;389 (10076):1299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lieberman DA, Williams JL, Holub JL, Morris CD, Logan JR, Eisen GM, et al. Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc. 2014;80(1):133–43. [DOI] [PubMed] [Google Scholar]
- 25. Lee JK, Jensen CD, Levin TR, Doubeni CA, Zauber AG, Chubak J, et al. Long‐term risk of colorectal cancer and related death after adenoma removal in a large, community‐based population. Gastroenterology. 2020;158(4):884–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He X, Hang D, Wu K, Nayor J, Drew DA, Giovannucci EL, et al. Long‐term risk of colorectal cancer after removal of conventional adenomas and serrated polyps. Gastroenterology. 2020;158 (4):852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pilonis ND, Bugajski M, Wieszczy P, Franczyk R, Didkowska J, Wojciechowska U, et al. Long‐term colorectal cancer incidence and mortality after single negative screening colonoscopy. Ann Intern Med. 2020:173:81‐91. [DOI] [PubMed] [Google Scholar]
- 28. Crockett SD, Nagtegaal ID. Terminology, molecular features, epidemiology, and management of serrated colorectal neoplasia. Gastroenterology. 2019;157(4):949–66. [DOI] [PubMed] [Google Scholar]
- 29. Kaminski MF, Thomas‐Gibson S, Bugajski M, Bretthauer M, Rees CJ, Dekker E, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. United Eur Gastroenterol J. 2017;5(3):309–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vemulapalli KC, Rex DK. Risk of advanced lesions at first follow‐up colonoscopy in high‐risk groups as defined by the United Kingdom post‐polypectomy surveillance guideline: data from a single U.S. center. Gastrointest Endosc. 2014;80 (2):299–306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


