Abstract
The increase in outbreaks of Chagas disease in Colombia has required the strengthening of entomological surveillance with the active participation of the affected communities and the monitoring of the natural infection of the collected kissing bugs recollected inside households. The natural infection with Trypanosoma cruzi of triatomines collected by inhabitants of some municipalities of the department of Antioquia in 2019 was evaluated by molecular methods. This study described the intradomiciliary presence of Panstrongylus geniculatus (Latreille, 1811) in four cities of Antioquia: Barbosa, Liborina, Ituango, and Puerto Triunfo. This vector is reported for the first time in the municipalities Liborina, Barbosa, and Ituango. Furthermore, the natural infection with T. cruzi, DTUI, was reported in Barbosa and Liborina. The epidemiological implications of these findings are analyzed within the context of recent reports of outbreaks of Chagas disease in Antioquia.
Keywords: Colombia, Chagas disease, DTUI, Triatomines, Trypanosoma cruzi
1. Introduction
Chagas disease is a neglected tropical disease that affects approximately six million people in Latin America, resulting in about 12,000 deaths annually (World Health Organization WHO, 2019). The etiological agent, Trypanosoma cruzi (Chagas, 1909) (Kinetoplastida, Trypanosomatidae), is a hemotrophic protozoan that exhibits broad intraspecific genetic diversity, and it is classified into six Discrete Typing Units (DTUs) named as TcI to TcVI (Zingales et al., 2009). In addition, there is also another genotype related to bats, designated as TcBat (World Health Organization WHO, 2019; Marcili et al., 2009; Ramirez et al., 2014). The circulation of all DTU's has been described in Colombia, with TcI being the most widely distributed DTU in Colombia (Guhl and Ramírez, 2013).
Trypanosoma cruzi is mainly transmitted by insects of the subfamily Triatominae (Hemiptera; Reduviidae). In Colombia, 26 triatomines species have been recorded, of which seventeen are naturally infected with the parasite, with species of the genera Rhodnius (Stål, 1859), Triatoma (Laporte, 1832), and Panstrongylus (Berg, 1879) being of the most significant epidemiologically (Guhl et al., 2007; Sandoval et al., 2007). Panstrongylus geniculatus (Latreille, 1811) is a triatomine with a wide geographic distribution in Colombia, reported in 28 departments and 268 municipalities, including Antioquia (Guhl et al., 2007; Ríos and Galeano, 2015). The department of Antioquia is located in the central region of the Colombian Andes and extends to the coast of the Caribbean Sea in the Gulf of Darien. Historically, it is considered to have low endemism for Chagas disease, with only isolated chronic cases found primarily in the Urabá region (Restrepo et al., 1999). However, in recent years, increasing reports of acute cases related to oral outbreaks have shown the need to perform systematic entomological surveillance for Chagas, especially on vectors that have been involved in oral transmission (Instituto Nacional de Salud (INS), 2019). The main goal of the present study was to provide current information regarding the presence of triatomines in four municipalities of the Antioquia department.
2. Materials and methods
Triatomine specimens were caught and brought to the outpatient SIU (acronym in Spanish of Sede de Investigación Universitaria -University Research Headquarters) unit of the University of Antioquia in 2019. When possible, precise instructions were delivered regarding biosecurity measures for the capture of specimens, and when they were present at the SIU, the scientific team instructed patients regarding several triatomine species present in the Antioquia department. Triatomine specimens were also caught and brought to the SIU by the inhabitants of four municipalities of the department: Liborina, which is located in the Western subregion (6°40′41″N, 75°48′44″W), Barbosa, located in the Aburrá Valley metropolitan area subregion of Medellín (6°26′15″N, 75°19′50″W), Puerto Triunfo, located in the northeast subregion (5°52′18.58″N, 74°38′24.57″W), and Ituango, located in the north subregion (7°10′15.78″N, 75°45′51.08″W) (Fig. 1). Triatomine specimens were transported to the laboratory, registered and identified using taxonomic keys (Lent and Wygodzinsky, 1979). The University of Antioquia has a permit from the national environmental authority to collect biological specimens for research purposes No 0524–27-05-2014. In addition, Ethical approval (Act No 113 of 2017) for analyzing triatomine species was obtained from the animal ethics committee of the Antioquia University.
Fig. 1.
Map of Colombia and the Antioquia Department, highlighting the municipality where samples of P. geniculatus were collected: Ituango (IT), Liborina (LI), Barbosa (BA), and Puerto Triunfo (PT).
All triatomines were evaluated for T. cruzi infection using molecular methods. Feces were obtained through abdominal compression and diluted in 300 μL of sterile PBS pH 7.2. Genomic DNA was extracted from 200 μL of feces using the Genomic DNA purification kit (Qiagen DNeasy Blood & Tissue kit, Germantown, MD USA) following the manufacturer's instructions. All DNA preparations were screened to test for T. cruzi using conventional PCR targeting satellite DNA. Positive T. cruzi samples were also analyzed for molecular discrimination of T. cruzi DTU's based on amplifying the spliced leader intergenic region (SL-IR) gene using the primers TCC, TC1, and TC2, as previously reported (Burgos et al., 2007) and 24Sα ribosomal RNA gene was achieved with the primers D71 and D72 as described in (Souto and Zingales, 1993). Amplification products were run on a 1.5% agarose gel stained with ethidium bromide and visualized under UV light.
3. Results and discussion
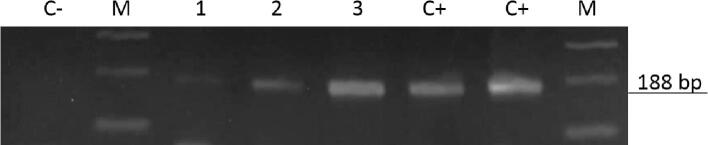
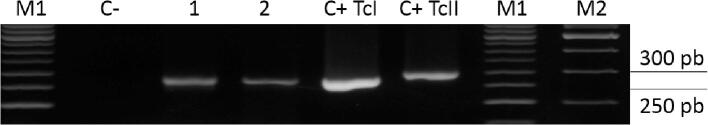
Five triatomines, including three females and two males, were captured: two at Puerto Triunfo, one at Liborina, one at Barbosa, and one at Ituango. All triatomines were taxonomically identified as P. geniculatus (Fig. 2). The specimens collected at Liborina and Barbosa were positive for T. cruzi DTU I, while the triatomine specimens collected at Puerto Triunfo and Ituango tested negative for T. cruzi infections (Fig. 3 and B). Regarding the P. geniculatus specimen captured at Liborina, it is noteworthy that it was collected in the urban area at the heart of the municipality. In contrast, the kissing bug from Barbosa was captured in a rural area in the village of Aguas Claras (6°29′25″N, 75°15′0.38″W). Finally, the triatomines from Puerto Triunfo were captured in a rural area, in a place called Rio Claro (5°53′58.80″N, 74°51′15.56″W) and the village of Las Aguitas, which is a rural area of Ituango (7°10′42.56″N, 75°36′40.86″W) (Fig. 1).
Fig. 2.

Dorsal view of two specimens of Panstrogylus geniculatus. A. Specimen captured in Liborina, length 23 mm. B. Specimen captured in Barbosa, length 27 mm.
Fig. 3.
A. Detection of T. cruzi satellite DNA in feces of triatomines caught in Liborina and Barbosa, Antioquia. C-: Negative amplification control, M: Molecular weight marker 100-bp, 1) P. geniculatus, 2) P. geniculatus Liborina, 3) P. geniculatus Barbosa, C+: Positive control (gDNA from T. cruzi Gal61 strain (a fragment of 188-bp). The products were separated on a 1.5% agarose gel and visualized using ethidium bromide staining. B. Detection of T. cruzi DTU I in feces of triatomines caught in Liborina and Barbosa, Antioquia using PCR amplification of 24Sα ribosomal RNA gene. C-: Negative control, M1: Molecular weight marker 50-bp, M2: Molecular weight marker 100-bp 1) P. geniculatus Liborina, 2) P. geniculatus Barbosa, C + TcI: Positive control DTUI, C + TcII: Positive control TcII-TcVI. The products were separated on a 1.5% agarose gel and visualized using ethidium bromide staining.
Nine triatomine species have been reported in 42 municipalities of the Antioquia department, including P. humeralis (Usinger, 1939), Panstrongylus rufotuberculatus (Champion, 1899), Rhodnius pallescens (Barber, 1932), Rhodnius prolixus (Stål, 1859), Triatoma dimidiata (Latreille, 1811), T. dispar (Lent, 1950), T. venosa (Stål, 1872) and Erathyrus cuspidatus (Stål, 1859) (Guhl et al., 2007; Ríos and Galeano, 2015). P. geniculatus is the vector species with the broadest distribution and has been reported in 35 municipalities of Antioquia, between 2 and 2500 m above sea level (m.a.s.l), with a greater abundance in the western zone (Ríos and Galeano, 2015). In the present study, its presence in the municipalities of Liborina, Barbosa, and Ituango, which are located 700, 920, and 1550 m above sea level (m.a.s.l), respectively, is recorded for the first time (Fig. 1). According to available literature, historical reports from the Antioquia department have shown an increase in the distribution of this species. The first reports from the 1930s showed the presence of P. geniculatus in Medellín. Later, the presence of this species was reported in 33 municipalities (Guhl et al., 2007; Molina et al., 2000). The most recent report of this species in the department of Antioquia, from 2015, confirmed its presence in 35 municipalities (Ríos and Galeano, 2015). The present study reports its expansion to three new municipalities. The greater distribution of this species concurs with recent theories regarding increased dispersion of P. geniculatus as a consequence of passive dispersal by human migration or by mammals such as Didelphis marsupialis (Linnaeus, 1758), Dasypus novemcinctus (Linnaeus, 1758), and chiropterans (Caicedo-Garzón et al., 2019). However, other authors have suggested that the increase of intrusion into households by P. geniculatus is principally due to its attraction to light, deforestation, and an increase in urban and rural construction (Reyes-Lugo, 2009). Finally, we must consider that the capture of P. geniculatus in these new municipalities may result from increased community participation in entomological surveillance. The authors of the present study posit that all theories should be considered.
T. cruzi infection in P. geniculatus in Antioquia is scarce. The reports from the 1930s did not confirm the infection, and the first reports of infection were recorded in the Amalfi municipality, in the northeast region of the department (Wolff et al., 1994). Subsequently, Molina et al. reported the infection of this vector in San Carlos, Puerto Berrío, Urabá, and Vegachí (Antioquia) (Molina et al., 2000). While this species was reported in 33 municipalities in 2007, the distribution of infected P. geniculatus (Guhl et al., 2007) was not specified. In addition, the latest update (2015) showed that infection with Trypanosoma spp. was only found in one municipality in the Urabá sub-region (Ríos and Galeano, 2015). The present study showed infection with T. cruzi in the municipalities of Liborina and Barbosa, where more than 63,000 people live (10,028 and 53,167, respectively). In addition, the presence of P. geniculatus in Rio Claro, the area with the highest tourist demand in the Antioquia department, is another relevant finding. Other authors have reported the presence of P. geniculatus in Puerto Triunfo (Guhl et al., 2007). However, in northeastern Antioquia, many gaps exist in informing about the intrusion and presence of this species in homes. The present study reports the intradomiciliary presence of two specimens in the village of Rio Claro without infection. This finding is of pivotal importance if we consider the occurrence in 2019 of an outbreak of oral transmission of T. cruzi in a nearby village of this municipality called El Alto de Pollo, which resulted in the infection of seven people (Instituto Nacional de Salud (INS), 2019). This outbreak could likely be related to the intrusion of this vector in the municipality. This theory should be corroborated through entomological surveillance.
TcI was the lineage of T. cruzi found in the present study and is the most widely distributed DTU in Colombia (Marcili et al., 2009). However, the presence of the TcII lineage has also been reported in P. geniculatus in the Antioquia department (Guhl et al., 2007). P. geniculatus is a vector with a robust adaptive ability to utilize different food sources (Reyes-Lugo, 2009). Therefore, the entomological surveillance of this species should be strengthened due to its eco-epidemiological importance and its relationship to disease outbreaks due to oral transmission (Hernández et al., 2016).
An increasing amount of evidence suggests that P. geniculatus might become a relevant vector for Chagas disease in the Antioquia department in the coming years for three reasons: its geographical distribution, a record of domiciliation, and its association with oral outbreaks in Colombia and Venezuela. Therefore, the results presented here demand the creation of entomological surveillance programs that involve the entire populations of these municipalities and the development of systematic entomological sampling and serological evaluation of residents. In this way, the risk of transmission in these areas and the role of P. geniculatus as a vector in the Antioquia department may be diminished.
Financial support
Thanks to the University of Antioquia for providing financial support for this study.
Authors statement
Conceptualization and design of study: O. Cantillo-Barraza, A Vélez-Mira and O. Triana-Chávez.
Acquisition of data: S. Zuluaga, P. Mejía, J. Quintero, A. Vélez-Mira and O. Cantillo-Barraza.
Analysis and/ interpretation of data: S. Zuluaga, O. Triana-Chávez and O. Cantillo-Barraza.
Revising the manuscript critically for important intellectual content: O. Cantillo-Barraza and O. Triana-Chávez.
Approval of the version of the manuscript to be published: S. Zuluaga, P. Mejía, J, Quintero, A. Vélez, O, Triana-Chávez, and O. Cantillo-Barraza.
Declaration of Competing Interest
Authors have no conflict of interest to declare.
Acknowledgments
We would like to give sincere thanks to our patients for their participation in this study.
References
- Burgos J.M., Altcheh J., Bisio M., Duffy T., Valadares H.M.S., Seidenstein M.E. Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. Int. J. Parasitol. 2007;37(12):1319–1327. doi: 10.1016/j.ijpara.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Caicedo-Garzón V., Salgado-Roa F.C., Sánchez-Herrera M., Hernández C., Arias-Giraldo L.M., García L. Genetic diversification of Panstrongylus geniculatus (Reduviidae: Triatominae) in northern South America. PLoS One. 2019;14(10):1–18. doi: 10.1371/journal.pone.0223963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guhl F., Ramírez J.D. Retrospective molecular integrated epidemiology of Chagas disease in Colombia. Infect. Genet. Evol. 2013;20(0):148–154. doi: 10.1016/j.meegid.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Guhl F., Aguilera G., Pinto N., Vergara D. Updated geographical distribution and ecoepidemiology of the triatomine fauna (Reduviidae: Triatominae) in Colombia. Biomedica. 2007;27:143–162. [PubMed] [Google Scholar]
- Hernández C., Salazar C., Brochero H., Teherán A., Buitrago L.S., Vera M. Untangling the transmission dynamics of primary and secondary vectors of Trypanosoma cruzi in Colombia: parasite infection, feeding sources and discrete typing units. Parasit. Vectors. 2016;9(620):1–14. doi: 10.1186/s13071-016-1907-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto Nacional de Salud (INS) INS; Bogotá: 2019. BES Boletín epidemiológico semanal, semana 33. [Internet]https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2019%20Bolet%C3%ADn%20epidemiol%C3%B3gico%20semana%2033.pdf 2 pp. [Cited 2020 February 22]. Available from: [Google Scholar]
- Lent H., Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas disease. Bull. Am. Mus. Nat. Hist. 1979;163(3):123–520. [Google Scholar]
- Marcili A., Lima L., Cavazzana M., Junqueira A.C., Veludo H.H., Maia Da Silva F., Campaner M., Paiva F., Nunes V.L., Teixeira M.M. A new genotype of Trypanosoma cruzi associated with bats evidenced by phylogenetic analyses using SSU rDNA, cytochrome b and Histone H2B genes and genotyping based on ITS1 rDNA. Parasitology. 2009 May;136(6):641–655. doi: 10.1017/S0031182009005861. [DOI] [PubMed] [Google Scholar]
- Molina J.A., Gualdrón L.E., Brochero H.L., Olano V.A., Barrios D., Guhl F. Distribución actual e importancia epidemiológica de las especies de triatominos (Reduviidae: Triatominae) en Colombia. Biomédica. 2000;20(4):344–360. [Google Scholar]
- Ramirez J.D., Hernandez C., Montilla M., Zambrano P., Florez A.C., Parra E. First report of human Trypanosoma cruzi infection attributed to TcBat genotype. Zoonoses Public Health. 2014;61(7):477–479. doi: 10.1111/zph.12094. Epub 2014/10/07. (PMID: 25285940) [DOI] [PubMed] [Google Scholar]
- Restrepo M., Restrepo B.N., Parra G.J. ICMT; Antioquia, Córdoba, Bolívar: 1999. Informe Final. Programa Nacional de Prevención y Control de la enfermedad de Chagas y la cardiopatía infantil; pp. 50–70. [Google Scholar]
- Reyes-Lugo M. Panstrongylus geniculatus Latreille 1811 (Hemiptera: Reduviidae: Triatominae), vector de la enfermedad de Chagas en el ambiente domiciliario del centro-norte de Venezuela. Biomédica. 2009;20(3):180–205. [Google Scholar]
- Ríos J.F., Galeano A. In: Vigilancia de Triatominae (Hemiptera: Reduviidae) en Colombia. 1st ed. Parra G.J., Flórez M., Angulo V.M., editors. Editorial Sic Editorial Ltda; Bogotá D.C. Colombia: 2015. Capítulo 1. Vigilancia de Triatominae (Hemiptera: Reduviidae) en Antioquia; pp. 6–13. [Google Scholar]
- Sandoval C.M., Pabon E., Jurberg J., Galvậo C. Belminus ferroae n. sp. from the Colombian north-east, with a key to the species of the genus (Hemiptera: Reduviidae: Triatominae) Zooataxa. 2007;1443:55–64. doi: 10.11646/zootaxa.1443.1.5. [DOI] [Google Scholar]
- Souto R.P., Zingales B. Sensitive detection and strain classification of Trypanosoma cruzi by amplification of a ribosomal RNA sequence. Mol. Biochem. Parasitol. 1993;62:45–52. doi: 10.1016/0166-6851(93)90176-x. [DOI] [PubMed] [Google Scholar]
- Wolff M., Arboleda J., González C., Manotas L., Rueda A. Estudio de Tripanosomiasis americana. Municipio de Amalfi, Vereda Montebello. Bol. Epidemiol. Antioquia. 1994;29:302–305. [Google Scholar]
- World Health Organization (WHO) Chagas disease (American trypanosomiasis) Epidemiology. 2019;90:33–44. https://www.who.int/chagas/en/ [Cited 2020 March 16]. Available from: [Google Scholar]
- Zingales B., Andrade S.G., Briones M.R.S., Campbell D.A., Chiari E., Fernandes O. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/S0074-02762009000700021. [DOI] [PubMed] [Google Scholar]